Metabolite Diversity in Pulp Segments, Peel, Leaves, and Bark of a Red-Fleshed ‘Baya Marisa’ Apple Cultivar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction of Individual Sugars and Organic Acids
2.3. HPLC Analysis of Individual Sugars and Organic Acids
2.4. Extraction of Individual Phenolic Compounds
2.5. HPLC–MS Analysis of Individual Phenolic Compounds
2.6. Chemicals
2.7. Statistical Analysis
3. Results and Discussion
3.1. Sugars and Organic Acids
3.2. Identification of Individual Phenolic Compounds
3.3. Distribution of Phenolic Compounds in Different Tissues
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Crops and Livestock Products. Available online: https://www.fao.org/faostat/en/#data/QCL/visualize (accessed on 17 April 2023).
- Średnicka-Tober, D.; Barański, M.; Kazimierczak, R.; Ponder, A.; Kopczyńska, K.; Hallmann, E. Selected Antioxidants in Organic vs. Conventionally Grown Apple Fruits. Appl. Sci. 2020, 10, 2997. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef]
- Bars-Cortina, D.; Macià, A.; Iglesias, I.; Romero, M.P.; Motilva, M.J. Phytochemical Profiles of New Red-Fleshed Apple Varieties Compared with Traditional and New White-Fleshed Varieties. J. Agric. Food Chem. 2017, 65, 1684–1696. [Google Scholar] [CrossRef]
- Jakopic, J.; Schmitzer, V.; Veberic, R.; Smrke, T.; Stampar, F. Metabolic Response of ‘Topaz’ Apple Fruit to Minimal Application of Nitrogen during Cell Enlargement Stage. Horticulturae 2021, 7, 266. [Google Scholar] [CrossRef]
- Hyson, D.A. A Comprehensive Review of Apples and Apple Components and Their Relationship to Human Health. Adv. Nutr. Int. Rev. J. 2011, 2, 408–420. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wei, Z.; Ma, F. The effects of fruit bagging on levels of phenolic compounds and expression by anthocyanin biosynthetic and regulatory genes in red-fleshed apples. Process. Biochem. 2015, 50, 1774–1782. [Google Scholar] [CrossRef]
- Shih, P.-H.; Chan, Y.-C.; Liao, J.-W.; Wang, M.-F.; Yen, G.-C. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. J. Nutr. Biochem. 2010, 21, 598–605. [Google Scholar] [CrossRef]
- Bizjak, J.; Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R. Changes in Primary Metabolites and Polyphenols in the Peel of “Braeburn” Apples (Malus domestica Borkh.) during Advanced Maturation. J. Agric. Food Chem. 2013, 61, 10283–10292. [Google Scholar] [CrossRef]
- Iglesias, I.; Echeverría, G.; Soria, Y. Differences in fruit colour development, anthocyanin content, fruit quality and consumer acceptability of eight ‘Gala’ apple strains. Sci. Hortic. 2008, 119, 32–40. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Stampar, F.; Veberic, R. Composition of Sugars, Organic Acids, and Total Phenolics in 25 Wild or Cultivated Berry Species. J. Food Sci. 2012, 77, C1064–C1070. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Stampar, F.; Veberic, R. Parameters of inner quality of the apple scab resistant and susceptible apple cultivars (Malus domestica Borkh.). Sci. Hortic. 2007, 114, 37–44. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Slatnar, A.; Schmitzer, V.; Stampar, F.; Veberic, R.; Koron, D. Chemical profile of black currant fruit modified by different degree of infection with black currant leaf spot. Sci. Hortic. 2013, 150, 399–409. [Google Scholar] [CrossRef]
- Medic, A.; Zamljen, T.; Grohar, M.C.; Slatnar, A.; Hudina, M.; Veberic, R. Using HPLC–MS/MS to Assess the Quality of Beet, Mizuna, Lettuce and Corn Salad after Juglone and Walnut Leaf Extract Treatments. Agronomy 2022, 12, 347. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Cheng, L. Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol. Plant. 2012, 148, 9–24. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Corollaro, M.L.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet taste in apple: The role of sorbitol, individual sugars, organic acids and volatile compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [Green Version]
- Cebulj, A.; Cunja, V.; Mikulic-Petkovsek, M.; Veberic, R. Importance of metabolite distribution in apple fruit. Sci. Hortic. 2017, 214, 214–220. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ‘Honeycrisp’ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Ma, B.; Chen, J.; Zheng, H.; Fang, T.; Ogutu, C.; Li, S.; Han, Y.; Wu, B. Comparative assessment of sugar and malic acid composition in cultivated and wild apples. Food Chem. 2015, 172, 86–91. [Google Scholar] [CrossRef]
- Hecke, K.; Herbinger, K.; Veberič, R.; Trobec, M.; Toplak, H.; Štampar, F.; Keppel, H.; Grill, D. Sugar-, acid- and phenol contents in apple cultivars from organic and integrated fruit cultivation. Eur. J. Clin. Nutr. 2006, 60, 1136–1140. [Google Scholar] [CrossRef] [Green Version]
- Dražeta, L.; Lang, A.; Hall, A.J.; Volz, R.K.; Jameson, P.E. Air volume measurement of ‘Braeburn’ apple fruit. J. Exp. Bot. 2004, 55, 1061–1069. [Google Scholar] [CrossRef] [Green Version]
- Etienne, A.; Génard, M.; Lobit, P.; Mbeguié-A-Mbéguié, D.; Bugaud, C. What controls fleshy fruit acidity? A review of malate and citrate accumulation in fruit cells. J. Exp. Bot. 2013, 64, 1451–1469. [Google Scholar] [CrossRef] [Green Version]
- De Souza, L.M.; Cipriani, T.R.; Iacomini, M.; Gorin, P.A.; Sassaki, G.L. HPLC/ESI-MS and NMR analysis of flavonoids and tannins in bioactive extract from leaves of Maytenus ilicifolia. J. Pharm. Biomed. Anal. 2008, 47, 59–67. [Google Scholar] [CrossRef]
- Bystrom, L.M.; Lewis, A.B.; Brown, D.L.; Rodriguez, E.; Obendorf, R.L. Characterisation of phenolics by LC–UV/Vis, LC–MS/MS and sugars by GC in Melicoccus bijugatus Jacq. ‘Montgomery’ fruits. Food Chem. 2008, 111, 1017–1024. [Google Scholar] [CrossRef] [Green Version]
- Jiménez, V.M.; Gruschwitz, M.; Schweiggert, R.M.; Carle, R.; Esquivel, P. Identification of phenolic compounds in soursop (Annona muricata) pulp by high-performance liquid chromatography with diode array and electrospray ionization mass spectrometric detection. Food Res. Int. 2014, 65, 42–46. [Google Scholar] [CrossRef]
- Sánchez-Rabaneda, F.; Jáuregui, O.; Lamuela-Raventós, R.M.; Viladomat, F.; Bastida, J.; Codina, C. Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun. Mass Spectrom. 2004, 18, 553–563. [Google Scholar] [CrossRef]
- Schieber, A.; Berardini, N.; Carle, R. Identification of Flavonol and Xanthone Glycosides from Mango (Mangifera indica L. Cv. “Tommy Atkins”) Peels by High-Performance Liquid Chromatography-Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 5006–5011. [Google Scholar] [CrossRef]
- Michodjehoun-Mestres, L.; Souquet, J.-M.; Fulcrand, H.; Bouchut, C.; Reynes, M.; Brillouet, J.-M. Monomeric phenols of cashew apple (Anacardium occidentale L.). Food Chem. 2009, 112, 851–857. [Google Scholar] [CrossRef]
- Vvedenskaya, I.O.; Rosen, R.T.; Guido, J.E.; Russell, D.J.; Mills, K.A.; Vorsa, N. Characterization of Flavonols in Cranberry (Vaccinium macrocarpon) Powder. J. Agric. Food Chem. 2003, 52, 188–195. [Google Scholar] [CrossRef]
- Navarro, M.; Moreira, I.; Arnaez, E.; Quesada, S.; Azofeifa, G.; Vargas, F.; Alvarado, D.; Chen, P. Polyphenolic Characterization and Antioxidant Activity of Malus domestica and Prunus domestica Cultivars from Costa Rica. Foods 2018, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Hu, X.; Chen, X.; Shi, S.; Jiang, X.; Liang, X.; Chen, W.; Zhang, S. Analysis and improved characterization of minor antioxidants from leaves of Malus doumeri using a combination of major constituents’ knockout with high-performance liquid chromatography–diode array detector–quadrupole time-of-flight tandem mass spectrometry. J. Chromatogr. A 2015, 1398, 57–65. [Google Scholar] [CrossRef]
- Lin, L.-Z.; Harnly, J.M. A Screening Method for the Identification of Glycosylated Flavonoids and Other Phenolic Compounds Using a Standard Analytical Approach for All Plant Materials. J. Agric. Food Chem. 2007, 55, 1084–1096. [Google Scholar] [CrossRef] [Green Version]
- Sanoner, P.; Guyot, S.; Marnet, N.; Molle, D.; Drilleau, J.-F. Polyphenol Profiles of French Cider Apple Varieties (Malus domestica sp.). J. Agric. Food Chem. 1999, 47, 4847–4853. [Google Scholar] [CrossRef]
- Flores, P.; Hernández, V.; Hellín, P.; Fenoll, J.; Cava, J.; Mestre, T.; Martínez, V. Metabolite profile of the tomato dwarf cultivar Micro-Tom and comparative response to saline and nutritional stresses with regard to a commercial cultivar. J. Sci. Food Agric. 2015, 96, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Cádiz-Gurrea, M.D.L.L.; Borrás-Linares, I.; Lozano-Sánchez, J.; Joven, J.; Fernández-Arroyo, S.; Segura-Carretero, A. Cocoa and Grape Seed Byproducts as a Source of Antioxidant and Anti-Inflammatory Proanthocyanidins. Int. J. Mol. Sci. 2017, 18, 376. [Google Scholar] [CrossRef] [Green Version]
- Wojdyło, A.; Oszmiański, J. Antioxidant Activity Modulated by Polyphenol Contents in Apple and Leaves during Fruit Development and Ripening. Antioxidants 2020, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Withouck, H.; Boeykens, A.; Luyten, W.; Lavigne, R.; Wagemans, J.; Broucke, M.V. Phenolic Composition, Antimicrobial and Antioxidant Properties of Belgian Apple Wood Extracts. J. Biol. Act. Prod. Nat. 2019, 9, 24–38. [Google Scholar] [CrossRef]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of phenolic compounds vs. variety, part of apple and cultivation model, extraction of phenolic compounds, biological properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Slatnar, A.; Stampar, F.; Veberic, R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef]
- Pontais, I.; Treutter, D.; Paulin, J.-P.; Brisset, M.-N. Erwinia amylovora modifies phenolic profiles of susceptible and resistant apple through its type III secretion system. Physiol. Plant. 2008, 132, 262–271. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Hammam, W.E.; El-Mahdy El-Tantawi, M.; Yassin, N.A.Z.; Kirollos, F.N.; Abdelhameed, M.F.; Abdelfattah, M.A.O.; Wink, M.; Sobeh, M. Apple leaves and their major secondary metabolite phlorizin exhibit distinct neuroprotective activities: Evidence from in vivo and in silico studies. Arab. J. Chem. 2021, 14, 103188. [Google Scholar] [CrossRef]
- Adamcová, A.; Horna, A.; Šatínský, D. Determination of Phloridzin and Other Phenolic Compounds in Apple Tree Leaves, Bark, and Buds Using Liquid Chromatography with Multilayered Column Technology and Evaluation of the Total Antioxidant Activity. Pharmaceuticals 2022, 15, 244. [Google Scholar] [CrossRef] [PubMed]
- Gosch, C.; Halbwirth, H.; Stich, K. Phloridzin: Biosynthesis, distribution and physiological relevance in plants. Phytochemistry 2010, 71, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Gosch, C.; Halbwirth, H.; Kuhn, J.; Milosic, S.; Stich, K. Biosynthesis of phloridzin in apple (Malus domestica Borkh.). Plant Sci. 2009, 176, 223–231. [Google Scholar] [CrossRef]
- Ehrenkranz, J.R.L.; Lewis, N.G.; Kahn, C.R.; Roth, J. Phlorizin: A review. Diabetes/Metab. Res. Rev. 2005, 21, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Liaudanskas, M.; Viškelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic Composition and Antioxidant Activity of Malus domestica Leaves. Sci. World J. 2014, 2014, 306217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picinelli, A.; Dapena, E.; Mangas, J.J. Polyphenolic Pattern in Apple Tree Leaves in Relation to Scab Resistance. A Preliminary Study. J. Agric. Food Chem. 1995, 43, 2273–2278. [Google Scholar] [CrossRef] [Green Version]
- Slatnar, A.; Mikulic Petkovsek, M.; Halbwirth, H.; Stampar, F.; Stich, K.; Veberic, R. Polyphenol metabolism of developing apple skin of a scab resistant and a susceptible apple cultivar. Trees 2012, 26, 109–119. [Google Scholar] [CrossRef]
- Petkovsek, M.M.; Stampar, F.; Veberic, R. Seasonal changes in phenolic compounds in the leaves of scab-resistant and susceptible apple cultivars. Can. J. Plant Sci. 2009, 89, 745–753. [Google Scholar] [CrossRef]
- Buer, C.S.; Muday, G.K.; Djordjevic, M.A. Flavonoids Are Differentially Taken Up and Transported Long Distances in Arabidopsis. Plant Physiol. 2007, 145, 478–490. [Google Scholar] [CrossRef] [Green Version]
- Gaucher, M.; de Bernonville, T.D.; Lohou, D.; Guyot, S.; Guillemette, T.; Brisset, M.-N.; Dat, J.F. Histolocalization and physico-chemical characterization of dihydrochalcones: Insight into the role of apple major flavonoids. Phytochemistry 2013, 90, 78–89. [Google Scholar] [CrossRef]
- Bujdei, A.; Ciceoi, R.; Stănică, F. The behavior of gala, jonagold, golden delicious and granny smith apple varieties in organic farming system. Sci. Pap.-Ser. B Hortic. 2018, 62, 191–195. [Google Scholar]
- Juhart, J.; Medic, A.; Veberic, R.; Hudina, M.; Jakopic, J.; Stampar, F. Phytochemical Composition of Red-Fleshed Apple Cultivar ‘Baya Marisa’ Compared to Traditional, White-Fleshed Apple Cultivar ‘Golden Delicious’. Horticulturae 2022, 8, 811. [Google Scholar] [CrossRef]
- Makarova, E.; Górnaś, P.; Konrade, I.; Tirzite, D.; Cirule, H.; Gulbe, A.; Pugajeva, I.; Seglina, D.; Dambrova, M. Acute anti-hyperglycaemic effects of an unripe apple preparation containing phlorizin in healthy volunteers: A preliminary study. J. Sci. Food Agric. 2014, 95, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Amogne, N.Y.; Ayele, D.W.; Tsigie, Y.A. Recent advances in anthocyanin dyes extracted from plants for dye sensitized solar cell. Mater. Renew. Sustain. Energy 2020, 9, 23. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Zhang, M.; Zhang, G.; Li, P.; Ma, F. Differential Regulation of Anthocyanin Synthesis in Apple Peel under Different Sunlight Intensities. Int. J. Mol. Sci. 2019, 20, 6060. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Che, F.; Wang, L.; Meng, R.; Zhang, X.; Zhao, Z. Fruit Coloration and Anthocyanin Biosynthesis after Bag Removal in Non-Red and Red Apples (Malus × domestica Borkh.). Molecules 2013, 18, 1549–1563. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.; Carle, R. Chemical quality parameters and anthocyanin pattern of red-fleshed Weirouge apples. J. Appl. Bot. Food Qual. 2006, 80, 82–87. [Google Scholar]
- He, Y.; Hu, Y.; Jiang, X.; Chen, T.; Ma, Y.; Wu, S.; Sun, J.; Jiao, R.; Li, X.; Deng, L.; et al. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells. J. Photochem. Photobiol. B Biol. 2017, 177, 24–31. [Google Scholar] [CrossRef]
- Brunetti, C.; Sebastiani, F.; Tattini, M. Review: ABA, flavonols, and the evolvability of land plants. Plant Sci. 2018, 280, 448–454. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Bat, K.B.; Vodopivec, B.M.; Eler, K.; Ogrinc, N.; Mulič, I.; Masuero, D.; Vrhovšek, U. Primary and secondary metabolites as a tool for differentiation of apple juice according to cultivar and geographical origin. LWT 2018, 90, 238–245. [Google Scholar] [CrossRef]
- Ngadze, E.; Icishahayo, D.; Coutinho, T.A.; van der Waals, J.E.; Insinga, J.K.; Alyokhin, A.; Hao, J.; Ge, T.; Marangoni, N.F.; Baron, A.; et al. Role of Polyphenol Oxidase, Peroxidase, Phenylalanine Ammonia Lyase, Chlorogenic Acid, and Total Soluble Phenols in Resistance of Potatoes to Soft Rot. Plant Dis. 2012, 96, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, S.; Cao, J.; Feng, Q.; Peng, J.; Hu, Y. Roles of chlorogenic acid on regulating glucose and Lipids Metabolism: A Review. Evid.-Based Complement. Altern. Med. 2013, 11, 801457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adyanthaya, I.; Kwon, Y.I.; Apostolidis, E.; Shetty, K. Health benefits of apple phenolics from postharvest stages for potential type 2 diabetes management using in vitro models. J. Food Biochem. 2008, 34, 31–49. [Google Scholar] [CrossRef]


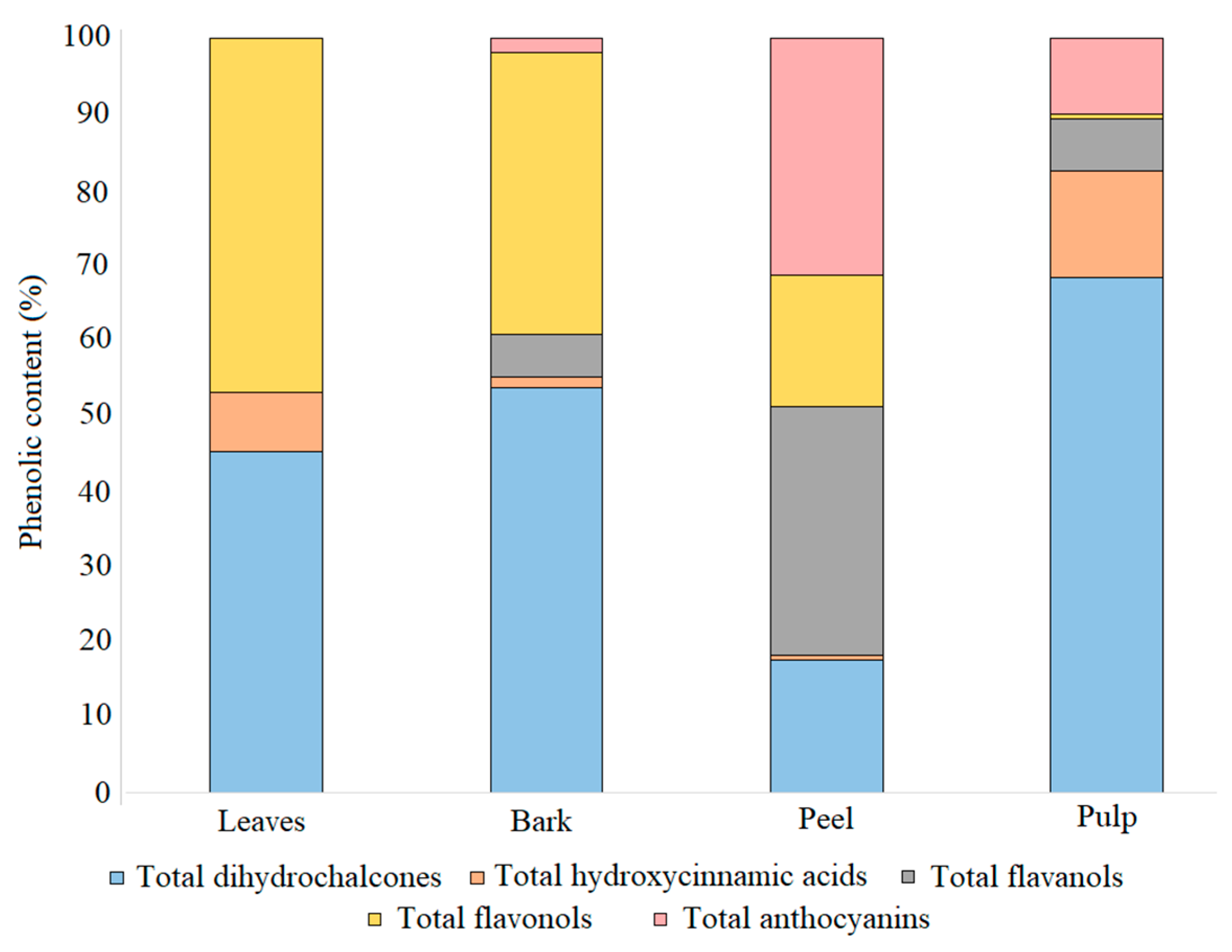
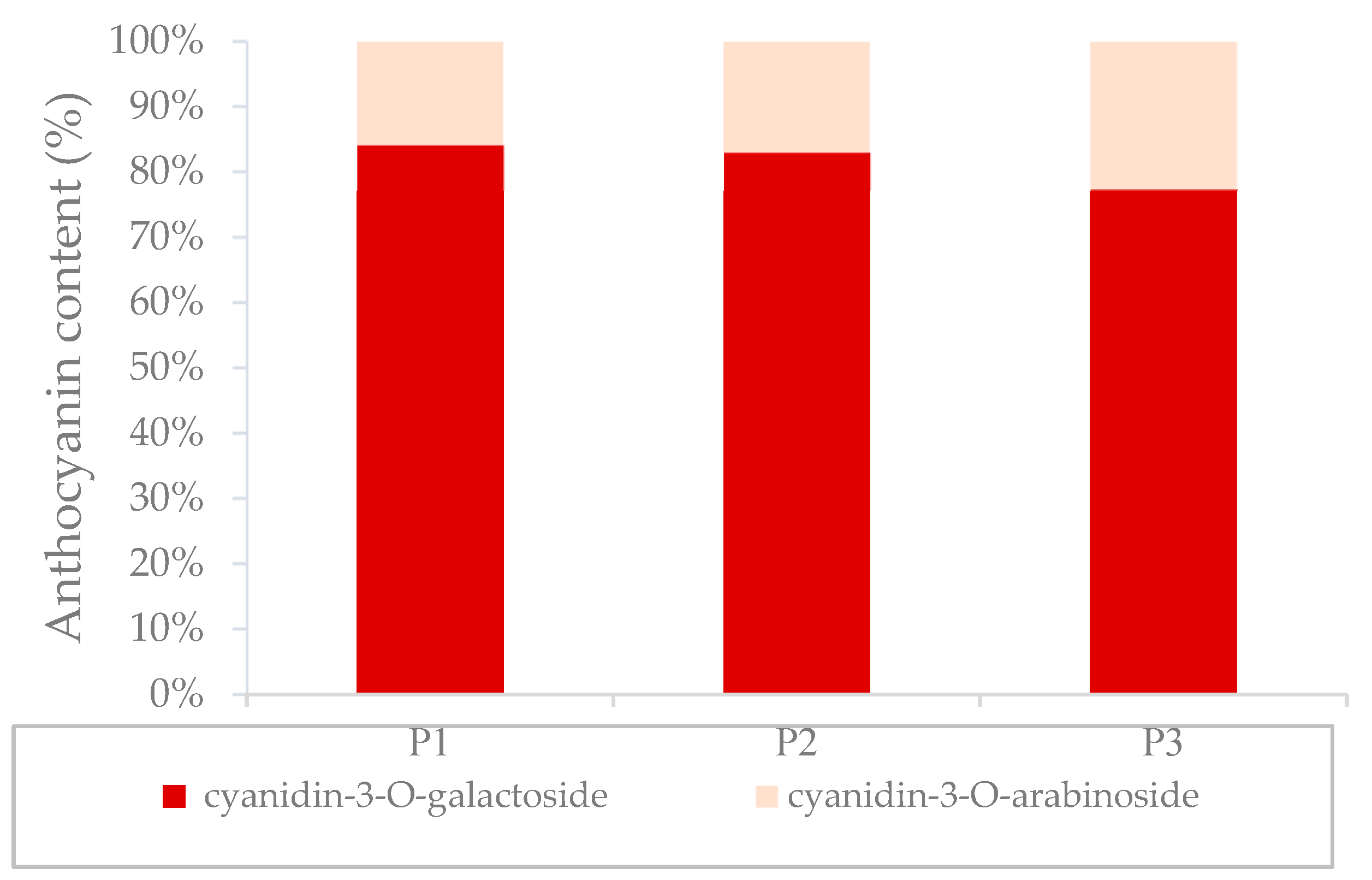
| Compound | P1 | P2 | P3 | |
|---|---|---|---|---|
| sugars | fructose | 55 ± 5 ab * | 62 ± 3 b | 52 ± 4 a |
| glucose | 21 ± 2 a | 25 ± 2 b | 24 ± 2 b | |
| sucrose | 14 ± 2 a | 18 ± 2 ab | 21 ± 4 b | |
| sorbitol | 4 ± 0.5 a | 4 ± 0.3 a | 5 ± 0.8 b | |
| sum of sugars | 96 ± 6 a | 108 ± 5 b | 105 ± 3 b | |
| organic acids | citric acid | 1.9 ± 0.03 b | 1.2 ± 0.10 a | 1.1 ± 0.1 a |
| malic acid | 12 ± 0.4 a | 13.1 ± 0.1 a | 14 ± 0.4 a | |
| sum of acids | 15 ± 1 a | 15 ± 1 a | 16 ± 2 a |
| Phenolic Compounds | Rt | [M − H]− | MS2 | MS3 | Expressed as | ||||
|---|---|---|---|---|---|---|---|---|---|
| (Min) | [M − H]+ (m/z) | (m/z) | (m/z) | Pulp | Peel | Leaves | Bark | ||
| cyanidin-3-O-galactoside | 8.93 | 449 | 287 | cyanidin-3-O-galactoside | X * | X | X | X | |
| p-coumaric acid hexoside derivative | 11.2 | 371 | 325, 163 | p-coumaric acid | X | ||||
| p-coumaric acid derivative 1 | 11.91 | 325 | 163, 145, 187 | p-coumaric acid | X | ||||
| cyanidin-3-O-arabinoside | 11.93 | 419 | 287 | cyanidin-arabinoside | X | X | X | X | |
| neochlorogenic acid | 12.43 | 353 | 191, 179 | neochlorogenic acid | X | ||||
| p-coumaric acid hexoside | 13.10 | 325 | 265, 235, 163 | p-coumaric acid | X | ||||
| chlorogenic acid | 13.12 | 353 | 191, 179 | chlorogenic acid | X | X | |||
| p-coumaric acid derivative 2 | 13.12 | 435 | 389 | 163, 179, 119 | p-coumaric acid | X | X | X | |
| p-coumaric acid derivative 3 | 13.12 | 435 | 389 | 163, 179, 119 | p-coumaric acid | X | |||
| procyanidin dimer | 13.80 | 577 | 451, 425, 407 | 289, 245 | procyanidin B1 | X | X | ||
| (-)epicatechin | 14.65 | 289 | 245, 205, 179 | epicatechin | X | X | X | ||
| myricetin hexoside | 14.90 | 479 | 317, 316 | myricetin-rhamnoside | X | ||||
| procyanidin trimer | 15.84 | 865 | 739, 695, 577 | procyanidin B1 | X | X | |||
| 4-p-coumaroylquinic acid | 15.97 | 337 | 191, 173, 163 | p-coumaric acid | X | X | |||
| myricetin pentoside | 17.38 | 463 | 317, 316 | myricetin-rhamnoside | X | ||||
| eriodictyol hexoside | 19.41 | 449 | 287 | kaempferol-3-O-galactoside | X | ||||
| (epi)catechin derivative | 19.70 | 583 | 289, 271 | 245, 205, 179 | epicatechin | X | |||
| quercetin-3-O-rutinoside | 19.72 | 609 | 301 | quercetin-3-O-rutinoside | X | X | |||
| quercetin-3-O-galactoside | 20.52 | 463 | 301 | quercetin-3-O-galactoside | X | X | X | ||
| quercetin-3-O-glucoside | 20.80 | 463 | 301 | quercetin-3-O-glucoside | X | X | X | ||
| (epi)catechin isomer | 20.93 | 289 | 245, 205, 179 | (-)epicatechin | X | ||||
| p-coumaric acid derivative 4 | 21.29 | 497 | 453, 325, 235, 163 | p-coumaric acid | X | ||||
| phloretin-2-O-xyloside | 21.54 | 567 | 273, 167 | phloridzin | X | ||||
| quercetin-3-O-xyloside | 21.59 | 433 | 301 | quercetin-3-O-xyloside | X | X | X | ||
| quercetin-3-O-arabinopyranoside | 21.80 | 433 | 301 | quercetin-3-O-arabinopyranoside | X | X | |||
| kaempferol derivative | 21.89 | 477 | 285, 284 | kaempferol-3-O-galactoside | X | ||||
| quercetin-3-O-arabinofuranoside | 22.40 | 433 | 301 | quercetin-3-O-arabinofuranoside | X | X | X | ||
| quercetin-3-O-rhamnoside | 22.50 | 447 | 301 | quercetin-3-O-rhamnoside | X | X | |||
| phloridzin | 23.33 | 481 | 435, 273 | phloridzin | X | X | X | X | |
| kaempferol-3-O-rhamnoside | 24.20 | 447 | 285 | kaempferol-3-O-galactoside | X | X | |||
| phloretin diglucoside | 29.33 | 597 | 273 | phloridzin | X | ||||
| phloretin | 30.12 | 273 | 167,179 | phloridzin | X | X | |||
| phloretin derivative 1 | 29.79 | 571 | 409 | 273 | phloridzin | X | X | ||
| phloretin derivative 2 | 30.63 | 581 | 419 | 273 | phloridzin | X | X |
| Phenolic Group | Compound | P1 | P2 | P3 | Peel |
|---|---|---|---|---|---|
| hydroxycinnamic acids | chlorogenic acid | 160 ± 1 a * | 189 ± 8 b | 169 ± 4 ab | ND |
| p-coumaric acid derivative | 0.82 ± 0.04 a | 0.87 ± 0.1 a | 1 ± 0.1 a | ND | |
| p-coumaric acid derivative | 1.4 ± 0.03 a | 1.3 ± 0.1 a | 1.1 ± 0.1 a | ND | |
| p-coumaric acid hexoside derivative | ND | ND | ND | 12 ± 1 | |
| 4-p-coumaroylquinic acid | 1.5 ± 0.1 a | 1.7 ± 0.1 a | 1.8 ± 0.1 a | ND | |
| flavanols | epicatechin isozyme | 17 ± 0.6 b | 12 ± 0.4 a | 10 ± 1.3 a | ND |
| Epicatechin | 29 ± 2 a | 16 ± 1 a | 6± 0.4 a | 309 ± 10 b | |
| epicatechin derivative | ND | ND | ND | 24 ± 2 | |
| procyanidin dimer | ND | ND | ND | 320 ± 8 | |
| procyanidin trimer | ND | ND | ND | 147 ± 6 | |
| flavonols | quercetin-3-O-arabinofuranoside | ND | ND | ND | 40 ± 3 |
| quercetin-3-O-glucoside | ND | ND | ND | 31 ± 3 | |
| quercetin-3-O-rhamnoside | 1.4 ± 0.03 a | 0.9 ± 0.03 a | 0.5 ± 0.02 a | 34 ± 2 b | |
| quercetin-3-O-arabinopyranoside | ND | ND | ND | 9 ± 1 | |
| quercetin-3-O-galactoside | ND | ND | ND | 82 ± 9 | |
| quercetin-3-O-rutinoside | ND | ND | ND | 23 ± 2 | |
| quercetin-3-O-xyloside | ND | ND | ND | 26 ± 3 | |
| anthocyanins | cyanidin-3-O-arabinoside | 11 ± 0.4 a | 3.2 ± 0.5 a | 2.2 ± 0.2 a | 74 ± 7 b |
| cyanidin-3-O-galactoside | 64 ± 0.7 a | 23 ± 3 a | 8 ± 0.7 a | 492 ± 45 b | |
| dihydrochalcones | phloretin | ND | ND | ND | 1.3 ± 0.09 |
| phloretin glucoside | 9 ± 0.2 a | 10 ± 0.3 a | 20 ± 2 b | ND | |
| phloridzin | 11 ± 0.2 a | 14 ± 1 a | 15 ± 0.6 a | 197 ± 20 b | |
| total dihydrochalcones | 21 ± 0.8 a | 28 ± 2 a | 46 ± 3 a | 339 ± 17 b | |
| total hydroxycinnamic acids | 164 ± 0.7 b | 182 ± 9 b | 172 ± 4 b | 11 ± 0.1 a | |
| total flavanols | 41 ± 2 a | 28 ± 2 a | 15 ± 0.7 a | 640 ± 11 b | |
| total flavonols | 2.7 ± 0.1 a | 1.8 ± 0.1 a | 1.7 ± 0.1 a | 337 ± 19 b | |
| total anthocyanins | 82 ± 1 a | 31 ± 2 a | 10 ± 0.7 a | 608 ± 60 b | |
| TAPC | 302 ± 3 a | 271 ± 3 a | 245 ± 3 a | 1934 ± 43 b |
| Phenolic Group | Compound | Leaves | Bark |
|---|---|---|---|
| hydroxycinnamic acids | chlorogenic acid | 67 ± 6 | ND * |
| p-coumaric acid derivative | 1076 ± 108 | 50 ± 5 | |
| p-coumaric acid derivative | 20.2 ± 0.9 | ND | |
| p-coumaric acid hexoside | ND | 36 ± 3 | |
| p-coumaric acid derivative | ND | 50± 5 | |
| neochlorogenic acid | 178.84 ± 11.91 | ND | |
| 4-p-coumaroylquinic acid | ND | 108 ± 9 | |
| flavanols | Epicatechin | ND | 320 ± 12 |
| procyanidin dimer | ND | 572 ± 27 | |
| procyanidin trimer | ND | 196 ± 14 | |
| flavanones | eriodictyol hexoside | ND | 226 ± 18 |
| flavonols | kaempferol derivative | ND | 1292 ± 53 |
| kaempferol-3-O-rhamnoside | 119.31 ± 7.10 | 65 ± 2 | |
| myricetin hexoside | ND | 245 ± 15 | |
| myricetin pentoside | ND | 261 ± 15 | |
| quercetin-3-O-arabinofuranoside | 841 ± 79 | 930 ± 163 | |
| quercetin-3-O-glucoside | 1289 ± 90 | 234 ± 16 | |
| quercetin-3-O-rhamnoside | 2310 ± 212 | 978 ± 16 | |
| quercetin-3-O-arabinopyranoside | ND | 499 ± 17 | |
| quercetin-3-O-galactoside | 2238 ± 149 | 1130 ± 240 | |
| quercetin-3-O-rutinoside | 1075 ± 122 | ND | |
| quercetin-3-O-xyloside | 175 ± 17 | 449 ± 24 | |
| anthocyanins | cyanidin-3-O-arabinoside | 2.7 ± 0.3 | 34 ± 3 |
| cyanidin-3-O-galactoside | 19 ± 3 | 344 ± 22 | |
| peonidin-3-O-galactoside | 0.64 ± 0.12 | 24 ± 2 | |
| dihydrochalcones | phloretin | 181 ± 15 | 179 ± 17 |
| phloretin derivative | 389 ± 18 | 76 ± 13 | |
| phloretin derivative | 63 ± 1 | ND | |
| phloretin diglucoside | ND | 240 ± 25 | |
| phloridzin | 7468 ± 88 | 8318 ± 61 | |
| total dihydrochalcones | 8039 ± 97 | 9031 ± 129 | |
| total hydroxycinnamic acids | 1421 ± 47 | 220 ± 6 | |
| total flavanols | ND | 959 ± 7 | |
| total flavonols | 8345 ± 285 | 6278 ± 192 | |
| total anthocyanins | 20.1 ± 0.6 | 339 ± 26 | |
| TAPC | 17,825 ± 178 | 16,828 ± 154 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schmitzer, V.; Medic, A.; Bordon, A.; Hudina, M.; Veberic, R.; Jakopic, J.; Stampar, F. Metabolite Diversity in Pulp Segments, Peel, Leaves, and Bark of a Red-Fleshed ‘Baya Marisa’ Apple Cultivar. Agriculture 2023, 13, 1564. https://doi.org/10.3390/agriculture13081564
Schmitzer V, Medic A, Bordon A, Hudina M, Veberic R, Jakopic J, Stampar F. Metabolite Diversity in Pulp Segments, Peel, Leaves, and Bark of a Red-Fleshed ‘Baya Marisa’ Apple Cultivar. Agriculture. 2023; 13(8):1564. https://doi.org/10.3390/agriculture13081564
Chicago/Turabian StyleSchmitzer, Valentina, Aljaz Medic, Aleks Bordon, Metka Hudina, Robert Veberic, Jerneja Jakopic, and Franci Stampar. 2023. "Metabolite Diversity in Pulp Segments, Peel, Leaves, and Bark of a Red-Fleshed ‘Baya Marisa’ Apple Cultivar" Agriculture 13, no. 8: 1564. https://doi.org/10.3390/agriculture13081564
APA StyleSchmitzer, V., Medic, A., Bordon, A., Hudina, M., Veberic, R., Jakopic, J., & Stampar, F. (2023). Metabolite Diversity in Pulp Segments, Peel, Leaves, and Bark of a Red-Fleshed ‘Baya Marisa’ Apple Cultivar. Agriculture, 13(8), 1564. https://doi.org/10.3390/agriculture13081564









