Fermented Feed in Broiler Diets Reduces the Antinutritional Factors, Improves Productive Performances and Modulates Gut Microbiome—A Review
Abstract
:1. Introduction
2. Fermentation Used in Feed Processing
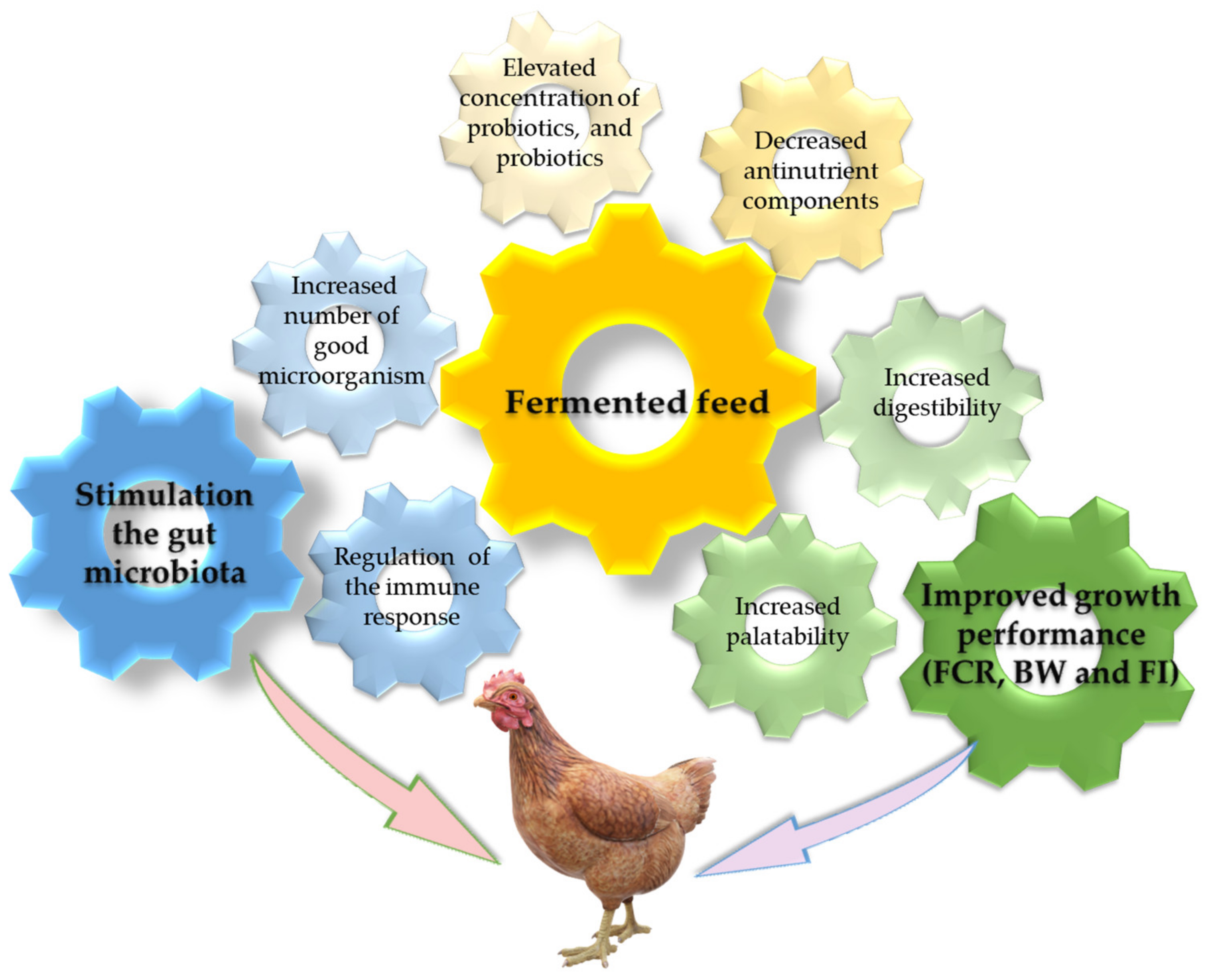
3. Effects of Fermented Feeds on Broilers Growth Performance and Gut Microbiome
3.1. Improved Growth Performance
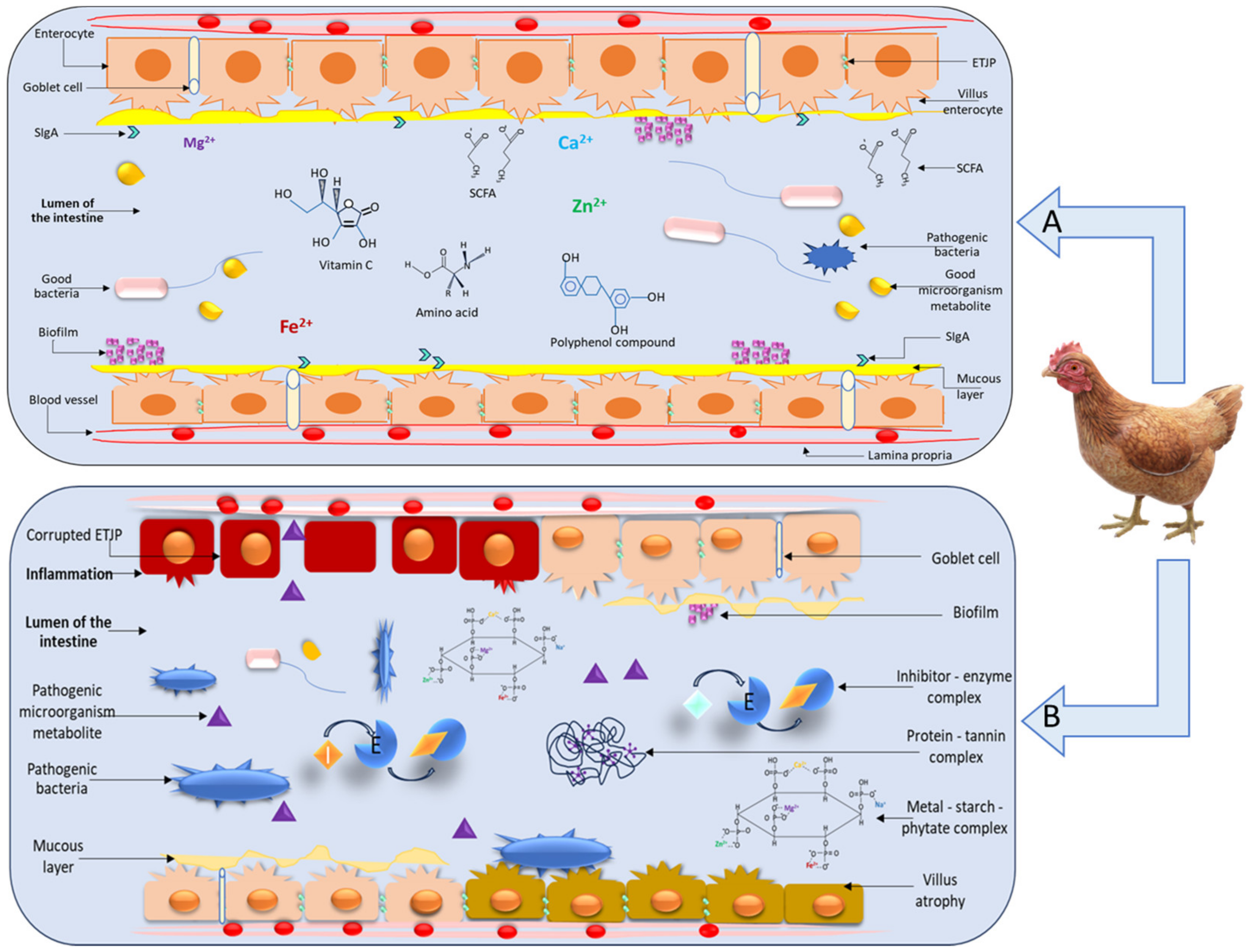
3.2. Gut Microbiota Modulation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Maharjan, P.; Martinez, D.A.; Weil, J.; Suesuttajit, N.; Umberson, C.; Mullenix, G.; Hilton, K.M.; Beitia, A.; Coon, C.N. Review: Physiological Growth Trend of Current Meat Broilers and Dietary Protein and Energy Management Approaches for Sustainable Broiler Production. Animal 2021, 15, 100284. [Google Scholar] [CrossRef]
- Zampiga, M.; Calini, F.; Sirri, F. Importance of feed efficiency for sustainable intensification of chicken meat production: Implications and role for amino acids, feed enzymes and organic trace minerals. J. World’s Poult. Sci. 2021, 77, 639–659. [Google Scholar] [CrossRef]
- Siegel, P.B. Evolution of the Modern Broiler and Feed Efficiency. Annu. Rev. Anim. Biosci. 2014, 2, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Desbruslais, A.; Wealleans, A.L. Oxidation in Poultry Feed: Impact on the Bird and the Efficacy of Dietary Antioxidant Mitigation Strategies. Poultry 2022, 1, 246–277. [Google Scholar] [CrossRef]
- Krysiak, K.; Konkol, D.; Korczyński, M. Overview of the Use of Probiotics in Poultry Production. Animals 2021, 11, 1620. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Kong, L.; Gao, H.; Cheng, X.; Wang, X. A Review of Current Bacterial Resistance to Antibiotics in Food Animals. Front. Microbiol. 2022, 13, 822689. [Google Scholar] [CrossRef]
- Selaledi, L.A.; Hassan, Z.M.; Manyelo, T.G.; Mabelebele, M. The Current Status of the Alternative Use to Antibiotics in Poultry Production: An African Perspective. Antibiotics 2020, 9, 594. [Google Scholar] [CrossRef]
- Panaite, T.D.; Saracila, M.; Papuc, C.P.; Predescu, C.N.; Soica, C. Influence of Dietary Supplementation of Salix alba Bark on Performance, Oxidative Stress Parameters in Liver and Gut Microflora of Broilers. Animals 2020, 10, 958. [Google Scholar] [CrossRef]
- Clavijo, V.; Morales, T.; Vives-Flores, M.J.; Reyes Muñoz, A. The gut microbiota of chickens in a commercial farm treated with a Salmonella phage cocktail. Sci. Rep. 2022, 12, 991. [Google Scholar] [CrossRef]
- Mangan, M.; Siwek, M. Strategies to combat heat stress in poultry production-A review. J. Anim. Physiol. Anim. Nutr. 2024, 108, 576–595. [Google Scholar] [CrossRef]
- Ivanova, S.; Sukhikh, S.; Popov, A.; Shishko, O.; Nikonov, I.; Kapitonova, E.; Krol, O.; Larina, V.; Noskova, S.; Babich, O. Medicinal Plants: A Source of Phytobiotics for the Feed Additives. J. Agric. Food Res. 2024, 16, 101172. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.E.; Shehata, A.M.; Alzahrani, S.O.; Shafi, M.E.; Mesalam, N.M.; Taha, A.E.; Swelum, A.A.; Arif, M.; Fayyaz, M.; El-Hack, M.E.A. The Role of Polyphenols in Poultry Nutrition. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V.; Stefan, G. Plant Polyphenols as Antioxidant and Antibacterial Agents for Shelf-Life Extension of Meat and Meat Products: Classification, Structures, Sources, and Action Mechanisms. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1243–1268. [Google Scholar] [CrossRef]
- Mamun, M.A.A.; Rakib, A.; Mandal, M.; Kumar, S.; Singla, B.; Singh, U.P. Polyphenols: Role in Modulating Immune Function and Obesity. Biomolecules 2024, 14, 221. [Google Scholar] [CrossRef] [PubMed]
- Masiala, A.; Vingadassalon, A.; Aurore, G. Polyphenols in Edible Plant Leaves: An Overview of Their Occurrence and Health Properties. Food Funct. 2024, 15, 6847–6882. [Google Scholar] [CrossRef]
- Bié, J.; Sepodes, B.; Fernandes, P.C.B.; Ribeiro, M.H.L. Polyphenols in Health and Disease: Gut Microbiota, Bioaccessibility, and Bioavailability. Compounds 2023, 3, 40–72. [Google Scholar] [CrossRef]
- Bešlo, D.; Golubić, N.; Rastija, V.; Agić, D.; Karnaš, M.; Šubarić, D.; Lučić, B. Antioxidant Activity, Metabolism, and Bioavailability of Polyphenols in the Diet of Animals. Antioxidants 2023, 12, 1141. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Q.; Jiang, J.; Khan, M.S. Tannin Complexation with Metal Ions and Its Implication on Human Health, Environment and Industry: An Overview. Int. J. Biol. Macromol. 2023, 253, 127485. [Google Scholar] [CrossRef]
- Wu, H.; Oliveira, G.; Lila, M.A. Protein-binding Approaches for Improving Bioaccessibility and Bioavailability of Anthocyanins. Compr. Rev. Food Sci. Food Saf. 2022, 22, 333–354. [Google Scholar] [CrossRef]
- Sugiharto, S.; Ranjitkar, S. Recent advances in fermented feeds towards improved broiler chicken performance, gastrointestinal tract microecology and immune responses: A review. Anim. Nutr. 2019, 5, 1–10. [Google Scholar] [CrossRef]
- Peng, W.; Talpur, M.Z.; Zeng, Y.; Xie, P.; Li, J.; Wang, S.; Wang, L.; Zhu, X.; Gao, P.; Jiang, Q.; et al. Influence of fermented feed additive on gut morphology, immune status, and microbiota in broilers. BMC Vet. Res. 2022, 18, 218. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Wu, H.; Xie, J.; Zeng, T.; Hao, L.; Xu, W.; Lu, L. The Effects of Fermented Feed on the Growth Performance, Antioxidant Activity, Immune Function, Intestinal Digestive Enzyme Activity, Morphology, and Microflora of Yellow-Feather Chickens. Animals 2023, 13, 3545. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Yang, Z.; Wu, G.; Xu, F.; Zhang, J.; Luo, X.; Ma, Y.; Pang, H.; Duan, Y.; Chen, J.; et al. Effects of Probiotic-Fermented Feed on the Growth Profile, Immune Functions, and Intestinal Microbiota of Bamei Piglets. Animals 2024, 14, 647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Tao, L.; Liu, H.; Yang, G. Effects of fermented feed on growth performance, immune organ indices, serum biochemical parameters, cecal odorous compound production, and the microbiota community in broilers. Poult. Sci. 2023, 102, 102629. [Google Scholar] [CrossRef]
- Leeuwendaal, N.K.; Stanton, C.; O’Toole, P.W.; Beresford, T.P. Fermented Foods, Health and the Gut Microbiome. Nutrients 2022, 14, 1527. [Google Scholar] [CrossRef]
- Sun, H.; Chen, D.; Cai, H.; Chang, W.; Wang, Z.; Liu, G.; Deng, X.; Chen, Z. Effects of Fermenting the Plant Fraction of a Complete Feed on the Growth Performance, Nutrient Utilization, Antioxidant Functions, Meat Quality, and Intestinal Microbiota of Broilers. Animals 2022, 12, 2870. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Xu, G.; Zhang, K.; Ahmad, S.; Wang, L.; Chen, F.; Liu, J.; Gu, X.; Li, J.; Zhang, J. Fermented Astragalus Powder, a New Potential Feed Additive for Broilers to Improve the Growth Performance and Health. Animals 2024, 14, 1628. [Google Scholar] [CrossRef]
- Dai, Z.; Cui, L.; Li, J.; Wang, B.; Guo, L.; Wu, Z.; Zhu, W.; Wu, G. Fermentation Techniques in Feed Production; Elsevier eBooks: Amsterdam, The Netherlands, 2020; pp. 407–429. [Google Scholar]
- Sokrab, A.M.; Mohamed Ahmed, I.A.; Babiker, E.E. Effect of fermentation on antinutrients, and total and extractable minerals of high and low phytate corn genotypes. J. Food Sci. Technol. 2014, 51, 2608–2615. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Salim, R.; Nehvi, I.B.; Mir, R.A.; Tyagi, A.; Ali, S.; Bhat, O.M. A review on anti-nutritional factors: Unraveling the natural gateways to human health. Front. Nutr. 2023, 10, 1215873. [Google Scholar] [CrossRef]
- Anderson, R.L.; Wolf, W.J. Compositional changes in trypsin inhibitors, phytic acid, saponins and isoflavones related to soybean processing. J. Nutr. 1995, 125, 581S–588S. [Google Scholar] [CrossRef]
- Adeyemo, S.M.; Onilude, A.A. Enzymatic Reduction of Anti-Nutritional Factors in Fermenting Soybeans by Lactobacillus plantarum Isolates from Fermenting Cereals. Niger. Food J. 2013, 31, 84–90. [Google Scholar] [CrossRef]
- Gogoi, P.; Sharma, P.; Mahajan, A.; Goudar, G.; Chandragiri, A.K.; Sreedhar, M.; Longvah, T. Exploring the nutritional potential, anti-nutritional components and carbohydrate fractions of Indian pigmented maize. Food Chem. Adv. 2023, 2, 100176. [Google Scholar] [CrossRef]
- Șonea, C.; Toader, M.; Năstase, I.P. The quality of maize grains in organic farming system. Rom Biotechnol. Lett. 2020, 25, 1781–1789. [Google Scholar] [CrossRef]
- Roger, T.; Léopold, T.N.; Funtong, M.C.M. Nutritional Properties and Antinutritional Factors of Corn Paste (Kutukutu) Fermented by Different Strains of Lactic Acid Bacteria. Int. J. Food Sci. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Emkani, M.; Oliete, B.; Saurel, R. Effect of Lactic Acid Fermentation on Legume Protein Properties, a Review. Fermentation 2022, 8, 244. [Google Scholar] [CrossRef]
- Etuk, E.B.; Ifeduba, A.V.; Okata, U.E.; Chiaka, I.; Charles, O.I.; Okeudo, N.J.; Esonu, B.O.; Udedibie, A.B.I.; Moreki, J.C. Nutrient Composition and Feeding Value of Sorghum for Livestock and Poultry: A Review. Anim. Sci. Adv. 2012, 2, 510–524. [Google Scholar]
- Aladeen, A.M.A.; Omer Elbashier, M. The Effect of Replacement of Sorghum with Millet on Broilers Performance. Int. J. Sci. Res. 2013, 6, 2319–7064. [Google Scholar]
- McCuistion, K.C.; Selle, P.H.; Liu, S.Y.; Goodband, R.D. Sorghum as a feed grain for animal production. In Sorghum and Millets. Chemistry, Technology, and Nutritional Attributes, 2nd ed.; Taylor, J., Duodu, K., Eds.; Elsevier Inc. in cooperation with AACC International: Amsterdam, The Netherlands, 2019; pp. 355–391. [Google Scholar]
- Gunawan, S.; Dwitasari, I.; Rahmawati, N.; Darmawan, R.; Wirawasista Aparamarta, H.; Widjaja, T. Effect of Process Production on Antinutritional, Nutrition, and Physicochemical Properties of Modified Sorghum Flour. Arab. J. Chem. 2022, 15, 104134. [Google Scholar] [CrossRef]
- Karunaratne, N.D.; Newkirk, R.W.; Ames, N.P.; Van Kessel, A.G.; Bedford, M.R.; Classen, H.L. Effects of exogenous β-glucanase on ileal digesta soluble β-glucan molecular weight, digestive tract characteristics, and performance of coccidiosis vaccinated broiler chickens fed hulless barley-based diets with and without medication. PLoS ONE 2021, 16, e0236231. [Google Scholar] [CrossRef]
- McNab, J.M.; Smithard, R.R. Barley β-Glucan: An Antinutritional Factor in Poultry Feeding. Nutr. Res. Rev. 1992, 5, 45–60. [Google Scholar] [CrossRef] [PubMed]
- Ram, S.N.; Narwal, S.; Gupta, O.P.; Pandey, V.; Singh, G. 4—Anti-nutritional factors and bioavailability: Approaches, challenges, and opportunities. In Wheat and Barley Grain Biofortification; Woodhead Publishing Series in Food Science, Technology and Nutrition; Gupta, O.P., Pandey, V., Narwal, S., Sharma, P., Ram, S., Singh, G.P., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 101–128. [Google Scholar] [CrossRef]
- Tanasković, S.J.; Šekuljica, N.; Jovanović, J.; Gazikalović, I.; Grbavčić, S.; Đorđević, N.; Sekulić, M.V.; Hao, J.; Luković, N.; Knežević-Jugović, Z. Upgrading of Valuable Food Component Contents and Anti-Nutritional Factors Depletion by Solid-State Fermentation: A Way to Valorize Wheat Bran for Nutrition. J. Cereal Sci. 2021, 99, 103159. [Google Scholar] [CrossRef]
- Alemayehu, G.F.; Forsido, S.F.; Tola, Y.B.; Teshager, M.A.; Assegie, A.A.; Amare, E. Proximate, mineral and anti-nutrient compositions of oat grains (Avena sativa) cultivated in Ethiopia: Implications for nutrition and mineral bioavailability. Heliyon 2021, 7, e07722. [Google Scholar] [CrossRef] [PubMed]
- Mehta, B.; Jood, S. Anti-Nutritional Factors and Mineral Content of Different Oat (Avena sativa L.) Varieties. Food Sci. Res. J. 2018, 9, 117–120. [Google Scholar] [CrossRef]
- Jasinska-Kuligowska, I.; Kuligowski, M.; Kolodziejczyk, P.; Michniewicz, J. Effect of fermentationextrusion and baking processes on content of fructans in rye products. Zywnosc-Nauka Technol. Jakosc 2013, 20, 129–141. [Google Scholar]
- Ismagilov, R.; Ayupov, D.; Nurlygayanov, R.; Ahiyarova, L.; Abdulloev, V. Ways to reduce anti-nutritional substances in winter rye grain. Physiol. Mol. Biol. Plants 2020, 26, 1067–1073. [Google Scholar] [CrossRef]
- Osman, A.; Hartung, C.B.; Lingens, J.B.; Rohn, K.; Schreiner, T.; Ahmed, M.F.E.; Hankel, J.; Abd El-Wahab, A.; Visscher, C. Fermentation Characteristics of Rye and Sorghum Depending on Water:Feed Ratio. Fermentation 2022, 8, 155. [Google Scholar] [CrossRef]
- Kumar, R. Anti-nutritional factors, the potential risks of toxicity and methods to alleviate them in Legume Trees and Other Fodder Trees as Protein Sources for Livestock. In Proceedings of the FAO Expert Consultation, Kuala Lumpur, Malaysia, 14–18 October 1991; FAO Animal and Health paper. Speedy, A., Pugliese, P., Eds.; FAO: Rome, Italy, 1991; p. 102. [Google Scholar]
- Shqueir, A.A.; Brown, D.L.; Taylor, S.J.; Rivkin, I.; Klasing, K.C. Effects of solvent extraction, heat treatments and added cholesterol on Sesbania sesban toxicity in growing chicks. Anim. Feed Sci. Technol. 1989, 27, 127–135. [Google Scholar] [CrossRef]
- Raharjo, Y.C.; Cheeke, P.R.; Patton, N.M. Effect of cecotrophy on the nutrient digestibility of alfalfa and black locust leaves. J. Appl. Rabbit Res. 1990, 613, 56–61. [Google Scholar]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Ramireddy, L.; Radhakrishnan, M. Cold Plasma Applications on Pulse Processing; Elsevier eBooks: Amsterdam, The Netherlands, 2021; pp. 295–307. [Google Scholar]
- Dittoe, D.K.; Olson, E.G.; Ricke, S.C. Impact of the Gastrointestinal Microbiome and Fermentation Metabolites on Broiler Performance. Poult. Sci. 2022, 101, 101786. [Google Scholar] [CrossRef] [PubMed]
- Saadi, S.; Saari, N.; Ghazali, H.M.; Abdulkarim, M.S. Mitigation of Antinutritional Factors and Protease Inhibitors of Defatted Winged Bean-Seed Proteins Using Thermal and Hydrothermal Treatments: Denaturation/Unfolding Coupled Hydrolysis Mechanism. Curr. Res. Food Sci. 2022, 5, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tao, L.; Zhang, R.; Yang, G. Effects of Fermented Feed on Growth Performance, Nutrient Metabolism and Cecal Micro-flora of Broilers. Anim. Biosci. 2022, 35, 596–604. [Google Scholar] [CrossRef]
- Feng, J.; Liu, X.; Xu, Z.R.; Wang, Y.Z.; Liu, J.X. Effects of Fermented Soybean Meal on Digestive Enzyme Activities and Intestinal Morphology in Broilers. Poult. Sci. 2007, 86, 1149–1154. [Google Scholar] [CrossRef]
- Haryati Supriyati, T.; Susanti, T.; Susana, I.W.R. Nutritional value of rice bran fermented by Bacillus amyloliquefaciens and humic substances and its utilization as a feed ingredient for broiler chickens. Asian Aust. J. Anim. Sci. 2015, 28, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Ogbuewu, I.P.; Mabelebele, M.; Mbajiorgu, C.A. Determination of performance response of broilers to fermented tropical leaf meal supplementation using meta-analytical method. Trop. Anim. Health Prod. 2024, 56, 98. [Google Scholar] [CrossRef]
- Chen, B.; Li, D.; Leng, D.; Kui, H.; Bai, X.; Wang, T. Gut microbiota and meat quality. Front. Microbiol. 2022, 13, 951726. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Li, Y.X.; Li, Y. Effect of Ginkgo biloba extract on growth performance, slaughter performance and immune index in broilers. J. Fujian Agric. For. Univ. 2008, 3, 295–298. [Google Scholar]
- Agboola, T.O.; Balogun, O.L.; Rahji, M.A.Y. Stock Size and Relative Efficiency in Broiler Production in SouthWestern Nigeria: A Normalized Profit Function Approach. IOSR J. Agric. Vet. Sci. 2014, 7, 43–50. [Google Scholar] [CrossRef]
- Castellone, V.; Bancalari, E.; Rubert, J.; Gatti, M.; Neviani, E.; Bottari, B. Eating Fermented: Health Benefits of LAB-Fermented Foods. Foods 2021, 10, 2639. [Google Scholar] [CrossRef]
- Irawan, A.; Ratriyanto, A.; Respati, A.N.; Ningsih, N.; Fitriastuti, R.; Suprayogi, W.P.S.; Hadi, R.F.; Setyono, W.; Akhirini, N.; Jayanegara, A. Effect of feeding fermented soybean meal on broiler chickens’ performance: A meta-analysis. Anim. Biosci. 2022, 35, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Saleh, A.A.; Shukry, M.; Farrag, F.; Soliman, M.M.; Abdel-Moneim, A.E. Effect of Feeding Wet Feed or Wet Feed Fermented by Bacillus licheniformis on Growth Performance, Histopathology and Growth and Lipid Metabolism Marker Genes in Broiler Chickens. Animals 2021, 11, 83. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, J.; Wang, Y.; Lv, J.; Li, J.; Guo, L.; Min, Y. Fermented Corn-Soybean Meal Mixed Feed Modulates Intestinal Morphology, Barrier Functions and Cecal Microbiota in Laying Hens. Animals 2021, 11, 3059. [Google Scholar] [CrossRef] [PubMed]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in animal nutrition—Production, impact and regulation. In FAO Animal Production and Health Paper; Makkar, H.P.S., Ed.; FAO: Rome, Italy, 2016; Volume 179. [Google Scholar]
- Fathima, S.; Shanmugasundaram, R.; Adams, D.; Selvaraj, R.K. Gastrointestinal Microbiota and Their Manipulation for Improved Growth and Performance in Chickens. Foods 2022, 11, 1401. [Google Scholar] [CrossRef]
- Pendleton, B. The regulatory environment. In Direct-Fed Microbial, Enzyme and Forage Additive Compendium; The Miller Publishing Company: Minnetonka, MN, USA, 1998; Volume 4, pp. 47–52. [Google Scholar]
- Medellin-Peña, M.J.; Wang, H.; Johnson, R.; Anand, S.; Griffiths, M.W. Probiotics affect virulence-related gene expression in Escherichia coli O157: H7. Appl. Environ. Microbiol. 2007, 73, 4259–4267. [Google Scholar] [CrossRef]
- Meng, Q.; Yan, L.; Ao, X.; Zhou, T.; Wang, J.S.; Garrido-Galand, A.; Asensio-Grau, J.; Calvo-Lerma, A.; Heredia, A. The potential of fermentation on nutritional and technological improvement of cereal and legume flours: A review. Int. Food Res. 2021, 145, 110398. [Google Scholar] [CrossRef]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Tropini, C.; Earle, K.A.; Huang, K.C.; Sonnenburg, J.L. The Gut microbiome: Connecting spatial organization to function. Cell Host Microbe 2017, 21, 433–442. [Google Scholar] [CrossRef]
- Farré, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Izadi, H.; Arshami, J.; Golian, A.; Raji, M.R. Effects of Chicory Root Powder on Growth Performance and Histomorphometry of Jejunum in Broiler Chicks. Dir. Open Access J. 2013, 4, 169–174. [Google Scholar]
- Raza, A.; Bashir, S.; Tabassum, R. An update on carbohydrases: Growth performance and intestinal health of poultry. Heliyon 2019, 5, e01437. [Google Scholar] [CrossRef] [PubMed]
- Vancamelbeke, M.; Vermeire, S. The Intestinal Barrier: A Fundamental Role in Health and Disease. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Dao, D.P.D.; Le, P.H. Histology, Goblet Cells. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553208/ (accessed on 23 September 2024).
- Horowitz, A.; Chanez-Paredes, S.D.; Haest, X.; Turner, J.R. Paracellular Permeability and Tight Junction Regulation in Gut Health and Disease. Nat. Rev. Gastroenterol. Hepatol. 2023, 20, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Ménard, S.; Cerf-Bensussan, N.; Heyman, M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol. 2010, 3, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, G.; Arnone, A.; Ciampaglia, R.; Tenore, G.C.; Novellino, E. Fermentation of Foods and Beverages as a Tool for Increasing Availability of Bioactive Compounds. Focus on Short-Chain Fatty Acids. Foods 2020, 9, 999. [Google Scholar] [CrossRef] [PubMed]
- Siddique, A.; Azim, S.; Ali, A.; Adnan, F.; Arif, M.; Imran, M.; Ganda, E.; Rahman, A. Lactobacillus reuteri and Enterococcus faecium from Poultry Gut Reduce Mucin Adhesion and Biofilm Formation of Cephalosporin and Fluoroquinolone-Resistant Salmonella enterica. Animals 2021, 11, 3435. [Google Scholar] [CrossRef]
- Iqbal, Y.; Cottrell, J.J.; Suleria, H.A.R.; Dunshea, F.R. Gut Microbiota-Polyphenol Interactions in Chicken: A Review. Animals 2020, 10, 1391. [Google Scholar] [CrossRef]

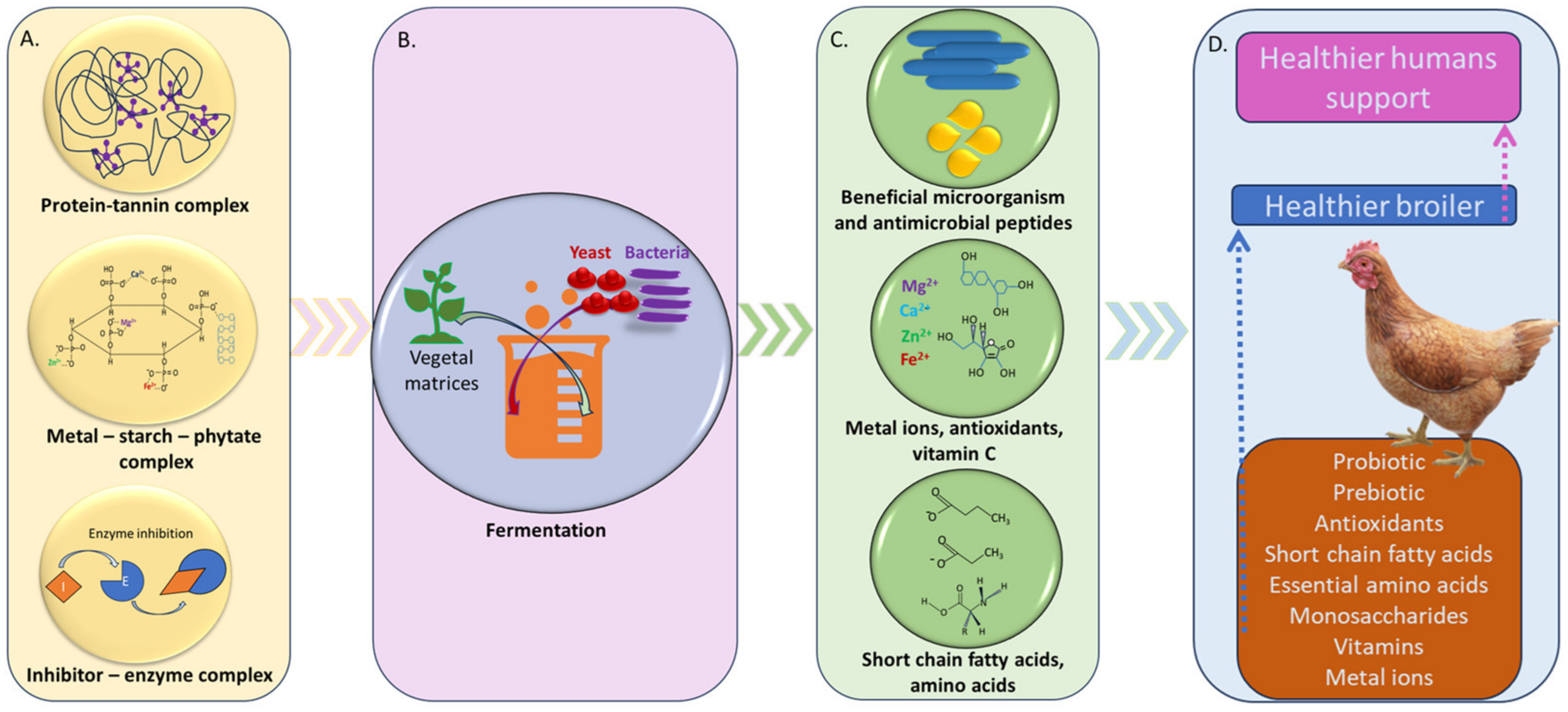
| Vegetal Matrices | Antinutrient Components (ANC) | Side Effects of Antinutrient Components | Biotechnological Solutions for Antinutrients Mitigation | References |
|---|---|---|---|---|
| Soybean | Trypsin inhibitors, phytic acid, saponins and lectins | Reduce the digestibility and absorption of the nutrients in the animal organism | Fermentation in the presence of Lactobacillus plantarum reduced the anti-nutritional factors in the milled soybeans | [31,32,33] |
| Corn | Phytate, tannins and polyphenols | Bind dietary minerals in the gastrointestinal tract, decreasing their bio-accessibility and bioavailability | Fermentation with L. plantarum A6, L. brevis G11, L. brevis G25, L. fermentum N33, L. fermentum N25, L. buchneri M11, and L. cellobiosus M41, reduced the antinutritional factors. | [29,34,35,36] |
| Sorghum | Tannin (phenolic compounds), and dhurrin | Affect the growth, feed intake, digestion of protein, egg production, and deformities of the legs of broiler chickens | The two-step procedure of sinking in a NaOH solution and fermentation (using L. bulgaricuss, L. casei, and L. brevis) lowers the ANC and enhances the nutritional composition. | [37,38,39,40,41] |
| Barley | β-glucan | Decrease digestibility, form gels in aqueous media and excretion of sticky droppings | Fermentation of barley with Rhizopus oligosporus, and supplementation with β-glucanase reduced ANC | [42,43] |
| Wheat | Phytate, protease inhibitors, tannins, lectins, alkaloids, and oxalate | Reduces the bioavailability of essential micronutrients such as iron and zinc | Fermentation (Bacillus sp. TMF–2) as well as genetic engineering led to the reduction of ANC | [44,45] |
| Oats | Phytate, tannin, and oxalate | Disrupt the stomach’s normal functioning and prevent nutrients from being absorbed. | Saccharomyces cerevisiae fermentation, germination, de-branning, autoclaving, soaking reduced ANC | [46,47] |
| Rye | Water-soluble pentosans | Clogged digestive tract with a viscous pentosan solution that prevents food nutrients being absorbed; esophageal and gastric blockages | Fermentation with 1k2079 L. plantarum, 1k2103 Pediococcus pentosaceus, and 1k2082 L. lactis enzymes seem to reduce ANC | [48,49,50] |
| Alfalfa | Saponins | Growth rate retardation | Fermentation with LAB reduced ANC | [51,52,53] |
| Fermented Feed Microorganisms | Vegetal Matrices | Effects of Fermented Feed Groups Compared to the Control Group | References | |
|---|---|---|---|---|
| Growth Performance Parameters | Other Parameters | |||
| Lactobacillus casei (C37M41) | Corn, soybean, and wheat, 6:2:2 (w:w:w) | - ↗ BW during the first 21 days days for broiler treat with fermented feed in concentration of 3 kg ∙ t−1 ↗ FCR during 0–42 days for broiler treat with fermented feed in concentration of 0.3 kg ∙ tone−1 | - ↙ pathogenic microorganisms (phylum Delsulfobacterota and class Desulfovibriona and Negativicutes) - ↗ beneficial microorganisms like Lactobacillaceae family | [21] |
| Bacillus amyloliquefaciens | Rice bran | - ↗ fermented feed consumption in the first three weeks, - ↗ FCR at the first three weeks of the feeding trial and BW | Zero mortality recorded during the experiment | [60] |
| Lactobacillus and Bacillus subtilis | Corn, soybean, cottonseed, and rapeseed (different ratio) | - ↗ growth performance after addition of 10% fermented feed, in the first 21 days - ↗ growth performance after addition of 5% fermented feed in 0–42 days of broiler life | - ↗ breast fat - ↙ cholesterol content in broiler meat - ↗ immune factors like IgM, IgG, and interferon-γ | [26] |
| Streptococcus alactolyticus, Lactobacillus acidophilus, Lactobacillus reuteri | Astragalus powder | - ↗ Final BW and ADG and ↙ F/G ratio for fermentation feed group compared to control and unfermented feed group | - ↗ serum IgA and IgG, serum albumin, serum total antioxidant capacity - ↙ serum and liver tissue malondialdehyde on day 28 and day 42. | [27] |
| Bacillus licheniformis | Corn–soybean (different ratio) | ↗ growth and digestibility for fermented feed group compared with other tested broiler groups | - ↗ duodenal and ileal villi heights - crypt depths weren’t not altered | [67] |
| Aspergillus oryzae 3.042 | Soybean | - ↗ Fermented feed broiler presented growth and feed conversion promotion compared to another tested group | - ↗ activities of trypsin, lipase, and protease in the intestinal content | [59] |
| Bacillus spp., Lactobacillus spp., Saccharomyces cerevisiae | Corn, soybean and wheat bran, 6:2:2 (w:w:w) | - ↗ nutrient absorption; - ↙the abdominal fat rate and the muscle fat | - ↙ feed pH value and anti-nutritional factor concentrations - ↗ good bacteria like Parasutterella, Butyricicoccus and Erysipelotrichaceae | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Predescu, N.C.; Stefan, G.; Rosu, M.P.; Papuc, C. Fermented Feed in Broiler Diets Reduces the Antinutritional Factors, Improves Productive Performances and Modulates Gut Microbiome—A Review. Agriculture 2024, 14, 1752. https://doi.org/10.3390/agriculture14101752
Predescu NC, Stefan G, Rosu MP, Papuc C. Fermented Feed in Broiler Diets Reduces the Antinutritional Factors, Improves Productive Performances and Modulates Gut Microbiome—A Review. Agriculture. 2024; 14(10):1752. https://doi.org/10.3390/agriculture14101752
Chicago/Turabian StylePredescu, Nicoleta Corina, Georgeta Stefan, Mihaela Petronela Rosu, and Camelia Papuc. 2024. "Fermented Feed in Broiler Diets Reduces the Antinutritional Factors, Improves Productive Performances and Modulates Gut Microbiome—A Review" Agriculture 14, no. 10: 1752. https://doi.org/10.3390/agriculture14101752






