Study on Revealing Peanut-Related Disease Prevention Gene Clusters via Whole Transcriptome Sequencing
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Determination of Chlorophyll Content
2.3. Determination of Soil Enzyme Activity
2.4. Transcriptome Sequencing of Peanut Root Tissue
2.5. Transcriptome Data Analysis Method
2.6. Statistical Analysis
3. Results
3.1. Picture of Peanut Plant Disease Resistance
3.2. Analysis of Chlorophyll and Protective Enzymes in Peanut Leaves
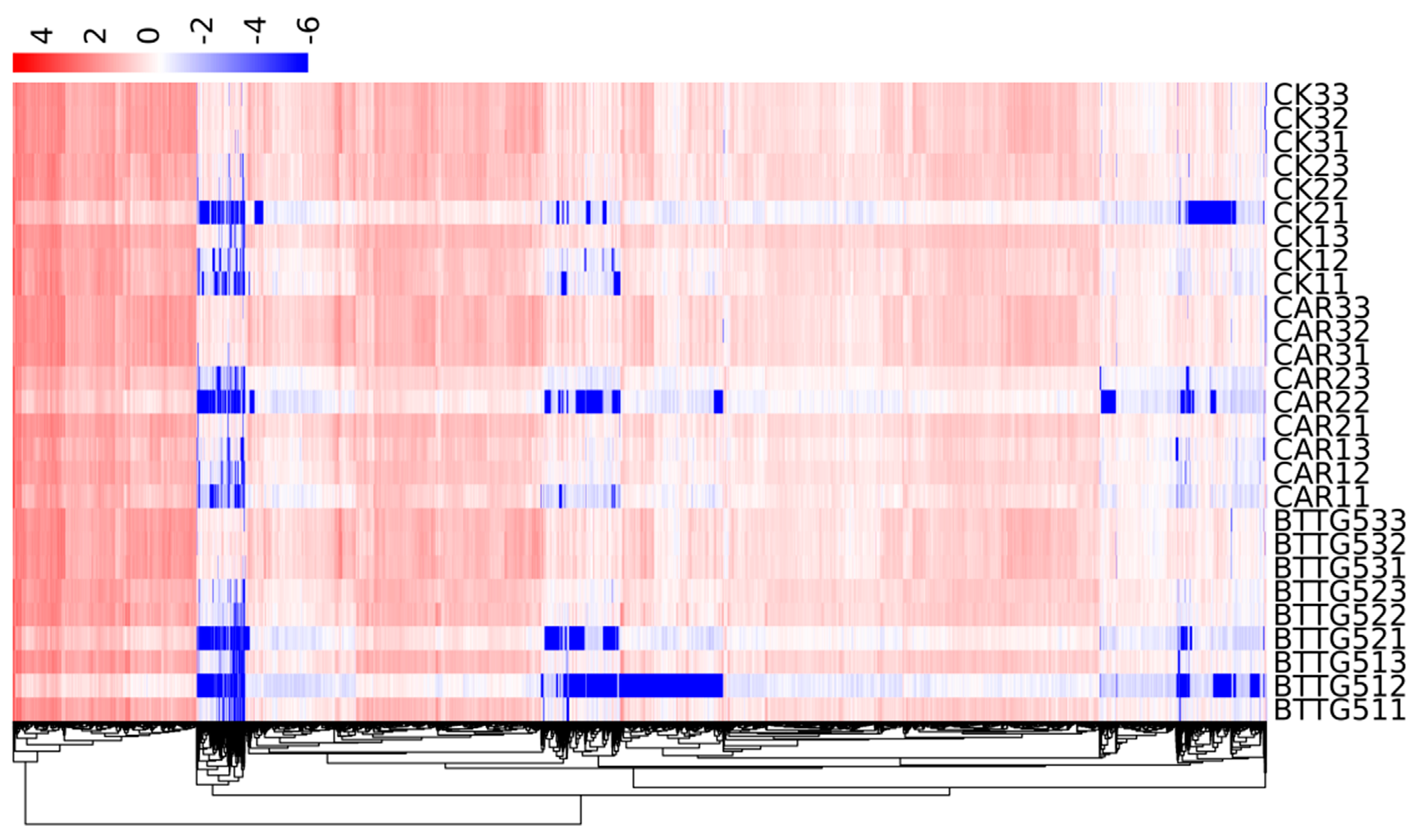
3.3. Sample Clustering and Principal Component Analysis
3.4. Gene Expression Level Analysis
3.5. Differential Gene Expression Cluster Analysis
3.6. Gene Ontology (GO) Analysis between TG5 Strains and Carbazim Treatment Groups
3.7. Gene Regulation Analysis in Biochemical Process
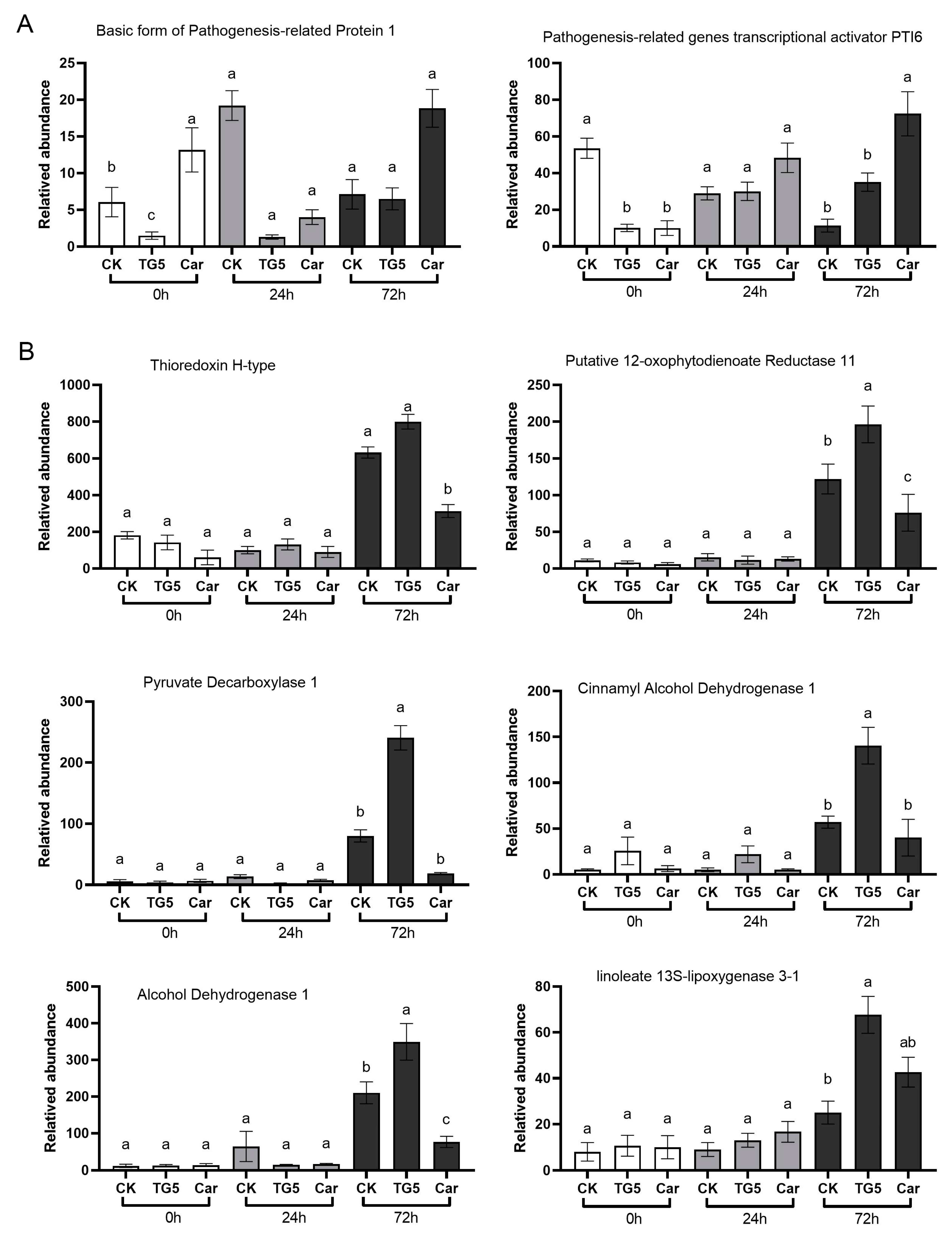
3.8. Pathogenesis-Related Regulatory Genes Analysis
3.9. TG5-Specific Expression Gene Analysis
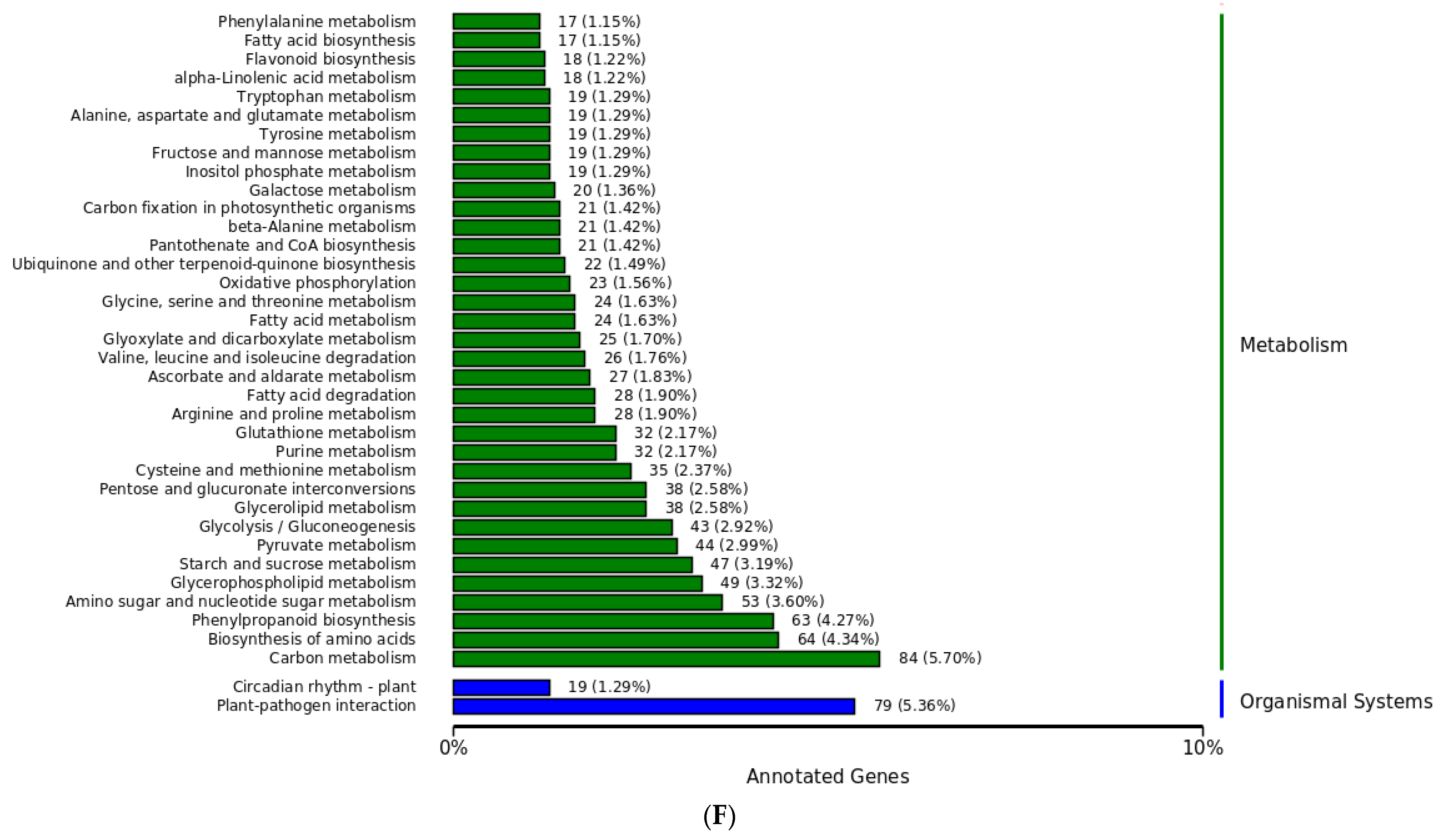
3.10. Difference Analysis in KEGG Metabolic Pathway between TG5 and Car Treatment Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Yuan, P.; Tanaka, K.; Poovaiah, B.W. Calcium/calmodulin-mediated defense signaling: What Is looming on the horizon for AtSR1/CAMTA3-mediated signaling in plant immunity. Front. Plant Sci. 2022, 12, 795353. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sun, J.; Zhang, X.; Ahmad, N.; Hou, L.; Zhao, C.; Pan, J.; Tian, R.; Wang, X.; Zhao, S. The Mechanisms Underlying Salt Resistance Mediated by Exogenous Application of 24-Epibrassinolide in Peanut. Int. J. Mol. Sci. 2022, 23, 6376. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Kumar, D.; Jain, M.; Chaudhary, P.; Deswal, R.; Sarin, N.B. Ectopic overexpression of a salt stress-induced pathogenesis-related class 10 protein (PR10) gene from peanut (Arachis hypogaea L.) affords broad spectrum abiotic stress tolerance in transgenic tobacco. Plant Cell Tissue Organ Cult. (PCTOC) 2012, 109, 19–31. [Google Scholar] [CrossRef]
- Senthilraja, G.; Anand, T.; Kennedy, J.; Raguchander, T.; Samiyappan, R. Plant growth promoting rhizobacteria (PGPR) and entomopathogenic fungus bioformulation enhance the expression of defense enzymes and pathogenesis-related proteins in groundnut plants against leafminer insect and collar rot pathogen. Physiol. Mol. Plant Pathol. 2013, 82, 10–19. [Google Scholar] [CrossRef]
- Kato, T.; Maeda, Y.; Hirukawa, T.; Namai, T.; Yoshioka, N. Lipoxygenase Activity Increment in Infected Tomato Leaves and Oxidation Product of Linolenic Acid by Its In Vitro Enzyme Reaction. Biosci. Biotechnol. Biochem. 1992, 56, 373–375. [Google Scholar] [CrossRef]
- An, J.-U.; Lee, I.-G.; Ko, Y.-J.; Oh, D.-K. Microbial Synthesis of Linoleate 9S-Lipoxygenase Derived Plant C18 Oxylipins from C18 Polyunsaturated Fatty Acids. J. Agric. Food Chem. 2019, 67, 3209–3219. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, C.; Yang, L.; Zhang, Y.; Wang, Y.; Fang, Z.; Lv, H. Genome-Wide Identification, Expression Profile of the TIFY Gene Family in Brassica oleracea var. capitata, and Their Divergent Response to Various Pathogen Infections and Phytohormone Treatments. Genes 2020, 11, 127. [Google Scholar] [CrossRef]
- Sen, S. Genome-wide TIFY family in Arachis hypogaeain the perspective of legume JAZs. Crop Sci. Biotechnol. 2022, 25, 465–488. [Google Scholar] [CrossRef]
- Zheng, L.L.; Wan, Q.; Wang, H.G.; Guo, C.L.; Niu, X.L.; Zhang, X.F.; Zhang, R.; Chen, Y.H.; Luo, K. Genome-wide identification and expression of TIFY family in cassava (Manihot esculenta Crantz). Front. Plant Sci. 2022, 13, 1017840. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Han, Z.; Chen, Y.; Huai, D.; Kang, Y.; Wang, Z.; Yan, L.; Jiang, H.; Lei, Y.; et al. Integrated Transcriptomics and Metabolomics Analysis Reveal Key Metabolism Pathways Contributing to Cold Tolerance in Peanut. Front. Plant Sci. 2021, 12, 752474. [Google Scholar] [CrossRef]
- Pramesti, I.; Shuhei, Y.; Pisanee, S. Comparative analysis of NADPH-Cytochrome P450 reductases from legumes for heterologous production of triterpenoids in transgenic saccharomyces cerevisiae. Front. Plant Sci. 2021, 12, 762546. [Google Scholar]
- Yoshimi, Y.; Ayaka, M. Functional characterization of nadph-cytochrome p450 reductase and cinnamic acid 4-hydroxylase encoding genes from Scoparia dulcis L. Bot. Stud. 2020, 61, 284. [Google Scholar]
- Muñoz, V.; Ibáñez, F.; Figueredo, M.S.; Fabra, A. An oxidative burst and its attenuation by bacterial peroxidase activity is required for optimal establishment of the Arachis hypogaea-Bradyrhizobium sp. symbiosis. J. Appl. Microbiol. 2016, 121, 244–253. [Google Scholar] [CrossRef]
- Radwan, D.E.M.; Fayez, K.A. Photosynthesis, antioxidant status and gas-exchange are altered by glyphosate application in peanut leaves. Photosynthetica 2016, 54, 307–316. [Google Scholar] [CrossRef]
- Monika, P.; Dhara, F.; Kumar, A.P. Silicon-induced mitigation of drought stress in peanut genotypes (Arachis hypogaea L.) through ion homeostasis, modulations of antioxidative defense system, and metabolic regulations. Plant Physiol. Biochem. PPB 2021, 166, 290–313. [Google Scholar]
- Raza, A.; Sharif, Y.; Chen, K.; Wang, L.; Fu, H.; Zhuang, Y.; Chitikineni, A.; Chen, H.; Zhang, C.; Varshney, R.K.; et al. Genome-Wide Characterization of Ascorbate Peroxidase Gene Family in Peanut (Arachis hypogea L.) Revealed Their Crucial Role in Growth and Multiple Stress Tolerance. Front. Plant Sci. 2022, 13, 962182. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Hou, L.; Ma, C.; Li, G.; Lin, R.; Zhao, Y.; Wang, X. Comparative transcriptome analysis of the peanut semi-dwarf mutant 1 reveals regulatory mechanism involved in plant height. Gene 2021, 791, 145722. [Google Scholar] [CrossRef]
- Zhang, R.; Jiang, Z.; Li, M.H.; Xi, P.G. Research progress on the pathogenic mechanism and related pathogenic genes of Fusarium oxysporum. Chin. J. Agric. 2011, 27, 254–258. [Google Scholar]
- Chen, P.; He, W.; Shen, Y.; Zhu, L.Y.; Yao, X.Z.; Sun, R.B.; Dai, C.C.; Sun, B.; Chen, Y. Interspecific neighbor stimulates peanut growth through modulating root endophytic microbial community construction. Front. Plant Sci. 2022, 13, 830666. [Google Scholar] [CrossRef]
- Basavaraju, S.; Devi, M.C. Mechanism of biological control of root rot of groundnut caused by Macrophomina phaseolina using Pseudomonas fluorescens. Indian Phytopathol. 2012, 65, 360–365. [Google Scholar]
- Kumar, C.J.; Priyanka, S.; Arpit, S. Microbial enzyme, 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase: An elixir for plant under stress. Physiol. Mol. Plant Pathol. 2021, 115, 101664. [Google Scholar]
- Torres-Cab, W.J.; Ruiz-Sanchez, E.; Reyes-Ramírez, A.; Lugo-García, G.A.; Tucuch-Haas, J.I.; Pierre, J.F. Field evaluation of microbial insecticides against fall armyworm, Spodoptera frugiperda (smith) and corn earworm, helicoverpa zea (boddie) (lepidoptera: Noctuidae), in maize. Arch. Phytopathol. Plant Prot. 2022, 55, 1713–1723. [Google Scholar] [CrossRef]
- Li, R.; Li, Y.; Kristiansen, K.; Wang, J. SOAP: Short oligonucleotide alignment program. Bioinformatics 2008, 24, 713–714. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.-C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat. Biotechnol. 2015, 33, 290–295. [Google Scholar] [CrossRef]
- Xu, J.X.; Li, Z.Y.; Lv, X.; Yan, H.; Zhou, G.-Y.; Cao, L.-X.; Yang, Q.; He, Y.-H. Isolation and characterization of Bacillus subtilis strain 1-L-29, an endophytic bacteria from Camellia oleifera with antimicrobial activity and efficient plant-root colonization. PLoS ONE 2020, 15, e0232096. [Google Scholar] [CrossRef]
- McKenna, A.; Hanna, M.; Banks, E.; Sivachenko, A.; Cibulskis, K.; Kernytsky, A.; Garimella, K.; Altshuler, D.; Gabriel, S.; Daly, M.; et al. The Genome Analysis Toolkit: A MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010, 20, 1297–1303. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Luo, X.; Wu, X.; Ren, J.; Huang, X.; Feng, S.; Lin, X.; Ren, M.; Dong, P. Antifungal activity and mechanism of thymol against Fusarium oxysporum, a pathogen of potato dry rot, and its potential application. Postharvest Biol. Technol. 2022, 192, 112025. [Google Scholar] [CrossRef]
- Zhu, H.; Jiang, Y.; Guo, Y.; Huang, J.B.; Zhou, M.H.; Tang, Y.Y.; Sui, J.M.; Wang, J.S.; Qiao, L.X. A novel salt inducible WRKY transcription factor gene, AhWRKY75, confers salt tolerance in transgenic peanut. Plant Physiol. Biochem. 2021, 160, 175–183. [Google Scholar] [CrossRef]
- Kurnool, K.; Gunupuru, L.R.; Merum, P.; Ambekar, N.K.; Vennapusa, A.R.; Uppala, L.; Boya, V.; Anthony Johnson, A.M.; Chinta, S. A Novel WRKY Transcription Factor, MuWRKY3 (Macrotyloma uniflorum Lam. Verdc.) Enhances Drought Stress Tolerance in Transgenic Groundnut (Arachis hypogaea L.) Plants. Front. Plant Sci. 2018, 9, 346. [Google Scholar]
- Xie, D.W.; Yang, X.; He, R.H.; Huo, H.; Ye, Z.C.; Ren, X.H.; Yuan, H.M.; Dai, Z.G.; Sun, J.; Su, J.G. Comprehensive analysis of the UDP glycosyltransferase gene family in flax [Linum usitatissimum L.] and functional verification of the role of LuUGT175 in the regulation of lignin biosynthesis. Ind. Crops Prod. 2022, 188, 115720. [Google Scholar] [CrossRef]
- Chávez, A.; Castillo, N.; López-Tubau, J.M.; Atanasov, K.E.; Fernandez-Crespo, E.; Camanes, G.; Altabella, T.; Ferrer, A. Tomato STEROL GLYCOSYL TRANSFERASE 1 silencing unveils a major role of steryl glycosides in plant and fruit development. Environ. Exp. Bot. 2023, 206, 105181. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Cui, H.; Yang, W.; Yu, B.; Zhang, C.; Wen, J.; Kang, J.; Wang, Z.; Yang, Q. The UDP-glycosyltransferase MtUGT84A1 regulates anthocyanin accumulation and plant growth via JA signaling in Medicago truncatula. Environ. Exp. Bot. 2022, 201, 104972. [Google Scholar] [CrossRef]
- Guo, F.; Zhu, X.; Zhao, C.; Zhao, S.; Pan, J.; Zhao, Y.; Wang, X.; Hou, L. Transcriptome Analysis and Gene Expression Profiling of the Peanut Small Seed Mutant Identified Genes Involved in Seed Size Control. Int. J. Mol. Sci. 2022, 23, 9726. [Google Scholar] [CrossRef] [PubMed]
- Gaobo, Y.; Qiusen, C.; Fengqiong, C. Glutathione Promotes Degradation and Metabolism of Residual Fungicides by Inducing UDP-Glycosyltransferase Genes in Tomato. Front. Plant Sci. 2022, 13, 893508. [Google Scholar]
- Yol, E.; Upadhyaya, H.D.; Uzun, B. Molecular diagnosis to identify new sources of resistance to sclerotinia blight in groundnut (Arachis hypogaea L.). Euphytica 2015, 203, 367–374. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Lu, N.; Li, C. Study on Revealing Peanut-Related Disease Prevention Gene Clusters via Whole Transcriptome Sequencing. Agriculture 2023, 13, 1608. https://doi.org/10.3390/agriculture13081608
Du H, Lu N, Li C. Study on Revealing Peanut-Related Disease Prevention Gene Clusters via Whole Transcriptome Sequencing. Agriculture. 2023; 13(8):1608. https://doi.org/10.3390/agriculture13081608
Chicago/Turabian StyleDu, Hongbo, Nan Lu, and Chuanrong Li. 2023. "Study on Revealing Peanut-Related Disease Prevention Gene Clusters via Whole Transcriptome Sequencing" Agriculture 13, no. 8: 1608. https://doi.org/10.3390/agriculture13081608
APA StyleDu, H., Lu, N., & Li, C. (2023). Study on Revealing Peanut-Related Disease Prevention Gene Clusters via Whole Transcriptome Sequencing. Agriculture, 13(8), 1608. https://doi.org/10.3390/agriculture13081608




