Characteristics and Migration Dynamics of Microplastics in Agricultural Soils
Abstract
1. Introduction
2. Environmental Characterization of Microplastics in Agricultural Soils
2.1. Literature Searching and Screening
2.2. Sources and Types of Microplastics in Agricultural Soils
2.3. Distribution of Microplastics in Agricultural Soils
3. Migration and Effects of Microplastics in Agricultural Soils
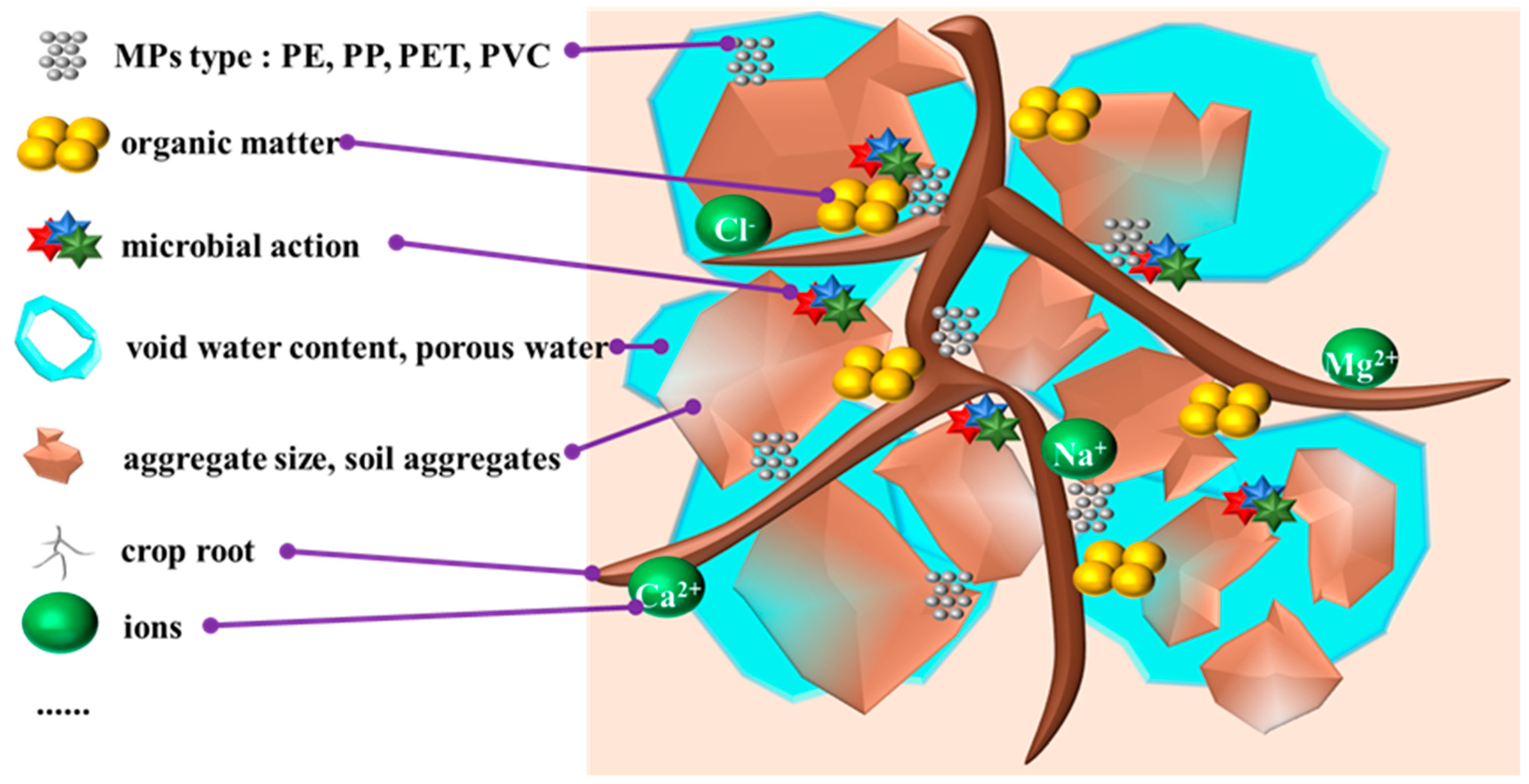
3.1. Migration of Microplastics in Agricultural Soils
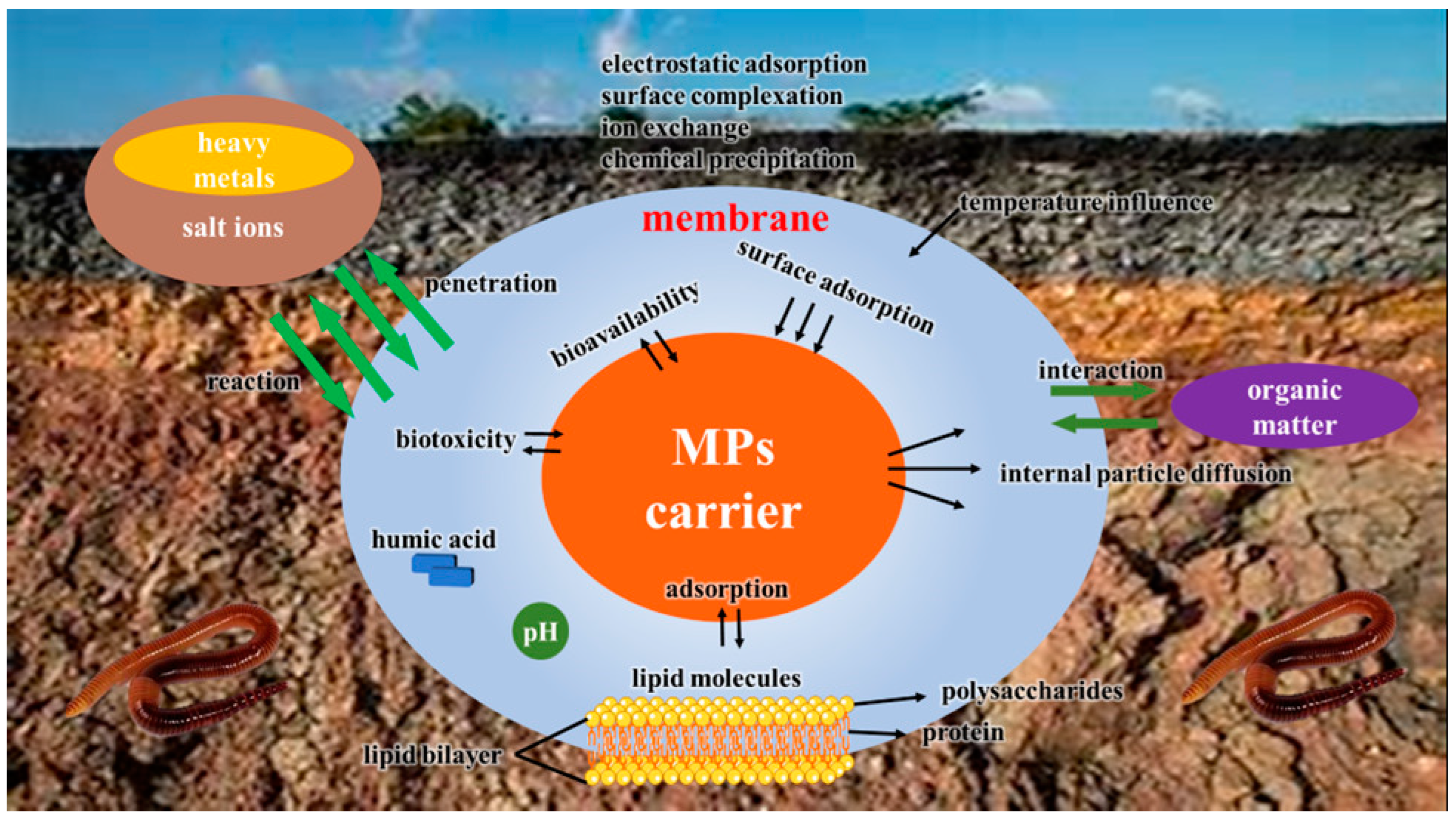
3.2. Effects of Microplastics on Agricultural Ecosystems
4. Biodegradation of Microplastics in Agricultural Soils
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CI | Carbonyl Index |
| DOM | Dissolved Organic Matter |
| EDA | Electron Donor-Acceptor |
| EU | European Union |
| FA | Fluviac Acid |
| FTIR | Fourier Transform Infrared Spectroscopy |
| GT | Goethite |
| NPs | Nanoplastics |
| MPs | Microplastics |
| O/C | Oxygen to Carbon Ratio |
| OTC | Oxytetracycline |
| PAHs | Polycyclic Aromatic Hydrocarbons |
| PBAT | Poly (butylene adipate-co-terephthalate) |
| PCL | Polycaprolactone |
| PE | Polyethylene |
| PHBV | Poly(-3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PET | Polyethylene Terephthalate |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl Chloride |
| ROS | Reactive Oxygen Species |
| SOC | Soil Organic Carbon |
| SMT | Sulfamethazine |
| XPS | X-ray Photoelectron Spectroscopy |
References
- Arthur, C.; Baker, J.; Bamford, H. Proceedings of the International Research Workshop on the Occurrence, Effects and Fate of Microplastic Marine Debris, Tacoma, WA, USA, 9–11 September 2008; National Oceanic and Atmospheric Administration Technical Memorandum NOS-OR&R-30; National Oceanic and Atmosphere Administration: Washington, DC, USA, 2009.
- Alimi, O.S.; Farner Budarz, J.; Hernandez, L.M.; Tufenkji, N. Microplastics and Nanoplastics in Aquatic Environments: Aggregation, Deposition, and Enhanced Contaminant Transport. Environ. Sci. Technol. 2018, 52, 1704–1724. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Microplastics in agricultural soils in China: Sources, impacts and solutions. Environ. Pollut. 2023, 322, 121235. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Contaminants in the Food Chain (CONTAM). Presence of microplastics and nanoplastics in food, with particular focus on seafood. EFSA J. 2016, 14, e04501. [Google Scholar]
- Zhou, B.; Wang, J.; Zhang, H.; Shi, H.; Fei, Y.; Huang, S.; Tong, Y.; Wen, D.; Luo, Y.; Barceló, D. Microplastics in agricultural soils on the coastal plain of Hangzhou Bay, east China: Multiple sources other than plastic mulching film. J. Hazard. Mater. 2020, 388, 121814. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wen, H.; Li, T.; Li, H.; Wu, C.; Zhang, G.; Yan, J. Distribution and Sources of Microplastics in Farmland Soil Along the Fenhe River. Environ. Sci. 2021, 42, 3894–3903. [Google Scholar]
- Yu, Y.; Flury, M. Current understanding of subsurface transport of micro- and nanoplastics in soil. Vadose Zone J. 2021, 20, e20108. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef]
- Nizzetto, L.; Futter, M.; Langaas, S. Are Agricultural Soils Dumps for Microplastics of Urban Origin? Environ. Sci. Technol. 2016, 50, 10777–10779. [Google Scholar] [CrossRef]
- Okeke, E.S.; Okoye, C.O.; Atakpa, E.O.; Ita, R.E.; Nyaruaba, R.; Mgbechidinma, C.L.; Akan, O.D. Microplastics in agroecosystems-impacts on ecosystem functions and food chain. Resour. Conserv. Recycl. 2022, 177, 105961. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total. Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef]
- Horton, A.; Walton, A.; Spurgeon, D.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Lim, X. Microplastics are everywhere—But are they harmful? Nature 2021, 593, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fu, D.; Gao, L.; Qi, H.; Su, Y.; Peng, L. Aged microplastics decrease the bioavailability of coexisting heavy metals to microalga Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2021, 217, 112199. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.S.; Dogiparthi, L.K.; Gangadhar, L.; Chakravorty, A.; Abhishek, N. Effects of microplastics and nanoplastics on marine environment and human health. Environ. Sci. Pollut. Res. Int. 2020, 27, 44743–44756. [Google Scholar] [CrossRef]
- Zhou, J.; Gui, H.; Banfield, C.C.; Wen, Y.; Zang, H.; Dippold, M.A.; Charlton, A.; Jones, D.L. The microplastisphere: Biodegradable microplastics addition alters soil microbial community structure and function. Soil Biol. Biochem. 2021, 156, 108211. [Google Scholar] [CrossRef]
- Patrício Silva, A.L.; Prata, J.C.; Mouneyrac, C.; Barcelò, D.; Duarte, A.C.; Rocha-Santos, T. Risks of COVID-19 face masks to wildlife: Present and future research needs. Sci. Total Environ. 2021, 792, 148505. [Google Scholar] [CrossRef]
- ESCAP. A Call for Healthy “Blue Oceans” in Asia and the Pacific; ESCAP: Bangkok, Thailand, 2019. [Google Scholar]
- European Union. Plastics Strategy; European Union: Brussels, Blegium, 2018. [Google Scholar]
- The National People’s Congress of the People’s Republic of China. Law of the People’s Republic of China on Prevention and Control of Soil Contamination; The National People’s Congress of the People’s Republic of China: Beijing, China, 2018.
- Dannis, M.L. Rubber Dust from the Normal Wear of Tires. Rubber Chem. Technol. 1974, 47, 1011–1037. [Google Scholar] [CrossRef]
- Napper, I.E.; Bakir, A.; Rowland, S.J.; Thompson, R.C. Characterisation, quantity and sorptive properties of microplastics extracted from cosmetics. Mar. Pollut. Bull. 2015, 99, 178–185. [Google Scholar] [CrossRef]
- Mahon, A.M.; O’Connell, B.; Healy, M.G.; O’Connor, I.; Officer, R.; Nash, R.; Morrison, L. Microplastics in Sewage Sludge: Effects of Treatment. Environ. Sci. Technol. 2017, 51, 810–818. [Google Scholar] [CrossRef]
- Scheurer, M.; Bigalke, M. Microplastics in Swiss Floodplain Soils. Environ. Sci. Technol. 2018, 52, 3591–3598. [Google Scholar] [CrossRef]
- Mahbub, M.S.; Shams, M. Acrylic fabrics as a source of microplastics from portable washer and dryer: Impact of washing and drying parameters. Sci. Total Environ. 2022, 834, 155429. [Google Scholar] [CrossRef]
- Sang, W.; Wang, X.; Wang, X.; Xiao, L. The source, occurance characteristics and migration behavior of microplastics in soil. J. Ecol. Rural Environ. 2021, 37, 1361–1367. [Google Scholar]
- Anthony, L.A. Plastics and the Environment; Wiley: Hoboken, NJ, USA, 2003. [Google Scholar]
- Sa’adu, I.; Farsang, A. Plastic contamination in agricultural soils: A review. Environ. Sci. Eur. 2023, 35, 1–11. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, R.; Lin, W.; Ouyang, G. Effect of salinity and humic acid on the aggregation and toxicity of polystyrene nanoplastics with different functional groups and charges. Environ. Pollut. 2019, 245, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Liu, S.; Li, H.; Chen, X.; Peng, C.; Zhang, P.; Liu, X. Distinct microplastic distributions in soils of different land-use types: A case study of Chinese farmlands. Environ. Pollut. 2021, 269, 116199. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Ren, S.-Y.; Ni, H.-G. Incidence of microplastics in personal care products: An appreciable part of plastic pollution. Sci. Total Environ. 2020, 742, 140218. [Google Scholar] [CrossRef]
- Patel, M.M.; Goyal, B.R.; Bhadada, S.V.; Bhatt, J.S.; Amin, A.F. Getting into the brain: Approaches to enhance brain drug delivery. CNS Drugs 2009, 23, 35–58. [Google Scholar] [CrossRef]
- Crawford, C.B.; Quinn, B. 4—Physiochemical properties and degradation. In Microplastic Pollutants; Crawford, C.B., Quinn, B., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 57–100. [Google Scholar]
- Fu, L.; Li, J.; Wang, G.; Luan, Y.; Dai, W. Adsorption behavior of organic pollutants on microplastics. Ecotoxicol. Environ. Saf. 2021, 217, 112207. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J. The chemical behaviors of microplastics in marine environment: A review. Mar. Pollut. Bull. 2019, 142, 1–14. [Google Scholar] [CrossRef]
- Li, H.; Li, S.; Nan, L.; Li, H.; Guo, Q. Meta-analysis of Effect of Plastic Film Mulching on Cotton Yield in China. Trans. Chin. Soc. Agric. Mach. 2017, 48, 228–235. [Google Scholar]
- Pei, X. Current Situation of Agricultural Plastic Sheeting Pollution in Henan Province and Its Countermeasures. Master’s Thesis, Henan Agricultural University, Zhengzhou, China, 2018. [Google Scholar]
- Mormile, P.; Stahl, N.; Malinconico, M. The World of Plasticulture. In Soil Degradable Bioplastics for a Sustainable Modern Agriculture; Green Chemistry and Sustainable Technology; Malinconico, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 1–21. [Google Scholar]
- Zhang, Q.-Q.; Ma, Z.-R.; Cai, Y.-Y.; Li, H.-R.; Ying, G.-G. Agricultural Plastic Pollution in China: Generation of Plastic Debris and Emission of Phthalic Acid Esters from Agricultural Films. Environ. Sci. Technol. 2021, 55, 12459–12470. [Google Scholar] [CrossRef] [PubMed]
- Brodhagen, M.; Goldberger, J.R.; Hayes, D.G.; Inglis, D.A.; Marsh, T.L.; Miles, C. Policy considerations for limiting unintended residual plastic in agricultural soils. Environ. Sci. Policy 2017, 69, 81–84. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, H.; Hu, W.; Qin, X.; Ma, X.; Yan, C.; Wang, H. The status and distribution characteristics of residual mulching film in Xinjiang, China. J. Integr. Agric. 2016, 15, 2639–2646. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, X.; Wen, H.; Zheng, X.; Niu, Q.; Kang, J. Research status and prospect of control technology for residual plastic film pollution in farmland. Trans. Chin. Soc. Agric. Eng. 2017, 48, 1–14. [Google Scholar]
- Yan, C.; Mei, X.; He, W.; Zheng, S. Present situation of residue pollution of mulching plastic film and controlling measures. Trans. Chin. Soc. Agric. Eng. 2006, 22, 269–272. [Google Scholar]
- Susanna, G. Plastic Pollution in Soil; Institute for European Environmental Policy: Brussels, Belgium, 2018. [Google Scholar]
- Isari, E.A.; Papaioannou, D.; Kalavrouziotis, I.K.; Karapanagioti, H.K. Microplastics in Agricultural Soils: A Case Study in Cultivation of Watermelons and Canning Tomatoes. Water 2021, 13, 2168. [Google Scholar] [CrossRef]
- Lusher, A.; Hurley, R.; Vogelsang, C.; Nizzetto, L.; Olsen, M. Mapping Microplastics in Sludge; Norsk Institutt for Vannforskning: Oslo, Norway, 2017. [Google Scholar]
- Gies, E.A.; LeNoble, J.L.; Noël, M.; Etemadifar, A.; Bishay, F.; Hall, E.R.; Ross, P.S. Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 133, 553–561. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Wang, P.-Y.; Wang, Y.-B.; Zhou, R.; Koskei, K.; Munyasya, A.N.; Liu, S.-T.; Wang, W.; Su, Y.-Z.; Xiong, Y.-C. Fate of plastic film residues in agro-ecosystem and its effects on aggregate-associated soil carbon and nitrogen stocks. J. Hazard. Mater. 2021, 416, 125954. [Google Scholar] [CrossRef]
- Yang, L.; Luo, W.; Zhao, P.; Zhang, Y.; Kang, S.; Giesy, J.P.; Zhang, F. Microplastics in the Koshi River, a remote alpine river crossing the Himalayas from China to Nepal. Environ. Pollut. 2021, 290, 118121. [Google Scholar] [CrossRef]
- Jiang, N.; Luo, W.; Zhao, P.; Ga, B.; Jia, J.; Giesy, J.P. Distribution of microplastics in benthic sediments of Qinghai Lake on the Tibetan Plateau, China. Sci. Total Environ. 2022, 835, 155434. [Google Scholar] [CrossRef]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Song, Y.; Cai, Y. Focus topics on microplastics in soil: Analytical methods, occurrence, transport, and ecological risks. Environ. Pollut. 2020, 257, 113570. [Google Scholar] [CrossRef] [PubMed]
- Huerta Lwanga, E.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Microplastics in the Terrestrial Ecosystem: Implications for Lumbricus terrestris (Oligochaeta, Lumbricidae). Environ. Sci. Technol. 2016, 50, 2685–2691. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Bonkowski, M. Microplastic and soil protists: A call for research. Environ. Pollut. 2018, 241, 1128–1131. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; van der Ploeg, M.; Lwanga, E.H.; Yang, X.; Zhang, S.; Ma, X.; Ritsema, C.J.; Geissen, V. Leaching of microplastics by preferential flow in earthworm (Lumbricus terrestris) burrows. Environ. Chem. 2019, 16, 31–40. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Gertsen, H.; Peters, P.; Salánki, T.; Geissen, V. A simple method for the extraction and identification of light density microplastics from soil. Sci. Total Environ. 2017, 616–617. [Google Scholar] [CrossRef]
- Choi, Y.R.; Kim, Y.-N.; Yoon, J.-H.; Dickinson, N.; Kim, K.-H. Plastic contamination of forest, urban, and agricultural soils: A case study of Yeoju City in the Republic of Korea. J. Soils Sediments 2021, 21, 1962–1973. [Google Scholar] [CrossRef]
- Bo, L.; Li, B.; Zhang, K.; Ma, R.; Li, Y.; Sun Bi Liu, Y. Distribution, Sources and Behavioral Characteristics of Microplastics in Farmland Soil. Environ. Sci. 2022, 44, 2375–2383. [Google Scholar]
- Dong, S.; Cai, W.; Xia, J.; Sheng, L.; Wang, W.; Liu, H. Aggregation kinetics of fragmental PET nanoplastics in aqueous environment: Complex roles of electrolytes, pH and humic acid. Environ. Pollut. 2021, 268, 115828. [Google Scholar] [CrossRef]
- Dong, S.; Xia, J.; Sheng, L.; Wang, W.; Liu, H.; Gao, B. Transport characteristics of fragmental polyethylene glycol terephthalate (PET) microplastics in porous media under various chemical conditions. Chemosphere 2021, 276, 130214. [Google Scholar] [CrossRef]
- Qi, K.; Lu, N.; Zhang, S.; Wang, W.; Wang, Z.; Guan, J. Uptake of Pb(II) onto microplastic-associated biofilms in freshwater: Adsorption and combined toxicity in comparison to natural solid substrates. J. Hazard. Mater. 2021, 411, 125115. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Luo, W.; Jiang, N.; Ga, B.; Pang, X.; Giesy, J.; Galloway, T. Distribution and Risks of Microplastics in Sediments of a Small Coastal River–Estuary System: Functional Areas, Wastewater Treatment Plants, and Dams. SSRN Electron. J. 2022, preprint. [Google Scholar] [CrossRef]
- Abdurahman, A.; Cui, K.; Wu, J.; Li, S.; Gao, R.; Dai, J.; Liang, W.; Zeng, F. Adsorption of dissolved organic matter (DOM) on polystyrene microplastics in aquatic environments: Kinetic, isotherm and site energy distribution analysis. Ecotoxicol. Environ. Saf. 2020, 198, 110658. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Qiu, Y.; Zhao, J.; Ouyang, X.; Zhao, Y.; Weng, L.; MDYasir, A.; Chen, Y.; Li, Y. Effect of Agricultural Organic Inputs on Nanoplastics Transport in Saturated Goethite-Coated Porous Media: Particle Size Selectivity and Role of Dissolved Organic Matter. Environ. Sci. Technol. 2022, 56, 3524–3534. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lin, L.; Wang, X.; Sun, X.; Yu, A. Adsorption of fulvic acid onto polyamide 6 microplastics: Influencing factors, kinetics modeling, site energy distribution and interaction mechanisms. Chemosphere 2021, 272, 129638. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, J.; Huang, W.; He, Y. Laboratory simulated aging methods, mechanisms and characteristic changes of microplastics: A review. Chemosphere 2023, 315, 137744. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Lang, M.; Yu, X.; Wu, R.; Yang, X.; Guo, X. Aging mechanism of microplastics with UV irradiation and its effects on the adsorption of heavy metals. J. Hazard. Mater. 2020, 393, 122515. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, T.; Tian, L.; Liu, X.; Qi, Z.; Ma, Y.; Ji, R.; Chen, W. Aging Significantly Affects Mobility and Contaminant-Mobilizing Ability of Nanoplastics in Saturated Loamy Sand. Environ. Sci. Technol. 2019, 53, 5805–5815. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, J.; Zhu, W.; Xue, X.; Zheng, L.; Yu, Y.; Wang, H. Adsorption of natural organic matter on original and aged polystyrene microplastics. Acta Sci. Circumstantiae 2023, 43, 181–191. [Google Scholar]
- Sander, T.; Gerke, H.H. Modelling field-data of preferential flow in paddy soil induced by earthworm burrows. J. Contam. Hydrol. 2009, 104, 126–136. [Google Scholar] [CrossRef]
- Bogner, C.; Borken, W.; Huwe, B. Impact of preferential flow on soil chemistry of a podzol. Geoderma 2012, 175–176, 37–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Niu, J.; Zheng, H. The preferential flow of soil: A widespread phenomenon in pedological perspectives. Eurasian Soil Sci. 2016, 49, 661–672. [Google Scholar] [CrossRef]
- Khan, M.A.; Huang, Q.; Khan, S.; Wang, Q.; Huang, J.; Fahad, S.; Sajjad, M.; Liu, Y.; Mašek, O.; Li, X.; et al. Abundance, spatial distribution, and characteristics of microplastics in agricultural soils and their relationship with contributing factors. J. Environ. Manag. 2023, 328, 117006. [Google Scholar] [CrossRef] [PubMed]
- Bradford, S.A.; Yates, S.R.; Bettahar, M.; Simunek, J. Physical factors affecting the transport and fate of colloids in saturated porous media. Water Resour. Res. 2002, 38, 63.1–63.12. [Google Scholar] [CrossRef]
- Hallaq, A. The impact of soil texture on nitrates leaching into groundwater in the north governorate, Gaza strip. J. Soc. Sci. 2010, 38, 11–35. [Google Scholar]
- Rahmatpour, S.; Mosaddeghi, M.R.; Shirvani, M.; Šimůnek, J. Transport of silver nanoparticles in intact columns of calcareous soils: The role of flow conditions and soil texture. Geoderma 2018, 322, 89–100. [Google Scholar] [CrossRef]
- Silori, R.; Shrivastava, V.; Mazumder, P.; Mootapally, C.; Pandey, A.; Kumar, M. Understanding the underestimated: Occurrence, distribution, and interactions of microplastics in the sediment and soil of China, India, and Japan. Environ. Pollut. 2023, 320, 120978. [Google Scholar] [CrossRef]
- Yu, L.; Zhang, J.; Liu, Y.; Chen, L.; Tao, S.; Liu, W. Distribution characteristics of microplastics in agricultural soils from the largest vegetable production base in China. Sci. Total Environ. 2021, 756, 143860. [Google Scholar] [CrossRef]
- Zhang, Z. Monitoring and Evaluation of Irrigation Water Utilization Coefficient under Different Irrigation Methods in Xiliaohe Well Irrigation District. Master’s Thesis, Changchun Institute of Technology, Changchun, China, 2020. [Google Scholar] [CrossRef]
- Hüffer, T.; Metzelder, F.; Sigmund, G.; Slawek, S.; Schmidt, T.C.; Hofmann, T. Polyethylene microplastics influence the transport of organic contaminants in soil. Sci. Total Environ. 2019, 657, 242–247. [Google Scholar] [CrossRef]
- Endo, S.; Droge ST, J.; Goss, K.-U. Polyparameter Linear Free Energy Models for Polyacrylate Fiber−Water Partition Coefficients to Evaluate the Efficiency of Solid-Phase Microextraction. Anal. Chem. 2011, 83, 1394–1400. [Google Scholar] [CrossRef]
- Liu, X.; Xu, J.; Zhao, Y.; Shi, H.; Huang, C.-H. Hydrophobic sorption behaviors of 17β-Estradiol on environmental microplastics. Chemosphere 2019, 226, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Bao, M.; Hong, M.; Zhao, B.; Xing, A.; Ye, H.; Shen, Q.; Wang, L. Distribution characteristics and influencing factors concerning residual quantity of agricultural mulch film in Hetao irrigation area, Inner Mongolia. J. Agric. Resour. Environ. 2023, 40, 45–54. [Google Scholar]
- Bryan, R.B. The development, use and efficiency of indices of soil erodibility. Geoderma 1968, 2, 5–26. [Google Scholar] [CrossRef]
- Boix-Fayos, C.; Calvo-Cases, A.; Imeson, A.C.; Soriano-Soto, M.D. Influence of soil properties on the aggregation of some Mediterranean soils and the use of aggregate size and stability as land degradation indicators. Catena 2001, 44, 47–67. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Till, J.; Kloas, W.; Lehmann, A.; Becker, R.; Rillig, M.C. Impacts of Microplastics on the Soil Biophysical Environment. Environ. Sci. Technol. 2018, 52, 9656–9665. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, A.; Fitschen, K.; Rillig, M.C. Abiotic and Biotic Factors Influencing the Effect of Microplastic on Soil Aggregation. Soil Syst. 2019, 3, 21. [Google Scholar] [CrossRef]
- Liang, Y.; Lehmann, A.; Yang, G.; Leifheit, E.F.; Rillig, M.C. Effects of Microplastic Fibers on Soil Aggregation and Enzyme Activities Are Organic Matter Dependent. Front. Environ. Sci. 2021, 9, 650155. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; van der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef]
- van der Heijden MG, A.; de Bruin, S.; Luckerhoff, L.; van Logtestijn RS, P.; Schlaeppi, K. A widespread plant-fungal-bacterial symbiosis promotes plant biodiversity, plant nutrition and seedling recruitment. ISME J. 2016, 10, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Lehmann, A.; de Souza Machado, A.A.; Yang, G. Microplastic effects on plants. New Phytol. 2019, 223, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Vallespir Lowery, N.; Ursell, T. Structured environments fundamentally alter dynamics and stability of ecological communities. Proc. Natl. Acad. Sci USA 2019, 116, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, Z.; Chen, L.; Cui, Q.; Cui, Y.; Song, D.; Fang, L. Review on migration, transformation and ecological impacts of microplastics in soil. Appl. Soil Ecol. 2022, 176, 104486. [Google Scholar] [CrossRef]
- Adeel, M.; Yang, Y.S.; Wang, Y.Y.; Song, X.M.; Ahmad, M.A.; Rogers, H.J. Uptake and transformation of steroid estrogens as emerging contaminants influence plant development. Environ. Pollut. 2018, 243, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Lei, C.; Xu, J.; Li, R. Foliar uptake and leaf-to-root translocation of nanoplastics with different coating charge in maize plants. J. Hazard. Mater. 2021, 416, 125854. [Google Scholar] [CrossRef] [PubMed]
- Schwab, F.; Zhai, G.; Kern, M.; Turner, A.; Schnoor, J.L.; Wiesner, M.R. Barriers, pathways and processes for uptake, translocation and accumulation of nanomaterials in plants—Critical review. Nanotoxicology 2016, 10, 257–278. [Google Scholar] [CrossRef]
- Azeem, I.; Adeel, M.; Ahmad, M.A.; Shakoor, N.; Jiangcuo, G.D.; Azeem, K.; Ishfaq, M.; Shakoor, A.; Ayaz, M.; Xu, M.; et al. Uptake and Accumulation of Nano/Microplastics in Plants: A Critical Review. Nanomaterials 2021, 11, 2935. [Google Scholar] [CrossRef]
- Hartmann, M.; Six, J. Soil structure and microbiome functions in agroecosystems. Nat. Rev. Earth Environ. 2023, 4, 4–18. [Google Scholar] [CrossRef]
- Fan, Y.; Zheng, K.; Zhu, Z.; Chen, G.; Peng, X. Distribution, sedimentary record, and persistence of microplastics in the Pearl River catchment, China. Environ. Pollut. 2019, 251, 862–870. [Google Scholar] [CrossRef]
- Bandow, N.; Will, V.; Wachtendorf, V.; Simon, F. Contaminant release from aged microplastic. Environ. Chem. 2017, 14, 394. [Google Scholar] [CrossRef]
- Cao, J.; Yang, Q.; Jiang, J.; Dalu, T.; Kadushkin, A.; Singh, J.; Fakhrullin, R.; Wang, F.; Cai, X.; Li, R. Coronas of micro/nano plastics: A key determinant in their risk assessments. Part. Fibre Toxicol. 2022, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; Barnoud, J.; Monticelli, L. Polystyrene Nanoparticles Perturb Lipid Membranes. J. Phys. Chem. Lett. 2014, 5, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Hale, R.C.; Seeley, M.E.; La Guardia, M.J.; Mai, L.; Zeng, E.Y. A global perspective on microplastics. J. Geophys. Res. Ocean 2020, 125, e2018JC014719. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Gu, J.; Yin, X.; Guo, L.; Qian, L.; Shi, L.; Guo, M.; Ji, G. The toxic effect of bisphenol AF and nanoplastic coexposure in parental and offspring generation zebrafish. Ecotoxicol. Environ. Saf. 2023, 251, 114565. [Google Scholar] [CrossRef] [PubMed]
- Vethaak, A.D.; Legler, J. Microplastics and human health. Science 2021, 371, 672–674. [Google Scholar] [CrossRef]
- He, L.; Li, Z.; Jia, Q.; Xu, Z. Soil microplastics pollution in agriculture. Science 2023, 379, 547. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Yu, Z.; Yan, C.; Qiu, D.; Zhang, X.; Wen, C.; Dong, S. Accumulation and ecotoxicological effects induced by combined exposure of different sized polyethylene microplastics and oxytetracycline in zebrafish. Environ. Pollut. 2023, 319, 120977. [Google Scholar] [CrossRef]
- Sendra, M.; Pereiro, P.; Figueras, A.; Novoa, B. An integrative toxicogenomic analysis of plastic additives. J. Hazard. Mater. 2021, 409, 124975. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, X.; Wu, A.; Tang, Z.; Niu, L.; Wu, F.; Wang, F.; Zhao, T.; Fu, Z. Aggregation and stability of sulfate-modified polystyrene nanoplastics in synthetic and natural waters. Environ. Pollut. 2021, 268, 114240. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Qian, L.; Wang, H.; Zhan, X.; Lu, K.; Gu, C.; Gao, S. New Insights into the Aging Behavior of Microplastics Accelerated by Advanced Oxidation Processes. Environ. Sci. Technol. 2019, 53, 3579–3588. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, P.; Shi, H.; Wang, H.; Huang, H.; Shi, Y.; Gao, S. Photo aging and fragmentation of polypropylene food packaging materials in artificial seawater. Water Res. 2021, 188, 116456. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, P.; Wu, X.; Shi, H.; Huang, H.; Wang, H.; Gao, S. Insight into chain scission and release profiles from photodegradation of polycarbonate microplastics. Water Res. 2021, 195, 116980. [Google Scholar] [CrossRef] [PubMed]
- Al Naggar, Y.; Brinkmann, M.; Sayes, C.M.; AL-Kahtani, S.N.; Dar, S.A.; El-Seedi, H.R.; Grünewald, B.; Giesy, J.P. Are Honey Bees at Risk from Microplastics? Toxics 2021, 9, 109. [Google Scholar] [CrossRef]
- Yu, Z.; Song, S.; Xu, X.; Ma, Q.; Lu, Y. Sources, migration, accumulation and influence of microplastics in terrestrial plant communities. Environ. Exp. Bot. 2021, 192, 104635. [Google Scholar] [CrossRef]
- Lwanga, E.H.; Gertsen, H.; Gooren, H.; Peters, P.; Salánki, T.; van der Ploeg, M.; Besseling, E.; Koelmans, A.A.; Geissen, V. Incorporation of microplastics from litter into burrows of Lumbricus terrestris. Environ. Pollut. 2017, 220, 523–531. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Kloas, W.; Zarfl, C.; Hempel, S.; Rillig, M.C. Microplastics as an emerging threat to terrestrial ecosystems. Glob. Chang. Biol. 2018, 24, 1405–1416. [Google Scholar] [CrossRef]
- Ma, J.; Chen, F.; Zhu, Y.; Li, X.; Yu, H.; Sun, Y. Joint effects of microplastics and ciprofloxacin on their toxicity and fates in wheat: A hydroponic study. Chemosphere 2022, 303, 135023. [Google Scholar] [CrossRef]
- Zhang, Z.; Cui, Q.; Chen, L.; Zhu, X.; Zhao, S.; Duan, C.; Zhang, X.; Song, D.; Fang, L. A critical review of microplastics in the soil-plant system: Distribution, uptake, phytotoxicity and prevention. J. Hazard. Mater. 2022, 424, 127750. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, P.; Wang, J.; Ding, L.; Li, L.; Jia, H.; Wang, T.; Guo, X.; Gao, S. Microplastics-derived dissolved organic matters accelerate photodegradation of sulfamethazine in wastewater ultraviolet disinfection process. Chem. Eng. J. 2023, 454, 140301. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Besseling, E.; Wegner, A.; Foekema, E.M. Plastic as a carrier of POPs to aquatic organisms: A model analysis. Environ. Sci. Technol. 2013, 47, 7812–7820. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.C.; da Costa, J.P.; Lopes, I.; Andrady, A.L.; Duarte, A.C.; Rocha-Santos, T. A One Health perspective of the impacts of microplastics on animal, human and environmental health. Sci. Total Environ. 2021, 777, 146094. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.C.; Rosenberg, M.; Cheng, L. Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol. Lett. 2012, 8, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Shen, M.; Zhang, Y.; Li, H.; Zeng, G. Microplastics and nanoplastics: Would they affect global biodiversity change. Environ. Sci. Pollut. Res. Int. 2019, 26, 19997–20002. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Chen, Z.; Zhu, F.; Zhu, C.; Wang, C.; Gu, C. Effect of Polyvinyl Chloride Microplastics on Bacterial Community and Nutrient Status in Two Agricultural Soils. Bull. Environ. Contam. Toxicol. 2021, 107, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Song, K.; Yeerken, S.; Geng, S.; Liu, D.; Dai, Z.; Xie, F.; Zhou, X.; Wang, Q. Effect evaluation of microplastics on activated sludge nitrification and denitrification. Sci. Total Environ. 2020, 707, 135953. [Google Scholar] [CrossRef]
- Krueger, M.C.; Harms, H.; Schlosser, D. Prospects for microbiological solutions to environmental pollution with plastics. Appl. Microbiol. Biotechnol. 2015, 99, 8857–8874. [Google Scholar] [CrossRef]
- Feng, W.; Deng, Y.; Cao, Y.; Liu, J.; Han, Y.; Liu, J.; Miao, Q.; Yang, F.; Zhu, Y.; Giesy, J.P. Biotechnology Remediation and Environmental Behavior of Microplastics in Soils: A Review. Rev. Environ. Contam. 2023, 261, 13. [Google Scholar] [CrossRef]
- Jin, Y.; Cai, F.; Wang, L.; Song, C.; Jin Sun, J.; Liu, G.; Chen, C. Advance in the degradation of biodegradable plastics in different environments. Chin. J. Biotech. 2022, 38, 1784–1808. [Google Scholar]
- Wang, M.; Yan, N.; Jiang, X.; Chen, G.; Liang, R.; Chen, Y.; Zhang, X.; Xu, L. Degradation performance of PBAT/PLA fully biodegradable mulching film and its effect on cotton growth in Shihezi reclamation areas in Xinjiang of China. Trans. Chin. Soc. Agric. Eng. 2022, 38, 273–282. [Google Scholar]
- Urbanek, A.K.; Rymowicz, W.; Strzelecki, M.C.; Kociuba, W.; Franczak, Ł.; Mirończuk, A.M. Isolation and characterization of Arctic microorganisms decomposing bioplastics. AMB Express 2017, 7, 148. [Google Scholar] [CrossRef] [PubMed]
- Witt, U.; Einig, T.; Yamamoto, M.; Kleeberg, I.; Deckwer, W.-D.; Müller, R.-J. Biodegradation of aliphatic–aromatic copolyesters: Evaluation of the final biodegradability and ecotoxicological impact of degradation intermediates. Chemosphere 2001, 44, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Liu Xi Dong, X.; Xie, Z.; Ma, X.; Luo, Y. Ecological Effects and Biodegradation of Microplastics in Soils. Acta Pedol. Sin. 2022, 59, 349–363. [Google Scholar]
- Helen, A.S.; Uche, E.C.; Hamid, F.S. Screening for Polypropylene Degradation Potential of Bacteria Isolated from Mangrove Ecosystems in Peninsular Malaysia. IJBBB 2017, 7, 245–251. [Google Scholar] [CrossRef]
- Peixoto, J.; Silva, L.P.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Tang, K.H.D.; Lock, S.S.M.; Yap, P.-S.; Cheah, K.W.; Chan, Y.H.; Yiin, C.L.; Ku AZ, E.; Loy AC, M.; Chin BL, F.; Chai, Y.H. Immobilized enzyme/microorganism complexes for degradation of microplastics: A review of recent advances, feasibility and future prospects. Sci. Total. Environ. 2022, 832, 154868. [Google Scholar] [CrossRef]
- Akutsu, Y.; Nakajima-Kambe, T.; Nomura, N.; Nakahara, T. Purification and properties of a polyester polyurethane-degrading enzyme from comamonas acidovorans tb-35. Appl. Environ. Microbiol. 1998, 64, 62–67. [Google Scholar] [CrossRef]
- Montazer, Z.; Habibi Najafi, M.B.; Levin, D.B. Challenges with verifying microbial degradation of polyethylene. Polymers 2020, 12, 123. [Google Scholar] [CrossRef]




| Mcroplastics | Chemical Formula | Polarity | Glass Transition Temperature (°C) | Density (g/cm3) | Resistance to UV Light | General Properties |
|---|---|---|---|---|---|---|
| PE | (C2H4)n | non-polar | −110 | 0.92–0.97 | Poor | With good water resistance, weather resistance and flexible chemical resistance |
| PP | (C3H6)n | non-polar | −49 to −20 | 0.88–1.23 | Fair | Resistance to a variety of acids and alkalis, low density, high stiffness, heat resistance and good transparency |
| PVC | (C2H3Cl)n | polar | 60–100 | 1.15–1.70 | Good | Flame retardancy, resistance to acids, alkalis and most inorganic chemicals |
| PS | (C8H8)n | non-polar | 90 | 1.04–1.50 | Poor | Crystal clear appearance, good impact resistance and toughness, and poor waterproofing and oxygen resistance |
| PET | (C10H8O4)n | polar | 73–78 | 1.30–1.50 | Fair | Light weight, aging resistance, strong impact and shatter resistant with good water retention and sealing properties. |
| PA6 | (C6H11NO)n | Strongly polar | −60 | 1.12–1.14 | Poor | Toughness, good tensile strength and good wear resistance |
| PA66 | (C12H22N2O2)n | 1.13–1.38 | Fair |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, Y.; Zeng, Z.; Feng, W.; Liu, J.; Yang, F. Characteristics and Migration Dynamics of Microplastics in Agricultural Soils. Agriculture 2024, 14, 157. https://doi.org/10.3390/agriculture14010157
Deng Y, Zeng Z, Feng W, Liu J, Yang F. Characteristics and Migration Dynamics of Microplastics in Agricultural Soils. Agriculture. 2024; 14(1):157. https://doi.org/10.3390/agriculture14010157
Chicago/Turabian StyleDeng, Yuxin, Zijie Zeng, Weiying Feng, Jing Liu, and Fang Yang. 2024. "Characteristics and Migration Dynamics of Microplastics in Agricultural Soils" Agriculture 14, no. 1: 157. https://doi.org/10.3390/agriculture14010157
APA StyleDeng, Y., Zeng, Z., Feng, W., Liu, J., & Yang, F. (2024). Characteristics and Migration Dynamics of Microplastics in Agricultural Soils. Agriculture, 14(1), 157. https://doi.org/10.3390/agriculture14010157









