Evaluation of Morpho-Physiological Traits of Oat (Avena sativa L.) under Drought Stress †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Root Morphology
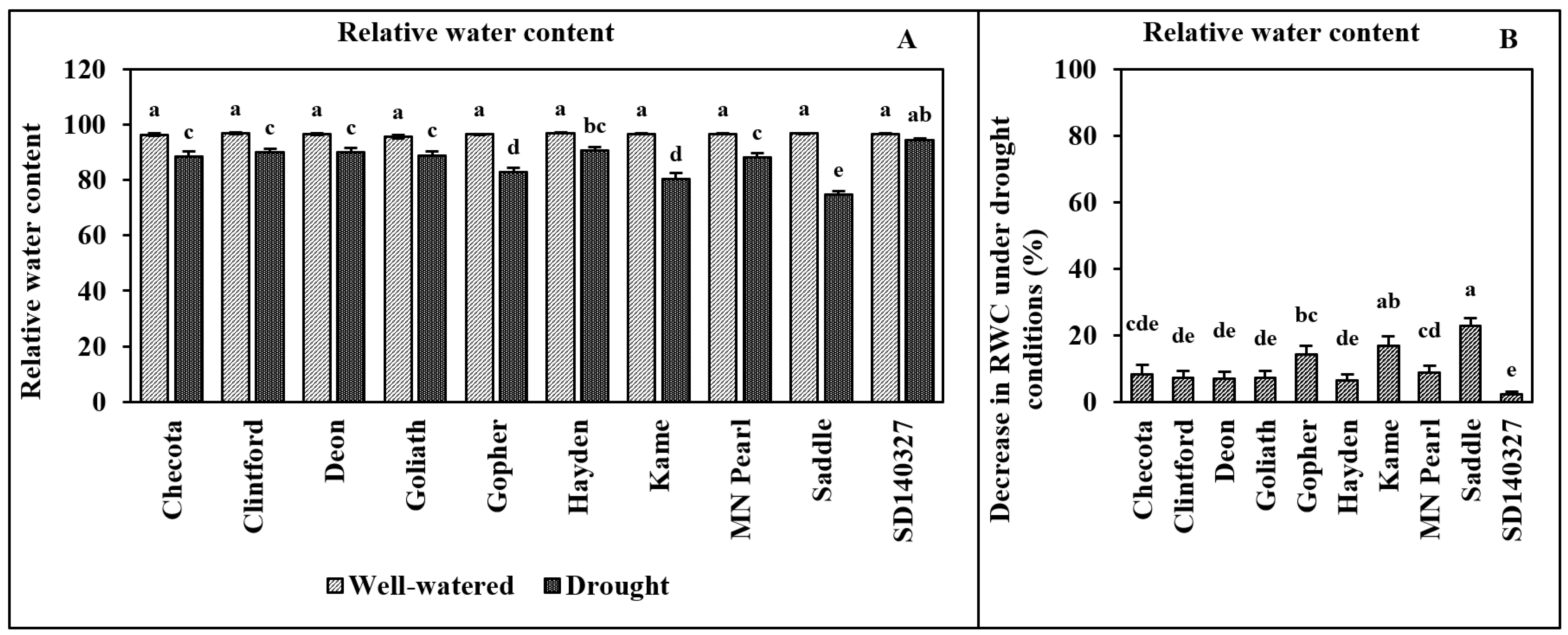
2.3. Relative Water Content (RWC)
- W—Sample fresh weight
- TW—Sample turgid weight
- DW—Sample dry weight.
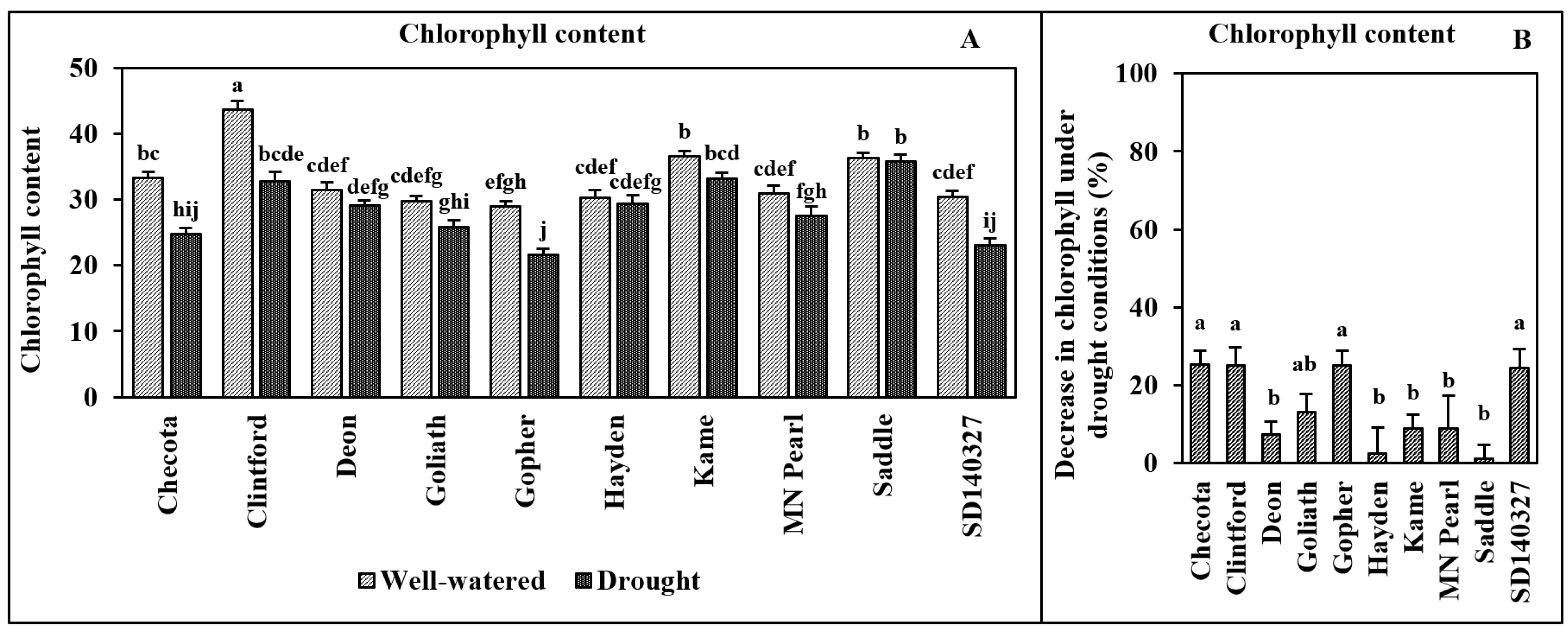
2.4. Chlorophyll Content
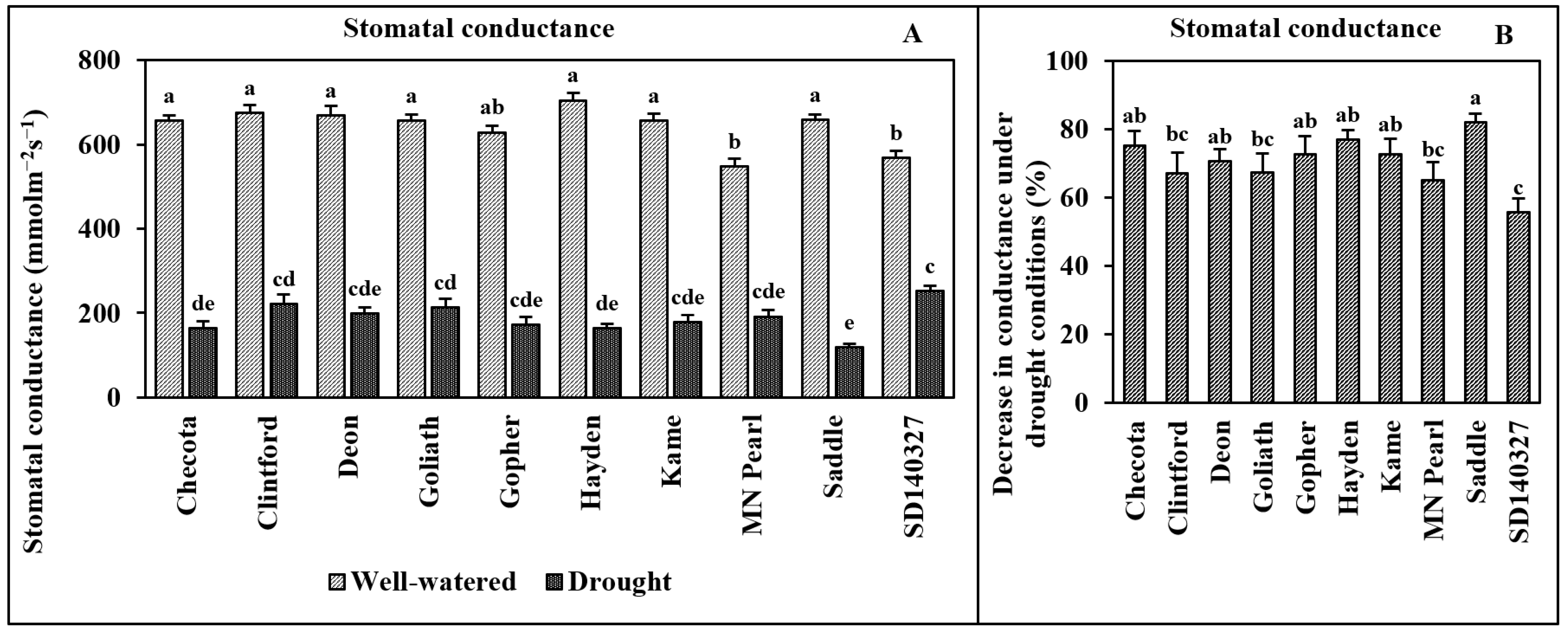
2.5. Stomatal Conductance
2.6. Stomata Number
2.7. Statistical Analysis
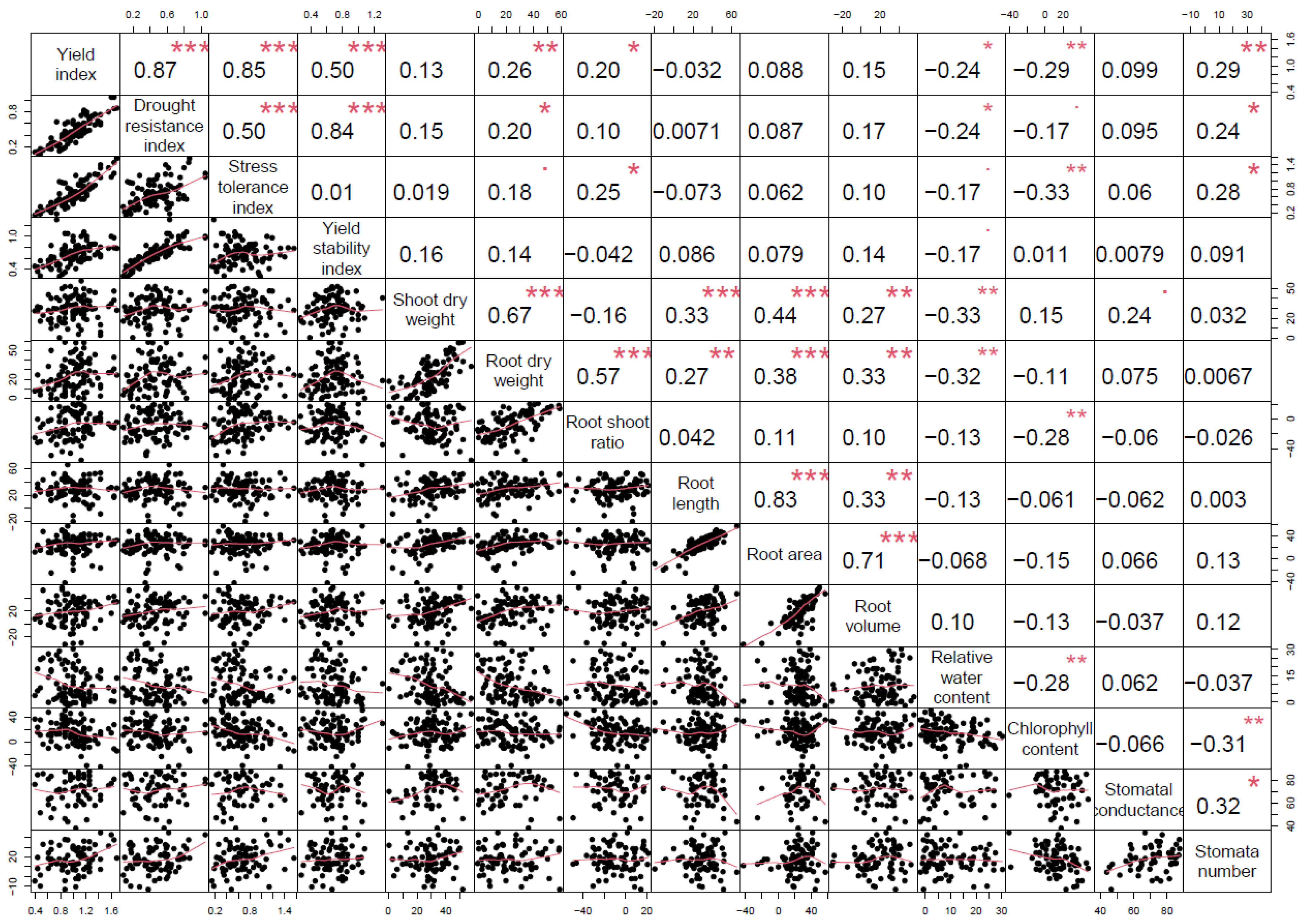
2.8. Drought Tolerance Indices
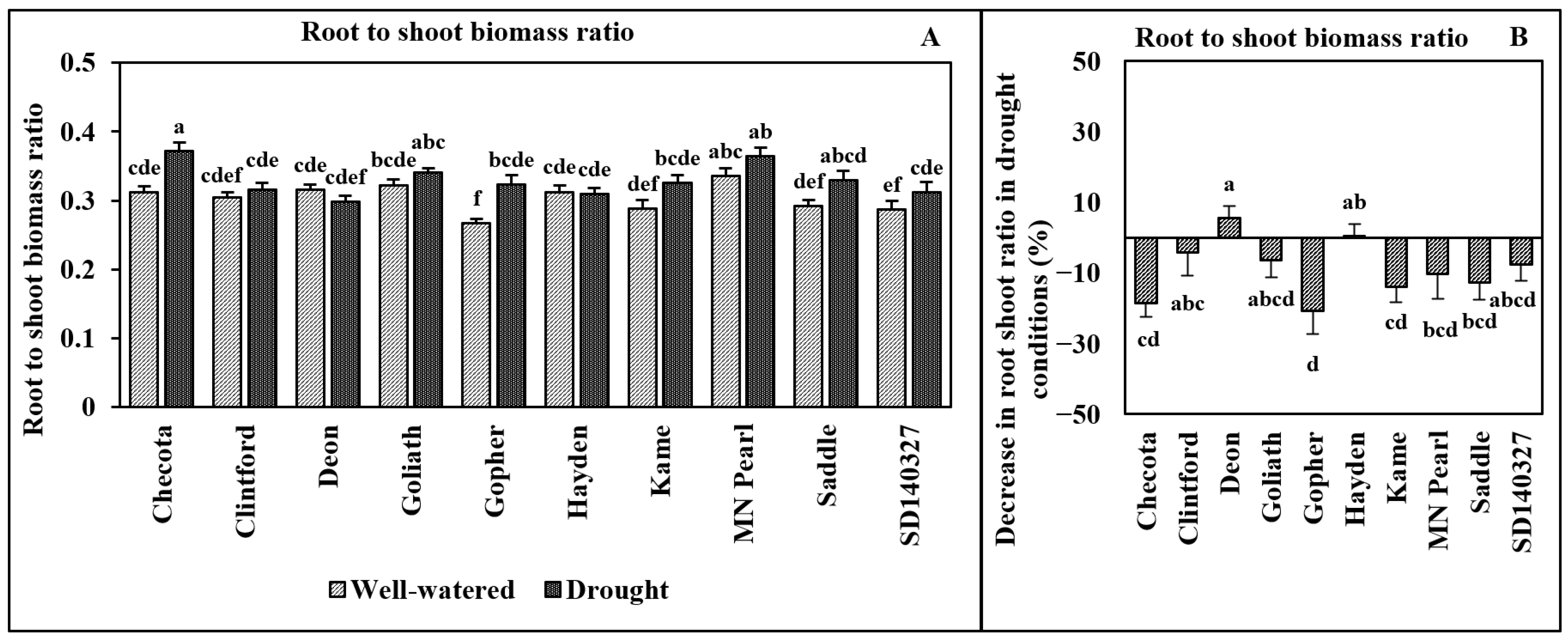
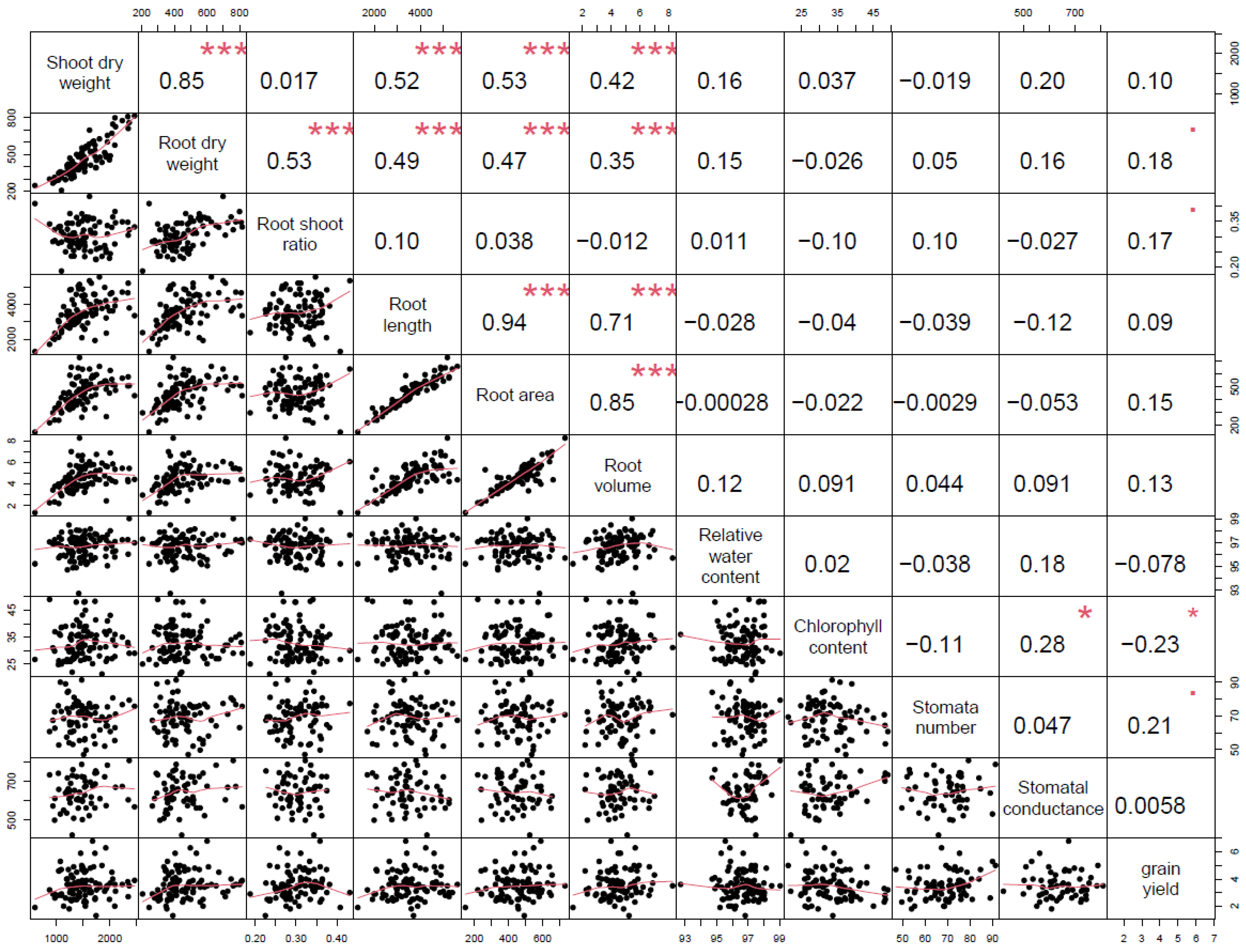
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clemens, R.; van Klinken, B.J. Oats, more than just a whole grain: An introduction. Br. J. Nutr. 2014, 112, S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Webster, H.H. Chapter 17: Oat Utilization: Past, Present, and Future. In Oats: Chemistry and Technology; Webster, H.H., Wood, P.J., Eds.; AAAC International Press: St. Paul, MN, USA, 2011; pp. 347–361. [Google Scholar]
- Broeck, H.C.; Londono, D.M.; Timmer, R.; Smulders, M.J.M.; Gilissen, L.J.W.J.; Meer, I.M. Profiling of nutritional and health-related compounds in oat varieties. Foods 2016, 5, 2. [Google Scholar] [CrossRef]
- Paudel, D.; Dhungana, B.; Caffe, M.; Krishnan, P. A Review of Health-Beneficial Properties of Oats. Foods 2021, 10, 2591. [Google Scholar] [CrossRef]
- Statista, Market Revenue Forecast of Oats Worldwide from 2018 to 2028. 2019. Available online: https://www.statista.com/statistics/1073693/global-oat-market-revenue (accessed on 1 October 2023).
- Isidro-Sánchez, J.; Prats, E.; Howarth, C.; Langdon, T.; Montilla-Bascón, G. Genomic Approaches for Climate Resilience Breeding in Oats. In Genomic Designing of Climate-Smart Cereal Crops; Kole, C., Ed.; Springer: Berlin/Heidelberg, Germany, 2020; pp. 133–169. [Google Scholar]
- Klink, K.; Crawford, C.J.; Wiersma, J.J.; Stuthman, D.D. Climate Variability and the Productivity of Barley and Oats in Minnesota. CURA Rep. 2011, 41, 12–18. Available online: http://www.cura.umn.edu/sites/cura.advantagelabs.com/files/publications/41-1-Klink_et_al_0.pdf (accessed on 1 October 2023).
- Statistics Canada, Estimated Areas, Yield, Production, Average Farm Price and Total Farm Value of Principal Field Crops, in Metric and Imperial Units, September 2023. 2023. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210035901 (accessed on 1 October 2023).
- Strychar, R.; Webster, F.H.; Wood, P.J. World oat production, trade, and usage. In Oats: Chemistry and Technology; Academic Press: Cambridge, MA, USA, 2011; pp. 77–94. [Google Scholar]
- USDA Foreign Agricultural Service, Grain: World Marktes and Trade. 2023. Available online: https://apps.fas.usda.gov/psdonline/circulars/grain.pdf (accessed on 1 September 2023).
- Mahadevan, M.; Calderini, D.F.; Zwer, P.K.; Sadras, V.O. The critical period for yield determination in oat (Avena sativa L.). Field Crops Res. 2016, 199, 109–116. [Google Scholar] [CrossRef]
- Akcura, M.; Ceri, S. Evaluation of drought tolerance indices for selection of Turkish oat (Avena sativa L.) landraces under various environmental conditions. Zemdirb. Agric. 2011, 98, 157–166. [Google Scholar]
- Mall, R.K.; Gupta, A.; Sonkar, G. Effect of Climate Change on Agricultural Crops. In Current Developments in Biotechnology and Bioengineering: Crop Modification, Nutrition, and Food Production; Dubey, S.K., Pandey, A., Sangwan, R.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 23–46. [Google Scholar]
- Sterk, R. Oat Prices Skyrocket as Supply Wanes Due to Drought, Demand. Food Business News. 2021. Available online: https://www.foodbusinessnews.net/articles/19903-oat-prices-skyrocket-as-supply-wanes-due-to-drought-demand#:~:text=KANSAS%20CITY%20%E2%80%94%20Oat%20supplies%20in,reported%20by%20Sosland%20Publishing%20Co (accessed on 1 October 2023).
- Hirtzer, M.; Carey, D. Drought Pushes U.S. Oat Crop to Lowest in Records Back to 1866. Bloomberg. 2021. Available online: https://www.bloomberg.com/news/articles/2021-07-12/drought-pushes-u-s-oat-crop-to-lowest-in-records-back-to-1866 (accessed on 1 September 2023).
- Zhang, X.; Liu, W.; Lv, Y.; Li, T.; Tang, J.; Yang, X.; Bai, J.; Jin, X.; Zhou, H. Effects of drought stress during critical periods on the photosynthetic characteristics and production performance of naked oat (Avena nuda L.). Sci. Rep. 2022, 12, 11199. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, B.-L.; Hu, Y.; Liu, J. Source–sink adjustment: A mechanistic understanding of the timing and severity of drought stress on photosynthesis and grain yields of two contrasting oat (Avena sativa L.) genotypes. J. Plant Growth Regul. 2021, 40, 263–276. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The Physiology of Plant Responses to Drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Canales, F.J.; Nagel, K.A.; Müller, C.; Rispail, N.; Prats, E. Deciphering Root Architectural Traits Involved to Cope with Water Deficit in Oat. Front. Plant Sci. 2019, 10, 1558. [Google Scholar] [CrossRef]
- Schachtman, D.P.; Goodger, J.Q. Chemical root to shoot signaling under drought. Trends Plant Sci. 2018, 13, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.M.; Gong, L.; Nie, Z.; Feng, X.; Ahammed, G.J.; Fang, X.W. ABA-induced stomatal movements in vascular plants during dehydration versus rehydration. Environ. Exp. Bot. 2021, 186, 104436. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Marcińska, I.; Janowiak, F.; Rzepka, A.; Hura, T. The relationship between seedling growth and grain yield under drought conditions in maize and triticale genotypes. Acta Physiol. Plant. 2012, 34, 1757–1764. [Google Scholar] [CrossRef]
- Patterson, F.; Schafer, J.; Roberts, J. Registration of Clintford Oat 1 Reg. No.(292). Crop Sci. 1979, 19, 294–295. [Google Scholar] [CrossRef]
- Caffe-Treml, M.; Hall, L.; Bauer, R.; Kleinjan, J.; Hall, N.; Ingemansen, J.A. Registration of oat cultivar ‘Hayden’. J. Plant Regist. 2017, 11, 95–99. [Google Scholar] [CrossRef]
- Edwards, L.H.; Smith, E.L.; Pass, H.; Evans, C.L. Registration of Checota Oats (Reg. No. 240). Crop Sci. 1971, 11, 134. [Google Scholar]
- Benlioglu, B.; Ozkan, U. The influence of salinity and drought stress on some oat cultivars (Avena sativa L.) by determining some stress indexes and growth performances at the germination stage. Fresen Environ. Bull. 2021, 30, 771–778. [Google Scholar]
- Wasson, A.P.; Richards, R.A.; Chatrath, R.; Misra, S.C.; Prasad, S.V.; Rebetzke, G.J.; Kirkegaard, J.A.; Christopher, J.; Watt, M. Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 2012, 63, 3485–3498. [Google Scholar] [CrossRef]
- Turner, N.C. Imposing and Maintaining Soil Water Deficits in Drought Studies in Pots. Plant Soil 2019, 439, 45–55. [Google Scholar] [CrossRef]
- Smart, R.E.; Bingham, G.E. Rapid Estimates of Relative Water Content. Plant Physiol. 1974, 53, 258–260. [Google Scholar] [CrossRef]
- Hilu, K.W.; Randall, J.L. Convenient method for studying grass leaf epidermis. Taxon 1984, 33, 413–415. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Package Version 1.3-5. 2021. Available online: https://www.vps.fmvz.usp.br/CRAN/web/packages/agricolae/vignettes/tutorial.pdf (accessed on 1 June 2023).
- Kay, M.; Wobbrock, J.O. ARTool: Aligned Rank Transform for Nonparametric Factorial ANOVAs. R Package Version 0.11.1. 2015. Available online: https://cran.r-project.org/web/packages/ARTool/ (accessed on 1 August 2023).
- Fernandez, G.C.J. Effective selection criteria for assessing plant stress tolerance. In Proceedings of the International Symposium on Adaptation of Vegetables and other Food Crops in Temperature and Water Stress, Shanhua, Taiwan, 13–16 August 1992. [Google Scholar]
- Mousavi, S.S.; Yazdi Samadi, B.; Naghavi, M.R.; Zali, A.A.; Dashti, H.; Pourshahbazi, A. Introduction of new indices to identify relative drought tolerance and resistance in wheat genotypes. Desert 2008, 12, 165–178. [Google Scholar]
- Gavuzzi, P.; Rizza, F.; Palumbo, M.; Campanile, R.G.; Ricciardi, G.L.; Borghi, B. Evaluation of field and laboratory predictors of drought and heat tolerance in winter cereals. Can. J. Plant Sci. 1997, 77, 523–531. [Google Scholar] [CrossRef]
- Bouslama, M.; Schapaugh, W.T. Stress Tolerance in Soybeans. I. Evaluation of Three Screening Techniques for Heat and Drought Tolerance. Crop Sci. 1984, 24, 933–937. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Fleury, D.; Jefferies, S.; Kuchel, H.; Langridge, P. Genetic and genomic tools to improve drought tolerance in wheat. J. Exp. Bot. 2010, 61, 3211–3222. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought—From genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2009, 89, 183–189. [Google Scholar] [CrossRef]
- Ghimire, K.; Gupta, S.; Geng, S.; Chen, S.; Boe, A.; Wu, Y. Identification of physiological and morphological traits governing high water use efficiency in alfalfa. J. Agron. Crop Sci. 2021, 207, 644–653. [Google Scholar] [CrossRef]
- Ahmed, H.G.M.; Sajjad, M.; Li, M.; Azmat, M.A.; Rizwan, M.; Maqsood, R.H.; Khan, S.H. Selection criteria for drought-tolerant bread wheat genotypes at seedling stage. Sustainability 2019, 11, 2584. [Google Scholar] [CrossRef]
- Almaghrabi, O.A. Impact of drought stress on germination and seedling growth parameters of some wheat cultivars. Life Sci. J. 2012, 9, 590–598. [Google Scholar]
- Lozano, Y.M.; Aguilar-Trigueros, C.A.; Flaig, I.C.; Rillig, M.C. Root trait responses to drought are more heterogeneous than leaf trait responses. Funct. Ecol. 2020, 34, 2224–2235. [Google Scholar] [CrossRef]
- Fang, Y.; Du, Y.; Wang, J.; Wu, A.; Qiao, S.; Xu, B.; Zhang, S.; Siddique, K.H.M.; Chen, Y. Moderate drought stress affected root growth and grain yield in old, modern and newly released cultivars of winter wheat. Front. Plant Sci. 2017, 8, 672. [Google Scholar] [CrossRef] [PubMed]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar] [CrossRef]
- Karcher, D.E.; Richardson, M.D.; Hignight, K.; Rush, D. Drought tolerance of tall fescue populations selected for high root/shoot ratios and summer survival. Crop Sci. 2008, 48, 771–777. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.V.; Kumari, J.; Rengel, Z. Root length and root lipid composition contribute to drought tolerance of winter and spring wheat. Plant Soil 2019, 439, 57–73. [Google Scholar] [CrossRef]
- Tolley, S.; Mohammadi, M. Variation in root and shoot growth in response to reduced nitrogen. Plants 2020, 9, 144. [Google Scholar] [CrossRef]
- Palta, J.; Chen, X.; Milroy, S.; Rebetzke, G.; Dreccer, M.; Watt, M. Large root systems: Are they useful in adapting wheat to dry environments? Funct. Plant Biol. 2011, 38, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Ma, B.L.; Shi, Y.; Liu, K.; Chen, W. Selection of oat (Avena sativa L.) drought-tolerant genotypes based on multiple yield-associated traits. J. Sci. Food Agric. 2023, 103, 4380–4391. [Google Scholar] [CrossRef] [PubMed]
- Soltys-Kalina, D.; Plich, J.; Strzelczyk-Zyta, D.; Sliwka, J.; Marczewski, W. The effect of drought stress on the leaf relative water content and tuber yield of a half-sib family of ‘Katahdin’-derived potato cultivars. Breed. Sci. 2016, 66, 328–331. [Google Scholar] [CrossRef]
- Rahman, H.; Ramanathan, V.; Nallathambi, J.; Duraialagaraja, S.; Muthurajan, R. Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol. 2016, 16, 7–20. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Osmotic adjustment is a prime drought stress adaptive engine in support of plant production. Plant Cell Environ. 2017, 40, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Gong, D.S.; Xiong, Y.C.; Ma, B.L.; Wang, T.M.; Ge, J.P.; Qin, X.L.; Li, P.F.; Kong, H.Y.; Li, Z.Z.; Li, F.M. Early activation of plasma membrane H+-ATPase and its relation to drought adaptation in two contrasting oat (Avena sativa L.) genotypes. Environ. Exp. Bot. 2010, 69, 1–8. [Google Scholar] [CrossRef]
- Ahmad, A.; Aslam, Z.; Javed, T.; Hussain, S.; Raza, A.; Shabbir, R.; Mora-Poblete, F.; Saeed, T.; Zulfiqar, F.; Ali, M.M.; et al. Screening of wheat (Triticum aestivum L.) genotypes for drought tolerance through agronomic and physiological response. Agronomy 2022, 12, 287. [Google Scholar] [CrossRef]
- Majid, K.; Roza, G. The effect of drought stress on leaf chlorophyll content and stress resistance in maize cultivars (Zea mays). Afr. J. Microbiol. Res. 2012, 6, 2844–2848. [Google Scholar]
- Canales, F.J.; Rispail, N.; Garcia, T.O.; Arbona, V.; Perez, L.A.; Prats, E. Drought resistance in oat involves ABA-mediated modulation of transpiration and root hydraulic conductivity. Environ. Exp. Bot. 2021, 182, 104333. [Google Scholar] [CrossRef]
- Changhai, S.; Baodi, D.; Yunzhou, Q.; Yuxin, L.; Mengyu, S.; Changhai, L.; Yuxin, L.; Lei, L.; Haipei, L. Physiological regulation of high transpiration efficiency in winter wheat under drought conditions. Plant Soil Environ. 2010, 56, 340–347. [Google Scholar] [CrossRef]
- Li, Y.; Li, H.; Li Zhang, S. Improving water-use efficiency by decreasing stomatal conductance and transpiration rate to maintain higher ear photosynthetic rate in drought-resistant wheat. Crop J. 2017, 5, 231–239. [Google Scholar] [CrossRef]
- Pirasteh-Anosheh, H.; Saed-Moucheshi, A.; Pakniyat, H.; Pessarakli, M. Stomatal responses to drought stress. In Water Stress and Crop Plants: A Sustainable Approach; Wiley Blackwell: Oxford, UK, 2016; Volume 1. [Google Scholar]
- Hughes, J.; Hepworth, C.; Dutton, C.; Dunn, J.A.; Hunt, L.; Stephens, J.; Waugh, R.; Cameron, D.D.; Gray, J.E. Reducing Stomatal Density in Barley Improves Drought Tolerance without Impacting on Yield. Plant Physiol. 2017, 174, 776–787. [Google Scholar] [CrossRef]
- Caine, R.S.; Yin, X.; Sloan, J.; Harrison, E.L.; Mohammed, U.; Fulton, T.; Biswal, A.K.; Dionora, J.; Chater, C.C.; Coe, R.A.; et al. Rice with reduced stomatal density conserves water and has improved drought tolerance under future climate conditions. New Phytol. 2019, 221, 371–384. [Google Scholar] [CrossRef]
- Raven, J.A. Speedy small stomata? J. Exp. Bot. 2014, 65, 1415–1424. [Google Scholar] [CrossRef] [PubMed]









| Genotype | Stress Tolerance Index | Yield Index | Drought Resistance Index | Yield Stability Index | Mean DTI |
|---|---|---|---|---|---|
| Checota | 0.70 | 1.05 | 0.46 | 0.67 | 0.72 |
| Clintford | 0.39 | 0.81 | 0.38 | 0.71 | 0.57 |
| Deon | 0.94 | 1.12 | 0.41 | 0.56 | 0.76 |
| Goliath | 0.92 | 1.09 | 0.39 | 0.54 | 0.74 |
| Gopher | 0.32 | 0.72 | 0.32 | 0.69 | 0.51 |
| Hayden | 1.14 | 1.44 | 0.73 | 0.78 | 1.02 |
| Kame | 0.59 | 1.00 | 0.47 | 0.72 | 0.70 |
| MN Pearl | 0.76 | 1.14 | 0.54 | 0.73 | 0.79 |
| Saddle | 0.50 | 0.80 | 0.28 | 0.54 | 0.53 |
| SD140327 | 0.47 | 0.82 | 0.33 | 0.62 | 0.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghimire, K.; McIntyre, I.; Caffe, M. Evaluation of Morpho-Physiological Traits of Oat (Avena sativa L.) under Drought Stress. Agriculture 2024, 14, 109. https://doi.org/10.3390/agriculture14010109
Ghimire K, McIntyre I, Caffe M. Evaluation of Morpho-Physiological Traits of Oat (Avena sativa L.) under Drought Stress. Agriculture. 2024; 14(1):109. https://doi.org/10.3390/agriculture14010109
Chicago/Turabian StyleGhimire, Krishna, Isabel McIntyre, and Melanie Caffe. 2024. "Evaluation of Morpho-Physiological Traits of Oat (Avena sativa L.) under Drought Stress" Agriculture 14, no. 1: 109. https://doi.org/10.3390/agriculture14010109






