Enhancing Nutritional Value of Rhubarb (Rheum rhaponticum L.) Products: The Role of Fruit and Vegetable Pomace
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Methods
2.2.1. Production of Rhubarb Products
2.2.2. Chemical Analysis
2.2.3. CIE (L*a*b*) Color Evaluation
2.2.4. Texture Analysis
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Ash and Minerals of Rhubarb Products
3.2. Antioxidant Properties and Antioxidant Activity of Rhubarb Products
3.3. Color Parameters of Rhubarb Products
3.4. Texture of Rhubarb Products
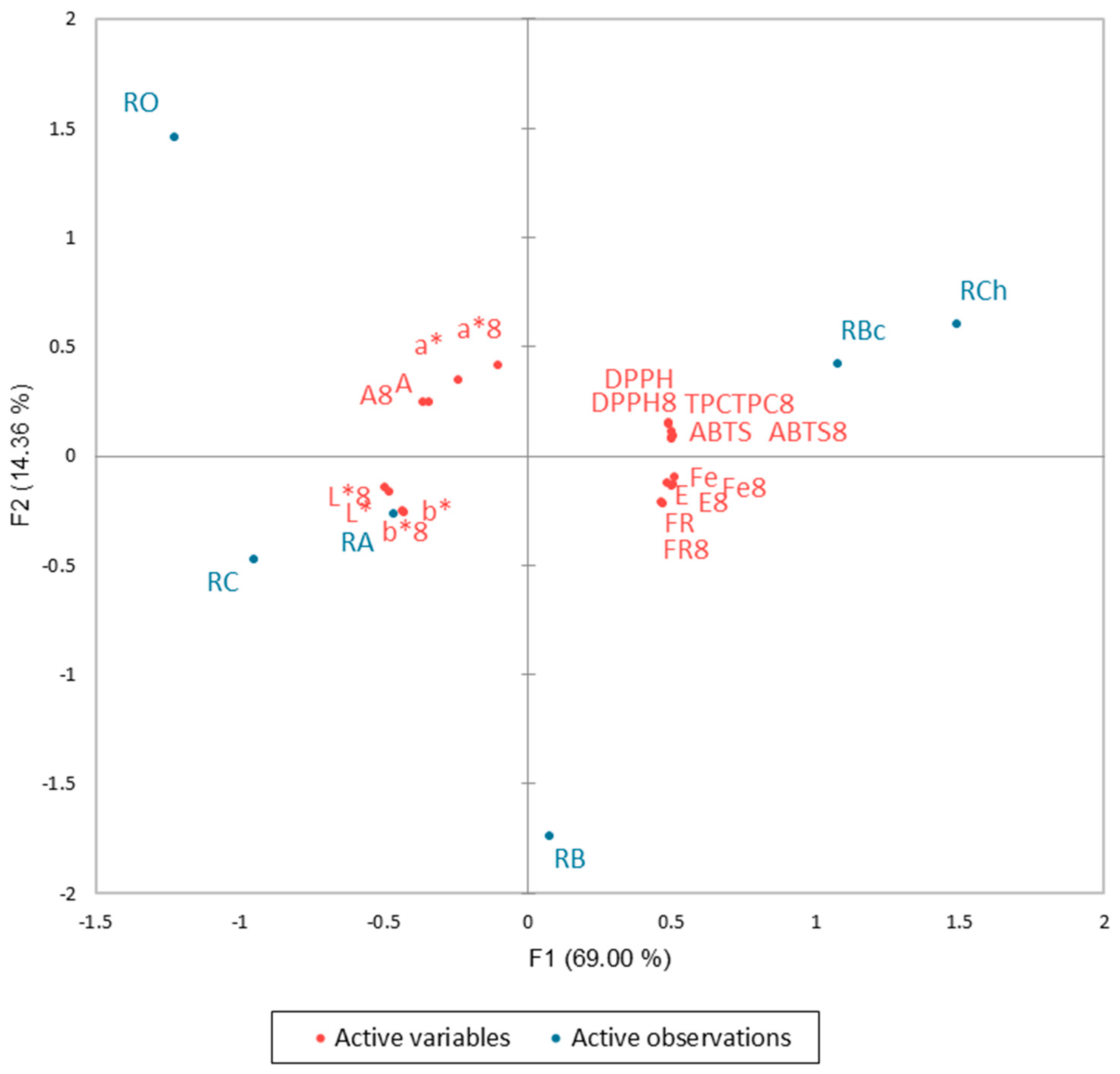
3.5. Principal Component Analysis (PCA)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Appleton, K.M.; Hemingway, A.; Saulais, L.; Dinnella, C.; Monteleone, E.; Depezay, L.; Morizet, D.; Armando Perez-Cueto, F.J.; Bevan, A.; Hartwell, H. Increasing vegetable intakes: Rationale and systematic review of published interventions. Eur. J. Nutr. 2016, 55, 869–896. [Google Scholar] [CrossRef]
- Sarac, I.; Butnariu, M. Food pyramid—The principles of a balanced diet. Int. J. Nutr. 2020, 5, 24–31. [Google Scholar] [CrossRef]
- World Health Organization. Healthy Diet. Available online: https://www.who.int/news-room/fact-sheets/detail/healthy-diet (accessed on 9 September 2024).
- Zhan, J.; Liu, Y.-J.; Cai, L.-B.; Xu, F.-R.; Xie, T.; He, Q.-Q. Fruit and vegetable consumption and risk of cardiovascular disease: A meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2017, 57, 1650–1663. [Google Scholar] [CrossRef]
- Aune, D.; Giovannucci, E.; Boffetta, P.; Fadnes, L.T.; Keum, N.; Norat, T.; Greenwood, D.C.; Riboli, E.; Vatten, L.J.; Tonstad, S. Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality-a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol. 2017, 46, 1029–1056. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Rodrigues, C.F.; Sharopov, F.; Docea, A.O.; Can Karaca, A.; Sharifi-Rad, M.; Kahveci Karıncaoglu, D.; Gülseren, G.; Şenol, E.; Demircan, E.; et al. Diet, lifestyle and cardiovascular diseases: Linking pathophysiology to cardioprotective effects of natural bioactive compounds. Int. J. Environ. Res. Public Health 2020, 17, 2326. [Google Scholar] [CrossRef]
- Key, T.J.; Bradbury, K.E.; Perez-Cornago, A.; Sinha, R.; Tsilidis, K.K.; Tsugane, S. Diet, nutrition, and cancer risk: What do we know and what is the way forward? BMJ 2020, 368, m511. [Google Scholar] [CrossRef]
- Zurbau, A.; Au-Yeung, F.; Blanco Mejia, S.; Khan, T.A.; Vuksan, V.; Jovanovski, E.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of different fruit and vegetable sources with incident cardiovascular outcomes: A systematic review and meta-analysis of prospective cohort studies. J. Am. Heart Assoc. 2020, 9, e017728. [Google Scholar] [CrossRef]
- Nawamawat, J.; Prasittichok, W.; Prompradit, T.; Chatchawanteerapong, S.; Sittisart, V. Prevalence and characteristics of risk factors for non-communicable diseases in semi-urban communities. J. Health Res. 2020, 34, 295–303. [Google Scholar] [CrossRef]
- Kalmpourtzidou, A.; Eilander, A.; Talsma, E.F. Global vegetable intake and supply compared to recommendations: A systematic review. Nutrients 2020, 12, 1558. [Google Scholar] [CrossRef]
- Hurtado-Barroso, S.; Tresserra-Rimbau, A.; Vallverdú-Queralt, A.; Lamuela-Raventós, R.M. Organic food and the impact on human health. Crit. Rev. Food Sci. Nutr. 2019, 59, 704–714. [Google Scholar] [CrossRef]
- Shyam, S.; Ramadas, A.; Chang, S.K. Isomaltulose: Recent evidence for health benefits. J. Funct. Foods 2018, 48, 173–178. [Google Scholar] [CrossRef]
- EU Commission Decision 2005/581/EC of 25th July 2005 Authorising the Placing on the Market of Isomaltulose as a Novel Food or Novel Food Ingredient under Regulation (EC) no 258/97 of the European Parliament and of the Council. 2007. Available online: http://data.europa.eu/eli/dec/2005/581/ (accessed on 17 September 2024).
- US GRAS Notification no 0184 on Isomaltulose. 2006. Available online: https://cfsanappsexternal.fda.gov/scripts/fdcc/index.cfm?set=GRASNotices&id=184 (accessed on 17 September 2024).
- von Rymon Lipinski, G.-W. Reduced-calorie sweeteners and caloric alternatives. In Optimising Sweet Taste in Foods, 1st ed.; Spillane, W.J., Ed.; Woodhead Publishing: Boca Raton, FL, USA, 2006; pp. 252–280. ISBN 9781845690083. [Google Scholar]
- Maresch, C.C.; Petry, S.F.; Theis, S.; Bosy-Westphal, A.; Linn, T. Low glycemic index prototype isomaltulose-update of clinical trials. Nutrients 2017, 9, 381. [Google Scholar] [CrossRef] [PubMed]
- de Groot, E.; Schweitzer, L.; Theis, S. Efficacy of isomaltulose compared to sucrose in modulating endothelial function in overweight adults. Nutrients 2020, 12, 141. [Google Scholar] [CrossRef] [PubMed]
- Keyhani-Nejad, F.; Kemper, M.; Schueler, R.; Pivovarova, O.; Rudovich, N.; Pfeiffer, A.F.H. Effects of palatinose and sucrose intake on glucose metabolism and incretin secretion in subjects with type 2 diabetes. Diabetes Care 2016, 39, e38–e39. [Google Scholar] [CrossRef]
- Foschia, M.; Peressini, D.; Sensidoni, A.; Brennan, C.S. The effects of dietary fibre addition on the quality of common cereal products. J. Cereal Sci. 2013, 58, 216–227. [Google Scholar] [CrossRef]
- Granato, D.; Barba, F.J.; Bursać Kovačević, D.; Lorenzo, J.M.; Cruz, A.G.; Putnik, P. Functional foods: Product development, technological trends, efficacy testing, and safety. Annu. Rev. Food Sci. Technol. 2020, 11, 93–118. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Sagar, N.A.; Pareek, S.; Sharma, S.; Yahia, E.M.; Lobo, M.G. Fruit and vegetable waste: Bioactive compounds, their extraction, and possible utilization. Compr. Rev. Food Sci. Food Saf. 2018, 17, 512–531. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Sieniawska, E.; Ortan, A.; Fierascu, I.; Xiao, J. Fruits by-products—A source of valuable active principles. A short review. Front. Bioeng. Biotechnol. 2020, 8, 319. [Google Scholar] [CrossRef]
- Coman, V.; Teleky, B.-E.; Mitrea, L.; Martău, G.A.; Szabo, K.; Călinoiu, L.-F.; Vodnar, D.C. Bioactive potential of fruit and vegetable wastes. Adv. Food Nutr. Res. 2020, 91, 157–225. [Google Scholar] [CrossRef]
- de Brito Nogueira, T.B.; Da Silva, T.P.M.; deAraújo Luiz, D.; de Andrade, C.J.; de Andrade, L.M.; Ferreira, M.S.L.; Fai, A.E.C. Fruits and vegetable-processing waste: A case study in two markets at Rio de Janeiro, RJ, Brazil. Environ. Sci. Pollut. Res. Int. 2020, 27, 18530–18540. [Google Scholar] [CrossRef] [PubMed]
- Cojocaru, A.; Munteanu, N.; Petre, B.A.; Stan, T.; Teliban, G.C.; Vintu, C.; Stoleru, V. Biochemical and production of rhubarb under growing technological factors. Rev. Chim. 2019, 70, 2000–2003. [Google Scholar] [CrossRef]
- Cao, Y.-J.; Pu, Z.-J.; Tang, Y.-P.; Shen, J.; Chen, Y.-Y.; Kang, A.; Zhou, G.-S.; Duan, J.-A. Advances in bio-active constituents, pharmacology and clinical applications of rhubarb. Chin. Med. 2017, 12, 36. [Google Scholar] [CrossRef]
- Xiang, H.; Zuo, J.; Guo, F.; Dong, D. What we already know about rhubarb: A comprehensive review. Chin. Med. 2020, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, H.; Kong, W.; Jin, C.; Zhao, Y.; Qu, Y.; Xiao, X. Microcalorimetric assay on the antimicrobial property of five hydroxyanthraquinone derivatives in rhubarb (Rheum palmatum L.) to Bifidobacterium adolescentis. Phytomedicine 2010, 17, 684–689. [Google Scholar] [CrossRef]
- Cojocaru, A.; Vlase, L.; Munteanu, N.; Stan, T.; Teliban, G.C.; Burducea, M.; Stoleru, V. Dynamic of phenolic compounds, antioxidant activity, and yield of rhubarb under chemical, organic and biological fertilization. Plants 2020, 9, 355. [Google Scholar] [CrossRef]
- Clapa, D.; Borsai, O.; Hârța, M.; Bonta, V.; Szabo, K.; Coman, V.; Bobiș, O. Micropropagation, genetic fidelity and phenolic compound production of Rheum rhabarbarum L. Plants 2020, 9, 656. [Google Scholar] [CrossRef]
- Takeoka, G.R.; Dao, L.; Harden, L.; Pantoja, A.; Kuhl, J.C. Antioxidant activity, phenolic and anthocyanin contents of various rhubarb (Rheum spp.) varieties. Int. J. Food Sci. Technol. 2013, 48, 172–178. [Google Scholar] [CrossRef]
- Horwitz, W.; Latimer, G.W. (Eds.) Official Methods of Analysis of AOAC International, 18th ed.; Current throug revision 1; AOAC International: Gaithersburg, MA, USA, 2006; ISBN 9780935584776. [Google Scholar]
- Paslawski, P.; Migaszewski, Z.M. The quality of element determinations in plant materials by instrumental methods. Polish J. Environ. Stud. 2006, 15, 154–164. [Google Scholar]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid tests to assess the antioxidant activity of Phaseolus vulgaris L. dry beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Csepregi, K.; Kocsis, M.; Hideg, E. On the spectrophotometric determination of total phenolic and flavonoid contents. Acta Biol. Hung. 2013, 64, 500–509. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Curr. Protocols Food Analyt. Chem. 2001, 1, F1.2.1–F1.2.13. [Google Scholar] [CrossRef]
- Plessi, M.; Bertelli, D.; Albasini, A. Distribution of metals and phenolic compounds as a criterion to evaluate variety of berries and related jams. Food Chem. 2007, 100, 419–427. [Google Scholar] [CrossRef]
- Nagata, M.; Yamashita, I. Simple method for simultaneous determination of chlorophyll and carotenoids in tomato fruit. Nippon Shokuhin Kogyo Gakkaishi 1992, 39, 925–928. [Google Scholar] [CrossRef]
- Pekkarinen, S.S.; Heinonen, I.M.; Hopia, A.I. Flavonoids quercetin, myricetin, kaemferol and (+)-catechin as antioxidants in methyl linoleate. J. Sci. Food Agric. 1999, 79, 499–506. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- CIE TC 1-85. In CIE 015:2018 Colorimetry, 4th ed.; International Commission on Illumination (CIE): Vienna, Austria, 2018; ISBN 978-3-902842-13-8.
- Genovese, D.B.; Ye, A.; Singh, H. High methoxyl pectin/apple particles composite gels: Effect of particle size and particle concentration on mechanical properties and gel structure. J. Texture Stud. 2010, 41, 171–189. [Google Scholar] [CrossRef]
- Arakaki, D.G.; Santos, V.S.D.; de Melo, E.P.; Pereira, H.; Figueiredo, P.S.; Cortês, M.R.; Carollo, C.A.; Silva de Oliveira, L.C.; Tschinkel, P.; Reis, F.; et al. Canjiqueira fruit: Are we losing the best of it? Foods 2020, 9, 521. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Ferrentino, G.; Asaduzzaman, M.; Scampicchio, M.M. Current technologies and new insights for the recovery of high valuable compounds from fruits by-products. Crit. Rev. Food Sci. Nutr. 2018, 58, 386–404. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Sharma, R.; Nepovimova, E.; Kuča, K.; Dhanjal, D.S.; Verma, R.; Bhardwaj, P.; Sharma, S.; Kumar, D. Fruit and vegetable peels: Utilization of high value horticultural waste in novel industrial applications. Molecules 2020, 25, 2812. [Google Scholar] [CrossRef]
- Umul, D.; Korus, A.; Ziobro, R. Extruded preparations with sour cherry pomace influence quality and increase the level of bioactive components in gluten-free breads. Int. J. Food Sci. 2020, 2020, 8024398. [Google Scholar] [CrossRef]
- Kalisz, S.; Oszmiański, J.; Kolniak-Ostek, J.; Grobelna, A.; Kieliszek, M.; Cendrowski, A. Effect of a variety of polyphenols compounds and antioxidant properties of rhubarb (Rheum rhabarbarum). LWT 2020, 118, 108775. [Google Scholar] [CrossRef]
- Mattioli, R.; Francioso, A.; Mosca, L.; Silva, P. Anthocyanins: A comprehensive review of their chemical properties and health effects on cardiovascular and neurodegenerative diseases. Molecules 2020, 25, 3809. [Google Scholar] [CrossRef]
- Raikos, V.; Ni, H.; Hayes, H.; Ranawana, V. Antioxidant properties of a yogurt beverage enriched with salal (Gaultheria shallon) berries and blackcurrant (Ribes nigrum) pomace during cold storage. Beverages 2019, 5, 2. [Google Scholar] [CrossRef]
- Toscano Martínez, H.; Gagneten, M.; Díaz-Calderón, P.; Enrione, J.; Salvatori, D.; Schebor, C.; Leiva, G. Natural food colorant from blackcurrant spray-dried powder obtained by enzymatic treatment: Characterization and acceptability. J. Food Process. Preserv. 2021, 45, e15011. [Google Scholar] [CrossRef]
- Kapci, B.; Neradová, E.; Čížková, H.; Voldřich, M.; Rajchl, A.; Capanoglu, E. Investigating the antioxidant potential of chokeberry (Aronia melanocarpa) products. J. Food Nutr. Res. 2013, 52, 219–229. [Google Scholar]
- Vagiri, M.; Jensen, M. Influence of juice processing factors on quality of black chokeberry pomace as a future resource for colour extraction. Food Chem. 2017, 217, 409–417. [Google Scholar] [CrossRef]
- Basegmez, H.I.O.; Povilaitis, D.; Kitrytė, V.; Kraujalienė, V.; Šulniūtė, V.; Alasalvar, C.; Venskutonis, P.R. Biorefining of blackcurrant pomace into high value functional ingredients using supercritical CO2, pressurized liquid and enzyme assisted extractions. J. Supercrit. Fluids 2017, 124, 10–19. [Google Scholar] [CrossRef]
- Azman, E.M.; Nor, N.D.M.; Charalampopoulos, D.; Chatzifragkou, A. Effect of acidified water on phenolic profile and antioxidant activity of dried blackcurrant (Ribes nigrum L.) pomace extracts. LWT 2022, 154, 112733. [Google Scholar] [CrossRef]
- Arfaoui, L. Dietary plant polyphenols: Effects of food processing on their content and bioavailability. Molecules 2021, 26, 2959. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Bober, I. The effect of addition of chokeberry, flowering quince fruits and rhubarb juice to strawberry jams on their polyphenol content, antioxidant activity and colour. Eur. Food Res. Technol. 2008, 227, 1043–1051. [Google Scholar] [CrossRef]
- Abdel-Hady, M.M.; Attia, G.Y.; Ali, A.M. Color stability of strawberry jam fortified by purple carrot puree. Egypt. J. Agric. Res. 2014, 92, 323–336. [Google Scholar] [CrossRef]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Surbhi, S.; Verma, R.C.; Deepak, R.; Jain, H.K.; Yadav, K.K. A review: Food, chemical composition and utilization of carrot (Daucus carota L.) pomace. Int. J. Chem. Stud. 2018, 6, 2921–2926. [Google Scholar]
- Sharma, M.; Usmani, Z.; Gupta, V.K.; Bhat, R. Valorization of fruits and vegetable wastes and by-products to produce natural pigments. Crit. Rev. Biotechnol. 2021, 41, 535–563. [Google Scholar] [CrossRef]
- Oszmiański, J.; Wojdyło, A. Polyphenol content and antioxidative activity in apple purées with rhubarb juice supplement. Int. J. Food Sci. Technol. 2008, 43, 501–509. [Google Scholar] [CrossRef]
- Kumar, A.; Madhumathi, C.; Sadarunnisa, S.; Srikanth, K. Standardization of protocol for best blending ratio of papaya cv. Red Lady and guava cv. Lalit fruit pulp for preparation of fruit bar. Plant Arch. 2017, 17, 59–68. [Google Scholar]
- Lafarga, T.; Aguiló-Aguayo, I.; Bobo, G.; Chung, A.V.; Tiwari, B.K. Effect of storage on total phenolics, antioxidant capacity, and physicochemical properties of blueberry (Vaccinium corymbosum L.) jam. J. Food Process. Preserv. 2018, 42, e13666. [Google Scholar] [CrossRef]
- Nafri, P.; Singh, A.K.; Sharma, A.; Sharma, I. Effect of storage condition on physiochemical and sensory properties of papaya jam. J. Pharmacogn. Phytochem. 2021, 10, 1296–1301. [Google Scholar] [CrossRef]
- Kopjar, M.; Pichler, A.; Turi, J.; Piližota, V. Influence of trehalose addition on antioxidant activity, colour and texture of orange jelly during storage. Int. J. Food Sci. Technol. 2016, 51, 2640–2646. [Google Scholar] [CrossRef]
- Meng, L.; Zhu, J.; Ma, Y.; Sun, X.; Li, D.; Li, L.; Bai, H.; Xin, G.; Meng, X. Composition and antioxidant activity of anthocyanins from Aronia melanocarpa cultivated in Haicheng, Liaoning, China. Food Biosci. 2019, 30, 100413. [Google Scholar] [CrossRef]
- Hurst, R.D.; Lyall, K.A.; Wells, R.W.; Sawyer, G.M.; Lomiwes, D.; Ngametua, N.; Hurst, S.M. Daily consumption of an anthocyanin-rich extract made from New Zealand blackcurrants for 5 weeks supports exercise recovery through the management of oxidative stress and inflammation: A randomized placebo controlled pilot study. Front. Nutr. 2020, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Hussein, A.M.; Kamil, M.M.; Hegazy, N.A.; Mahmoud, K.F.; Ibrahim, M.A. Utilization of some fruits and vegetables by-products to produce high dietary fiber jam. Food Sci. Qual. Manag. 2015, 37, 39–45. [Google Scholar]
- Chávez-Santoscoy, R.A.; Lazo-Vélez, M.A.; Serna-Sáldivar, S.O.; Gutiérrez-Uribe, J.A. Delivery of flavonoids and saponins from black bean (Phaseolus vulgaris) seed coats incorporated into whole wheat bread. Int. J. Mol. Sci. 2016, 17, 222. [Google Scholar] [CrossRef]
- Maner, S.; Sharma, A.K.; Banerjee, K. Wheat flour replacement by wine grape pomace powder positively affects physical, functional and sensory properties of cookies. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 87, 109–113. [Google Scholar] [CrossRef]
- Banaś, A.; Korus, A.; Korus, J. Texture, color, and sensory features of low-sugar gooseberry jams enriched with plant ingredients with prohealth properties. J. Food Qual. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Kirca, A.; Özkan, M.; Cemeroğlu, B. Storage stability of strawberry jam color enhanced with black carrot juice concentrate. J. Food Process. Preserv. 2007, 31, 531–545. [Google Scholar] [CrossRef]
- Dordoni, R.; Duserm Garrido, G.; Marinoni, L.; Torri, L.; Piochi, M.; Spigno, G. Enrichment of whole wheat cocoa biscuits with encapsulated grape skin extract. Int. J. Food Sci. 2019, 2019, 9161840. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour difference ΔE—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Touati, N.; Tarazona-Díaz, M.P.; Aguayo, E.; Louaileche, H. Effect of storage time and temperature on the physicochemical and sensory characteristics of commercial apricot jam. Food Chem. 2014, 145, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Vukoja, J.; Pichler, A.; Kopjar, M. Stability of anthocyanins, phenolics and color of tart cherry jams. Foods 2019, 8, 255. [Google Scholar] [CrossRef] [PubMed]
- Kopjar, M.; Piližota, V.; Nedić Tiban, N.; Šubarić, D.; Babić, J.; Ačkar, Đ.; Sajdl, M. Strawberry jams: Influence of different pectins on colour and textural properties. Czech J. Food Sci. 2009, 27, 20–28. [Google Scholar] [CrossRef]
- Nourmohammadi, A.; Ahmadi, E.; Heshmati, A. Optimization of physicochemical, textural, and rheological properties of sour cherry jam containing stevioside by using response surface methodology. Food Sci. Nutr. 2021, 9, 2483–2496. [Google Scholar] [CrossRef]
- Mohammadi-Moghaddam, T.; Firoozzare, A.; Daryadar, S.; Rahmani, Z. Black plum peel jam: Physicochemical properties, sensory attributes, and antioxidant capacity. Int. J. Food Prop. 2020, 23, 1737–1747. [Google Scholar] [CrossRef]
- Mousavi, M.; Heshmati, A.; Daraei Garmakhany, A.; Vahidinia, A.; Taheri, M. Texture and sensory characterization of functional yogurt supplemented with flaxseed during cold storage. Food Sci. Nutr. 2019, 7, 907–917. [Google Scholar] [CrossRef]
- Tolve, R.; Pasini, G.; Vignale, F.; Favati, F.; Simonato, B. Effect of grape pomace addition on the technological, sensory, and nutritional properties of durum wheat pasta. Foods 2020, 9, 354. [Google Scholar] [CrossRef]
- Begum, Y.A.; Chakraborty, S.; Deka, S.C. Bread fortified with dietary fibre extracted from culinary banana bract: Its quality attributes and in vitro starch digestibility. Int. J. Food Sci. Technol. 2020, 55, 2359–2369. [Google Scholar] [CrossRef]
- Teixeira, F.; Santos, B.A.D.; Nunes, G.; Soares, J.M.; Amaral, L.A.d.; de Souza, G.H.O.; de Resende, J.T.V.; Menegassi, B.; Rafacho, B.P.M.; Schwarz, K.; et al. Addition of orange peel in orange jam: Evaluation of sensory, physicochemical, and nutritional characteristics. Molecules 2020, 25, 1670. [Google Scholar] [CrossRef]
- Younis, K.; Islam, R.U.; Jahan, K.; Yousuf, B.; Ray, A. Effect of addition of mosambi (Citrus limetta) peel powder on textural and sensory properties of papaya jam. Cogent Food Agric. 2015, 1, 1023675. [Google Scholar] [CrossRef]
- Abolila, R.M.; Barakat, H.; El-Tanahy, H.A.; El-Mansy, H.A. Chemical, nutritional and organoleptical characteristics of orange-based formulated low-calorie jams. Food Nutr. Sci. 2015, 06, 1229–1244. [Google Scholar] [CrossRef]
- Rababah, T.M.; Al-u’datt, M.H.; Al-Mahasneh, M.A.; Feng, H.; Alothman, A.M.; Almajwal, A.; Yang, W.; Kilani, I.; Alhamad, M.N.; Ereifej, K.; et al. Effect of storage on the physicochemical properties, total phenolic, anthocyanin, and antioxidant capacity of strawberry jam. J. Food Agric. Environ. 2011, 9, 101–105. [Google Scholar]
- Sutwal, R.; Dhankhar, J.; Kindu, P.; Mehla, R. Development of low calorie jam by replacement of sugar with natural sweetener stevia. Int. J. Curr. Res. Rev. 2019, 11, 9–16. [Google Scholar] [CrossRef]
- Morris, G.A.; Castile, J.; Smith, A.; Adams, G.G.; Harding, S.E. The effect of different storage temperatures on the physical properties of pectin solutions and gels. Polym. Degrad. Stab. 2010, 95, 2670–2673. [Google Scholar] [CrossRef]
| Type of Product b | Ingredients a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| R | AP | ChP | BcP | CP | BP | Isomaltulose | Pectin | Citric Acid | Water | |
| RO | 550 | 190 | 14 | 1.2 | 244.8 | |||||
| RA | 550 | 200 | 177 | 12 | 2.5 | 58.5 | ||||
| RCh | 550 | 200 | 150 | 12 | 2.5 | 85.5 | ||||
| RBc | 550 | 200 | 185 | 12 | 2.0 | 51.0 | ||||
| RC | 550 | 200 | 185 | 12 | 4.5 | 48.5 | ||||
| RB | 550 | 200 | 186 | 12 | 4.5 | 47.5 | ||||
| Type of Product a | Ash | Potassium | Calcium | Magnesium | Phosphorus | Sulfur | Sodium |
|---|---|---|---|---|---|---|---|
| RO | 0.268 a ± 0.021 | 94 a ± 4 | 44.6 a ± 1.3 | 6.3 a ± 0.4 | 5.6 a ± 0.2 | 5.5 a ± 0.1 | 2.8 a ± 0.2 |
| RA | 0.345 bc ± 0.006 | 123 b ± 4 | 60.0 c ± 2.1 | 9.5 c ± 0.4 | 8.8 b ± 0.4 | 13.2 b ± 1.9 | 3.4 b ± 0.2 |
| RBc | 0.366 c ± 0.007 | 173 e ± 3 | 65.7 d ± 1.4 | 11.0 d ± 1.1 | 11.7 c ± 1.1 | 29.4 d ± 5.4 | 4.0 c ± 0.2 |
| RCh | 0.351 bc ± 0.006 | 182 f ± 5 | 63.0 cd ± 2.6 | 9.8 c ± 0.3 | 15.2 e ± 1.2 | 20.0 c ± 1.2 | 4.2 c ± 0.5 |
| RB | 0.442 d ± 0.009 | 164 d ± 3 | 49.3 b ± 1.8 | 9.6 c ± 0.5 | 13.4 d ± 0.2 | 15.5 b ± 1.7 | 7.1 d ± 0.2 |
| RC | 0.339 b ± 0.006 | 134 c ± 6 | 48.8 b ± 1.1 | 7.7 b ± 0.1 | 9.8 b ± 0.6 | 15.3 b ± 1.1 | 7.3 d ± 0.3 |
| Type of Product a | Zinc mg/100 g | Manganese mg/100 g | Iron mg/100 g | Boron µg/100 g | Copper µg/100 g | Molybdenum µg/100 g | Chromium µg/100 g |
|---|---|---|---|---|---|---|---|
| RO | 0.163 a ± 0.100 | 0.131 a ± 0.100 | 0.126 a ± 0.100 | 17.4 a ± 0.4 | 15.9 a ± 0.4 | 3.3 a ± 0.3 | 2.2 a ± 0.1 |
| RA | 0.305 b ± 0.005 | 0.333 e ± 0.010 | 0.132 a ± 0.009 | 51.8 b ± 0.8 | 24.1 b ± 1.6 | 7.1 c ±1.3 | 2.4 a ± 0.2 |
| RBc | 0.598 d ± 0.047 | 0.189 c ± 0.012 | 0.363 c ± 0.054 | 56.6 bc ± 2.9 | 45.4 c ± 3.6 | 8.5 d ± 0.5 | 2.1 a ± 0.5 |
| RCh | 0.361 b ± 0.015 | 0.241 d ± 0.008 | 0.153 a ± 0.047 | 60.9 c ± 3.1 | 28.0 b ± 5.1 | 11.8 e ± 0.5 | 3.1 b ± 0.3 |
| RB | 0.707 e ± 0.018 | 0.177 bc ± 0.013 | 0.231 b ± 0.011 | 73.1 d ± 7.1 | 27.1 b ± 1.2 | 5.3 b ± 0.6 | 2.4 a ± 0.2 |
| RC | 0.438 c ± 0.054 | 0.169 b ± 0.009 | 0.142 a ± 0.005 | 50.9 b ± 1.6 | 18.7 a ± 1.7 | 7.5 cd ± 0.7 | 2.4 a ± 0.4 |
| Type of Product a | Total Polyphenols (mg EC b/100 g) | Total Flavonoids (mg EC/100 g) | Total Anthocyanins (mg Cyanidin-3-Glucoside Equivalent/100 g) | Beta-Carotene (µg/100 g) | ABTS (µmol Tx/g) | DPPH (µmol Tx/g) |
|---|---|---|---|---|---|---|
| 0 months stored: | ||||||
| RO | 18.2 b ± 1.1 | 8.3 b ± 0.3 | 9.4 cd ± 0.3 | 74 a ± 10 | 16.1 b ± 0.5 | 13.4 b ± 0.5 |
| RA | 49.3 f ± 2.5 | 18.8 ef ± 1.9 | 11.8 d ± 2.3 | 107 cd ± 6 | 34.2 e ± 0.2 | 25.3 e ± 0.7 |
| RBc | 87.1 i ± 1.5 | 26.2 g ± 0.9 | 76.4 h ± 4.1 | 77 ab ± 4 | 67.8 h ± 4.1 | 46.8 h ± 1.5 |
| RCh | 93.1 j ± 3.7 | 39.7 i ± 1.8 | 62.5 g ± 3.5 | 139 e ± 17 | 76.3 i ± 1.8 | 55.9 i ± 0.9 |
| RB | 37.8 e ± 2.6 | 17.1 e ± 0.7 | 9.7 d ± 0.6 | 109 cd ± 6 | 27.6 d ± 0.2 | 17.4 d ± 0.2 |
| RC | 29.7 d ± 1.9 | 14.9 d ± 1.7 | 10.5 d ± 0.5 | 397 g ± 29 | 25.3 cd ± 1.0 | 15.4 c ± 1.1 |
| 8 months stored: | ||||||
| RO | 13.5 a ± 1.3 | 6.3 a ± 0.5 | 5.4 a ± 0.4 | 65 a ± 7 | 12.2 a ± 0.8 | 8.8 a ± 0.5 |
| RA | 40.1 e ± 1.5 | 15.0 d ± 0.6 | 8.9 bcd ± 1.1 | 98 bc ± 4 | 27.9 d ± 2.1 | 15.0 c ± 0.5 |
| RBc | 72.5 g ± 1.5 | 22.4 f ± 1.1 | 52.0 f ± 2.4 | 72 a ± 6 | 55.1 f ± 2.1 | 34.7 f ± 1.5 |
| RCh | 79.6 h ± 1.5 | 32.8 h ± 1.6 | 45.8 e ± 2.5 | 125 de ± 19 | 62.0 g ± 2.0 | 43.4 g ± 2.3 |
| RB | 30.3 d ± 3.1 | 14.4 cd ± 0.6 | 6.0 ab ± 0.3 | 100 c ± 12 | 24.3 c ± 1.2 | 12.4 b ± 0.5 |
| RC | 24.3 c ± 1.4 | 12.9 c ± 1.0 | 6.3 abc ± 0.4 | 364 f ± 9 | 18.3 b ± 0.4 | 12.2 b ± 0.4 |
| Type of Product a | L* | a* | b* | C* | h° | ΔE* |
|---|---|---|---|---|---|---|
| 0 months stored: | ||||||
| RO | 30.45 e ± 0.41 | 18.24 g ± 0.29 | 16.96 c ± 0.05 | 24.90 ± 0.20 | 42.92 c ± 0.51 | |
| RA | 33.06 f ± 1.19 | 13.56 d ± 0.76 | 24.38 e ± 1.09 | 27.90 f ± 1.15 | 60.9 e ± 1.37 | 9.21 a ± 1.25 |
| RBc | 17.17 c ± 0.58 | 11.36 bc ± 0.37 | 1.87 a ± 0.27 | 11.52 a ± 0.41 | 9.30 a ± 1.02 | 21.20 c ± 0.71 |
| RCh | 7.81 a ± 0.68 | 15.90 ef ± 0.68 | 4.17 b ± 0.51 | 16.44 b ± 0.79 | 14.65 b ± 1.12 | 26.11 d ± 1.02 |
| RB | 27.93 d ± 1.54 | 10.54 b ± 0.46 | 21.28 d ± 1.86 | 23.76 ± 1.72 | 63.57 f ± 2.00 | 9.33 a ± 0.65 |
| RC | 37.54 h ± 0.60 | 16.53 f ± 0.80 | 25.71 f ± 0.23 | 30.94 g ± 0.85 | 56.25 d ± 1.73 | 11.42 b ± 0.58 |
| 8 months stored: | ||||||
| RO | 31.19 e ± 0.21 | 15.61 ef ± 0.37 | 16.64 c ± 0.24 | 19.22 c ± 0.39 | 60.00 e ± 0.66 | |
| RA | 33.80 f ± 0.79 | 11.86 c ± 0.75 | 24.28 e ± 1.02 | 27.04 f ± 0.57 | 63.94 f ± 2.40 | 10.32 a ± 0.43 |
| RBc | 9.77 b ± 0.20 | 10.96 b ± 0.02 | 3.66 b ± 0.05 | 18.30 c ± 0.03 | 10.38 a ± 0.11 | 25.65 d ± 0.32 |
| RCh | 7.70 a ± 1.18 | 15.08 e ± 0.08 | 4.09 b ± 0.19 | 16.60 b ± 0.11 | 14.26 b ± 0.59 | 26.33 d ± 0.76 |
| RB | 26.69 d ± 0.16 | 9.30 a ± 0.04 | 20.09 d ± 0.27 | 22.14 ± 0.26 | 65.17 f ± 0.25 | 10.20 a ± 0.36 |
| RC | 36.11 g ± 0.23 | 13.63 d ± 0.97 | 23.52 e ± 0.33 | 27.19 ± 0.78 | 59.93 e ± 1.42 | 9.85 a ± 0.35 |
| Type of Product a | Gel Strength (Fe) N | Rupture Strength (FR) N | Energy of Penetration (E) N s | Adhesiveness (A) N s |
|---|---|---|---|---|
| 0 months stored: | ||||
| RO | 0.99 b ± 0.19 | 1.48 a ± 0.36 | 12.80 a ± 1.32 | −0.78 d ±0.18 |
| RA | 1.32 def ± 0.13 | 1.71 abc ± 0.11 | 15.78 abc ± 1.30 | −0.96 cd ± 0.10 |
| RBc | 1.59 h ± 0.36 | 2.59 d ± 0.20 | 19.08 cd ± 1.03 | −0.99 bcd ± 0.12 |
| RCh | 1.71 i ± 0.14 | 2.55 d ± 0.36 | 21.05 d ± 2.71 | −1.56 a ± 0.10 |
| RB | 1.43 fg ± 0.30 | 2.45 cd ± 0.10 | 18.43 cd ± 1.08 | −1.46 a ± 0.31 |
| RC | 1.21 cd ± 0.31 | 1.55 a ± 0.30 | 15.47 abc ± 1.71 | −1.04 bcd ± 0.09 |
| 8 months stored: | ||||
| RO | 0.74 a ± 0.53 | 0.98 a ± 0.42 | 12.49 a ± 1.20 | −0.63 d ± 0.15 |
| RA | 1.21 cd ± 0.31 | 1.59 ab ± 0.32 | 15.49 abc ± 1.33 | −0.87 cd ± 0.08 |
| RBc | 1.45 h ± 0.18 | 2.36 cd ± 0.19 | 18.37 cd ± 1.07 | −0.90 cd ± 0.10 |
| RCh | 1.57 h ± 0.45 | 2.33 bcd ± 0.83 | 19.46 cd ± 6.89 | −1.42 ab ± 0.19 |
| RB | 1.39 efg ± 0.14 | 2.32 bcd ± 0.30 | 17.51 bcd ± 2.43 | −1.28 abc ± 0.09 |
| RC | 1.16 c ± 0.10 | 1.44 a ± 0.38 | 14.01 ab ± 2.09 | −0.95 cd ± 0.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korus, A.; Korus, J. Enhancing Nutritional Value of Rhubarb (Rheum rhaponticum L.) Products: The Role of Fruit and Vegetable Pomace. Agriculture 2024, 14, 1784. https://doi.org/10.3390/agriculture14101784
Korus A, Korus J. Enhancing Nutritional Value of Rhubarb (Rheum rhaponticum L.) Products: The Role of Fruit and Vegetable Pomace. Agriculture. 2024; 14(10):1784. https://doi.org/10.3390/agriculture14101784
Chicago/Turabian StyleKorus, Anna, and Jarosław Korus. 2024. "Enhancing Nutritional Value of Rhubarb (Rheum rhaponticum L.) Products: The Role of Fruit and Vegetable Pomace" Agriculture 14, no. 10: 1784. https://doi.org/10.3390/agriculture14101784
APA StyleKorus, A., & Korus, J. (2024). Enhancing Nutritional Value of Rhubarb (Rheum rhaponticum L.) Products: The Role of Fruit and Vegetable Pomace. Agriculture, 14(10), 1784. https://doi.org/10.3390/agriculture14101784





