Excellent Canopy Structure in Soybeans Can Improve Their Photosynthetic Performance and Increase Yield
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
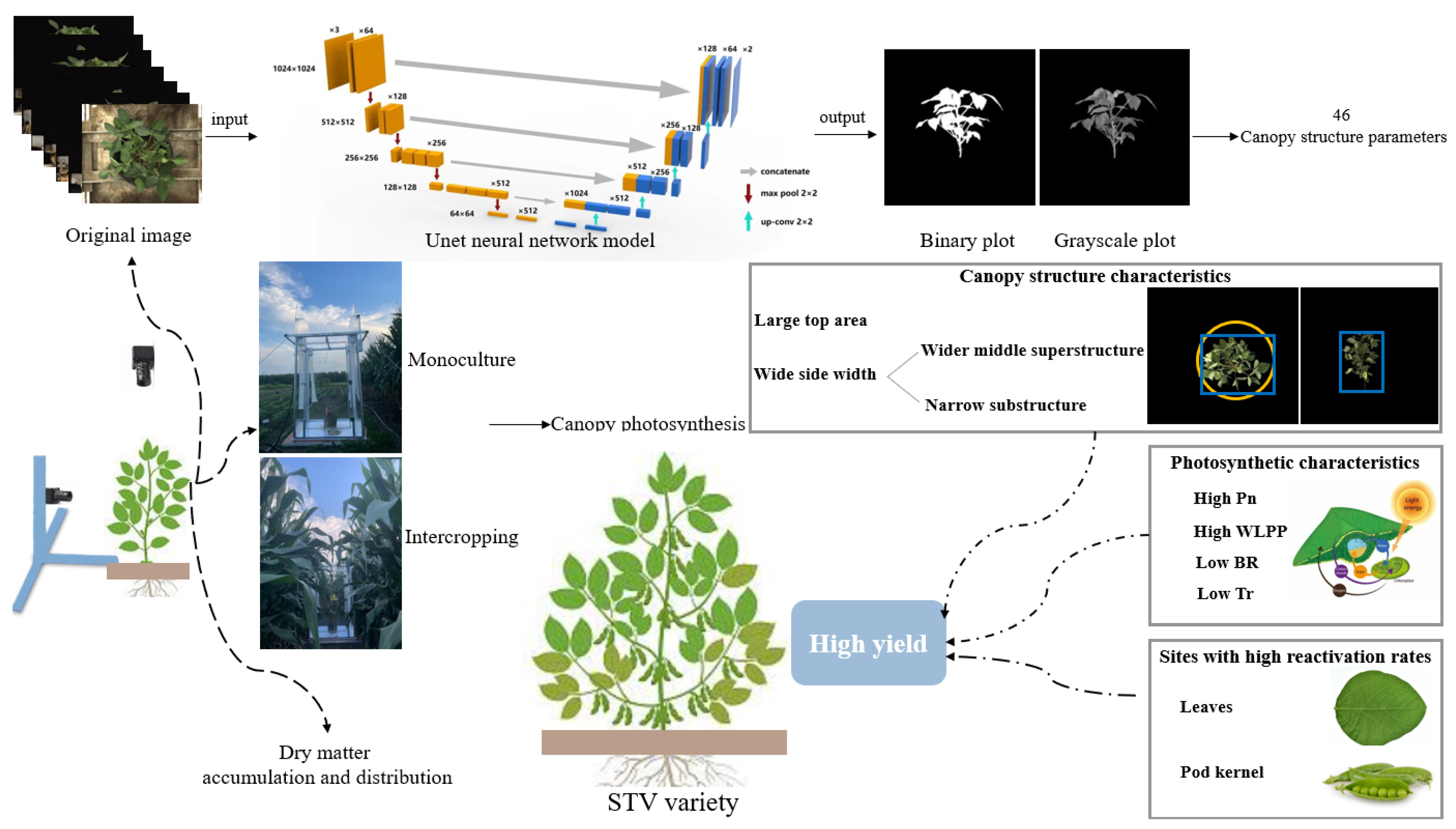
2.2. Canopy Structure Acquisition
2.3. Single-Leaf Photosynthesis Measurement
2.4. Canopy Photosynthetic Rate Measurement
2.5. Analysis of Plant Growth and Development
2.6. Data Analysis and Processing
3. Results
3.1. Growth and Development of Different Shade-Tolerant Soybeans
3.1.1. Accumulation of Dry Matter in Above-Ground Organs
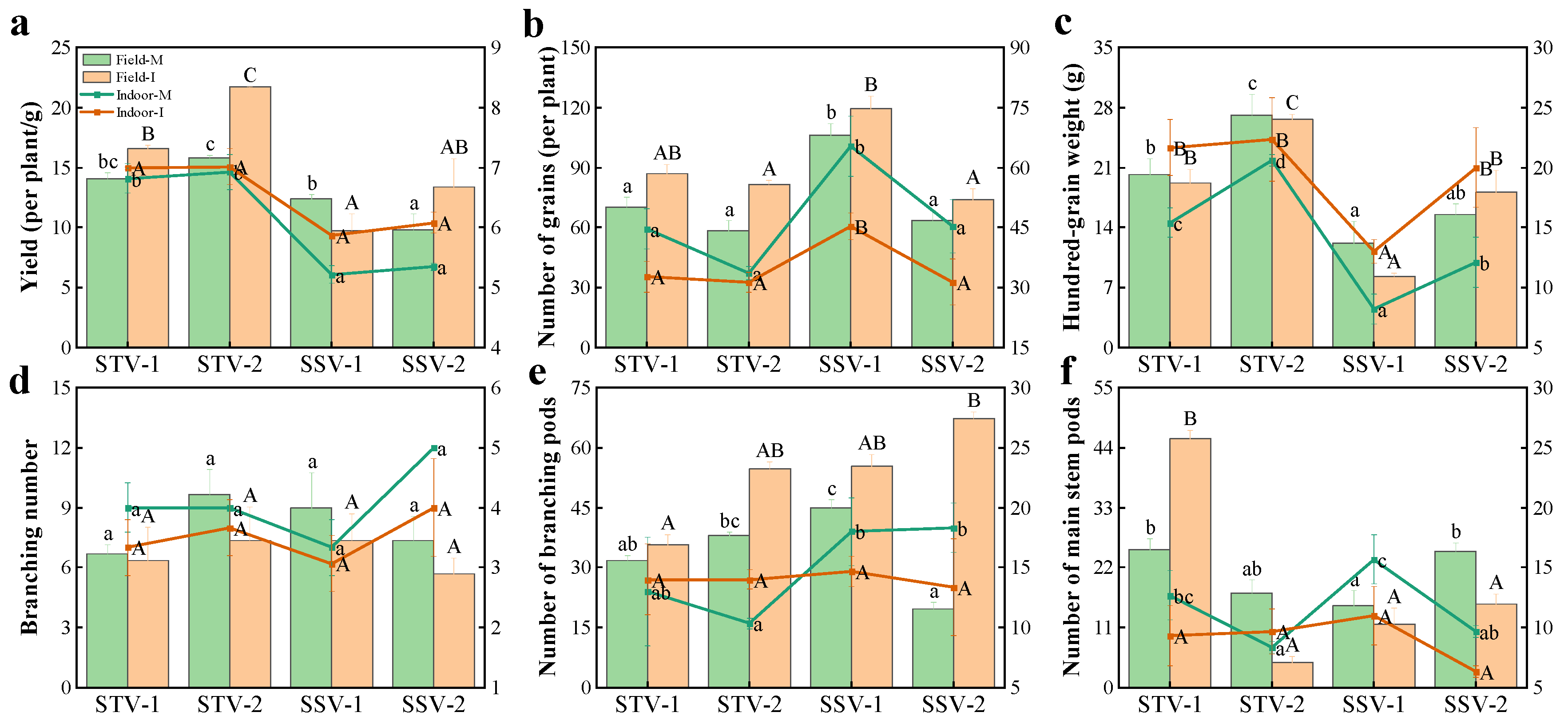
3.1.2. Yield and Yield Composition of Different Shade-Tolerant Soybeans
3.2. Single-Leaf Photosynthetic Characteristics of Different Shade-Tolerant Soybeans
3.3. Canopy Photosynthetic Characteristics of Different Shade-Tolerant Soybeans
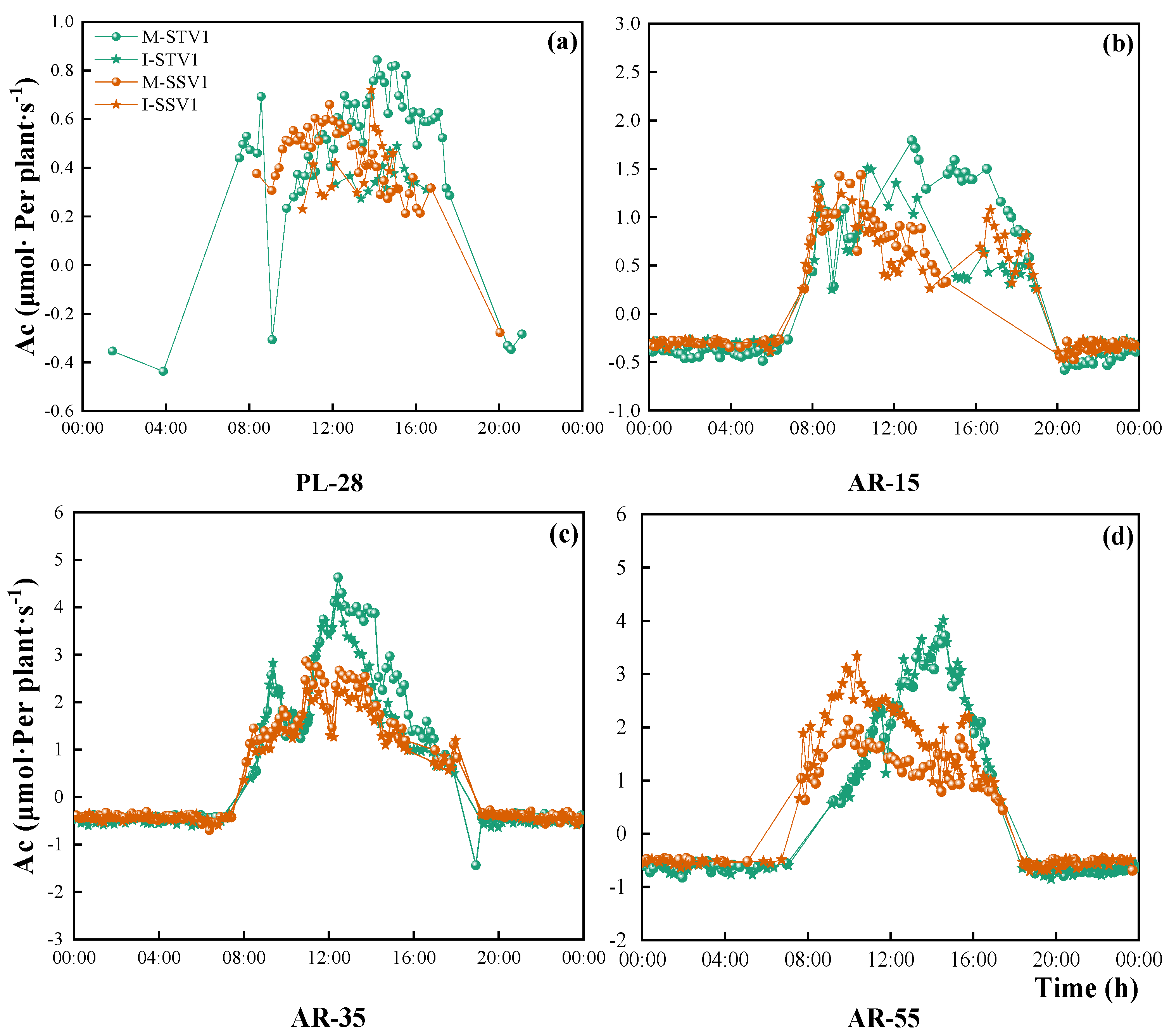
3.3.1. Diurnal Variation of Photosynthetic Rate
3.3.2. Optical Response Curve
3.3.3. Whole-Leaf Photosynthetic Potential
3.4. Selection of Canopy Structure Parameters
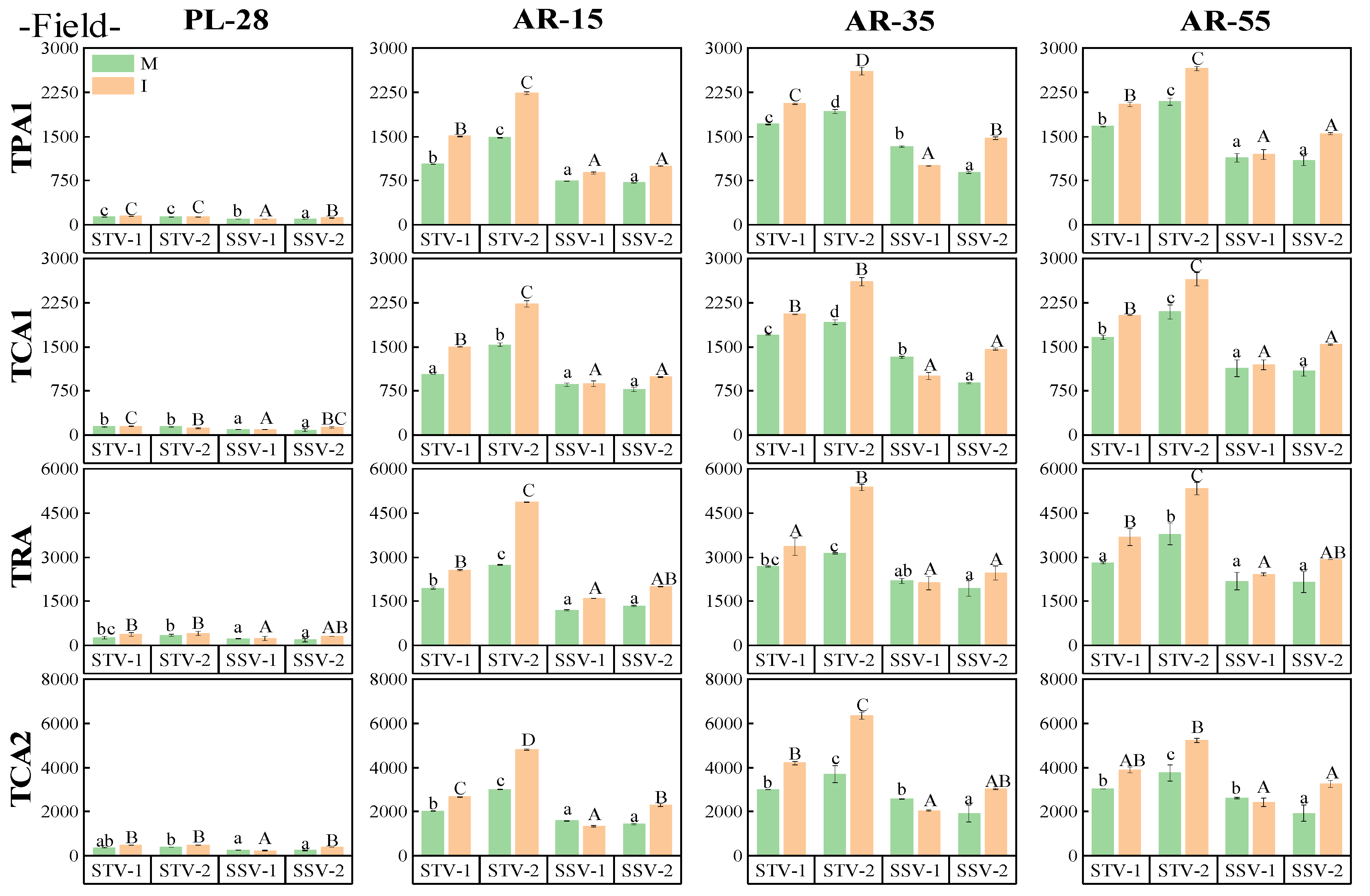
3.4.1. Relationship between the Canopy Structure and Canopy Photosynthesis
3.4.2. Regression Analysis between Canopy Structure Parameters and Yield
3.5. The Canopy Structure of Different Shade-Tolerant Soybeans
3.5.1. Top Parameters
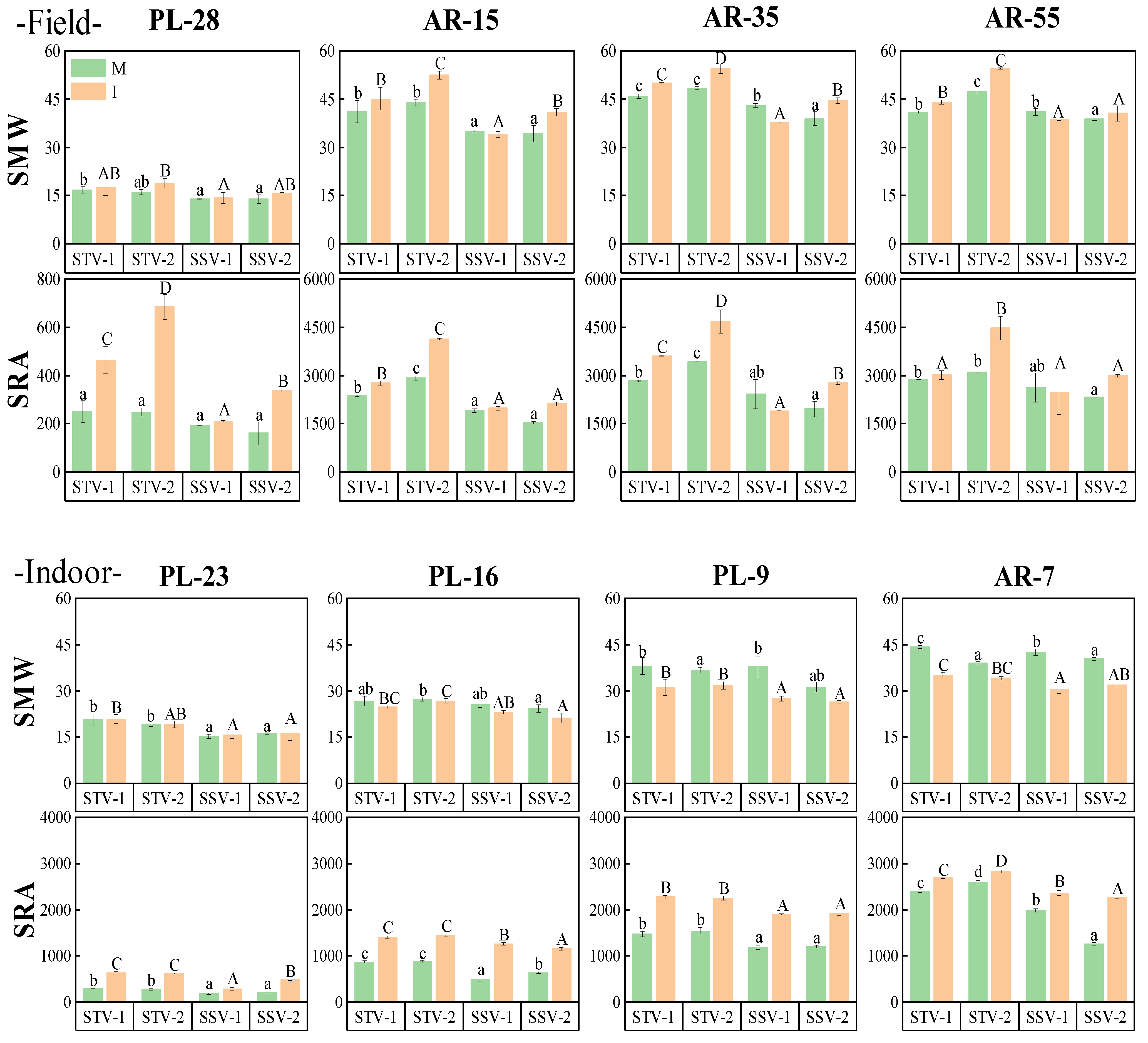
3.5.2. Side Parameters
3.5.3. The Canopy Overlap Ratio
4. Discussion
4.1. The High Biomass Productivity in the Source and Reservoir Provided Advantages for the High Yield of STV Varieties
4.2. High Photosynthesis Provides the Power for Biomass Productivity of STV Varieties
4.3. Excellent Canopy Structure Provided a Guarantee for High Photosynthesis of STV Varieties
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fu, J.J.; Lyu, D.; Sun, J. China’s Grain Trade Research Based on DEA model of National Food Security Perspective: Soybean as an Example. Teh. Vjesn. Tech. Gaz. 2021, 28, 609–615. [Google Scholar]
- Sun, J.; Yang, L.; Zhao, F.Q.; Wu, W.B. Domestic dynamics of crop production in response to international food trade: Evidence from soybean imports in China. J. Land Use Sci. 2020, 15, 91–98. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, B.; Zhou, T.; Jing, S.Z.; Liu, R.J.; Gao, Y.; Deng, C.Y.; Ye, W.W.; Luo, Z.G.; Raza, A.; et al. Quantifying the effects of plant density on soybean lodging resistance and growth dynamics in maize-soybean strip intercropping. Front. Plant Sci. 2023, 14, 1264378. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, L.; Gao, Y.; Guo, Y.K.; Zheng, N.W.; Xu, X.Y.; Xu, M.; Wang, W.Y.; Liu, C.Y.; Liu, W.G.; et al. Genome-Wide Identification of SMXL Gene Family in Soybean and Expression Analysis of GmSMXLs under Shade Stress. Plant 2022, 11, 2410. [Google Scholar] [CrossRef]
- Liu, S.P.; Wang, L.X.; Chang, L.; Khan, I.; Nadeem, F.; Rehman, A.; Suo, R. Evaluating the influence of straw mulching and intercropping on nitrogen uptake, crop growth, and yield performance in maize and soybean. Front. Plant Sci. 2023, 14, 1280382. [Google Scholar] [CrossRef]
- Robert, P.; Gouis, J.L.; Consortium, T.B.; Rincent, R. Combining Crop Growth Modeling With Trait-Assisted Prediction Improved the Prediction of Genotype by Environment Interactions. Front. Plant Sci. 2020, 11, 827. [Google Scholar] [CrossRef]
- Onogi, A. Integration of Crop Growth Models and Genomic Prediction. Genom. Predict. Complex Trait. Methods Protoc. 2022, 2467, 359–396. [Google Scholar]
- Choudhury, S.D.; Samal, A.; Awada, T. Leveraging Image Analysis for High-Throughput Plant Phenotyping. Front. Plant Sci. 2019, 10, 508. [Google Scholar] [CrossRef]
- Song, P.; Wang, J.L.; Guo, X.Y.; Yang, W.N.; Zhao, C.J. High-throughput phenotyping: Breaking through the bottleneck in future crop breeding. Crop J. 2021, 9, 633–645. [Google Scholar] [CrossRef]
- He, S.Y.; Li, X.N.; Chen, M.G.; Tang, F.D.; Gong, T.; Xu, M.; Yang, W.Y.; Liu, W.G. Crop HTP Technologies: Applications and Prospects. Agriculture 2024, 14, 723. [Google Scholar] [CrossRef]
- Adachi, S.; Tanaka, Y.; Miyagi, A.; Kashima, M.; Tezuka, A.; Toya, Y.; Kobayashi, S.; Ohkubo, S.; Shimizu, H.; Kawai-Yamada, M.; et al. High-yielding rice Takanari has superior photosynthetic response to a commercial rice Koshihikari under fluctuating light. J. Exp. Bot. 2019, 70, 5287–5297. [Google Scholar] [CrossRef] [PubMed]
- Russell, G. Canopy architecture. Encycl. Appl. Plant Sci. 2017, 3, 13–17. [Google Scholar] [CrossRef]
- Song, Q.F.; Xiao, H.; Xiao, X.L.; Zhu, X.G. A new canopy photosynthesis and transpiration measurement system (CAPTS) for canopy gas exchange research. Agric. For. Meteorol. 2016, 217, 101–107. [Google Scholar] [CrossRef]
- Xu, C.L.; Li, R.D.; Song, W.W.; Wu, T.T.; Sun, S.; Han, T.F.; Wu, C.X. High Density and Uniform Plant Distribution Improve Soybean Yield by Regulating Population Uniformity and Canopy Light Interception. Agronomy 2021, 11, 1880. [Google Scholar] [CrossRef]
- Zhao, W.; Ren, T.H.; Huang, X.Y.; Xu, Z.; Zhou, Y.Z.; Yin, C.L.; Zhao, R.; Liu, S.B.; Ning, T.Y.; Li, G. Leaf shape, planting density, and nitrogen application affect soybean yield by changing direct and diffuse light distribution in the canopy. Plant Physiol. Biochem. 2023, 204, 108071. [Google Scholar] [CrossRef]
- Ye, Z.P.; Yu, Q. Comparison of photocooperative photoresponse models. Chin. J. Plant Ecol. 2008, 32, 1356–1361. (In Chinese) [Google Scholar]
- Zhang, H.Y.; Tian, P.; Mei, N.; Sui, P.X.; Zhang, W.K.; Qi, H. Effects of Light Stress and Light Recovery on Two Maize (Zea mays L.) Cultivars. Bangladesh J. Bot. 2019, 48, 513–520. [Google Scholar] [CrossRef]
- Puteh, A.B.; Mondal, N.M.A.; Ismail, M.R.; Latif, M.A. Grain Sterility in relation to Dry Mass Production and Distribution in Rice (Oryza sativa L.). BioMed Res. Int. 2014, 2014, 302179. [Google Scholar] [CrossRef]
- Chen, B.L.; Wang, Q.H.; Tang, X.X.; Ye, Z.P.; Stiles, S.; Feng, G. Growth and sink-source relationship of cotton (Gossypium hirsutum L.) under mulched drip irrigation in response to phosphorus fertilization and cultivar. J. Plant Nutr. 2021, 45, 259–272. [Google Scholar] [CrossRef]
- Zhu, X.G.; Ort, D.R.; Parry, M.A.J.; von Caemmerer, S. A wish list for synthetic biology in photosynthesis research. J. Exp. Bot. 2020, 71, 2219–2225. [Google Scholar] [CrossRef]
- Du, W.G.; Gai, J.Y. Discussion on research progress of soybean breeding for super high yield. Soils Crop. 2014, 3, 81–92. (In Chinese) [Google Scholar]
- Suo, R.Z.; Wang, M.J.; Zhao, T.Q. Contribution of Photosynthetic, Root and Phenotypic Traits to Soybean Plant Height. Sustainability 2024, 16, 2886. [Google Scholar] [CrossRef]
- Virdi, K.S.; Sreekanta, S.; Dobbels, A.; Haaning, A.; Jarquin, D.; Stupar, R.M.; Lorenz, A.J.; Muehlbauer, G.J. Branch angle and leaflet shape are associated with canopy coverage in soybean. Plant Genome 2023, 16, e20304. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.J.; Zhang, Y.; Du, J.J.; Guo, X.Y.; Wen, W.L.; Gu, S.H.; Wang, J.L.; Fan, J.C. Crop Phenomics: Current Status and Perspectives. Front. Plant Sci. 2019, 10, 714. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.C.; Li, Y.L.; Yu, S.; Gou, W.B.; Zhao, C.J. Application of Internet of Things to Agriculture—The LQ-FieldPheno Platform: A High-Throughput Platform for Obtaining Crop Phenotypes in Field. Sci. Partn. J. 2023, 6, 0059. [Google Scholar] [CrossRef]
- Tillier, L.C.; Murchie, E.H.; Sparkes, D.L. Does canopy angle influence radiation use efficiency of sugar beet? Field Crop Res. 2023, 293, 108841. [Google Scholar] [CrossRef]
- Chang, T.G.; Zhao, H.L.; Wang, N.; Song, Q.F.; Xiao, Y.; Qu, M.N.; Zhu, X.G. A three-dimensional canopy photosynthesis model in rice with a complete description of the canopy architecture, leaf physiology, and mechanical properties. J. Exp. Bot. 2019, 70, 2479–2490. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Xu, S.J.; Wei, Q.R.; Yang, Y.X.; Pan, H.Q.; Fu, X.L.; Fan, Z.H.; Qin, B.T.; Wang, X.C.; Ma, X.M.; et al. Variation in Leaf Type, Canopy Architecture, and Light and Nitrogen Distribution Characteristics of Two Winter Wheat (Triticum aestivum L.) Varieties with High Nitrogen-Use Efficiency. Agronomy 2022, 12, 2411. [Google Scholar] [CrossRef]
- Hussain, S.; Pang, T.; Lqbal, N.; Shafiq, I.; Skalicky, M.; Brestic, M.; Safdar, M.E.; Mumtaz, M.; Ahmad, A.; Asghar, M.A. Acclimation strategy and plasticity of different soybean genotypes in intercropping. Funct. Plant Biol. 2020, 47, 592–610. [Google Scholar] [CrossRef]
- Li, X.; Chen, M.; He, S.; Xu, X.; He, L.; Wang, L.; Gao, Y.; Tang, F.; Gong, T.; Wang, W.; et al. Estimation of soybean yield based on high-throughput phenotyping and machine learning. Front. Plant Sci. 2024, 15, 1395760. [Google Scholar] [CrossRef]
- Liu, N.; Li, L.; Li, H.T.; Liu, Z.M.; Lu, Y.; Shao, L.W. Selecting maize cultivars to regulate canopy structure and light interception for high yield. Agron. J. 2022, 115, 770–780. [Google Scholar] [CrossRef]
- Cheng, Y.X.; Xiao, F.; Huang, D.Y.; Yang, Y.; Cheng, W.D.; Jin, S.C.; Li, G.H.; Ding, Y.F.; Paul, M.J.; Liu, Z.H. High canopy photosynthesis before anthesis explains the outstanding yield performance of rice cultivars with ideal plant architecture. Field Crop. Res. 2024, 306, 109223. [Google Scholar] [CrossRef]
- Furbank, R.T.; Jimenez-Berni, J.A.; George-Jaeggli, B.; Potgieter, A.B.; Deery, D.M. Field crop phenomics: Enabling breeding for radiation use efficiency and biomass in cereal crops. New Phytol. 2019, 223, 1714–1727. [Google Scholar] [CrossRef]
- Ma, X.D.; Zhu, K.X.; Guan, H.O.; Feng, J.R.; Yu, S.; Liu, G. High-Throughput Phenotyping Analysis of Potted Soybean Plants Using Colorized Depth Images Based on A Proximal Platform. Remote Sens. 2019, 11, 1085. [Google Scholar] [CrossRef]
- Li, X.N.; Xu, X.Y.; Xiang, S.; Chen, M.G.; He, S.Y.; Wang, W.Y.; Xu, M.; Liu, C.Y.; Yu, L.; Liu, W.G.; et al. Soybean leaf estimation based on RGB images and machine learning methods. Plant Methods 2023, 19, 59. [Google Scholar] [CrossRef]
- Sreekanta, S.; Haaning, A.; Dobbels, A.; O’Neill, R.; Hofstad, A.; Virdi, K.; Katagiri, F.; Stupar, R.M.; Muehlbauer, G.J.; Lorenz, A.J. Variation in shoot architecture traits and their relationship to canopy coverage and light interception in soybean (Glycine max). BMC Plant Biol. 2024, 24, 194. [Google Scholar] [CrossRef] [PubMed]
- Falchi, R.; Bonghi, C.; Drincovich, M.F.; Famiani, F.; Lara, M.V.; Walker, R.P.; Vizzotto, G. Sugar Metabolism in Stone Fruit: Source-Sink Relationships and Environmental and Agronomical Effects. Front. Plant Sci. 2020, 11, 573982. [Google Scholar] [CrossRef]
- Yan, S.C.; Wu, Y.; Fan, J.L.; Zhang, F.C.; Zheng, J.; Guo, J.J.; Lu, J.S.; Wu, L.F.; Qiang, S.C.; Xiang, Y.Z. Source-sink relationship and yield stability of two maize cultivars in response to water and fertilizer inputs in northwest China. Agric. Water Manag. 2022, 262, 107331. [Google Scholar] [CrossRef]
- Lu, M.Z.; Snyder, R.; Grant, J.; Tegeder, M. Manipulation of sucrose phloem and embryo loading affects pea leaf metabolism, carbon and nitrogen partitioning to sinks as well as seed storage pools. Plant J. 2019, 101, 217–236. [Google Scholar] [CrossRef]
- Fu, Z.D.; Chen, P.; Zhang, X.N.; Du, Q.; Zheng, B.C.; Yang, H.; Luo, K.; Lin, P.; Li, Y.L.; Pu, T.; et al. Maize-legume intercropping achieves yield advantages by improving leaf functions and dry matter partition. BMC Plant Biol. 2023, 23, 438. [Google Scholar] [CrossRef]
- Egashira, C.; Hashiguchi, Y.; Kurauchi, E.; Tatsumi, Y.; Nakagawa, A.C.S.; Hamaoka, N.; Yuasa, T.; Iwaya-inoue, M.Y.; Ishibashi, Y. A rapid translocation of photoassimilates from source organs maintains grain yield in cowpea subjected to drought stress during grain filling. Biol. Plant. 2019, 64, 529–534. [Google Scholar] [CrossRef]
- Panda, D.; Dash, G.K.; Mohanty, S.; Sekhar, S.; Roy, A.; Tudu, C.; Behera, L.; Tripathy, B.C.; Baig, M.J. Phytochrome A mediated modulation of photosynthesis, development and yield in rice (Oryza sativa L.) in fluctuating light environment. Environ. Exp. Bot. 2023, 206, 105183. [Google Scholar] [CrossRef]
- Yang, H.K.; Zhao, J.R.; Ma, H.L.; Shi, Z.Q.; Huang, X.L.; Fan, G.Q. Shading affects the starch structure and digestibility of wheat by regulating the photosynthetic light response of flag leaves. Int. J. Biol. Macromol. 2023, 236, 123972. [Google Scholar] [CrossRef]
- Zheng, B.; Zhao, W.; Ren, T.H.; Zhang, X.H.; Ning, T.Y.; Liu, P.; Li, G. Low Light Increases the Abundance of Light Reaction Proteins: Proteomics Analysis of Maize (Zea mays L.) Grown at High Planting Density. Int. J. Mol. Sci. 2022, 23, 3015. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Shi, L.; Shi, A.P.; Zhang, Q.X. Photosynthetic responses of four Hosta cultivars to shade treatments. Photosyntheti 2004, 42, 213–218. [Google Scholar] [CrossRef]
- Fan, Y.H.; Lv, Z.Y.; Qin, B.Y.; Yang, J.H.; Ren, K.M.; Liu, Q.X.; Jiang, F.Y.; Zhang, W.J.; Ma, S.Y.; Ma, C.X.; et al. Night warming at the vegetative stage improves pre-anthesis photosynthesis and plant productivity involved in grain yield of winter wheat. Plant Physiol. Biochem. 2022, 186, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.S.; Guo, Z.P.; Xiao, J.X.; Dong, K.; Dong, Y. Effects of Applied Ratio of Nitrogen on the Light Environment in the Canopy and Growth, Development and Yield of Wheat When Intercropped. Front. Plant Sci. 2021, 12, 719850. [Google Scholar] [CrossRef]
- Deng, F.; Li, B.; Yuan, Y.J.; He, C.Y.; Zhou, X.; Li, Q.P.; Zhu, Y.Y.; Huang, X.F.; He, Y.X.; Ai, X.F.; et al. Increasing the number of seedlings per hill with reduced number of hills improves rice grain quality by optimizing canopy structure and light utilization under shading stress. Field Crop. Res. 2022, 287, 108668. [Google Scholar] [CrossRef]
- Liang, F.B.; Yang, C.X.; Sui, L.L.; Xu, S.Z.; Yao, H.S.; Zhang, W.F. Flumetralin and dimethyl piperidinium chloride alter light distribution in cotton canopies by optimizing the spatial configuration of leaves and bolls. J. Integr. Agric. 2020, 19, 1777–1788. [Google Scholar] [CrossRef]
- Oikawa, S.; Ainsworth, E.A. Changes in leaf area, nitrogen content and canopy photosynthesis in soybean exposed to an ozone concentration gradient. Environ. Pollut. 2016, 215, 347–355. [Google Scholar] [CrossRef]
- Li, S.W.; van Der Werf, W.; Zhu, J.Q.; Guo, Y.; Li, B.G.; Ma, Y.T.; Evers, J.B. Estimating the contribution of plant traits to light partitioning in simultaneous maize/soybean intercropping. J. Exp. Bot. 2021, 72, 3630–3646. [Google Scholar] [CrossRef] [PubMed]
- Gou, F.; van Ittersum, M.K.; Simon, E.; Leffelaar, P.A.; van der Putten, P.E.L.; Zhang, L.Z.; van der Werf, W. Intercropping wheat and maize increases total radiation interception and wheat RUE but lowers maize RUE. Eur. J. Agron. 2017, 84, 125–139. [Google Scholar] [CrossRef]
- Bi, Y.L.; Zhou, H.L. Changes in peanut canopy structure and photosynthetic characteristics induced by an arbuscular mycorrhizal fungus in a nutrient-poor environment. Sci. Rep. 2021, 11, 14832. [Google Scholar] [CrossRef] [PubMed]
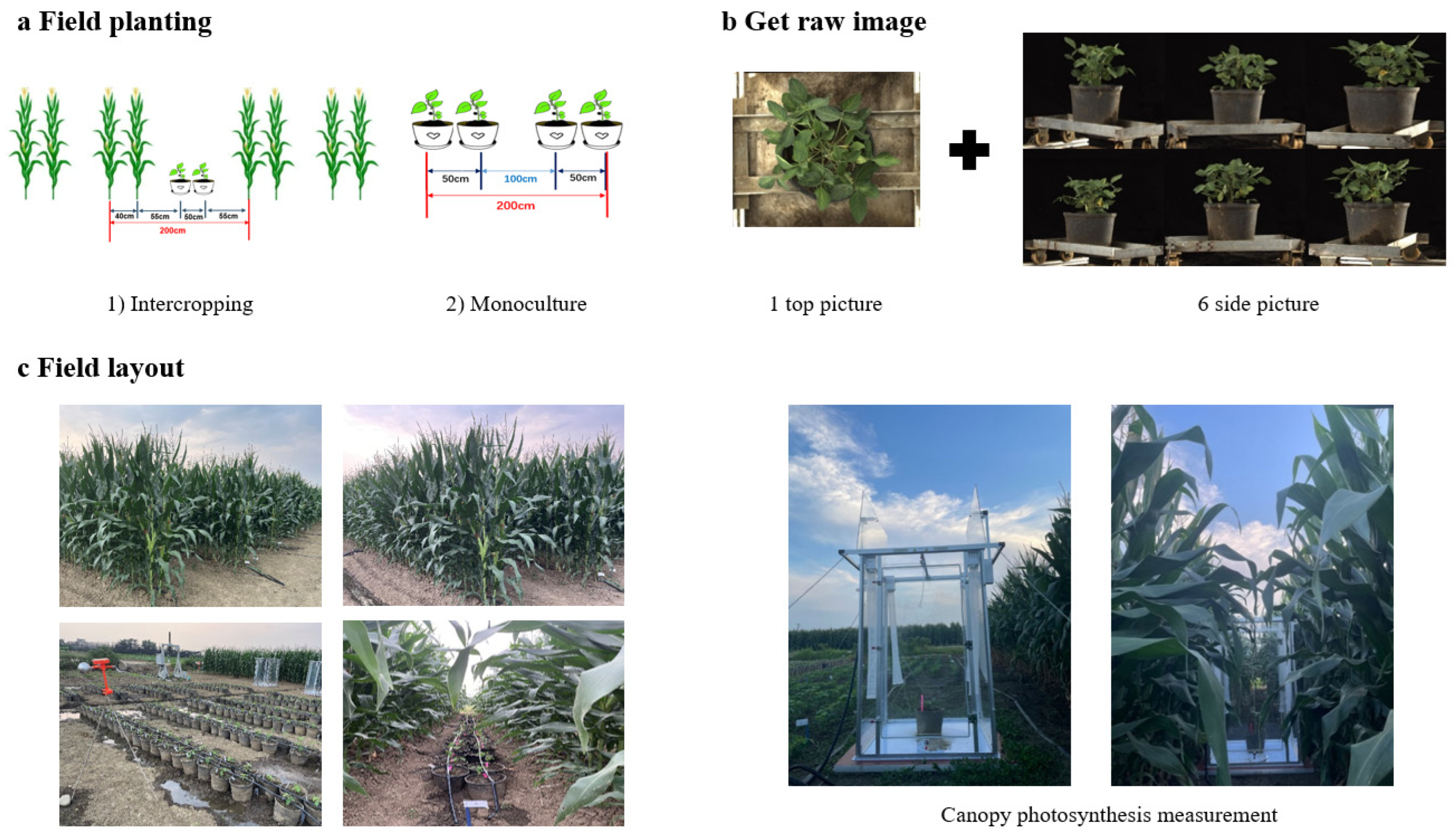





| Index | Variety | Environment | Measurement Period |
|---|---|---|---|
| Canopy structure | STV-1,2; SSV-1,2 | Field, | PL-28d; AR-15, 35, 55d |
| Indoor | PL-23, 16, 9d; AR-7d | ||
| Single-leaf photosynthesis | STV-1,2; SSV-1,2 | Indoor | PL-23, 16, 9d; AR-7d |
| Canopy photosynthesis | STV-1, SSV-1 | Field | PL-28d; AR-15, 35, 55d |
| Growth and development monitoring | STV-1,2; SSV-1,2 | Field | Period V1-R5 |
| Treatment | Variety | Planting Pattern | RR (%) | CR (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Leaves | Stem | Branch | Pods | Synthetic Sort | ||||
| Filed | STV-1 | M | 32.4 b | 2.5 a | 2.8 a | 6.3 b | 4 | 59.15 bc |
| STV-2 | 30.9 b | 3.0 a | 4.3 a | 6.5 b | 3 | 61.80 c | ||
| SSV-1 | 20.0 a | 4.6 a | 1.9 a | 4.0 ab | 6 | 58.81 b | ||
| SSV-2 | 19.4 a | 4.8 a | 3.0 a | 3.3 a | 7 | 22.92 a | ||
| STV-1 | I | 36.3 BC | 3.2 A | 4.0 B | 8.9 C | 2 | 74.61 A | |
| STV-2 | 46.4 C | 4.8 A | 4.6 B | 10.4 C | 1 | 62.76 A | ||
| SSV-1 | 19.7 A | 3.9 A | 1.2 A | 2.0 A | 8 | 53.86 A | ||
| SSV-2 | 29.5 AB | 5.2 A | 3.1 AB | 4.9 B | 5 | 55.42 A | ||
| Indoor | STV-1 | M | 0.34 c | 0.25 b | 0.68 c | 0.25 c | 4 | 76.85 c |
| STV-2 | 0.47 d | 0.16 a | 0.82 d | 0.30 d | 3 | 88.48 d | ||
| SSV-1 | 0.21 b | 0.35 c | 0.35 a | 0.17 a | 8 | 59.45 b | ||
| SSV-2 | 0.15 a | 0.36 c | 0.40 b | 0.22 b | 7 | 52.18 a | ||
| STV-1 | I | 0.38 C | 0.29 B | 0.71 C | 0.41 C | 2 | 44.92 C | |
| STV-2 | 0.51 D | 0.14 A | 0.75 D | 0.46 D | 1 | 72.99 D | ||
| SSV-1 | 0.20 B | 0.34 C | 0.66 B | 0.22 A | 6 | 37.32 B | ||
| SSV-2 | 0.05 A | 0.52 D | 0.62 A | 0.25 B | 5 | 29.45 A | ||
| Index | Period | Treatment | STV-1 | STV-2 | SSV-1 | SSV-2 |
|---|---|---|---|---|---|---|
| Pn µmol·m−2·s−1 | PL-23 | NL | 15.09 ± 0.45 c | 14.70 ± 1.04 c | 12.95 ± 1.18 b | 10.92 ± 0.15 a |
| ST | 8.34 ± 1.09 C | 7.38 ± 1.36 BC | 5.14 ± 0.68 A | 5.98 ± 0.63 AB | ||
| PL-16 | NL | 18.48 ± 0.06 b | 18.91 ± 0.83 b | 14.60 ± 1.21 a | 17.80 ± 0.32 b | |
| ST | 17.19 ± 0.79 C | 16.87 ± 0.14 BC | 14.44 ± 0.53 A | 16.00 ± 0.65 B | ||
| PL-9 | NL | 15.82 ± 0.14 c | 14.65 ± 0.72 b | 13.31 ± 0.13 a | 12.68 ± 0.06 a | |
| ST | 15.54 ± 0.72 C | 14.53 ± 1.55 BC | 13.06 ± 0.14 AB | 12.22 ± 0.55 A | ||
| AR-7 | NL | 8.38 ± 0.22 ab | 9.14 ± 0.22 b | 8.92 ± 0.18 ab | 8.08 ± 0.99 a | |
| ST | 7.91 ± 2.58 AB | 8.82 ± 1.52 B | 8.44 ± 0.72 B | 5.07 ± 1.43 A | ||
| Tr mmol·m−2·s−1 | PL-23 | NL | 5.11 ± 0.07 b | 4.12 ± 0.31 a | 3.40 ± 0.83 a | 3.28 ± 0.55 a |
| ST | 2.55 ± 0.94 B | 1.52 ± 0.57 AB | 1.38 ± 0.30 AB | 1.12 ± 0.64 A | ||
| PL-16 | NL | 3.72 ± 0.26 a | 3.99 ± 0.36 ab | 4.07 ± 0.54 ab | 4.76 ± 0.78 b | |
| ST | 5.69 ± 0.31 AB | 4.47 ± 0.39 A | 6.95 ± 0.62 B | 6.16 ± 1.92 AB | ||
| PL-9 | NL | 3.39 ± 0.23 b | 2.74 ± 0.59 ab | 2.96 ± 0.18 ab | 2.47 ± 0.13 a | |
| ST | 4.20 ± 0.71 A | 3.36 ± 1.26 A | 4.02 ± 0.13 A | 4.20 ± 2.08 A | ||
| AR-7 | NL | 1.67 ± 0.62 ab | 1.12 ± 0.38 a | 2.02 ± 0.44 b | 0.93 ± 0.18 a | |
| ST | 1.15 ± 0.81 A | 1.44 ± 0.16 A | 1.41 ± 0.29 A | 0.86 ± 0.47 A | ||
| Ci µmol·m−2·s−1 | PL-23 | NL | 333.47 ± 1.2 c | 322.07 ± 19.8 ab | 298.61 ± 18.6 a | 317.95 ± 12.8 ab |
| ST | 327.93 ± 23.9 B | 296.24 ± 14.6 A | 301.66 ± 1.5 AB | 295.88 ± 11.5 A | ||
| PL-16 | NL | 295.22 ± 6.46 a | 296.02 ± 13.05 a | 320.01 ± 7.73 b | 312.42 ± 12.66 ab | |
| ST | 352.40 ± 7.78 A | 330.92 ± 18.85 A | 353.04 ± 6.44 A | 336.90 ± 26.90 A | ||
| PL-9 | NL | 311.93 ± 14.48 b | 262.55 ± 30.49 a | 295.54 ± 16.34 ab | 253.32 ± 27.71 a | |
| ST | 320.22 ± 17.90 A | 286.96 ± 38.11 A | 306.85 ± 5.50 A | 313.89 ± 33.10 A | ||
| AR-7 | NL | 276.46 ± 28.63 b | 218.03 ± 26.50 a | 258.65 ± 10.79 b | 203.88 ± 8.48 a | |
| ST | 217.64 ± 48.11 A | 241.89 ± 7.39 A | 233.64 ± 31.66 A | 232.02 ± 55.36 A | ||
| Gs µmol·m−2·s−1 | PL-23 | NL | 0.50 ± 0.05 b | 0.37 ± 0.05 a | 0.28 ± 0.08 a | 0.27 ± 0.05 a |
| ST | 0.24 ± 0.14 B | 0.11 ± 0.04 AB | 0.08 ± 0.01 A | 0.09 ± 0.05 AB | ||
| PL-16 | NL | 0.31 ± 0.03 a | 0.33 ± 0.04 a | 0.34 ± 0.05 a | 0.41 ± 0.09 a | |
| ST | 0.62 ± 0.09 B | 0.34 ± 0.09 A | 0.66 ± 0.09 B | 0.57 ± 0.22 AB | ||
| PL-9 | NL | 0.34 ± 0.06 b | 0.19 ± 0.05 a | 0.26 ± 0.03 ab | 0.17 ± 0.04 a | |
| ST | 0.41 ± 0.04 A | 0.26 ± 0.12 A | 0.33 ± 0.03 A | 0.36 ± 0.24 A | ||
| AR-7 | NL | 0.12 ± 0.04 b | 0.08 ± 0.02 ab | 0.13 ± 0.01 b | 0.06 ± 0.02 a | |
| ST | 0.08 ± 0.06 A | 0.10 ± 0.01 A | 0.10 ± 0.02 A | 0.06 ± 0.03 A |
| Canopy Structure | Meaning | Pn | Significance | |
|---|---|---|---|---|
| Top parameter | Top perimeter | TP | 0.80 | *** |
| Top projected area | TPA1 | 0.91 | *** | |
| Top contour area | TCA1 | 0.92 | *** | |
| Top rectangular width | TRW | 0.86 | *** | |
| Top rectangular height | TRH | 0.91 | *** | |
| Top outer circle radius | TCR | 0.90 | *** | |
| Top rectangular area | TRA | 0.91 | *** | |
| Top circle area | TCA2 | 0.91 | *** | |
| Top circle compactness | TCC | 0.46 | ns | |
| The ratio of the area of the top projection to the area of the outer rectangle | TPRA | −0.17 | ns | |
| Ratio of top height to width | THW | 0.45 | ns | |
| Ratio of top circumference to area | TPA2 | −0.84 | *** | |
| Top convex hull area | TCA3 | 0.89 | *** | |
| Number of top convex hull vertices | TCN | −0.57 | * | |
| Top shape rate compactness | TSC | 0.91 | *** | |
| Top round rate compactness | TRC | −0.45 | ns | |
| Side parameter | Side perimeter | SP | 0.77 | *** |
| Side projection area | SPA1 | 0.90 | *** | |
| Side profile area | SCA1 | 0.90 | *** | |
| 1_5 area ratio | 1_5AR | −0.51 | * | |
| 2_5 area ratio | 2_5AR | 0.04 | ns | |
| 3_5 area ratio | 3_5AR | 0.43 | ns | |
| 4_5 area ratio | 4_5AR | 0.54 | * | |
| 5_5 area ratio | 5_5AR | 0.001 | ns | |
| 1_5 maximum width | 1_5MW | 0.93 | *** | |
| 2_5 maximum width | 2_5MW | 0.94 | *** | |
| 3_5 maximum width | 3_5MW | 0.89 | *** | |
| 4_5 maximum width | 4_5MW | 0.83 | *** | |
| 5_5 maximum width | 5_5MW | 0.62 | ** | |
| Side mean width | SMW | 0.87 | *** | |
| Crown height | CH | 0.54 | * | |
| Crown height/plant height | CPH | −0.28 | ns | |
| Crown breadth | TC2 | 0.91 | *** | |
| Minimum side width | SMIW | 0.90 | *** | |
| Side external rectangular height | SRH | 0.71 | ** | |
| Radius of the side circumscribed circle | SCR | 0.73 | ** | |
| Side rectangle area | SRA | 0.92 | *** | |
| Side circumscribed circular area | SCA2 | 0.72 | ** | |
| Side shape rate compactness | SSC | 0.92 | *** | |
| Side circularity compactness | SRC | −0.23 | ns | |
| Compactness of side circumferential circle | SCC | 0.61 | * | |
| Side projected area/external rectangular area | SPRA | 0.61 | * | |
| Side height/width | SHW | −0.20 | ns | |
| Side circumference/area | SPA2 | −0.89 | *** | |
| Area of convex hull | SCA | 0.87 | *** | |
| Number of vertices of convex hull | SCN | 0.43 | ns | |
| COR (%) | M | I | M | I | M | I | M | I | |
|---|---|---|---|---|---|---|---|---|---|
| Treatment | PL-28 | AR-15 | AR-35 | AR-55 | |||||
| Field | STV-1 | 40.03 c | 8.12 A | 27.54 a | 5.07 A | 24.32 a | 19.50 A | 84.44 b | 62.03 B |
| STV-2 | 23.46 a | 11.55 B | 55.38 c | 23.35 B | 54.53 b | 23.04 B | 105.02 d | 60.34 B | |
| SSV-1 | 51.63 d | 13.75 C | 52.48 b | 24.43 B | 70.52 c | 26.40 C | 100.15 c | 23.34 A | |
| SSV-2 | 32.18 b | 14.11 C | 54.73 c | 48.15 C | 69.27 c | 90.37 D | 71.65 a | 82.85 C | |
| PL-23 | PL-16 | PL-9 | AR-7 | ||||||
| Indoor | STV-1 | 85.53 a | 120.60 B | 229.38 b | 103.29 B | 106.05 a | 97.53 B | 128.79 b | 123.84 B |
| STV-2 | 86.78 a | 86.56 A | 155.32 a | 96.39 A | 155.59 b | 74.69 A | 121.00 a | 121.86 A | |
| SSV-1 | 252.02 c | 180.79 D | 387.73 c | 123.29 C | 313.32 d | 135.50 C | 336.74 d | 171.49 D | |
| SSV-2 | 219.75 b | 152.62 C | 398.32 d | 122.23 C | 166.53 c | 145.70 D | 176.32 c | 153.26 C | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, S.; Li, X.; Chen, M.; Xu, X.; Zhang, W.; Chi, H.; Shao, P.; Tang, F.; Gong, T.; Guo, M.; et al. Excellent Canopy Structure in Soybeans Can Improve Their Photosynthetic Performance and Increase Yield. Agriculture 2024, 14, 1783. https://doi.org/10.3390/agriculture14101783
He S, Li X, Chen M, Xu X, Zhang W, Chi H, Shao P, Tang F, Gong T, Guo M, et al. Excellent Canopy Structure in Soybeans Can Improve Their Photosynthetic Performance and Increase Yield. Agriculture. 2024; 14(10):1783. https://doi.org/10.3390/agriculture14101783
Chicago/Turabian StyleHe, Shuyuan, Xiuni Li, Menggen Chen, Xiangyao Xu, Wenjing Zhang, Huiling Chi, Panxia Shao, Fenda Tang, Tao Gong, Ming Guo, and et al. 2024. "Excellent Canopy Structure in Soybeans Can Improve Their Photosynthetic Performance and Increase Yield" Agriculture 14, no. 10: 1783. https://doi.org/10.3390/agriculture14101783
APA StyleHe, S., Li, X., Chen, M., Xu, X., Zhang, W., Chi, H., Shao, P., Tang, F., Gong, T., Guo, M., Xu, M., Yang, W., & Liu, W. (2024). Excellent Canopy Structure in Soybeans Can Improve Their Photosynthetic Performance and Increase Yield. Agriculture, 14(10), 1783. https://doi.org/10.3390/agriculture14101783






