Abstract
The aim of this work was to determine the influence of chosen biostimulants and microbiological preparations on the yield of sweet corn and the occurrence of Ostrinia nubilalis Hbn, and diseases. In both years of the study, the preparations used in this experiment did not have a statistically significant effect on marketable yield; however, in 2017, the highest weight was observed in the cobs of plants treated with Rizocore and Polyversum WP while the lowest in the cobs treated with RhizoVital 42. The biostimulant Asahi SL and the biological fungicide Serenade ASO proved to be the most effective in protecting sweet corn against cob and shoot infections by fungi of the genus Fusarium. All the preparations reduced the development of the common smut in corn, especially on the cobs. There were no statistically significant differences in cob infection by the O. nubilalis in the combinations treated with different preparations, although the lowest number of cobs damaged by pest in both years were observed on plots treated with Serenade ASO and RhizoVital 42, while the highest on plots treated with Goëmar BM.
1. Introduction
Sweet corn is one of the eight most consumed vegetables in the world. The world leader in corn production is the United States, where this crop covers an area of about 120,000 hectares and its consumption is estimated at 10 kg per capita. In Poland, sweet corn is grown both for the fresh market and for the processing industry, with an annual area of about 10,000 ha and an annual consumption of 0.5 kg per capita [1].
The gradually increasing consumption of this vegetable is due not only to its high nutritional value, but also to its health-promoting properties. Sweet corn is a rich source of vitamin E, selenium, antioxidants (lutein and zeaxanthin), and dietary fibre, among others. Moreover, its grains do not contain gluten, so sweet corn is a valuable component of gluten-free diets [1].
The dynamically increasing size of this vegetable crop has prompted the search for methods to improve its productivity per unit area. One of these is the use of biostimulants, which, unlike standard chemicals, have no negative impact on the environment [2]. Biostimulants belong to a group of compounds containing active substances that, when applied in small doses, improve plant metabolism by stimulating the synthesis and activity of natural hormones, increasing nutrient uptake, stimulating root system development and increasing resistance to unfavourable environmental factors. Biostimulants may include hormones, enzymes, proteins, ammo acids, vitamins, trace elements and other biologically active compounds [3,4,5]. Recently, many articles have been published on the use of biostimulants, which indicate their positive or negative effects on plant growth, chemical composition, and the yield of different vegetables [6,7,8,9,10], but the number of articles on their usefulness in sweet corn cultivation remains quite limited. Among others, Das et al. [11] conducted a field experiment to investigate the effect of organic and standard sources of fertilizer on the yield and quality of sweet corn in West Bengal, India; Al-Temimi and Al-Hilfy [12] investigated the application of a combination of certain mineral fertilizers and seaweed extract on the number of cobs per plant, number of rows per cob, number of grains per row, and the grain field; Tadros et al. [13] used a foliar application of amino acid biostimulants at a chosen growth stage of the sweet corn plants.
Sweet corn can be infected by a number of pathogens during the growing season, the most damaging of which are fungi of the genus Fusarium Link. These pathogens can cause two forms of disease: corn cob fusariosis, as well as root rot and stem base rot. Fusarium spp. fungi can infect various parts of plants, starting from the seedling stage, but the most harmful infections affect corn cobs. Infected cobs become deformed and the seeds may get damaged. A coating of white, yellowish, or reddish (salmon-coloured) mycelium, the colour of which depends on the predominant fungus species, develops on the infected tissues. Fungi of the genus Fusarium adversely affect human health, as some species produce harmful mycotoxins [14,15]. Fusarium spp. are polyphagous fungi and can also occur on other plants such as cereals and many vegetables. They are easily transmitted by conidial spores produced in large numbers and survive in the soil for several years in the form of spores—chlamydospores [16,17,18].
Fusarium root rot and stem base rot, often abbreviated as ‘stem fusariosis’, is a serious disease capable of causing the death of entire plants. The first symptoms of the disease are visible on the ground side of the stems, on which rot spots can be seen, and sometimes the wilting and drying of the leaves from the bottom upwards can be observed. In the final stage of the disease, further localized drying and dying of entire plants may occur [16,17,18].
Common smut in corn, caused by Ustilago maydis (DC.) Corda, can be quite often observed mainly on the cobs in sweet corn plantations. This pathogen does not produce mycotoxins and therefore does not pose a threat to human health in the same way as the fungi Fusarium spp. The main sources of infestation can be infected seed and corn residues in the soil. Significant damage, due to the high level of infection, is associated with damage caused by pests such as thrips, aphids, the fruit fly, and the European corn borer. The most important symptom of the disease is growths in the form of nodules with a mass of compact, black-grey spores inside. Earlier, the growths are bright and hard, but as the spores mature inside, the inside of the nodules darkens, and their surface wrinkles and may crack. Early infected plants may not produce cobs at all. Infection by the pathogen at a later stage of cob development, reduces their yield and quality [16,17,19].
A disease of lesser importance is the small leaf spot of corn caused by the fungus Kabatiella zeae Narita and Y. Hirats (syn. Aureobasidium zeae). The main source of the pathogen is post-harvest corn, and infection is promoted by wet leaves during rainy weather, or long-lasting dew. Optimal conditions for the causative agent are high humidity above 75% and temperatures of 18–25 °C. Symptoms on the leaves are small, initially chlorotic spots, which enlarge over time and become necrotic inside. At high severity, the spots can cover the entire leaves and cause a decrease in yield quantity and quality [14].
The most effective preparations for the protection of sweet corn against disease are strobilurin and triazole fungicides, which are used either preventively or curatively. Unfortunately, the number of synthetic plant protection products currently available for vegetable protection is decreasing annually, due to the non-renewal of the registration of certain chemical preparations in the European Union member states.
This is in line with one of the strategies of the European Green Deal, which promotes the use of biological plant protection methods and aims to reduce the number of registered pesticides [14]. Consequently, in line with the principles of integrated plant protection, research should focus on the evaluation of the effectiveness of biological and biotechnical preparations, as well as supportive measures to enhance plant health, including new fertilizer formulations.
Corn is also attacked by a number of insects, one of the most harmful being the European corn borer (Ostrinia nubilalis Hubner). This is a widespread pest known to feed on 250 different kinds of plants, including sweet corn and grain corn. The larvae eat leaves, mine all parts of the stalks and ears, and feed on cobs, causing serious yield loss [20,21].
Insecticides are commonly applied to protect corn plants against damage [22,23]. The research for innovative and alternative methods is an increasingly growing reality [24]. The use of cultural practices, host plant resistance, biological control and biostimulants has been shown to improve many agronomic characteristics of plants and protect them against pests and diseases [25,26,27,28,29].
Seaweed (marine algae) extracts are the new type of products currently used in plant cultivations. Microalgae are a rich source of bioactive substances that can find many applications in agriculture [30,31]. Primary and secondary metabolites are of great importance in medicine and agriculture [32,33]. Seaweeds are classified as the most important organism that can be used in plant production as biostimulants of plant growth [26,29,34,35,36,37,38,39] and as biostimulants or microbiological preparations for the control of pests and diseases [26,27,32,33,40,41].
Another substance that can be used in alternative pest control is silicon. This element acts as a mechanical barrier limiting the damage caused by pests. It also strengthens the induced chemical defence of plants after insect feeding [42,43,44]. In general, silicon is involved in plant resistance against insect-caused damage via two major defence mechanisms: physical defence and induced biochemical (chemical) defence [45,46,47].
The aim of this work was to determine the influence of chosen biostimulants and microbiological preparations on the yield of sweet corn and the occurrence of pests and diseases.
2. Material and Methods
2.1. Research Site
The experiment was carried out in 2016 and 2017 at the experimental station of the Agricultural University in Mydlniki near Krakow, located in southern Poland (50°04′ N, 19°51′ E). According to Koppen’s classification, the climate is humid continental (Dfb). According to the classification of the Food and Agriculture Organization of the United Nations [48], the soil type is a Fluvic Cambisol (Humic).
2.2. Methods
Seeds of the sweet corn (Zea mays L. ssp. saccharata Kcke) cultivar Sweet Wonder F1 (Agri Frimeko) were sown in the middle of May, at 75 × 20 cm spacing and covered for three weeks with non-woven fabric (Agryl PP, 19 g m−2). The plants were treated four times (foliar spray) at weekly intervals with biostimulants and microbiological preparations (Table 1). The control were unsprayed plants.

Table 1.
Doses and dates of application in the experiment in years 2016–2017.
Each treatment combination was performed in 4 replications. Each plot consisted of 40 plants sown in 4 rows where 2 of them served as protective rows. Standard cultivation practices were performed, including weed control, fertilization and irrigation. The doses of fertilizers were calculated on the basis of soil analyses using the universal method according to Nowosielski [49] to achieve nutrient contents of 120 mg N (NH4 + NO3), 60 mg P, 200 mg K, 70 mg Mg, and 1500 mg Ca in 1 dm3 of soil.
The biostimulants and microbiological preparations used in this experiment are as follows:
AS-Asahi SL (UPL, formerly Arysta LifeScience, Warszawa, Poland) is a biostimulator based on three active substances from the group of nitrophenols naturally occurring in plants. It also improves the tolerance of crops to stress factors unfavourable to plant growth and development. The content of active substances is as follows: sodium para-nitrophenolate—3 g/dm3 (0.3%), sodium ortho-nitrophenolate—2 g/dm3 (0.2%), 5-sodium nitroguaiacolate—1 g/dm3 (0.1%);
TY—Tytanit® (Intermag, Okusz, Poland) is a liquid mineral growth stimulator. It increases the content of chlorophyll in the leaves, which intensifies photosynthesis. It significantly improves the efficiency of the pollination process and increases the uptake of nutrients from the soil, especially potassium (K), nitrogen (N), magnesium (Mg), calcium (Ca), and iron (Fe). It strengthens the natural resistance of plants to stress factors. Furthermore, it contains titanium (Ti) calculated as TiO2 (8.5 g/dm3; 0.8% m/m);
OSI—Optysil® (Intermag, Olkusz, Poland) is a liquid silicon anti-stress agent that activates the natural immune systems of plants and stimulates their growth and development. It increases plants’ tolerance to unfavourable growing conditions, e.g., drought, and reduces the impact of biotic stress caused by pathogens and/or pest attack. It contains silicon (Si) calculated as SiO2 (200 g/dm3; 16.5% m/m);
BM—Goëmar BM 86 (UPL, formerly Arysta LifeScience, Warszawa, Poland) is a liquid preparation recommended for the cultivation of vegetables whose edible part is fruit. It activates mineral nutrition and ensures optimal flowering. It contains biologically active filtrate GA 142 obtained from Ascophyllum nodosum (sea alga), with the following composition: boron—2.03% (m/m), molybdenum—0.02% (m/m), magnesium—4.8% (m/m);
POL—Polyversum WP (Biopreparaty Praque, Czech Republic) is a biological agent based on the fungus-like organism Pythium oligandrum (concentration 106 oospores/g). It is a parasite of some species of oomycetes and pathogenic fungi. Pythium oligandrum decomposes pathogen hyphae through enzymatic decomposition, while stimulating the immune mechanisms of the protected plant by introducing phytohormones, phosphorus and sugars into them. The dose used is 0.05%;
RhV—RhizoVital 42 (ABiTEP, Berlin, Germany) is a biostimulator that contains the beneficial bacterium Bacillus amyloliquefaciens FZB42, naturally occurring in the soil (concentration above 2.5 × 1010 spores per mL). It supports the plant in building a strong and efficient root system, improving its tolerance towards stress (e.g., water deficiency, salinity) caused by unfavourable climatic conditions and field management. The bacteria germinate and colonize the young growing roots and release hormones and enzymes, which stimulate plant growth and mobilize nutrients, which increases plant growth and promotes a higher yield. The preparation is used at a dose of 0.5 L/ha.
RI—Rizocore (Biogard, Grassobbio, Italy) is a microbiological soil preparation with a unique composition of three ingredients: Bacillus megaterium (concentration 104 CFU/g), Trichoderma harzianum NAT11 (concentration 1010 CFU/g), and mycorrhizal fungi Glomus spp. (5%). The preparation improves the microbiological composition of the soil, increases the vigor and health of seedlings and young plants, causes better development of the roots in young plants and the growth and health of plants, and increases the resistance of mature plants in defence processes in the presence of pathogens; mycorrhiza also increases the absorbent surface of roots. The preparation is used at a dose of 60 g/ha.
SE—Serenade ASO (Bayer AG, Leverkusen, Germany) is biological preparation based on the beneficial soil bacterium Bacillus subtilis QST 713 (concentration 13.96 g/L, 1.34%, 1.042 × 1012 CFU/L). It is intended for use against diseases caused by fungi and bacteria. The bacterium occurs naturally and has fungicidal and fungistatic properties. It works by disrupting the development of mycelium as a result of contact with the pathogen on the plant surface and producing substances that disrupt the functioning of fungal cell membranes. Bacillus subtilis QST 713 also competes with pathogens for living space and nutrients as well as induces the systemic immunity of the plant. Its effect on bacterial diseases is to block the synthesis of the bacterial cell wall. The preparation was used at a dose of 8 L/ha.
A study on the effect of applied preparations on the health of the shoot base, leaves, and cobs of sweet corn was performed just before harvest. The severity of the disease symptoms was evaluated on 30 randomly selected leaves, shoots, and cobs of maize from each plot, respectively. During direct observations of the plants, the severity of the disease symptoms was visually assessed according to the following scale: 0—no symptoms, 1—light infestation (spot area of up to 5% of the leaf blade), 2—medium infestation (spot area of 6–25%), 3—heavy infestation (spot area of 26–50%), 4—very heavy infestation (more than 51% of the area). The infestation scale was also applied to the area of rot spots on a 30 cm section, measured from the base, of shoots infected by Fusarium-induced stem base rot and the area of cobs attacked by the nodular head [17]. Based on the obtained data, an infestation index was calculated for each combination replicate. The infestation index expressed as a percentage was calculated according to the formula:
where Ip—infestation index; a—number of plants infested at a given scale level; b—scale level; N—total number of plants analysed; n—the highest scale level.
Ip= ∑(a × b) × 100/N × n
The effects of the preparations on the plant crop and the occurrence of O. nubilalis were estimated after harvesting the cobs at the end of August. Fifteen cobs from each combination were weighed and measured, and the number of cobs damaged by O. nubilalis Hbn was noted.
2.3. Meteorological Data
The data source is a meteorological station located on the research site.
There was less rainfall in each month of the growing season (May–August) of 2016 than in the corresponding period of 2017 (Figure 1). However, the distribution of rainfall on individual days was different—in 2017 rain events were less frequent, but more intense, while 2016 had more regular rainfall. This means that in the second year of research there were many periods of drought during the growing season. Such a pattern was unfavourable for the development of pathogens, including fungal ones. The highest air temperatures in 2016 were noted in June and July, whereas in 2017 they were noted in May and July.
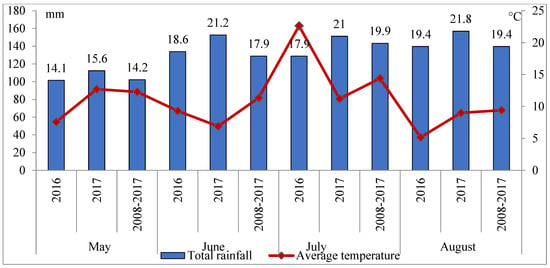
Figure 1.
Rainfall and air temperature in 2016–2017—Mydlniki.
2.4. Statistical Analysis
Data were analysed using a one-way analysis of variance ANOVA with Statistica software v. 13.3 (Tibco Software Inc., Palo Alto, CA, USA); calculations were conducted for each season separately. The values expressed as a percentage were transformed according to the Bliss function (y = arcsin ). Tukey’s HSD test for the results of the analyses concerning yield and cob parameters, Duncan’s MRT test for the results concerning the occurrence of disease symptoms, and Fisher’s LSD test for cob infestation by O. nubilalis were used to determine the significance of differences between mean values at the p = 0.05 significance level.
3. Results
Considering the cob parameters studied, it should be noted that the results are inconclusive (Table 2). On average, the highest corn cob weight was obtained and recorded in the first year of the study, while the cob diameter and length were slightly bigger in 2017. In 2017, the cobs of plants treated with Rizocore and Polyversum WP had the heaviest weight (but, similar to the control and plants treated with Asahi SL), while the cobs treated with RhizoVital 42 were the lightest, but their weight was similar to those treated with Tytanit and Optysil. No statistically significant differences were found for the cob weight in 2017, the cob diameter in both years, and the cob length in 2016. The control and RhizoVital 42-treated plants produced the shortest cobs in 2017, but their length was comparable to that of the cobs from the other treatments.

Table 2.
Effect of the treatment on corn cob parameters in years 2016–2017.
Figure 2 demonstrates that in both years of the study, the preparations used in this experiment did not have a statistically significant effect on the marketable yield. In 2016, it ranged from 14.97 (RhizoVital 42) to 17.00 t ha−1 (Rizocore), while in the following year, from 13.30 (Goëmar BM) to 15.33 t ha−1 (Polyversum WP).
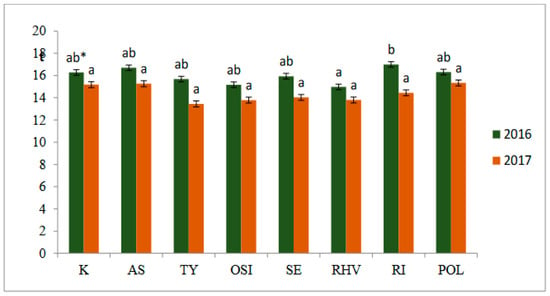
Figure 2.
Marketable yield (t ha−1) of sweet corn depending on preparation in years 2016–2017. * means that for each year marked with the same letter they do not differ significantly at p = 0.05, Tukey’s HSD test. K-Control, AS—Asahi SL, TY—Tytanit, OSI—Optysil, SE—Serenade ASO, RHV—RhizoVital 42, RI—Rizocore, POL—Polyversum.
In the first year of the study, all the preparations used effectively limited the development of fungi of the Fusarium genus on sweet corn cobs compared to the control. The most effective were Goëmar BM 86, Asahi SL and Serenade ASO, and the weakest protective effect was demonstrated by RhizoVital 42, Tytanit and Optysil (Table 3). In the next year, the following products most effectively protected corn against Fusarium spp. infection: Asahi SL, Serenade ASO, Goëmar BM 86, and Polyversum WP. The remaining preparations were ineffective and limited the development of Fusarium rot on corn cobs to a small extent only.

Table 3.
Indices of infection of sweet corn cobs with Fusarium fungi [%].
During the 2016 growing season, the infection of the base of corn shoots in all the combinations was greater than in the next year of research (Table 4). In 2016, the most effective protective effect on shoots was observed on plants sprayed with Asahi SL and Serenade ASO, and the weakest in combinations with Tytanit, RhizoVital 42, and Polyversum WP. In 2017, similar results were obtained, although Polyversum WP turned out to be one of the most effective preparations.

Table 4.
Indices of infection of the base of sweet corn shoots with Fusarium fungi [%].
In the first year of the study, all the preparations used effectively reduced infections with the causative agent of common smut in corn (Table 5). The most effective were Goëmar BM 86; RhizoVital 42; and Asahi SL. In 2017, corn infection by Ustilago maydis was at a very low level; in some combinations practically no disease symptoms were observed. Tytanit did not significantly improve the health of corn cobs; the number of infected cobs was only slightly lower than in the control.

Table 5.
Intensity of infection of sweet corn cobs with the fungus Ustilago maydis—the cause of common smut in corn—infection indices (%).
In both years of the experiments, among the applied agents, only Tytanit and Optysil fertilizers did not significantly reduce leaf infection by Kabatiella zeae compared to the control (Table 6). The most effective preparation was Goëmar BM 86; Asahi SL, RhizoVital 42 and Serenade ASO quite effectively protected corn leaves against the pathogen causing small leaf spot in both growing seasons.

Table 6.
Infection of sweet corn leaves with the fungus Kabatiella zeae (syn. Aureobasidium zeae)—the cause of small corn leaf spot—infection indices [%].
The results indicate that the level of infestation by the European corn borer differed between the study years. In 2016, a greater number of damaged cobs was recorded compared to 2017 (Table 7).

Table 7.
Mean number of cobs infested with O. nubilalis depending on the treatment in years 2016–2017.
There were no statistically significant differences in cob infection by the O. nubilalis in the combinations treated with different biostimulants and microbiological preparations, although the least number of cobs damaged by pests in both years was observed in plots treated with Serenade (2.0 in 2016 and 2.5 in 2017) and RhizoVital (2.75 in both years) while the highest (5.0 in 2016 and 4.0 in 2017) in plots treated with Goëmar BM. On control plots, an average of 4.75 in 2016 and 3.0 in 2017 damaged cobs were recorded (Table 7, Figure 3).
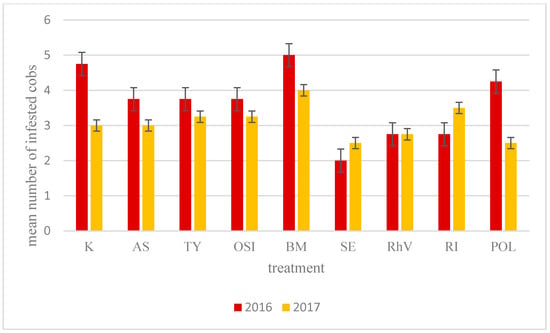
Figure 3.
Mean number of cobs damaged by O. nubilalis. K—Control, AS—Asahi SL, TY—Tytanit, OSI—Optysil, BM—Goëmar BM 86, SE—Serenade ASO, RHV—RhizoVital 42, RI—Rizocore, POL—Polyversum.
4. Discussion
Seaweeds are a popular choice among farmers for encouraging plant growth and improving crop yields in terms of both quality and quantity. In our experiment in both years of the study, no statistically significant differences were found in the marketable yield of corn.
Waligóra and Majchrzak [50] reported that in 2016–2017, the yield of the tested cultivars in Poland ranged from 7.8 to 21.4 t ha−1 depending on the cultivar and the year of the study, and a yield of 15 t ha−1 is considered profitable, which corresponds with the results achieved in this experiment. Das et al. [10] used vermicompost, an extract from the seaweed Ascophyllum nodosum, and a mineral fertilizer, and observed that the application of a full dose of the mineral fertilizer alone or in combination with vermicompost resulted in a higher yield of sweet corn as compared to treatments solely with the seaweed, which confirms the results obtained in this experiment, i.e., the lack of a significant effect of Goëmar BM 86 on sweet corn yield. Similarly, Al-Temimi and Al-Hilfy [12] found out that the application of a seaweed extract resulted in the highest number of cobs per plant, rows per cob, and grains per row, and a higher grain yield only when combined with mineral fertilizers. In the research conducted by Tadros et al. [13], the goal was to determine the optimal concentration and timing of foliar applications of amino acid biostimulants to achieve more efficient use in sweet corn cultivation. The plants were sprayed with four different concentrations: 1, 2, 3, and 4 mL/L of amino acids biostimulant liquid (commercial product PerfectoseTM), at three different growth stages: the seventh leaf, the tasselling and milk stage, and while the control plants were sprayed with distilled water only. The results indicated that the corn plant height; leaf water potential; chlorophyll content; and nitrogen, phosphorus, potassium, and protein content were significantly affected by the foliar application treatments, while the cob length was not significant. Similar effects of biostimulants on yield have been observed in other vegetables. Kunicki et al. [7] noted that spraying with Aminoplant had no effect on the yield of two cultivars of spinach. In an experiment conducted by Grabowska and Kunicki [6], there was no significant effect of Aminoplant and Goëmar Goteo preparations on the yield of broccoli, despite their positive effect on increasing the aboveground weight of seedlings. Similarly, the biostimulants Asahi SL, Biozyme, and Goëmar BM 86 did not contribute to an increase in the yield of the tomato grown in the open field [10], whereas an experiment with carrots showed an increase in the yield after the application of Aminoplant at a dose of 1.5 dm3 ha−1, but only in the first year of the study [9]. The study by Xu et al. [51] on the effectiveness of different foliar sprays, two forms of silicon and two kinds of organic nitrogenous compounds, showed that the treatments improved the marketable yield of tomatoes.
The use of preparations based on seaweed extracts, mainly containing laminarin, can significantly improve the physiological parameters and condition of sweet corn. Seaweed extract based preparations will keep the plants in good condition and less susceptible to pathogen infections [52,53]. In our experiment, Goëmar BM 86 was quite effective in protecting corn against Fusarium spp. and at the same time was most effective in reducing the development of the pathogens responsible for small leaf spot and nodular head.
The formulation Rizocore, which contains both beneficial microorganisms and arbuscular mycorrhizal fungi, did not show during the trials any significant efficacy in reducing dangerous pathogens, especially soil-borne pathogens such as Fusarium spp. The lack of effectiveness could be due to a short growing season, which might be insufficient for the mycorrhizal process to develop, or because of unfavourable soil conditions, especially soil drought, during the summer period. Subaedah et al. [54] showed that mycorrhiza applied on sweet corn had a positive effect on improving the yield and quality of sweet corn. The lower effectiveness of Rizocore may have been duem not only to a too-short growing season for sweet corn, but also due to soil moisture that was insufficient in promoting the development of beneficial soil microorganisms. The development of arbuscular mycorrhizal fungi requires a longer period than a few months, especially under varying weather conditions. The development of bacteria of the genus Bacillus contained in the fungicides Serenade ASO and RhizoVital 42 is faster than that of fungi under optimal conditions, and they can develop, not only in the rhizosphere, but also in the phyllosphere of the protected plants. This may be one of the reasons why these beneficial bacteria are more effective in protecting sweet corn from pathogens, compared to the microbial complex contained in Rizocore. This points to the need for continued research in future years, under different weather conditions, to gain more information on this subject.
Antagonistic bacteria, actinomycetes, oomycetes as well as fungi can effectively reduce the occurrence of some pathogens, including Fusarium fungi. Ridout et al. [15] showed that the presence of some beneficial bacteria as well as fungi on corn grains can reduce the number of pathogens of the genus Fusarium as well as reduce the content of some mycotoxins produced by these fungi. In a study, the Pythium oligandrum fungus-like organism contained in Polyversum WP effectively inhibited, especially in the second year, the occurrence of disease symptoms of Fusarium head rot on cobs, as well as on sweet corn stems.
The tested fertilizer Tytanit proved ineffective in reducing sweet corn pathogens in both years of the experiments. Pangaribuan et al. [55] and Mohammed et al. [56] showed that the quality and yield of sweet corn cobs were positively affected by urea as well as organic fertilizers. In addition, the applied fertilizers had a beneficial effect on stimulating beneficial microbial communities in the soil. Similar results were reported by Fahrurrozi et al. [57] on several sweet corn cultivars. Organic fertilization can affect soil structure, improving conditions for sweet corn growth [58]. By contrast, Canatoy [59] showed that the organic fertilization of sweet corn was less effective than mineral fertilization in improving the quality and quantity of cob yield.
The variation in the occurrence of the symptoms of common smut in corn between the years of the experiments was due, among other things, to different weather conditions during the growing season. In 2017, the symptoms were not severe, which was perhaps also related to the better health of the seeds used in the experiment. The high relevance of sweet corn cultivar and meteorological conditions prevailing during the growing season in the degree of plant infection by Ustilago maydis was shown by Waligóra et al. [19] and Frommer et al. [60] Szőke et al. [61] found in their research, that the use of auxin, cytokinin, and gibberellin reduced the negative effects of Ustilago maydis infection on sweet corn and that these phytohormones may be a new method of protecting plants against common smut in corn.
In 2016, there were longer periods of higher air and soil moisture during the growing season compared to 2017, which resulted from lower but more frequent rainfalls. Air humidity is one of the most important factors influencing the growth of bacteria and fungi, both those that are beneficial and those that are pathogenic to plants. Lower air humidity during the summer of 2017 may have had a limiting effect on the development of fungi that is pathogenic to sweet corn. The disease symptoms were less severe in 2017, with no common smut symptoms observed on corn in some plots. In addition to weather conditions, the presence of sources of infection with a particular pathogen is essential for pathogen development. Fungi of the genus Fusarium, as polyphages, can develop from many sources, not only from crop residues or infected seeds, but also from other, neighbouring crops. Therefore, they also pose a very high threat to corn every year. In the case of Ustilago maydis, there are fewer inoculum sources [16]. Dry, hot days and scarcity of water in the soil adversely affect the application of biopreparations, especially those containing living organisms. The development of beneficial microorganisms is inhibited under such conditions, leading to a significant reduction in their effectiveness.
The high efficacy of Serenade ASO in the protection of sweet corn against pathogens during our tests confirms the information contained, among other things, on the label of the product. The Bacillus subtilis bacterium, which is the main component of this biofungicide, can directly affect certain plant pathogens by producing important antibiotic-like substances and thus inhibiting the growth of other microorganisms. Additionally, this beneficial bacterium plays an important role in stimulating induced systemic resistance to pathogenic microorganisms. The biosynthesis of phenylpropanoids, flavonoids, and flavonols is important in this process [62]. Currently, the use of plant growth-promoting bacteria [PGPB], which include B. subtilis contained in the fungicide Serenade ASO and B. megaterium in Rizocore, is gaining popularity in agricultural production. Different strains of beneficial bacterial species have been found to have a great diversity of action mechanisms affecting crop plants in manifold ways. Katsenios et al. [63] showed the significant effect of some tested PGPB strains, including B. subtilis, in sweet corn crops on yield increase as well as on grain quality by increasing protein and fibre content in the grains.
When using biological preparations, it is important to bear in mind several factors that determine their effectiveness. The timing of their application is very important; biopreparations should be applied as early as possible so that the beneficial microorganisms have time to develop on the surface of plant organs (phyllosphere and rhizosphere) and activate their multidirectional action on pathogens. Protective treatments should be carried out under conditions ensuring the proper development and survival of beneficial microorganisms in the environment; crops should be sprayed during cloudy weather with moderate temperatures and high air and soil humidity. Biopreparations should not be applied in bright sunshine and periods of drought with high air temperatures because such conditions are unfavourable for the development of beneficial microorganisms and, at the same time, inhibit or even block their effect on pathogens [64]. The low effectiveness of biopreparations is most often the result of their application late in the growing season, sometimes already on infected plants, or under unfavourable climatic conditions. The fungi most dangerous to sweet corn are those of the genus Fusarium, which can infect plants already in the early stages of plant development, even in the seedling stage [16]. The use of biopreparations on infected plants is pointless in such a case. Although seaweeds are registered as plant growth stimulants, little is known about their effect against plant pests. In our experiment, no statistically significant differences were found in cob infection by the O. nubilalis in the combinations treated with different preparations; however, less pest-damaged cobs were noted in plots treated with biostimulants and microbiological preparations compared to the control, except Goëmar BM (seaweeds), where the highest infestation was observed. Similar information was provided by Warner [65], who studied the influence of seaweeds on the occurrence of orchards’ pests. The study revealed that seaweed treatment had no effect on fruit damage caused by Hoplocampa testudinea, Conotrachelus nenuphar, Cydia pomonella, Grapholita molesta, Grapholita prunivora, or tortricids and also noted that those extracts could slightly limit the occurrence of spider mites compared to the control. Yu et al. [32] observed the potential of seaweeds and their isolated components, such as polysaccharides, phenolics, proteins, terpenes, lipids, and halogenated compounds against eggs, larvae, pupae, and adult mosquitoes. They also found out that seaweed compounds affected the growth, development, and reproduction of adults and larvae. Also, Bianco et al. [66] and Cetin et al. [67] concluded that seaweed contained substances with larvicidal properties against mosquitoes. According to Ishii et al. [33], compounds from red alga (seaweeds) showed strong toxicity against Sithophilus zeamais, and termites. Asharaja and Sahayaray [68], while working on the biocidal activity of the water extracts (100, 200, 400, 600 and 800 ppm) of brown algae Sargassum wightii and Padina pavonica against Dysdercus cingulatus, noted that extracts from seaweeds controlled the nymphs and adults of this pest on cotton, negatively influencing their biological parameters by, e.g., increasing nymphal mortality, significantly prolonging the mating period, and highly reducing fecundity and hatchability.
Thawfeeq et al. [69] and Thawfeeq et al. [70] studied the insecticidal activity of brown seaweed (Sargassum tenerrimum) and red seaweed (Gracilaria corticata) against Aphis craccivora adults on cowpea. Methanolic extracts of both seaweeds showed insecticidal activity against aphids (at 0.1, 0.2, 0.4, 0.8 and 1.6%) and caused a mortality ratio of over 60% within 72 h after the treatment against A. craccivora. The authors concluded that seaweeds could be an alternative ecological pesticide in the control of aphids. Similarly, Osmundae pinnatifida showed insecticidal activity against five different pests Callosobruchus analis, Sitophilus oryzae, Tribolium castaneum, Rhyzopertha dominica, and Trogoderma granarium [71]. In addition, seaweed extracts from Plocamium cartilagineum could be used to manage several agricultural pests, such as aphids, bugs, weevils, or moths [72]. Gonzalez-Castro et al. [41] found out that seaweed extract caused a 100% mortality rate in Diaphorina citri (Asian citrus psyllid) after 24 h of exposure and suggested that seaweed extracts offered an applicable alternative method for pest control. However, Machado et al. [27] stated that the promising results of the application of seaweed extracts to pest control obtained under laboratory or greenhouse conditions do not always reflect the actual weather conditions affecting plantations.
In our studies no effect of silicon on the plant infestation by O. nubilalis was found, whereas Alhousari and Greger [44], who tested two types of mechanisms (physical or mechanical barriers and biochemical mechanisms) limiting pest feeding, found out that Si played an important role in strengthening direct and indirect protection against many insects. They noted that Si acted in plants both below and above ground, inducing lignin accumulation in the roots, increasing toughness and, eventually, resistance to pest attack. Han et al. [45] showed that silicon application at 0.16 and 0.32 g Si/kg soil made a susceptible rice variety resistant to the Cnaphalocrocis medinalis. The authors noted that silicon significantly prolonged larval development and reduced the larval survival and pupation rate. High doses of silicon negatively influenced the development of third instar larvae and the weight of pupae. In addition, silicon reduced the intrinsic rate of increase, the finite rate of increase, and the net reproduction rate of the C. medinalis population. According to Lang et al. [46], the modification of plants by adding silicone increased the plants’ physical barrier and changed the insect behaviours, causing enhanced resistance in Si-amended plants. Si application to rice had an effect on plant defence responses induced by the feeding of Nilaparvata lugens. Si supplement improved the silicification of leaf sheaths. It was noted that silicon affected direct and indirect plant defence against chewing and sucking insects by influencing the development time, larvae survival, and population increase rate. The authors suggested that, due to its protective plant defence effects, silicon could be an eco-friendly alternative to chemical pesticides in plant protection.
5. Conclusions
The presented study on the effects of biostimulants and microbiological preparations on the yield and occurrence of selected diseases and pests on sweet corn has shown inconclusive results. Thus, further investigations are necessary in the future in order to clarify the impact of such substances on the quantity and quality of the yield of sweet corn and the occurrence of O. nubilalis.
The biostimulant Asahi SL and the biological fungicide Serenade ASO proved to be the most effective in protecting sweet corn against cob and shoot infections by fungi of the genus Fusarium in both years of the study. The biostimulant Goëmar BM 86 and the biofungicide Polyversum WP were effective in 2017. All the preparations reduced the development of the common smut in corn, especially on the cobs. Tytanit was the least effective fertilizer, and it did not significantly affect the health of sweet corn compared to the control in the second year of the study. The fertilizer Titanite and the biostimulant Optysil were completely ineffective in protecting sweet corn against small leaf spots in both years of the experiment. The biostimulants Goëmar BM 86 and Asahi SL were the most effective, and the biopreparations RhizoVital 42 and Serenade ASO were moderately effective in this regard.
No statistically significant differences in cob infection by the O. nubilalis were noted between the respective plots, although the lowest number of cobs damaged by pest were observed in both years on plots treated with Serenade and RhizoVital while the highest on plots treated with Goëmar BM.
Further research is needed on the effects of different application techniques, doses or mixtures of the biostimulants and microbiological preparations under alternative environmental conditions.
Author Contributions
All the authors contributed to this work. E.W.-Ż. designed and performed the experiments, analysed the data, and wrote the paper; J.N. designed and performed the experiments, analysed the data, and wrote the paper; and E.K. designed and performed the experiments, analysed the data, and wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Polish Ministry of Science and Higher Education and under the research subsidy granted to the University of Agriculture in Krakow.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Warzecha, R. Sweet corn—A valuable crop for vegetable processing and the fresh market. Agro Serwis 2019, 10, 52–54. [Google Scholar]
- Paradikovic, N.; Vinkovic, T.; Vrcek, I.; Zuntar, I.; Bojic, M.; Medic, M. Effect of natural biostimulants on yield and nutritional quality: An example of sweet yellow pepper (Capsicum annuum L.) plants. Sci. Food Agric. 2011, 91, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.; Craigie, J.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Reg. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Ojeda-Barrios, D.L.; Cruz-Alvarez, O.; Sánchez-Chavez, E.; Ciscomani-Larios, J.P. Effect of foliar application of zinc on annual productivity, foliar nutrients, bioactive compounds and oxidative metabolism in pecan. Folia Hort. 2023, 35, 179–192. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E. The influence of selected biopreparations on the yield of broccoli in spring cultivation. Zesz. Probl. Postep. Nauk. Roln 2009, 539, 193–197. [Google Scholar]
- Kunicki, E.; Grabowska, A.; Sękara, A.; Wojciechowska, R. The effect of cultivar type, time of cultivation, and biostimulant treatment on the field of spinach (Spinacia oleracea L.). Folia Hort. 2010, 22/2, 9–13. [Google Scholar] [CrossRef]
- Guinan, K.J.; Sujeeth, N.; Copeland, R.B.; Jones, P.W.; O’Brien, N.M.; Sharma, H.S.S.; Prouteau, P.F.J.; O’Sullivan, J.T. Discrete roles for extracts of Ascophyllum nodosum in enhancing plant growth and tolerance to abiotic and biotic stresses. Acta Hort. 2013, 1009, 127–135. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E.; Sękara, A.; Kalisz, A.; Wojciechowska, R. The effect of cultivar and biostimulant treatment on the carrot yield and its quality. Veget. Crop. Res. Bull. 2012, 77, 37–48. [Google Scholar] [CrossRef]
- Grabowska, A.; Kunicki, E.; Sękara, A.; Kalisz, A.; Jezdinský, A.; Gintrowicz, K. The effect of biostimulants on the quality parameters of tomato grown for the processing industry. Agrochimica 2015, 59, 203–217. [Google Scholar] [CrossRef]
- Das, A.; Murmu, K.; Bandopadhyay, P.; Roy, M. Seaweed and its role in enhancing yield and antioxidant properties in sweet corn. Res. Sq. 2022, 1–17. Available online: https://www.researchgate.net/publication/360059422_Seaweed_and_its_role_in_enhancing_yield_and_antioxidant_properties_in_sweet_corn/fulltext/636c6041431b1f5300863bd7/Seaweed-and-its-role-in-enhancing-yield-and-antioxidant-properties-in-sweet-corn.pdf (accessed on 15 June 2024).
- Al-Temimi, A.H.M.; Al-Hilfy, I.H.H. Effect of the application of biostimulants on the agronomic parameters of corn varieties. Rev. Bras. Cienc. Agrar. Recife 2021, 16, e9257. [Google Scholar] [CrossRef]
- Tadros, M.J.; Omari, H.J.; Turk, M.A. The morphological, physiological and biochemical responses of sweet corn to foliar application of amino acids biostimulants sprayed at three growth stages. AJCS 2019, 13, 412–417. [Google Scholar]
- Korbas, M. Common smuts of maize and other diseases—Harmfulness and possibilities of their control. Prog. Plant Prot. 2006, 46, 354–357. [Google Scholar]
- Ridout, M.E.; Godfrey, B.; Newcombe, G. Effects of antagonists on mycotoxins of seedborne Fusarium spp. in sweet corn. Toxins 2019, 1, 438. [Google Scholar] [CrossRef]
- Koike, T.S.; Gladders, P.; Paulus, O.A. Vegetable Diseases: A Colour Handbook; Manson Publishing: London, UK, 2007. [Google Scholar]
- Bereś, P.K.; Siekaniec, Ł.; Kontowski, Ł. Occurrence of maize (Zea mays L.) diseases caused by fungi on long-term monoculture fields in south-eastern Poland in 2010–2021. Prog. Plant Prot. 2022, 62, 117–127. [Google Scholar] [CrossRef]
- Filho, R.C.; Guimarães, M.; de Abreu, V.P.; Rocha, G.A.; de Carvalho Menezes, R.; Dias, V.D.; da Cunha, M.G. Fusarium sacchari associated with stem rot in sweet corn in Brazil. Pesq. Agropec. Trop. Goiânia 2023, 53, e74263. [Google Scholar] [CrossRef]
- Waligóra, H.; Sawinska, Z.; Skrzypczak, W.; Idziak, R.; Weber, A.; Głowicka-Wołoszyn, R. Susceptibility of sweet corn varieties to Ustilago maydis (DC). Fragm. Agron. 2016, 33, 76–86. [Google Scholar]
- Hudon, M.; LeRoux, E.J.; Harcourt, D.G. Seventy years of European corn borer (Ostrinia nubilalis) research in North America. Agric Zool Rev. 1989, 3, 53–96. [Google Scholar]
- Capinera, J.L. European corn borer Ostrinia nubilalis (Hübner) (Insecta: Lepidoptera: Crambidae) Distribution—Life Cycle and Description—Host Plants—Natural Enemies—Weather—Damage—Management; Publication Number EENY-156. 2000. Available online: https://edis.ifas.ufl.edu/publication/IN313 (accessed on 15 June 2024).
- Nault, B.A.; Kennedy, G.G. Timing insecticide applications for managing European corn borer (Lepidoptera: Pyralidae) infestations in potato. Crop Prot. 1996, 15, 465–471. [Google Scholar] [CrossRef]
- Toninato, A.; Burkness, E.C.; Hutchison, W.D. Insecticidal Control of Corn Earworm in Minnesota Sweet Corn. Arthropod Manag. Tests 2021, 46, 1–2. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Ye, S.; Ding, Y.; Guo, S.; Ningning, F. Inhibitory action of ginkgolic acid against pathogenic fungi and characterisation of its inhibitory activities on Nigrospora oryzae. Folia Hortic. 2023, 35, 49–59. [Google Scholar] [CrossRef]
- Rice, M.E.; Pilcher, C.D. Potential benefits and limitations of transgenic Bt corn for management of the European corn borer. Am. Entomol. 1998, 44, 75–78. [Google Scholar] [CrossRef]
- Tuhy, Ł.; Chowańska, J.; Chojnacka, K. Seaweed extracts as biostimulants of plant growth: Review. Chemik 2013, 67, 636–641. [Google Scholar]
- Machado, L.P.; de Godoy, M.C.; Gasparoto; Alves, N.; Filho, S.; Pavarini, R. Seaweeds in the Control of Plant Diseases and Insects. In Seaweeds as Plant Fertilizer, Agricultural Biostimulants and Animal Fodder; CRC Press: Boca Raton, FL, USA, 2019; pp. 98–124. ISBN 9780429487156. [Google Scholar] [CrossRef]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.V.; Willett, D.S. The Effects of Biostimulants on Induced Plant Defense. Front. Agron. 2021, 3, 630596. [Google Scholar] [CrossRef]
- Martínez-Gutiérrez, A.; Zamudio-González, B.; Tadeo-Robledo, M.; Espinosa-Calderón, A.; Cardoso-Galvão, J.C.; Vázquez-Carrillo, M.G. Yield of corn hybrids in response to foliar fertilization with biostimulants. Rev. Mex. Cienc. Agríc 2022, 13, 2. [Google Scholar] [CrossRef]
- Aziz, A.; Poinssot, B.; Daire, X.; Adrian, M.; Bézier, A.; Lambert, B.; Joubert, J.-M.; Pugin, A. Laminarin elicits defense responses in grapevine and induces protection against Botrytis cinerea and Plasmopara viticola. Mol. Plant Microbe Interact. 2003, 16, 1118–1128. [Google Scholar] [CrossRef]
- Chandía, N.P.; Matsuhiro, B. Characterization of a fucoidan from Lessonia vadosa (Phaeophyta) and its anticoagulant and elicitor properties. Int. J. Biol. Macromol. 2008, 1, 235–240. [Google Scholar] [CrossRef]
- Yu, K.-X.; Jantan, I.; Ahmad, R.; Wong, C.-L. The major bioactive components of seaweeds and their mosquitocidal potential. Parasitol Res 2014, 113, 3121–3141. [Google Scholar] [CrossRef]
- Ishii, T.; Nagamine, T.; Nguyen, B.C.Q.; Tawata, S. Insecticidal and Repellent Activities of Laurinterol from the Okinawan Red Alga Laurencia nidifica. Rec. Nat. Prod. 2017, 11, 63–68. [Google Scholar]
- El-Naggar, A.H.; Osman, M.E.H.; El-Sheekh, M.; Gheda, S.F. Influence of the aqueous extracts of Ulva lactuca and Chlorella kessleri on growth and yield of Vicia faba. Algol. Stud. 2005, 116, 213–229. [Google Scholar] [CrossRef]
- Salah El Din, R.; Elbakry, A.; Ghazi, S.; Abdel Hamid, O. Effect of seaweed extract on the growth and yield of faba bean (Vicia faba L.). Egypt. J. Phycol. 2008, 9, 25–38. [Google Scholar] [CrossRef]
- Zodape, S.; Kawarkhe, V.; Patolia, J.; Warade, A. Effect of liquid seaweed fertilizer on yield and quality of okra (Abelmoschus esculentus L.). J. Sci. Ind. Res 2009, 67, 1115–1117. [Google Scholar]
- Zodape, S.T.; Mukhopadhyay, S.; Eswaran, K.; Reddy, M.P.; Chikara, J. Enhanced yield and nutritional quality in green gram (Phaseolus radiata L) treated with seaweed (Kappaphycus alvarezii) extract. J. Sci. Ind. Res. 2010, 69, 468–471. [Google Scholar]
- Kavipriya, R.; Dhanalakshmi, P.K.; Jayashree, S.; Thangaraju, N. Seaweed extract as a biostimulant for legume crop, green gram. J. Ecobiotech. 2011, 3, 16–19. [Google Scholar]
- Zodape, S.T.; Gupta, A.; Bhandari, S.C.; Rawat, U.S.; Chaudhary, D.R.; Eswaran, K.; Chikara, J. Foliar application of seaweed sap as biostimulant for enhancement of yield and quality of tomato (Lycopersicon esculentum Mill). J. Sci. Ind. Res 2011, 70, 215–219. [Google Scholar]
- Baloch, G.N.; Tariq, S.; Ehteshamul-Haque, S.; Athar, M.; Sultana, V.; Ara, J. Management of root diseases of eggplant and watermelon with the application of asafoetida and seaweeds. J Appl Bot Food 2013, 86, 138–142. [Google Scholar] [CrossRef]
- González-Castro, A.; Muñoz-Ochoa, M.; Hernandez-Carmona, G.; López-Vivas, J. Evaluation of seaweed extracts for the control of the Asian citrus psyllid Diaphorina citri. J. Appl. Phycol. 2019, 31, 3815–3821. [Google Scholar] [CrossRef]
- Fauteux, F.; Rémus-Borel, W.; Menzies, J.G.; Bélanger, R.R. Silicon and plant disease resistance against pathogenic fungi. FEMS Microbiol. Lett. 2005, 249, 1–6. [Google Scholar] [CrossRef]
- Ali, J.G.; Agrawal, A.A. Specialist versus generalist insect herbivores and plant defense. Trends Plant Sci. 2012, 17, 293–302. [Google Scholar] [CrossRef]
- Alhousari, F.; Greger, M. Silicon and Mechanisms of Plant Resistance to Insect Pests. Plants 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lei, W.; Wen, L.; Hou, M. Silicon-mediated resistance in a susceptible rice variety to the rice leaf folder, Cnaphalocrocis medinalis Guenée (Lepidoptera: Pyralidae). PLoS ONE 2015, 10, e0120557. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Han, Y.Q.; Li, P.; Li, F.; Ali, S.; Hou, M.L. Silicon amendment is involved in the induction of plant defense responses to a phloem feeder. Sci. Rep. 2017, 7, 4232. [Google Scholar]
- Liang, Y.; Nikolic, M.; Belanger, R.; Gong, H.; Song, A. Silicon and Insect Pest Resistance. In Silicon in Agriculture; Springer: Dordrecht, The Netherlands, 2015; pp. 197–207. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources; FAO: Rome, Italy, 2014. [Google Scholar]
- Nowosielski, O. Principles for Developing Fertilizer Recommendations in Horticulture; PWRiL: Warszawa, Poland, 1978; pp. 250–280. (In Polish) [Google Scholar]
- Waligóra, H.; Majchrzak, L. Yield of sweet corn cultivars under conditions in Wielkopolska, Poland. Fragm. Agron. 2019, 36, 8–14. [Google Scholar]
- Xu, X.; Lei, X.; Liao, S.; Li, Y.; Sun, Y. Foliar application of potassium silicate, potassium fulvate and betaine improve summer-time tomato yield by promoting plant nitrogen and potassium uptake. Folia Hort. 2022, 34, 125–138. [Google Scholar] [CrossRef]
- Skwarek, M.; Pipiak, P. Maize pathogenic diseases and the role of biostimulators in their control. Technol. I Jakość Wyr. 2020, 65, 129–143. [Google Scholar]
- Villaver, J.P. Physiological and yield parameters of sweet corn (Zea mays L. var. rogusa) in response to biofertilizer application under low elevation conditio. Int. J. Agric. Techno 2022, 18, 2307–2314. [Google Scholar]
- Subaedah, S.; Edy, E.; Numba, S.; Sabahannur, S.; Fausiah, R. Effectiveness of arbuscular mycorrhizal fungi and NPK fertilizer in increasing the production of sweet corn. Plant Asian J. Plant Sci. 2023, 21, 685–692. [Google Scholar] [CrossRef]
- Pangaribuan, D.H.; Hendarto, K.; Elzhivago, S.R.; Yulistiani, A. The effect of organic fertilizer and urea fertilizer on growth, yield and quality of sweet corn and soil health. Asian J. Agri. Biol. 2018, 6, 335–344. [Google Scholar]
- Mohammed, A.A.; Majid, Z.M.; Kasnazany, S.A.S.; Rashis, Z.O.; Hama, B.M. Evaluation of growth, yield and some quality characteristics of sweet corn under effect of different nutrient sources. J. Zankoy Sulaimani 2018, 561–568. [Google Scholar] [CrossRef]
- Fahrurrozi, F.; Muktamar, Z.; Dwatmadji, D.; Setyowati, N.; Sudjatmiko, S.; Chozin, M. Growth and yield responses of three sweet corn (Zea mays L. var. saccharata) varieties to local-based liquid organic fertilizer. Int. J. Adv. Sci. Eng. Inf. Techno 2016, 6, 318–323. [Google Scholar] [CrossRef]
- Intansari, R.S.I. The effectiveness of organic fertilizer granules for increasing sweet corn production on acid dryland in Bogor district. J. Soilsc. Agric 2022, 1, 40–52. [Google Scholar] [CrossRef]
- Canatoy, R.C. Growth and yield response of sweet corn (Zea mays L. var. saccharata) as affected by tillage operations and fertilizer applications. Int. J. Educ. 2018, 6, 265–276. [Google Scholar]
- Frommer, D.; Veres, S.; Radócz, L. Susceptibility of stem infected sweet corn hybrids to common smut disease. Acta Agrar. Debrec. 2018, 7, 55–57. [Google Scholar] [CrossRef]
- Szőke, L.; Moloi, M.J.; Kovács, G.E.; Biró, G.; Radócz, L.; Hájos, M.T.; Kovács, B.; Rácz, D.; Danter, M.; Toth, B. The Application of Phytohormones as Biostimulants in Corn Smut Infected Hungarian Sweet and Fodder Corn Hybrids. Plants 2021, 10, 1822. [Google Scholar] [CrossRef]
- Shao, M.; Chen, Y.; Gong, Q.; Miao, S.; Li, C.; Sun, Y.; Qin, D.; Guo, X.; Yan, X.; Cheng, P.; et al. Biocontrol endophytes Bacillus subtilis R31 influence the quality, transcriptome and matabolome of sweet corn. PeerJ 2023, 11, e14967. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Kasimatis, C.N.; Kakabouki, I.; Leonidakis, D.; Danalatos, N.; et al. Assessment of plant growth promoting bacteria strains on growth, yield and quality of sweet corn. Sci. Rep. 2022, 12, 11598. [Google Scholar] [CrossRef] [PubMed]
- Pięta, D.; Patkowska, E.; Pastucha, A. The protective effect of biopreparations applied as the dressing for common bean (Phaseolus vulgaris L.) and pea (Pisum sativum L.). Acta Sci. Pol. Hort. Cult. 2005, 4, 59–67. [Google Scholar]
- Warner, G. Seaweeds tested for pest control. Insects and mites. Good Fruit Grow 2012, 1, 1–5. [Google Scholar]
- Bianco, E.; Pires, L.; Santos, G.; Dutra, K.; Reis, T.; Vasconcelos, E.; Cocentino, A.; Navarro, D. Larvicidal activity of seaweeds from northeastern Brazil and of a halogenated sesquiterpene against the dengue mosquito (Aedes aegypti). Ind. Crop. Prod. 2013, 43, 270–275. [Google Scholar] [CrossRef]
- Cetin, H.; Gokoglu, M.; Oz, E. Larvicidal Activity of the Extract of Seaweed, Caulerpa scalpelliformis, against Culex pipiens. J. Am. Mosq. Control Assoc. 2010, 26, 433–435. [Google Scholar] [CrossRef] [PubMed]
- Asharaja, A.; Sahayaraj, K. Screening of insecticidal activity of brown macroalgal extracts against Dysdercus cingulatus (Fab.) (Hemiptera: Pyrrhocoridae). J. Biopest. 2013, 6, 193–203. [Google Scholar] [CrossRef]
- Thawfeeq, A.; Srinivasan, G.; Shanthi, M.; Mini, M.L. Insecticidal activity of brown and red seaweed extracts against cowpea bean aphid, Aphis craccivora Koch. J. Pharm Innov. 2022, 11, 4223–4225. [Google Scholar]
- Thawfeeq, A.J.; Shanthi, M.; Mini, M.L.; Srinivasan, G. Multifaceted effects of seaweed extracts against cowpea aphid, Aphis craccivora Koch, by evaluating four macroalgae. J. Appl Phycol. 2023, 35, 1–10. [Google Scholar]
- Rizvi, M.A.; Shameela, M. Biological activity and Elementology of Benthic Algae from Karachi coast. Pak. J. Bot. 2003, 35, 717–729. [Google Scholar]
- Argadoña, V.; Del Pozo, T.; San-Martín, A.; Rovirosa, J. Insecticidal activity of Plocamium cartilagineum monoterpenes. J. Chil. Chem. Soc. 2000, 45, 371–376. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).