Autonecrotic Tomato (Solanum lycopersicum L.) Line as a Potential Model for Applications in Proximal Sensing of Biotic and Abiotic Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Tomato Material
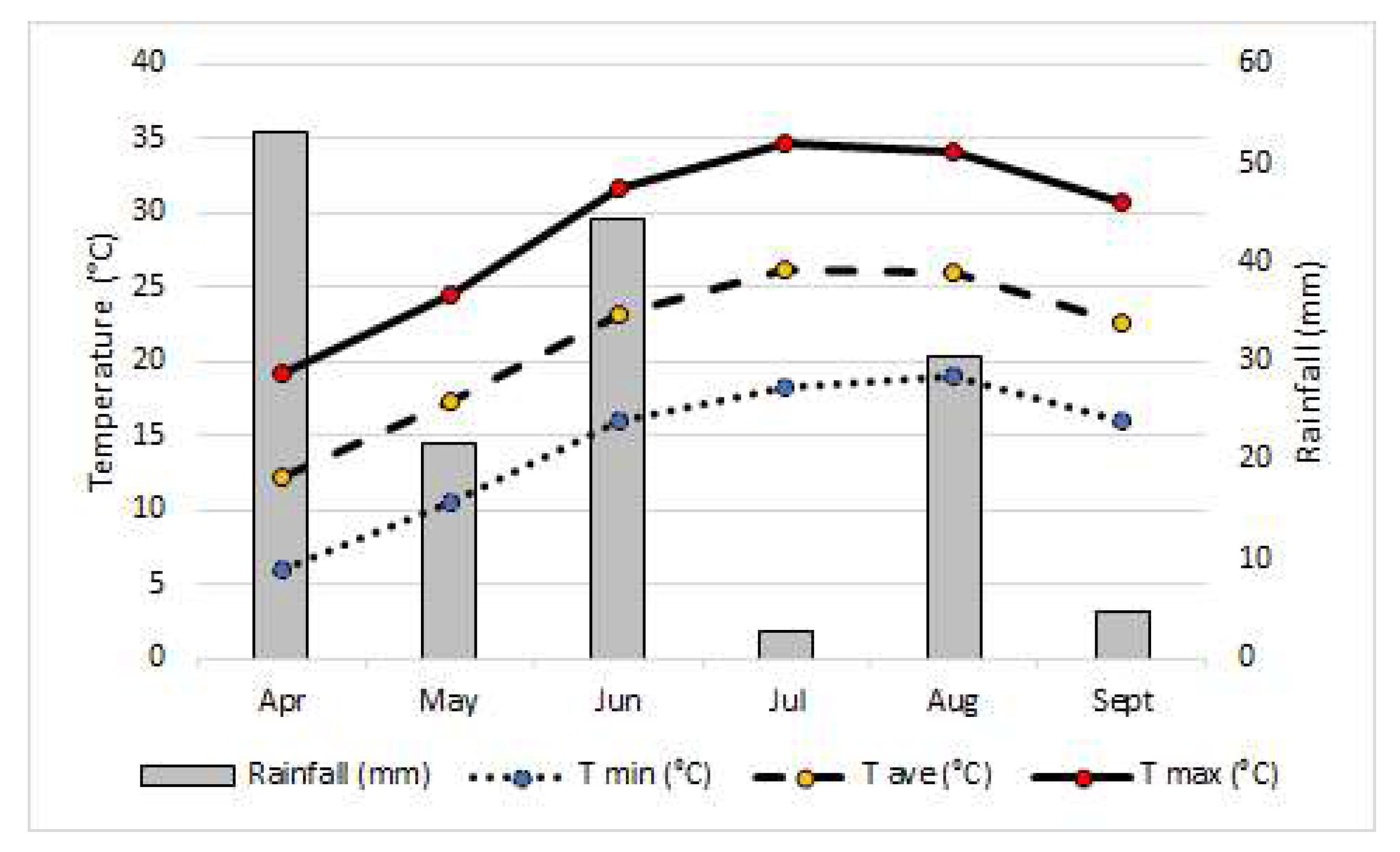
2.2. Growth Conditions
2.3. Morphological Characterization of the Autonecrotic Mutant
2.4. VIS/NIR Analysis of the Leaves
2.5. Statistical Analysis
3. Results
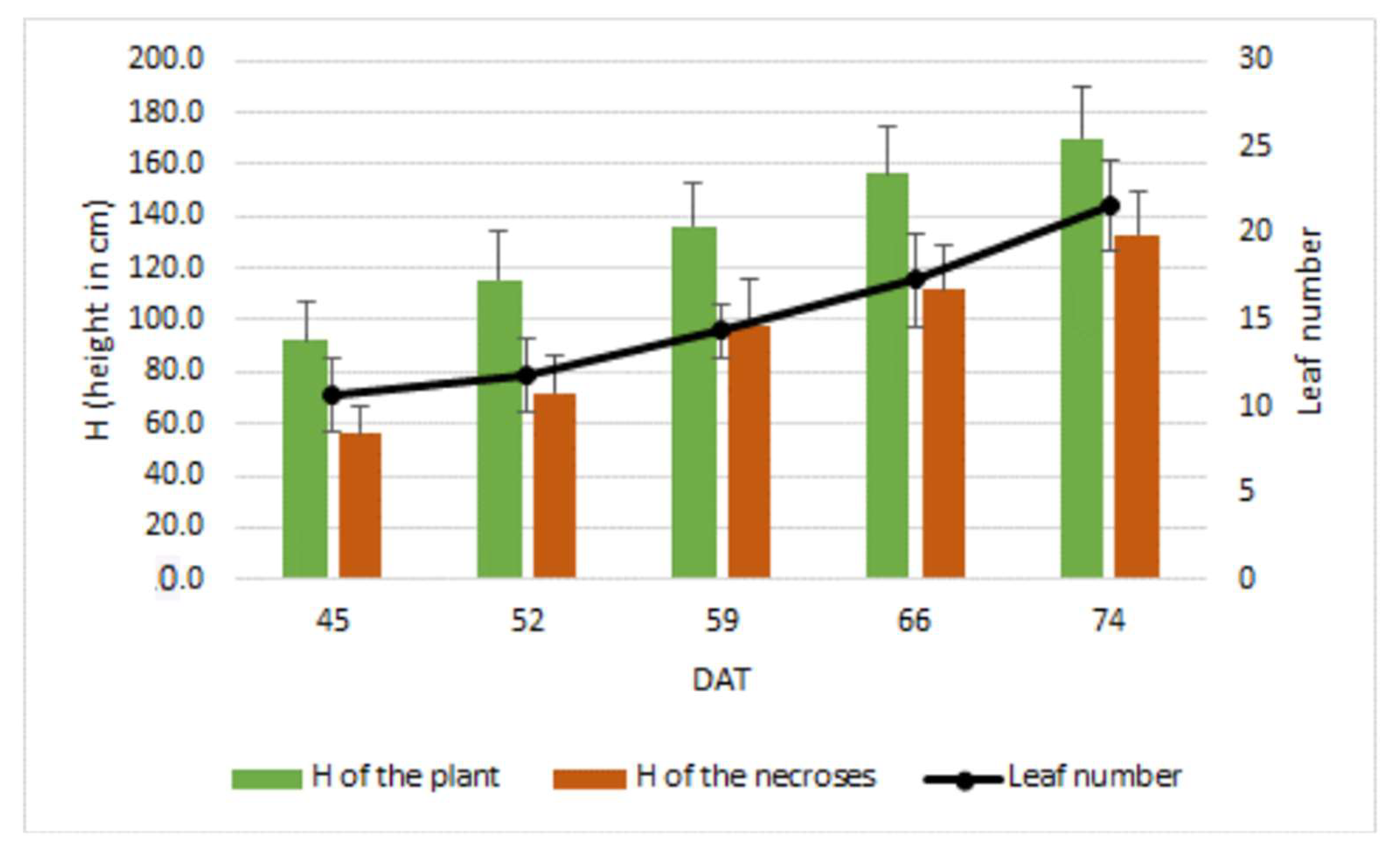
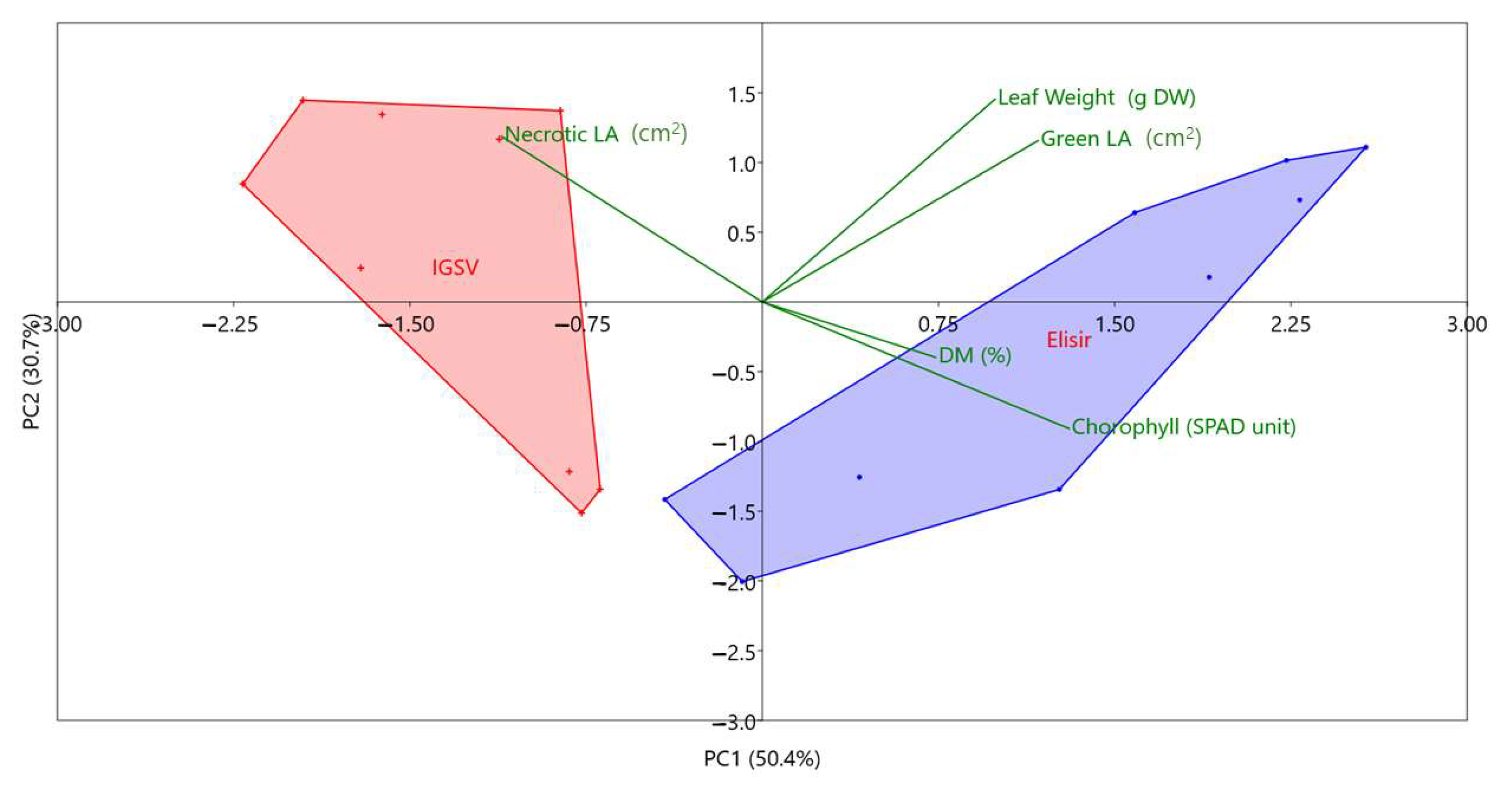
3.1. Morphological Characterization of the Autonecrotic Mutant
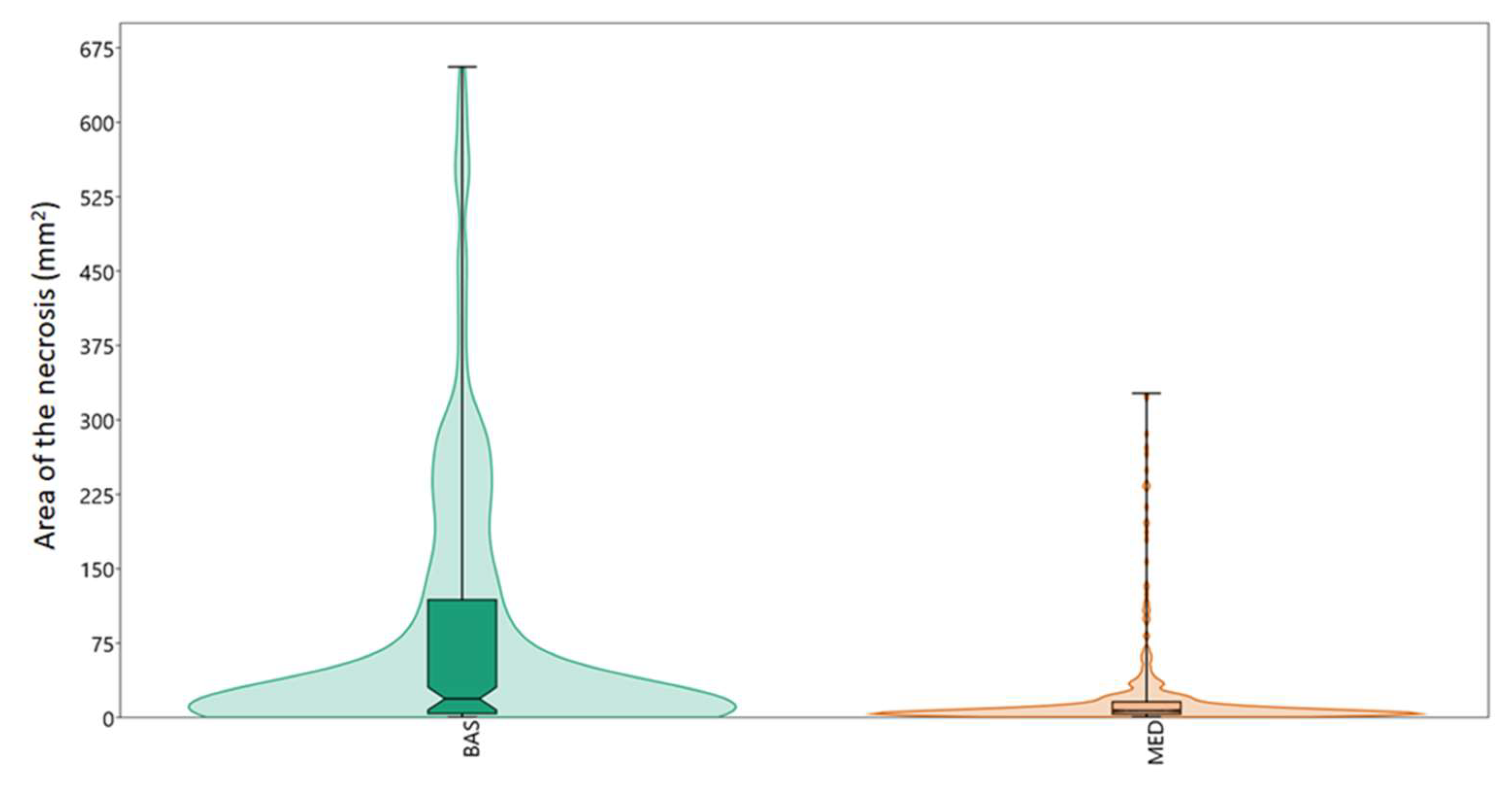
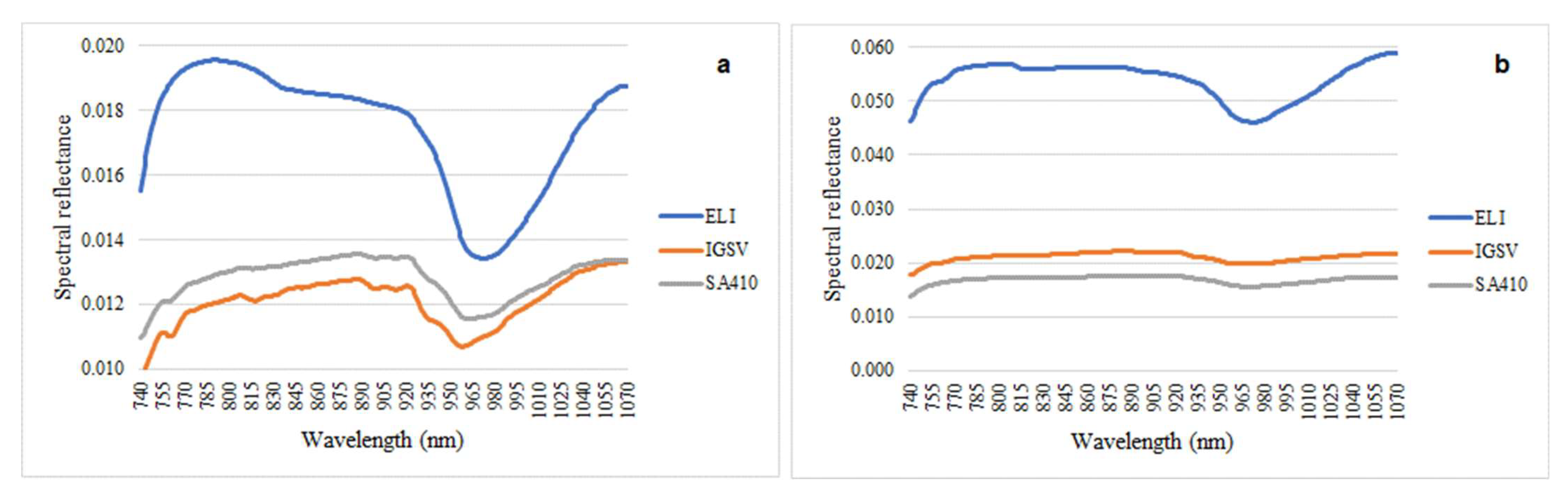
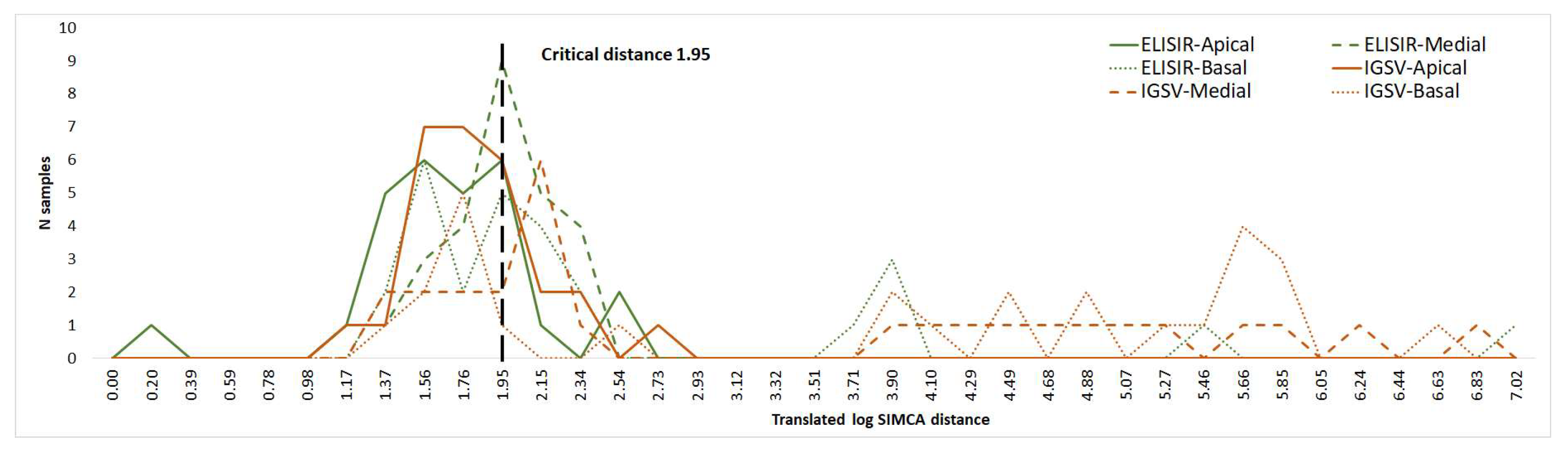
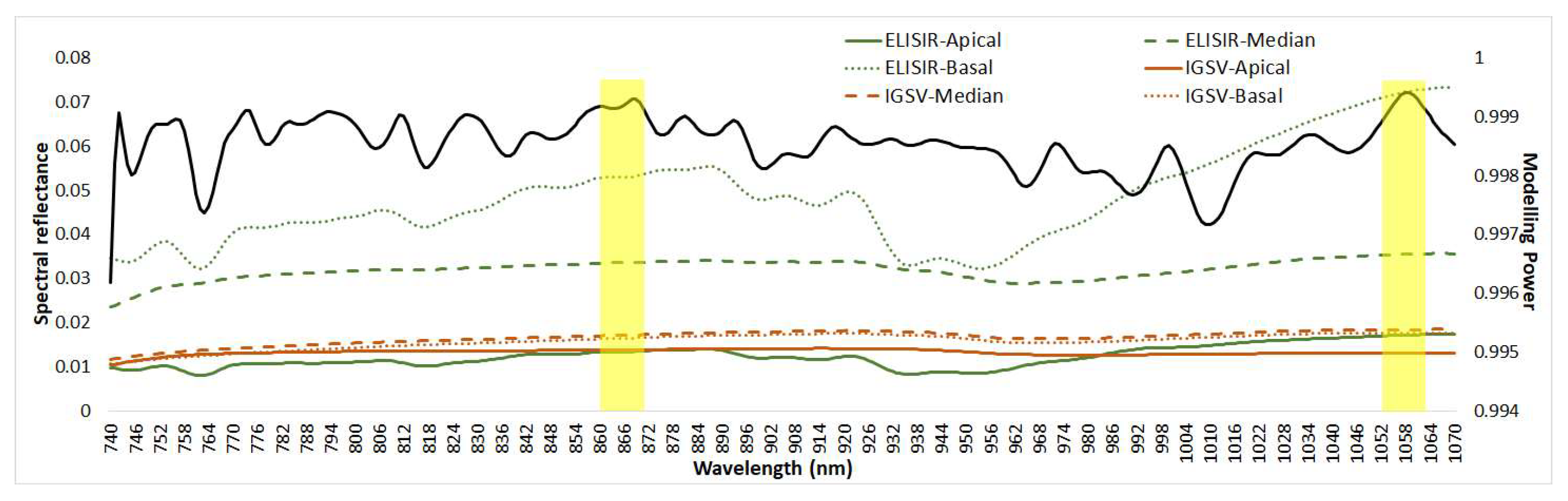
3.2. VIS/NIR Analysis of the Leaves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dangl, J.L.; Dietrich, R.A.; Richberg, M.H. death don’t have no mercy: Cell death programs in plant-microbe interactions. Plant Cell 1996, 8, 1793–1807. [Google Scholar] [CrossRef]
- Hammond-Kosack, K.E.; Jones, J.D. Resistance gene-dependent plant defense responses. Plant Cell 1996, 8, 1773–1791. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The oxidative burst in plant disease resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Beltran, P.M.J.; Federspiel, J.D.; Sheng, X.; Cristea, I.M. Proteomics and integrative omic approaches for understanding host–pathogen interactions and infectious diseases. Mol. Syst. Biol. 2017, 13, 922. [Google Scholar] [CrossRef]
- Peyraud, R.; Dubiella, U.; Barbacci, A.; Genin, S.; Raffaele, S.; Roby, D. Advances on plant–pathogen interactions from molecular toward systems biology perspectives. Plant J. 2017, 90, 720–737. [Google Scholar] [CrossRef]
- Jones, J.; Dangl, J. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Dangl, J.L.; Horvath, D.M.; Staskawicz, B.J. Pivoting the plant immune system from dissection to deployment. Science 2013, 341, 746–751. [Google Scholar] [CrossRef]
- Dixon, M.S.; Golstein, C.; Thomas, C.M.; van der Biezen, E.A.; Jones, J.D.G. Genetic complexity of pathogen perception by plants: The example of Rcr3, a tomato gene required specifically by Cf-2. Proc. Natl. Acad. Sci. USA 2000, 97, 8807–8814. [Google Scholar] [CrossRef]
- Rooney, H.C.; Van’t Klooster, J.W.; van der Hoorn, R.A.; Joosten, M.H.; Jones, J.D.; de Wit, P.J. Cladosporium Avr2 Inhibits Tomato Rcr3 Protease Required for Cf-2-Dependent Disease Resistance. Science 2005, 308, 1783–1786. [Google Scholar] [CrossRef] [PubMed]
- Kourelis, J.; Malik, S.; Mattinson, O.; Krauter, S.; Kahlon, P.S.; Paulus, J.K.; van der Hoorn, R.A.L. Evolution of a guarded decoy protease and its receptor in solanaceous plants. Nat. Commun. 2020, 11, 4393. [Google Scholar] [CrossRef] [PubMed]
- Krüger, J.; Thomas, C.M.; Golstein, C.; Dixon, M.S.; Smoker, M.; Tang, S.; Mulder, L.; Jones, J.D. A tomato cysteine protease required for Cf-2-dependent disease resistance and suppression of autonecrosis. Science 2002, 296, 744–747. [Google Scholar] [CrossRef]
- Wan, W.; Kim, S.; Castel, B.; Charoennit, N.; Chae, E. Genetics of autoimmunity in plants: An evolutionary genetics perspective. New Phytol. 2021, 229, 1215–1233. [Google Scholar] [CrossRef] [PubMed]
- Bomblies, K.; Weigel, D. Hybrid necrosis: Autoimmunity as a potential gene-flow barrier in plant species. Nat. Rev. Genet. 2007, 8, 382–393. [Google Scholar] [CrossRef]
- Moeder, W.; Yoshioka, K. Lesion mimic mutants. Plant Signal. Behav. 2008, 3, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Bruggeman, Q.; Raynaud, C.; Benhamed, M.; Delarue, M. To die or not to die? Lessons from lesion mimic mutants. Front. Plant Sci. 2015, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Mahlein, A.-K. Plant disease detection by imaging sensors—Parallels and specific demands for precision agriculture and plant phenotyping. Plant Dis. 2016, 100, 241–251. [Google Scholar] [CrossRef]
- Pallottino, F.; Antonucci, F.; Costa, C.; Bisaglia, C.; Figorilli, S.; Menesatti, P. Optoelectronic proximal sensing vehicle-mounted technologies in precision agriculture: A review Partial Least Squares Regression. Comput. Electron. Agric. 2019, 162, 859–873. [Google Scholar] [CrossRef]
- Pane, C.; Manganiello, G.; Nicastro, N.; Ortenzi, L.; Pallottino, F.; Cardi, T.; Costa, C. Machine learning applied to canopy hyperspectral image data to support biological control of soil-borne fungal diseases in baby leaf vegetables. Biol. Control 2021, 164, 104784. [Google Scholar] [CrossRef]
- Navarro, A.; Nicastro, N.; Costa, C.; Pentangelo, A.; Cardarelli, M.; Ortenzi, L.; Pallottino, F.; Cardi, T.; Pane, C. Sorting biotic and abiotic stresses on wild rocket by leaf-image hyperspectral data mining with an artificial intelligence model. Plant Methods 2022, 18, 45. [Google Scholar] [CrossRef]
- Violino, S.; Figorilli, S.; Ferrigno, M.; Manganiello, V.; Pallottino, F.; Costa, C.; Menesatti, P. A data-driven bibliometric review on precision irrigation. Smart Agric. Technol. 2023, 5, 100320. [Google Scholar] [CrossRef]
- Mahlein, A.-K.; Kuska, M.; Behmann, J.; Polder, G.; Walter, A. Hyperspectral sensors and imaging technologies in phytopathology: State of the art. Annu. Rev. Phytopathol. 2018, 56, 535–558. [Google Scholar] [CrossRef]
- Martinelli, F.; Scalenghe, R.; Davino, S.; Panno, S.; Scuderi, G.; Ruisi, P.; Villa, P.; Stroppiana, D.; Boschetti, M.; Goulart, R.L.; et al. Advanced methods of plant disease detection: A review. Agron. Sustain. Dev. 2015, 35, 1–25. [Google Scholar] [CrossRef]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Zhang, M.; Qin, Z.; Liu, X.; Ustin, S.L. Detection of stress in tomatoes induced by late blight disease in California, USA, using hyperspectral remote sensing. Int. J. Appl. Earth Obs. Geoinf. 2003, 4, 295–310. [Google Scholar] [CrossRef]
- Oerke, E.-C.; Herzog, K.; Toepfer, R. Hyperspectral phenotyping of the reaction of grapevine genotypes to Plasmopara viticola. J. Exp. Bot. 2016, 67, 5529–5543. [Google Scholar] [CrossRef]
- Yao, Z.; Lei, Y.; He, D. Early visual detection of wheat stripe rust using visible/near-infrared hyperspectral imaging. Sensors 2019, 19, 952. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Hu, Y.; Zhang, P.; Hou, L. Potato Late Blight Severity and Epidemic Period Prediction Based on Vis/NIR Spectroscopy. Agriculture 2022, 12, 897. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, D.; Liu, Y.; Zhou, H.; Sun, Y. Measurement of early disease blueberries based on vis/nir hyper-spectral imaging system. Sensors 2020, 20, 5783. [Google Scholar] [CrossRef]
- Jiang, Q.; Wu, G.; Tian, C.; Li, N.; Yang, H.; Bai, Y.; Zhang, B. Hyperspectral imaging for early identification of strawberry leaves diseases with machine learning and spectral fingerprint features. Infrared Phys. Technol. 2021, 118, 103898. [Google Scholar] [CrossRef]
- Li, J.; Huang, W.; Tian, X.; Wang, C.; Fan, S.; Zhao, C. Fast detection and visualization of early decay in citrus using Vis-NIR hyperspectral imaging. Comput. Electron. Agric. 2016, 127, 582–592. [Google Scholar] [CrossRef]
- Chu, X.; Zhang, K.; Wei, H.; Ma, Z.; Fu, H.; Miao, P.; Jiang, H.; Liu, H. A Vis/NIR spectra-based approach for identifying bananas infected with Colletotrichum musae. Front. Plant Sci. 2023, 14, 1180203. [Google Scholar] [CrossRef]
- Bagheri, N.; Mohamadi-Monavar, H. Early detection of fire blight disease of pome fruit trees using visible-nir spec-trometry and dimensionality reduction methods. J. Agric. Mach. 2020, 10, 37–48. [Google Scholar]
- Najjar, K. Sensing Botrytis cinerea in Tomato Using Visible/Near-Infrared (VIS/NIR) Spectroscopy. Ph.D. Dissertation, Palestine Technical University—Kadoorie, Tulkarm, Palestine, 2020. [Google Scholar]
- Berardo, N.; Pisacane, V.; Battilani, P.; Scandolara, A.; Pietri, A.; Marocco, A. Rapid detection of kernel rots and mycotoxins in maize by near-infrared reflectance spectroscopy. J. Agric. Food Chem. 2005, 53, 8128–8134. [Google Scholar] [CrossRef]
- Morellos, A.; Tziotzios, G.; Orfanidou, C.; Pantazi, X.E.; Sarantaris, C.; Maliogka, V.; Alexandridis, T.K.; Moshou, D. Non-destructive early detection and quantitative severity stage classification of Tomato Chlorosis Virus (ToCV) infection in young tomato plants using vis–NIR Spectroscopy. Remote Sens. 2020, 12, 1920. [Google Scholar] [CrossRef]
- Santangelo, E.; Fonzo, V.; Astolfi, S.; Zuchi, S.; Caccia, R.; Mosconi, P.; Mazzucato, A.; Soressi, G.P. The Cf-2/Rcr3esc gene interaction in tomato (Lycopersicon esculentum) induces autonecrosis and triggers biochemical markers of oxidative burst at cellular level. Funct. Plant Biol. 2003, 30, 1117–1125. [Google Scholar] [CrossRef]
- Violino, S.; Taiti, C.; Marone, E.; Pallottino, F.; Costa, C. A statistical tool to determine the quality of extra virgin olive oil (EVOO). Eur. Food Res. Technol. 2022, 248, 2825–2832. [Google Scholar] [CrossRef]
- Forina, M.; Oliveri, P.; Casale, M.; Lanteri, S. Multivariate range modeling, a new technique for multivariate class modeling: The uncertainty of the estimates of sensitivity and specificity. Anal. Chim. Acta 2008, 622, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, D.; Antonucci, F.; Costa, C.; Talento, C.; Ciccoritti, R. An artificial class modelling approach to identify the most largely diffused cultivars of sweet cherry (Prunus avium L.) in Italy. Food Chem. 2020, 333, 127515. [Google Scholar] [CrossRef]
- Barry, C.S.; Aldridge, G.M.; Herzog, G.; Ma, Q.; McQuinn, R.P.; Hirschberg, J.; Giovannoni, J.J. Altered chloroplast development and delayed fruit ripening caused by mutations in a zinc metalloprotease at the lutescent2 locus of tomato. Plant Physiol. 2012, 159, 1086–1098. [Google Scholar] [CrossRef] [PubMed]
- Regione Emilia-Romagna. Pomodoro da Industria. Parte Agronomica. 2019. Available online: http://agricoltura.regione.emilia-romagna.it/produzioni-agroalimentari/temi/bio-agro-climambiente/agricoltura-integrata/disciplinari-produzione-integrata-vegetale/Collezione-dpi/2019/orticole-2019 (accessed on 16 November 2023).
- Fan, X.-X.; Xu, Z.-G.; Liu, X.-Y.; Tang, C.-M.; Wang, L.-W.; Han, X.-L. Effects of light intensity on the growth and leaf development of young tomato plants grown under a combination of red and blue light. Sci. Hortic. 2013, 153, 50–55. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Montanari, M. Statistica ambientale. Analisi Multivariata. Metodologie di Ordinamento; SISSAD Snc: Trieste, Italy, 2012. [Google Scholar]
- Zanetti, M.; Costa, C.; Greco, R.; Grigolato, S.; Aalmo, G.O.; Cavalli, R. How wood fuels’ quality relates to the standards: A class-modelling approach. Energies 2017, 10, 1455. [Google Scholar] [CrossRef]
- Kennard, R.; Stone, L. Computer aided design of experiments. Technometrics 1969, 11, 137–148. [Google Scholar] [CrossRef]
- Sgarbossa, A.; Costa, C.; Menesatti, P.; Antonucci, F.; Pallottino, F.; Zanetti, M.; Grigolato, S.; Cavalli, R. A multivariate SIMCA index as discriminant in wood pellet quality assessment. Renew. Energy 2015, 76, 258–263. [Google Scholar] [CrossRef]
- De Biasi, M.G.; Astolfi, S.; Acampora, A.; Zuchi, S.; Fonzo, V.; Santangelo, E.; Caccia, R.; Badiani, M.; Soressi, G.P. A H2O2-forming peroxidase rather than a NAD(P)H-dependent O2•—Synthase may be the major player in cell death responses controlled by the Pto—Fen complex following fenthion treatment. Funct. Plant Biol. 2003, 30, 409–417. [Google Scholar] [CrossRef]
- Jeuken, M.J.; Zhang, N.W.; McHale, L.K.; Pelgrom, K.; Boer, E.D.; Lindhout, P.; Michelmore, R.W.; Visser, R.G.; Niks, R.E. Rin4 causes hybrid necrosis and race-specific resistance in an interspecific lettuce hybrid. Plant Cell 2009, 21, 3368–3378. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Fang, L.; Zhu, X.; Zhou, B.; Zhang, T. A CC-NBS-LRR gene induces hybrid lethality in cotton. J. Exp. Bot. 2019, 70, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.-Q.; Zhao, Y.-R.; Zhu, F.-L.; Li, X.-L.; He, Y. Mapping of chlorophyll and SPAD distribution in pepper leaves during leaf senescence using visible and near-infrared hyperspectral imaging. Trans. ASABE 2016, 59, 13–24. [Google Scholar] [CrossRef]
- Rinaldi, M. Variation of Specific Leaf Area for Sugar Beet Depending on Sowing Date and Irrigation. Ital. J. Agron. 2003, 7, 23–32. [Google Scholar]
- Aguirre-Becerra, H.; García-Trejo, J.F.; Vázquez-Hernández, C.; Alvarado, A.M.; Feregrino-Pérez, A.A.; Contreras-Medina, L.M.; Guevara-Gonzalez, R.G. Effect of extended photoperiod with a fixed mixture of light wavelengths on tomato seedlings. HortScience 2020, 55, 1832–1839. [Google Scholar] [CrossRef]
- Evans, J.R.; Poorter, H. Photosynthetic acclimation of plants to growth irradiance: The relative importance of specific leaf area and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ. 2001, 24, 755–767. [Google Scholar] [CrossRef]
- van Esse, H.P.; Klooster, J.W.V.; Bolton, M.D.; Yadeta, K.A.; van Baarlen, P.; Boeren, S.; Vervoort, J.; de Wit, P.J.; Thomma, B.P. The Cladosporium fulvum virulence protein Avr2 inhibits host proteases required for basal defense. Plant Cell 2008, 20, 1948–1963. [Google Scholar] [CrossRef]
- Fei, W.; Liu, Y. Biotrophic Fungal Pathogens: A Critical Overview. Appl. Biochem. Biotechnol. 2023, 195, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wahabzada, M.; Mahlein, A.-K.; Bauckhage, C.; Steiner, U.; Oerke, E.-C.; Kersting, K. Metro maps of plant disease dynamics—Automated mining of differences using hyperspectral images. PLoS ONE 2015, 10, e0116902. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- Xie, C.; Shao, Y.; Li, X.; He, Y. Detection of early blight and late blight diseases on tomato leaves using hyperspectral imaging. Sci. Rep. 2015, 5, 16564. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Antonucci, F.; Pallottino, F.; Aguzzi, J.; Sun, D.-W.; Menesatti, P. Shape analysis of agricultural products: A review of recent research advances and potential application to computer vision. Food Bioprocess Technol. 2011, 4, 673–692. [Google Scholar] [CrossRef]
- Violino, S.; Benincasa, C.; Taiti, C.; Ortenzi, L.; Pallottino, F.; Marone, E.; Mancuso, S.; Costa, C. AI-based hyperspectral and VOCs assessment approach to identify adulterated extra virgin olive oil. Eur. Food Res. Technol. 2021, 247, 1013–1022. [Google Scholar] [CrossRef]
- Janni, M.; Cocozza, C.; Birilli, F.; Pignatelli, S.; Vurro, F.; Coppede, N.; Bettelli, M.; Calestani, D.; Loreto, F.; Zappettini, A. Real-time monitoring of Arundo donax response to saline stress through the application of in vivo sensing technology. Sci. Rep. 2021, 11, 18598. [Google Scholar] [CrossRef]
- Janni, M.; Coppede, N.; Bettelli, M.; Briglia, N.; Petrozza, A.; Summerer, S.; Vurro, F.; Danzi, D.; Cellini, F.; Marmiroli, N.; et al. In vivo phenotyping for the early detection of drought stress in tomato. Plant Phenomics 2019, 2019, 6168209. [Google Scholar] [CrossRef]
- Coatsworth, P.; Gonzalez-Macia, L.; Collins, A.S.P.; Bozkurt, T.; Güder, F. Continuous monitoring of chemical signals in plants under stress. Nat. Rev. Chem. 2023, 7, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Presti, D.L.; Di Tocco, J.; Massaroni, C.; Cimini, S.; De Gara, L.; Singh, S.; Raucci, A.; Manganiello, G.; Woo, S.L.; Schena, E.; et al. Current understanding, challenges and perspective on portable systems applied to plant monitoring and precision agriculture. Biosens. Bioelectron. 2023, 222, 115005. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhang, L.; Cai, X.; Li, X.; Bian, L.; Luo, Z.; Li, Z.; Chen, Z.; Xin, Z. (E)-Nerolidol is a volatile signal that induces defenses against insects and pathogens in tea plants. Hortic. Res. 2020, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Aratani, Y.; Uemura, T.; Hagihara, T.; Matsui, K.; Toyota, M. Green leaf volatile sensory calcium transduction in Arabidopsis. Nat. Commun. 2023, 14, 6236. [Google Scholar] [CrossRef]
- Razo-Belman, R.; Ozuna, C. Volatile Organic Compounds: A Review of Their Current Applications as Pest Biocontrol and Disease Management. Horticulturae 2023, 9, 441. [Google Scholar] [CrossRef]




| #. | Plant Disease | Technique | References |
|---|---|---|---|
| 1 | Biocontrol of Trichoderma spp. by estimating disease severity in young small leafy vegetable plants during specific plant-pathogen-antagonist interactions | VIS-NIR spectroscopy and machine learning | [18] |
| 2 | Late blight caused by Phytophthora infestans in potato production | Visible/near-infrared (VIS/NIR) spectroscopy with machine learning (ML) and chemometric methods | [27] |
| 3 | Early disease in blueberries | Hyperspectral imaging between the spectral range of 400–1000 nm | [28] |
| 4 | Anthracnose and gray in the strawberries | Hyperspectral imaging between the spectral range of 400–1000 nm | [29] |
| 5 | Fungal infection in citrus fruit | VIS-NIR spectroscopy with range between 325–1100 nm | [30] |
| 6 | Anthracnose of banana caused by Colletotrichum species | VIS-NIR spectroscopy | [31] |
| 7 | Fire blight (FB) of pear trees | Visible-NIR spectrometry method | [32] |
| 8 | Gray mold disease caused by Botrytis cinerea in tomato | VIS-NIR spectroscopy with range between 550–1100 nm | [33] |
| 9 | Micotoxigenic fungi and their toxic metabolites produced in naturally and artificially contaminated products in maize | NIR spectroscopy | [34] |
| 10 | Tomato chlorosis virus (ToCV) | VIS-NIR in healthy and diseased leaves at a pre-symptomatic stage | [35] |
| 11 | Abiotic and biotic stresses in wild rocket (Diplotaxis tenuifolia) | ANN coupled with VIS-NIR and NIR | [19] |
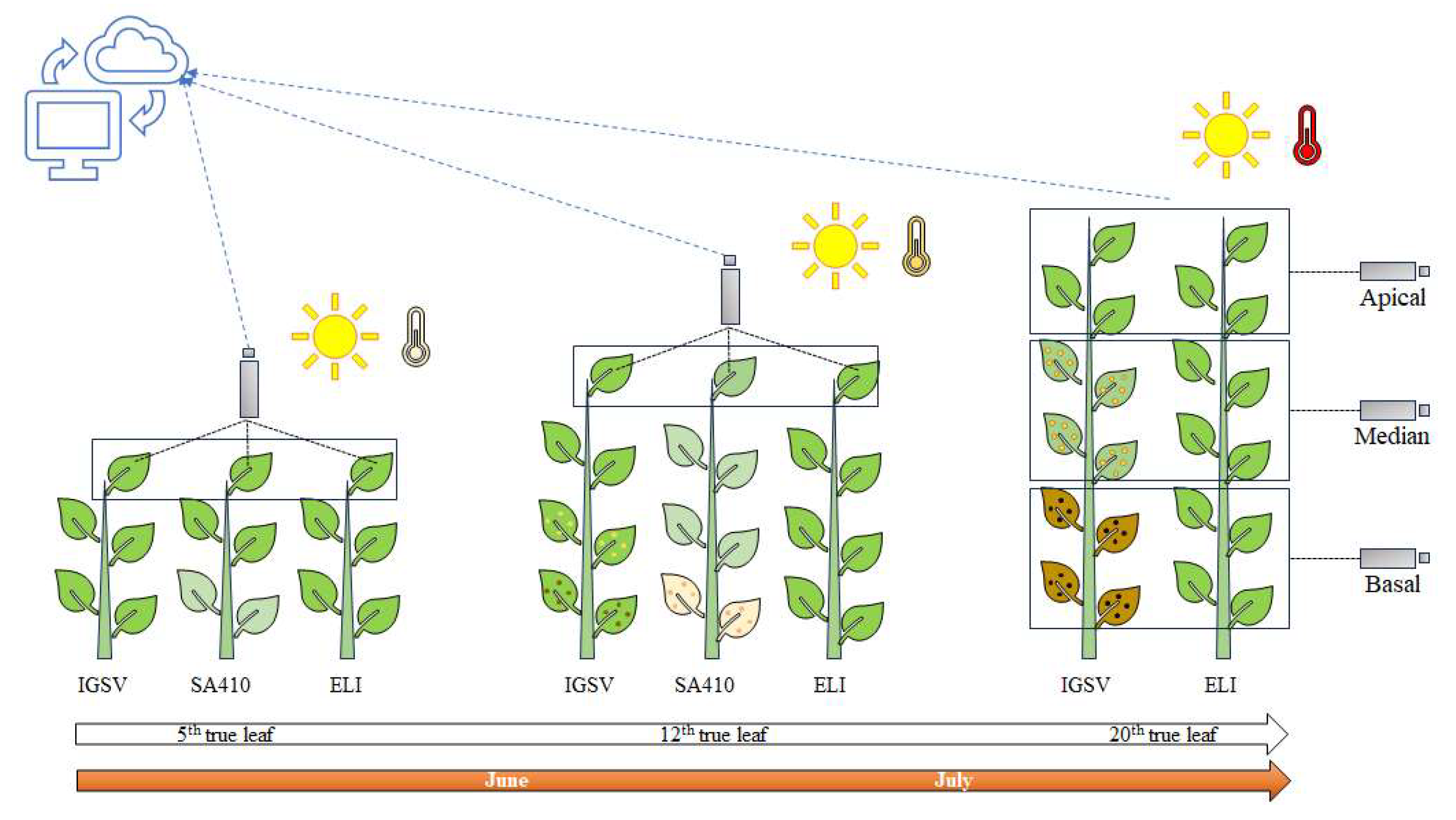
| True Leaf | Date of Analysis | DAT * | Leaf Position | Genotype |
|---|---|---|---|---|
| 5th | 10 June | 27 | Apical | Elisir, IGSV, SA410 |
| 12th | 25 June | 42 | Apical | Elisir, IGSV, SA410 |
| 20th | 20 July | 67 | Basal, median, apical | Elisir, IGSV |
| Leaf | Genotype | Chlorophyll (Spad Unit) | Dry Matter (%) | Leaf Weight (gDW) | Green LA * (cm−2) | LA | |
|---|---|---|---|---|---|---|---|
| Necrotic (%) | Specific (cm2 g−1DW) | ||||||
| Basal | IGSV | 27.0 c | 16.97 b | 1.83 | 109.03 | 34.17 | 60.49 b |
| Elisir | 56.6 a | 15.86 b | 2.58 | 218.13 | 0.45 | 86.16 a | |
| Median | IGSV | 43.5 b | 14.17 c | 2.22 | 193.42 | 14.11 | 87.18 a |
| Elisir | 56.4 a | 18.05 a | 3.74 | 275.21 | 0.00 | 76.14 a | |
| Apical | IGSV | 55.5 a | 14.90 c | 0.61 | 61.96 | 0.00 | 101.68 a |
| Elisir | 54.8 a | 18.16 a | 1.19 | 86.96 | 0.00 | 72.04 b | |
| Cultivar | Leaf | N. Accepted | N. Rejected | Accepted (%) |
|---|---|---|---|---|
| ELISIR | Apical | 24 | 3 | 88.9 |
| Median | 18 | 9 | 66.7 | |
| Basal | 15 | 12 | 55.6 | |
| IGSV | Apical | 22 | 5 | 81.5 |
| Median | 8 | 19 | 29.6 | |
| Basal | 9 | 18 | 33.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santangelo, E.; Giudice, A.D.; Figorilli, S.; Violino, S.; Costa, C.; Bascietto, M.; Bergonzoli, S.; Beni, C. Autonecrotic Tomato (Solanum lycopersicum L.) Line as a Potential Model for Applications in Proximal Sensing of Biotic and Abiotic Stress. Agriculture 2024, 14, 136. https://doi.org/10.3390/agriculture14010136
Santangelo E, Giudice AD, Figorilli S, Violino S, Costa C, Bascietto M, Bergonzoli S, Beni C. Autonecrotic Tomato (Solanum lycopersicum L.) Line as a Potential Model for Applications in Proximal Sensing of Biotic and Abiotic Stress. Agriculture. 2024; 14(1):136. https://doi.org/10.3390/agriculture14010136
Chicago/Turabian StyleSantangelo, Enrico, Angelo Del Giudice, Simone Figorilli, Simona Violino, Corrado Costa, Marco Bascietto, Simone Bergonzoli, and Claudio Beni. 2024. "Autonecrotic Tomato (Solanum lycopersicum L.) Line as a Potential Model for Applications in Proximal Sensing of Biotic and Abiotic Stress" Agriculture 14, no. 1: 136. https://doi.org/10.3390/agriculture14010136
APA StyleSantangelo, E., Giudice, A. D., Figorilli, S., Violino, S., Costa, C., Bascietto, M., Bergonzoli, S., & Beni, C. (2024). Autonecrotic Tomato (Solanum lycopersicum L.) Line as a Potential Model for Applications in Proximal Sensing of Biotic and Abiotic Stress. Agriculture, 14(1), 136. https://doi.org/10.3390/agriculture14010136











