Changes in Enzyme Activities in Salt-Affected Soils during Incubation Study of Diverse Particle Sizes of Rice Straw
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site and Soil
2.2. Analysis of Plant Material
2.3. Treatments and Experimental Procedure
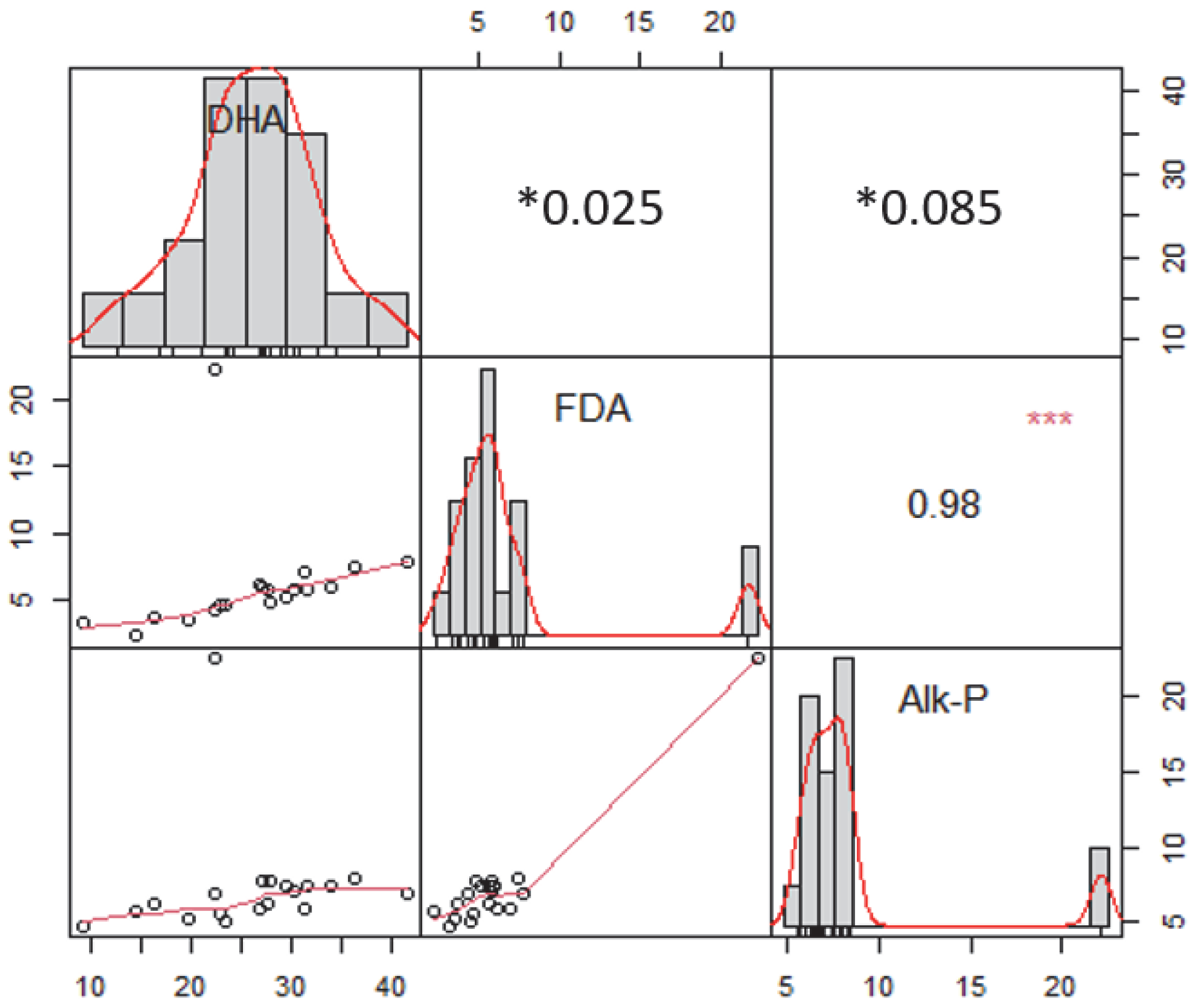
2.4. Statistical Analysis
3. Results
3.1. Biochemical Quality of Rice Residue
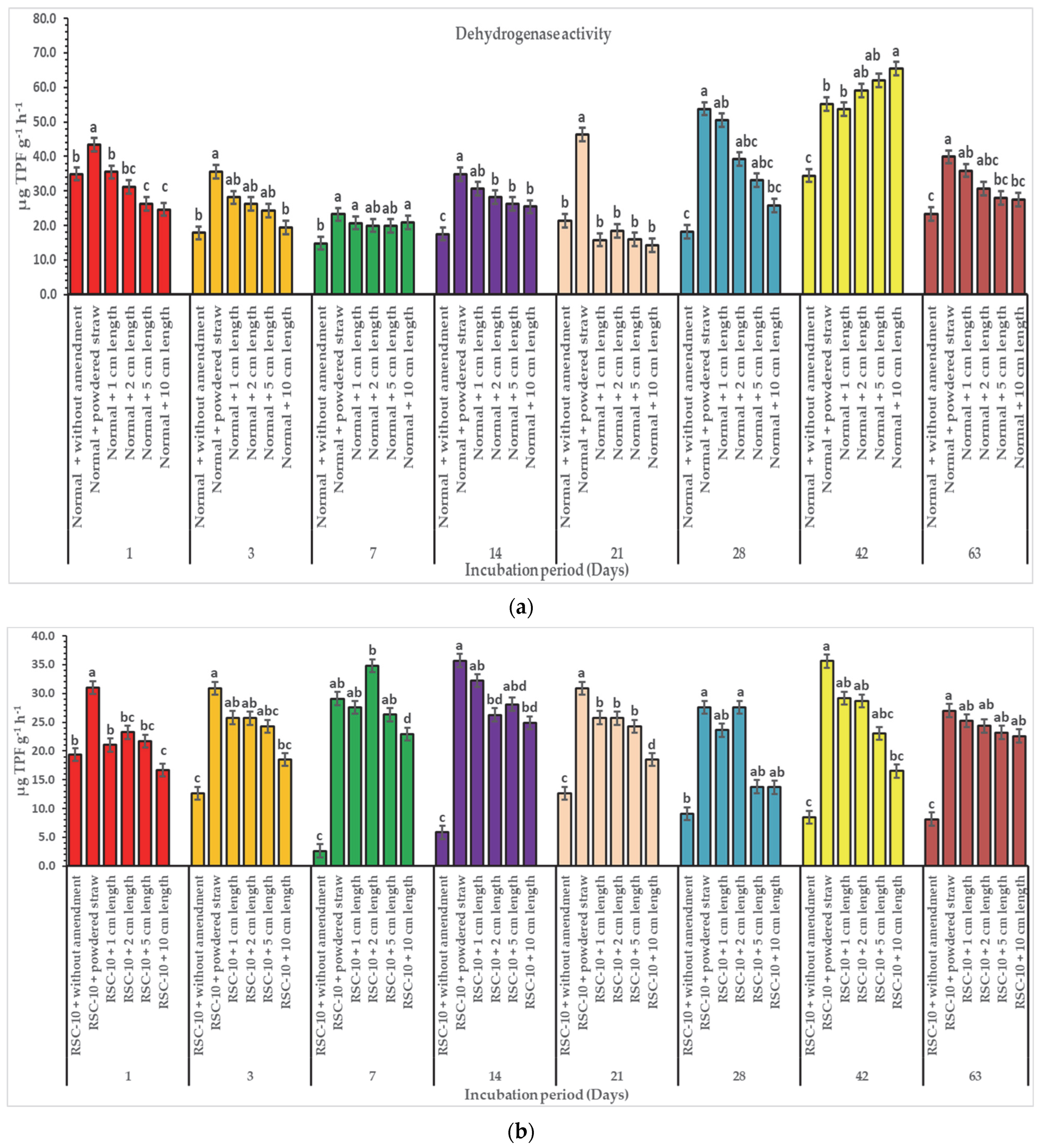
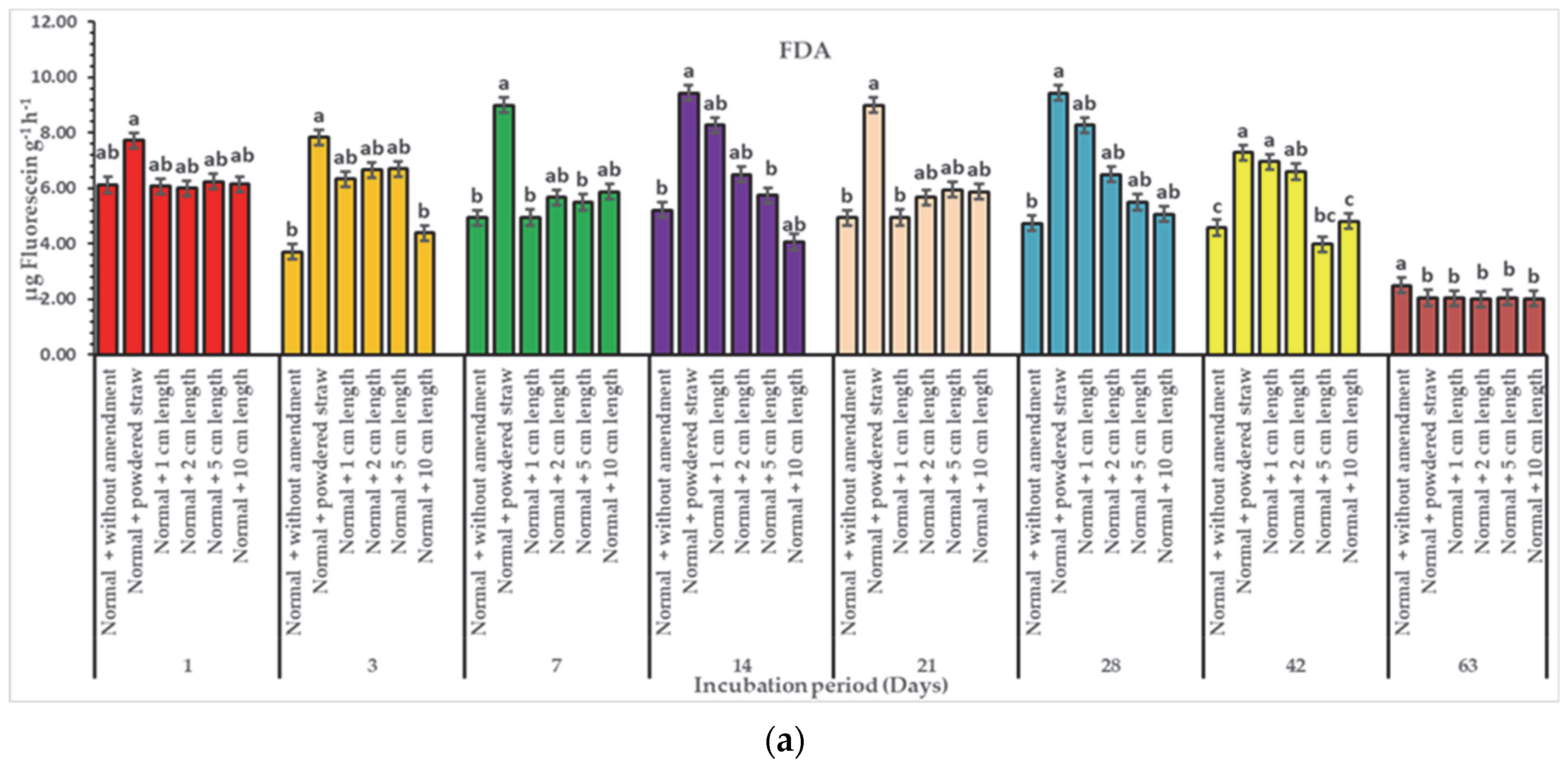
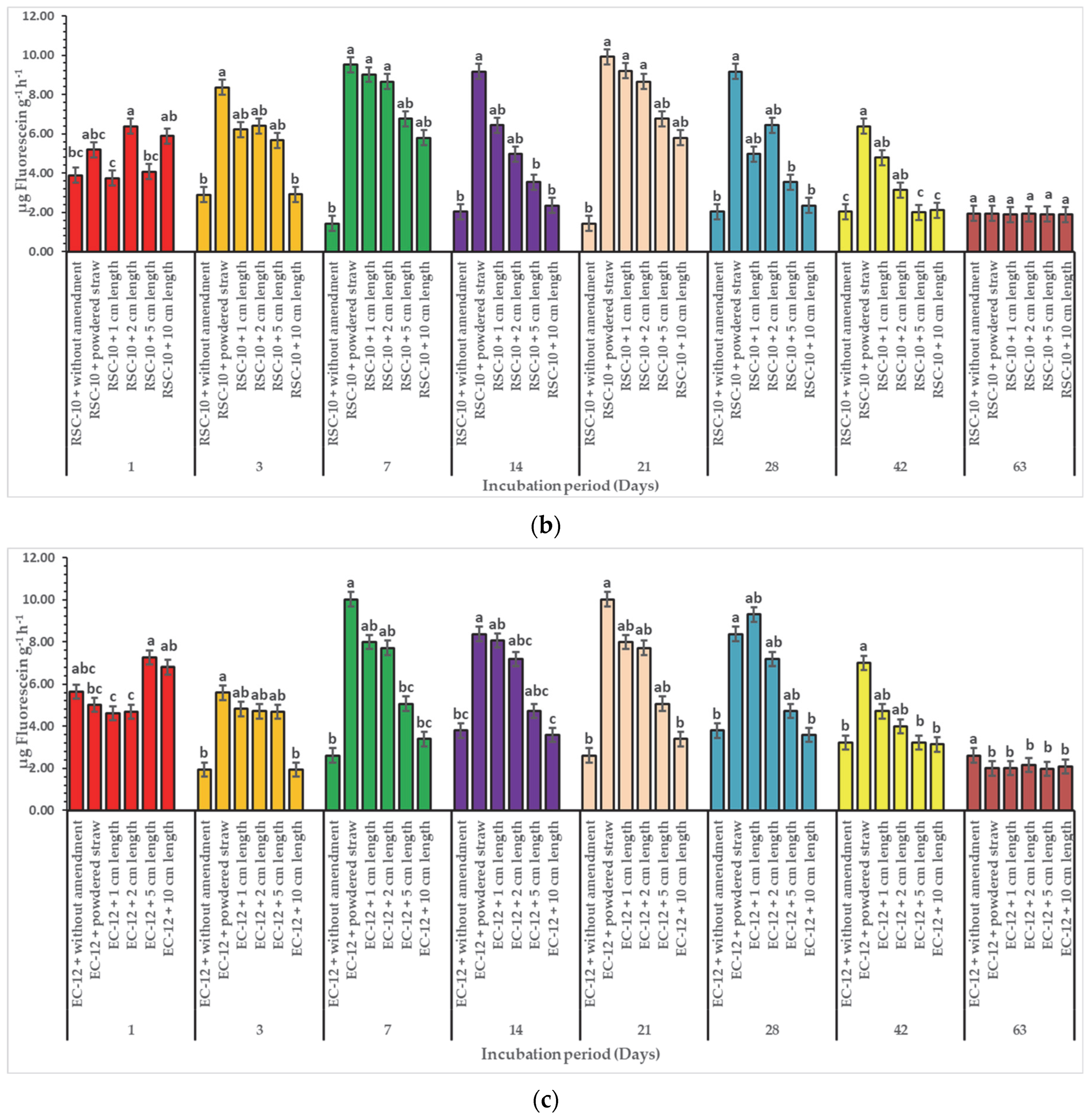
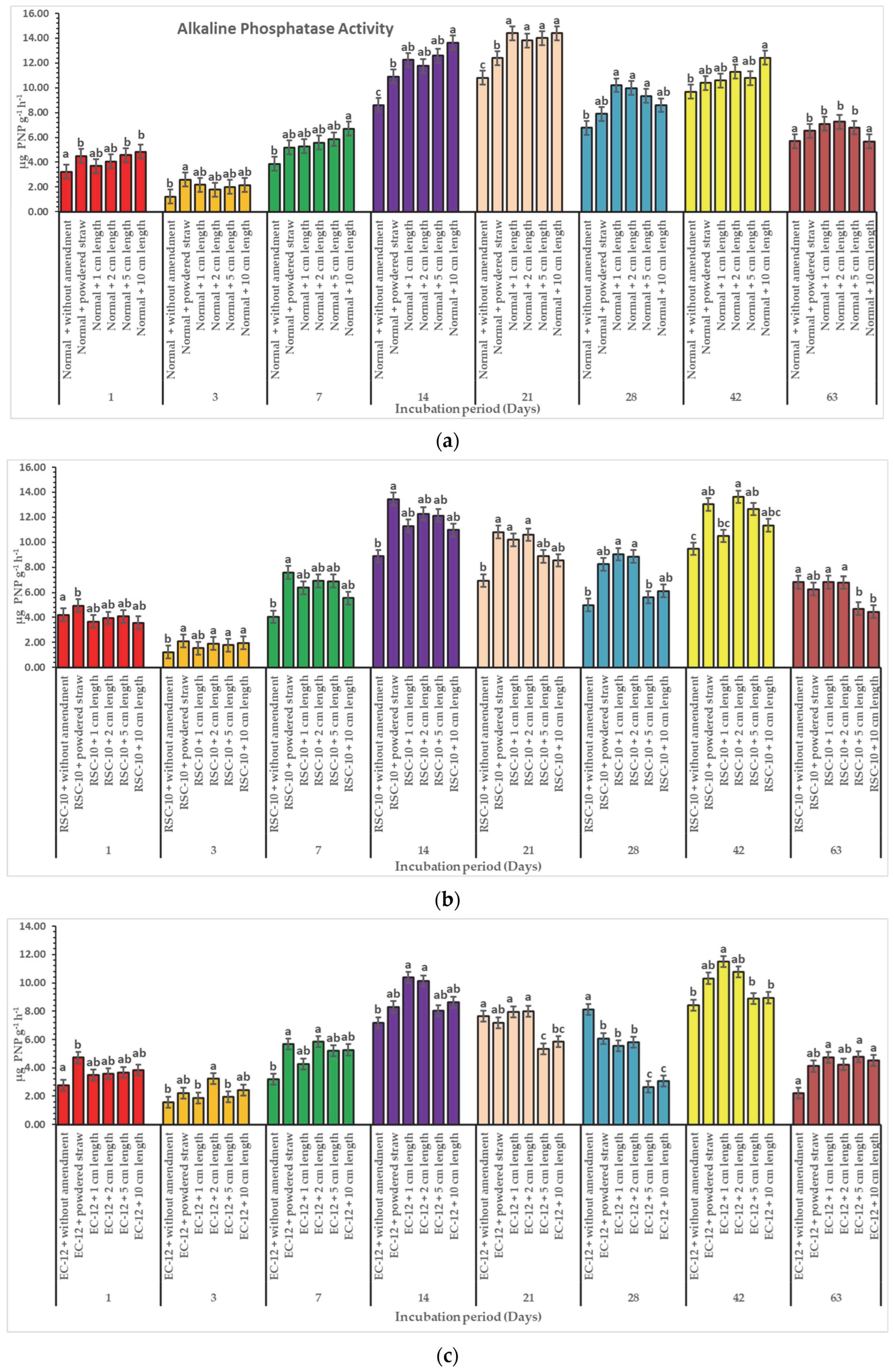
3.2. Effect of Diverse Particle Sizes of Rice Straw on Enzymatic Activities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Tang, J.; Li, Z.; Liu, Y.; Zhou, Z.; Wang, J.; Dai, Z. Carbon mineralization under different saline—Alkali stress conditions in paddy fields of Northeast China. Sustainability 2020, 12, 2921. [Google Scholar] [CrossRef]
- Zhang, S.J.; Zhang, G.; Wang, D.J.; Liu, Q. Abiotic and biotic effects of long-term straw retention on reactive nitrogen runoff losses in a rice–wheat cropping system in the Yangtze Delta region. Agric. Ecosyst. Environ. 2021, 305, 107162. [Google Scholar] [CrossRef]
- Buckley, S.; Allen, D.; Brackin, R.; Jamtgard, S.; Nasholm, T.; Schmidt, S. Microdialysis as an in situ technique for sampling soil enzymes. Soil Biol. Biochem. 2019, 135, 20–27. [Google Scholar] [CrossRef]
- Mazzon, M.; Cavani, L.; Margon, A.; Sorrenti, G.; Ciavatta, C.; Marzadori, C. Changes in soil phenol oxidase activities due to long-term application of compost and mineral N in a walnut orchard. Geoderma 2018, 316, 70–77. [Google Scholar] [CrossRef]
- Ma, L.; Zhao, B.; Guo, Z.; Wang, D.; Li, D.; Xu, J.; Zhang, J. Divergent responses of bacterial activity, structure, and co-occurrence patterns to long-term unbalanced fertilization without nitrogen, phosphorus, or potassium in a cultivated vertisol. Environ. Sci. Pollut. Res. 2019, 26, 12741–12754. [Google Scholar] [CrossRef]
- Das, S.K.; Varma, A. Role of enzymes in maintaining soil health. In Soil Enzymology; Shukla, G., Varma, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 25–42. [Google Scholar]
- Sun, R.; Dsouza, M.; Gilbert, J.A.; Guo, X.; Wang, D.; Guo, Z.; Ni, Y.; Chu, H. Fungal community composition in soils subjected to long-term chemical fertilization is most influenced by the type of organic matter. Environ. Microbiol. 2016, 18, 5137–5150. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Goh, K.M. Crop residue management: Effects on soil quality, soil nitrogen dynamics, crop yield, and nitrogen recovery. Adv. Agron. 2000, 68, 197–319. [Google Scholar]
- Wang, J.B.; Chen, Z.H.; Chen, L.J.; Zhu, A.N.; Wu, Z.J. Surface soil phosphorus and phosphatase activities affected by tillage and crop residue input amounts. Plant Soil Environ. 2011, 6, 251–257. [Google Scholar] [CrossRef]
- Ahmad, R.; Jilani, G.; Arshad, M.; Zahir, Z.A.; Khalid, A. Bio-conversion of organic wastes for their recycling in agriculture: An overview of perspectives and prospects. Ann. Microbiol. 2007, 57, 471–479. [Google Scholar] [CrossRef]
- Alghamdi, R.S.; Cihacek, L. Do post-harvest crop residues in no-till systems provide for nitrogen needs of following crops? Agron. J. 2022, 114, 835–852. [Google Scholar] [CrossRef]
- Magid, J.; De Neergaard, A.; Brandt, M. Heterogeneous distribution may substantially decrease initial decomposition, long-term microbial growth and N-immobilization from high C-to-N ratio resources. Eur. J. Soil Sci. 2006, 57, 517–529. [Google Scholar] [CrossRef]
- Guerif, J.; Richard, G.; Durr, C.; Machet, J.M.; Recous, S.; Roger-Estrade, J. A review of tillage effects on crop residue management, seedbed conditions and seedling establishment. Soil Tillage Res. 2001, 61, 13–32. [Google Scholar] [CrossRef]
- Cogle, A.L.; Strong, W.M.; Saffigna, P.G.; Ladd, J.N.; Amato, M. Wheat straw decomposition in subtropical Australia. II.* effect of straw placement on decomposition and recovery of added “N-urea. Aust. J. Soil Res. 1987, 25, 481–490. [Google Scholar] [CrossRef]
- Nyhan, J.W. Decomposition of Carbon-14 labeled plant materials in a grassland soil under field conditions. Soil Proc. Sci. Soc. Am. 1975, 39, 643–648. [Google Scholar] [CrossRef]
- Amato, M.; Jackson, R.B.; Butler, J.H.A.; Ladd, J.N. Decomposition of plant material in Australian soils. II. Residual organic 14C and 15N from legume plant parts decomposing under field and laboratory conditions. Aust. J. Soil Res. 1984, 22, 331–341. [Google Scholar] [CrossRef]
- Sørensen, P.; Ladd, J.N.; Amato, M. Microbial assimilation of14C of ground and unground plant materials decomposing in aloamy sand and a clay soil. Soil Biol. Biochem. 1996, 28, 1425–1434. [Google Scholar] [CrossRef]
- Thomas, B.S.; Yang, J.; Mo, K.M.; Abdalla, J.A.; Hawileh, R.A.; Ariyachandra, E. Biomass ashes from agricultural wastes as supplementary cementitious materials or aggregate replacement in cement/geopolymer concrete: A comprehensive review. J. Build. Eng. 2021, 40, 102332. [Google Scholar] [CrossRef]
- Hailu, B.; Mehari, H. Impacts of soil salinity/sodicity on soil-water relations and plant growth in dry land areas: A review. J. Nat. Sci. Res. 2021, 12, 1–10. [Google Scholar]
- Litalien, A.; Zeeb, B. Curing the earth: A review of anthropogenic soil salinization and plant based strategies for sustainable mitigation. Sci. Total Environ. 2020, 698, 134235. [Google Scholar] [CrossRef]
- Hayat, K.; Bundschuh, J.; Jan, F.; Menhas, S.; Hayat, S.; Haq, F.; Shah, A.; Chaudhary, H.J.; Ullah, A.; Zhang, D.; et al. Combating soil salinity with combining saline agriculture and phyto management with salt accumulating plants. Crit. Rev. Environ. Sci. Technol. 2020, 11, 1085–1115. [Google Scholar] [CrossRef]
- Wicke, B.; Smeets, E.; Dornburg, V.; Vashev, B.; Gaiser, T.; Turkenburg, W.; Faaij, A. The global technical and economic potential of bioenergy fromsalt-affected soils. Energy Environ. Sci. 2011, 4, 2669–2681. [Google Scholar] [CrossRef]
- Dendooven, L.; Alcántara-Hernández, R.J.; Valenzuela-Encinas, C.; Luna-Guido, M.; Perez-Guevara, F.; Marsh, R. Dynamics of carbon and nitrogen in an extreme alkaline saline soil: A review. Soil Biol. Biochem. 2010, 42, 865–877. [Google Scholar] [CrossRef]
- Yan, N.; Marshner, P. Response of microbial activity and biomass to increasing salinity depends on the final salinity, not the original salinity. Soil Biol. Biochem. 2012, 53, 50–55. [Google Scholar] [CrossRef]
- Singh, K.; Singh, B.; Singh, R.R. Changes in physico-chemical, microbial and enzymatic activities during restoration of degraded sodic land: Ecological suitability of mixed forest over monoculture plantation. Catena 2012, 96, 57–67. [Google Scholar] [CrossRef]
- Zhang, X.; Dippold, M.A.; Kuzyakov, Y.; Razavi, B.S. Spatial pattern of enzyme activities depends on root exudate composition. Soil Biol. Biochem. 2019, 133, 83–93. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Renella, G.; Wirth, S.; Islam, R. Secondary salinity effects on soil microbial biomass. Biol. Fertil. Soils 2010, 46, 445–449. [Google Scholar] [CrossRef]
- Makoi, J.H.J.R.; Ndakidemi, P.A. Reclamation of sodic soils in northern Tanzania, using locally available organic and inorganic resources. Afr. J. Biotechnol. 2007, 6, 1926–1931. [Google Scholar]
- Garcıa-Gil, J.C.; Plaza, C.; Soler-Rovira, P.; Polo, A. Long-term effects of municipal solid waste compost application on soil enzyme activities and microbial biomass. Soil Biol. Biochem. 2000, 32, 1907–1913. [Google Scholar] [CrossRef]
- Ansari, M.A.; Ravishankar, N.; Ansari, M.H.; Babu, S.; Layek, J.; Panwar, A.S. Integrating conservation agriculture with intensive crop diversification in the maize-based organic system: Impact on sustaining food and nutritional security. Front. Nutr. 2023, 10, 1137247. [Google Scholar] [CrossRef]
- Yang, X.; Liu, J.; McGrouther, K.; Huang, H.; Lu, K.; Guo, X.; He, L.; Lin, X.; Che, L.; Ye, Z.; et al. Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb and Zn) and enzyme activity in soil. Environ. Sci. Pollut. Res. 2016, 23, 974–984. [Google Scholar] [CrossRef]
- Pérez-Hidalgo, M.; Guerra-Hernández, E.; García-Villanova, B. Determination of insoluble dietary fiber compounds: Cellulose, hemicellulose and lignin in legumes. ARS Pharm. 1997, 38, 357–364. [Google Scholar]
- Camina, F.; Trasar, C.C.; Sotres, G.F.; Leiros, C. Measurement of dehydrogenase activity in acid soil rich in organic matter. Soil Biol. Biochem. 1998, 33, 1005–1011. [Google Scholar] [CrossRef]
- Casida, L.E.; Kalien, D.A., Jr.; Sartoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremmer, J.M. Use of p-nitrophenyl phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Adam, G.; Duncan, H. Development of a sensitive and rapid method for the measurement of total microbial activity using fluorescein diacetate (FDA) in a range of soils. Soil Biol. Biochem. 2001, 33, 943–951. [Google Scholar] [CrossRef]
- R Core Team. R Language Definition; R Foundation for Statistical Computing: Vienna, Austria, 2000; Volume 3. [Google Scholar]
- Wu, J.P.; Liu, Z.F.; Sun, Y.X.; Zhou, L.X.; Lin, Y.B.; Fu, S.F. Introduced Eucalyptus urophylla plantations change the composition of the microbial community in subtropical China. Land Degrad. Dev. 2013, 24, 400–406. [Google Scholar] [CrossRef]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The effect of salinity on soil chemical characteristics, enzyme activity and bacterial community composition in rice rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef] [PubMed]
- Lemanowicz, J.; Gawlińska, K.; Siwik-Ziomek, A. Impact of technogenic saline soils on some chemical properties and on the activity of selected enzymes. Energies 2021, 14, 4882. [Google Scholar] [CrossRef]
- Singh, P.; Chaudhary, O.P.; Mavi, M.S. Irrigation-induced salinization effects on soil chemical and biological properties under Cotton-Wheat rotation on loamy sand soil in Northwest India. J. Ind. Soc. Soil Sci. 2018, 66, 386–391. [Google Scholar] [CrossRef]
- Yan, N.; Marshner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Whalen, J.K. Managing soil biota-mediated decomposition and nutrient mineralization in sustainable agroecosystems. Adv. Agric. 2014, 2014, 384604. [Google Scholar] [CrossRef]
- Tejada, M.; Benitez, C. Effects of crushed maize straw residues on soil biological properties and soil restoration. Land Degrad. Dev. 2014, 25, 501–509. [Google Scholar] [CrossRef]
- Sharma, S.; Saikia, R.; Thind, H.S.; Singh, Y.; Jat, M.L. Tillage, green manure and residue management accelerate soil carbon pools and hydrolytic enzymatic activities for conservation agriculture based rice-wheat systems. Commun. Soil Sci. Plant Anal. 2020, 52, 470–486. [Google Scholar] [CrossRef]
- Sisodia, R.S.; Lal, M.; Vardhan, A.D.; Singh, R.B.; Mandal, A.; Manna, M.C.; Singh, V.K.; Brajendra. Effect of manure and chemical amelioration on crop yields and soil biological activities in saline soils of semiarid Indo-Gangetic alluvium (Typic ustrochrepts) type in India. Ind. J. Agric. Sci. 2013, 83, 1031–1037. [Google Scholar]
- Dzionek, A.; Dzik, J.; Wojcieszyńska, D.; Guzik, U. Fluorescein diacetate hydrolysis using the whole biofilm as a sensitive tool to evaluate the physiological state of immobilized bacterial cells. Catalysts 2018, 8, 434. [Google Scholar] [CrossRef]
- Fagodiya, R.K.; Malyan, S.K.; Singh, D.; Kumar, A.; Yadav, R.K.; Sharma, P.C.; Pathak, H. Greenhouse gas emissions from salt-affected soils: Mechanistic understanding of interplay factors and reclamation approaches. Sustainability 2022, 14, 11876. [Google Scholar] [CrossRef]
- Perucci, P. Effect of the addition of municipal solid-waste compost on microbial biomass and enzyme activities in soil. Biol. Fertil. Soils 1990, 10, 221–226. [Google Scholar] [CrossRef]
- Pathak, H.; Rao, D.L.N. Carbon and nitrogen mineralization from added organic matter in saline and alkali soils. Soil Biol. Biochem. 1998, 30, 695–702. [Google Scholar] [CrossRef]
- Ambus, P.; Jensen, E.S. Nitrogen mineralization and denitrification as influenced by crop residue particle size. Plant Soil 1997, 197, 261–270. [Google Scholar] [CrossRef]
- Azadi, N.; Raiesi, F. Salinization depresses soil enzyme activity in metal-polluted soils through increases in metal mobilization and decreases in microbial biomass. Ecotoxicology 2021, 30, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Neemisha; Sharma, S. Soil enzymes and their role in nutrient cycling. In Structure and Functions of Pedosphere; Springer Nature Singapore: Singapore, 2022; pp. 173–188. [Google Scholar]
- Tabatabai, M.A. Soil Enzymes. In Methods of Soil Analysis Part 2 Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]
- Liang, C.; Gasco, G.; Fu, S.; Méndez, A.; Paz-Ferreiro, J. Biochar from pruning residues as a soil amendment: Effects of pyrolysis temperature and particle size. Soil Tillage Res. 2016, 164, 3–10. [Google Scholar] [CrossRef]
- Zahran, H.H. Diversity, adaptation and activity of the bacterial flora in saline environments. Biol. Fert. Soil 1997, 25, 211–223. [Google Scholar] [CrossRef]
- Harrison, A.F. Relationship between intensity of phosphatase activity and physico-chemical properties in woodland soils. Soil Biol. Biochem. 1983, 15, 93–99. [Google Scholar] [CrossRef]
- Mishra, P.; Mishra, J.; Arora, N.K. Plant growth promoting bacteria for combating salinity stress in plants–Recent developments and prospects: A review. Microbiol. Res. 2021, 252, 126861. [Google Scholar] [CrossRef]

| Parameters | Normal Soil | Sodic Soil | Saline Soil |
|---|---|---|---|
| pH | 7.82 | 8.86 | 7.05 |
| EC | 0.406 | 2.10 | 2.45 |
| Ca2+ + Mg2+ (meq L−1) | 4.3 | 1.9 | 1.4 |
| HCO3− (meq L−1) | 2.5 | 6.5 | 3.8 |
| Chlorine (meq L−1) | 3.5 | 16.8 | 17.1 |
| Na+ (meq L−1) | 7.88 | 31.25 | 30.7 |
| K+ (meq L−1) | 2.88 | 1.44 | 0.961 |
| SAR | 7.43 | 44.0 | 51.0 |
| Organic carbon (%) | 0.44 | 0.42 | 0.43 |
| Available phosphorus (kg ha−1) | 24.18 | 22.04 | 4.76 |
| Available potassium (kg ha−1) | 160 | 150 | 70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Gupta, N.; Chakkal, A.S.; Sharma, N.; Alamri, S.; Siddiqui, M.H.; Haider, F.U. Changes in Enzyme Activities in Salt-Affected Soils during Incubation Study of Diverse Particle Sizes of Rice Straw. Agriculture 2023, 13, 1694. https://doi.org/10.3390/agriculture13091694
Sharma S, Gupta N, Chakkal AS, Sharma N, Alamri S, Siddiqui MH, Haider FU. Changes in Enzyme Activities in Salt-Affected Soils during Incubation Study of Diverse Particle Sizes of Rice Straw. Agriculture. 2023; 13(9):1694. https://doi.org/10.3390/agriculture13091694
Chicago/Turabian StyleSharma, Sandeep, Nihar Gupta, Anmoldeep Singh Chakkal, Neha Sharma, Saud Alamri, Manzer H. Siddiqui, and Fasih Ullah Haider. 2023. "Changes in Enzyme Activities in Salt-Affected Soils during Incubation Study of Diverse Particle Sizes of Rice Straw" Agriculture 13, no. 9: 1694. https://doi.org/10.3390/agriculture13091694









