Abstract
This study examined the potential of using the endophytic bacteria Bacillus subtilis (10-4 and 26D) to enrich hydroponically grown potato seed minitubers (Solanum tuberosum L. cv. Bashkirsky) to improve plant growth, photosynthetic pigments, yield, and quality parameters, including nutritional value (i.e., macro-/microelements, vitamin C, anthocyanins). Potato seed minitubers, obtained from in-vitro-grown microplants in a hydroponic system, were inoculated with endophytic B. subtilis and subsequently grown in pots under controlled conditions. The results demonstrated the successful colonization of seed minitubers by B. subtilis, with subsequent distribution into growing plants (roots, shoots). The endophytes accelerated the plant’s phenological shifts, resulting in earlier emergence of sprouts, budding, and flowering compared with control plants. They also had increased leaf photosynthetic pigments (chlorophyll (Chl) a, Chl b, and carotenoids), total leaf area, and positively influenced leaf proline contents. The height of plants and number of stems per plant did not change significantly upon endophyte treatment, but improved root growth was observed throughout the experiment. As a result of endophyte application, there was an increase in stolon weight, number and size of tubers, and overall tuber yield. There were no significant differences in terms of total dry matter and starch content of the tubers compared to the control group, but the sugar levels decreased and the size of the starch grains was larger in endophyte-treated tubers. Furthermore, endophyte treatment resulted in an increased accumulation of nutrients including N, P, K, Cu, and Fe, as well as vitamin C and anthocyanins in harvested tubers. These findings indicate that colonization of hydroponically grown potato seed minitubers with endophytic B. subtilis (10-4 and 26D) before planting has great potential as an eco-friendly approach to obtain higher-quality seeds and to increase tuber yield and nutritional value in field conditions.
1. Introduction
Potato (Solanum tuberosum L., Solanaceae) is a major staple food crop, ranking fourth in global production after maize, wheat, and rice. It plays a crucial role in providing essential nutrition to millions of people worldwide [1]. Potato tubers are consumed primarily because they are a significant source of carbohydrates (primary starch), high-quality protein, macro- and microelements, and bioactive ingredients [2,3,4]. The world’s leaders in potato production are China, India, and Russia [4]. It is expected that the world’s population will grow, on average, by about 100 million people per year over the next two decades [1]. Ensuring food security for the current and future generations by increasing potato productivity and tuber quality while preserving natural resources is a significant challenge for the international community [1,2,3,4]. Potato production and product systems are currently hindered by various technical and developmental issues that impact potato crop productivity and tuber quality. Various biotic and abiotic factors and the use of low-quality planting material can adversely affect both potato yield and tuber quality [4,5]. Hence, the search for novel and efficient approaches to enhance plant resilience and boost crop productivity is a relevant task. Most studies on potatoes are based on crop yields of different varieties, disease resistance, and quality requirements for tuber processing. The potato industry has benefited from major recent discoveries in plant genetics, physiology, and pathology, which simplify the creation of improved varieties. However, many determinants of yield are still limited by seed quality [5]. The obligatory stage of potato seed production is the clonal micropropagation of plants free from all types of pathogens. This involves cultivating apical meristems in vitro and subsequently obtaining seed minitubers using aeroponic or hydroponic systems. These minitubers are then planted in fields to obtain pre-basic and basic seeds. However, despite being sterile and free from diseases, these seed minitubers quickly become populated by various soil microbes, including phytopathogens (i.e., disease-causing agents). This can harm plant physiology, tuber yield, and tuber quality, as well as post-harvest storage viability and market appearance [4,5]. Thus, the classical method of seed tuber production is insufficient to meet the needs of potato producers, as it forces them to use infected and degraded seed potatoes year after year [5]. High-quality tuber seeds should ensure the cultivation of healthy and robust crops throughout the growing season, as well as during post-harvest storage and subsequent generations. Therefore, it is crucial to prioritize the production of high-quality potato seeds and develop breeding methods that are both applicable and accessible to potato seed producers [5].
The colonization of plants with useful microflora is one of the most promising eco-friendly approaches to improve potato growth, development, and stress resilience. Several studies have documented the ability of rhizosphere plant-growth-promoting (PGP) bacteria and entomopathogenic fungi to stimulate potato growth and improve productivity, including within the seed production system [6,7,8,9,10,11,12,13]. It was reported that, at all stages of cultivation, including in vitro and in vivo, rhizobacteria had a positive effect on plant growth and adaptive capacity when planting microplants in non-sterile conditions ex vitro [14,15,16,17]. Rhizobacteria such as Azospirillum baldaniorum Sp245 [6] and Ochrobactrum cytisi IPA7.2 [7] have demonstrated growth-stimulating properties in both in vitro and ex vitro cultivation, leading to improved planting material. The efficacy of rhizobacteria application is high under optimal in vitro conditions but decreases when plants are grown in field conditions [16,17,18]. In such cases, the colonization of initially sterile seed minitubers by beneficial endophytic bacteria, such as Bacillus subtilis, may prove more effective. B. subtilis is generally recognized as a safe microorganism for use in the food industry and has been shown to improve and maintain seed quality for extended periods. The growth-promoting and protective effects of the endophytic bacteria B. subtilis have been well-documented in various plant species under different biotic and abiotic stresses [19,20,21,22,23,24]. Because they colonize and live within plant tissues, endophytes are less reliant on external environmental factors compared with rhizospheric microbial strains while also providing beneficial attributes to host plants [22,25,26,27]. Once implanted in plant tissues, endophytes can exert a prolonged influence on plant physiology throughout the entire growing season [28]. The positive effects of endophytic B. subtilis on the post-harvest physiology of various fruits and vegetables, including potatoes, have been well-documented [23,29,30,31,32]. Moreover, B. subtilis produces spores that are resistant to various physical and chemical influences, such as heat, desiccation, organic solvents, and UV irradiation. These properties make these bacteria an excellent foundation for the development of commercial biological products [29]. Despite progress in this field, many questions remain regarding the interactions between endophytic bacteria and potato plants, as well as the optimal conditions for their use, which hampers the development of commercial products. The known mechanisms of action include direct and indirect methods, such as the production of a wide range of biologically active substances (including antibacterial and insecticidal components, siderophores and chelators, phytohormones and enzymes, 1-aminocyclopropane-1-carboxylate (ACC) deaminase), effective competition with phytopathogenic microbes [14,29,33,34,35,36], reduction of the ethylene level in plants [26], improvement of macro-/micronutrient availability [34], the formation of microbial communities in the rhizosphere and phyllosphere of plants [27,28], and the induction of systemic resistance/tolerance in host plant against diseases and abiotic stresses [12,19,24,36]. While several studies have been published on the application of different rhizobacteria in high-quality potato seed production [6,7,8,11,14,15,16], there is no information concerning the use of bacterial endophytes for obtaining high-quality seed tubers. Most studies have focused on the application of endophytic PGP microorganisms using methods such as pre-planting inoculation of non-sterile seeds or inoculation of vegetative plants via spraying, with assessment primarily focused on growth, general yield, and quality attributes [37,38,39]. Less attention has been given to understanding the underlying physiological, biochemical, and molecular mechanisms responsible for establishing effective plant–microbe interactions. Therefore, the instability of microbial-based biopreparations often leads to challenges in the dissemination of this innovation and the establishment of Bacillus application in potato production, despite its numerous benefits [12]. Our hypothesis was that the settlement of healthy (pathogens-free) hydroponically (or aeroponically) grown seed minitubers with the beneficial endophyte, B. subtilis, could help improve seed quality, tuber yield, and nutritional value while also exhibiting a prolonged beneficial effect on plant physiology. In earlier studies, we discovered that the endophyte B. subtilis (strains 10-4 and 26D) possesses several PGP traits (i.e., produces auxins, siderophores, fix atmospheric nitrogen) and is capable of enhancing the resistance of hydroponically grown potato tubers to post-harvest diseases, as well as prolonging product shelf life when applied immediately before storage [23,30,31].
In this study, to assess the potential of the endophyte B. subtilis to obtain high-quality seed tubers for further use in field conditions, we analyzed the effect of pre-planting enrichment (treatment) of healthy, hydroponically grown (disease-free) seed minitubers with the endophyte B. subtilis (strains 10-4 and 26D) on plant growth, photosynthetic pigments, leaf area, proline content, tuber yield, and quality parameters (total dry matter, starch, reducing sugars), as well as the accumulation of macro-/micronutrients, vitamin C, and anthocyanins in pot experiments under controlled conditions.
2. Material and Methods
2.1. Bacterial Strains and Inoculum Preparation
The endophyte B. subtilis, strain 10-4, was earlier isolated from the arable soils (Republic of Bashkortostan, Russia). In our previous work [19], strain 10-4 was identified as B. subtilis (99%) based on the sequencing analysis of the variable regions of genes encoding 16S rRNA and PCR analysis using species-specific primers (secYsubF TTATATCACGGCTTCGAT, secYsubR CGGTAGTTTCGTTTCACCA) and deposited in the National Bio-Resource Center of the All-Russian Collection of Industrial Microorganisms (VKPM reg. num. B-12988). Strain 10-4 produces IAA, siderophores, and catalase, fixes atmospheric N, promotes plant growth, ameliorates environmental stresses, and colonizes internal plant tissues (i.e., endophytes) [19,20]. As a positive control (standard), we used the endophytic bacterial strain B. subtilis 26D (VKPM reg. num. 016-02-2491-1) based on the commercial biological Phytosporin-M (BashInkom Innovation & Research Enterprise Ltd., Ufa, Russia), which was kindly provided by the Microbiological Laboratory of BashInkom Innovation & Research Enterprise Ltd. (Ufa, Russia).
To obtain the inoculums, the bacterial cells (strains 10-4 and 26D) were incubated in liquid Luria-Bertani (LB) medium for 24 h at 37 °C (180 rpm) until the concentration reached 108 cells per mL (cells mL−1). The bacterial cell concentrations were determined according to the 0.5 McFarland Turbidity Standard and, additionally, their optical densities were monitored at 600 nm (OD600) using a SmartSpecTM Plus spectrophotometer (Bio-Rad, Hercules, CA, USA).
2.2. Plant Material
The experiments were carried out on potato plants (Solanum tuberosum L., cv. Bashkirsky) grown from healthy, hydroponically grown seed minitubers, which were provided by the Laboratory of Potato Breeding and Seed Production of the Bashkir Research Institute of Agriculture UFRC RAS (Ufa, Russia). Healthy (sterile) minitubers were obtained using the scheme described earlier [30]. Briefly, healthy potato microplants (obtained in vitro using a clonal micropropagation technology) were placed in the hydroponic equipment KD-10 (DokaGene, Moscow, Russia) and grown in a continuously supplied nutrient solution containing fertilizer Novalon (Doktor Tarsa, Antalya, Turkey) (19-19-19 + 2MgO+ME, pH 5.6 moL L−1) in the following concentrations: 0.4–0.6% during the first week; 0.8% during the second week; 1.2–1.4% during the third week; and 1.5–1.8% from the fourth week until the end of the growing season (which was about 65 d). The lighting mode was divided into three main periods with intensities of 120,000 Lux, 150,000 Lux, and 80,000 Lux, respectively. The freshly harvested healthy hydroponic potato minitubers (5–6 g per minituber) had an oval-rounded shape with medium-depth eyes, smooth red skin, and white flesh.
2.3. Potato Seed Minitubers Treatment and Growth Conditions
Before planting, the healthy (sterile) potato minitubers were immersed in aqueous solutions containing B. subtilis 10-4 (108 cells mL−1) (test), B. subtilis 26D (108 cells mL−1) (standard), and water (control) for 30 min. Thereafter, the solutions were merged and the minitubers were air-dried for 2 d at room temperature and planted in plastic pots (40 cm × 20 cm × 17 cm) with universal soil (pH 6.7) (LLC FASKO+, Solnechnogorsk, Russia). The composition of the universal soil was a mixture of peats, sand, limestone (dolomite) flour, and a complex of mineral fertilizers (N—350 mg kg−1, P2O5—400 mg kg−1, K2O—500 mg kg−1). Three minitubers were planted in each pot (n = 3, 3 replicates) and grown in a climatic chamber Spectr KR (Bashkir State Agrarian University, Ufa, Russia) with a controlled temperature regime, cooling system, ventilation, and relative humidity (RH). During the growth of plants, the photoperiod was 16 h day/8 h night, the temperature was 22 °C day/18 °C night, and 70% RH. In the first half of the growing period (from planting to the end of flowering), the light intensity was 39,000 Lux, and in the second half of the growing period (tuber formation period) it was 23,000 Lux. The plants were watered twice a week with equal portions of H2O per pot (200 mL from planting to sprouting stage; 300 mL from the end of sprouting to budding stage; 500 mL from the end of budding to the end of flowering stage and until harvesting).
The physiological and biochemical parameters of the plants (plant height, root length, photosynthetic pigments, total leaf area, and proline) were dynamically assessed at 3 points: (I)—15-day-old plants (corresponds to the sprouting–leaf development stage); (II)—30-day-old plants (corresponds to the budding stage); (III)—45-day-old plants (corresponds to the flowering stage). The photosynthetic pigments were analyzed in the fresh leaves. To assess the concentrations of proline, freshly harvested leaves were immediately frozen in liquid nitrogen and stored in a freezer prior to analysis. Yield components (plant height, above-ground biomass, weight of the stolones, and the number of tubers, their weights, and sizes) and tuber quality parameters (total dry matter, starch, reducing sugars, and vitamin C) were determined in 65-day-old plants. The content of macroelements (N, P, K), microelements (Mn, Cu, Zn, Fe, and Co), and total anthocyanins was determined both in leaves and the tubers harvested from 65-day-old plants.
2.4. Potato Seed Minitubers and Sprouts Colonization Assay
The ability of B. subtilis (10-4 and 26D) to colonize the internal tissues of potato seed minitubers was determined using surface-sterilized minitubers 2 d and 14 d after inoculation with these bacteria. Bacteria-inoculated and non-inoculated (control) seed minitubers were immersed in 70% ethanol for 5 min, washed with sterile water, and then air-dried and aseptically cut in half. The cut minitubers were laid out in Petri dishes with LB medium and kept for 1 h at 37 °C; then, the cut tubers were removed and the dishes were left for 24 h at 28 °C for bacterial growth.
The ability of B. subtilis (10-4 and 26D) to colonize potato sprouts (roots, shoots) grown from inoculated seed minitubers was registered at 14 d after inoculation and planting. Briefly, the surface-sterilized seed minitubers were inoculated with B. subtilis 10-4 or 26D (108 cells mL−1) and planted in plastic pots (10 × 8 × 5 cm) with sterile sand and cultivated at 22–24 °C for 14 d. The plants were watered twice a week with equal portions of sterile H2O per pot. Then, 14-day-old sprouts were immersed in 0.2% diacid for 15 min, washed with sterile H2O, immersed in 70% ethanol for 5 min, washed with sterile H2O, cut in segments, and laid out in Petri dishes with LB medium. The dishes were cultivated for 24 h at 28 °C for bacterial growth.
The identities of the bacteria grown on the places of surface-sterilized minituber cut prints and around the segments of sprouts to native strains 10-4 and 26D were determined using a random amplification of polymorphic DNA (RAPD) polymerase chain rection (PCR) analysis as described earlier [20]. To control the surface sterilization, the last flush was seeded into the LB medium, and we were convinced of the lack of bacterial growth after 7 d at 28 °C. The number of colony-forming units (CFU) of endophytes within the plant tissues was determined using surface-sterilized potato seed minitubers (2 d and 14 d after inoculation) and sprouts (roots and shoots) (14 d after inoculation and planting). The surface-sterilized plant material was homogenized in sterile conditions. Then, their aliquot was distributed over the surface of solid LB medium and incubated at 28 °C for 24 h. The number of bacteria was counted after the third dilution via 1 g of fresh plant biomass (CFU g−1 FW).
2.5. Bacterial DNA Extraction
The extraction of DNA from bacteria was carried out using a lysis buffer [20]. The genetic polymorphism of bacterial strains was evaluated based on the results of RAPD-PCR of total DNA using AFK primers (50-gcgtccattc-30). Amplification was carried out on the equipment Terzik (DNA-Technology, Moscow, Russia). The analysis and visualization of RAPD-analysis products were carried out with horizontal electrophoresis in PAAG (1.5%) in the chamber SE-2 (Helicon, Moscow, Russia) (25 kV, 1 h). The gel was colored with EtBr and the results were recorded using the Gel Doc XR (Bio-Rad, Hercules, CA, USA).
2.6. Plant Growth Parameters and Tubers Yield Components
The length of plants (above-ground parts and roots) was assessed by classical methods [40]. The plants were harvested 65 days after planting. The growth-promoting effects of endophytes were evaluated by determining plant germination (%), plant length (cm), tubers number per plant, tuber weight (g) and size (cm), weight of the aboveground plant parts (g), the number of stolons and their weight (g), and the yield of tubers (g per m2) [37].
2.7. Photosynthetic Pigments and Total Leaf Area
The concentrations of photosynthetic pigments chlorophyll (Chl) a, Chl b, and carotenoids (Car) were determined in fresh leaves from the middle nodes of the plants by the spectrophotometric method [41]. Leaf samples (0.05 g) were ground in 96% ethanol (10 mL) with the addition of CaCO3 and then filtered. The filtered extracts were analyzed to record the absorbance at 665 nm (Chl a), 649 nm (Chl b), and 470 nm (Car) using a UV spectrophotometer u-Violet DB (SILab, Beijing, China). The pigment concentration was expressed in mg g−1 FW.
The total leaf area was evaluated by a scanner image analysis. Plant leaves were placed in an optical scanner HP laser MFP 135w (HP Inc., Paolo Alto, CA, USA) and scanned (200 dpi, black and white halftone). The leaf area obtained in pixels was converted to cm2 (http://csaa.ru/opredelenie-ploshhadi-listev-metodom-skanirovanija/ (accessed on 31 March 2023)). In each variant, all leaves of plants in three replicates were evaluated.
2.8. Proline
Total free proline content was assessed in leaves by the spectrophotometric method using a ninhydrin reagent [42]. Leaf samples (0.5 g) were filled with boiling dH2O and incubated in a water bath (100 °C, 30 min), and then the extract was cooled. Thereafter, the obtained extract (1 mL), ninhydrin solution (1 mL), and glacial acetic acid (1 mL) were mixed. The mixture was incubated in a water bath (100 °C, 1 h) and cooled. The intensity of proline coloring with ninhydrin was determined at 522 nm using a UV spectrophotometer u-Violet DB (SILab, Beijing, China). The proline content was determined using a calibration curve prepared using chemically pure L-proline (Sigma Aldrich, Burlington, MA, USA) as a standard. The concentration of proline was expressed in µmoL g−1 FW.
2.9. Total Anthocyanins
The content of total anthocyanins was determined in the fresh leaves and periderm of freshly harvested tubers at 530 nm using a UV spectrophotometer u-Violet DB (SILab, Beijing, China). Plant samples (1 g) were extracted in an acid–ethanol solvent (containing 95% C2H6O/1.5 N HCl (85:15, v:v)) (5 mL) for 24 h at 4 °C in the dark. The anthocyanins concentration was expressed in mg 100 g−1 FW [43].
2.10. Macro- and Microelements
The contents of macroelements N, P, and K were assessed according to GOST 13496.4-93 [44], GOST 26657-97 [45], and GOST 30504-97, respectively [46]. The contents of microelements (Mn, Cu, Zn, Fe, and Co) were determined using the atomic absorption spectroscopy method [47]. Plant samples (1 g) (leaves or tubers) were placed into the muffle furnace for 20 h at 550 °C. Thereafter, the obtained ash was incubated with 0.1M HNO3 (50 mL) and filtered. The contents of microelements (Mn, Cu, Zn, Fe, and Co) were determined in the filtered extracts using an atomic absorption spectrophotometer (Shimadzu AA-6300, Shimadzu, Kyoto, Japan) with an electrothermal atomizer GFA EX-7. The contents of macro-/and microelements were expressed as % DW and mg kg−1 DW, respectively.
2.11. Tubers’ Quality Parameters
The contents of starch and total dry matter were assessed by a specific weight of potato tubers in air and water [48,49]. The visualization of starch granules was carried out using a fluorescence scanning microscope (Biozero BZ-8100E, Keyence Co., Osaka, Japan). The content of reducing sugars was determined using Samner’s reagent [48]. Vitamin C content was determined by the titration method according to GOST 24556-89 [50].
2.12. Statistical Analysis
All experiments were carried out in three biological and three analytical replicates. Statistical analysis was performed using the computer program STATISTICA 6.0 (StatSoft, Inc., Tulsa, OK, USA). The figures and tables show the averages (M) and their standard errors (±SE) at p ≤ 0.05.
3. Results
3.1. Potato Minituber Seed Treatment with Endophytic B. subtilis Allows Establishing Close Microbe–Plant Relationships and Has the Potential to Modulate Plant Growth
The colonization of inner plant tissues by bacteria serves as crucial evidence of their endophytic properties and plays a significant role in influencing biological activity within plant–microbial relationships. Preliminary analysis was carried out to assess the ability of B. subtilis 10-4 and 26D to colonize the internal tissues of healthy, hydroponically grown seed minitubers and the transmission and colonization of the potato plantlets (roots, shoots). This approach can obtain high-quality seeds enriched with beneficial endophytes capable of having a potentially prolonged influence on plant growth and physio-biochemical parameters related to tuber yield and quality.
3.1.1. Bacterial Colonization
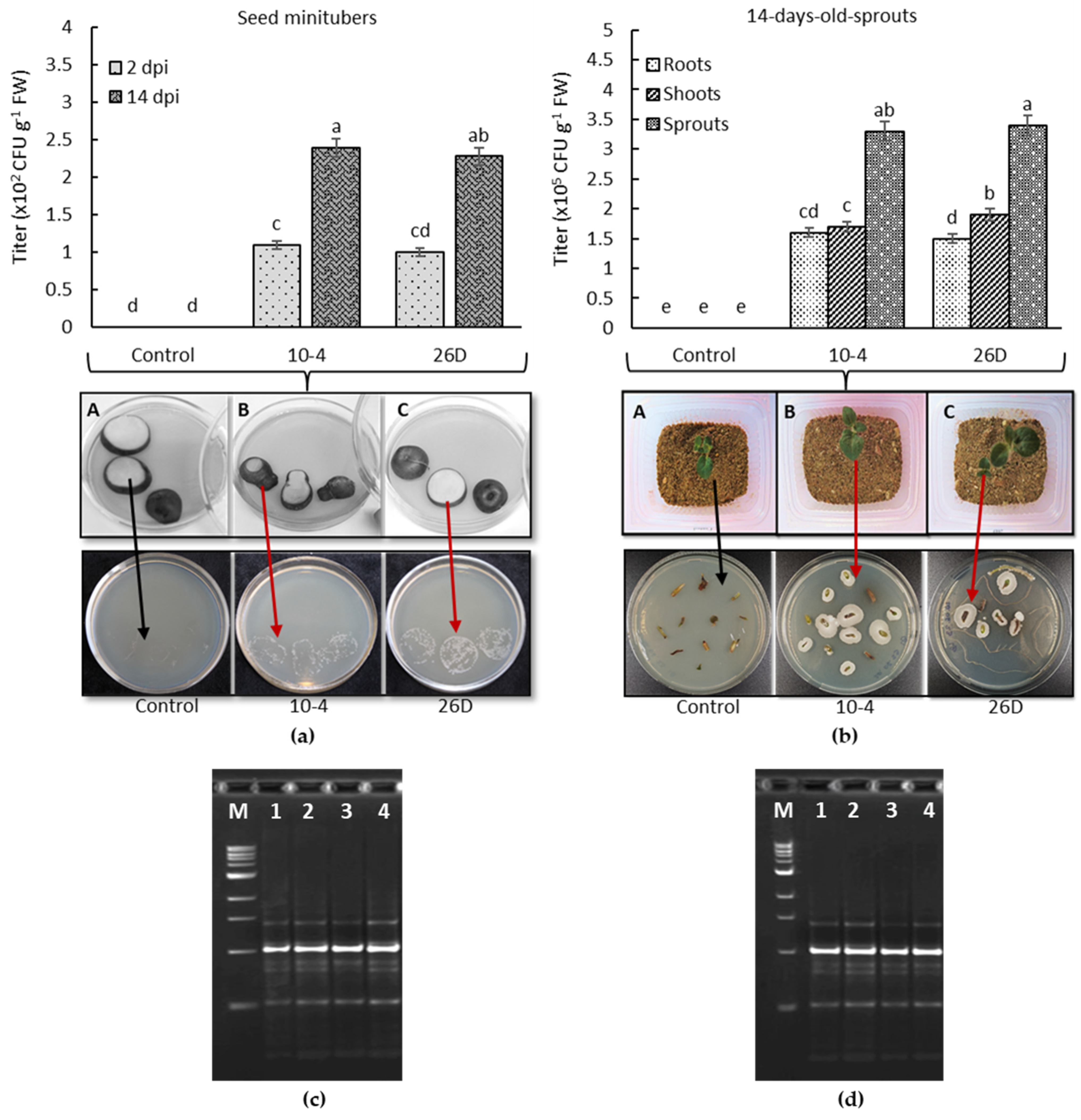
The bacterial colonization of seed potato minitubers was recorded in 2 d and 14 d after their treatment and storage at room temperature. Using the prints of the slices of surface-sterilized seed minitubers and quantitative accounting of bacteria (B. subtilis titer), it was shown that strains 10-4 and 26D successfully penetrated the internal tissues of minitubers and colonized them from the inside (Figure 1a). In addition, both bacteria continued to colonize inner tissues more intensely over time, as evidenced by bacterial titer growth within minitubers after 14 d (Figure 1a). The capability of the bacteria to transmit and colonize potato plantlets, grown from the bacteria-inoculated seed minitubers, was evaluated 14 d after planting. The results showed that both B. subtilis 10-4 and 26D continued to colonize of roots and leaves of potato plantlets, which grew from the bacteria-inoculated seed minitubers (Figure 1b). Growth of bacteria with morphological and cultural properties was observed as similar to B. subtilis 10-4 and 26D around the surface-sterilized segments of plantlets (Figure 1b) and prints of cut tubers (Figure 1a). Further, a RAPD-PCR analysis was conducted to determine the identity of the pure cultures of isolates obtained from the surface-sterilized and cut tuber prints and plantlet segments in relation to the original strains. The results of RAPD-PCR analysis confirmed the identity of the bacteria that grew around the surface-sterilized segments of the plantlet’s roots and shoots (Figure 1b,d), as well as in the places of the prints of the cut seed tubers (Figure 1a,c), as originated from the bacterial strains 10-4 and 26D with which the seeds were inoculated. In control seed minitubers and plantlets (roots, shoots) grown from them, no bacterial growth was recorded after 24 h of incubation in LB medium at 28 °C (Figure 1a,b).
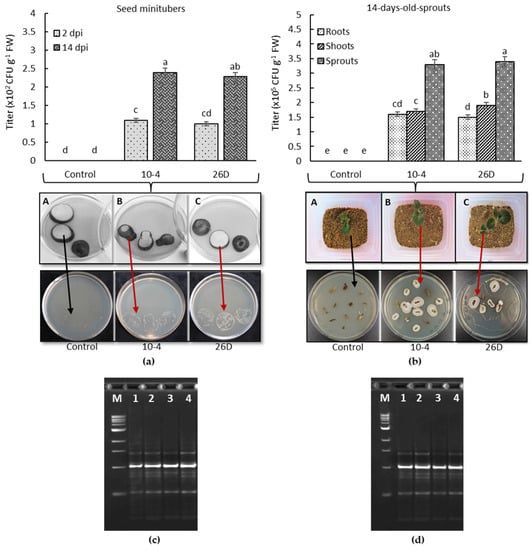
Figure 1.
The capability of 10-4 and 26D to colonize internal tissues of seed minitubers (a) and sprouts (roots and shoots) (b) of potatoes grown from the bacteria-inoculated seed minitubers; (c,d) electrophoregrams in PAAG after RAPD–PCR analysis: M—DNA marker; 1 and 3—DNA of 10-4 and 26D origins, respectively, used for the pre-planting treatment of seed minitubers; 2 and 4—DNA of bacteria isolated from the bacteria grown in the places of cut tuber prints and around potato sprout segments in LB medium after 24 h incubation at 28 °C. Data are the mean of three replicates; different letters show significant differences at p ≤ 0.05 and bars represent standard errors (±SE). Red arrows indicate bacterial growth in Petri dishes with LB medium in places of surface-sterilized and cut seed tuber prints and around the segments of potato sprouts grown from bacteria-inoculated seed tubers; black arrows indicate the absence of bacterial growth in Petri dishes with LB medium in control (non-treated) cut seed tuber print places and around the segments of sprouts grown from control (non-treated) seeds. dpi—days post-bacterial-inoculation.
Thus, the treatment of seed minitubers with B. subtilis 10-4 and 26D allowed the formation of compatible plant–microbial relationships since these introduced bacteria successfully colonized both the minitubers themselves and the plantlets (roots, shoots) as they grew, thereby indicating that these bacterial strains can influence potato metabolism from inside and potentially may have a prolonged influence on plants during the growing season.
3.1.2. Morphological Parameters of Plants Grown from Endophyte-Colonized Seed Minitubers
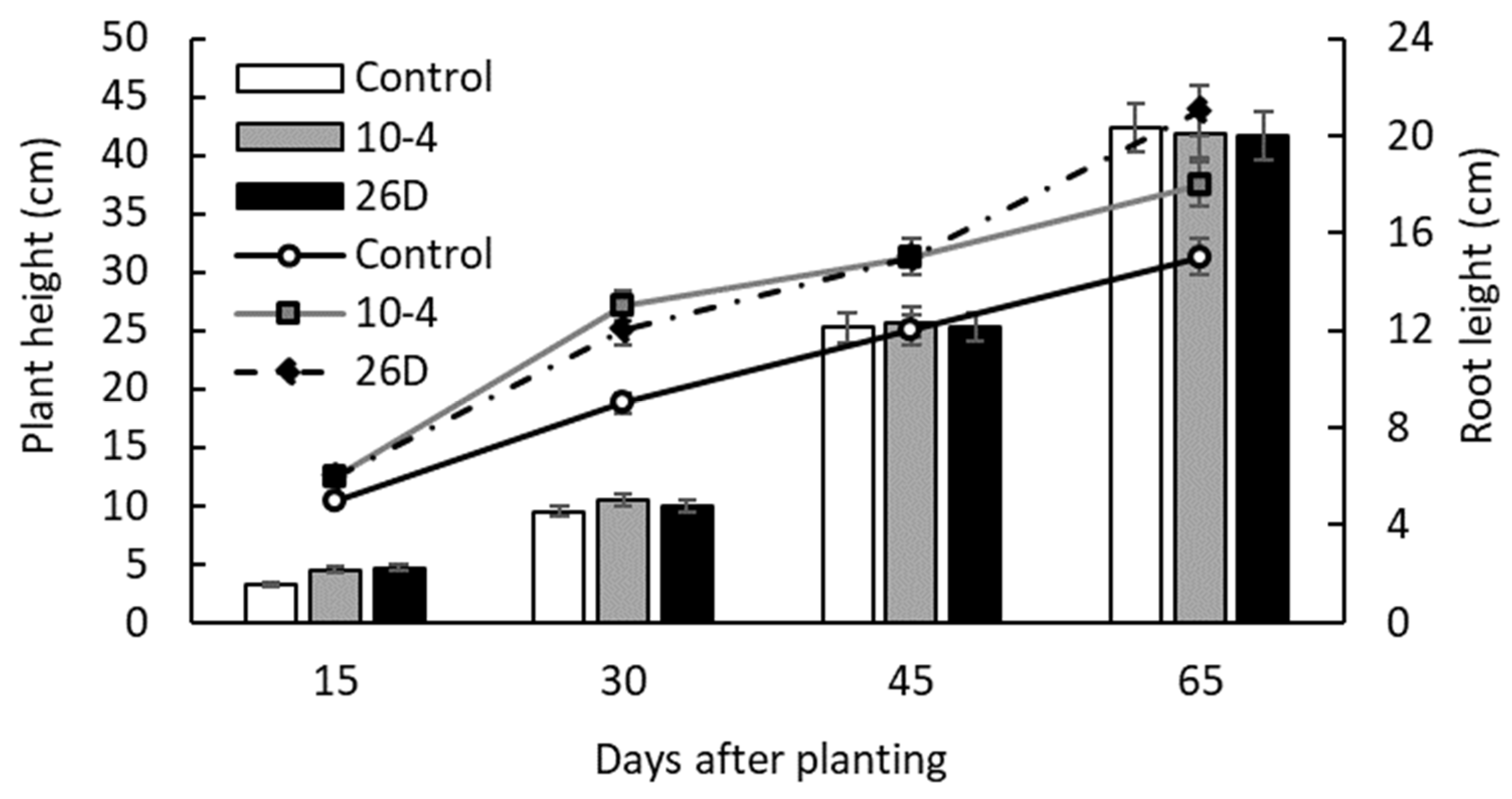
The results of pot experiments showed that seed minituber treatment with endophyte B. subtilis strains 10-4 and 26D resulted in the earlier emergence of potato sprouts and better plantlet growth (i.e., plant height and root length) during the first 15–30 d after planting in comparison with non-inoculated controls (Figure 2). Fifteen days after planting, there were 62.5% and 57.5% of sprouts in strain 10-4- and 26D-treated groups, respectively, while in the control there was 40% of sprouts. Similarly, the height of 15-day-old plants was higher with strain 10-4 (8.6 cm or +32%) and 26D (7.3 cm or +12%) treatments in comparison with the control (6.5 cm). However, over time, the average height of control plants became comparable to endophyte-treated ones; there were no significant differences observed in the heights of bacteria-inoculated and non-inoculated groups until the end of vegetation (65 d). At the same time, the positive influence of endophytes on the formation of roots and their length remained throughout the experiment (Figure 2).
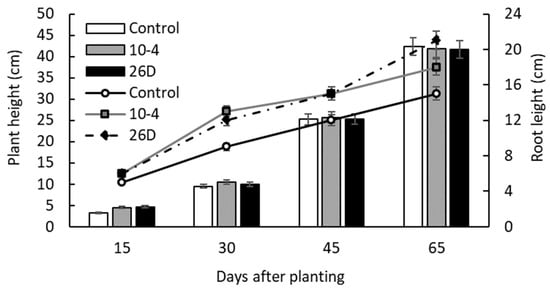
Figure 2.
Influence of pre-planting seed minitubers treatment with the endophyte B. subtilis strain 10-4 (10-4) and B. subtilis strain 26D (26D) on the height of the aboveground and underground parts (roots) of potato plants in pot experiments. Control—plants grown from non-inoculated seed minitubers. Data are the mean of three replicates; different letters show significant differences at p ≤ 0.05 and bars represent standard errors (±SE).
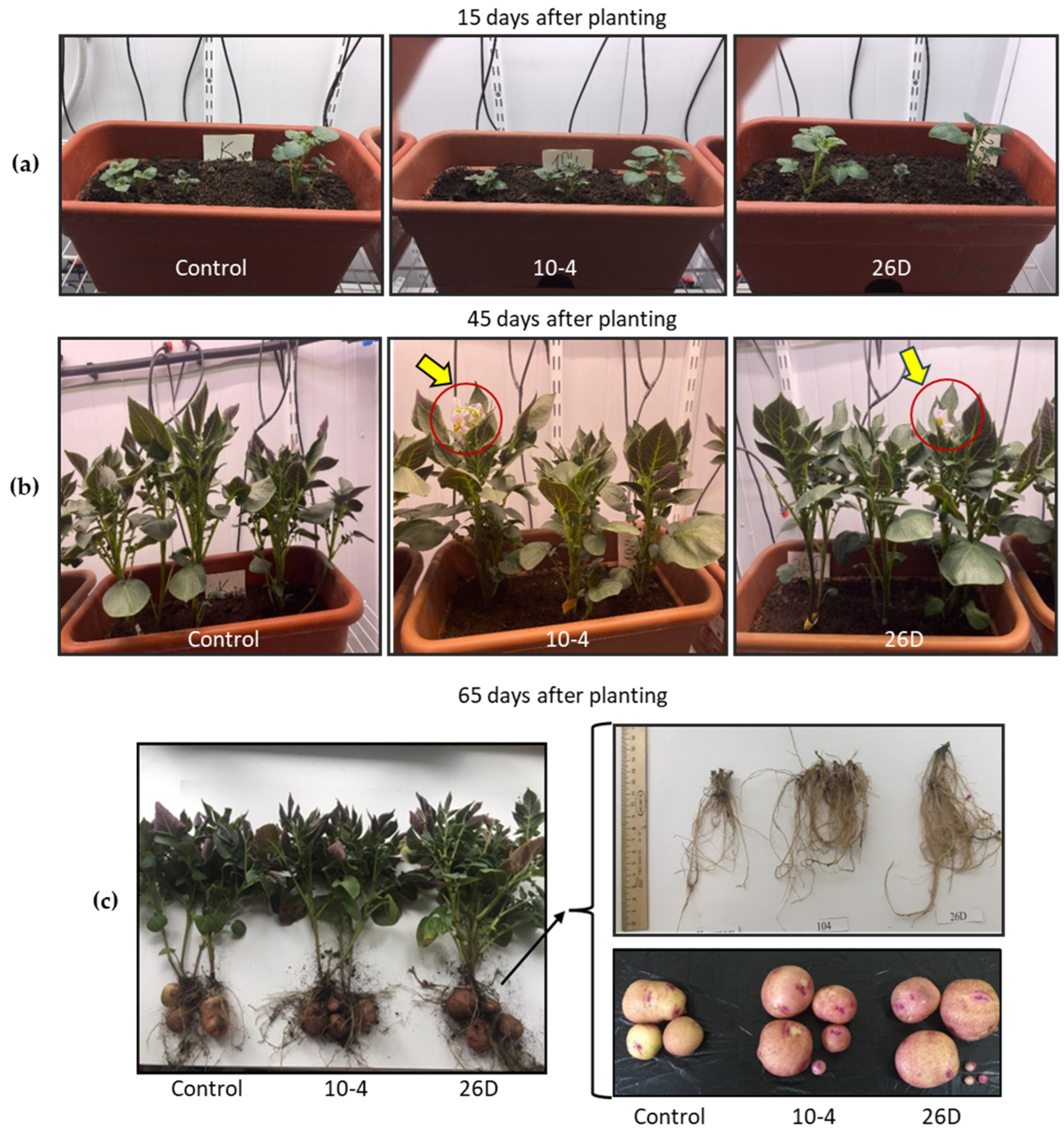
The number of stems did not change upon endophyte application; on average, three stems per bush (plant) in all groups were observed. Additionally, it was noticed that 26D-treated plants had larger stem widths compared with the others. In general, the endophytes accelerated plant phenology and resulted not only in earlier sprout emergence but also earlier budding and flowering of potatoes (Table 1, Figure 3a–c), wilting and dying off of the tops, and physiological maturation of the tubers. Endophyte-treated plants bloomed and completed flowering earlier than the untreated controls. When flowering was completed in bacteria-inoculated plants, about 30% of plants were still in the flowering stage in the control group (not presented).

Table 1.
Phenological growth stages of potato plants in 45 days after planting in pot experiments. Control—plants grown from non-inoculated seed minitubers; 10-4—plants grown from B. subtilis strain-10-4-inoculated seed minitubers; 26D—plants grown from B. subtilis strain-26D-inoculated seed minitubers. In the table, standard errors (±SE) of the means in triplicate (6 plants per replicate) are represented. Different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.
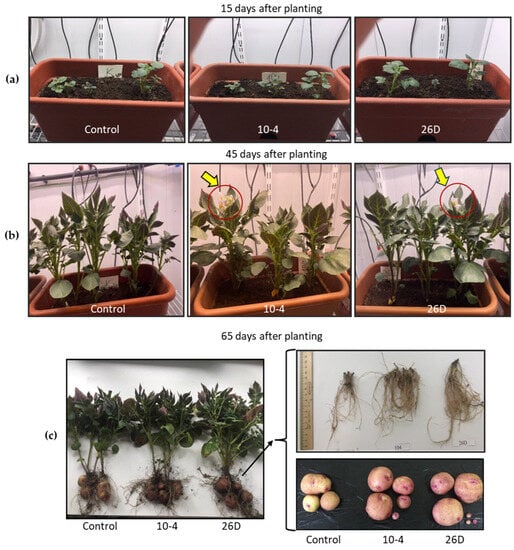
Figure 3.
Representative images of the visual appearance of potato plants 15 days (a), 45 days (b), and 65 days (c) after planting and grown in pots under controlled conditions. Control—plants grown from non-inoculated seed minitubers; 10-4—plants grown from B. subtilis strain-10-4-inoculated seed minitubers; 26D—plants grown from B. subtilis strain-26D-inoculated seed minitubers. Yellow arrows indicate flowers on plants.
3.2. Photosynthetic Pigments and Total Leaf Area
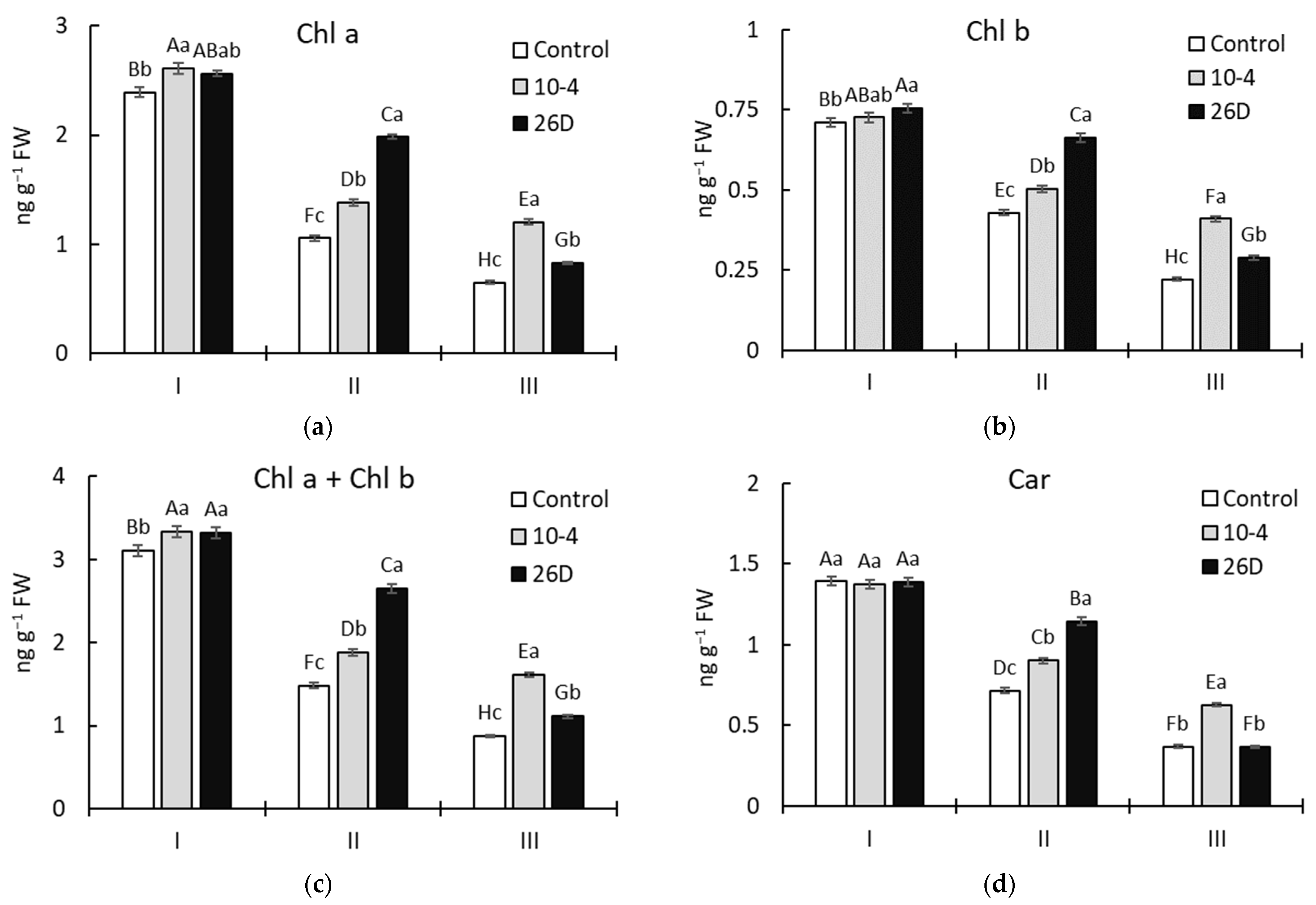
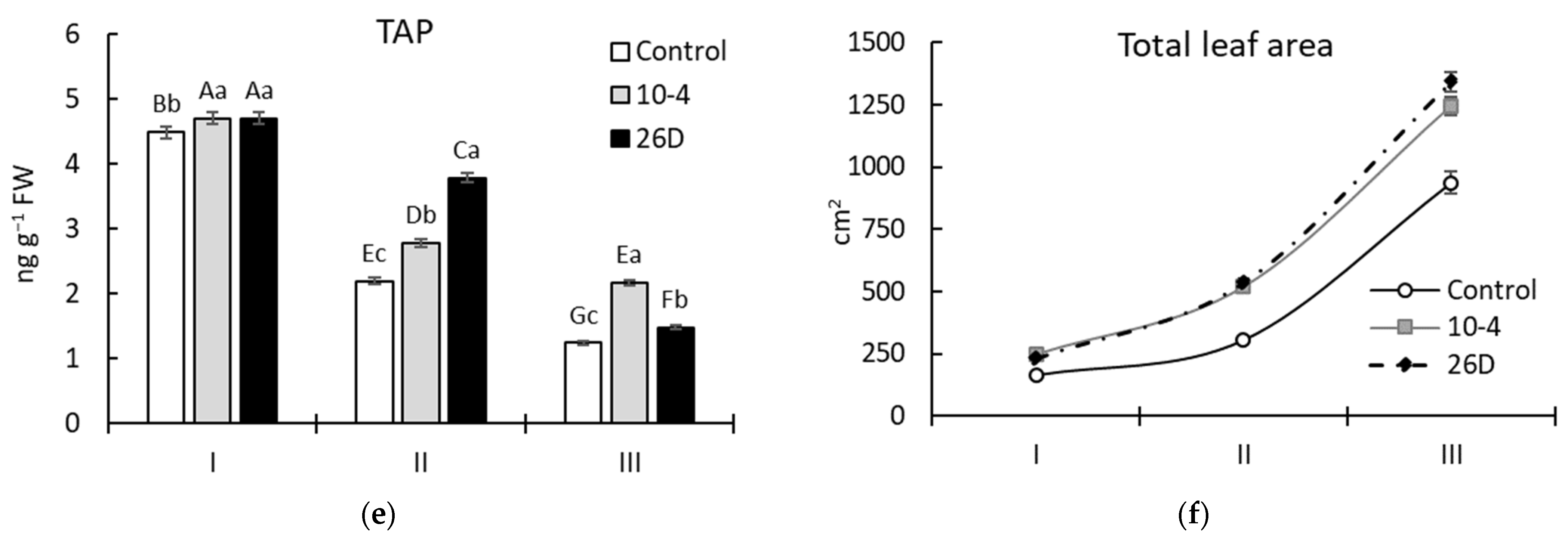
A reliable increase in the levels (in comparison to the control) of the photosynthetic pigments Chl a, Chl b, and Car in both strain 10-4- and 26D-inoculated potato plants was revealed during vegetation (Figure 4). However, the pattern of changes varied in a bacterial-strain-dependent manner, with higher differences observed after 30 d (corresponding to phenological stage II—budding) and 45 d (corresponding to stage III—flowering) after planting. Therefore, after 15 d of planting, treatment with either strain increased the leaf chlorophyll content (i.e., Chl a, Chl b, and Chl a + Chl b) and TAP by up to 5–8% (Figure 4a–c), while no changes in Car content (Figure 4d) were revealed. Furthermore, after 30 d and 45 d of planting, the content of chlorophyll (Chl a, Chl b, and Chl a + Chl b) was increased, respectively, by up to 18–31% and 85–86% (for strain 10-4) and up to 53–88% and 26–32% (for strain 26D). The contents of Car and TAPs were changed similarly, except for 26D-treated leaves at stage III, wherein the content of Car was at the level of the control plants (Figure 4d). It was also revealed that plants inoculated with both strains (10-4 and 26D) were characterized by a significantly larger total leaf area throughout the experiment (Figure 4f).

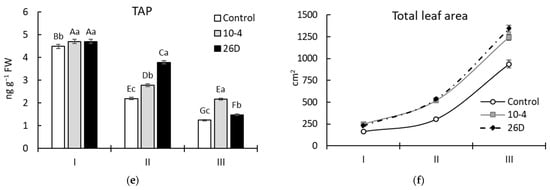
Figure 4.
Changes in the content of leaf photosynthetic pigment chlorophyll (Chl) a (a), Chl b (b), Chl a + Chl b (c), carotenoids (Car) (d), the total amount of pigments (TAP) (e), and leaf area (f) of potatoes grown from non-inoculated seed minitubers (Control) and those inoculated with the endophyte B. subtilis strain 10-4 (10-4) and B. subtilis strain 26D (26D). I—15-day-old plants (corresponds to the leaf development stage); II—30-day-old plants (corresponds to the budding stage); III—45-day-old plants (corresponds to the flowering stage). Error bars in the figures represent standard errors (±SE) of the means in triplicate (n = 3). The various letters show significant differences between the averages of different groups at p ≤ 0.05.
3.3. Proline
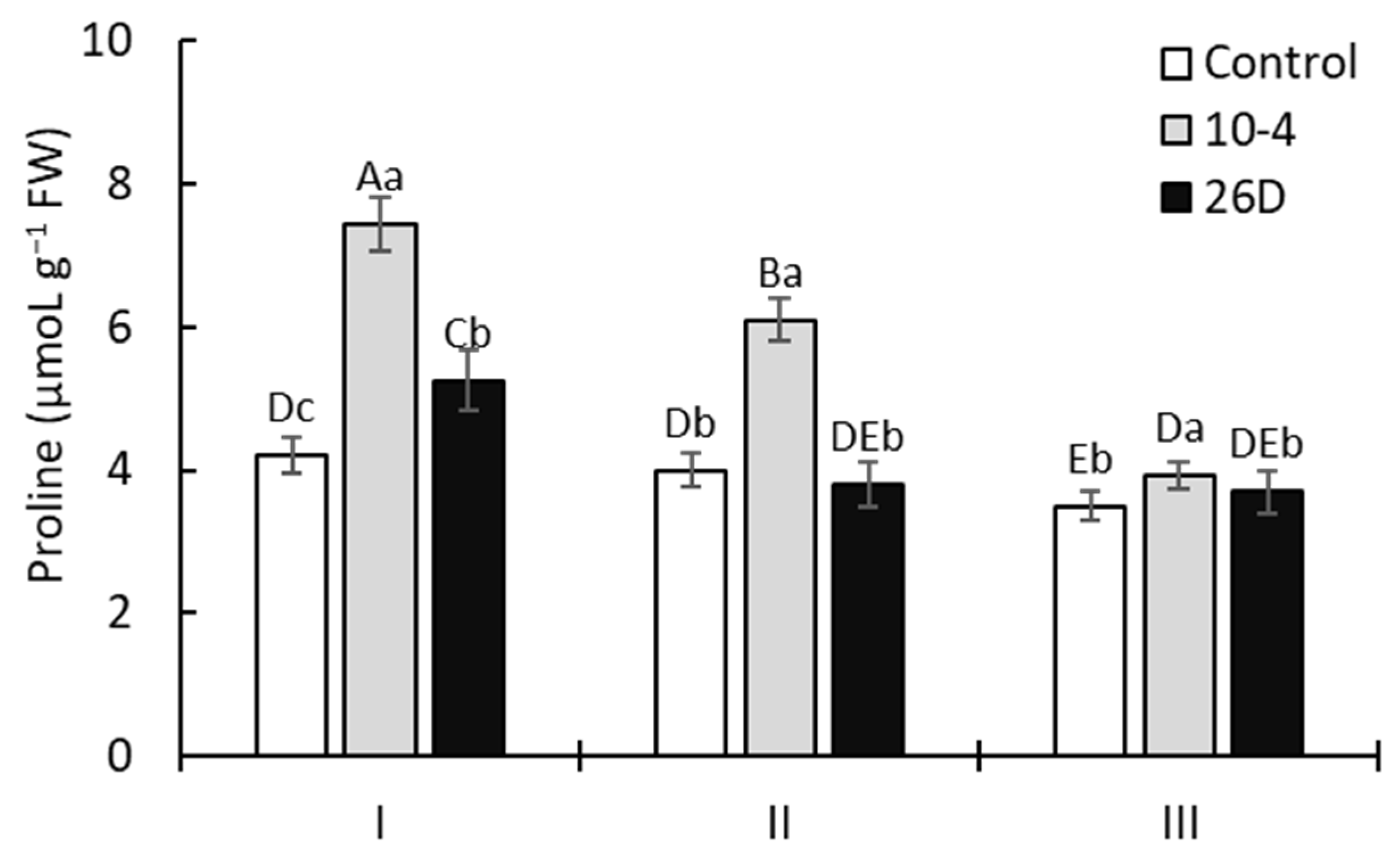
Figure 5 illustrates that treatment with strains 10-4 and 26D enhanced the proline accumulation in the leaves of 15-day-old potato plants by up to 76% and 26%, respectively, in comparison with the control. Furthermore, a gradual decrease in the content of proline was observed for all bacteria-inoculated plants. However, upon treatment with strain 10-4, the proline content remained higher than control values by up to 52% and 11%, respectively, in 30- and 45-day-old plants. At the same time, the content of proline in strain-26D-treated plants declined, almost to control values, in 30- and 45-day-old plants.
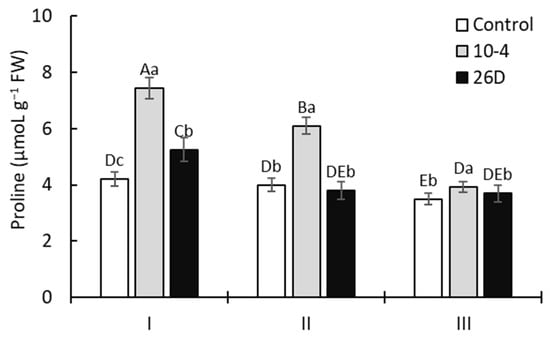
Figure 5.
The effect of treatment with B. subtilis 10-4 (10-4) and B. subtilis 26D (26D) on the free proline concentration in potato leaves in pre-planting seed minitubers. Control—leaves of plants grown from non-inoculated seed minitubers. I—15-day-old plants (corresponds to the leaf development stage); II—30-day-old plants (corresponds to the budding stage); III—45-day-old plants (corresponds to the flowering stage). Error bars represent standard errors (±SE) (n = 3, three replicates); different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.
3.4. Tubers’ Yield Parameters
The data on the productivity of tubers serve as a total indicator of physio-biochemical processes over the entire growing period. The results showed that pre-planting seed treatment with the endophyte B. subtilis, strains 10-4 and 26D, increased the number of tubers per plant (by up to 36–43%) and increased the weight of the tubers and their yield per square meter (by up to 20–28%) in comparison with the control (Table 2, Figure 6). With that, greater tuber yield was observed upon the application of strain 26D. There were no significant differences compared to the control in the height and weight of the aboveground part of the plants upon treatment with either strain 10-4 or 26D, but the biomass of the stolons was increased by up to 22% and 16%, respectively.

Table 2.
Biometric parameters and tubers yield of potato plants 65 days after planting and grown in pots. Control—plants grown from non-inoculated seed minitubers; 10-4—plants grown from B. subtilis 10-4 inoculated seed minitubers; 26D—plants grown from B. subtilis 26D inoculated seed minitubers. In the table, standard errors (±SE) of the means in triplicate (n = 3) are represented. Different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.

Figure 6.
Representative photograph of the visual appearance of whole potato plants with tubers grown for 65 days in pots under controlled conditions. Control—plants grown from non-inoculated seed minitubers; 10-4—plants grown from strain-10-4-inoculated seed minitubers; 26D—plants grown from strain-26D-inoculated seed minitubers.
The results also demonstrated that strain 10-4 led to the formation of a higher number of tubers with average sizes (in comparison with 26D) (Table 3), which is appropriate for potato seed tuber production. However, upon the application of strain 26D, there was a greater number of tubers with large sizes (in comparison with strain 10-4 and the control), which are more appropriate for marketable potato production.

Table 3.
Potato fractional composition. Control—tubers of plants grown from non-inoculated seed minitubers; 10-4—tubers of plants grown from B. subtilis strain-10-4-inoculated seed minitubers; 26D—tubers of plants grown from B. subtilis strain-26D-inoculated seed minitubers. In the table, standard errors (±SE) of the means in triplicate (n = 3 plants) are represented. Different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.
3.5. Tubers’ Quality Parameters
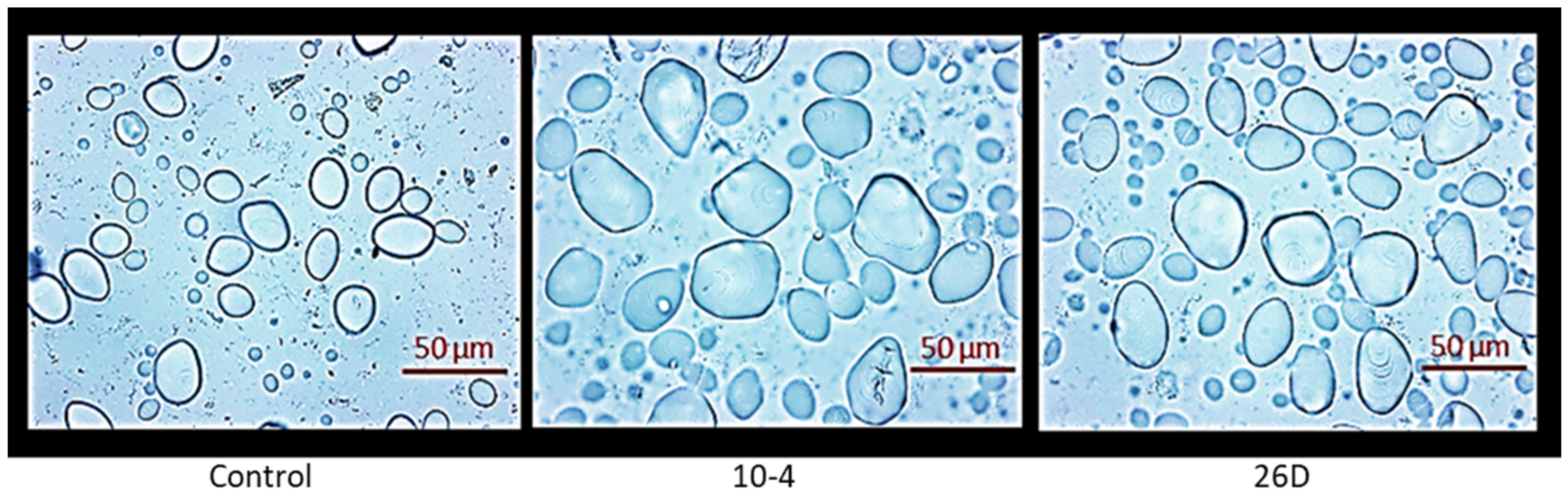
It was revealed that the application of B. subtilis strains 10-4 and 26D did not significantly change the contents of total dry matter and starch, and decreased reducing sugars (by 11–16%) in harvested tubers in comparison with the control (indicating that endophytes, probably, colonize tubers and feed on sugars) (Table 4). However, in endophyte-treated tubers, the sizes of starch granules were larger in comparison with the control (Figure 7). It was also found that the contents of vitamin C and total anthocyanins in endophyte-treated tubers were increased, respectively, by up to 7% and 12% (strain 10-4) and up to 16% and 11% (strain 26D) in comparison with the control. It should be noted that upon treatment with either endophyte strain, a significant increase in total anthocyanins was observed in potato leaves as well (i.e., total anthocyanins accounted for an average of 9.18 mg% (strain 10-4), 9.01 mg% (strain 26D), and 8.64 mg% (control)) (data not presented).

Table 4.
The changes in the contents of total dry matter, starch, reducing sugars, vitamin C, and total anthocyanins in potato tubers grown from endophytic B. subtilis 10-4 (10-4) and 26D (26D) colonized seed minitubers. Control—tubers of plants grown from non-inoculated seed minitubers. In the table, standard errors (±SE) of the means in triplicate (n = 6) are represented. Different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.

Figure 7.
Influence of seed minituber treatment with endophytic B. subtilis 10-4 (10-4) and B. subtilis 26D (26D) on the size of starch grains in harvested potato tubers. Control—tubers obtained from the control plant (without bacterial treatment).
3.6. Macro- and Microelements Content
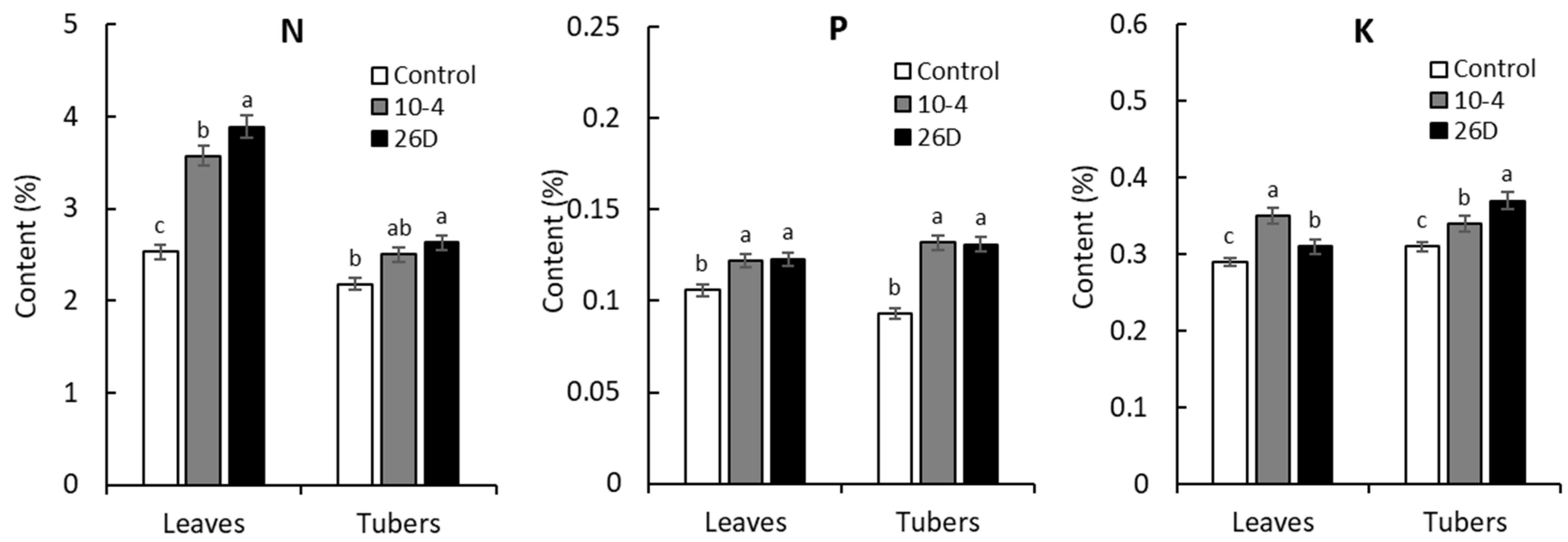
The contents of major macroelements (N, P, and K) and microelements (Mn, Cu, Zn, Fe, and Co) in aboveground and underground (tubers) parts of 65-days-old potato plants upon treatment with B. subtilis strains 10-4 and 26D were analyzed. The results showed that endophytes (strains 10-4 and 26D) increased the content of N (by up to 41% and 54%), P (by up to 15% and 16%), and K (by up to 21% and 7%) in leaves. Similarly, in harvested tubers upon the application of strains 10-4 and 26D, we found increased contents of N (by up to 15% and 21%), P (by up to 42% and 41%), and K (by up to 10% and 19%) in comparison with control tubers (Figure 8).
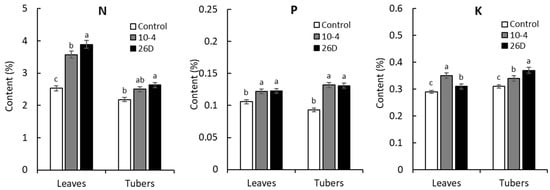
Figure 8.
The effect of pre-planting seed minituber treatment with B. subtilis strains 10-4 (10-4) and B. subtilis strain 26D (26D) on the content of macronutrients nitrogen (N), phosphorus (P), and potassium (K) in the leaves and tubers of potato plants grown for 65 days in pots under controlled conditions. Error bars represent standard errors (±SE) (n = 6, three replicates); different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.
The results represented in Table 5 demonstrated that upon the application of endophytes (strains 10-4 and 26D), there were no statistically significant differences in the contents of Mn, Zn, and Co in harvested tubers; however, the contents of Cu and Fe were increased, respectively, by up to 13% and 10% (for strain 10-4) and by up to 7% and 4% (for strain 26D) in comparison with the control. As for leaves, application of strain 10-4 increased the contents of Mn (+5%), Cu (+13%), Zn (+18%), and Co (+2%), while the content of Fe was decreased (−11%). At the same time, upon application of strain 26D, we observed an increased leaf content of Cu (+2%), Zn (+7%), Fe (+2%), and decreased contents of Mn (−2%) and Co (-13%). In general, pre-planting inoculation of seed minitubers with both endophytes (10-4, 26D) resulted in the accumulation of N, P, and K in leaves and tubers of 65-day-old potato plants. However, the contents of microelements changed differently and depended on the applied strain as well (i.e., changed in a bacterial-strain-dependent manner). Both strains increased Cu in leaves and tubers, Zn in leaves, Fe in tubers, and decreased Co in tubers; however, they differently influenced Mn in leaves and tubers (i.e., 10-4 increased, 26D decreased) and Co in leaves (i.e., 10-4 increased, 26D decreased).

Table 5.
The effect of pre-planting seed minituber treatment with B. subtilis strain 10-4 (10-4) and B. subtilis strain 26D (26D) on the content of microelements manganese (Mn), cuprum (Cu), zinc (Zn), iron (Fe), and cobalt (Co) in the leaves and tubers of potato plants grown for 65 days in pots under controlled conditions. In the table, standard errors (±SE) of the means in triplicate (n = 6) are represented. Different letters indicate a significant difference between the averages of different groups at p ≤ 0.05.
Thus, taken together, the results indicated that B. subtilis strains 10-4 and 26D successfully colonized seed minitubers and plants (roots, shoots), thereby intensifying sprout germination, speeding up plant phenological phases, enhancing the synthesis of photosynthetic pigments, increasing total leaf area and endogenous proline, improving root development, increasing the weight of stolones, the number and size of tubers, their weight, the size of starch grains, and the content of vitamin C, and decreasing reducing sugars; the height of plants, the content of starch, and the total dry matter of tubers were comparable to the non-inoculated control plants. Endophytes also contribute to the accumulation of anthocyanins, macroelements N, P, and K, and some microelements (Cu, Fe) in aboveground plant parts and tubers.
4. Discussion
Improving the production of high-quality potato tubers both for seeds and consumption with PGP endophytic bacteria is a promising, yet underexplored direction in agronomy research. Several studies showed that treatment of tubers pre-planting with microbiological preparations based on endophytic bacteria has a positive influence on potato yield [37,38,39]. For example, the endophytes P. xylanexedens N40 and B. thuregiensis W65 increased tuber yield and considerably decreased, in a variety-specific manner, the prevalence of phytophthorosis, fusariosis, and common scab on tubers [37]. Our results, for the first time, demonstrated that the enrichment of healthy, hydroponically grown seed minitubers with endophytic B. subtilis strains 10-4 and 26D improves tuber yield (Table 2 and Table 3) and some quality parameters, including essential nutrient uptake (Figure 8; Table 5), vitamin C, and anthocyanin accumulation, decreases reducing sugars (Table 4), increases the size of starch grains (Figure 7) without a significant influence on the height of plants (Figure 2, Table 2), and increases the contents of starch and total dry matter in harvested tubers (Table 4). Additionally, the findings revealed a strain-dependent manner of endophyte influences on potatoes: particularly, strain 26D resulted in the formation of a greater number of large tubers, which is preferable for marketable potatoes; however, upon treatment with strain 10-4, there was a greater number of tubers with average sizes, which is preferable for seed production (Table 3). Interestingly, in a recent study, Chebotar et al. [39] reported the variety-dependent influence of endophytic bacteria Bacillus sp. X20 and B. thuringiensis W65 on potato yield. Particularly, the yield of the Charoit variety increased due to an increase in the average weight of one tuber, while the yield of the Gusar variety increased due to an increase in the number of tubers per plant [39]. The current knowledge suggests that to fully unlock the potential of endophytes in potato productions, exploring the underlying mechanisms responsible for effective plant–endophyte interactions is required.
It is known that the colonization of endophytes is the first and most crucial step in providing benefits to host plants [34,35]. It involves the penetration, growth, and reproduction of endophyte populations within the plant. While there are several hypotheses regarding the colonization pathways used by endophytes [35], the mechanism by which plants recruit endophytes is still largely unknown. The initial stage of colonization involves the attachment or adhesion of bacterial cells to the plant surface, allowing them to explore potential penetration sites to gain access to the plant’s internal tissues. The specific mechanisms by which bacterial endophytes attach to the plant surface are not well explored, but it is believed that factors such as endophyte-produced exopolysaccharides and bacterial structures such as flagella, fimbria, or surface polysaccharides play a key role in this process [35]. Endophytic bacteria can enter the host plant through natural ruptures such as the root apex, root hairs, stomata, wounds, and glandular trichomes [25]. Some endophytes may use cellulolytic enzymes to degrade the plant cell wall, allowing them to penetrate and distribute within the plant tissues [25]. Bacterial mobility, along with the synthesis of cellulolytic enzymes, can contribute to the spread of endophytes to above-ground plant parts such as leaves and stems [35,51,52]. The colonization of plants by endophytic bacteria can involve dynamic changes in gene expression in both bacteria and plants, potentially mediated by various biomolecules [52]. For example, the production of surfactin by endophytic B. subtilis 26D was found to be important for colonization of potato plantlets, and salicylic acid was shown to influence this mechanism [51]. Endophytes can colonize various parts of the plants, including roots, leaves, stems, flowers, and seeds, often colonizing intercellular spaces that are rich in nutrients (carbohydrates, amino acids, and inorganic compounds) [25,26,53,54,55]. Intracellular colonization by bacteria has also been reported in certain cases [56,57]. Colonization can occur at the tissue level or spread systematically throughout the plant. The distribution of endophytes within the plant can vary, with stronger colonization observed in specific regions such as the root cortex, xylem vessels, and the base of lateral roots. During germination and early growth, endophytes can colonize and grow in developing germs [53,54,55]. The number of bacterial endophytes from surface-sterilized aboveground and underground tissues is used to estimate the internal endophyte population in the host plant [51]. In our study, after two days of treating seed minitubers, bacilli were found within them, indicating their colonization. In addition, our findings indicate that the titer of the endophytic bacteria strains 10-4 and 26D increased over time, suggesting their continued translocation into the roots and leaves (Figure 1a,b). This colonization of internal tissues was observed in both seed minitubers and sprouts. Notably, the bacterial titer was higher in the shoots, particularly in the stems, compared to the roots of 14-day-old sprouts. This difference may be attributed to the stems being richer in nutrients at that stage compared to the roots and leaves. Similar results were reported by Sorokan et al. [51], who employed a different method of bacterial inoculation. In their study, a significantly higher amount of B. subtilis 26D per gram of fresh weight was found in the shoots compared to the roots of sterile test-tube potato microplants on the seventh day post-inoculation [51]. It can be hypothesized that as plants develop, the preferred sites for endophyte localization may change. Therefore, by the time the tubers mature and the foliage dies, the bacteria may have concentrated in the nutrient-enriched tubers. Further detailed investigations are needed to provide more insight into this matter. In any case, our results showed the principal ability of the endophyte B. subtilis strains 10-4 and 26D to colonize inner seed minitubers and plantlets using this method of inoculation.
The observed modulation of potato growth induced by endophytes (Figure 2 and Figure 3) is most likely associated with the ability of strains 10-4 and 26D to synthesize phytohormones, including indole-3-acetic acid (IAA) [19,20] or by regulating the level of endogenous hormones in the plant [25,52,58]. IAA produced by endophytes within plants increases the number of lateral and subventional roots, facilitating access to nutrients and improving root exudation, offering soil microbes more resources for root interaction [58,59]. Many studies have reported increased growth by increasing plant height and/or biomass when plants were inoculated by bacterial endophytes capable of producing IAA [52,58]. In addition, strains 10-4 and 26D of the bacterial endophyte B. subtilis secrete siderophores and solubilize phosphorus in soil [19,20], which contribute to initiating symbiotic interactions with host plants [25,59]. Siderophores are organic compounds secreted by microorganisms and plants in Fe-limited environments that allow them to chelate Fe from the environment for absorption by microbial and plant cells [25,60]. Similarly, P- P-solubilizing bacteria can dissolve stationary phosphorus in soil that is potentially available for uptake by plants, which is an important sign for stimulating plant growth [25,61]. The ability to fix N is also an essential trait to produce higher tuber yield and good quality while not having detrimental impacts on the environment. The revealed ability of B. subtilis strains 10-4 and 26D to increase the length of roots entire growing season and intensify the growth of the above-ground parts of plants during the first 15–30 days after planting (Figure 2) is most likely associated with the capability of these strains to produce IAA, siderophores, fix atmospheric N [20], and improve water (Figure 5) and nutrient uptake (Figure 8, Table 5) by plants. As a result, B. subtilis strains 10-4 and 26D contributed to a more accelerated (than control) onset of plant phenophases (Table 1). Plants with bacteria bloomed and completed flowering earlier than the non-inoculated control (Table 1, Figure 3). This indicates that the processes in bacteria-inoculated variants were faster and ahead of the development of control plants. It should be noted that in other studies carried out in field conditions, inoculation with endophytic Bacillus sp. X20 and B. thuringiensis W65 did not affect the duration of the phenophases from germination to flowering but increased the duration of flowering by 8–13 days compared to the control [39]. The revealed absence of differences in height of endophyte-colonized and control potato plants during the second half of vegetation (Figure 2) is consistent with other studies [39,62].
The process of photosynthesis is crucial for plant productivity and is directly related to primary plant metabolism; however, it is still unclear how endophytes influence the photosynthetic activity of potatoes. Our results demonstrated that the enrichment of seed minitubers with endophytic B. subtilis 10-4 and 26D led to an increase in the content of leaf photosynthetic pigments during the vegetation period (Figure 4). It should be noted that, currently, the known effects of microbes on photosynthetic pigment composition of different plants are contradictory in the available literature [9,20,63,64]. Some studies have reported decreased [9] chlorophyll (Chl) contents in potatoes following entomopathogenic fungi application. On the other hand, increased Chl content has been observed in sorghum during colonization by Beauveria bassiana and Metarhizium brunneum [64] in tomatoes and wheat after seed inoculation with B. bassiana [63,64], and in bean plants after seed priming with Bacillus subtilis 10-4 and 26D [20]. The increase in Chl availability in endophyte-colonized plants has been attributed to the production of siderophores by the endophytes, leading to enhanced iron (Fe) uptake by plants (Table 5) [20]. However, some studies [65,66] have shown that colonization of maize and tomato plants with Trichoderma spp. did not affect photosynthetic pigment content under normal humidity conditions, but significantly increased total Chl content under dry conditions compared to non-colonized plants. The influence of endophytes on photosynthetic pigments and composition may also depend on factors such as the method of inoculation, duration of colonization, substrate composition for plant growth, and certain endophyte–plant systems.
Proline acts as an osmoprotectant and antioxidant, playing a crucial role in cellular metabolism and exhibiting regulatory functions during protein synthesis. In addition, proline serves as a signaling molecule during plant development [67]. The ability of endophytic PGPMs to modulate proline content in various plants has been previously reported [19,20,21,68], but limited information is available specifically regarding potato plants and the endophyte B. subtilis [24]. Previous studies have shown that certain endophytes, such as B. cereus BST YS1_42 and B. marisfavi CHR JH 203, can increase proline content in bean plants under normal conditions, while decreasing it under abiotic stress, thereby exerting a protective effect on stressed plants [68]. In the case of potato plants, a significant accumulation of proline was observed upon spraying 15-day-old plants with a suspension of endophytic B. subtilis, and this increase was positively correlated with disease resistance [24]. Similarly, our results demonstrate that pre-planting seed inoculation with endophytes B. subtilis (10-4 and 26D) increased endogenous proline levels in potato plants under normal growth conditions, particularly during the first half of the vegetation period, with a stronger effect observed with strain 10-4 (Figure 5). This proline accumulation likely reflects the plant’s response to initial endophyte colonization, and it may be involved in the formation of microbial-induced plant resistance, providing a pre-adaptive effect against potential subsequent stressors.
The yield and quality of potato tubers, including their biochemical parameters, are essential factors determining the effectiveness of the entire production process for both seed and food potatoes. Starch synthesis and accumulation in amyloplasts, in the form of starch grains, are important biochemical processes during tuberization [69]. They significantly contribute to the quality and nutritional value of potato tubers. PGPMs can influence photosynthetic activity (Figure 4), which, in turn, promotes starch synthesis and accumulation [69]. As a rule, the size of starch grains in cells correlates with the starch content in potato tubers [69]. Several studies have reported that the application of rhizobacteria can increase the starch content and grain size in potato tubers. For example, inoculating aeroponically grown potato plants with the rhizobacteria A. brasilense Sp245 improved the regulation of starch grain formation and the activity of enzymes involved in starch synthesis [8]. In our study, the pre-planting enrichment of sterile seed minitubers with endophytic B. subtilis 10-4 and 26D did not significantly alter the starch content in harvested tubers, although a slight decrease was noticed (Table 4). However, an increase in the size of starch grains was observed (Figure 7). We supposed that the revealed phenomenon of a slight decrease in starch may be due to endophytes colonizing the internal tissues of the tubers, possibly utilizing starch as a nutrient substrate and/or producing hydrolytic enzymes that can degrade starch. Similar findings were observed in our previous studies on stored tubers [23,31], where these endophytes exhibited the same trend. However, these endophytes positively regulated other plant mechanisms, resulting in improved resistance to post-harvest diseases and preservation of stored tubers quality during long-term storage [23,31].
The size of starch grains, the mass fractions of total dry matter, and reducing sugars are crucial indicators for determining the quality of potatoes intended for processing [70,71,72,73]. Larger starch grains contribute to digestion resistance and result in creamier and richer dishes with a smoother and more uniform consistency in processed foods such as purees, soups, or sauces. A higher total dry matter content in potatoes leads to increased yield in potato products due to lower oil absorption during the cooking process [74] and enhanced resistance to darkening of the raw pulp [75,76,77]. A low mass fraction of reducing sugars in potatoes helps prevent the darkening of the final product and the development of a bitter aftertaste, which can negatively impact consumer perception [78,79,80]. These quality indicators are influenced by various factors, including genotype, climatic conditions, soil type, fertilizer use, and the duration of the growing season [71,81]. For processing purposes, tubers should meet specific requirements, such as a total dry matter content of 20–25%, reducing sugars at 0.2–0.5% [82], starch content at 16% [83], and glycoalkaloids not exceeding 200 mg kg −1 [84]. In our previous studies [23,31], we demonstrated that the endophyte B. subtilis strains 10-4 and 26D, when applied prior to tuber storage, reduced the accumulation of glycoalkaloids (α-solanine and α-chaconine) in long-term-stored potatoes. The revealed decrease in reducing sugars in harvested tubers compared to the control group (Table 4), under the influence of endophytes, may be attributed to the endophytes colonizing the internal tissues of the tubers and utilizing sugars as a nutrient source. Overall, the findings of increased starch grain sizes and decreased reducing sugars under the influence of B. subtilis indicate that these endophytes have a positive effect on the consumer qualities of tubers in terms of these indicators.
Our findings also demonstrated that the endophyte B. subtilis plays a significant role in enhancing vitamin C, anthocyanins (Table 4), and macro-/microelements (N, P, K, Fe) (Figure 8, Table 5) in tubers compared to the control. The mechanisms behind such enhancement of vitamin C and anthocyanins possibly may involve endophyte-induced improvement of nutrients uptake and assimilation (Figure 8, Table 5), the expression of genes related to vitamin C and anthocyanin biosynthesis pathways [85], and hormonal interactions that also can influence the biosynthesis pathways, thereby leading to enhanced production of these compounds. Furthermore, B. subtilis can also trigger signaling pathways and activate specific enzymes involved in the synthesis of vitamin C and anthocyanins [85,86]. These findings, in addition to increased nutritional value, may also suggest increased resistance of endophyte-colonized plants to possible stressful situations. Anthocyanin-rich potato cultivars are known for their heightened disease resistance and tolerance to abiotic stresses [86,87]. Anthocyanins, as natural phenolic compounds, provide color to plant organs and possess potent antioxidant, antihypertensive, antimutagenic, and anticarcinogenic properties [85,88]. The content of anthocyanins in potatoes is typically correlated with the total amount of soluble phenolic compounds and antioxidant activity [85,89]. Anthocyanin pigments can be synthesized in various parts of potato plants, including the skin, flesh, flowers, leaves, stems, and eyes. These pigments serve as photoprotective agents, shielding and safeguarding the photosynthetic apparatus by absorbing excessive visible and ultraviolet light and neutralizing free radicals [86]. Acting as antioxidants, anthocyanins prevent lipid peroxidation and maintain membrane integrity, which slows down cell aging and can play an important role in improving post-harvest vegetable quality [88]. Increased vitamin C in harvested potato tubers upon endophyte application (Table 4) is in accordance with the results of other reports [23,32,38].
At the beginning of our study, we did not find information in the available literature about the influence of the endophytic bacteria B. subtilis on the contents of essential macro-/micronutrients in potato plants and tubers. Our findings demonstrated that both endophytic strains 10-4 and 26D led to an increased content of macroelements N, P, and K (Figure 8) and certain microelements (Table 5) in harvested tubers and the aboveground parts of the plants. This is probably associated with the ability of the endophytes to produce metabolites responsible for improved nutrient bioavailability and uptake. In our previous research [20], it was revealed that strains 10-4 and 26D have the capability for atmospheric N fixation and siderophore production, which improves Fe bioavailability for plants [25,60]. Therefore, the increase in anthocyanins, vitamin C, and macro-/micronutrients detected by us for the first time in potato plants (both in the upper part and in tubers) under the influence of endophytes may indicate enhanced antioxidant properties and nutritional value of endophytes-colonized potatoes. This could contribute to plant survival under stressful conditions and enrich the beneficial properties of potatoes for human consumption. It is important to note that the level of essential nutrient accumulation may be more pronounced in field conditions, as our current study was conducted in containers with limited areas and using a universal soil. Recent research by Lee et al. [90] demonstrated that potato growth and yield are significantly influenced by container type and size, with the best results obtained in large boxes. Therefore, further detailed investigation under field conditions is also necessary to assess all parameters in real agricultural settings, where plants are exposed to unpredictable climatic conditions, diseases, temperature fluctuations, and high or low humidity. The results of our current study, conducted in controlled conditions using pots, highlighted the significant potential of enriching healthy hydroponically grown potato seed minitubers with the endophytic bacteria B. subtilis strains 10-4 and 26D prior to planting. This approach shows promise in enhancing tuber yield, nutritional value, and overall consumption properties.
5. Conclusions
Thus, the results indicated that settlement (colonization) of healthy hydroponically grown seed minitubers with the endophytic bacteria B. subtilis strains 10-4 and 26D improves yield and some quality parameters of tubers. This includes improved uptake of essential nutrients (N, P, K, Fe, and Cu), increased accumulation of vitamin C and anthocyanins, decreased levels of reducing sugars, and larger size of starch grains. These effects were observed without a significant influence on plant height, starch content, and total dry matter in harvested tubers. Such endophyte-caused prolonged positive influences on potato plants can be associated with the revealed increase in photosynthetic pigments, leaf area, proline levels, root system development, and enhanced nutrient availability. The study also revealed a strain-dependent effect of endophyte influence on potatoes; particularly, strain 26D resulted in the formation of a greater number of larger-sized tubers, which is preferable for marketable potato production; strain 10-4 promoted the development of a higher number of tubers with average sizes, which is preferable for seed tuber production. Overall, these findings demonstrate that the enrichment of hydroponically grown healthy seed minitubers pre-planting with endophytic B. subtilis has a great potential as a low-cost, eco-friendly approach for producing high-yielding and nutrient-rich potatoes. However, further detailed investigations under field conditions are needed, considering the diversity of endophytic bacterial strains, potato varieties, and ecological conditions, as these factors may influence the effectiveness of this approach.
Author Contributions
Conceptualization, methodology, O.L., L.P. and S.E.; software, L.P. and O.L.; validation, L.P. and O.L.; formal analysis, L.P., O.L. and S.E.; investigation, L.P. and O.L.; resources, O.L. and L.P.; data curation, O.L. and L.P.; writing—original draft preparation, L.P. and O.L.; writing—review and editing, O.L. and S.E.; visualization, O.L. and L.P.; supervision, L.P.; project administration, L.P.; funding acquisition, L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Russian Science Foundation, grant number 23-26-00262, https://rscf.ru/en/project/23-26-00262/.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to staff of Analytical Laboratory of Bashkir Research Institute of Agriculture UFRC RAS for technical support and other colleagues and students who contributed to the research. The experiments were carried out using the instrument park of the RCCU “Agidel” and “KODINK” UFRC RAS.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Food and Agriculture Organization of the United Nations. World Food and Agriculture—Statistical Yearbook 2022. Available online: https://www.fao.org/3/cc2211en/cc2211en.pdf (accessed on 21 June 2023).
- Zia, M.A.B.; Demirel, U.; Nadeem, M.A.; Ali, F.; Dawood, A.; Ijaz, M.; Caliskan, M.E. Genome-Wide Association Studies (GWAS) Revealed a Genetic Basis Associated with Floral Traits in Potato Germplasm. Turk. J. Agric. For. 2022, 46, 90–103. [Google Scholar] [CrossRef]
- Kordabovskiy, V.Y. Biochemical Composition of Potato Tubers of Magadan Selection. Int. Res. J. 2017, 5, 208–209. [Google Scholar] [CrossRef]
- Shen, C.; Sun, J.B.; Wu, J.Z.; Zhou, X.Y. World Potato Production, Consumption and Trade Pattern and Evolution Analysis. Shandong Agric. Sci. 2021, 2, 127–132. [Google Scholar] [CrossRef]
- Ishibwela Obedi, N. Production of Potato Quality Seeds in Mountainous Region of Central Africa. In Advances in Root Vegetables Research; Kaushik, P., Ed.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Tkachenko, O.V.; Evseeva, N.V.; Boikova, N.V.; Matora, L.Y.; Burygin, G.L.; Lobachev, Y.V.; Shchyogolev, S.Y. Improved Potato Microclonal Reproduction with the Plant Growth-Promoting Rhizobacteria Azospirillum. Agron. Sustain. Dev. 2015, 35, 1167–1174. [Google Scholar] [CrossRef]
- Burygin, G.L.; Kargapolova, K.Y.; Kryuchkova, Y.V.; Avdeeva, E.S.; Gogoleva, N.E.; Ponomaryova, T.S.; Tkachenko, O.V. Ochrobactrum cytisi IPA7.2 Promotes Growth of Potato Microplants and Is Resistant to Abiotic Stress. World J. Microbiol. Biotechnol. 2019, 35, 55. [Google Scholar] [CrossRef]
- Tkachenko, O.V.; Evseeva, N.V.; Terentyeva, E.V.; Burygin, G.L.; Shirokov, A.A.; Burov, A.M.; Matora, L.Y.; Shchyogolev, S.Y. Improved Production of High-Quality Potato Seeds in Aeroponics with Plant Growth-Promoting Rhizobacteria. Potato Res. 2021, 64, 55–66. [Google Scholar] [CrossRef]
- Tomilova, O.G.; Kryukova, N.A.; Efimova, M.V.; Kovtun, I.S.; Kolomeichuk, L.V.; Kryukov, V.Y.; Glupov, V.V. Early Physiological Response of Potato Plants to Entomopathogenic Fungi under Hydroponic Conditions. Horticulturae 2021, 7, 217. [Google Scholar] [CrossRef]
- Oswald, A.; Calvo, V.P.; Davila, D.Z.; Pineda, J.A. Evaluating Soil Rhizobacteria for Their Ability to Enhance Plant Growth and Tuber Yield in Potato. Ann. Appl. Biol. 2010, 157, 259–271. [Google Scholar] [CrossRef]
- Naqqash, T.; Hameed, S.; Imran, A.; Hanif, M.K.; Majeed, A.; van Elsas, J.D. Differential Response of Potato Toward Inoculation with Taxonomically Diverse Plant Growth Promoting Rhizobacteria. Front. Plant Sci. 2016, 7, 144. [Google Scholar] [CrossRef]
- Devi, A.R.; Kotoky, R.; Pandey, P.; Sharma, G.D. Application of Bacillus spp. for Sustainable Cultivation of Potato (Solanum tuberosum L.) and the Benefits. In Bacilli and Agrobiotechnology Cham; Islam, M., Rahman, M., Pandey, P., Jha, C., Aeron, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 185–211. [Google Scholar] [CrossRef]
- Song, J.; Kong, Z.-Q.; Zhang, D.-D.; Chen, J.-Y.; Dai, X.-F.; Li, R. Rhizosphere Microbiomes of Potato Cultivated under Bacillus subtilis Treatment Influence the Quality of Potato Tubers. Int. J. Mol. Sci. 2021, 22, 12065. [Google Scholar] [CrossRef]
- Belimov, A.A.; Dodd, I.C.; Safronova, V.I.; Shaposhnikov, A.I.; Azarova, T.S.; Makarova, N.M.; Davies, W.J.; Tikhonovich, I.A. Rhizobacteria That Produce Auxins and Contain 1-Amino-Cyclopropane1-Carboxylic Acid Deaminase Decrease Amino Acid Concentrations in the Rhizosphere and Improve Growth and Yield of Well-Watered and Water-Limited Potato (Solanum tuberosum). Ann. Appl. Biol. 2015, 167, 11–25. [Google Scholar] [CrossRef]
- Santiago, C.D.; Yagi, S.; Ijima, M.; Nashimoto, T.; Sawada, M.; Ikeda, S.; Asano, K.; Orikasa, Y.; Ohwada, T. Bacterial Compatibility in Combined Inoculations Enhances the Growth of Potato Seedlings. Microbes Environ. 2017, 32, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Soumare, A.; Diédhiou, A.G.; Arora, N.K.; Al-Ani, L.K.T.; Ngom, M.; Fall, S.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L.; Sy, M.O. Potential Role and Utilization of Plant Growth Promoting Microbes in Plant Tissue Culture. Front. Microbiol. 2021, 12, 649878. [Google Scholar] [CrossRef]
- Oswald, A.; Calvo, P. Using Rhizobacteria to Improve Productivity of Potato. In Proceedings of the 15th Triennial International Society for Tropical Root Crops (ISTRC), Lima, Peru, 2–7 November 2009; pp. 29–33. [Google Scholar]
- Kargapolova, K.Y.; Burygin, G.L.; Tkachenko, O.V.; Evseeva, N.V.; Pukhalskiy, Y.V.; Belimov, A.A. Effectiveness of Inoculation of In Vitro Grown Potato Microplants with Rhizosphere Bacteria of the Genus Azospirillum. Plant Cell Tissue Organ Cult. 2020, 141, 351–359. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on Some Physiological and Biochemical Parameters of Triticum aestivum L. (Wheat) under Salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed Priming with Endophytic Bacillus subtilis Strain-Specifically Improves Growth of Phaseolus vulgaris Plants Under Normal and Salinity Conditions and Exerts Anti-Stress Effect Through Induced Lignin Deposition in Roots and Decreased Oxidative and Osmotic Damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef]
- Garipova, S.R.; Markova, O.V.; Fedorova, K.A.; Dedova, M.A.; Iksanova, M.A.; Kamaletdinova, A.A.; Lastochkina, O.V.; Pusenkova, L.I. Malondialdehyde and Proline Content in Bean Cultivars Following the Inoculation with Endophytic Bacteria. Acta Physiol. Plant. 2022, 44, 89. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; SeifiKalhor, M.; Bosacchi, M.; Maslennikova, D.; Lubyanova, A. Novel Approaches for Sustainable Horticultural Crop Production: Advances and Prospects. Horticulturae 2022, 8, 910. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Garshina, D.; Kasnak, C.; Palamutoglu, R.; Shpirnaya, I.; Mardanshin, I.; Maksimov, I. Improving the Biocontrol Potential of Endophytic Bacteria Bacillus subtilis with Salicylic Acid against Phytophthora infestans-Caused Postharvest Potato Tuber Late Blight and Impact on Stored Tubers Quality. Horticulturae 2022, 8, 117. [Google Scholar] [CrossRef]
- Yarullina, L.G.; Tsvetkov, V.O.; Burkhanova, G.F.; Cherepanova, E.A.; Sorokan, A.V.; Zaikina, E.A.; Mardanshin, I.S.; Kalatskaya, J.N.; Balyuk, N.V. Effect of Bacillus subtilis and Signaling Molecules on the State of the Pro/Antioxidant System and the Expression of Protective Protein Genes in Potato Plants Upon Phytophthorosis and a Moisture Deficit. Appl Biochem Microbiol 2021, 57, 760–769. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The hidden world within Plants: Ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Alaylar, B. Isolation and characterization of culturable endophytic plant growth-promoting Bacillus species from Mentha longifolia L. Turk. J. Agric. For. 2022, 46, 73–82. [Google Scholar] [CrossRef]
- Pusenkova, L.I.; Il’yasova, E.Y.; Lastochkina, O.V.; Maksimov, I.V.; Leonova, S.A. Changes in The Species Composition of The Rhizosphere and Phyllosphere of Sugar Beet Under the Impact of Biological Preparations Based on Endophytic Bacteria and Their Metabolites. Eurasian Soil Sci. 2016, 49, 1136–1144. [Google Scholar] [CrossRef]
- Lastochkina, O.; Seifikalhor, M.; Aliniaeifard, S.; Baymiev, A.; Pusenkova, L.; Garipova, S.; Kulabuhova, D.; Maksimov, I. Bacillus spp.: Efficient Biotic Strategy to Control Postharvest Diseases of Fruits and Vegetables. Plants 2019, 8, 97. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Baymiev, A.; Shayahmetova, A.; Garshina, D.; Koryakov, I.; Shpirnaya, I.; Pusenkova, L.; Mardanshin, I.; Kasnak, C.; Palamutoglu, R. Effects of Endophytic Bacillus subtilis and Salicylic Acid on Postharvest Diseases (Phytophthora infestans, Fusarium oxysporum) Development in Stored Potato Tubers. Plants 2020, 9, 76. [Google Scholar] [CrossRef] [PubMed]
- Lastochkina, O.; Pusenkova, L.; Garshina, D.; Yuldashev, R.; Shpirnaya, I.; Kasnak, C.; Palamutoglu, R.; Mardanshin, I.; Garipova, S.; Sobhani, M.; et al. The Effect of Endophytic Bacteria Bacillus subtilis and Salicylic Acid on Some Resistance and Quality Traits of Stored Solanum tuberosum L. Tubers Infected with Fusarium Dry Rot. Plants 2020, 9, 738. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Kiprushkina, E.I. Application of Microbial Preparations in Potato Storage Technologies. Achiev. Sci. Techn. AIC 2015, 29, 33–35. (In Russian) [Google Scholar]
- Leelasuphakul, W.; Sivanunsakul, P.; Phongpaichit, S. Purification, Characterization and Synergistic Activity of B1,3-Glucanase and Antibiotic Extract from An Antagonistic Bacillus subtilis NSRS 89-24 Against Rice Blast and Sheath Blight Pathogens. Enzyme Microb. Technol. 2006, 38, 990–997. [Google Scholar] [CrossRef]
- Pandey, P.K.; Singh, M.C.; Singh, S.S.; Kumar, A.K.; Pathak, M.M.; Shakywar, R.C.; Pandey, A.K. Inside the plants: Endophytic Bacteria and Their Functional Attributes for Plant Growth Promotion. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 11–21. [Google Scholar] [CrossRef]
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 25, 77. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of High Salinity Stress Damage by Plant Growth-Promoting Bacterial Endophytes That Contain ACC Deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Chebotar, V.; Zaplatkin, A.; Komarova, O.; Baganova, M.; Chizhevskaya, E.; Polunin, N.; Balakina, S. Endophytic Bacteria for Development of Microbiological Preparations for Increasing Productivity and Protection of New Potato Varieties. Res. Crops 2021, 22, 104–107. [Google Scholar] [CrossRef]
- Pusenkova, L.I.; Garipova, S.R.; Lastochkina, O.V.; Fedorova, K.A.; Mardanshin, I.S. Influence of Endophytic Bacteria Bacillus subtilis on Harvest, Quality of Tubes and Post-Harvest Diseases of Potato. Agrochem. Her. J. 2021, 5, 73–79. [Google Scholar] [CrossRef]
- Chebotar, V.K.; Zaplatkin, A.N.; Balakina, S.V.; Gadzhiev, N.M.; Lebedeva, V.A.; Khiutti, A.V.; Chizhevskaya, E.P.; Filippova, P.S.; Keleinikova, O.V.; Baganova, M.E.; et al. The Effect of Endophytic Bacteria Bacillus thuringiensis W65 and B. amyloliquefaciens P20 on the Yield and the Incidence of Potato Rhizoctoniosis and Late Blight. Agricul. Biol. 2023, 58, 429–446. [Google Scholar] [CrossRef]
- Mokronosova, A.T. Small Workshop on Plant Physiology; Moscow State University: Moscow, Russia, 1994; p. 184. [Google Scholar]
- Jeffrey, S.; Humphrey, G. New Spectrophotometric Equations for Determining Chlorophylls A, B, C1 and C2 in Higher Plants, Algae and Natural Phytoplankton. Biochem. Physiol. Pfl. 1975, 167, 191–194. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldern, R.P.; Teare, D. Rapid Determination of Free Proline for Water-Stress Studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Islam, M.Z.; Lee, Y.-T.; Mele, M.A.; Choi, I.-L.; Kang, H.-M. The Effect of Phosphorus and Root Zone Temperature on Anthocyanin of Red Romaine Lettuce. Agronomy 2019, 9, 47. [Google Scholar] [CrossRef]
- GOST 13496.4-93. Methods of Nitrogen and Crude Protein Determination. Izdatelstvo Standartov. 1993. Available online: https://docs.cntd.ru/document/1200024323 (accessed on 23 January 2023).
- GOST 26657-97. Methods for Determination of Phosphorus Content. Izdatelstvo Standartov. 1997. Available online: https://docs.cntd.ru/document/1200024370 (accessed on 23 January 2023).
- GOST 30504-97. Flame Photometric Method for Determination of Potassium Content. Izdatelstvo Standartov. 1997. Available online: https://docs.cntd.ru/document/1200024417 (accessed on 23 January 2023).
- GOST 56372-2015. Determination of Mass Fraction of Iron, Manganese, Zinc, Cobalt, Copper, Molybdenum and Selenium by Atomic Absorption Spectroscopy Method. Izdatelstvo Standartov. 2016. Available online: https://docs.cntd.ru/document/1200119647 (accessed on 23 January 2023).
- Vasanthan, T.; Bergthaller, W.; Driedger, D.; Yeung, J.; Sporus, P. Starch from Alberta Potatoes: Wet Isolation and Some Physicochemical Proprieties. Food Res. Int. 1999, 32, 355–365. [Google Scholar] [CrossRef]
- Widmann, N.; Goian, M.; Ianculov, I.; Dumbravă, D.; Moldovan, C. Method to Starch Content Determination from Plants by Specific Weight. Sci. Papers Zootech. Biotech. 2008, 41, 814–818. [Google Scholar]
- GOST 24556-89. Products of Fruits and Vegetables Processing. Methods for Determination of Vitamin C. Izdatelstvo Standartov. 2003. Available online: http://docs.cntd.ru/document/gost-24556-89 (accessed on 23 January 2023).
- Sorokan, A.; Burkhanova, G.; Gordeev, A.; Maksimov, I. Exploring the Role of Salicylic Acid in Regulating the Colonization Ability of Bacillus subtilis 26D in Potato Plants and Defense against Phytophthora infestans. Int. J. Plant Biol. 2023, 14, 242–253. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; del Carmen Orozco-Mosqueda, M.; Glick, B.R. Plant Growth-Promoting Bacterial Endophytes. Microbiol. Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Truyens, S.; Weyens, N.; Cuypers, A.; Vangronsveld, J. Bacterial Seed Endophytes: Genera, Vertical Transmission and Interaction with Plants. Environ. Microbiol. Rep. 2015, 7, 40–50. [Google Scholar] [CrossRef]
- Nelson, E.B. The Seed microbiome: Origins, Interactions, and Impacts. Plant Soil 2017, 422, 7–34. [Google Scholar] [CrossRef]
- Shade, A.; Jacques, M.A.; Barret, M. Ecological Patterns of Seed Microbiome Diversity, Transmission, and Assembly. Curr. Opin. Microbiol. 2017, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Frank, A.; Saldierna Guzmán, J.; Shay, J. Transmission of Bacterial Endophytes. Microorganisms 2017, 5, 70. [Google Scholar] [CrossRef]
- De Almeida, C.V.; Andreote, F.D.; Yara, R.; Tanaka, F.A.O.; Azevedo, J.L.; de Almeida, M. Bacteriosomes in Axenic Plants: Endophytes as Stable Endosymbionts. World J. Microbiol. Biotechnol. 2009, 25, 1757–1764. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J. Auxin and Plant-Microbe Interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, 1–13. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Mechanisms Used by Plant Growth-Promoting Bacteria. In Bacteria in Agrobiology: Plant Nutrient Management; Maheshwari, D.K.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 17–47. [Google Scholar]
- Ahmed, E.; Holmström, S.J.M. Siderophores in Environmental Research: Roles and Applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Joe, M.M.; Devaraj, S.; Benson, A.; Sa, T. Isolation of Phosphate Solubilizing Endophytic Bacteria from Phyllanthus amarus Schum & Thonn: Evaluation of Plant Growth Promotion and Antioxidant Activity Under Salt Stress. J. Appl. Res. Med. Aromat. Plants. 2016, 3, 71–77. [Google Scholar] [CrossRef]
- Derevyagina, М.К.; Vasil’eva, S.V.; Zejruk, V.N.; Belov, G.L. Biological and Chemical Protection of Potato from Diseases. Agrochem. Herald 2018, 5, 65–68. (In Russian) [Google Scholar]
- Sánchez-Rodríguez, A.R.; del Campillo, M.C.; Quesada-Moraga, E. Beauveria bassiana: An Entomopathogenic Fungus Alleviates Fe Chlorosis Symptoms in Plants Grown on Calcareous Substrates. Sci. Hortic. 2015, 197, 193–202. [Google Scholar] [CrossRef]
- Raya-Díaz, S.; Sánchez-Rodríguez, A.R.; Segura-Fernández, J.M.; Campillo, M.D.C.D.; Quesada-Moraga, E. Entomopathogenic Fungi-Based Mechanisms for Improved Fe Nutrition in Sorghum Plants Grown on Calcareous Substrates. PLoS ONE 2017, 12, e0185903. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N.; Karaoglu, S.A.; Guzel, S.; Bozdeveci, A. Trichoderma atroviride ID20G Inoculation Ameliorates Drought Stress-Induced Damages by Improving Antioxidant Defence in Maize Seedlings. Acta Physiol. Plant. 2016, 38, 132. [Google Scholar] [CrossRef]
- Racić, G.; Vukelić, I.; Prokić, L.; Ćurčić, N.; Zorić, M.; Jovanović, L.; Panković, D. The Influence of Trichoderma brevicompactum Treatment and Drought on Physiological Parameters, Abscisic Acid Content and Signalling Pathway Marker Gene Expression in Leaves and Roots of Tomato. Ann. Appl. Biol. 2018, 173, 213–221. [Google Scholar] [CrossRef]
- Kolupaev, Y.E.; Vayner, A.A.; Yastreb, T.O. Proline: Physiological Functions and Regulation of Content in Plants under Stress Conditions. Bull Kharkiv National Univ 2014, 32, 6–22. [Google Scholar]
- Gupta, A.; Bano, A.; Rai, S.; Kumar, M.; Ali, J.; Sharma, S.; Pathak, N. ACC Deaminase Producing Plant Growth Promoting Rhizobacteria Enhance Salinity Stress Tolerance in Pisum sativum. Biotech. 2021, 11, e514. [Google Scholar] [CrossRef]
- Gerrits, N.; Turk, S.; van Dun, K.; Hulleman, S.; Visser, R.; Weisbeek, P.J.; Smeekens, S.C.M. Sucrose Metabolism in Plastids. Plant Physiol 2001, 125, 926–934. [Google Scholar] [CrossRef]
- Torikov, V.E.; Kotikov, M.V.; Bogomaz, O.A. Evaluation of Tubers of Different Varieties of Potatoes for Suitability for Processing inro French Fries and Chips. Vest. Bryansk State Agric. Acad. 2008, 3, 34–40. (In Russian) [Google Scholar]
- Vershinina, Y.A.; Anoshkina, L.S. Original Material for Potato Breeding to Suitability for Industrial Processing to Chips, Starch and Alcohol. Achiev. Sci. Techn. AIC 2009, 9, 15–17. [Google Scholar]
- Abong, G.O.; Okoth, M.W.; Imungi, J.K.; Kabira, J.N. Evaluation of Selected Kenyan Potato Cultivars for Processing into French Fries. Agric. Biol. J. North America 2010, 1, 886–893. [Google Scholar] [CrossRef]
- Nezakonova, L.V.; Pingol, A.P. Improving the Effectiveness of the Selection of Potato Genotypes for Their Suitability for Processing into Crispy Potatoes at the Early Stages of Breeding. Potato Protection 2011, 1, 8–13. (In Russian) [Google Scholar]
- Molyavko, A.A.; Marukhlenko, A.V.; Erenkova, L.A.; Borisova, N.P.; Belous, N.M.; Torikov, V.E. The Quality of Potatoes and Potato Products Depends on The Mineral Nutrition. Vest. Bryansk State Agric. Academy 2019, 5, 10–15. (In Russian) [Google Scholar]
- Cherezov, S.N.; Gizatullina, A.T.; Stashevsky, Z. Evaluation of Potato Breeding Material: Determination of the Suitability of Tubers for Industrial Processing. Sci. Notes Kazan Univ. 2010, 152, 207–216. (In Russian) [Google Scholar]
- Rommens, C.M.; Shakya, R.; Heap, M.; Fessenden, K. Tastier and Healthier Alternatives to French Fries. J. Food Sci. 2010, 75, H109–H115. [Google Scholar] [CrossRef] [PubMed]
- Gaizatullin, A.S.; Mityushkin, A.V.; Zhuravlev, A.A.; Mityushkin, A.V.; Salyukov, S.S.; Ovechkin, S.V.; Simakov, E.A. Selection and Evaluation of the Source Material in Potato Breeding for Suitability for Processing. Potatoes Veget. 2019, 7, 36–40. (In Russian) [Google Scholar]
- Wang-Pruski, G.; Nowak, J. Potato After-Cooking Darkening. Amer. J. Potato Res. 2004, 81, 7–16. [Google Scholar] [CrossRef]
- Pshechenkov, K.A.; Maltsev, S.V. Evaluation of Potato Varieties of VNIIKH Selection for Suitability for Industrial Processing. Potato Prot. 2011, 1, 38–40. (In Russian) [Google Scholar]
- Davidenko, A.; Podpryatov, G.; Shevchenko, A. The Influence of Tuber Conditioning Modes on the Quality of Chips. Modern Sci. 2017, 4, 158–165. (In Russian) [Google Scholar]
- Rubtsov, S.L.; Bakunov, A.L.; Milekhin, A.V.; Dmitrieva, N.N. Analysis of Biochemical Parameters of Potato Minitubers Grown on Various Biotechnological Installations. Izv. Samar. Nauchn. Tsentra Ross. Akad. Nauk. 2017, 19, 648–649. (In Russian) [Google Scholar]
- Simakov, E.A.; Mityushkin, L.V.; Mitryushkin, A.V.; Zhuravlev, A.A. Modern Requirements for Potato Varieties of Various Intended Use. Achiev. Sci. Techn. Agric. 2016, 30, 45–48. (In Russian) [Google Scholar]
- Serderov, V.K.; Alilov, M.M.; Khanbabaev, T.G. Selection of Potato Varieties for Industrial Processing. Bull. Sci. Practice 2018, 4, 144–148. [Google Scholar]
- Zarzecka, K.; Gugała, M. Changes in the Content of Glycoalkaloids in Potato Tubers According to Soil Tillage and Weed Control Methods. Plant Soil Environ. 2007, 53, 247–251. [Google Scholar] [CrossRef]
- Polivanova, O.B.; Gins, E.M. Antioxidant Activity of Potatoes (Solanum tuberosum L.) and Anthocyanin Content, its Biosynthesis and Physiological Role. Veg. Crops Russ. 2019, 6, 84–90. (In Russian) [Google Scholar] [CrossRef]
- Guo, J.; Han, W.; Wang, M.H. Ultraviolet and Environmental Stresses Involved in the Induction and Regulation of Anthocyanin Biosynthesis: A Review. Afr. J. Biotechnol. 2008, 7, 4966–4972. [Google Scholar]
- Wegener, C.B.; Jansen, G. Soft-rot Resistance of Coloured Potato Cultivars (Solanum tuberosum L.): The Role of Anthocyanins. Potato Res. 2007, 50, 31–44. [Google Scholar] [CrossRef]
- Jiao, Y.; Jiang, Y.; Zhai, W.; Yang, Z. Studies on Antioxidant Capacity of Anthocyanin Extract from Purple Sweet Potato (Ipomoea batatas L.). Afr. J. Biotechnol. 2012, 11, 7046–7054. [Google Scholar] [CrossRef]
- Brown, C.R.; Durst, R.W.; Wrolstad, R.; De Jong, W. Variability of Phytonutrient Content of Potato in Relation to Growing Location and Cooking Method. Potato Res. 2008, 51, 259–270. [Google Scholar] [CrossRef]
- Lee, G.B.; Park, H.J.; Cheon, C.G.; Choi, J.G.; Seo, J.H.; Im, J.S.; Park, Y.E.; Cho, J.H.; Chang, D.C. Effect of Plant Container Type on Seed Potato (Solanum tuberosum L.) Growth and Yield in Substrate Culture. Potato Res. 2022, 65, 105–117. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).