Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review
Abstract
1. Introduction
2. Essential Oils
3. Essential Oils for Pest Management
3.1. Essential Oils as Botanical-Sourced Pesticides
| Order | Target | EO Botanical Family | EO | Plant Part | Application Type | Reference |
|---|---|---|---|---|---|---|
| Acari | Tetranychus cinnabarinus (carmine spider mite) | Lamiaceae | Lavandula stoechas (lavender) | Leaves and stems | Fumigant | [72] |
| Lamiaceae | Mentha spicata (spearmint) | Leaves and stems | Fumigant | [72] | ||
| Lamiaceae | Origanum onites (oregano) | Leaves and stems | Fumigant | [72] | ||
| Lamiaceae | Thymbra spicata (thyme) | Leaves and stems | Fumigant | [72] | ||
| Zingiberaceae | Curcuma longa (turmeric) | Rhizome | Spray | [77] | ||
| Acari | Tetranychus turkestani (strawberry spider mite) | Lamiaceae | Satureja hortensis (summer savory) | Leaves | Fumigant | [71] |
| Lamiaceae | Zataria multiflora | Leaves | Fumigant | [71] | ||
| Acari | Tetranychus urticae (two-spotted spider mite) | Asteraceae | Chamomilla recutita (chamomile) | Whole plant | Spray on host | [67] |
| Lamiaceae | Majorana hortensis (marjoram) | Whole plant | Spray on host | [67] | ||
| Lamiaceae | Rosmarinus officinalis (rosemary) | Aerial | Contact | [68] | ||
| Lamiaceae | Salvia officinalis (sage) | Aerial | Contact | [68] | ||
| Lamiaceae | Lavandula angustifolia (lavender) | Leaves | Fumigant | [69] | ||
| Lamiaceae | Salvia fruticose (Greek sage) | Leaves | Fumigant | [69] | ||
| Lamiaceae | Mentha spicata (spearmint) | Commercial, NS | Fumigant | [78] | ||
| Lamiaceae | Ocimum basilicum (basil) | Commercial, NS | Fumigant | [78] | ||
| Myrtaceae | Callistemon viminalis (callistemo) | Leaves and twigs | Contact | [79] | ||
| Myrtaceae | Eucalyptus bicostata (Victorian blue gum) | Leaves | Contact | [79] | ||
| Myrtaceae | Eucalyptus maidenii (Maiden’s gum) | Leaves | Contact | [79] | ||
| Myrtaceae | Eucalyptus sideroxylm (red ironbark) | Leaves | Contact | [79] | ||
| Myrtaceae | Eucalyptus approximans (Barren Mountain mallee) | Leaves | Contact | [79] | ||
| Verbenaceae | Lippia origanoides (Colombian oregano) | Leaves | Fumigant | [80] | ||
| Verbenaceae | Lippia sidoides (oregano) | Leaves | Fumigant | [81] | ||
| Coleoptera | Callosobruchus chinensis (adzuki bean weevil) | Apiaceae | Coriandum sativum (coriander) | Industrial, NS | Fumigant/contact | [82] |
| Myrtaceae | Eucalyptus obliqua (eucalyptus) | Industrial, NS | Fumigant/contact | [82] | ||
| Pinaceae | Pinus langifolia (pine) | Industrial, NS | Fumigant/contact | [82] | ||
| Coleoptera | Callosobruchus maculatus (cowpea weevil) | Lamiaceae | Rosmarinus officinalis (rosemary) | Aerial | Fumigant | [83] |
| Lamiaceae | Mentha piperita (peppermint) | Aerial | Fumigant | [83] | ||
| Rutaceae | Citrus sinensis (sweet orange) | Peel | Fumigant | [84] | ||
| Coleoptera | Lasioderma serricone (cigarette beetle) | Cruciferae | Cocholeria armoracia (horseradish) | Commercial, NS | Fumigant | [85] |
| Lauraceae | Cinnamomum cassia (cinnamon) | Commercial, NS | Contact | [85] | ||
| Coleoptera | Sitophilus granarius (granary weevil) | Lauraceae | Cinnamomum zeylanicum (cinammon) | Commercial, NS | Contact | [54] |
| Myrtaceae | Syzygium aromaticum (clove) | Commercial, NS | Contact | [54] | ||
| Coleoptera | Ulomoides dermestoides (peanut beetle) | Poaceae | Cymbopogon citratus (lemongrass) | Commercial, NS | Contact | [86] |
| Diptera | Ceratitis capitata (Mediterranean fruit fly | Lamiaceae | Rosmarinus officinalis (rosemary) | Leaves | Fumigant/Topical | [87] |
| Lamiaceae | Lavandula angustifolia (lavender) | Leaves | Fumigant/Topical | [87] | ||
| Diptera | Drosophila melanogaster (fruit fly) | Lamiaceae | Mentha pulegium (pennyroyal) | NS | Contact | [31] |
| Lamiaceae | Mentha spicata (spearmint) | NS | Contact | [31] | ||
| Diptera | Drosophila suzukii (spotted wing drosophila) | Lamiaceae | Mentha piperita (peppermint) | Aerial flowering | Fumigant | [88] |
| Lamiaceae | Perilla frutescens (perilla) | Leaves | Fumigant | [88] | ||
| Hemiptera | Aphis forbesi (strawberry aphid) | Fabaceae | Tephrosia vogelii (Vogel’s tephrosia) | Flowers | Spray | [89] |
| Hemiptera | Bemisia tabaci (silverleaf whitefly) | Amaryllidaceae | Allium sativum | NS | Fumigant/contact | [90] |
| Lamiaceae | Micromeria fruticosa (white savory) | Aerial | Fumigant | [91] | ||
| Lamiaceae | Nepeta racemose (dwarf catnip) | Aerial | Fumigant | [91] | ||
| Lamiaceae | Origanum vulgare (oregano) | Aerial | Fumigant | [91] | ||
| Lamiaceae | Thymus vulgaris (thyme) | Leaves | Contact | [92] | ||
| Rutaceae | Citrus aurantium (bitter orange) | Peel | Fumigant | [93] | ||
| Hemiptera | Hyadaphis foeniculi (fly honeysuckle aphid, fennel aphid) | Lamiaceae | Hyptis suaveolens (alfazema) | NS | Topical | [66] |
| Hemiptera | Myzus persicae (green peach aphid) | Apiaceae | Cuminum cyminum (cumin) | Schizocarp | Spray on host | [94] |
| Asteraceae | Santolina chamaecyparissus (cotton lavender) | Aerial | Spray | [95] | ||
| Asteraceae | Achillea millefolium (yarrow) | Aerial | Spray | [96] | ||
| Cannabaceae | Cannabis sativa (hemp) | Inflorescences | Spray on host | [96] | ||
| Lamiaceae | Mentha pulegium (pennyroyal) | Aerial | Fumigant | [97] | ||
| Lamiaceae | Mentha pulegium (pennyroyal) | Aerial | Spray | [98] | ||
| Lamiaceae | Origanum majorana (marjoram) | Aerial | Spray | [98] | ||
| Lamiaceae | Melissa officinalis (lemon balm) | Aerial | Spray | [98] | ||
| Hemiptera | Rhopolasiphum maidis (corn leaf aphid) | Myrtaceae | Syzygium aromaticum (clove) | Buds | Fumigant | [99] |
| Hemiptera | Trialeurodes vaporariorum (greenhouse whitefly) | Asteraceae | Eupatorium buniifolium (chilca) | NS | Spray on host | [100] |
| Hemiptera | Trialeurodes vaporariorum (greenhouse whitefly) | Atherospermataceae | Laurelia sempervirens (Chilean laurel) | Leaves | Fumigant | [101] |
| Hymenoptera | Acromyrmex balzani (leaf-cutter ant) | Lamiaceae | Eplingiella fruticosa | Leaves | Fumigant | [102] |
| Myrtaceae | Myrcia lundiana | Leaves | Fumigant | [103] | ||
| Lepidoptera | Mediterranean flour moth | Lamiaceae | Origanum onites L. (oregano) | Leaves | Fumigant | [104] |
| Lamiaceae | Satureja thymbra L. (savory) | Leaves | Fumigant | [104] | ||
| Lepidoptera | Indian meal moth | Lamiaceae | Origanum onites L. (oregano) | Leaves | Fumigant | [104] |
| Lamiaceae | Satureja thymbra L. (savory) | Leaves | Fumigant | [104] | ||
| Lepidoptera | Spodoptera littoralis (cotton leafworm) | Lamiaceae | Thymus algeriensis (Thyme) | Aerial | Fumigant | [35] |
| Apiaceae | Pimpinella anisum (anise) | Schizocarp | Topical | [94] | ||
| Apiaceae | Crithmum maritimum (sea fennel) | Seeds/aereal | Topical | [105] | ||
| Euphorbiaceae | Ricinus communis (castor bean) | Commercia, NS | Fumigant | [106] | ||
| Lamiaceae | Ocimun gratissimum (white wild basil) | Aerial | Topical | [107] | ||
| Orthoptera | Schistocerca gregaria (desert locust) | Amaryllidaceae | Allium cepa (onion) | Leaves | Topical | [108] |
| Apiaceae | Petroselinum sativum (parsley) | Seeds | Topical | [108] | ||
| Geraniaceae | Pelargonium radula (geranium) | Whole plant | Topical | [108] | ||
| Amaryllidaceae | Allium sativum (garlic) | Commercial, NS | Spray | [109] |
3.2. Mechanism of Action of Essential Oils as Insecticides
3.2.1. Inhibition of Enzyme Acetylcholinesterase (AChE)
3.2.2. Modification of GABA Receptors
3.2.3. Interference on Octopamine Receptors
3.3. Repellency and Sublethal Activities
3.3.1. Repellency
3.3.2. Antifeedant
3.3.3. Effects on Reproductive Conduct
3.4. Non-Target Safety
4. Encapsulation of EOs
4.1. Materials and Techniques for the Encapsulation of EOs
4.1.1. Emulsification
4.1.2. Ionic Gelation
4.1.3. Complex Coacervation
| Material | Encapsulated EO | Method | Particle Type | Part. Size | EE | Application | Observations | References |
|---|---|---|---|---|---|---|---|---|
| Biopolymers | ||||||||
| Chitosan | Citronella (Cymbopogon spp.) | Ionic gelation | Microcapsules | 11–225 µm | 94.7–98.2% | − | Sustained release | [202] |
| Satureja hortensis | Ionic gelation | Microcapsules | 192 nm | 96.17% | Acaricidal | Sustained release Prolonged bioactivity | [204] | |
| Citrus oils | Ionic gelation | Microcapsules | 289.3–8843.2 nm | 61.9–68.1% | − | Good release rates. Particle size and EE dependent on the surfactant. | [191] | |
| Coriandrum sativum | Ionic gelation | Nanocapsules | 50–80 nm | 26.5–75.99% | Storage product preservation | Controlled release Enhanced bioactivity | [222] | |
| Eugenia caryophyllata | Ionic gelation | Nanocapsules | 40–100 nm | 31–45.77% | Antifungal | Controlled release Higher bioactivity | [223] | |
| Cinnamomum zeylanicum | Ionic gelation | Microcapsules | 100–200 nm | 88.6% | Antimicrobial | Prolonged stability Improved antimicrobial capacity | [224] | |
| Mentha piperita | Ionic gelation | Microcapsules | ≤563.3 nm | 64–70% | Stored food pest control | Improved AChEI and fumigant toxicity | [225] | |
| Piper nigrum | Ionic gelation | Microcapsules | Av. 527.5 nm | 35–40% | Stored food pest control | Improved AChEI and insecticidal activity | [226] | |
| Eryngium campestre | Ionic gelation | Microcapsules | Av. 157.8 nm | 61–75% | Storage product preservation | Improved bioactivity | [227] | |
| Chitosan-Caffeic acid | Cuminum cyminum | EO in nanogel | Nanogel | ≤100 nm | 85% | Antifungal | Improved antifungal performance by sustained release | [228] |
| Chitosan–cashew gum | Lippia siodes | Complex coacervation | Nanogel | 335–558 nm | 70% | Insecticidal | Controlled release Improvement in bioactivity | [217] |
| Chitosan-Cinnamic acid | Mentha piperita | EO in nanogel | Nanogel | ≤100 nm | − | Antifungal | Improved antifungal performance by sustained release | [229] |
| Bunium persicum | EO in nanogel | Nanogel | ≤100 nm | 41–43% | Antifungal and antioxidant | Enhanced antifungal and antioxidant activities | [230] | |
| β-cyclodextrin | Commercial thyme oil (Thymus spp.) | Complexation (kneading) | Complex | 582.9 nm | 82.55 g/100 g | Antimicrobial | Enhanced antimicrobial activity | [231] |
| Complexation (freeze drying) | 3226.7 nm | 71.27 g/100 g | ||||||
| Lippia berlanderi | Complexation | Complex | − | 71% | Antimicrobial | High entrapment Increased stability | [232] | |
| Eugenia caryophyllata | − | 61% | ||||||
| β-cyclodextrin (HP-β-CD) | Piper nigrum | Complexation (inclusion complex formation) | Complex | − | 50.55% | Antioxidant | Increased stability Higher antioxidant activity | [233] |
| β- & γ-cyclodextrin | Lippia graveolens (thymol and carvacrol chemotypes) | Complexation | Complex | 6–13.22 µm | 7.4–63.4% | − | Sustained release | [234] |
| Cornstarch | Thymus vulgaris | Thermoplastic extrusion | Particles | 0.01–0.5 cm | 69.07% | Repellent | Prolonged residual effect. Improved bioactivity. | [235] |
| Zein | Syzygium aromaticum | Antisolvent precipitation | Nanoparticles | 125 nm | 91.4% | Insecticide | Low toxicity to a non-target organism, Caenorhaabditis. elegans Potentialized the bioinsectide activity against Drosophila melanogaster | [236] |
| Gelatin-gum Arabic (Glutaraldehyde as wall hardener) | Camphor oil with added oil-soluble polystyrene | Complex coacervation | Particles | 85.7–299.7 µm | 99.6% | − | Sustained release | [211] |
| Gelatin-chia mucilage | Oregano oil (Lippia spp.) | Complex coacervation/spray dry | Microcapsules | 1.65–8.85 µm | 82.15–95.6% | − | Enhanced bioactivity Particle size-dependent EE | [209] |
| Gelatin-Persian gum, Persian gum, and gum Arabic | Satureja hortensis | Complex coacervation | Nanocapsules | 81–208 nm | 72.1–92.8% | Herbicidal | Improvement in herbicidal activity on encapsulated oil | [161] |
| Gelatin-sodium alginate | Cymbopogon winterianus | Complex coacervation | Complex | 434.06 µm | 83.5% | − | Controlled release Usage of waste product gelatin | [215] |
| Piper nigrum | Complex coacervation | Complex | − | 49.13–82.36% | − | Good retention capacity and preservation of composition | [210] | |
| Maltodextrin-gum Arabic | Schinus molle | Spray drying | Microparticles | 0.2–40 µm | 96–100% | Insecticidal | Slow release, over 366 h Prolonged insecticidal effect | [237] |
| Maltodextrin-casein | Thymus vulgaris | Spray drying | Microparticles | Av. 0.87 µm | 88.9% | Food preservation | Thermal stability | [238] |
| Sodium alginate (crosslinker CaCl2) | Satureja hortensis | Ionic gelation | Microparticles | 47–117 µm | 52–66% | Antibacterial Antioxidant | Controlled release Improved bioactivity | [207] |
| Whey protein- mesquite gum | Salvia hispanica | Spray drying | Microcapsules | 2.54–3.35 µm | 71.4–80.7% | − | Good encapsulation efficiency | [239] |
| Whey protein- gum Arabic | 2.33–3.09 µm | 70.7–79.8% | ||||||
| Emulsions | ||||||||
| o/w Tween 80 as surfactant | Eucalyptus spp. | Sonication | Droplets | Av. 3.8 nm | − | Antimicrobial (topical) | Improvement in wound-healing effectivity | [240] |
| o/w Tween 20/Triton x−100 as surfactant | Azadiractha indica- Cymbopogon nardus | Constant stir | Droplets | 2.8–17.8 nm | − | Antifungal | Stability of components Potent antifungal activity | [64] |
| Isopropanol Tween 80 as surfactant | Curcuma longa | Sonication | Microemulsion | − | − | Acaricidal | Controlled release Improved acaricidal activity | [77] |
| o/w/o Palm oil Sunflower oil Soy lecithin as surfactant | Terpene mixture | High-pressure homogenization | Droplets | 75–175 nm | − | Antimicrobial | Enhanced activity | [196] |
| o/w/ polysorbate | Crithmum maritimum | Emulsion phase inversion | Droplets | 50–70 nm | − | Agriculture and domestic pest control | Increased anti-ovipository and toxic activity | [241] |
| Octenyl succinic anhydride (OSA)—starch | Rosmarinus officinalis Zataria multiflora | Spray drying | Microcapsules | 461–854 nm | − | Insecticidal | More effective than non-formulated in a long time | [242] |
| Chitosan-cellulose nanofibers | Citronella essential oil | Ultrasonication | Nanoparticles | 426.9 nm | 90.8% | Insecticidal | Nano-systems increased the insecticidal activity | [243] |
| Nanocarriers | ||||||||
| SiO2 | Crithmum maritimum | Loading of hollow capsule | Nanocapsules | 20–78 nm | − | Agriculture and domestic pest control | Increased anti-ovipository and toxic activity | [241] |
| Zein sta-bilized with Tween 80 | Eucalyptus staigeriana Litsea cubeba | Ultrasound-assisted nano-precipitation | Nanoparticles | 200 nm | − | Antifungal | Activity against Colletotrichum lindemuthianum | [244] |
4.2. Prospects for Application in Agriculture
4.2.1. Chitosan
4.2.2. Gelatin-Gum Arabic
4.2.3. Cyclodextrins
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Buchbauer, G.; Jirovetz, L.; Jäger, W. Aromatherapy: Evidence for Sedative Effects of the Essential Oil of Lavender after Inhalation. Z. Für. Naturforschung C 1991, 46, 1067–1072. [Google Scholar] [CrossRef] [PubMed]
- Woronuk, G.; Demissie, Z.; Rheault, M.; Mahmoud, S. Biosynthesis and Therapeutic Properties of Lavandula Essential Oil Constituents. Planta Med. 2011, 77, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.; Al-Wabel, N.A.; Shams, S.; Ahamad, A.; Khan, S.A.; Anwar, F. Essential Oils Used in Aromatherapy: A Systemic Review. Asian Pac. J. Trop. Biomed. 2015, 5, 601–611. [Google Scholar] [CrossRef]
- Plant, R.M.; Dinh, L.; Argo, S.; Shah, M. The Essentials of Essential Oils. Adv. Pediatr. 2019, 66, 111–122. [Google Scholar] [CrossRef]
- Yuan, R.; Zhang, D.; Yang, J.; Wu, Z.; Luo, C.; Han, L.; Yang, F.; Lin, J.; Yang, M. Review of Aromatherapy Essential Oils and Their Mechanism of Action against Migraines. J. Ethnopharmacol. 2021, 265, 113326. [Google Scholar] [CrossRef] [PubMed]
- Burfield, T.; Reekie, S. Mosquitoes, Malaria and Essential Oils. Int. J. Aromather. 2005, 15, 30–41. [Google Scholar] [CrossRef]
- Tisgratog, R.; Sanguanpong, U.; Grieco, J.P.; Ngoen-Kluan, R.; Chareonviriyaphap, T. Plants Traditionally Used as Mosquito Repellents and the Implication for Their Use in Vector Control. Acta Trop. 2016, 157, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Gallucci, S.; Neto, A.P.; Porto, C.; Barbizan, D.; Costa, I.; Marques, K.; Benevides, P.; Figueiredo, R. Essential Oil of Eugenia uniflora L.: An Industrial Perfumery Approach. J. Essent. Oil Res. 2010, 22, 176–179. [Google Scholar] [CrossRef]
- Smith, R.L.; Cohen, S.M.; Doull, J.; Feron, V.J.; Goodman, J.I.; Marnett, L.J.; Portoghese, P.S.; Waddell, W.J.; Wagner, B.M.; Hall, R.L.; et al. A Procedure for the Safety Evaluation of Natural Flavor Complexes Used as Ingredients in Food: Essential Oils. Food Chem. Toxicol. 2005, 43, 345–363. [Google Scholar] [CrossRef] [PubMed]
- Khorshidian, N.; Yousefi, M.; Khanniri, E.; Mortazavian, A.M. Potential Application of Essential Oils as Antimicrobial Preservatives in Cheese. Innov. Food Sci. Emerg. Technol. 2018, 45, 62–72. [Google Scholar] [CrossRef]
- Philippe, S.; Souaïbou, F.; Paulin, A.; Issaka, Y.; Dominique, S. In Vitro Antifungal Activities of Essential Oils Extracted from Fresh Leaves of Cinnamomum zeylanicum and Ocimum gratissimum against Foodborne Pathogens for Their Use as Traditional Gheese Wagashi Conservatives. Res. J. Recent Sci. 2012, 1, 67–73. [Google Scholar]
- Vergis, J.; Gokulakrishnan, P.; Agarwal, R.K.; Kumar, A. Essential Oils as Natural Food Antimicrobial Agents: A Review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1320–1323. [Google Scholar] [CrossRef]
- Bajpai, V.K.; Baek, K.-H.; Kang, S.C. Control of Salmonella in Foods by Using Essential Oils: A Review. Food Res. Int. 2012, 45, 722–734. [Google Scholar] [CrossRef]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Melo Coutinho, H.D.; et al. Combination of Essential Oils in Dairy Products: A Review of Their Functions and Potential Benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Abdel-Maksoud, G.; El-Amin, A.R. A Review on the Materials Used during the Mummification Processes in Ancient Egypt. Mediterr. Archaeol. Ar. 2011, 11, 129–150. [Google Scholar]
- Barnes, K.M.; Whiffin, A.L.; Bulling, M.T. A Preliminary Study on the Antibacterial Activity and Insect Repellent Properties of Embalming Fluids from the 18th Dynasty (1550–1292 BCE) in Ancient Egypt. J. Archaeol. Sci. Rep. 2019, 25, 600–609. [Google Scholar] [CrossRef]
- Łucejko, J.; Connan, J.; Orsini, S.; Ribechini, E.; Modugno, F. Chemical Analyses of Egyptian Mummification Balms and Organic Residues from Storage Jars Dated from the Old Kingdom to the Copto-Byzantine Period. J. Archaeol. Sci. 2017, 85, 1–12. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and Therapeutic Potentials of Essential Oils and Their Individual Volatile Constituents: A Review. Phytother. Res. 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Bajalan, I.; Rouzbahani, R.; Pirbalouti, A.G.; Maggi, F. Antioxidant and Antibacterial Activities of the Essential Oils Obtained from Seven Iranian Populations of Rosmarinus officinalis. Ind. Crops Prod. 2017, 107, 305–311. [Google Scholar] [CrossRef]
- Kumar, V.; Mathela, C.S.; Kumar, M.; Tewari, G. Antioxidant Potential of Essential Oils from Some Himalayan Asteraceae and Lamiaceae species. Med. Drug Discov. 2019, 1, 100004. [Google Scholar] [CrossRef]
- Goudjil, M.B.; Zighmi, S.; Hamada, D.; Mahcene, Z.; Bencheikh, S.E.; Ladjel, S. Biological Activities of Essential Oils Extracted from Thymus capitatus (Lamiaceae). S. Afr. J. Bot. 2020, 128, 274–282. [Google Scholar] [CrossRef]
- Sharma, K.; Guleria, S.; Razdan, V.K.; Babu, V. Synergistic Antioxidant and Antimicrobial Activities of Essential Oils of Some Selected Medicinal Plants in Combination and with Synthetic Compounds. Ind. Crops Prod. 2020, 154, 112569. [Google Scholar] [CrossRef]
- Jahani, M.; Pira, M.; Aminifard, M.H. Antifungal Effects of Essential Oils against Aspergillus niger in Vitro and in Vivo on Pomegranate (Punica granatum) Fruits. Sci. Hortic. 2020, 264, 109188. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; Coppola, R.; Feo, V. De Essential Oils and Antifungal Activity. Pharmaceuticals 2017, 10, 86. [Google Scholar] [CrossRef]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal Effect of Peppermint Oil on the Enveloped Viruses Herpes simplex Virus Type 1 and Type 2 in Vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef]
- Sarmento-Neto, J.; Do Nascimento, L.; Felipe, C.; De Sousa, D. Analgesic Potential of Essential Oils. Molecules 2015, 21, 20. [Google Scholar] [CrossRef]
- Funk, J.L.; Frye, J.B.; Oyarzo, J.N.; Chen, J.; Zhang, H.; Timmermann, B.N. Anti-Inflammatory Effects of the Essential Oils of Ginger (Zingiber officinale Roscoe) in Experimental Rheumatoid Arthritis. PharmaNutrition 2016, 4, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liang, X.; Wang, B.; Lin, Z.; Ye, M.; Ma, R.; Zheng, M.; Xiang, H.; Xu, P. Six Herbs Essential Oils Suppressing Inflammatory Responses via Inhibiting COX-2/TNF-α/IL-6/NF-ΚB Activation. Microchem. J. 2020, 156, 104769. [Google Scholar] [CrossRef]
- Akhtar, Y.; Pages, E.; Stevens, A.; Bradbury, R.; da Camara, C.A.G.; Isman, M.B. Effect of Chemical Complexity of Essential Oils on Feeding Deterrence in Larvae of the Cabbage looper. Physiol. Entomol. 2012, 37, 81–91. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations, FAO. Concerted Action Needed to Stop Diseases and Pests from Ravaging the Food Chain. Available online: https://www.fao.org/newsroom/detail/Concerted-action-needed-to-stop-diseases-and-pests-from-ravaging-the-food-chain/es (accessed on 10 October 2020).
- Franzios, G.; Mirotsou, M.; Hatziapostolou, E.; Kral, J.; Scouras, Z.G.; Mavragani-Tsipidou, P. Insecticidal and Genotoxic Activities of Mint Essential Oils. J. Agric. Food Chem. 1997, 45, 2690–2694. [Google Scholar] [CrossRef]
- Štefanidesová, K.; Škultéty, Ľ.; Sparagano, O.A.E.; Špitalská, E. The Repellent Efficacy of Eleven Essential Oils against Adult Dermacentor reticulatus Ticks. Ticks Tick Borne. Dis. 2017, 8, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Katiki, L.M.; Barbieri, A.M.E.; Araujo, R.C.; Veríssimo, C.J.; Louvandini, H.; Ferreira, J.F.S. Synergistic Interaction of Ten Essential Oils against Haemonchus contortus in Vitro. Vet. Parasitol. 2017, 243, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R. Insecticidal Activity of Some Essential Oils against Larvae of Spodoptera littoralis. Fitoterapia 2005, 76, 691–696. [Google Scholar] [CrossRef] [PubMed]
- Ben El Hadj Ali, I.; Chaouachi, M.; Bahri, R.; Chaieb, I.; Boussaïd, M.; Harzallah-Skhiri, F. Chemical Composition and Antioxidant, Antibacterial, Allelopathic and Insecticidal Activities of Essential Oil of Thymus algeriensis Boiss. et Reut. Ind. Crops Prod. 2015, 77, 631–639. [Google Scholar] [CrossRef]
- Jafari, S.M. Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–34. [Google Scholar]
- Wu, C.-C.; Chung, J.G.; Tsai, S.-J.; Yang, J.H.; Sheen, L.Y. Differential Effects of Allyl Sulfides from Garlic Essential Oil on Cell Cycle Regulation in Human Liver Tumor Cells. Food Chem. Toxicol. 2004, 42, 1937–1947. [Google Scholar] [CrossRef] [PubMed]
- Pellizzeri, V.; Costa, R.; Grasso, E.; Dugo, G. Valuable Products from the Flowers of Lemon (Citrus limon (L.) Osbeck) and Grapefruit (Citrus paradisi Macfad.) Italian Trees. Food Bioprod. Process. 2020, 123, 123–133. [Google Scholar] [CrossRef]
- Ríos, J.-L. Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 3–10. [Google Scholar]
- dos Santos, C.P.; de Oliveira, T.C.; Pinto, J.A.O.; Fontes, S.S.; Cruz, E.M.O.; de Fátima Arrigoni-Blank, M.; Andrade, T.M.; de Matos, I.L.; Alves, P.B.; Innecco, R.; et al. Chemical Diversity and Influence of Plant Age on the Essential Oil from Lippia sidoides Cham. Germplasm. Ind. Crops Prod. 2015, 76, 416–421. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Upadhyay, S.; Bhuiyan, M.; Bhattacharya, P.R. A Review on Prospects of Essential Oils as Biopesticide in Insect-Pest Management. J. Pharmacogn. Phytother. 2009, 1, 52–063. [Google Scholar]
- Rehman, R.; Asif Hanif, M. Biosynthetic Factories of Essential Oils: The Aromatic Plants. Nat. Prod. Chem. Res. 2016, 4, 1000227. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods—A Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Dima, C.; Dima, S. Essential Oils in Foods: Extraction, Stabilization, and Toxicity. Curr. Opin. Food Sci. 2015, 5, 29–35. [Google Scholar] [CrossRef]
- Zhou, W.; Li, J.; Wang, X.; Liu, L.; Li, Y.; Song, R.; Zhang, M.; Li, X. Research Progress on Extraction, Separation, and Purification Methods of Plant Essential Oils. Separations 2023, 10, 596. [Google Scholar] [CrossRef]
- Khan, S.; Abdo, A.A.A.; Shu, Y.; Zhang, Z.; Liang, T. The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review. Foods 2023, 12, 4169. [Google Scholar] [CrossRef] [PubMed]
- Stratakos, A.C. Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2016; pp. 31–38. [Google Scholar]
- Souiy, Z. Essential Oil Extraction Process. In Book Essential Oils—Recent Advances, New Perspectives and Applications; Jonas, V., Ed.; IntechOpen: London, UK, 2024; Volume 1, Available online: https://www.intechopen.com/chapters/88433 (accessed on 22 September 2024).
- Kant, R.; Kumar, A. Review on essential oil extraction from aromatic and medicinal plants: Techniques, performance and economic analysis. Sustain. Chem. Pharm. 2022, 30, 100829. Available online: https://www.sciencedirect.com/science/article/pii/S2352554122002339?via%3Dihub (accessed on 22 September 2024). [CrossRef]
- Kim, S.-W.; Kang, J.; Park, I.-K. Fumigant Toxicity of Apiaceae Essential Oils and Their Constituents against Sitophilus oryzae and Their Acetylcholinesterase Inhibitory Activity. J. Asia Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Andrés, M.F.; Rossa, G.E.; Cassel, E.; Vargas, R.M.F.; Santana, O.; Díaz, C.E.; González-Coloma, A. Biocidal Effects of Piper hispidinervum (Piperaceae) Essential Oil and Synergism among Its Main Components. Food Chem. Toxicol. 2017, 109, 1086–1092. [Google Scholar] [CrossRef]
- Cao, J.-Q.; Guo, S.-S.; Wang, Y.; Pang, X.; Geng, Z.-F.; Du, S.-S. Toxicity and Repellency of Essential Oil from Evodia lenticellata Huang Fruits and Its Major Monoterpenes against Three Stored-Product Insects. Ecotoxicol. Environ. Saf. 2018, 160, 342–348. [Google Scholar] [CrossRef]
- Lerdau, M.; Litvak, M.; Monson, R. Plant Chemical Defense: Monoterpenes and the Growth-Differentiation Balance Hypothesis. Trends Ecol. Evol. 1994, 9, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid Constituents of Cinnamon and Clove Essential Oils Cause Toxic Effects and Behavior Repellency Response on Granary Weevil, Sitophilus Granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- Abdelgaleil, S.A.M.; Badawy, M.E.I.; Mahmoud, N.F.; Marei, A.E.-S.M. Acaricidal Activity, Biochemical Effects and Molecular Docking of Some Monoterpenes against Two-Spotted Spider Mite (Tetranychus urticae Koch). Pestic. Biochem. Physiol. 2019, 156, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.-T.; Feng, Y.-X.; Zhang, D.; Guo, S.-S.; Pang, X.; Geng, Z.-F.; Xi, C.; Du, S.-S. Comparative Evaluation of the Chemical Composition and Bioactivities of Essential Oils from Four Spice Plants (Lauraceae) against Stored-Product Insects. Ind. Crops Prod. 2019, 140, 111640. [Google Scholar] [CrossRef]
- de Andrade Brito, F.; Bacci, L.; da Silva Santana, A.; da Silva, J.E.; de Castro Nizio, D.A.; de Lima Nogueira, P.C.; de Fatima Arrigoni-Blank, M.; Melo, C.R.; de Melo, J.O.; Blank, A.F. Toxicity and Behavioral Alterations Caused by Essential Oils of Croton tetradenius and Their Major Compounds on Acromyrmex balzani. Crop Prot. 2020, 137, 105259. [Google Scholar] [CrossRef]
- Li, A.S.; Iijima, A.; Huang, J.; Li, Q.X.; Chen, Y. Putative Mode of Action of the Monoterpenoids Linalool, Methyl Eugenol, Estragole, and Citronellal on Ligand-Gated Ion Channels. Engineering 2020, 6, 541–545. [Google Scholar] [CrossRef]
- Walker, K.; Frederick, R. Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier Science: Philadelphia, PA, USA, 2011; pp. 306–314. ISBN 0444522735. [Google Scholar]
- Sawicka, B.; Egbuna, C. Pests of Agricultural Crops and Control Measures. In Natural Remedies for Pest, Disease and Weed Control; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–16. [Google Scholar]
- U.S. Food and Drug Administration, FDA. Substances Generally Recognized as Safe (GRAS). Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?FR=182.20 (accessed on 22 September 2024).
- da Silva, J.C.P.; Campos, V.P.; Barros, A.F.; Pedroso, L.A.; de Freitas Silva, M.; de Souza, J.T.; Pedroso, M.P.; de Medeiros, F.H.V. Performance of Volatiles Emitted from Different Plant Species against Juveniles and Eggs of Meloidogyne incognita. Crop Prot. 2019, 116, 196–203. [Google Scholar] [CrossRef]
- de Freitas Silva, M.; Paulo Campos, V.; Barros, A.F.; da Silva, J.C.P.; Pedroso, M.P.; de Jesus Silva, F.; Gomes, V.A.; Justino, J.C. Medicinal Plant Volatiles Applied against the Root-Knot Nematode Meloidogyne incognita. Crop Prot. 2020, 130, 105057. [Google Scholar] [CrossRef]
- Osman Mohamed Ali, E.; Shakil, N.A.; Rana, V.S.; Sarkar, D.J.; Majumder, S.; Kaushik, P.; Singh, B.B.; Kumar, J. Antifungal Activity of Nano Emulsions of Neem and Citronella Oils against Phytopathogenic Fungi, Rhizoctonia solani and Sclerotium rolfsii. Ind. Crops Prod. 2017, 108, 379–387. [Google Scholar] [CrossRef]
- Regnier, T.; Combrinck, S.; Veldman, W.; Du Plooy, W. Application of Essential Oils as Multi-Target Fungicides for the Control of Geotrichum citriaurantii and Other Postharvest Pathogens of Citrus. Ind. Crops Prod. 2014, 61, 151–159. [Google Scholar] [CrossRef]
- Abramson, C.I.; Wanderley, P.A.; Wanderley, M.J.A.; Mina, A.J.S.; Souza, O.B. de Effect of Essential Oil from Citronella and Alfazema on Fennel Aphids Hyadaphis foeniculi Passerini (Hemiptera: Aphididae) and Its Predator Cycloneda sanguinea L. (Coleoptera: Coccinelidae). Am. J. Environ. Sci. 2007, 3, 9–10. [Google Scholar] [CrossRef]
- Afify, A.E.-M.M.; Ali, F.S.; Turky, A. Control of Tetranychus urticae Koch by Extracts of Three Essential Oils of Chamomile, Marjoram and Eucalyptus. Asian Pac. J. Trop. Biomed. 2012, 2, 24–30. [Google Scholar] [CrossRef]
- Laborda, R.; Manzano, I.; Gamón, M.; Gavidia, I.; Pérez-Bermúdez, P.; Boluda, R. Effects of Rosmarinus officinalis and Salvia officinalis Essential Oils on Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2013, 48, 106–110. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of Water Stress on Lavender and Sage Biomass Production, Essential Oil Composition and Biocidal Properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Basaid, K.; Mayad, E.H.; Bouharroud, R.; Furze, J.N.; Benjlil, H.; de Oliveira, A.L.; Chebli, B. Biopesticidal Value of Senecio glaucus Subsp. Coronopifolius Essential Oil against Pathogenic Fungi, Nematodes, and Mites. Mater. Today Proc. 2020, 27, 3082–3090. [Google Scholar] [CrossRef]
- Zandi-Sohani, N.; Ramezani, L. Evaluation of Five Essential Oils as Botanical Acaricides against the Strawberry Spider Mite Tetranychus turkestani Ugarov and Nikolskii. Int. Biodeterior. Biodegrad. 2015, 98, 101–106. [Google Scholar] [CrossRef]
- Sertkaya, E.; Kaya, K.; Soylu, S. Acaricidal Activities of the Essential Oils from Several Medicinal Plants against the Carmine Spider Mite (Tetranychus Cinnabarinus Boisd.) (Acarina: Tetranychidae). Ind. Crops Prod. 2010, 31, 107–112. [Google Scholar] [CrossRef]
- Kalaiselvi, D.; Mohankumar, A.; Shanmugam, G.; Thiruppathi, G.; Nivitha, S.; Sundararaj, P. Altitude-Related Changes in the Phytochemical Profile of Essential Oils Extracted from Artemisia nilagirica and Their Nematicidal Activity against Meloidogyne incognita. Ind. Crops Prod. 2019, 139, 111472. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Naik, S.N. Biopesticidal Value of Selected Essential Oils against Pathogenic Fungus, Termites, and Nematodes. Int. Biodeterior. Biodegrad. 2011, 65, 703–707. [Google Scholar] [CrossRef]
- Onifade, A.K.; Fatope, M.O.; Deadman, M.L.; Al-Kindy, S.M.Z. Nematicidal Activity of Haplophyllum tuberculatum and Plectranthus cylindraceus Oils against Meloidogyne javanica. Biochem. Syst. Ecol. 2008, 36, 679–683. [Google Scholar] [CrossRef]
- Kang, J.S.; Kim, E.; Lee, S.H.; Park, I.-K. Inhibition of Acetylcholinesterases of the Pinewood Nematode, Bursaphelenchus xylophilus, by Phytochemicals from Plant Essential Oils. Pestic. Biochem. Physiol. 2013, 105, 50–56. [Google Scholar] [CrossRef]
- Cheng, Z.-H.; Fan, F.-F.; Zhao, J.-Z.; Li, R.; Li, S.-C.; Zhang, E.-J.; Liu, Y.-K.; Wang, J.-Y.; Zhu, X.-R.; Tian, Y.-M. Optimization of the Microemulsion Formulation of Curcuma Oil and Evaluation of Its Acaricidal Efficacy against Tetranychus cinnabarinus (Boisduval) (Acari: Tetranychidae). J. Asia Pac. Entomol. 2020, 23, 1014–1022. [Google Scholar] [CrossRef]
- Pavela, R.; Stepanycheva, E.; Shchenikova, A.; Chermenskaya, T.; Petrova, M. Essential Oils as Prospective Fumigants against Tetranychus urticae Koch. Ind. Crops Prod. 2016, 94, 755–761. [Google Scholar] [CrossRef]
- Roh, H.S.; Lee, B.H.; Park, C.G. Acaricidal and Repellent Effects of Myrtacean Essential Oils and Their Major Constituents against Tetranychus urticae (Tetranychidae). J. Asia Pac. Entomol. 2013, 16, 245–249. [Google Scholar] [CrossRef]
- Mar, J.M.; Silva, L.S.; Azevedo, S.G.; França, L.P.; Goes, A.F.F.; dos Santos, A.L.; Bezerra, J.d.A.; Nunomura, R.d.C.S.; Machado, M.B.; Sanches, E.A. Lippia origanoides Essential Oil: An Efficient Alternative to Control Aedes aegypti, Tetranychus urticae and Cerataphis lataniae. Ind. Crops Prod. 2018, 111, 292–297. [Google Scholar] [CrossRef]
- Cavalcanti, S.C.H.; dos, S. Niculau, E.; Blank, A.F.; Câmara, C.A.G.; Araújo, I.N.; Alves, P.B. Composition and Acaricidal Activity of Lippia sidoides Essential Oil against Two-Spotted Spider Mite (Tetranychus urticae Koch). Bioresour. Technol. 2010, 101, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Rani, P.U. Fumigant and Contact Toxic Potential of Essential Oils from Plant Extracts against Stored Product Pests. J. Biopestic. 2011, 5, 120–128. [Google Scholar] [CrossRef]
- Mahmoudvand, M.; Abbasipour, H.; Hosseinpour, M.H.; Rastegar, F.; Basij, M. Using Some Plant Essential Oils as Natural Fumigants against Adults of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae). Munis Entomol. Zool. 2011, 6, 150–154. [Google Scholar]
- Oyedeji, A.O.; Okunowo, W.O.; Osuntoki, A.A.; Olabode, T.B.; Ayo-folorunso, F. Insecticidal and Biochemical Activity of Essential Oil from Citrus sinensis Peel and Constituents on Callosobrunchus maculatus and Sitophilus zeamais. Pestic. Biochem. Physiol. 2020, 168, 104643. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-I.; Park, C.; Ohh, M.-H.; Cho, H.-C.; Ahn, Y.-J. Contact and Fumigant Activities of Aromatic Plant Extracts and Essential Oils against Lasioderma serricorne (Coleoptera: Anobiidae). J. Stored Prod. Res. 2003, 39, 11–19. [Google Scholar] [CrossRef]
- Plata-Rueda, A.; Martínez, L.C.; da Silva Rolim, G.; Coelho, R.P.; Santos, M.H.; de Souza Tavares, W.; Zanuncio, J.C.; Serrão, J.E. Insecticidal and Repellent Activities of Cymbopogon citratus (Poaceae) Essential Oil and Its Terpenoids (Citral and Geranyl Acetate) against Ulomoides dermestoides. Crop Prot. 2020, 137, 105299. [Google Scholar] [CrossRef]
- Benelli, G.; Flamini, G.; Canale, A.; Cioni, P.L.; Conti, B. Toxicity of Some Essential Oil Formulations against the Mediterranean Fruit Fly Ceratitis capitata (Wiedemann) (Diptera tephritidae). Crop Prot. 2012, 42, 223–229. [Google Scholar] [CrossRef]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and Acetylcholinesterase Inhibitory Activities of Lamiaceae Plant Essential Oils and Their Major Components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Bravim dos Santos, A.T.; Zanuncio Junior, J.S.; Parreira, L.A.; Pedra de Abreu, K.M.; de Oliveira Bernardes, C.; Romário de Carvalho, J.; Menini, L. Chemical Identification and Insecticidal Effect of Tephrosia vogelii Essential Oil against Cerosipha forbesi in Strawberry Crop. Crop Prot. 2021, 139, 105405. [Google Scholar] [CrossRef]
- Kim, S.; Chae, S.; Youn, H.; Yeon, S.; Ahn, Y. Contact and Fumigant Toxicity of Plant Essential Oils and Efficacy of Spray Formulations Containing the Oils against B- and Q-biotypes of Bemisia tabaci. Pest Manag. Sci. 2011, 67, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Çalmaşur, Ö.; Aslan, İ.; Şahin, F. Insecticidal and Acaricidal Effect of Three Lamiaceae Plant Essential Oils against Tetranychus urticae Koch and Bemisia tabaci Genn. Ind. Crops Prod. 2006, 23, 140–146. [Google Scholar] [CrossRef]
- Yang, N.-W.; Li, A.-L.; Wan, F.-H.; Liu, W.-X.; Johnson, D. Effects of Plant Essential Oils on Immature and Adult Sweetpotato Whitefly, Bemisia tabaci Biotype B. Crop Prot. 2010, 29, 1200–1207. [Google Scholar] [CrossRef]
- Zarrad, K.; Hamouda, A.B.; Chaieb, I.; Laarif, A.; Jemâa, J.M.-B. Chemical Composition, Fumigant and Anti-Acetylcholinesterase Activity of the Tunisian Citrus aurantium L. Essential Oils. Ind. Crops Prod. 2015, 76, 121–127. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Canale, A.; Senthil-Nathan, S.; Maggi, F. Not Just Popular Spices! Essential Oils from Cuminum cyminum and Pimpinella anisum Are Toxic to Insect Pests and Vectors without Affecting Non-Target Invertebrates. Ind. Crops Prod. 2018, 124, 236–243. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G.; Sprawka, I.; Sytykiewicz, H. Aphicidal Activity of Selected Asteraceae Essential Oils and Their Effect on Enzyme Activities of the Green Peach Aphid, Myzus persicae (Sulzer). Pestic. Biochem. Physiol. 2018, 145, 84–92. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The Essential Oil from Industrial Hemp (Cannabis Sativa L.) by-Products as an Effective Tool for Insect Pest Management in Organic Crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Kimbaris, A.C.; Papachristos, D.P.; Michaelakis, A.; Martinou, A.F.; Polissiou, M.G. Toxicity of Plant Essential Oil Vapours to Aphid Pests and Their Coccinellid Predators. Biocontrol. Sci. Technol. 2010, 20, 411–422. [Google Scholar] [CrossRef]
- Petrakis, E.A.; Kimbaris, A.C.; Perdikis, D.C.; Lykouressis, D.P.; Tarantilis, P.A.; Polissiou, M.G. Responses of Myzus persicae (Sulzer) to Three Lamiaceae Essential Oils Obtained by Microwave-Assisted and Conventional Hydrodistillation. Ind. Crops Prod. 2014, 62, 272–279. [Google Scholar] [CrossRef]
- Toledo, P.F.S.; Viteri Jumbo, L.O.; Rezende, S.M.; Haddi, K.; Silva, B.A.; Mello, T.S.; Della Lucia, T.M.C.; Aguiar, R.W.S.; Smagghe, G.; Oliveira, E.E. Disentangling the Ecotoxicological Selectivity of Clove Essential Oil against Aphids and Non-Target Ladybeetles. Sci. Total Environ. 2020, 718, 137328. [Google Scholar] [CrossRef] [PubMed]
- Umpiérrez, M.L.; Paullier, J.; Porrini, M.; Garrido, M.; Santos, E.; Rossini, C. Potential Botanical Pesticides from Asteraceae Essential Oils for Tomato Production: Activity against Whiteflies, Plants and Bees. Ind. Crops Prod. 2017, 109, 686–692. [Google Scholar] [CrossRef]
- Zapata, N.; Vargas, M.; Latorre, E.; Roudergue, X.; Ceballos, R. The Essential Oil of Laurelia sempervirens Is Toxic to Trialeurodes vaporariorum and Encarsia formosa. Ind. Crops Prod. 2016, 84, 418–422. [Google Scholar] [CrossRef]
- Silva, D.C.; de Fátima Arrigoni-Blank, M.; Bacci, L.; Blank, A.F.; Nunes Faro, R.R.; Oliveira Pinto, J.A.; Garcia Pereira, K.L. Toxicity and Behavioral Alterations of Essential Oils of Eplingiella fruticosa Genotypes and Their Major Compounds to Acromyrmex balzani. Crop Prot. 2019, 116, 181–187. [Google Scholar] [CrossRef]
- Melo, C.R.; Blank, A.F.; Oliveira, B.M.S.; Santos, A.C.C.; Cristaldo, P.F.; Araújo, A.P.A.; Bacci, L. Formicidal Activity of Essential Oils of Myrcia lundiana Chemotypes on Acromyrmex balzani. Crop Prot. 2021, 139, 105343. [Google Scholar] [CrossRef]
- Ayvaz, A.; Sagdic, O.; Karaborklu, S.; Ozturk, I. Insecticidal Activity of the Essential Oils from Different Plants Against Three Stored-Product Insects. J. Insect Sci. 2010, 10, 1–13. [Google Scholar] [CrossRef]
- Pavela, R.; Maggi, F.; Lupidi, G.; Cianfaglione, K.; Dauvergne, X.; Bruno, M.; Benelli, G. Efficacy of Sea Fennel (Crithmum maritimum L., Apiaceae) Essential Oils against Culex quinquefasciatus Say and Spodoptera littoralis (Boisd.). Ind. Crops Prod. 2017, 109, 603–610. [Google Scholar] [CrossRef]
- Ali, A.M.; Ibrahim, A.M.A. Castor and Camphor Essential Oils Alter Hemocyte Populations and Induce Biochemical Changes in Larvae of Spodoptera littoralis (Boisduval) (Lepidoptera: Noctuidae). J. Asia Pac. Entomol. 2018, 21, 631–637. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Maggi, F.; Nkuimi Wandjou, J.G.; Yvette Fofie, N.G.B.; Koné-Bamba, D.; Sagratini, G.; Vittori, S.; Caprioli, G. Insecticidal Activity of the Essential Oil and Polar Extracts from Ocimum gratissimum Grown in Ivory Coast: Efficacy on Insect Pests and Vectors and Impact on Non-Target Species. Ind. Crops Prod. 2019, 132, 377–385. [Google Scholar] [CrossRef]
- Mansour, S.A.; El-Sharkawy, A.Z.; Abdel-Hamid, N.A. Toxicity of Essential Plant Oils, in Comparison with Conventional Insecticides, against the Desert Locust, Schistocerca gregaria (Forskål). Ind. Crops Prod. 2015, 63, 92–99. [Google Scholar] [CrossRef]
- Abdelatti, Z.A.S.; Hartbauer, M. Plant Oil Mixtures as a Novel Botanical Pesticide to Control Gregarious locusts. J. Pest Sci. 2020, 93, 341–353. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal Activity of Essential Oils: Octopaminergic Sites of Action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System—A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Lee, S.H. Invertebrate Acetylcholinesterases: Insights into Their Evolution and Non-Classical Functions. J. Asia Pac. Entomol. 2018, 21, 186–195. [Google Scholar] [CrossRef]
- Fukuto, T.R. Mechanism of Action of Organophosphorus and Carbamate Insecticides. Environ. Health Perspect. 1990, 87, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, Y.H.; Kwon, D.H.; Cha, D.J.; Kim, J.H. Mutation and Duplication of Arthropod Acetylcholinesterase: Implications for Pesticide Resistance and Tolerance. Pestic. Biochem. Physiol. 2015, 120, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Karakaya, S.; Yılmaz, S.V.; Koca, M.; Demirci, B.; Sytar, O. Screening of Non-Alkaloid Acetylcholinesterase Inhibitors from Extracts and Essential Oils of Anthriscus nemorosa (M.Bieb.) Spreng. (Apiaceae). S. Afr. J. Bot. 2019, 125, 261–269. [Google Scholar] [CrossRef]
- Orhan, I.; Şenol, F.S.; Gülpinar, A.R.; Kartal, M.; Şekeroglu, N.; Deveci, M.; Kan, Y.; Şener, B. Acetylcholinesterase Inhibitory and Antioxidant Properties of Cyclotrichium niveum, Thymus praecox Subsp. caucasicus Var. caucasicus, Echinacea purpurea and E. pallida. Food Chem. Toxicol. 2009, 47, 1304–1310. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum Oil, a Potential Insecticide against Rice Weevil with Anti-Acetylcholinesterase Activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Castillo-Morales, R.M.; Carreño Otero, A.L.; Mendez-Sanchez, S.C.; Da Silva, M.A.N.; Stashenko, E.E.; Duque, J.E. Mitochondrial Affectation, DNA Damage and AChE Inhibition Induced by Salvia officinalis Essential Oil on Aedes aegypti Larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2019, 221, 29–37. [Google Scholar] [CrossRef]
- El Euch, S.K.; Hassine, D.B.; Cazaux, S.; Bouzouita, N.; Bouajila, J. Salvia officinalis Essential Oil: Chemical Analysis and Evaluation of Anti-Enzymatic and Antioxidant Bioactivities. S. Afr. J. Bot. 2019, 120, 253–260. [Google Scholar] [CrossRef]
- Perry, N.S.L.; Houghton, P.J.; Jenner, P.; Keith, A.; Perry, E.K. Salvia lavandulaefolia Essential Oil Inhibits Cholinesterase in Vivo. Phytomedicine 2002, 9, 48–51. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Prakash, B. Toxicity and Biochemical Efficacy of Chemically Characterized Rosmarinus Officinalis Essential Oil against Sitophilus oryzae and Oryzaephilus surinamensis. Ind. Crops Prod. 2015, 74, 817–823. [Google Scholar] [CrossRef]
- Seo, S.-M.; Kim, J.; Kang, J.; Koh, S.-H.; Ahn, Y.-J.; Kang, K.-S.; Park, I.-K. Fumigant Toxicity and Acetylcholinesterase Inhibitory Activity of 4 Asteraceae Plant Essential Oils and Their Constituents against Japanese Termite (Reticulitermes speratus Kolbe). Pestic. Biochem. Physiol. 2014, 113, 55–61. [Google Scholar] [CrossRef]
- López, M.D.; Pascual-Villalobos, M.J. Mode of Inhibition of Acetylcholinesterase by Monoterpenoids and Implications for Pest Control. Ind. Crops Prod. 2010, 31, 284–288. [Google Scholar] [CrossRef]
- Lummis, S.C.R.; Sattelle, D.B. Insect Central Nervous System γ-Aminobutyric Acid. Neurosci. Lett. 1985, 60, 13–18. [Google Scholar] [CrossRef]
- Robinson, T.; MacAllan, D.; Lunt, G.; Battersby, M. Γ-Aminobutyric Acid Receptor Complex of Insect CNS: Characterization of a Benzodiazepine Binding Site. J. Neurochem. 1986, 47, 1955–1962. [Google Scholar] [CrossRef]
- Ashby, J.A.; McGonigle, I.V.; Price, K.L.; Cohen, N.; Comitani, F.; Dougherty, D.A.; Molteni, C.; Lummis, S.C.R. GABA Binding to an Insect GABA Receptor: A Molecular Dynamics and Mutagenesis Study. Biophys. J. 2012, 103, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Priestley, C.M.; Williamson, E.M.; Wafford, K.A.; Sattelle, D.B. Thymol, a Constituent of Thyme Essential Oil, Is a Positive Allosteric Modulator of Human GABA A Receptors and a Homo-oligomeric GABA Receptor from Drosophila melanogaster. Br. J. Pharmacol. 2003, 140, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- García, D.A.; Bujons, J.; Vale, C.; Suñol, C. Allosteric Positive Interaction of Thymol with the GABAA Receptor in Primary Cultures of Mouse Cortical Neurons. Neuropharmacology 2006, 50, 25–35. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Effects of Monoterpenoid Insecticides on [3H]-TBOB Binding in House Fly GABA Receptor and 36Cl− Uptake in American Cockroach Ventral Nerve Cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Waliwitiya, R.; Belton, P.; Nicholson, R.A.; Lowenberger, C.A. Effects of the Essential Oil Constituent Thymol and Other Neuroactive Chemicals on Flight Motor Activity and Wing Beat Frequency in the Blowfly Phaenicia sericata. Pest Manag. Sci. 2010, 66, 277–289. [Google Scholar] [CrossRef]
- Gashout, H.A.; Guzman-Novoa, E.; Goodwin, P.H.; Correa-Benítez, A. Impact of Sublethal Exposure to Synthetic and Natural Acaricides on Honey Bee (Apis mellifera) Memory and Expression of Genes Related to Memory. J. Insect Physiol. 2020, 121, 104014. [Google Scholar] [CrossRef] [PubMed]
- Atwood, H.L.; Klose, M.K. Neuromuscular Transmission Modulation at Invertebrate Neuromuscular Junctions. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2009; pp. 671–690. [Google Scholar]
- Hana, S.; Lange, A.B. Octopamine and Tyramine Regulate the Activity of Reproductive Visceral Muscles in the Adult Female Blood-Feeding Bug, Rhodnius prolixus. J. Exp. Biol. 2017, 220, 1830–1836. [Google Scholar] [CrossRef]
- Livingstone, M.S.; Harris-Warrick, R.M.; Kravitz, E.A. Serotonin and Octopamine Produce Opposite Postures in Lobsters. Science 1980, 208, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Kostyukovsky, M.; Rafaeli, A.; Gileadi, C.; Demchenko, N.; Shaaya, E. Activation of Octopaminergic Receptors by Essential Oil Constituents Isolated from Aromatic Plants: Possible Mode of Action against Insect Pests. Pest Manag. Sci. 2002, 58, 1101–1106. [Google Scholar] [CrossRef]
- Price, D.N.; Berry, M.S. Comparison of Effects of Octopamine and Insecticidal Essential Oils on Activity in the Nerve Cord, Foregut, and Dorsal Unpaired Median Neurons of Cockroaches. J. Insect Physiol. 2006, 52, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Neckameyer, W.S.; Leal, S.M. Non-Mammalian Hormone-Behavior Systems. In Hormones, Brain and Behavior; Pfaff, D.W., Joëls, M., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 1, pp. 3–365. [Google Scholar]
- Mizunami, M.; Matsumoto, Y.; Watanabe, H.; Nishino, H. Olfactory and Visual Learning in Cockroaches and Crickets; Elsevier: Amsterdam, The Netherlands, 2013; pp. 549–560. [Google Scholar]
- Lee, K.H.; Lee, J.-S.; Kim, E.S.; Lee, H.G. Preparation, Characterization, and Food Application of Rosemary Extract-Loaded Antimicrobial Nanoparticle Dispersions. LWT 2019, 101, 138–144. [Google Scholar] [CrossRef]
- Soares de Oliveira, M.A.; Melo Coutinho, H.D.; Jardelino de Lacerda Neto, L.; Castro de Oliveira, L.C.; Bezerra da Cunha, F.A. Repellent Activity of Essential Oils against Culicids: A Review. Sustain. Chem. Pharm. 2020, 18, 100328. [Google Scholar] [CrossRef]
- de Andrade Dutra, K.; de Oliveira, J.V.; Navarro, D.M. do A.F.; Barbosa, D.R. e S.; Santos, J.P.O. Control of Callosobruchus maculatus (FABR.) (Coleoptera: Chrysomelidae: Bruchinae) in Vigna Unguiculata (L.) WALP. with Essential Oils from Four Citrus Spp. Plants. J. Stored Prod. Res. 2016, 68, 25–32. [Google Scholar] [CrossRef]
- Al-Sarar, A.S.; Hussein, H.I.; Abobakr, Y.; Al-Zabib, A.A.S.; Bazeyad, A.Y. Mosquitocidal and Repellent Activities of Essential Oils against Culex pipiens L. Entomol. Res. 2020, 50, 182–188. [Google Scholar] [CrossRef]
- Sheikh, Z.; Amani, A.; Basseri, H.R.; Kazemi, S.H.M.; Sedaghat, M.M.; Azam, K.; Azizi, M.; Amirmohammadi, F. Repellent Efficacy of Eucalyptus globulus and Syzygium aromaticum Essential Oils against Malaria Vector, Anopheles stephensi (Diptera: Culicidae). Iran. J. Public Health 2021, 50, 1668. [Google Scholar] [CrossRef]
- Akeumbiwo Tchumkam, C.; Kojom Foko, L.P.; Ndo, C.; Essangui Same, E.; Cheteug Nguetsa, G.; Eya’Ane Meva, F.; Ayong, L.; Eboumbou Moukoko, C.E. Chemical Composition and Repellent Activity of Essential Oils of Tithonia Diversifolia (Asteraceae) Leaves against the Bites of Anopheles coluzzii. Sci. Rep. 2023, 13, 6001. [Google Scholar] [CrossRef] [PubMed]
- Haris, A.; Azeem, M.; Abbas, M.G.; Mumtaz, M.; Mozūratis, R.; Binyameen, M. Prolonged Repellent Activity of Plant Essential Oils against Dengue Vector, Aedes aegypti. Molecules 2023, 28, 1351. [Google Scholar] [CrossRef] [PubMed]
- Catani, L.; Grassi, E.; Cocozza di Montanara, A.; Guidi, L.; Sandulli, R.; Manachini, B.; Semprucci, F. Essential Oils and Their Applications in Agriculture and Agricultural Products: A Literature Analysis through VOSviewer. Biocatal. Agric. Biotechnol. 2022, 45, 102502. [Google Scholar] [CrossRef]
- Danna, C.; Malaspina, P.; Cornara, L.; Smeriglio, A.; Trombetta, D.; De Feo, V.; Vanin, S. Eucalyptus Essential Oils in Pest Control: A Review of Chemical Composition and Applications against Insects and Mites. Crop Prot. 2024, 176, 106319. [Google Scholar] [CrossRef]
- Lacotte, V.; Rey, M.; Peignier, S.; Mercier, P.-E.; Rahioui, I.; Sivignon, C.; Razy, L.; Benhamou, S.; Livi, S.; da Silva, P. Bioactivity and Chemical Composition of Forty Plant Essential Oils against the Pea Aphid Acyrthosiphon pisum Revealed Peppermint Oil as a Promising Biorepellent. Ind. Crops Prod. 2023, 197, 116610. [Google Scholar] [CrossRef]
- Saıfı, R.; Saıfı, H.; Akca, İ.; Benabadelkader, M.; Askın, A.K.; Belghoul, M. Insecticidal and Repellent Effects of Mentha longifolia L. Essential Oil against Aphis craccivora Koch (Hemiptera: Aphididae). Chem. Biol. Technol. Agric. 2023, 10, 18. [Google Scholar] [CrossRef]
- Singh, D.; Singh, A.K. Repellent and Insecticidal Properties of Essential Oils against Housefly, Musca domestica L. Int. J. Trop. Insect Sci. 1991, 12, 487–491. [Google Scholar] [CrossRef]
- Choi, W.S.; Park, B.S.; Ku, S.K.; Lee, S.E. Repellent Activities of Essential Oils and Monoterpenes against Culex pipiens pallens. J. Am. Mosq. Control. Assoc. 2002, 18, 348–351. [Google Scholar] [PubMed]
- Park, B.-S.; Choi, W.-S.; Kim, J.-H.; Kim, K.-H.; Lee, S.-E. Monoterpenes from Thyme (Thymus vulgaris) as Potential Mosquito Repellents. J. Am. Mosq. Control. Assoc. 2005, 21, 80–83. [Google Scholar] [CrossRef]
- Odalo, J.O.; Omolo, M.O.; Malebo, H.; Angira, J.; Njeru, P.M.; Ndiege, I.O.; Hassanali, A. Repellency of Essential Oils of Some Plants from the Kenyan Coast against Anopheles gambiae. Acta Trop. 2005, 95, 210–218. [Google Scholar] [CrossRef]
- Erler, F.; Ulug, I.; Yalcinkaya, B. Repellent Activity of Five Essential Oils against Culex pipiens. Fitoterapia 2006, 77, 491–494. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, V.; Tripathi, A.; Aggarwal, K.; Khanuja, S. Insecticidal, Repellent and Oviposition-Deterrent Activity of Selected Essential Oils against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus. Bioresour. Technol. 2005, 96, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Hussein, H.S.; Salem, M.Z.M.; Soliman, A.M. Repellent, Attractive, and Insecticidal Effects of Essential Oils from Schinus terebinthifolius Fruits and Corymbia citriodora Leaves on Two Whitefly Species, Bemisia tabaci, and Trialeurodes ricini. Sci. Hortic. 2017, 216, 111–119. [Google Scholar] [CrossRef]
- Costa, E.C.C.; Christofoli, M.; de Souza Costa, G.C.; Peixoto, M.F.; Fernandes, J.B.; Forim, M.R.; de Castro Pereira, K.; Silva, F.G.; de Melo Cazal, C. Essential Oil Repellent Action of Plants of the Genus Zanthoxylum against Bemisia tabaci Biotype B (Homoptera: Aleyrodidae). Sci. Hortic. 2017, 226, 327–332. [Google Scholar] [CrossRef]
- Liu, C.H.; Mishra, A.K.; Tan, R.X.; Tang, C.; Yang, H.; Shen, Y.F. Repellent and Insecticidal Activities of Essential Oils from Artemisia princeps and Cinnamomum camphora and Their Effect on Seed Germination of Wheat and Broad Bean. Bioresour. Technol. 2006, 97, 1969–1973. [Google Scholar] [CrossRef]
- Reyes, E.I.M.; Farias, E.S.; Silva, E.M.P.; Filomeno, C.A.; Plata, M.A.B.; Picanço, M.C.; Barbosa, L.C.A. Eucalyptus resinifera Essential Oils Have Fumigant and Repellent Action against Hypothenemus hampei. Crop Prot. 2019, 116, 49–55. [Google Scholar] [CrossRef]
- Chiluwal, K.; Kim, J.; Bae, S.D.; Park, C.G. Essential Oils from Selected Wooden Species and Their Major Components as Repellents and Oviposition Deterrents of Callosobruchus chinensis (L.). J. Asia Pac. Entomol. 2017, 20, 1447–1453. [Google Scholar] [CrossRef]
- Taban, A.; Saharkhiz, M.J.; Khorram, M. Formulation and Assessment of Nano-Encapsulated Bioherbicides Based on Biopolymers and Essential Oil. Ind. Crops Prod. 2020, 149, 112348. [Google Scholar] [CrossRef]
- Parreira, D.S.; Alcántara-de la Cruz, R.; Dimaté, F.A.R.; Batista, L.D.; Ribeiro, R.C.; Ferreira, G.A.R.; Zanuncio, J.C. Bioactivity of Ten Essential Oils on the Biological Parameters of Trichogramma pretiosum (Hymenoptera: Trichogrammatidae) Adults. Ind. Crops Prod. 2019, 127, 11–15. [Google Scholar] [CrossRef]
- Baccari, W.; Znati, M.; Zardi-Bergaoui, A.; Chaieb, I.; Flamini, G.; Ascrizzi, R.; Ben Jannet, H. Composition and Insecticide Potential against Tribolium castaneum of the Fractionated Essential Oil from the Flowers of the Tunisian Endemic Plant Ferula Tunetana nomel Ex Batt. Ind. Crops Prod. 2020, 143, 111888. [Google Scholar] [CrossRef]
- Tak, J.-H.; Isman, M.B. Acaricidal and Repellent Activity of Plant Essential Oil-Derived Terpenes and the Effect of Binary Mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). Ind. Crops Prod. 2017, 108, 786–792. [Google Scholar] [CrossRef]
- Isman, M.B. Plant Essential Oils for Pest and Disease Management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Sousa, R.M.O.F.; Rosa, J.S.; Oliveira, L.; Cunha, A.; Fernandes-Ferreira, M. Activities of Apiaceae Essential Oils against Armyworm, Pseudaletia unipuncta (Lepidoptera: Noctuidae). J. Agric. Food Chem. 2013, 61, 7661–7672. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Colorado, B.E.; Pino-Benitez, N.; González-Coloma, A. Volatile Composition and Biocidal (Antifeedant and Phytotoxic) Activity of the Essential Oils of Four Piperaceae species from Choco-Colombia. Ind. Crops Prod. 2019, 138, 111463. [Google Scholar] [CrossRef]
- Formisano, C.; Rigano, D.; Senatore, F.; Arnold, N.A.; Simmonds, M.S.J.; Rosselli, S.; Bruno, M.; Loziene, K. Essential Oils of Three Species of Scutellaria and Their Influence on Spodoptera littoralis. Biochem. Syst. Ecol. 2013, 48, 206–210. [Google Scholar] [CrossRef]
- Santos, G.K.N.; Dutra, K.A.; Barros, R.A.; da Câmara, C.A.G.; Lira, D.D.; Gusmão, N.B.; Navarro, D.M.A.F. Essential Oils from Alpinia purpurata (Zingiberaceae): Chemical Composition, Oviposition Deterrence, Larvicidal and Antibacterial Activity. Ind. Crops Prod. 2012, 40, 254–260. [Google Scholar] [CrossRef]
- Soonwera, M.; Phasomkusolsil, S. Adulticidal, Larvicidal, Pupicidal and Oviposition Deterrent Activities of Essential Oil from Zanthoxylum limonella Alston (Rutaceae) against Aedes aegypti (L.) and Culex quinquefasciatus (Say). Asian Pac. J. Trop. Biomed. 2017, 7, 967–978. [Google Scholar] [CrossRef]
- Thanigaivel, A.; Chanthini, K.M.-P.; Karthi, S.; Vasantha-Srinivasan, P.; Ponsankar, A.; Sivanesh, H.; Stanley-Raja, V.; Shyam-Sundar, N.; Narayanan, K.R.; Senthil-Nathan, S. Toxic Effect of Essential Oil and Its Compounds Isolated from Sphaeranthus amaranthoides Burm. f. against Dengue Mosquito Vector Aedes aegypti Linn. Pestic. Biochem. Physiol. 2019, 160, 163–170. [Google Scholar] [CrossRef]
- Mbata, G.N.; Payton, M.E. Effect of Monoterpenoids on Oviposition and Mortality of Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) under Hermetic Conditions. J. Stored Prod. Res. 2013, 53, 43–47. [Google Scholar] [CrossRef]
- Papanastasiou, S.A.; Ioannou, C.S.; Papadopoulos, N.T. Oviposition-deterrent Effect of Linalool—A Compound of Citrus Essential Oils—On Female Mediterranean Fruit Flies, Ceratitis capitata (Diptera: Tephritidae). Pest Manag. Sci. 2020, 76, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Silva, C.T.; Wanderley-Teixeira, V.; da Cunha, F.M.; de Oliveira, J.V.; de Andrade Dutra, K.; do Amaral Ferraz Navarro, D.M.; Teixeira, Á.A.C. Biochemical Parameters of Spodoptera frugiperda (J. E. Smith) Treated with Citronella Oil (Cymbopogon winterianus Jowitt Ex Bor) and Its Influence on Reproduction. Acta Histochem. 2016, 118, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.P.; Oliveira, E.E.; Tschoeke, P.H.; Pinheiro, R.G.; Maia, A.M.S.; Aguiar, R.W.S. Potential Use of Negramina (Siparuna guianensis Aubl.) Essential Oil to Control Wax Moths and Its Selectivity in Relation to Honey Bees. Ind. Crops Prod. 2017, 109, 151–157. [Google Scholar] [CrossRef]
- Iglesias, A.; Mitton, G.; Szawarski, N.; Cooley, H.; Ramos, F.; Meroi Arcerito, F.; Brasesco, C.; Ramirez, C.; Gende, L.; Eguaras, M.; et al. Essential Oils from Humulus lupulus as Novel Control Agents against Varroa destructor. Ind. Crops Prod. 2020, 158, 113043. [Google Scholar] [CrossRef]
- Rossini, C.; Rodrigo, F.; Davyt, B.; Umpiérrez, M.L.; González, A.; Garrido, P.M.; Cuniolo, A.; Porrini, L.P.; Eguaras, M.J.; Porrini, M.P. Sub-Lethal Effects of the Consumption of Eupatorium buniifolium Essential Oil in Honeybees. PLoS ONE 2020, 15, e0241666. [Google Scholar] [CrossRef]
- Sabahi, Q.; Hamiduzzaman, M.M.d.; Barajas-Pérez, J.S.; Tapia-Gonzalez, J.M.; Guzman-Novoa, E. Toxicity of Anethole and the Essential Oils of Lemongrass and Sweet Marigold to the Parasitic Mite Varroa destructor and Their Selectivity for Honey Bee (Apis mellifera) Workers and Larvae. Psyche A J. Entomol. 2018, 2018, 6196289. [Google Scholar] [CrossRef]
- Vasantha-Srinivasan, P.; Senthil-Nathan, S.; Ponsankar, A.; Thanigaivel, A.; Chellappandian, M.; Edwin, E.-S.; Selin-Rani, S.; Kalaivani, K.; Hunter, W.B.; Duraipandiyan, V.; et al. Acute Toxicity of Chemical Pesticides and Plant-Derived Essential Oil on the Behavior and Development of Earthworms, Eudrilus eugeniae (Kinberg) and Eisenia fetida (Savigny). Environ. Sci. Pollut. Res. 2018, 25, 10371–10382. [Google Scholar] [CrossRef]
- Pavela, R. Essential Oils from Foeniculum Vulgare Miller as a Safe Environmental Insecticide against the Aphid Myzus persicae Sulzer. Environ. Sci. Pollut. Res. 2018, 25, 10904–10910. [Google Scholar] [CrossRef]
- George, D.R.; Sparagano, O.A.E.; Port, G.; Okello, E.; Shiel, R.S.; Guy, J.H. Repellence of Plant Essential Oils to Dermanyssus gallinae and Toxicity to the Non-Target Invertebrate Tenebrio Molitor. Vet. Parasitol. 2009, 162, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Benelli, G. Essential Oils as Ecofriendly Biopesticides? Challenges and Constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Tabari, M.A.; Youssefi, M.R.; Maggi, F.; Benelli, G. Toxic and Repellent Activity of Selected Monoterpenoids (Thymol, Carvacrol and Linalool) against the Castor Bean Tick, Ixodes ricinus (Acari: Ixodidae). Vet. Parasitol. 2017, 245, 86–91. [Google Scholar] [CrossRef]
- Corrêa-Filho, L.C.; Lourenço, M.M.; Moldão-Martins, M.; Alves, V.D. Microencapsulation of β-Carotene by Spray Drying: Effect of Wall Material Concentration and Drying Inlet Temperature. Int. J. Food Sci. 2019, 2019, 8914852. [Google Scholar] [CrossRef] [PubMed]
- Locali-Pereira, A.R.; Lopes, N.A.; Menis-Henrique, M.E.C.; Janzantti, N.S.; Nicoletti, V.R. Modulation of Volatile Release and Antimicrobial Properties of Pink Pepper Essential Oil by Microencapsulation in Single- and Double-Layer Structured Matrices. Int. J. Food Microbiol. 2020, 335, 108890. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Ismail, A.; Karam, L. Advances in Essential Oils Encapsulation: Development, Characterization and Release Mechanisms. Polym. Bull. 2024, 81, 3837–3882. [Google Scholar] [CrossRef]
- Maes, C.; Bouquillon, S.; Fauconnier, M.-L. Encapsulation of Essential Oils for the Development of Biosourced Pesticides with Controlled Release: A Review. Molecules 2019, 24, 2539. [Google Scholar] [CrossRef]
- Naseema, A.; Kovooru, L.; Behera, A.K.; Kumar, K.P.P.; Srivastava, P. A Critical Review of Synthesis Procedures, Applications and Future Potential of Nanoemulsions. Adv. Colloid Interface Sci. 2021, 287, 102318. [Google Scholar] [CrossRef]
- Simonazzi, A.; Cid, A.G.; Villegas, M.; Romero, A.I.; Palma, S.D.; Bermúdez, J.M. Nanotechnology Applications in Drug Controlled Release. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 81–116. [Google Scholar]
- Ruiz-Montañez, G.; Ragazzo-Sanchez, J.A.; Picart-Palmade, L.; Calderón-Santoyo, M.; Chevalier-Lucia, D. Optimization of Nanoemulsions Processed by High-Pressure Homogenization to Protect a Bioactive Extract of Jackfruit (Artocarpus heterophyllus Lam). Innov. Food Sci. Emerg. Technol. 2017, 40, 35–41. [Google Scholar] [CrossRef]
- Li, Y.; Wu, C.-L.; Liu, J.; Zhu, Y.; Zhang, X.-Y.; Jiang, L.-Z.; Qi, B.-K.; Zhang, X.-N.; Wang, Z.-J.; Teng, F. Soy Protein Isolate-Phosphatidylcholine Nanoemulsions Prepared Using High-Pressure Homogenization. Nanomaterials 2018, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Gu, J.; McClements, D.J. Fabrication of Oil-in-Water Nanoemulsions by Dual-Channel Microfluidization Using Natural Emulsifiers: Saponins, Phospholipids, Proteins, and Polysaccharides. Food Hydrocoll. 2016, 61, 703–711. [Google Scholar] [CrossRef]
- Perazzo, A.; Preziosi, V.; Guido, S. Phase Inversion Emulsification: Current Understanding and Applications. Adv. Colloid Interface Sci. 2015, 222, 581–599. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Grumezescu, A.M. Nanoemulsion Preparation, Characterization, and Application in the Field of Biomedicine. In Nanoarchitectonics in Biomedicine; Elsevier: Amsterdam, The Netherlands, 2019; pp. 169–188. [Google Scholar]
- Ren, G.; Sun, Z.; Wang, Z.; Zheng, X.; Xu, Z.; Sun, D. Nanoemulsion Formation by the Phase Inversion Temperature Method Using Polyoxypropylene Surfactants. J. Colloid Interface Sci. 2019, 540, 177–184. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of Essential Oils to Enhance Their Antimicrobial Activity in Foods. LWT-Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Al-Tayyar, N.A.; Youssef, A.M.; Al-Hindi, R.R. Edible Coatings and Antimicrobial Nanoemulsions for Enhancing Shelf Life and Reducing Foodborne Pathogens of Fruits and Vegetables: A review. Sustain. Mater. Technol. 2020, 26, e00215. [Google Scholar] [CrossRef]
- Xiong, Y.; Li, S.; Warner, R.D.; Fang, Z. Effect of Oregano Essential Oil and Resveratrol Nanoemulsion Loaded Pectin Edible Coating on the Preservation of Pork Loin in Modified Atmosphere Packaging. Food Control. 2020, 114, 107226. [Google Scholar] [CrossRef]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation Mechanism of Monodisperse, Low Molecular Weight Chitosan Nanoparticles by Ionic Gelation Technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pant, A.; Negi, J.S. Novel Controlled Ionic Gelation Strategy for Chitosan Nanoparticles Preparation Using TPP-β-CD Inclusion Complex. Eur. J. Pharm. Sci. 2018, 112, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Remuñán-López, C.; Vila-Jato, J.L.; Alonso, M.J. Novel Hydrophilic Chitosan-Polyethylene Oxide Nanoparticles as Protein Carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Hsieh, W.-C.; Chang, C.-P.; Gao, Y.-L. Controlled Release Properties of Chitosan Encapsulated Volatile Citronella Oil Microcapsules by Thermal Treatments. Colloids Surf. B Biointerfaces 2006, 53, 209–214. [Google Scholar] [CrossRef]
- Giri, T.K. Nanoarchitectured Polysaccharide-Based Drug Carrier for Ocular Therapeutics. In Nanoarchitectonics for Smart De-livery and Drug Targeting; Holban, A.M., Grumezescu, A.M., Eds.; William Andrew Publishing: New York, NY, USA, 2016; Volume 1, pp. 119–141. [Google Scholar]
- Ahmadi, Z.; Saber, M.; Akbari, A.; Mahdavinia, G.R. Encapsulation of Satureja hortensis L. (Lamiaceae) in Chitosan/TPP Nanoparticles with Enhanced Acaricide Activity against Tetranychus urticae Koch (Acari: Tetranychidae). Ecotoxicol. Environ. Saf. 2018, 161, 111–119. [Google Scholar] [CrossRef]
- Carvalho, E.L.S.; Grenha, A.; Remuñán-López, C.; Alonso, M.J.; Seijo, B. Chapter 15 Mucosal Delivery of Liposome–Chitosan Nanoparticle Complexes. Methods Enzymol. 2009, 465, 289–312. [Google Scholar] [PubMed]
- Vaezifar, S.; Razavi, S.; Golozar, M.A.; Karbasi, S.; Morshed, M.; Kamali, M. Effects of Some Parameters on Particle Size Distribution of Chitosan Nanoparticles Prepared by Ionic Gelation Method. J. Clust. Sci. 2013, 24, 891–903. [Google Scholar] [CrossRef]
- Hosseini, S.M.; Hosseini, H.; Mohammadifar, M.A.; Mortazavian, A.M.; Mohammadi, A.; Khosravi-Darani, K.; Shojaee-Alibadi, S.; Dehghan, S.; Khaksar, R. Incorporation of Essential Oil in Alginate Microparticles by Multiple Emulsion/Ionic Gelation Process. Int. J. Biol. Macromol. 2013, 62, 582–588. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, H.; Hu, X.; Bao, S.; Huang, H. Synthesis and Release Studies of Microalgal Oil-Containing Microcapsules Prepared by Complex Coacervation. Colloids Surf. B Biointerfaces 2012, 89, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Nava, R.; López-Malo, A.; Palou, E.; Ramírez-Corona, N.; Jiménez-Munguía, M.T. Encapsulation of Oregano Essential Oil (Origanum vulgare) by Complex Coacervation between Gelatin and Chia Mucilage and Its Properties after Spray Drying. Food Hydrocoll. 2020, 109, 106077. [Google Scholar] [CrossRef]
- Bastos, L.P.H.; Vicente, J.; dos Santos, C.H.C.; de Carvalho, M.G.; Garcia-Rojas, E.E. Encapsulation of Black Pepper (Piper nigrum L.) Essential Oil with Gelatin and Sodium Alginate by Complex Coacervation. Food Hydrocoll. 2020, 102, 105605. [Google Scholar] [CrossRef]
- Chang, C.-P.; Leung, T.-K.; Lin, S.-M.; Hsu, C.-C. Release Properties on Gelatin-Gum Arabic Microcapsules Containing Camphor Oil with Added Polystyrene. Colloids Surf. B Biointerfaces 2006, 50, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Shaddel, R.; Hesari, J.; Azadmard-Damirchi, S.; Hamishehkar, H.; Fathi-Achachlouei, B.; Huang, Q. Use of Gelatin and Gum Arabic for Encapsulation of Black Raspberry Anthocyanins by Complex Coacervation. Int. J. Biol. Macromol. 2018, 107, 1800–1810. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Nicoletti, V.R. Complex Coacervation Assisted by a Two-Fluid Nozzle for Microencapsulation of Ginger Oil: Effect of Atomization Parameters. Food Res. Int. 2020, 138, 109828. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, M.; Rao, K.P. Pectin–Gelatin and Alginate–Gelatin Complex Coacervation for Controlled Drug Delivery: Influence of Anionic Polysaccharides and Drugs Being Encapsulated on Physicochemical Properties of Microcapsules. Carbohydr. Polym. 2010, 80, 808–816. [Google Scholar] [CrossRef]
- de Matos, E.F.; Scopel, B.S.; Dettmer, A. Citronella Essential Oil Microencapsulation by Complex Coacervation with Leather Waste Gelatin and Sodium Alginate. J. Environ. Chem. Eng. 2018, 6, 1989–1994. [Google Scholar] [CrossRef]
- Mohseni, F.; Goli, S.A.H. Encapsulation of Flaxseed Oil in the Tertiary Conjugate of Oxidized Tannic Acid-Gelatin and Flaxseed (Linum usitatissimum) Mucilage. Int. J. Biol. Macromol. 2019, 140, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Abreu, F.O.M.S.; de Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/Cashew Gum Nanogels for Essential Oil Encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef]
- de Oliveira, W.Q.; Wurlitzer, N.J.; de Oliveira Araújo, A.W.; Comunian, T.A.; do Socorro Rocha Bastos, M.; de Oliveira, A.L.; Magalhães, H.C.R.; Ribeiro, H.L.; de Figueiredo, R.W.; de Sousa, P.H.M. Complex Coacervates of Cashew Gum and Gelatin as Carriers of Green Coffee Oil: The Effect of Microcapsule Application on the Rheological and Sensorial Quality of a Fruit Juice. Food Res. Int. 2020, 131, 109047. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, K.; Nagao, H. Microencapsulation of Oil Droplets Using Freezing-Induced Gelatin–Acacia Complex Coacervation. Colloids Surf. A Physicochem. Eng. Asp. 2012, 411, 129–139. [Google Scholar] [CrossRef]
- Aloys, H.; Korma, S.A.; Alice, T.M.; Chantal, N.; Ali, A.H.; Abed, S.M. Microencapsulation by Complex Coacervation: Methods, Techniques, Benefits, and Applications—A Review. Am. J. Food Sci. Nut. Res. 2016, 1, 188–192. [Google Scholar]
- Timilsena, Y.P.; Akanbi, T.O.; Khalid, N.; Adhikari, B.; Barrow, C.J. Complex Coacervation: Principles, Mechanisms and Applications in Microencapsulation. Int. J. Biol. Macromol. 2019, 121, 1276–1286. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Singh, V.K.; Dwivedy, A.K.; Chaudhari, A.K.; Upadhyay, N.; Singh, P.; Sharma, S.; Dubey, N.K. Encapsulation in Chitosan-Based Nanomatrix as an Efficient Green Technology to Boost the Antimicrobial, Antioxidant and in Situ Efficacy of Coriandrum sativum Essential Oil. Int. J. Biol. Macromol. 2019, 133, 294–305. [Google Scholar] [CrossRef] [PubMed]
- Hasheminejad, N.; Khodaiyan, F.; Safari, M. Improving the Antifungal Activity of Clove Essential Oil Encapsulated by Chitosan Nanoparticles. Food Chem. 2019, 275, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, A.; Hosseini, S.M.; Hashemi, M. Emerging Chitosan Nanoparticles Loading-System Boosted the Antibacterial Activity of Cinnamomum zeylanicum Essential Oil. Ind. Crops Prod. 2020, 155, 112824. [Google Scholar] [CrossRef]
- Rajkumar, V.; Gunasekaran, C.; Paul, C.A.; Dharmaraj, J. Development of Encapsulated Peppermint Essential Oil in Chitosan Nanoparticles: Characterization and Biological Efficacy against Stored-Grain Pest Control. Pestic. Biochem. Physiol. 2020, 170, 104679. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, V.; Gunasekaran, C.; Dharmaraj, J.; Chinnaraj, P.; Paul, C.A.; Kanithachristy, I. Structural Characterization of Chitosan Nanoparticle Loaded with Piper nigrum Essential Oil for Biological Efficacy against the Stored Grain Pest Control. Pestic. Biochem. Physiol. 2020, 166, 104566. [Google Scholar] [CrossRef] [PubMed]
- Arabpoor, B.; Yousefi, S.; Weisany, W.; Ghasemlou, M. Multifunctional Coating Composed of Eryngium campestre L. Essential Oil Encapsulated in Nano-Chitosan to Prolong the Shelf-Life of Fresh Cherry Fruits. Food Hydrocoll. 2021, 111, 106394. [Google Scholar] [CrossRef]
- Zhaveh, S.; Mohsenifar, A.; Beiki, M.; Khalili, S.T.; Abdollahi, A.; Rahmani-Cherati, T.; Tabatabaei, M. Encapsulation of Cuminum cyminum Essential Oils in Chitosan-Caffeic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus flavus. Ind. Crops Prod. 2015, 69, 251–256. [Google Scholar] [CrossRef]
- Beyki, M.; Zhaveh, S.; Khalili, S.T.; Rahmani-Cherati, T.; Abollahi, A.; Bayat, M.; Tabatabaei, M.; Mohsenifar, A. Encapsulation of Mentha Piperita Essential Oils in Chitosan–Cinnamic Acid Nanogel with Enhanced Antimicrobial Activity against Aspergillus flavus. Ind. Crops Prod. 2014, 54, 310–319. [Google Scholar] [CrossRef]
- Yadav, A.; Kujur, A.; Kumar, A.; Singh, P.P.; Gupta, V.; Prakash, B. Encapsulation of Bunium persicum Essential Oil Using Chitosan Nanopolymer: Preparation, Characterization, Antifungal Assessment, and Thermal Stability. Int. J. Biol. Macromol. 2020, 142, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Hill, L.E.; Peng, Y.; Gomes, C.L. Synthesis and Characterization of β-Cyclodextrin Inclusion Complexes of Thymol and Thyme oil for Antimicrobial Delivery Applications. LWT—Food Sci. Technol. 2014, 59, 247–255. [Google Scholar] [CrossRef]
- Anaya-Castro, M.A.; Ayala-Zavala, J.F.; Muñoz-Castellanos, L.; Hernández-Ochoa, L.; Peydecastaing, J.; Durrieu, V. β-Cyclodextrin Inclusion Complexes Containing Clove (Eugenia caryophyllata) and Mexican oregano (Lippia berlandieri) Essential Oils: Preparation, Physicochemical and Antimicrobial Characterization. Food Packag. Shelf Life 2017, 14, 96–101. [Google Scholar] [CrossRef]
- Rakmai, J.; Cheirsilp, B.; Mejuto, J.C.; Torrado-Agrasar, A.; Simal-Gándara, J. Physico-Chemical Characterization and Evaluation of Bio-Efficacies of Black Pepper Essential Oil Encapsulated in Hydroxypropyl-Beta-Cyclodextrin. Food Hydrocoll. 2017, 65, 157–164. [Google Scholar] [CrossRef]
- Barbieri, N.; Sanchez-Contreras, A.; Canto, A.; Cauich-Rodriguez, J.V.; Vargas-Coronado, R.; Calvo-Irabien, L.M. Effect of Cyclodextrins and Mexican Oregano (Lippia graveolens Kunth) Chemotypes on the Microencapsulation of Essential Oil. Ind. Crops Prod. 2018, 121, 114–123. [Google Scholar] [CrossRef]
- Maia, J.D.; La Corte, R.; Martinez, J.; Ubbink, J.; Prata, A.S. Improved Activity of Thyme Essential Oil (Thymus vulgaris) against Aedes aegypti Larvae Using a Biodegradable Controlled Release System. Ind. Crops Prod. 2019, 136, 110–120. [Google Scholar] [CrossRef]
- Saraiva, N.R.; Roncato, J.F.F.; Pascoli, M.; e Sousa, J.M.F.M.; Windberg, L.F.; Rossatto, F.C.P.; de Jesus Soares, J.; Denardin, E.L.G.; Puntel, R.L.; Zimmer, K.R.; et al. Clove Oil-Loaded Zein Nanoparticles as Potential Bioinsecticide Agent with Low Toxicity. Sustain. Chem. Pharm. 2021, 24, 100554. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal Activity of Microencapsulated Schinus molle Essential Oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Radünz, M.; dos Santos Hackbart, H.C.; Camargo, T.M.; Nunes, C.F.P.; de Barros, F.A.P.; Dal Magro, J.; Filho, P.J.S.; Gandra, E.A.; Radünz, A.L.; da Rosa Zavareze, E. Antimicrobial Potential of Spray Drying Encapsulated Thyme (Thymus vulgaris) Essential Oil on the Conservation of Hamburger-like Meat Products. Int. J. Food Microbiol. 2020, 330, 108696. [Google Scholar] [CrossRef]
- Rodea-González, D.A.; Cruz-Olivares, J.; Román-Guerrero, A.; Rodríguez-Huezo, M.E.; Vernon-Carter, E.J.; Pérez-Alonso, C. Spray-Dried Encapsulation of Chia Essential Oil (Salvia hispanica L.) in Whey Protein Concentrate-Polysaccharide Matrices. J. Food Eng. 2012, 111, 102–109. [Google Scholar] [CrossRef]
- Sugumar, S.; Ghosh, V.; Nirmala, M.J.; Mukherjee, A.; Chandrasekaran, N. Ultrasonic Emulsification of Eucalyptus Oil Nanoemulsion: Antibacterial Activity against Staphylococcus aureus and Wound Healing Activity in Wistar Rats. Ultrason. Sonochem. 2014, 21, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Suresh, U.; Murugan, K.; Panneerselvam, C.; Aziz, A.T.; Cianfaglione, K.; Wang, L.; Maggi, F. Encapsulation of Sea Fennel (Crithmum maritimum) Essential Oil in Nanoemulsion and SiO2 Nanoparticles for Treatment of the Crop Pest Spodoptera litura and the Dengue Vector Aedes aegypti. Ind. Crops Prod. 2020, 158, 113033. [Google Scholar] [CrossRef]
- Ahsaei, S.M.; Rodríguez-Rojo, S.; Salgado, M.; Cocero, M.J.; Talebi-Jahromi, K.; Amoabediny, G. Insecticidal Activity of Spray Dried Microencapsulated Essential Oils of Rosmarinus officinalis and Zataria multiflora against Tribolium Confusum. Crop Prot. 2020, 128, 104996. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Abou-Elseoud, W.S.; Elbehery, H.H.; Hassan, M.L. Chitosan-Cellulose Nanoencapsulation Systems for Enhancing the Insecticidal Activity of Citronella Essential Oil against the Cotton Leafworm Spodopteral littoralis. Ind. Crops Prod. 2022, 184, 115089. [Google Scholar] [CrossRef]
- Barros, J.M.H.F.; Santos, A.A.; Stadnik, M.J.; da Costa, C. Encapsulation of Eucalyptus and Litsea cubeba Essential Oils Using Zein Nanopolymer: Preparation, Characterization, Storage Stability, and Antifungal Evaluation. Int. J. Biol. Macromol. 2024, 278, 134690. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture, USDA. The National List of Allowed and Prohibited Substances. Available online: https://www.ams.usda.gov/rules-regulations/national-list-allowed-and-prohibited-substances (accessed on 9 November 2020).
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Pal, A.; Nakashima, K.; Yadav, B.K. Applications of Chitosan in Environmental Remediation: A review. Chemosphere 2021, 266, 128934. [Google Scholar] [CrossRef]
- Rashki, S.; Asgarpour, K.; Tarrahimofrad, H.; Hashemipour, M.; Ebrahimi, M.S.; Fathizadeh, H.; Khorshidi, A.; Khan, H.; Marzhoseyni, Z.; Salavati-Niasari, M.; et al. Chitosan-Based Nanoparticles against Bacterial Infections. Carbohydr. Polym. 2021, 251, 117108. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.-J.; Xia, S.-Q.; Hua, S.; Hayat, K.; Zhang, X.-M.; Xu, S.-Y. Optimization of Cross-Linking Parameters during Production of Transglutaminase-Hardened Spherical Multinuclear Microcapsules by Complex Coacervation. Colloids Surf. B Biointerfaces 2008, 63, 41–47. [Google Scholar] [CrossRef]
- Lv, Y.; Yang, F.; Li, X.; Zhang, X.; Abbas, S. Formation of Heat-Resistant Nanocapsules of Jasmine Essential Oil via Gelatin/Gum Arabic Based Complex Coacervation. Food Hydrocoll. 2014, 35, 305–314. [Google Scholar] [CrossRef]
- Prata, A.S.; Zanin, M.H.A.; Ré, M.I.; Grosso, C.R.F. Release Properties of Chemical and Enzymatic Crosslinked Gelatin-Gum Arabic Microparticles Containing a Fluorescent Probe Plus Vetiver Essential Oil. Colloids Surf. B Biointerfaces 2008, 67, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ma, Y.; Hayat, K.; Jia, C.; Xia, S.; Zhang, X. Morphology and Release Profile of Microcapsules Encapsulating Peppermint Oil by Complex Coacervation. J. Food Eng. 2011, 104, 455–460. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins Enhance the Antioxidant Activity of Essential Oils from Three Lamiaceae species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- da Rocha Neto, A.C.; da Rocha, A.B.D.O.; Maraschin, M.; Di Piero, R.M.; Almenar, E. Factors Affecting the Entrapment Efficiency of β-Cyclodextrins and Their Effects on the Formation of Inclusion Complexes Containing Essential Oils. Food Hydrocoll. 2018, 77, 509–523. [Google Scholar] [CrossRef]
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in Micro and Nano-Encapsulation of Bioactive Compounds Using Biopolymer and Lipid-Based Transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Tarhini, M.; Greige-Gerges, H.; Elaissari, A. Protein-based Nanoparticles: From Preparation to Encapsulation of Active Molecules. Int. J. Pharm. 2017, 522, 172–197. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, S.; Jafari, S.M.; Assadpour, E.; Khomeiri, M. Production of Pectin-Whey Protein Nano-Complexes as Carriers of Orange Peel Oil. Carbohydr. Polym. 2017, 177, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Weisany, W.; Yousefi, S.; Tahir, N.A.; Golestanehzadeh, N.; McClements, D.J.; Adhikari, B.; Ghasemlou, M. Targeted Delivery and Controlled Released of Essential Oils Using Nanoencapsulation: A Review. Adv. Colloid Interface Sci. 2022, 303, 102655. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.S.; Carvalho, S.G.; Bertoli, L.D.; Villanova, J.C.O.; Pinheiro, P.F.; dos Santos, D.C.M.; Yoshida, M.I.; de Freitas, J.C.C.; Cipriano, D.F.; Bernardes, P.C. β-Cyclodextrin Inclusion Complexes with Essential Oils: Obtention, Characterization, Antimicrobial Activity and Potential Application for Food Preservative Sachets. Food Res. Int. 2019, 119, 499–509. [Google Scholar] [CrossRef]
- Hill, L.E.; Gomes, C.; Taylor, T.M. Characterization of Beta-Cyclodextrin Inclusion Complexes Containing Essential Oils (Trans-Cinnamaldehyde, Eugenol, Cinnamon Bark, and Clove Bud Extracts) for Antimicrobial Delivery Applications. LWT—Food Sci. Technol. 2013, 51, 86–93. [Google Scholar] [CrossRef]
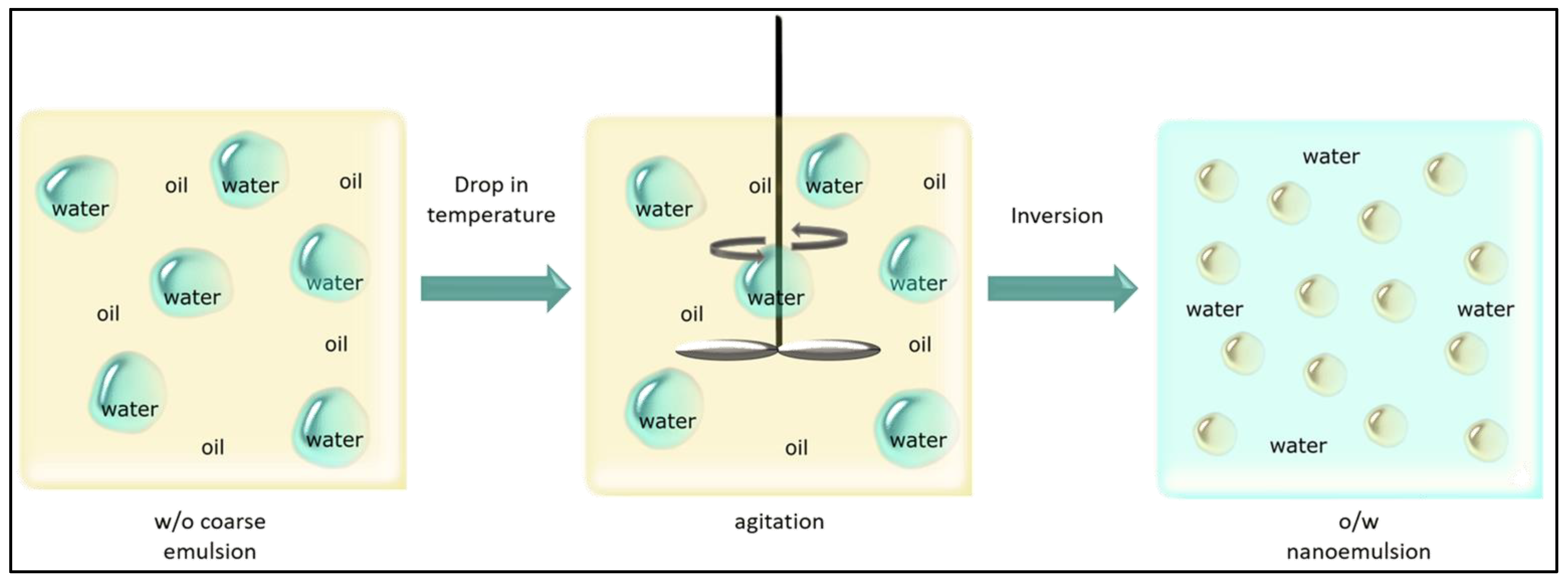
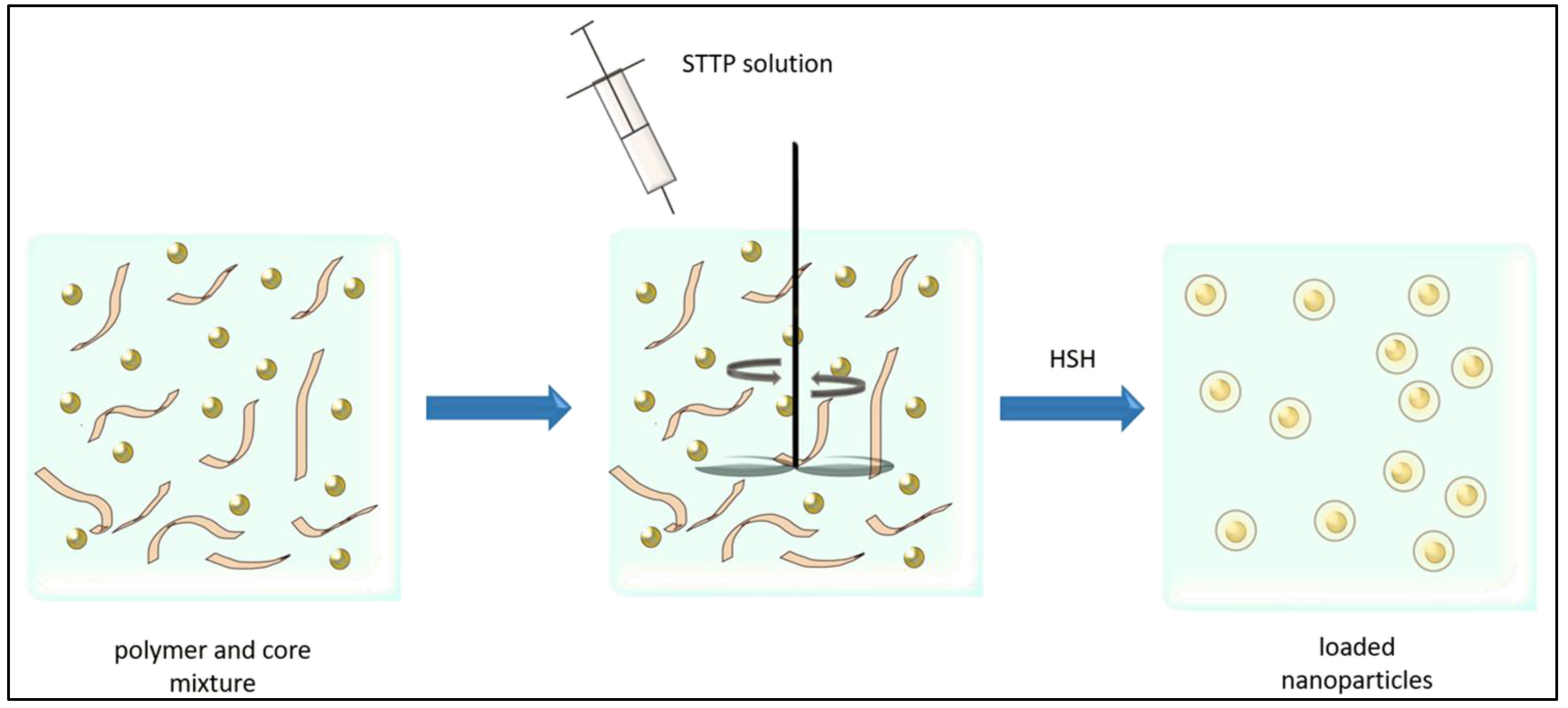
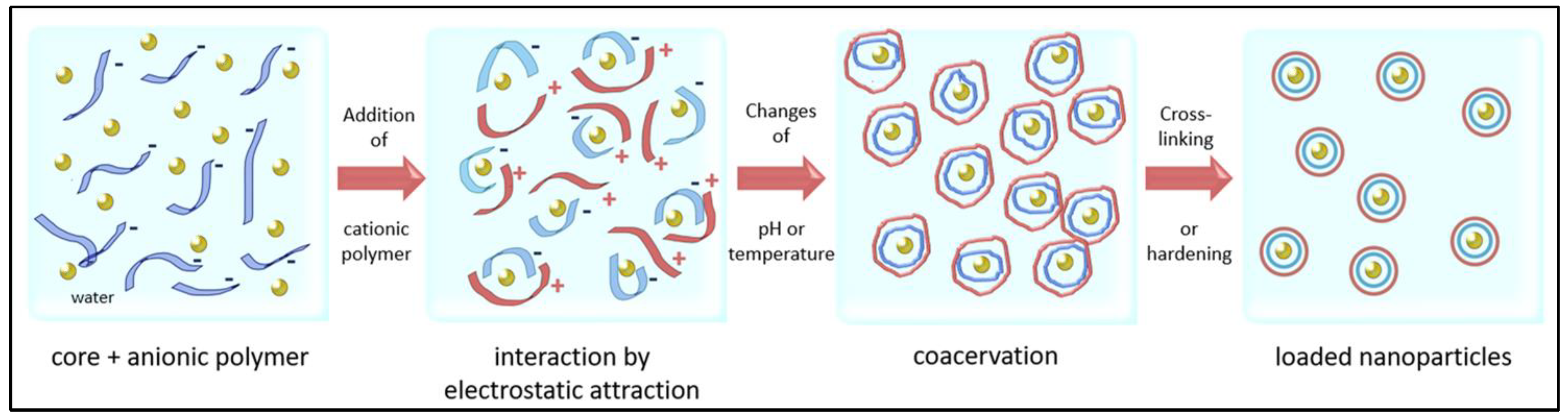
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ayllón-Gutiérrez, R.; Díaz-Rubio, L.; Montaño-Soto, M.; Haro-Vázquez, M.d.P.; Córdova-Guerrero, I. Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review. Agriculture 2024, 14, 1766. https://doi.org/10.3390/agriculture14101766
Ayllón-Gutiérrez R, Díaz-Rubio L, Montaño-Soto M, Haro-Vázquez MdP, Córdova-Guerrero I. Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review. Agriculture. 2024; 14(10):1766. https://doi.org/10.3390/agriculture14101766
Chicago/Turabian StyleAyllón-Gutiérrez, Rocío, Laura Díaz-Rubio, Myriam Montaño-Soto, María del Pilar Haro-Vázquez, and Iván Córdova-Guerrero. 2024. "Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review" Agriculture 14, no. 10: 1766. https://doi.org/10.3390/agriculture14101766
APA StyleAyllón-Gutiérrez, R., Díaz-Rubio, L., Montaño-Soto, M., Haro-Vázquez, M. d. P., & Córdova-Guerrero, I. (2024). Applications of Plant Essential Oils in Pest Control and Their Encapsulation for Controlled Release: A Review. Agriculture, 14(10), 1766. https://doi.org/10.3390/agriculture14101766







