Abstract
Different content of Zn in the soil and organic fertilization can affect micronutrient uptake by plants. A pot experiment was carried out to determine the impact of increasing Zn application rates, i.e., 200, 400 and 600 Zn mg·kg−1, in combination with bovine and chicken manures and mushroom substrate on Cu and Ni content, uptake and bioaccumulation factor in cocksfoot (Dactylis glomerata L.). Control objects without Zn and organic fertilizers and after application of only different Zn doses and only organic fertilizers were also tested. Application of Zn at 400 and 600 mg·kg−1 significantly decreased the content, uptake and value of bioaccumulation factor of Cu in the grass. Different Zn doses were not found to influence the content and bioaccumulation factor of Ni in cocksfoot, but application at 200 Zn mg·kg−1 increased Ni uptake. Chicken manure increased the content and bioaccumulation factor of Cu and Ni in the test plant, and all the organic fertilizers increased their uptake. Cocksfoot showed no tendency to excessive bioaccumulation of Cu and Ni.
1. Introduction
To obtain high yields of good quality in agricultural production, it is necessary not only to provide an adequate supply of macroelements to crop plants, but to meet their demand for microelements as well. Essential microelements in plant nutrition include Zn, Cu, Ni and others (e.g., B, Fe, Mn, Mo) [1,2,3]. Their source for plants can be mineral and organic fertilizers or waste substances used in fertilization and industrial human activity [4]. However, taken up by plants in excessive amounts, these metals can have toxic effects, disturbing metabolic processes and decreasing yields [5,6,7,8].
Zn is a catalytic and structural cofactor of many enzymes, influencing the activity of enzymes such as hydrogenase and carbonic anhydrase [9,10]. It also performs crucial structural functions in the domains of proteins which interact with other molecules [11,12]. In addition, Zn increases crop resistance to drought [13]. Zn deficiency inhibits the activity of enzymes involved in photosynthesis [14] and also reduces accumulation of amino acids and protein synthesis. An excessive amount of this metal inhibits photosynthesis and chlorophyll biosynthesis, resulting in reduced whole mass of plants [15,16].
Cu is a catalyst in respiration and photosynthesis and plays an important role in hormonal signalling and cell wall metabolism [17,18]. Cu ions are cofactors in many enzymes, e.g., cytochrome c oxidase, Cu/Zn superoxide dismutase (SOD), amine oxidase, polyphenol oxidase, plastocyanin and laccase [19]. Cu deficiency disturbs metabolism, reduces stress resistance, damages enzyme systems and, in effect, reduces yields [20]. At high concentrations in tissues, Cu inhibits plant growth, disturbs photosynthetic electron transport and causes chlorosis, necrosis and dwarfism [21,22].
Ni is an element that activates urease, which catalyses hydrolysis of urea in plants [23,24]. It is also a component of other metalloenzymes, such as NiFe hydrogenase, superoxide dismutase, carbon monoxide dehydrogenase, methyl-coenzyme M reductase, hydrogenase and RNase A, acetyl-coenzyme-A synthase. Ni deficiency reduces urease activity, disturbs N assimilation and limits scavenging of superoxide free radicals. Excessive amounts of this metal adversely affect nutrient absorption by the roots, disturb plant metabolism, and inhibit photosynthesis and transpiration [25,26].
The uptake of heavy metals into plants’ roots occurs mainly through two pathways: the apoplastic pathway (passive diffusion) and the symplastic pathway (active transport against electrochemical potential gradients and concentrations across the plasma membrane [27]. Uptake of Zn, Ni and Cu by plants depends on numerous soil factors, including pH, content of organic matter, redox conditions and the antagonistic or synergistic interactions between these elements [28,29].
Studies carried out by many researchers have shown that nearly half of the arable soils in the world have a low content of available Zn, resulting in a deficiency of this element in crop plants [2,28,30,31,32,33]. Application of this microelement in the form of mineral and organic fertilizer is the most practical means of preventing the effects of its deficiencies [34]. However, a high content of Zn in the soil may reduce its biological activity and cause the accumulation of this metal in plants [8]. At the same time, it can reduce the uptake of other elements by plants, in relation to which it is an antagonist. In such conditions, the organic fertilizers used may be a source of, e.g., Cu and Ni for plants.
The presented studies show that the content of zinc in Polish soils may range from 12 to 632 mg Zn·kg−1 of soil, which was taken into account when determining its doses in the experiment [35]. Therefore, a study was undertaken to determine the impact of varied levels of Zn applied together with organic materials, e.g., bovine and chicken manures or mushroom substrate, on the content, uptake and bioaccumulation factor of Cu and Ni in cocksfoot.
Grasses occupy a special place among fodder crops. When adequately fertilized, they have high yield potential for dry matter, protein and carbohydrates. Cocksfoot is one of the most important fodder crops in Europe and in the world [36,37]. It attains high yields and is disease-resistant [36], as well as highly resistant to drought [38]. Due to its high quality as fodder, it is used for production of hay and silage and for grazing [36]. The National List of varieties in Poland includes eight cocksfoot cultivars: Amera, Bepra, Berta, Crown Royale, Dika, Minora, Trerano and Tukan [39]. The Amera cultivar, the test plant in the present study, is the earliest variety of cocksfoot, with early onset of growth, a high rate of spring growth and rapid regrowth. It produces high yields of palatable and digestible biomass.
It was hypothesized that increasing doses of Zn reduce the content and bioaccumulation factor of Cu and Ni in cocksfoot, while organic fertilization causes their increase.
2. Materials and Methods
2.1. Experimental Design
A pot experiment was carried out in the years 2014–2016 in a greenhouse in Siedlce (52°10′ N, 22°17′ E, 155 m a.s.l.).
Pots with a capacity of 10 L were filled with 12 kg of soil. The soil was Luvisols consisting of 71% sand, 24% silt and 5% clay.
The experiment was planned in a randomized design in triplicate, with two factors:
I—Zn application amount
II—organic fertilizer.
The experimental design is shown in Table 1.

Table 1.
Design of the pot experiment.
Zn and organic fertilizer were applied once, only in the first year of the study, two weeks before sowing of the grass. Zn was applied as an aqueous solution of ZnSO4·5H2O. Organic fertilizers were applied in amounts that introduced 2 g Corg·kg−1 to the soil. In three years of the study, the test plant was the grass cocksfoot (Dactylis glomerata L.), the Amera cultivar, which was sown in the first decade of May of each year. The green parts of the grass were collected every 30 days, four times a year. During the growing period, the soil moisture in the pots was kept at 60–70% of the total water capacity.
2.2. Laboratory Analyses
2.2.1. Soil
Before starting the experiment, the soil was analysed for pH by potentiometric titration in 1 mol·dm−3 of KCl solution [40]; organic C by a modification of the Tyurin method [41]; total N content by CHNS (automatic CHN analyser with an IDC detector, Series II 2400, Perkin-Elmer, Valencia, CA, USA); and content of total K, P, Zn, Cu and Ni by ACP-AES (Optima 3200 RL spectrometer, Perkin-Elmer, Waltham, MA, USA), following wet mineralization in a mixture of concentrated HCl and HNO3 (3:1 ratio) [42]. Available K and P were determined by the Egner–Riehm method [43]. Selected properties of soil are shown in Table 2.

Table 2.
Some soil properties before the start of the experiment.
2.2.2. Organic Fertilizers
The organic materials used in the experiment (bovine and chicken manures and mushroom substrate) were analysed for dry matter (DW at 105 °C) organic C by the Tyurin method [41]; total N content by CHNS; and content of P, K, Zn, Cu and Ni by ICP-AES following dry mineralization of the samples at 500 °C [42]. The results of the chemical analyses are given in Table 3.

Table 3.
Chemical composition of organic fertilizers.
The contents of dry matter, total N and K were highest in the mushroom substrate, organic C content was highest in the bovine manure, and the contents of P, Zn, Cu and Ni were highest in chicken manure.
2.2.3. Plant Material
The aerial parts of cocksfoot were analysed for content of Cu and Ni by inductively coupled plasma atomic emission spectroscopy (ICP-AES). Prior to the analysis, the samples were subjected to dry mineralization at 450 °C and dissolved in 10% HCl solution [44].
2.3. Calculations
Uptake of Cu and Ni by cocksfoot was calculated according to the formulas given by Bokhari et al. [45]:
where:
CuUP = Y × Cuplant/1000,
CuUP—Cu uptake by grass (accumulation), Cu mg·pot−1
Y—yield of grass, g·pot−1
Cuplant—Cu content in grass, Cu mg·kg−1.
where:
NiUP = Y × Niplant/1000,
NiUP—Ni uptake by grass (accumulation), Ni mg·pot−1
Y—yield of grass, g·pot−1
Niplan—Ni content in grass, Ni mg·kg−1.
In addition, the bioaccumulation factor of Cu and Ni [46,47] was calculated according to the following formulas:
where:
BFCu = Cuplant/Cusoil,
BFCu—Bioaccumulation factor of Cu
Cuplant—Cu content in grass, Cu mg·kg−1
Cusoil—total Cu content in the soil, Cu mg·kg−1
where:
BFNi = Niplant/Nisoil,
BFNi—Bioaccumulation factor of Ni
Niplant—Ni content in grass, Ni mg·kg−1
Nisoil—total Ni content in the soil, Ni mg·kg−1.
2.4. Statistical Analysis
The content, uptake and bioaccumulation factor obtained in subsequent years of the experiment were analysed by analysis of variance using Statistica 13 PL software (ver. 13.1, StatSoft, Tulsa, OK, USA).
where µ—mean from all treatments; ai—effect of Zn level; bj—effect of organic fertilizer type; ck—effect of year (third source of variation); abij—interaction of Zn level and fertilizer type; acik—interaction of Zn level and year; bcjk—interaction of fertilizer type and year; abcijd—interaction of Zn level and fertilizer type and year; eijkp—sampling error.
yijk = µ + ai + bj + ck + abij +acik + bcjk + abcijk + eijkp
The total Cu and Ni uptake over a period of 3 years was also calculated in this application according to the equation:
where µ—mean from all treatments; ai—effect of Zn level; bj—effect of organic fertilizer type; abij—interaction of Zn level and fertilizer type; eijp—sampling error.
yijk = µ + ai + bj + abij + eijp
The significance of the experimental factors was determined on the basis of the Fisher–Snedecor distribution. Least significant difference (LSD) values were calculated by Tukey’s test. The calculations were performed for a significance level of p = 0.05. In addition, Pearson’s linear correlation coefficient was calculated.
3. Results
The content of Cu in the biomass of cocksfoot ranged from 2.35 to 5.24 mg·kg−1 DM (on average, 3.34 Cu mg·kg−1 DM) (Table 4). It was significantly reduced by application of Zn at 400 and 600 mg·kg−1, by 10.6% and 12.0%, respectively, relative to the control. The content of Cu in the grass was not significantly affected by application of 200 Zn mg·kg−1, bovine manure or mushroom substrate. The highest content of this metal was noted in the cocksfoot fertilized with chicken manure; it was 32.5% higher than in the plants from the control treatment and 24.2% and 28.2% higher, respectively, than following application of mushroom substrate and bovine manure. The Cu content in the biomass of the grass significantly decreased in successive years of the experiment. In the second and third years, it was 7.8% and 31.7% lower, respectively, than in the first year of the experiment.

Table 4.
Cu content in grass biomass (Cu mg·kg−1 DM).
All experimental factors also significantly influenced Cu uptake by cocksfoot, calculated as the mean from the three years of the experiment (Table 5) and the total for the 3-year cycle (Figure 1 and Figure 2). In the first year of the study, Cu accumulation was highest following application of 200 Zn mg·kg−1 and lowest following application of 600 Zn mg·kg−1. In the subsequent years of the experiment, increasing application of Zn did not significantly affect Cu uptake by the test plant. The organic fertilizers, irrespective of their origin, increased Cu uptake by cocksfoot. In all years of the study, its accumulation by the plants was significantly the highest following application of chicken manure. Cu uptake by the grass decreased in successive years. In the second and third years of the experiment, it was 57.0% and 31.4%, respectively, of the amount taken up in the first year.

Table 5.
Cu uptake by grass (Cu mg·pot−1).
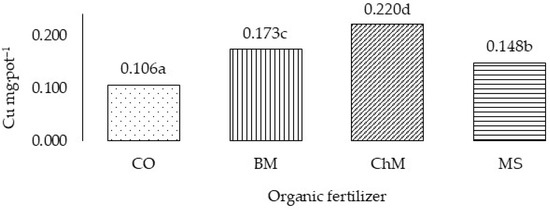
Figure 1.
Total Cu uptake by grass in three years (Cu mg·pot–1)—variability caused by different organic fertilizers. CO—without organic fertilization, BM—bovine manure, ChM—chicken manure, MS—mushroom substrate. a,b,c,d—means investigated factors with different letters are significantly different.
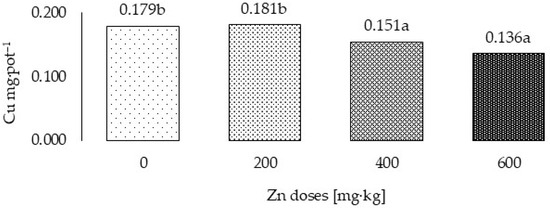
Figure 2.
Total Cu uptake by grass in three years (Cu mg·pot–1)—variability caused by different zinc doses. a,b—means investigated factors with different letters are significantly different.
Ni content in tested grass ranged from 1.34 to 6.40 Ni mg·kg−1 DM (on average, 3.20 Ni mg·kg−1 DM) and did not depend on the amount of Zn applied to the soil (Table 6). It was significantly increased by application of chicken manure, but only in the first year of the study. Mushroom substrate and bovine manure were not shown to significantly influence this parameter. The content of Ni in grass significantly decreased in successive years of the experiment. In the second and third years of the experiment, it was 43.1% and 62.7% lower, respectively, than in the first year.

Table 6.
Ni content in grass biomass (Ni mg·kg−1 DM).
Uptake of Ni by cocksfoot, calculated as the mean from the 3 years of the study (Table 7) and the total for the 3-year cycle (Figure 3 and Figure 4), was significantly dependent on the level of Zn applied to the soil, organic fertilizer, and the year of the experiment. In the 3-year cycle, following application of Zn at 200 mg·kg−1 of soil, the plants accumulated 13.7% more Ni than the plants from the control treatment and 14.4% and 27.5% more, respectively, than following application of 400 and 600 Zn mg·kg−1. No significant differences were noted in accumulation of Ni in the plants from the treatments without Zn application or with application at 400 and 600 Zn mg·kg−1. Bovine and chicken manures and mushroom substrate increased uptake of nickel by the grass by 63.2%, 107.2% and 39.6%, respectively, relative to the control treatment. Significantly, the highest uptake of this metal was noted following application of chicken manure. It was 105.5% higher than in the control plants and 20.4% and 42.7% higher, respectively, than following application of bovine manure and mushroom substrate. During the three years of the experiment, Ni uptake by grass was highest in the first year and lowest in the third year. Uptake in the second and third year was 34.5% and 15.0%, respectively, of the level of uptake in the first year.

Table 7.
Ni uptake by grass (Ni mg·pot−1).
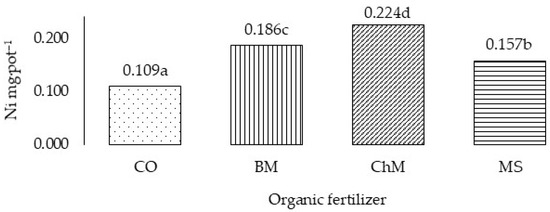
Figure 3.
Total Ni uptake by grass in three years (Ni mg·pot–1)—variability caused by different organic fertilizers. CO—without organic fertilization, BM—bovine manure, ChM—chicken manure, MS—mushroom substrate. a,b,c,d—means investigated factors with different letters are significantly different.
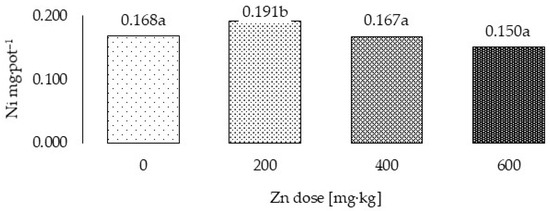
Figure 4.
Total Ni uptake by grass in three years (Ni mg·pot–1)—variability caused by different zinc doses. a,b—means investigated factors with different letters are significantly different.
The application of 400 and 600 Zn mg·kg−1 reduced the factor of Cu bioaccumulation in cocksfoot (Figure 5). There was no significant effect of all doses of Zn on the bioaccumulation factor of Ni in this grass.
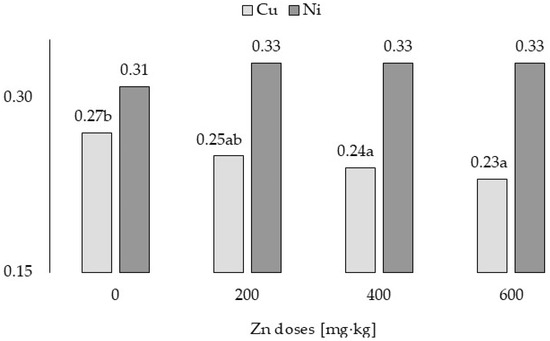
Figure 5.
Bioaccumulation factor of Cu and Ni in grass—variability caused by different zinc doses. a,b for Cu—means investigated factors with different letters are significantly different.
Mushroom substrate and bovine manure did not significantly change the bioaccumulation factor of Cu and Ni, whereas chicken manure increased its value (Figure 6).
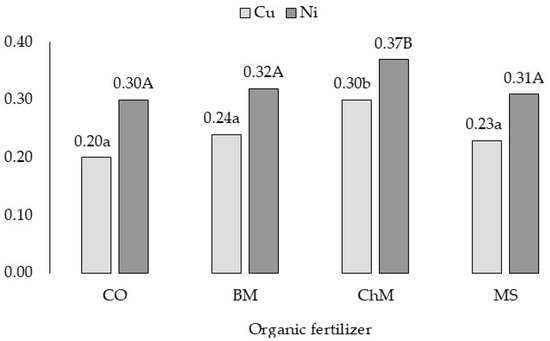
Figure 6.
Bioaccumulation factor of Cu and Ni in grass—variability caused by different organic fertilizers. CO—without organic fertilization, BM—bovine manure, ChM—chicken manure, MS—mushroom substrate. a,b for Cu and A,B for Ni—means investigated factors with different letters are significantly different.
In the subsequent years of the study, the bioaccumulation factor of Cu and Ni decreased (Figure 7).
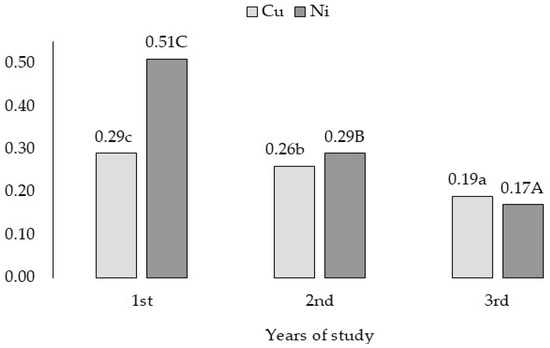
Figure 7.
Bioaccumulation factor of Cu and Ni in grass—variability caused by years of study. a,b,c for Cu and A,B,C for Ni—means investigated factors with different letters are significantly different.
The correlation coefficients showed no clear effect of increasing Zn application (200, 400 and 600 Zn mg·kg−1) on the content and uptake of Cu and Ni by grass (Table 8). They also showed no significant relationship between the content of these two metals and the content of Zn in the grass given by Kuziemska et al. [48]. At the same time, they indicated a significant relationship between the uptake and content of Cu and Ni and the yield of this plant reported in the study cited above.

Table 8.
Linear correlation coefficients between some properties of cultivated grass.
4. Discussion
The biological value of crops is influenced not only by their content of macronutrients and microelements, but also by their proportions. Interactions between elements in crops occur when the supply of one element affects the absorption and use of other elements. Interaction of this type is most common when one element is present in the soil in excessive amounts. Antagonistic and synergistic relationships between plant nutrients cause disturbances in their chemical composition. According to Rietra et al. [49], knowledge of the interactions between nutrients can help to optimize fertilization strategies to achieve high yields of good biological value.
In the present study, the average Cu content in the cocksfoot biomass was 3.34 Cu mg·kg−1 DM. Łukowski et al. [50] reported content of this metal ranging from 2.2 to 21 Cu mg·kg−1 DM in various grass species cultivated in Poland. In a study by Gawryluk et al. [51], the average Cu content in grasses ranged from 8.6 to 16.2 Cu mg·kg−1 DM. Zn application at 400 and 600 mg·kg−1 reduced Cu content, uptake and bioaccumulation factor in cocksfoot. Decreases in Cu content is indicative of antagonistic relationships between these metals. Chaudhry et al. [52] investigated the effect of various levels of application of Zn and Cu on their uptake by rice (Oryza sativa L.) and rice yield. They showed that Zn application reduced the content of Cu in the biomass of the plant. Similar tendencies were obtained by Imtiaz et al. [53] for the relationship between Zn and Cu. In a pot experiment, they tested the effect of increasing Zn application (0, 5, 10, 15 and 20 Zn mg·ml−1) on the content of Cu in wheat. The authors found that Zn, irrespective of the rate of application, decreased the content of Cu in the test plant. Zhao et al. [54] tested the effect of Zn, Mn and Cu on plant growth and accumulation of these elements in Phytolacca americana. They showed that excessive Zn application leads to chlorosis, disturbs elemental homeostasis and reduces the Cu concentration in the plant. Antagonistic relationships between Zn and Cu have also been reported by Abou Seeda et al. [3], Mousavi et al. [55] and Liščáková et al. [56].
Contrasting results for the interrelationships between Zn and Cu were obtained by Malinowska et al. [57], who tested the content of these metals in selected plants growing along a motorway. They found no significant correlations between their content in the plants. The results obtained by the cited authors and the results of our own research indicate that the relationship between the content of Zn and Cu in plants probably depends on their species.
The present study showed no significant effect of bovine manure or mushroom substrate on the content and bioaccumulation factor of Cu by cocksfoot. Application of chicken manure, with which the largest quantity of this metal was applied to the soil, significantly increased its content in the biomass of the grass. In addition, all of the organic fertilizers increased Cu uptake by the test plant. Sumner [58] and Wuana and Okieimen [59] described bovine manure and chicken manure as potential sources of Cu for plants. At the same time, Wuana and Okieimen [59] reported that Cu is added to livestock fodder as a growth promoter, which may result in an increase in its content in animal waste and in the biomass of fertilized crops. Velusami et al. [60] tested the fertilizer value of mushroom substrates and showed that they are a valuable source of macro- and microelements, including Cu, for plants. The correlation coefficients indicate that the increase in Cu accumulation by cocksfoot under the influence of chicken manure, bovine manure and mushroom substrate is associated with stimulation of yield by these fertilizers, discussed in a previous paper [48].
The average content of Ni in cocksfoot was 3.20 Ni mg·kg−1 DM. Wowkowicz et al. [61] tested the content of heavy metals in crops grown on permanent grassland in the vicinity of Warsaw. The Ni content in the grasses ranged from 0.8 to 2.28 Ni mg·kg−1 DM. Contrasting results were presented by Łukowski et al. [50], who found highly varied content of this metal in fodder grasses, ranging from 4.55 to 39.60 Ni mg·kg−1 DM.
The present study showed no significant effect of increasing soil application of Zn (200, 400 and 600 Zn mg·kg−1) on the content and bioaccumulation factor of Ni in the biomass of cocksfoot. The results do not indicate an interaction between Zn and Ni. Previous studies also showed no antagonistic or synergistic relationships between these metals in cocksfoot cultivation [62,63]. Contrasting results were obtained by Dalir et al. [64], who studied the relationship between the uptake of Zn and Ni by spring wheat grown in a water medium containing varied concentrations of both metals. The authors found that the interactions were dependent on their content in the medium. Zn at low concentrations did not affect Ni uptake, but higher Zn content in the medium reduced Ni uptake by wheat. Antagonistic relationships between Zn and Ni in cultivation of Arabidopsis thaliana (L.) were also reported by Nishida et al. [65]. At the same time, application of 200 Zn mg·kg−1 soil caused a significant increase in the uptake of Ni in the test plant. Analysis of the correlation coefficients indicates that this is linked to the increase in the yield of cocksfoot.
In our study, the highest amount of Ni was applied to the soil with chicken manure, which caused a significant increase in its content and bioaccumulation factor in the cocksfoot biomass. Earlier research on the chemical composition of selected organic fertilizers (pig, bovine and chicken manure) showed that chicken manure was the best source of Ni for plants [66]. The organic fertilizers, irrespective of their origin, increased uptake of this metal by the test plant. Analysis of the correlation coefficients indicates that this is directly linked to their positive effect on the yield of cocksfoot. The values of the bioaccumulation factor provide information about the ability of plants to absorb elements from the soil. In research conducted by Łukowski et al. [50], the average bioaccumulation factor of Cu and Ni in Polish grasses was 0.8 and 1.8, respectively. Small values (much lower than 1) of this factor for Cu and Ni obtained in our own research indicate that cocksfoot does not tend to excessive accumulation of these heavy metals [46].
5. Conclusions
Zn applied to the soil at 200 mg·kg−1 did not influence the content, uptake and bioaccumulation factor of Cu in cocksfoot, whereas higher application rates—400 and 600 Zn mg·kg−1—significantly reduced them. Decrease in the copper content indicates antagonistic relationships between Zn and Cu. Application of Zn, irrespective of the application rate, did not affect the content and bioaccumulation factor of Ni in the grass, but it increased its uptake at 200 Zn mg·kg−1, as a result of increasing the yield. Bovine manure and mushroom substrate did not affect the contents and bioaccumulation factor of Cu or Ni in the grass. The chicken manure application, with which the largest amount of Cu and Ni was introduced, resulted in a significant increase in their content and bioaccumulation coefficient in tested grass. All of the organic fertilizers increased the uptake of Ni and Cu by the biomass of the test grass. Uptake of both of these metals was highest in the grass fertilized with chicken manure. Low values of bioaccumulation factors of Cu and Ni indicate a low potential of cocksfoot for their excessive accumulation.
Author Contributions
Conceptualization, B.K., A.W. and P.K.; methodology, B.K., P.K. and A.W.; resources, B.K., P.K. and A.W.; writing—original draft preparation, B.K. and A.W.; writing—review and editing, B.K. and A.W.; visualization, A.W. and B.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish Ministry of Science and Higher Education, grant numbers 314/12/S, 105/14/MN and 158/23/B.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Fageria, N.K.; Baligar, V.C.; Clark, R.B. Micronutrients in Crop Production Sparks. In Advances in Agronomy; Donald, L., Ed.; Academic Press: Cambridge, MA, USA, 2002; Volume 77, pp. 185–268. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrients and crop production: An introduction. In Micronutrient Deficiencies in Global Crop Production; Alloway, B.J., Ed.; Springer: Dordrecht, The Netherlands, 2008; pp. 1–39. [Google Scholar] [CrossRef]
- Abou Seeda, M.A.; Abou El-Nour, E.A.A.; Yassen, A.A.; Gad Mervat, M.; Sahar, M.Z. Interaction of copper, zinc, and their importance in plant physiology: Review, Acquisition and Transport. Middle East J. Appl. Sci. 2020, 10, 407–434. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Menzies, N.W.; de Jonge, M.D.; McKenna, B.A.; Donner, E.; Webb, R.I.; Paterson, D.J.; Howard, D.L.; Ryan, C.G.; Glover, C.J.; et al. In situ distribution and speciation of toxic copper, nickel, and zinc in hydrated roots of cowpea. Plant Physiol. 2011, 156, 663–673. [Google Scholar] [CrossRef] [PubMed]
- Arif, N.; Yadav, V.; Singh, S.; Singh, S.; Ahmad, P.; Mishra, R.K.; Sharma, S.; Tripathi, D.K.; Dubey, N.K.; Chauhan, D.K. Influence of high and low levels of plant-beneficial heavy metal ions on plant growth and development. Front. Environ. Sci. 2016, 4, 69. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Qureshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risks and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef]
- Angulo-Bejarano, P.I.; Puente-Rivera, J.; Cruz-Ortega, R. Metal and metalloid toxicity in plants: An overview on molecular Aspects. Plants 2021, 10, 635. [Google Scholar] [CrossRef]
- Kaur, H.; Garg, N. Zinc toxicity in plants: Review. Planta 2021, 253, 129. [Google Scholar] [CrossRef]
- Hambidge, M.; Cousins, R.J.; Costello, R.B. Zinc and health: Current status and future directions. J. Nutr. 2020, 130, 1437S–1446S. [Google Scholar] [CrossRef]
- Hafeez, B.; Khanif, Y.; Saleem, M. Role of zinc in plant nutrition—A review. Am. J. Exp. Agric. 2013, 3, 374–391. [Google Scholar] [CrossRef]
- Ma, D.; Sun, D.; Wang, C.; Ding, H.; Qin, H.; Hou, J.; Huang, X.; Xie, Y.; Guo, T. Physiological responses and yield of wheat plants in zinc-Mediated alleviation of drought stress. Front. Plant Sci. 2017, 8, 860. [Google Scholar] [CrossRef]
- Cabot, C.; Martos, S.; Llugany, M.; Gallego, B.; Tolrà, R.; Poschenrieder, C.A. Role for zinc in plant defense against pathogens and herbivores. Front. Plant Sci. 2019, 10, 1171. [Google Scholar] [CrossRef]
- Umair Hassan, M.; Aamer, M.; Umer Chattha, M.; Haiying, T.; Shahzad, B.; Barbanti, L.; Nawaz, M.; Rasheed, A.; Afzal, A.; Liu, Y.; et al. The critical role of zinc in plants facing the drought stress. Agriculture 2020, 10, 396. [Google Scholar] [CrossRef]
- Brown, P.H.; Cakmak, I.; Zhang, Q. Form and function of zinc plants. In Zinc in Soils and Plants: Developments in Plant and Soil Sciences; Robson, A.D., Ed.; Springer: Dordrecht, The Netherlands, 1993; Volume 55, pp. 93–106. [Google Scholar] [CrossRef]
- Stoyanova, Z.; Doncheva, S. The effect of zinc supply and succinate treatment on plant growth and mineral uptake in pea plant. Braz. J. Plant Physiol. 2002, 14, 111–116. [Google Scholar] [CrossRef]
- Balafrej, H.; Bogusz, D.; Triqui, Z.E.A.; Guedira, A.; Bendaou, N.; Smouni, A.; Fahr, M. Zinc hyperaccumulation in plants: A review. Plants 2020, 9, 562. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Pilon, M.; Shikanai, T. How do plants respond to copper deficiency? Plant Signal Behav. 2008, 3, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Wairich, A.; De Conti, L.; Lamb, T.I.; Keil, R.; Neves, L.O.; Brunetto, G.; Sperotto, R.A.; Ricachenevsky, F.K. Throwing copper around: How plants control uptake, distribution, and accumulation of copper. Agronomy 2022, 12, 994. [Google Scholar] [CrossRef]
- Aguirre, A.J.; Meyers, R.M.; Weir, B.A.; Vazquez, F.; Zhang, C.Z.; Ben-David, U.; Cook, A.; Ha, G.; Harrington, W.F.; Doshi, M.B.; et al. Genomic copy number dictates a gene-independent cell response to CRISPR/Cas9 targeting. Cancer Discov. 2016, 6, 914–929. [Google Scholar] [CrossRef]
- Hippler, F.W.R.; Boaretto, R.M.; Quaggio, J.A.; Mattos Júnior, D. Copper in citrus production: Required but avoided. Citrus Res. Technol. 2017, 38, 99–106. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant. Physiol. 2005, 17, 185–268. [Google Scholar] [CrossRef]
- Pietrini, F.; Carnevale, M.; Beni, C.; Zacchini, M.; Gallucci, F.; Santangelo, E. Effect of different copper levels on growth and morpho-physiological parameters in giant reed (Arundo donax L.) in semi-hydroponic mesocosm experiment. Water 2019, 11, 1837. [Google Scholar] [CrossRef]
- Fabiano, C.C.; Tezotto, T.; Favarin, J.L.; Polacco, J.C.; Mazzafera, P. Essentiality of nickel in plants: A role in plant stresses. Front. Plant Sci. 2015, 6, 754. [Google Scholar] [CrossRef]
- Harasim, P.; Filipek, T. Nickel in the environment. J. Elem. 2015, 20, 525–534. [Google Scholar] [CrossRef]
- Ahmad, M.S.; Ashraf, M. Essential roles and hazardous effects of nickel in plants. Rev. Environ. Contam. Toxicol. 2011, 214, 125–167. [Google Scholar] [CrossRef]
- Shahzad, B.; Tanveer, M.; Rehman, A.; Cheema, S.A.; Fahad, S.; Rehman, S.; Sharma, A. Nickel; whether toxic or essential for plants and environment—A review. Plant Physiol. Biochem. 2018, 132, 641–651. [Google Scholar] [CrossRef]
- Yan, A.; Wang, Y.; Tan, S.N.; Mohd Yusof, M.L.; Ghosh, S.; Chen, Z. Phytoremediation: A promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 2020, 11, 359. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Shivay, Y.; Kumar, D. Interactions of zinc with other nutrients in soils and plants—A Review. Indian J. Fert. 2016, 12, 16–26. [Google Scholar]
- Fan, T.T.; Wang, Y.J.; Li, C.B.; He, J.Z.; Gao, J.; Zhou, D.M.; Friedman, S.P.; Sparks, D.L. Effect of organic matter on sorption of Zn on soil: Elucidation by wien effect measurements and EXAFS spectroscopy. Environ. Sci. Technol. 2016, 50, 2931–2937. [Google Scholar] [CrossRef]
- Naik, S.K.; Das, D.K. Relative performance of chelated zinc and zinc sulphate for lowland rice (Oryza sativa L.). Nutr. Cycl. Agroecosyst. 2008, 81, 219–227. [Google Scholar] [CrossRef]
- Sharma, A.; Patni, B.; Shankhdhar, D.; Shankhdhar, S.C. Zinc—An indispensable micronutrient. Physiol. Mol. Biol. Plants. 2013, 19, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef]
- Doolette, C.L.; Read, T.L.; Li, C.; Scheckel, K.G.; Donner, E.; Kopittke, P.M.; Schjoerring, J.K.; Lombi, E. Foliar application of zinc sulphate and zinc EDTA to wheat leaves: Differences in mobility, distribution, and speciation. J. Exp. Bot. 2018, 69, 4469–4481. [Google Scholar] [CrossRef]
- Kumar, S.; Verma, G.; Dhaliwal, S.S. Zinc phasing and fertilization effects on soil properties and some agromorphological parameters in maize–wheat cropping system. Commun. Soil Sci. Plant Anal. 2022, 53, 453–462. [Google Scholar] [CrossRef]
- Arasimowicz, M.; Niemiec, M.; Wiśniowska-Kielian, B. Zinc, copper and chromium content in soils and needles of the scots pine (Pinus silvestris L.) from the Krakow agglomeration terrain. Ecol. Chem. Eng. A 2010, 17, 1543–1552. [Google Scholar]
- Last, L.; Widmer, F.; Fjellstad, W.; Stoyanova, S.; Kölliker, R. Genetic diversity of natural orchardgrass (Dactylis glomerata L.) populations in three regions in Europe. BMC Genet. 2013, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Šiaudinis, G.; Jasinskas, A.; Karčauskienė, D.; Šarauskis, E.; Lekavičienė, K.; Repšienė, R. The dependence of cocksfoot productivity of liming and nitrogen application and the assessment of qualitative parameters and environmental impact using biomass for biofuels. Sustainability 2020, 12, 8208. [Google Scholar] [CrossRef]
- Zhouri, L.; Kallida, R.; Shaimi, N.; Barre, P.; Volaire, F.; Gaboun, F.; Douaik, A.; Fakiri, M. Characterization of cocksfoot (Dactylis glomerata L.) population for growth traits and summer dormancy. J. Mater. Environ. Sci. 2017, 8, 4378–4384. [Google Scholar] [CrossRef]
- National Register of Crop Varieties. Available online: https://coboru.gov.pl/pl/szczegoly_odmiany?nrodm=1990 (accessed on 25 May 2023).
- Stępień, M.; Bodecka, E.; Gozdowski, D.; Wijata, M.; Groszyk, J.; Studnicki, M.; Sobczyński, G.; Rozbicki, J.; Samborski, S. Compatibility of granulometric groups determined based on standard BN-78/9180-11 and granulometric groups according to PTG 2008 and USDA texture classes. Soil Sci. Ann. 2018, 69, 223–233. [Google Scholar] [CrossRef]
- Barančíková, G.; Makovníková, J. Comparison of two methods of soil organic carbon determination. Pol. J. Soil Sci. 2015, 48, 48–56. [Google Scholar] [CrossRef]
- Adamczyk, D.; Jankiewicz, B. Effects of thiuram on uptake of copper, zinc and manganese by Valeriana officinalis. Pol. J. Environ. Stud. 2008, 17, 823–826. [Google Scholar]
- Egnér, H.; Riehm, H.; Domingo, W.R. Studies on chemical soil analysis as a basis for assessing the nutrient status of soils. II. Chemical extraction methods for phosphorus and potassium determination. K. Lantbrukshögskolans Ann. 1960, 26, 199–215. [Google Scholar]
- Malinowska, E.; Jankowski, K.; Wiśniewska-Kadżajan, B.; Skrzyczyńska, J.; Sosnowski, J. Cobalt and arsenic concentration in herbs growing in field pond areas in Poland. Appl. Ecol. Environ. Res. 2018, 16, 3805–3814. [Google Scholar] [CrossRef]
- Bokhari, S.H.; Ahmad, I.; Mahmood-Ul-Hassan, M.; Mohammad, A. Phytoremediation potential of Lemna minor L. for heavy metals. Int. J. Phytoremediation 2016, 18, 25–32. [Google Scholar] [CrossRef]
- Netty, S.; Wardiyati, T.; Maghfoer, M.D.; Handayanto, E. Bioaccumulation of nickel by five wild plant species on nickel contaminated soil. IOSR J. Eng. 2013, 3, 1–6. [Google Scholar] [CrossRef]
- Aladesanmi, O.T.; Oroboade, J.G.; Osisiogu, C.P.; Osewole, A.O. Bioaccumulation factor of selected heavy metals in Zea mays. J. Health Pollut. 2019, 9, 191207. [Google Scholar] [CrossRef] [PubMed]
- Kuziemska, B.; Klej, P.; Wysokinski, A.; Jaremko, D.; Pakuła, K. Yielding and bioaccumulation of zinc by cocksfoot under conditions of different doses of this metal and organic fertilization. Agronomy 2022, 12, 686. [Google Scholar] [CrossRef]
- Rietra, R.P.J.J.; Heinen, M.; Dimkpa, C.O.; Prem, S.; Bindraban, P.S. Effects of nutrient antagonism and synergism on yield and fertilizer use efficiency. Commun. Soil Sci. Plant Anal. 2017, 48, 1895–1920. [Google Scholar] [CrossRef]
- Łukowski, A.; Wiater, J.; Dymko, A. Bioaccumulation of heavy metals in forage grasses. Ecol. Eng. Environ. Technol. 2017, 18, 149–158. [Google Scholar] [CrossRef]
- Gawryluk, A.; Wyłupek, T.; Wolański, P. Assessment of Cu, Pb and Zn content in selected species of grasses and in the soil of the roadside embankment. Ecol. Evol. 2020, 10, 9841–9852. [Google Scholar] [CrossRef]
- Chaudhry, F.M.; Sharif, M.; Latif, A.; Qureshi, R.H. Zinc-copper antagonism in the nutrition of rice (Oryza sativa L.). Plant Soil 1973, 38, 573–580. [Google Scholar] [CrossRef]
- Imtiaz, M.; Alloway, B.J.; Shah, K.H.; Siddiqui, S.H.; Memon, M.Y.; Aslam, M.; Khan, P. Zinc nutrition of wheat: II: Interaction of zinc with other trace elements. Asian J. Plant Sci. 2003, 2, 156–160. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Chai, T.; Zhang, Y.; Tan, J.; Ma, S. The effects of copper, manganese and zinc on plant growth and elemental accumulation in the manganese-hyperaccumulator Phytolacca americana. J. Plant Physiol. 2012, 169, 1243–1252. [Google Scholar] [CrossRef]
- Mousavi, S.R.; Galavi, M.; Rezaei, M. The interaction of zinc with other elements in plants: A review. Indian J. Adv. Chem. Sci. 2012, 4, 1881–1884. [Google Scholar]
- Liščáková, P.; Nawaz, A.; Molnárová, M. Reciprocal effects of copper and zinc in plants. Int. J. Environ. Sci. Technol. 2022, 19, 9297–9312. [Google Scholar] [CrossRef]
- Malinowska, E.; Jankowski, K.; Wiśniewska-Kadżajan, B.; Sosnowski, J.; Kolczarek, R.; Jankowska, J.; Ciepiela, G.A. Content of zinc and copper in selected plants growing along a motorway. Bull. Environ. Contam. Toxicol. 2015, 95, 638–643. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sumner, M.E. Beneficial use of effluents, wastes, and biosolids. Commun. Soil Sci. Plant Anal. 2008, 31, 1701–1715. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Velusami, B.; Jordan, S.N.; Curran, T.; Grogan, H. Fertiliser characteristics of stored spent mushroom substrate as a sustainable source of nutrients and organic matter for tillage, grassland and agricultural soils. Ir. J. Agric. Food Res. 2021, 60, 1–11. [Google Scholar] [CrossRef]
- Wowkonowicz, P.; Malowaniec, B.; Niesiobędzka, K. Heavy metals in soil and plants on grassland around Warsaw. Environ. Prot. Natur. Resour. 2011, 49, 309–319. [Google Scholar]
- Kuziemska, B.; Kalembasa, D.; Kalembasa, S. Effect of liming and organic materials on content of selected metals in of cocksfoot grown in soil contaminated with nickel. Acta Agroph. 2014, 21, 293–304. [Google Scholar]
- Kuziemska, B.; Kalembasa, S.; Kalembasa, D. Effect of liming and use of organic materials on the contents of cooper adn zinic in of cock’s-foot grown in soil contaminated with nickel. Acta Agroph. 2017, 24, 263–270. [Google Scholar]
- Dalir, N.; Tandy, S.; Gramlich, A.; Khoshgoftarmanesh, A.; Schulin, R. Effects of nickel on zinc uptake and translocation in two wheat cultivars differing in zinc efficiency. Environ. Exp. Bot. 2017, 134, 96–101. [Google Scholar] [CrossRef]
- Nishida, S.; Kato, A.; Tsuzuki, C.; Yoshida, J.; Mizuno, T. Induction of nickel accumulation in response to zinc deficiency in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 9420–9430. [Google Scholar] [CrossRef] [PubMed]
- Kuziemska, B.; Jaremko, D.; Wysokiński, A. Content of macroelements and fractions of nickel in selected organic fertilizers. Fresenius Env. Bull. 2019, 28, 1417–1422. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).