Quantitative Analysis of Vertical and Temporal Variations in the Chlorophyll Content of Winter Wheat Leaves via Proximal Multispectral Remote Sensing and Deep Transfer Learning
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Experimental Site
2.2. Field Data Acquisition and Preprocessing
2.2.1. Leaf Sampling and Image Collection
2.2.2. Chlorophyll Measurement
2.2.3. Image Preprocessing
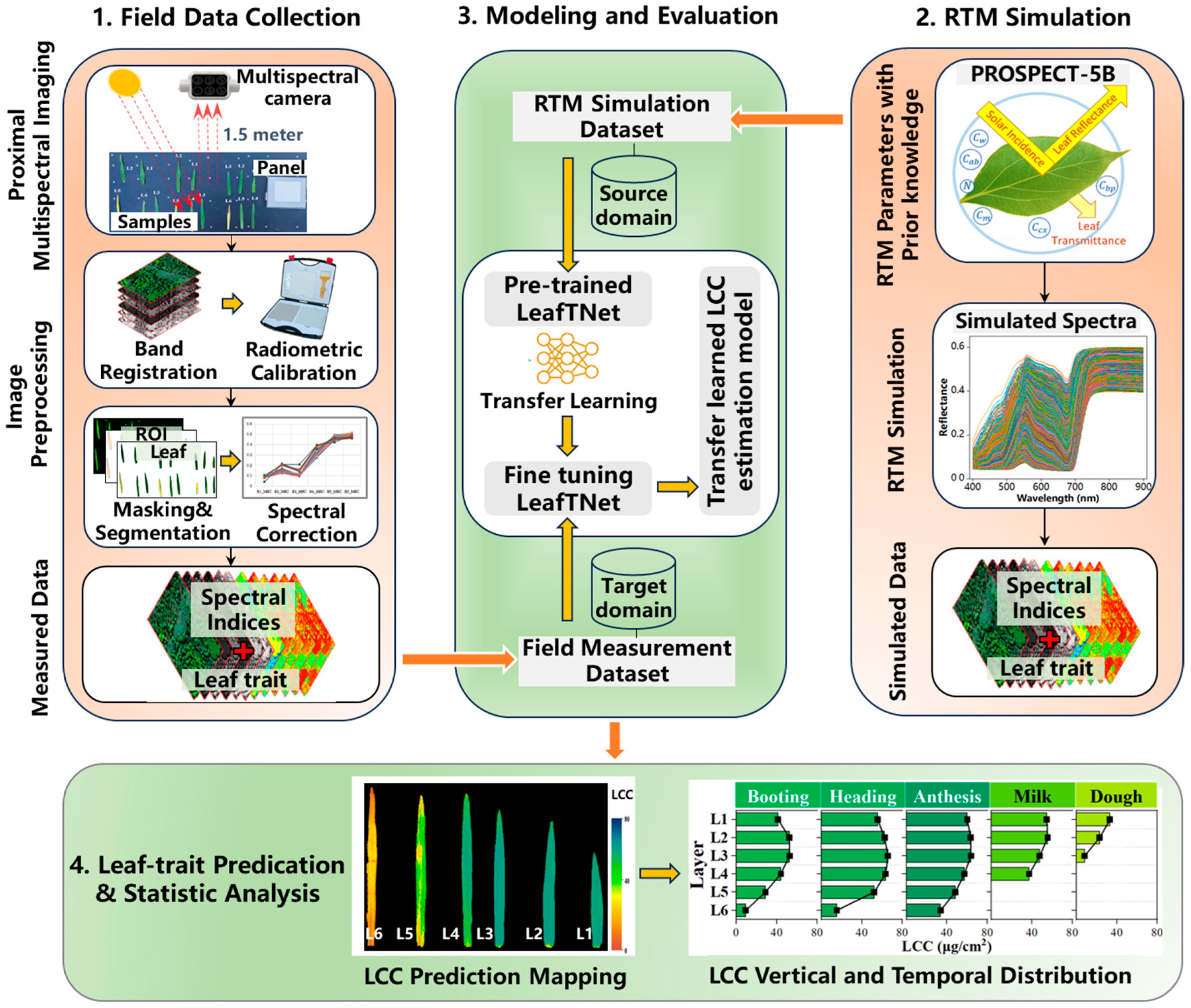
2.3. Model Establishment
2.3.1. RTM Simulation Training Dataset Generation
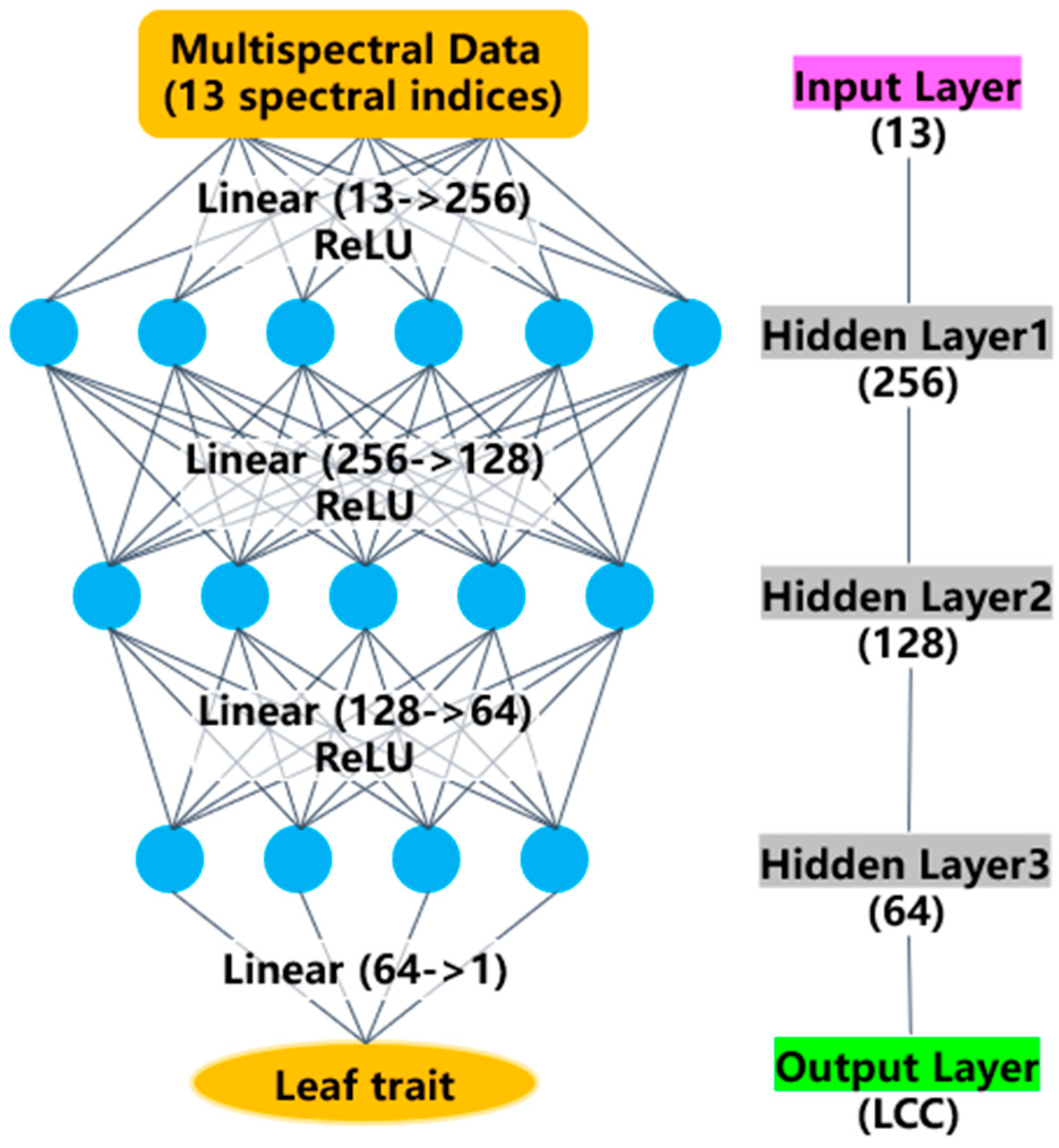
2.3.2. LeafTNet Based on Tapering Network Concept
2.3.3. Model Training
- (1)
- Pre-Training of the LeafTNet Network
- (2)
- Fine-tuning of the LeafTNet Network
2.4. Statistical Regression Approach
2.4.1. Empirical Statistical Model Based on Spectral Index
2.4.2. Partial Least-Squares Regression
2.5. Model Evaluation
3. Results
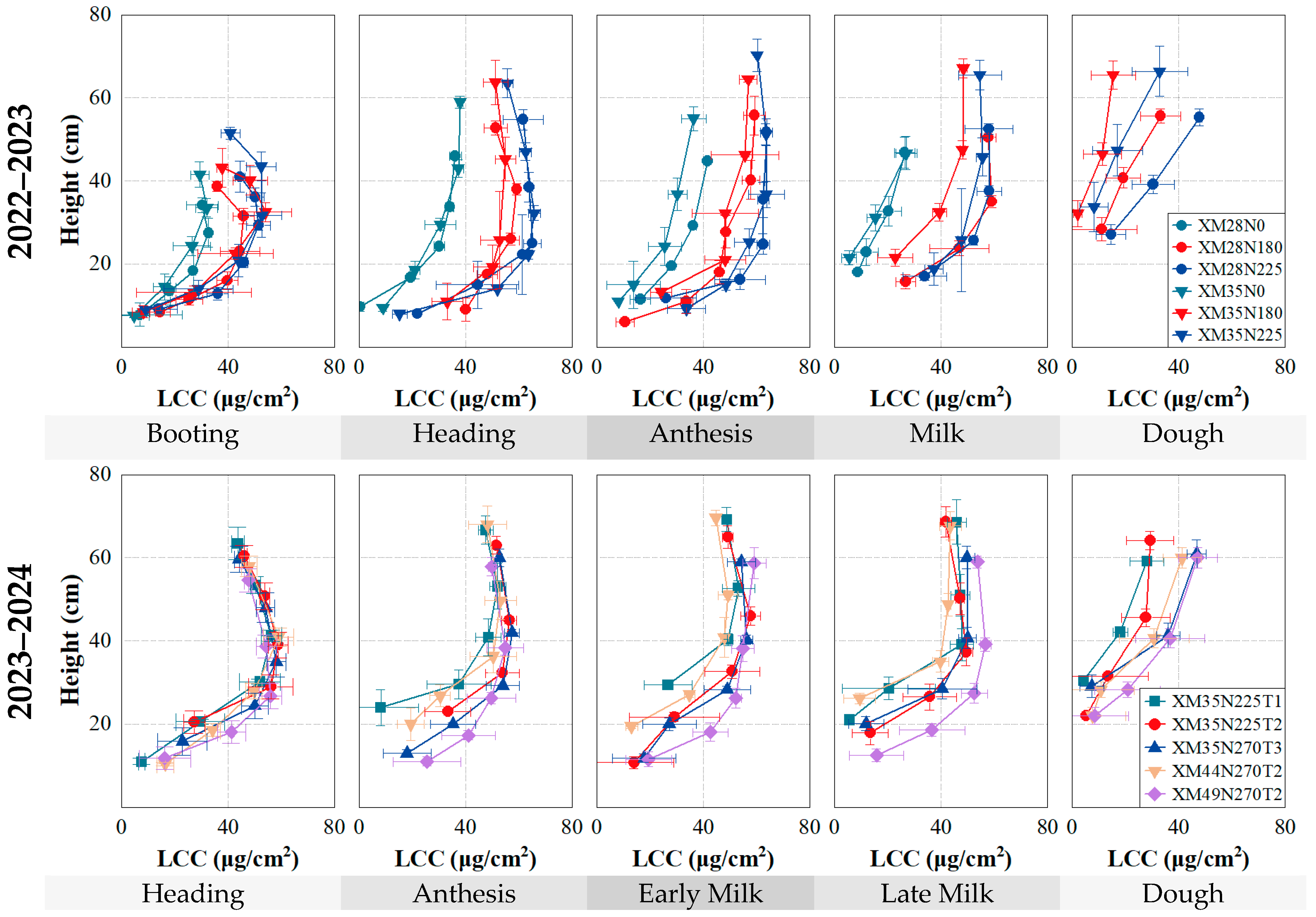
3.1. The Vertical and Temporal Variations of LCC within Winter Wheat Canopies
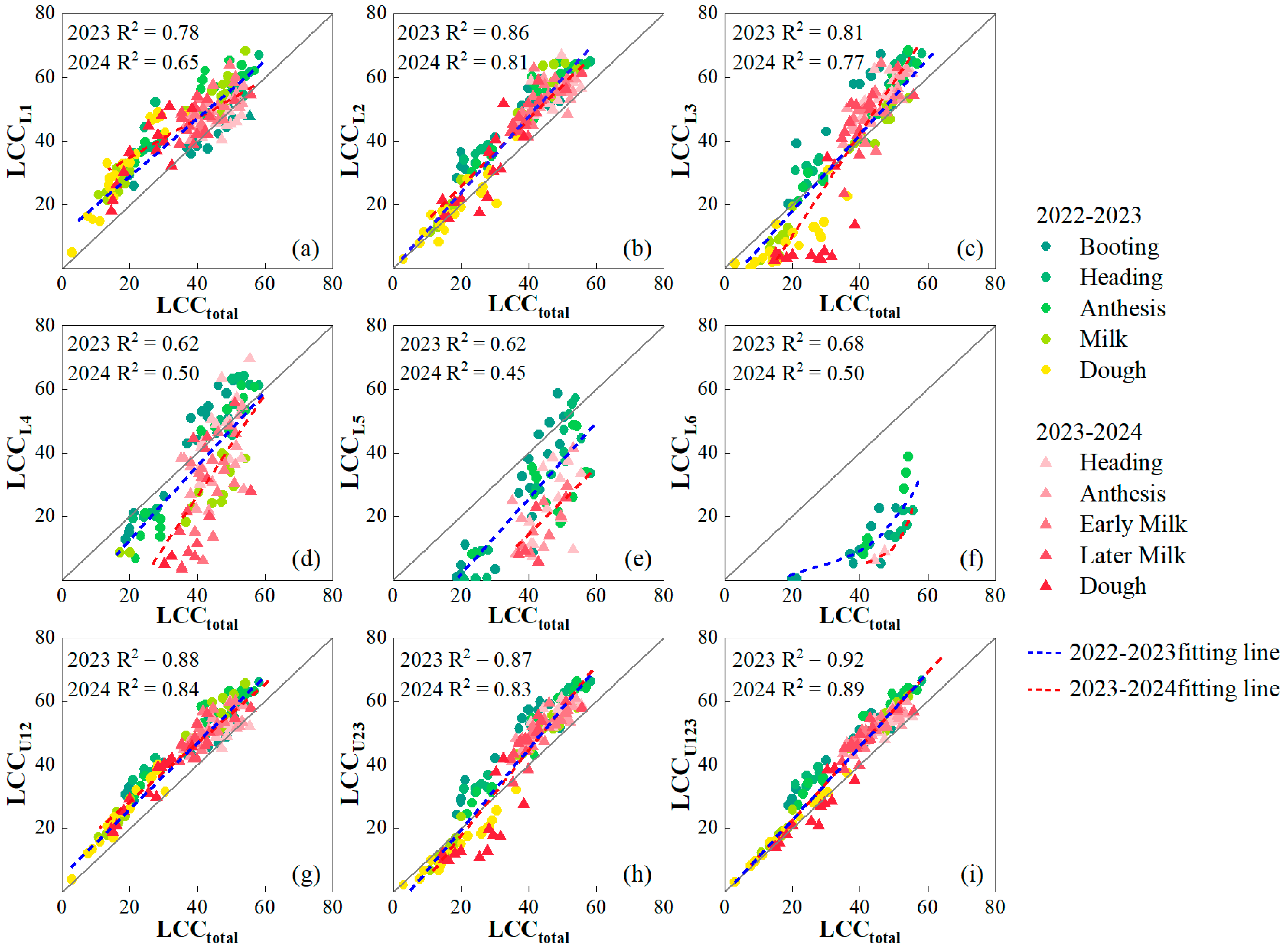
3.2. The Relationship of LCCtotal with LCCLi,Ui
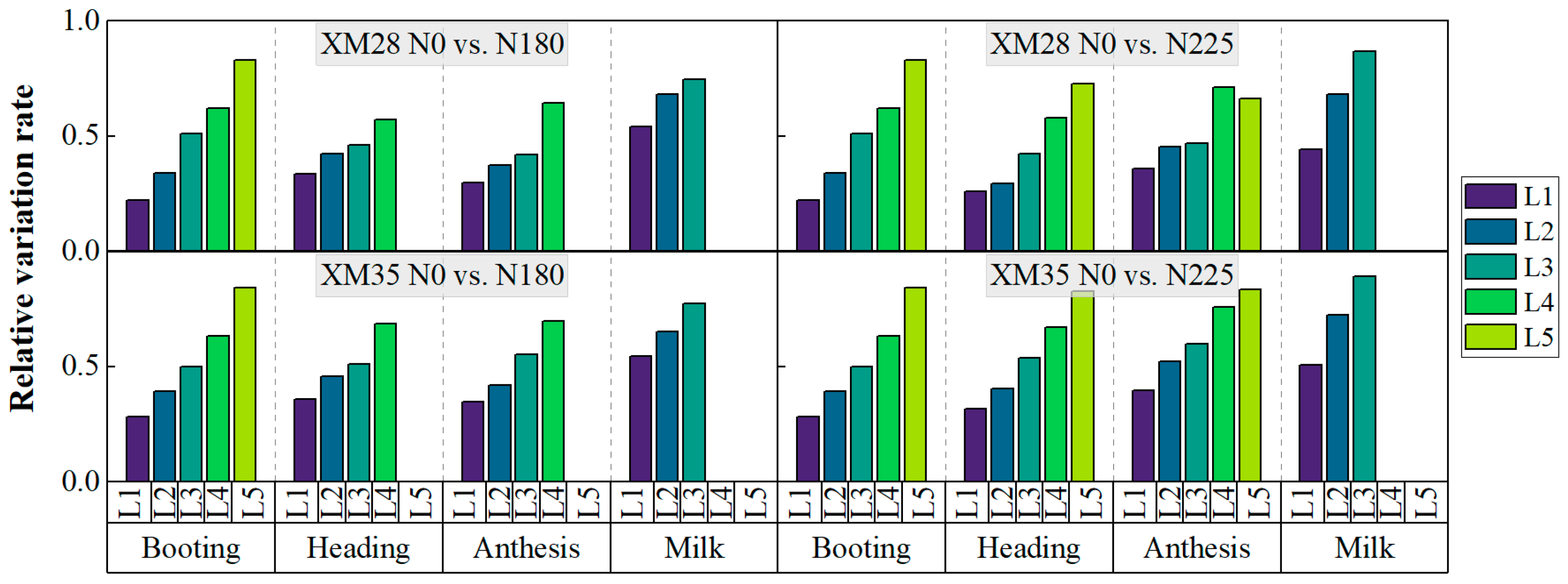
3.3. Effects of Different Nitrogen Fertilization Treatments on the LCCLi
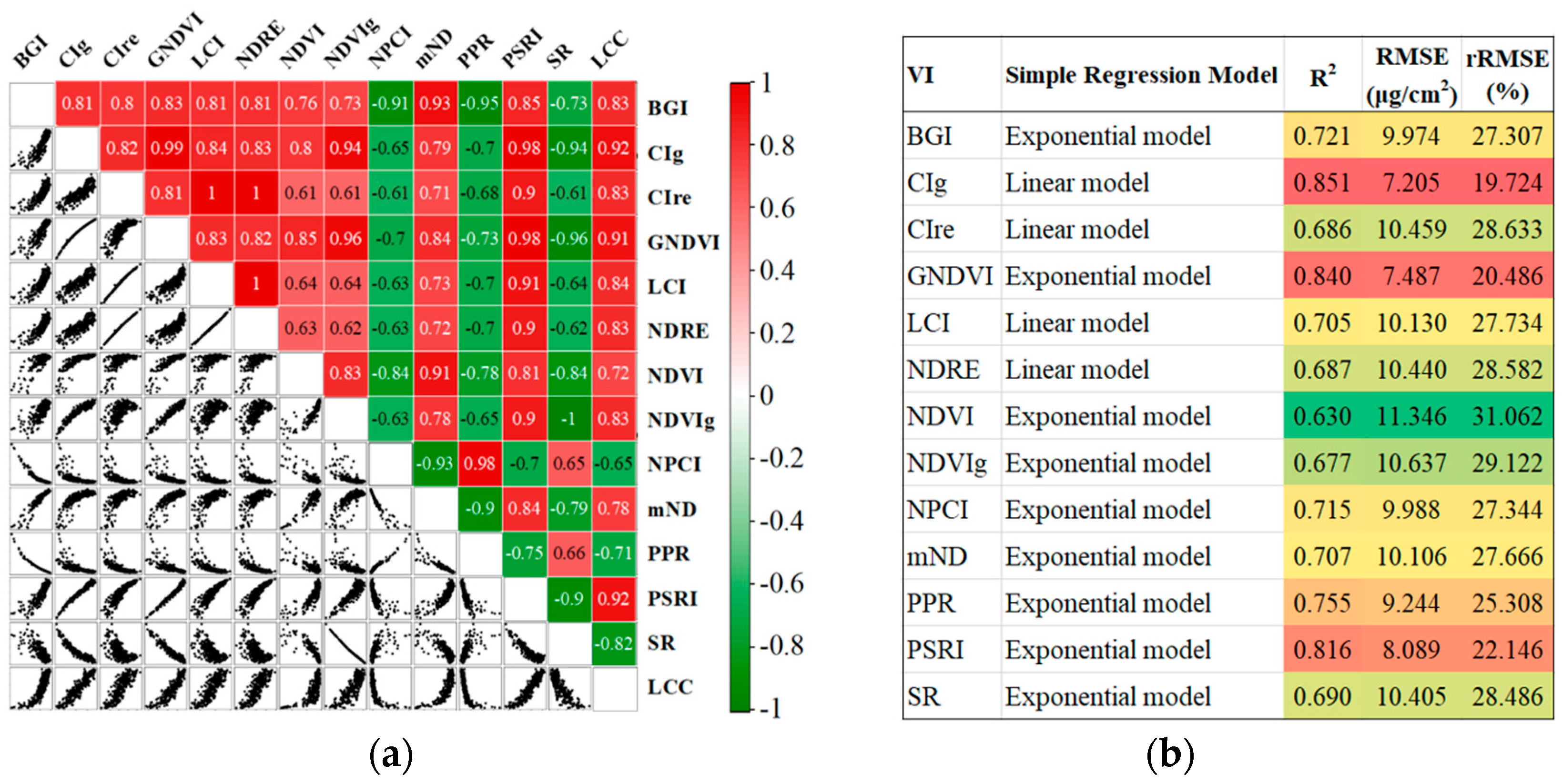
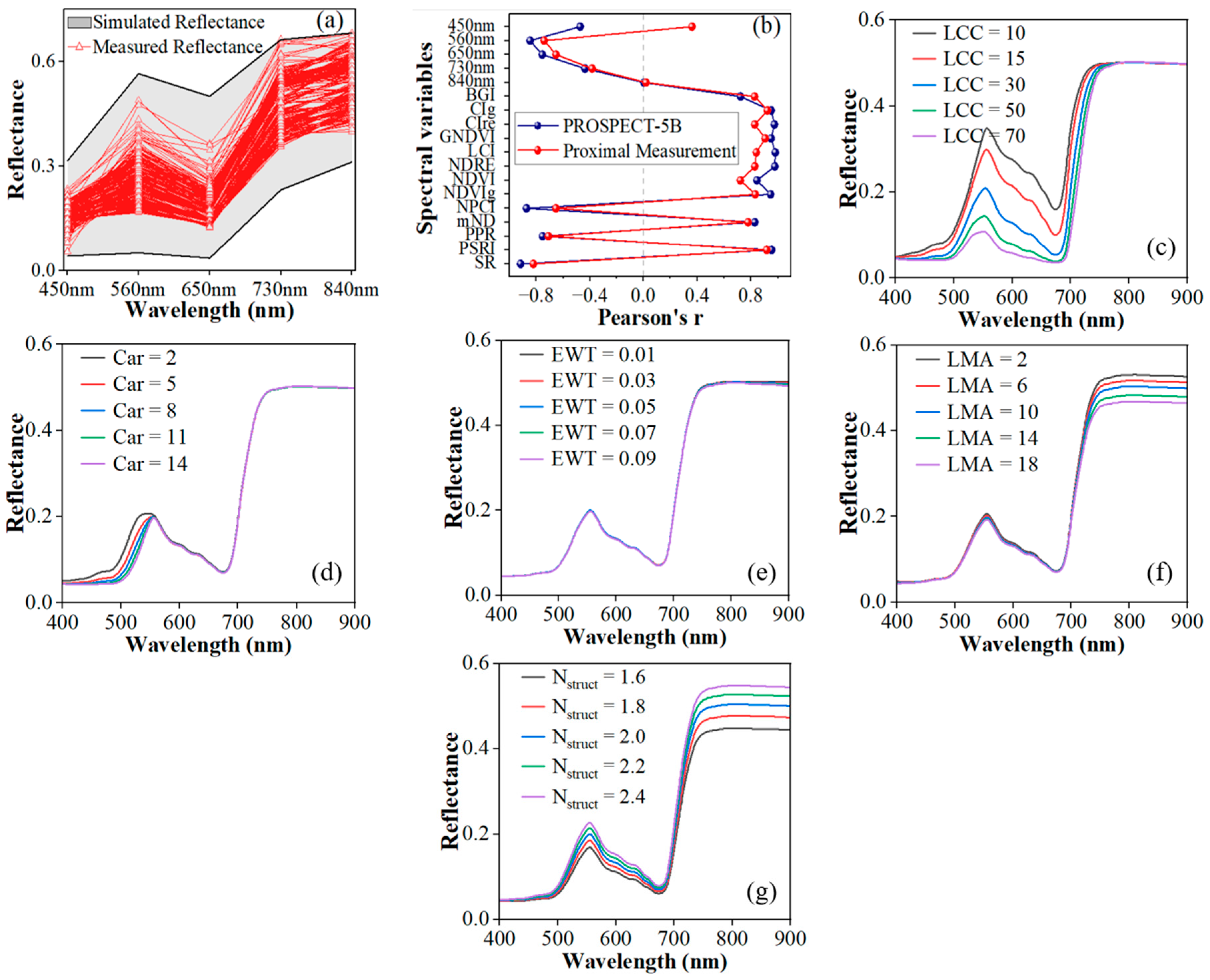
3.4. Characteristics and Sensitivity of Wheat Leaf Spectrum
3.5. Retrieved LCC with LeafTNet and Transfer Learning
3.6. Visual Mapping of LCC Trait
4. Discussion
4.1. Vertical Distribution of LCC within the Winter Wheat Canopies
4.2. Influencing Factors on Winter Wheat LCCLi Estimation Using Proximal Imaging Data
4.3. Feasibility of Transfer Learned LeafTNet
4.3.1. Sensitivity of LCC Inversion to Nstruct Vatiation
4.3.2. Annual Transferability of the Deep-Learning-Based LeafTNet
4.4. Limitations and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Battude, M.; Bitar, A.A.; Morin, D.; Cros, J.; Huc, M.; Sicre, C.M.; Dantec, V.L.; Demarez, V. Estimating Maize Biomass and Yield over Large Areas Using High Spatial and Temporal Resolution Sentinel-2 like Remote Sensing Data. Remote Sens. Environ. 2016, 184, 668–681. [Google Scholar] [CrossRef]
- Darvishzadeh, R.; Skidmore, A.; Schlerf, M.; Atzberger, C. Inversion of a Radiative Transfer Model for Estimating Vegetation LAI and Chlorophyll in a Heterogeneous Grassland. Remote Sens. Environ. 2008, 112, 2592–2604. [Google Scholar] [CrossRef]
- Yu, K.; Lenz-Wiedemann, V.; Chen, X.; Bareth, G. Estimating Leaf Chlorophyll of Barley at Different Growth Stages Using Spectral Indices to Reduce Soil Background and Canopy Structure Effects. ISPRS-J. Photogramm. Remote Sens. 2014, 97, 58–77. [Google Scholar] [CrossRef]
- Croft, H. The Global Distribution of Leaf Chlorophyll Content. Remote Sens. Environ. 2020, 236, 111479. [Google Scholar] [CrossRef]
- Xiao, Q.; Tang, W.; Zhang, C.; Zhou, L.; Feng, L.; Shen, J.; Yan, T.; Gao, P.; He, Y.; Wu, N. Spectral Preprocessing Combined with Deep Transfer Learning to Evaluate Chlorophyll Content in Cotton Leaves. Plant Phenomics 2022, 2022, 9813841. [Google Scholar] [CrossRef]
- Jay, S. Exploiting the Centimeter Resolution of UAV Multispectral Imagery to Improve Remote-Sensing Estimates of Canopy Structure and Biochemistry in Sugar Beet Crops. Remote Sens. Environ. 2018, 231, 110898. [Google Scholar] [CrossRef]
- Sanaeifar, A.; Yang, C.; De La Guardia, M.; Zhang, W.; Li, X.; He, Y. Proximal Hyperspectral Sensing of Abiotic Stresses in Plants. Sci. Total Environ. 2023, 861, 160652. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Huang, W.; Yang, G. Non-Uniform Vertical Nitrogen Distribution within Plant Canopy and Its Estimation by Remote Sensing: A Review. Field Crops Res. 2013, 142, 75–84. [Google Scholar] [CrossRef]
- He, J.; Zhang, X.; Guo, W.; Pan, Y.; Yao, X.; Cheng, T.; Zhu, Y.; Cao, W.; Tian, Y. Estimation of Vertical Leaf Nitrogen Distribution within a Rice Canopy Based on Hyperspectral Data. Front. Plant Sci. 2020, 10, 1802. [Google Scholar] [CrossRef]
- Hikosaka, K. Optimal Nitrogen Distribution within a Leaf Canopy under Direct and Diffuse Light. Plant Cell Environ. 2014, 37, 2077–2085. [Google Scholar] [CrossRef]
- Ye, H.; Huang, W.; Huang, S.; Wu, B.; Dong, Y.; Cui, B. Remote Estimation of Nitrogen Vertical Distribution by Consideration of Maize Geometry Characteristics. Remote Sens. 2018, 10, 1995. [Google Scholar] [CrossRef]
- Kellomäki, S.; Wang, K.-Y. Effects of Long-Term CO, and Temperature Elevation on Crown Nitrogen Distribution and Daily Photosynthetic Performance of Scats Pine. For. Ecol. Manag. 1997, 99, 309–326. [Google Scholar] [CrossRef]
- Kattge, J.; Knorr, W. Temperature Acclimation in a Biochemical Model of Photosynthesis: A Reanalysis of Data from 36 Species. Plant Cell Environ. 2007, 9, 1176–1190. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Huang, W.; Zhou, X.; Ye, H.; Dong, Y.; Casa, R. Off-Nadir Hyperspectral Sensing for Estimation of Vertical Profile of Leaf Chlorophyll Content within Wheat Canopies. Sensors 2017, 17, 2711. [Google Scholar] [CrossRef]
- Wu, B.; Huang, W.; Ye, H.; Luo, P.; Ren, Y.; Kong, W. Using Multi-Angular Hyperspectral Data to Estimate the Vertical Distribution of Leaf Chlorophyll Content in Wheat. Remote Sens. 2021, 13, 1501. [Google Scholar] [CrossRef]
- Duan, D.; Zhao, C.; Li, Z.; Yang, G.; Yang, W. Estimating Total Leaf Nitrogen Concentration in Winter Wheat by Canopy Hyperspectral Data and Nitrogen Vertical Distribution. J. Integr. Agric. 2019, 18, 1562–1570. [Google Scholar] [CrossRef]
- Wang, C. Remotely Assessing FIPAR of Different Vertical Layers in Field Wheat. Field Crops Res. 2023, 297, 108932. [Google Scholar] [CrossRef]
- Li, H.; Zhao, C.; Yang, G.; Feng, H. Variations in Crop Variables within Wheat Canopies and Responses of Canopy Spectral Characteristics and Derived Vegetation Indices to Different Vertical Leaf Layers and Spikes. Remote Sens. Environ. 2015, 169, 358–374. [Google Scholar] [CrossRef]
- Zhang, C.; Xue, Y. Estimation of Biochemical Pigment Content in Poplar Leaves Using Proximal Multispectral Imaging and Regression Modeling Combined with Feature Selection. Sensors 2024, 24, 217. [Google Scholar] [CrossRef]
- Gara, T.; Darvishzadeh, R.; Skidmore, A.; Wang, T. Impact of Vertical Canopy Position on Leaf Spectral Properties and Traits across Multiple Species. Remote Sens. 2018, 10, 346. [Google Scholar] [CrossRef]
- Verrelst, J.; Malenovský, Z.; Van der Tol, C.; Camps-Valls, G.; Gastellu-Etchegorry, J.-P.; Lewis, P.; North, P.; Moreno, J. Quantifying Vegetation Biophysical Variables from Imaging Spectroscopy Data: A Review on Retrieval Methods. Surv. Geophys. 2019, 40, 589–629. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Song, X.; Yang, G.; Du, X.; Mei, X.; Yang, X. Remote Sensing Monitoring of Rice and Wheat Canopy Nitrogen: A Review. Remote Sens. 2022, 14, 5712. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.; Wang, L.; Zhang, X.; Chen, S.; Su, Z.; Zhang, S.; Xue, Y. Estimation of the Bio-Parameters of Winter Wheat by Combining Feature Selection with Machine Learning Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Images. Remote Sens. 2024, 16, 469. [Google Scholar] [CrossRef]
- Huang, W.; Yang, Q.; Pu, R.; Yang, S. Estimation of Nitrogen Vertical Distribution by Bi-Directional Canopy Reflectance in Winter Wheat. Sensors 2014, 14, 20347–20359. [Google Scholar] [CrossRef]
- Luo, J.; Ma, R.; Feng, H.; Li, X. Estimating the Total Nitrogen Concentration of Reed Canopy with Hyperspectral Measurements Considering a Non-Uniform Vertical Nitrogen Distribution. Remote Sens. 2016, 8, 789. [Google Scholar] [CrossRef]
- Dreccer, M.F. Dynamics of Vertical Leaf Nitrogen Distribution in a Vegetative Wheat Canopy. Impact on Canopy Photosynthesis. Ann. Bot. 2000, 86, 821–831. [Google Scholar] [CrossRef]
- Sun, Q. Monitoring Maize Canopy Chlorophyll Density under Lodging Stress Based on UAV Hyperspectral Imagery. Comput. Electron. Agric. 2022, 193, 106671. [Google Scholar] [CrossRef]
- Feng, Z.; Guan, H.; Yang, T.; He, L.; Duan, J.; Song, L.; Wang, C.; Feng, W. Estimating the Canopy Chlorophyll Content of Winter Wheat under Nitrogen Deficiency and Powdery Mildew Stress Using Machine Learning. Comput. Electron. Agric. 2023, 211, 107989. [Google Scholar] [CrossRef]
- Geladi, P.; MacDougall, D.; Martens, H. Linearization and Scatter-Correction for Near-Infrared Reflectance Spectra of Meat. Appl. Spectrosc. 1985, 39, 491–500. [Google Scholar] [CrossRef]
- Bian, X.; Wang, K.; Tan, E.; Diwu, P.; Zhang, F.; Guo, Y. A Selective Ensemble Preprocessing Strategy for Near-Infrared Spectral Quantitative Analysis of Complex Samples. Chemom. Intell. Lab. Syst. 2020, 197, 103916. [Google Scholar] [CrossRef]
- Main, R.; Cho, M.A.; Mathieu, R.; O’Kennedy, M.M.; Ramoelo, A.; Koch, S. An Investigation into Robust Spectral Indices for Leaf Chlorophyll Estimation. ISPRS-J. Photogramm. Remote Sens. 2011, 66, 751–761. [Google Scholar] [CrossRef]
- Index Data Base (IDB). Available online: https://www.indexdatabase.de/ (accessed on 15 August 2024).
- Zarco-Tejada, P.J.; Miller, J.R.; Noland, T.L.; Mohammed, G.H.; Sampson, P.H. Scaling-up and Model Inversion Methods with Narrowband Optical Indices for Chlorophyll Content Estimation in Closed Forest Canopies with Hyperspectral Data. IEEE Trans. Geosci. Remote Sens. 2001, 39, 1491–1507. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Remote Estimation of Leaf Area Index and Green Leaf Biomass in Maize Canopies. Geophys. Res. Lett. 2003, 30, 1248. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS-MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Datt, B. Remote Sensing of Water Content in Eucalyptus Leaves. Aust. J. Bot. 1999, 47, 909. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E. Coincident Detection of Crop Water Stress, Nitrogen Status, and Canopy Density Using Ground Based Multispectral Data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Tucker, C.J.; Elgin, J.H.; McMurtrey, J.E.; Fan, C.J. Monitoring Corn and Soybean Crop Development with Hand-Held Radiometer Spectral Data. Remote Sens. Environ. 1979, 8, 237–248. [Google Scholar] [CrossRef]
- Metternicht, G. Vegetation Indices Derived from High-Resolution Airborne Videography for Precision Crop Management. Int. J. Remote Sens. 2003, 24, 2855–2877. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Fredeen, A.L.; Merino, J.; Field, C.B. Reflectance Indices Associated with Physiological Changes in Nitrogen- and Water-Limited Sunflower Leaves. Remote Sens. Environ. 1994, 48, 135–146. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Gitelson, A.A.; Chivkunova, O.B.; Rakitin, V.Y. Non-destructive Optical Detection of Pigment Changes during Leaf Senescence and Fruit Ripening. Physiol. Plant. 1999, 106, 135–141. [Google Scholar] [CrossRef]
- Shibayama, M.; Salli, A.; Heino, S.; Alanen, M.; Morinaga, S.; Inoue, Y.; Akiyama, T. Detecting Phenophases of Subarctic Shrub Canopies by Using Automated Reflectance Measurements. Remote Sens. Environ. 1999, 67, 160–180. [Google Scholar] [CrossRef]
- Jacquemoud, S.; Baret, F. PROSPECT: A Model of Leaf Optical Properties Spectra. Remote Sens. Environ. 1990, 34, 75–91. [Google Scholar] [CrossRef]
- Wan, L.; Liu, Y.; He, Y.; Cen, H. Prior Knowledge and Active Learning Enable Hybrid Method for Estimating Leaf Chlorophyll Content from Multi-Scale Canopy Reflectance. Comput. Electron. Agric. 2023, 214, 108308. [Google Scholar] [CrossRef]
- Liang, L.; Di, L.; Zhang, L.; Deng, M.; Qin, Z.; Zhao, S.; Lin, H. Estimation of Crop LAI Using Hyperspectral Vegetation Indices and a Hybrid Inversion Method. Remote Sens. Environ. 2015, 165, 123–134. [Google Scholar] [CrossRef]
- Zhao, C.; Li, H.; Li, P.; Yang, G.; Gu, X.; Lan, Y. Effect of Vertical Distribution of Crop Structure and Biochemical Parameters of Winter Wheat on Canopy Reflectance Characteristics and Spectral Indices. IEEE Trans. Geosci. Remote Sens. 2017, 55, 236–247. [Google Scholar] [CrossRef]
- Pan, S.J.; Yang, Q. A Survey on Transfer Learning. IEEE Trans. Knowl. Data Eng. 2010, 22, 1345–1359. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.J.; Krupnik, T.; Six, J.; Van Kessel, C. Efficiency of Fertilizer Nitrogen in Cereal Production: Retrospects and Prospects. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2005; Volume 87, pp. 85–156. [Google Scholar]
- Tomás, M.; Flexas, J.; Copolovici, L.; Galmés, J.; Hallik, L.; Medrano, H.; Ribas-Carbó, M.; Tosens, T.; Vislap, V.; Niinemets, Ü. Importance of Leaf Anatomy in Determining Mesophyll Diffusion Conductance to CO2 across Species: Quantitative Limitations and Scaling up by Models. J. Exp. Bot. 2013, 64, 2269–2281. [Google Scholar] [CrossRef] [PubMed]
- Ouk, R.; Oi, T.; Sugiura, D.; Taniguchi, M. Structural Changes of Mesophyll Cells in the Rice Leaf Tissue in Response to Salinity Stress Based on the Three-Dimensional Analysis. AoB Plants 2024, 16, plae016. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Jákli, B.; Lu, P.; Ren, T.; Ming, J.; Liu, S.; Wang, S.; Lu, J. Assessing Leaf Nitrogen Concentration of Winter Oilseed Rape with Canopy Hyperspectral Technique Considering a Non-Uniform Vertical Nitrogen Distribution. Ind. Crops Prod. 2018, 116, 1–14. [Google Scholar] [CrossRef]
- Shen, X.; Cao, L.; Coops, N.C.; Fan, H.; Wu, X.; Liu, H.; Wang, G.; Cao, F. Quantifying Vertical Profiles of Biochemical Traits for Forest Plantation Species Using Advanced Remote Sensing Approaches. Remote Sens. Environ. 2020, 250, 112041. [Google Scholar] [CrossRef]
- Yang, H.; Ming, B.; Nie, C.; Xue, B.; Xin, J.; Lu, X.; Xue, J.; Hou, P.; Xie, R.; Wang, K.; et al. Maize Canopy and Leaf Chlorophyll Content Assessment from Leaf Spectral Reflectance: Estimation and Uncertainty Analysis across Growth Stages and Vertical Distribution. Remote Sens. 2022, 14, 2115. [Google Scholar] [CrossRef]
- Hikosaka, K. Leaf Canopy as a Dynamic System: Ecophysiology and Optimality in Leaf Turnover. Ann. Bot. 2004, 95, 521–533. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Zhao, C.; Zhao, M.; Huang, W.; Wang, C. Vertical Distribution of Nitrogen in Different Layers of Leaf and Stem and Their Relationship with Grain Quality of Winter Wheat. J. Plant Nutr. 2005, 28, 73–91. [Google Scholar] [CrossRef]
- Chen, J.L.; Reynolds, J.F.; Harley, P.C.; Tenhunen, J.D. Coordination Theory of Leaf Nitrogen Distribution in a Canopy. Oecologia 1993, 93, 63–69. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, Y.; Jiang, H.; Cao, W. Positional Differences in Nitrogen and Sugar Concentrations of Upper Leaves Relate to Plant N Status in Rice under Different N Rates. Field Crops Res. 2006, 96, 224–234. [Google Scholar] [CrossRef]
- Berger, K.; Verrelst, J.; Féret, J.-B.; Hank, T.; Wocher, M.; Mauser, W.; Camps-Valls, G. Retrieval of Aboveground Crop Nitrogen Content with a Hybrid Machine Learning Method. Int. J. Appl. Earth Obs. Geoinf. 2020, 92, 102174. [Google Scholar] [CrossRef]
- Kayad, A.; Rodrigues, F.A., Jr.; Naranjo, S.; Sozzi, M.; Pirotti, F.; Marinello, F.; Schulthess, U.; Defourny, P.; Gerard, B.; Weiss, M. Radiative Transfer Model Inversion Using High-Resolution Hyperspectral Airborne Imagery—Retrieving Maize LAI to Access Biomass and Grain Yield. Field Crops Res. 2022, 282, 108449. [Google Scholar] [CrossRef]
- Vilfan, N.; Van Der Tol, C.; Yang, P.; Wyber, R.; Malenovský, Z.; Robinson, S.A.; Verhoef, W. Extending Fluspect to Simulate Xanthophyll Driven Leaf Reflectance Dynamics. Remote Sens. Environ. 2018, 211, 345–356. [Google Scholar] [CrossRef]
- Féret, J.-B.; Berger, K.; De Boissieu, F.; Malenovský, Z. PROSPECT-PRO for Estimating Content of Nitrogen-Containing Leaf Proteins and Other Carbon-Based Constituents. Remote Sens. Environ. 2021, 252, 112173. [Google Scholar] [CrossRef]
- Jurado, J.M. Remote Sensing Image Fusion on 3D Scenarios: A Review of Applications for Agriculture and Forestry. Int. J. Appl. Earth Obs. Geoinf. 2022, 112, 102856. [Google Scholar] [CrossRef]
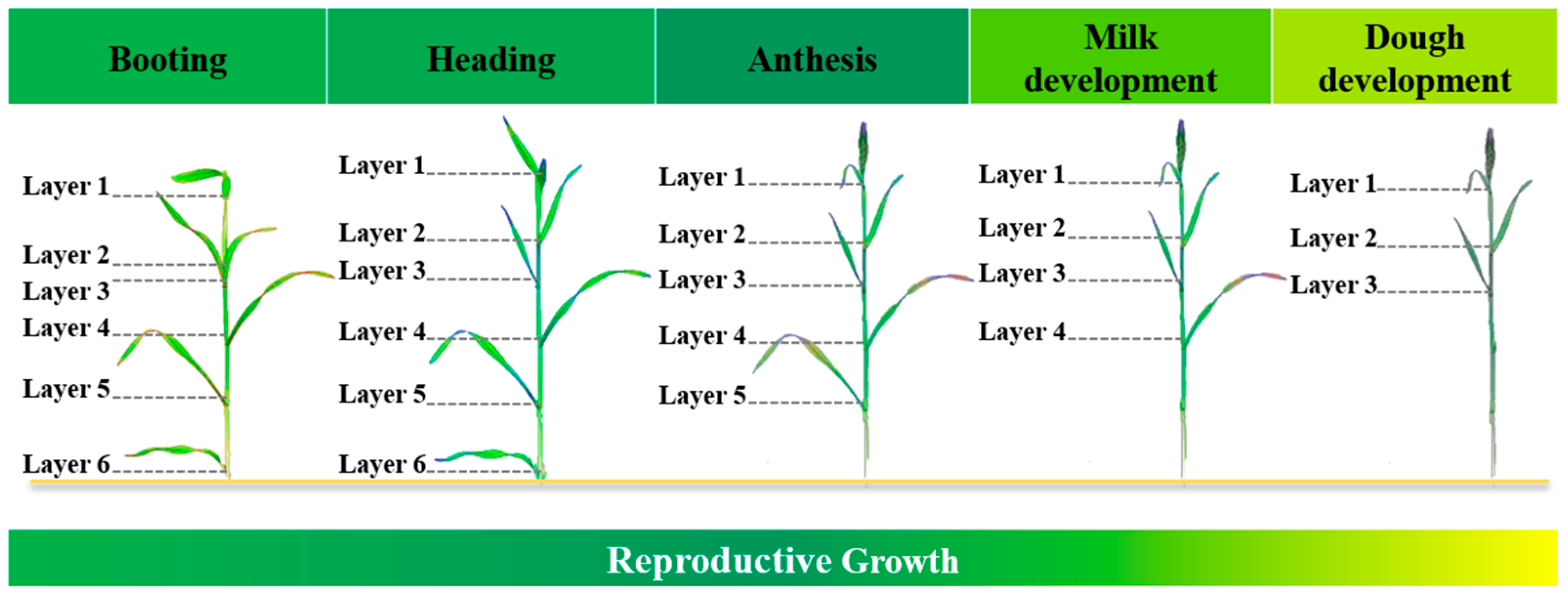

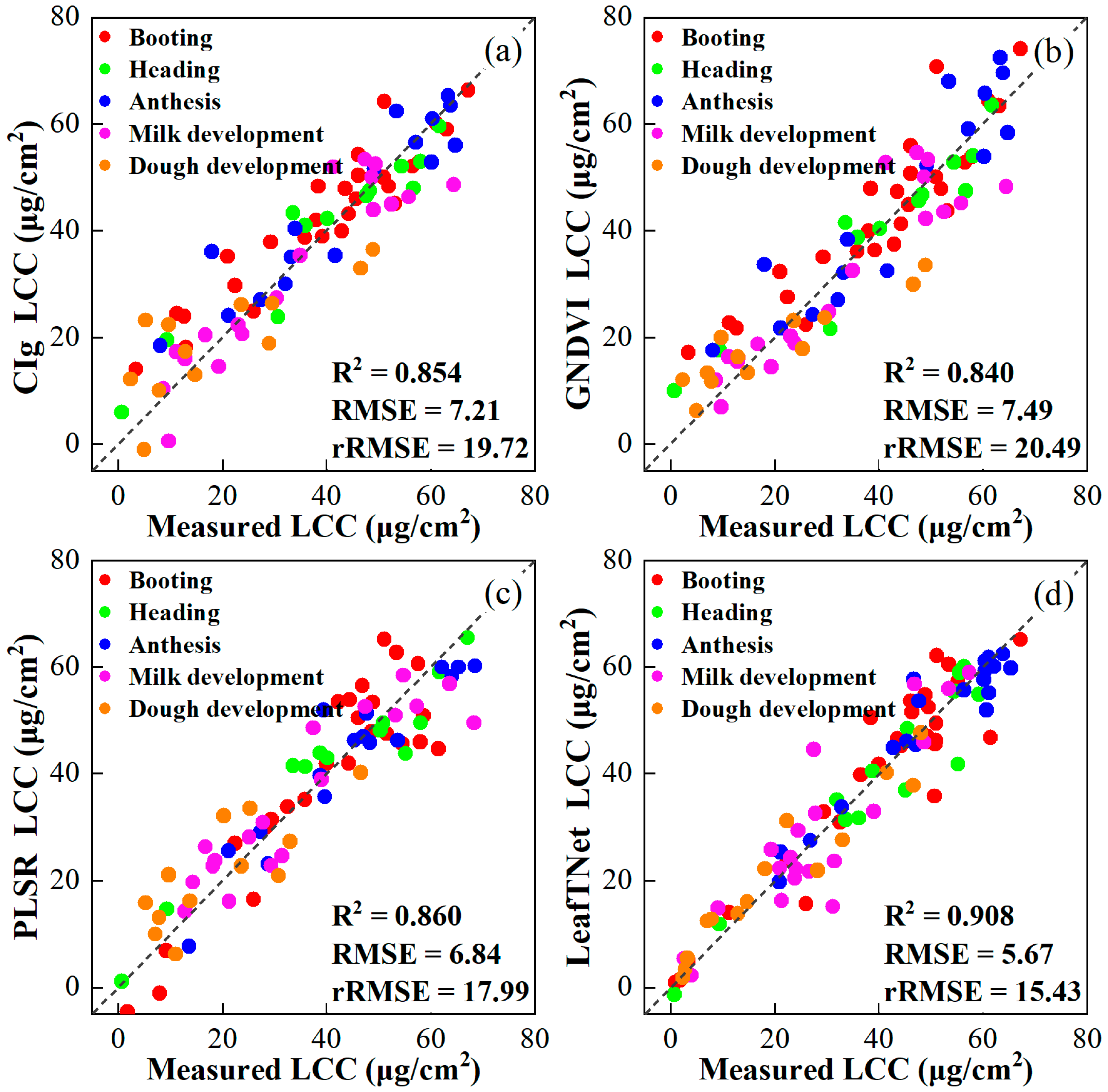

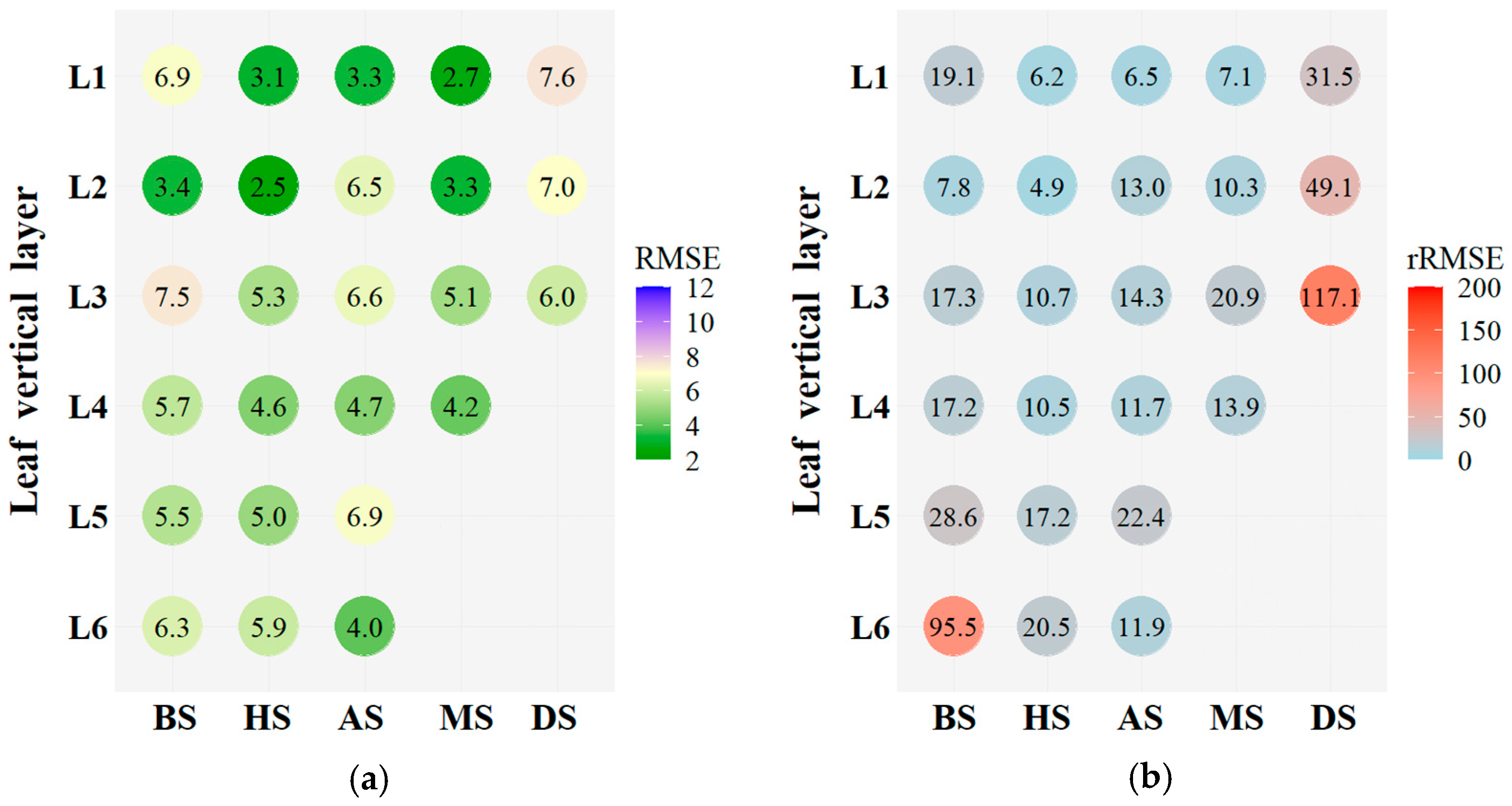
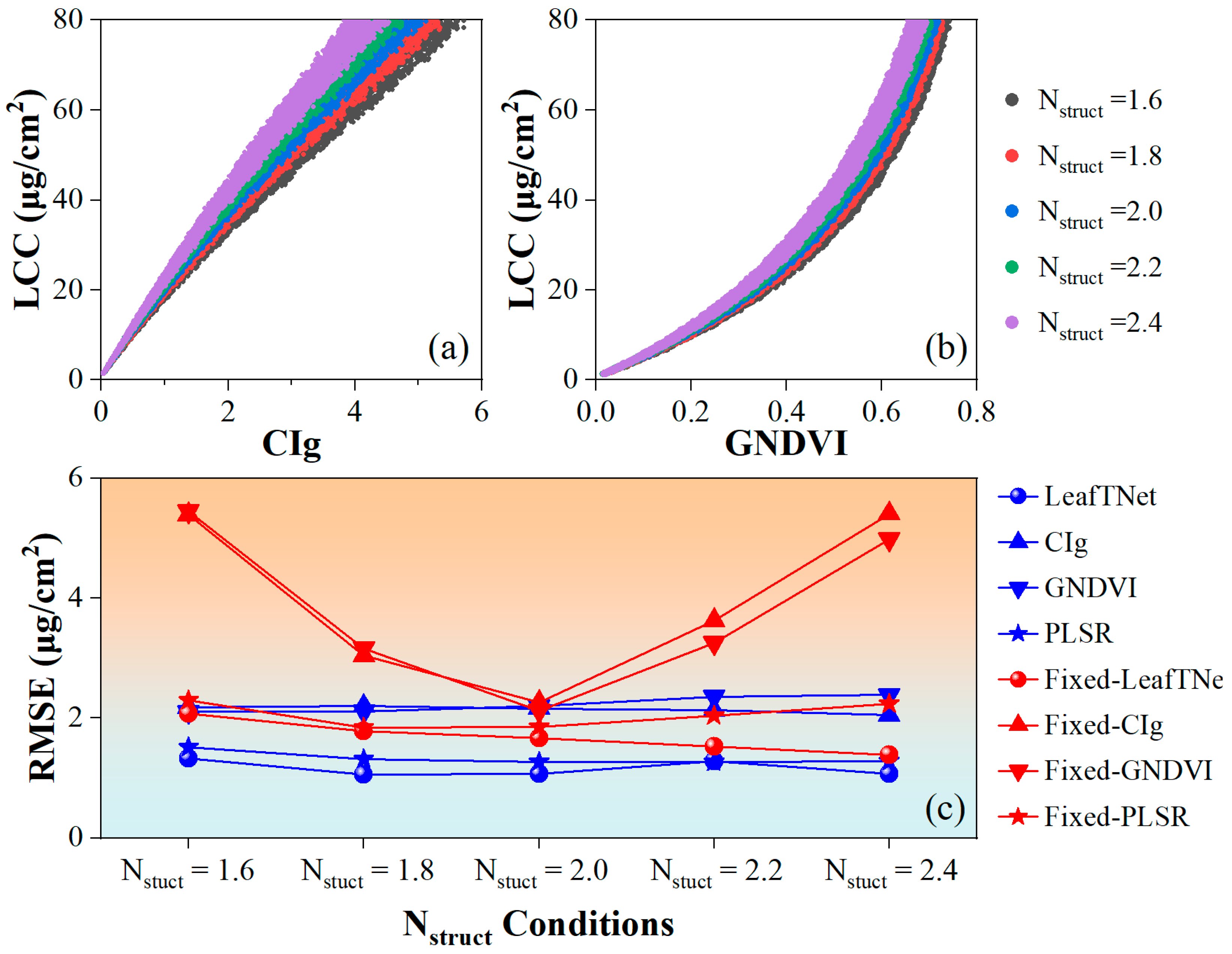
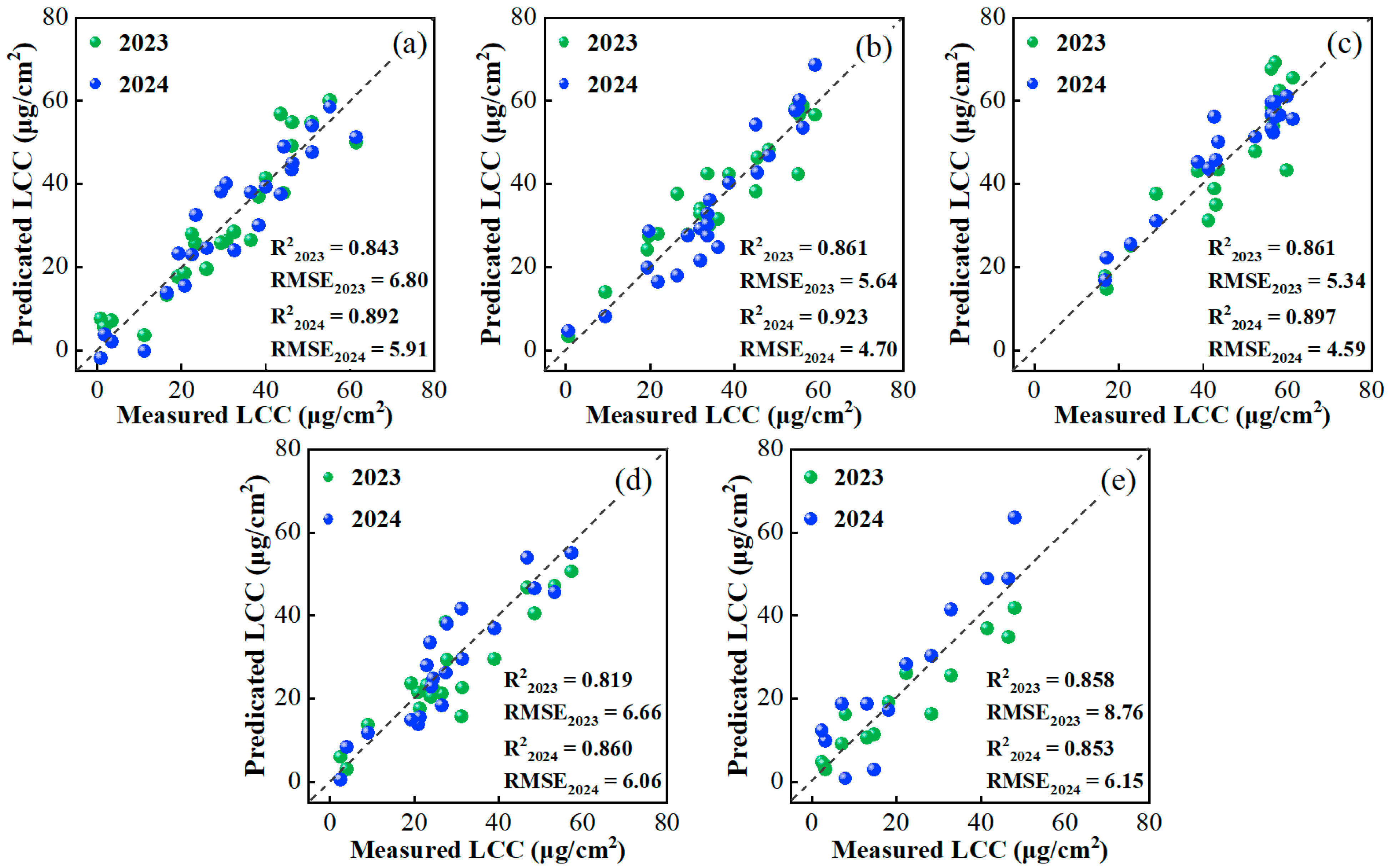
| Year | Variety | N Rate (kg/ha) | Date | Growth Stage Description |
|---|---|---|---|---|
| 2022–2023 | XM28, XM35 (Medium Gluten Wheat) | 0 | 9 April | Booting Stage |
| 180 | 19 April | Heading Stage | ||
| 225 | 27 April | Anthesis Stage | ||
| 13 May | Milk Stage | |||
| 20 May | Dough Stage | |||
| 2023–2024 | XM35 (Medium Gluten Wheat) XM44, XM49 (Strong Gluten Wheat) | 225 | 12 April | Heading Stage |
| 270 | 24 April | Anthesis Stage | ||
| 2 May | Early Milk Stage | |||
| 10 May | Late Milk Stage | |||
| 20 May | Dough Stage |
| Index (Abbreviation) | Formula | References |
|---|---|---|
| Blue Green Pigment Index (BGI) | Blue/Green | [33] |
| Chlorophyll Index using Green Reflectance (CIg) | (NIR/Green) − 1 | [34] |
| Chlorophyll Index using Red Edge Reflectance (CIre) | (NIR/RE) − 1 | [34] |
| Green Normalized Difference Vegetation Index (GNDVI) | (NIR − Green)/(NIR + Green) | [35] |
| Leaf chlorophyll index (LCI) | (NIR − RE)/(NIR + Red) | [36] |
| Normalized Difference Red Edge Index (NDRE) | (NIR − RE)/(NIR + RE) | [37] |
| Normalized Difference Vegetation Index (NDVI) | (NIR − Red)/(NIR + Red) | [38] |
| Green NDVI (NDVIg) | (RE − Green)/(RE + Green) | [39] |
| Normalized Pigment Chlorophyll Index (NPCI) | (Red − Blue)/(Red + Blue) | [40]] |
| Modified Normalized Difference (mND) | (NIR − Red)/(NIR + Red − 2 × Blue) | [31] |
| Plant Pigment Ratio (PPR) | (Green − Blue)/(Green + Blue) | [39] |
| Plant Senescence Reflectance Index (PSRI) | (NIR − Green)/RE | [41] |
| Simple Ratio Index (SR) | Green/RE | [42] |
| Parameter | Symbol | Units | Range |
|---|---|---|---|
| Leaf mesophyll structure index | Nstruct | - | 1.5–2.5 |
| Leaf chlorophyll content | LCC | μg·cm−2 | 0–80 |
| Leaf carotenoids content | Car | μg·cm−2 | 0–15 |
| Equivalent water thickness | EWT | cm | 0.001–0.1 |
| Leaf mass per area | LMA | mg.cm−2 | 2–20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yi, Y.; Zhang, S.; Li, P. Quantitative Analysis of Vertical and Temporal Variations in the Chlorophyll Content of Winter Wheat Leaves via Proximal Multispectral Remote Sensing and Deep Transfer Learning. Agriculture 2024, 14, 1685. https://doi.org/10.3390/agriculture14101685
Zhang C, Yi Y, Zhang S, Li P. Quantitative Analysis of Vertical and Temporal Variations in the Chlorophyll Content of Winter Wheat Leaves via Proximal Multispectral Remote Sensing and Deep Transfer Learning. Agriculture. 2024; 14(10):1685. https://doi.org/10.3390/agriculture14101685
Chicago/Turabian StyleZhang, Changsai, Yuan Yi, Shuxia Zhang, and Pei Li. 2024. "Quantitative Analysis of Vertical and Temporal Variations in the Chlorophyll Content of Winter Wheat Leaves via Proximal Multispectral Remote Sensing and Deep Transfer Learning" Agriculture 14, no. 10: 1685. https://doi.org/10.3390/agriculture14101685
APA StyleZhang, C., Yi, Y., Zhang, S., & Li, P. (2024). Quantitative Analysis of Vertical and Temporal Variations in the Chlorophyll Content of Winter Wheat Leaves via Proximal Multispectral Remote Sensing and Deep Transfer Learning. Agriculture, 14(10), 1685. https://doi.org/10.3390/agriculture14101685






