Foliar Fertilization Improves the Nitrogen Nutrition of Sugarcane
Abstract
:1. Introduction
2. Material and Methods
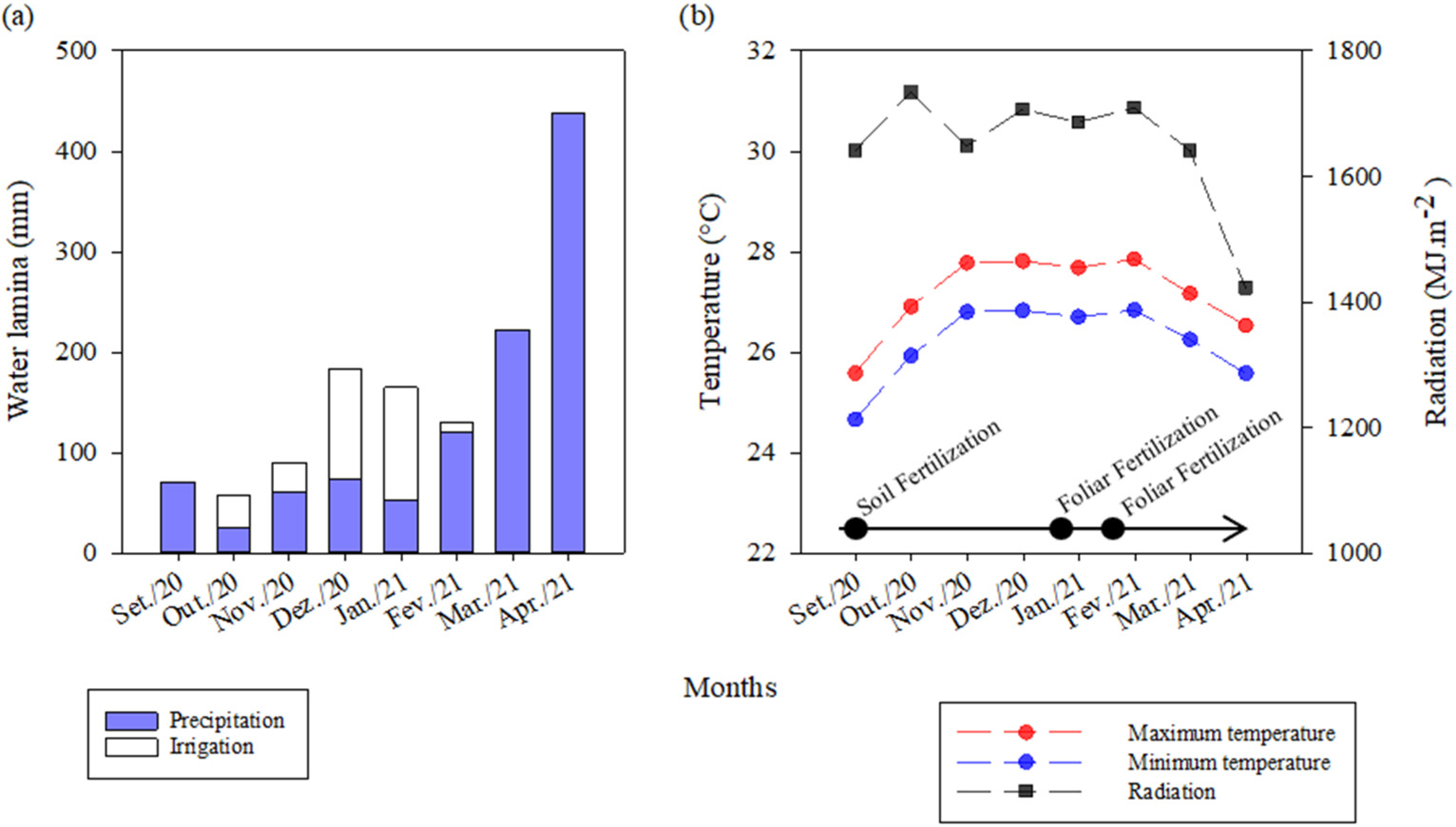
2.1. Description of Treatments and Experimental Conduct
2.2. Biomass Accumulation and Nitrogen Recovery in the Aerial Part
2.3. Data Analysis
3. Results
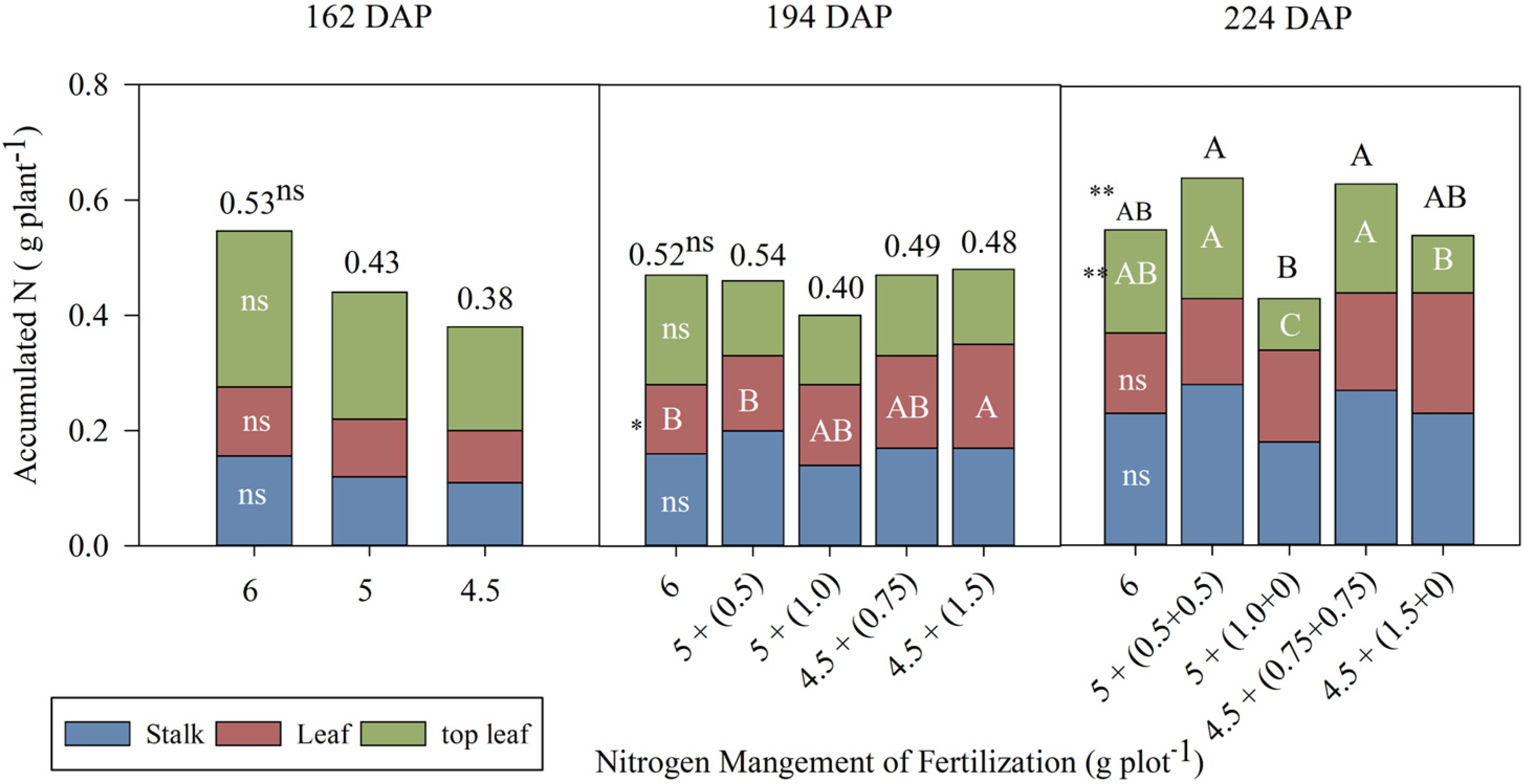
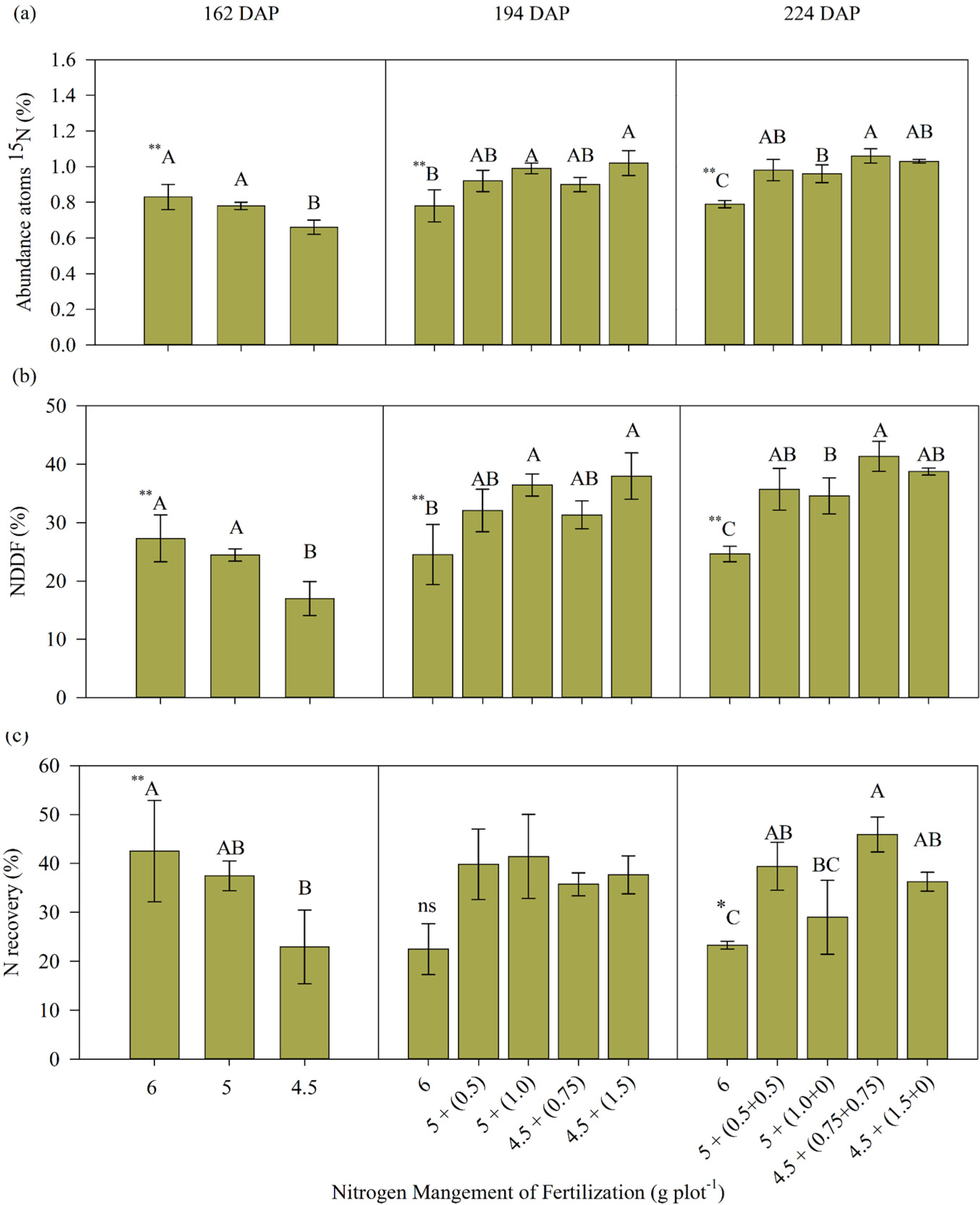
3.1. Accumulation and Recovery of N Fertilizer in the Aerial Part of Sugarcane
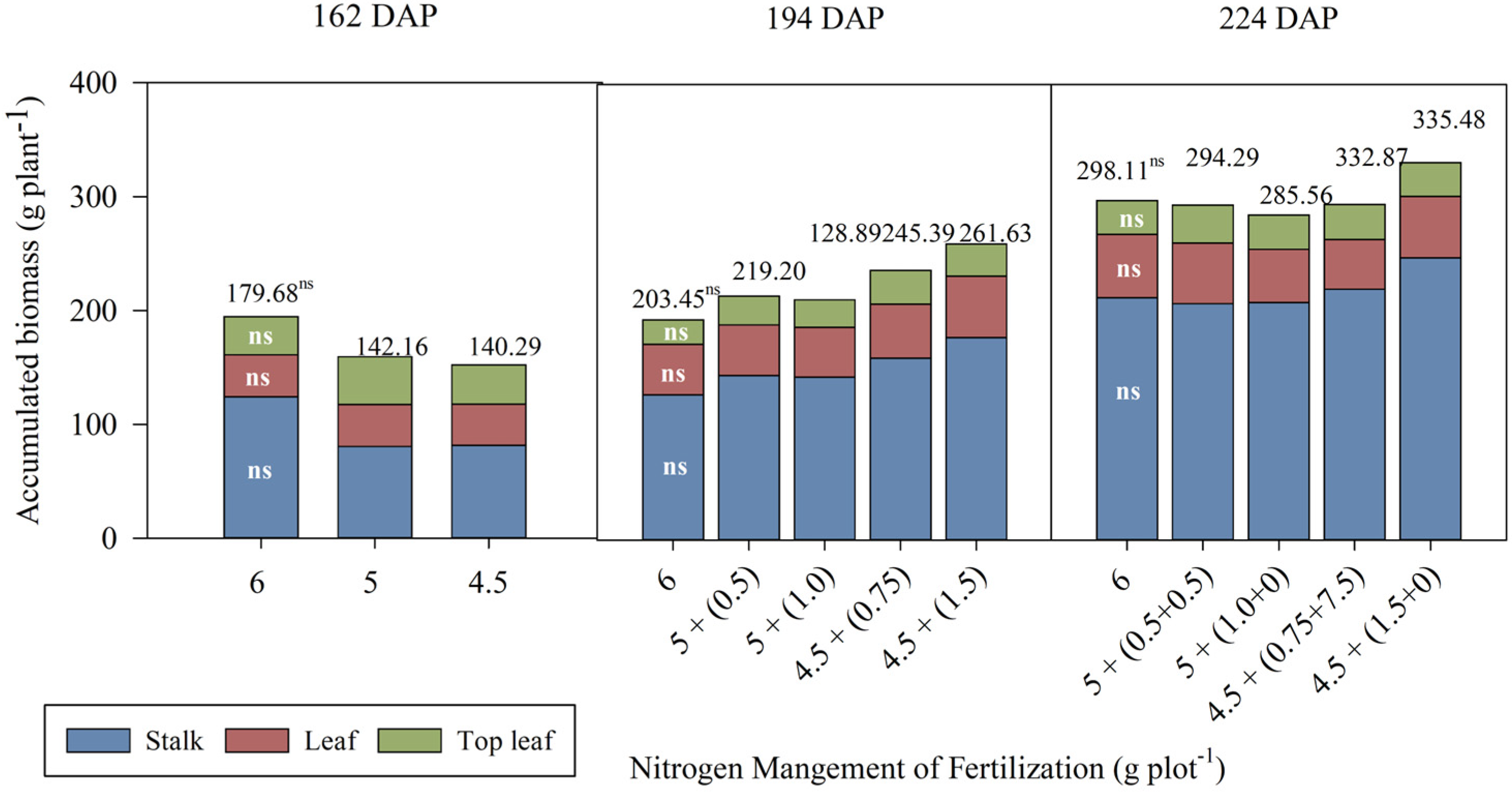
3.2. Biomass Accumulation in the Aerial Part of Sugarcane
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Do Vale, D.W.; Prado, R.D.M.; Avalhães, C.C.; Hojo, R.H. Omissão de macronutrientes na nutrição e no crescimento da cana-de-açúcar cultivada em solução nutritiva. Rev. Bras. Ciênc Agrár. 2011, 6, 189–196. [Google Scholar] [CrossRef]
- Cherubin, M.R.; Lisboa, I.P.; Silva, A.G.B.; Varanda, L.L.; Bordonal, R.O.; Carvalho, J.L.N.; Otto, R.; Pavinato, P.S.; Soltangheisi, A.; Cerri, C.E.P. Sugarcane Straw Removal: Implications to Soil Fertility and Fertilizer Demand in Brazil. Bioenergy Res. 2019, 12, 888–900. [Google Scholar] [CrossRef]
- Oliveira, E.C.A.d.; Freire, F.J.; de Oliveira, R.I.; de Oliveira, A.C.; dos Santos Freire, M.B.G. Acúmulo e alocação de nutrientes em cana-de-açúcar. Rev. Cienc. Agron. 2011, 42, 579–588. [Google Scholar]
- Shrivastava, A.K.; Solomon, S.; Rai, R.K.; Singh, P.; Chandra, A.; Jain, R.; Shukla, S.P. Physiological Interventions for Enhancing Sugarcane and Sugar Productivity. Sugar Tech 2015, 17, 215–226. [Google Scholar] [CrossRef]
- Otto, R.; Souza-Netto, G.J.M.d.; Ferraz-Almeida, R.; Altarugio, L.M.; Favarin, J.L. Multisite response of sugarcane to nitrogen rates and split application under Brazilian field conditions. Agron. J. 2021, 113, 419–435. [Google Scholar] [CrossRef]
- Trivelin, P.C.O.; Vitti, G.C.; Oliveira, M.W.; Gava, G.J.C.; Sarriés, G.A. Utilização de nitrogênio e produtividade da cana-de-açúcar (cana-planta) em solo arenoso com incorpotação de resíduos da cultura. Rev. Bras. Cienc. Solo 2002, 26, 637–646. [Google Scholar] [CrossRef]
- Franco, H.C.J.; Otto, R.; Faroni, C.E.; Vitti, A.C.; Almeida de Oliveira, E.C.; Trivelin, P.C.O. Nitrogen in sugarcane derived from fertilizer under Brazilian field conditions. Field Crops Res. 2011, 121, 29–41. [Google Scholar] [CrossRef]
- Yang, L.; Zhou, Y.; Meng, B.; Zhan, J.; Xi, M.; Deng, Y.; Wu, W.; Lakshmanan, P.; Chen, X.; Zhang, F. High sugarcane yield and large reduction in reactive nitrogen loss can be achieved by lowering nitrogen input. Agric. Ecosyst. Environ. 2024, 369, 109032. [Google Scholar] [CrossRef]
- Otto, R.; Castro, S.A.Q.; Mariano, E.; Castro, S.G.Q.; Franco, H.C.J.; Trivelin, P.C.O. Nitrogen Use Efficiency for Sugarcane-Biofuel Production: What Is Next? Bioenergy Res. 2016, 9, 1272–1289. [Google Scholar] [CrossRef]
- Boschiero, B.N.; Mariano, E.; Trivelin, P.C.O. “Preferential” ammonium uptake by sugarcane does not increase the 15N recovery of fertilizer sources. Plant Soil 2018, 429, 253–269. [Google Scholar] [CrossRef]
- Moreira, L.A.; Otto, R.; Cantarella, H.; Junior, J.L.; Azevedo, R.A.; de Mira, A.B. Urea-Versus Ammonium Nitrate–Based Fertilizers for Green Sugarcane Cultivation. J. Soil Sci. Plant Nutr. 2021, 21, 1329–1338. [Google Scholar] [CrossRef]
- Carvalho, J.L.N.; Oliveira, B.G.; Cantarella, H.; Chagas, M.F.; Gonzaga, L.C.; Lourenço, K.S.; Bordonal, R.O.; Bonomi, A. Implications of regional N2O–N emission factors on sugarcane ethanol emissions and granted decarbonization certificates. Renew. Sustain. Energy Rev. 2021, 149, 111423. [Google Scholar] [CrossRef]
- Vera, J.C.; Portocarrero, R.; Piñeiro, G.; Acreche, M.M. Increases in nitrogen use efficiency decrease nitrous oxide emissions but can penalize yield in sugarcane. Nutr. Cycl. Agroecosyst. 2022, 122, 41–57. [Google Scholar] [CrossRef]
- Gava, G.J.C.; Trivelin, P.C.O.; Vitti, A.C.; Oliveira, M.W. Recovery of nitrogen (N-15) from urea and cane trash by sugar cane ratoon (Saccharum spp.). Rev. Bras. Cienc. Solo 2003, 27, 621–630. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Oliveira, D.M.d.A.; Santos, R.V.d.S.; de Moura, M.J.A.; Guedes, V.H.d.F.; Barbosa, J.d.A.; Lopes, N.R.d.C.; Costa, L.G.d.A.F.; da Silva, J.L.F.; da Costa Santos, M.B.; et al. Nitrate Reductase Activity, Productivity and Technological Quality of Sugarcane Under Molybdenum and Nitrogen Fertilization. Sugar Tech 2022, 24, 463–472. [Google Scholar] [CrossRef]
- Castro, S.A.Q.; Sermarini, R.A.; Rossi, M.L.; Castro, R.R.L.; Trivelin, P.C.O.; Linhares, F.S. Optimizing foliar N-fertilization in sugarcane depends on plant genotype. Physiol. Plant. 2023, 175, 14085. [Google Scholar] [CrossRef]
- Cassim, B.M.A.R.; Lisboa, I.P.; Besen, M.R.; Otto, R.; Cantarella, H.; Inoue, T.T.; Batista, M.A. Nitrogen: From discovery, plant assimilation, sustainable usage to current enhanced efficiency fertilizers technologies—A review. Rev. Bras. Cienc. Solo 2024, 48, e0230037. [Google Scholar] [CrossRef]
- Uscola, M.; Villar-Salvador, P.; Oliet, J.; Warren, C.R. Foliar absorption and root translocation of nitrogen from different chemical forms in seedlings of two Mediterranean trees. Environ. Exp. Bot. 2014, 104, 34–43. [Google Scholar] [CrossRef]
- Checa, R.R.; Pérez-Jordán, H.; García-Gómez, H.; Prieto-Benítez, S.; Gónzalez-Fernández, I.; Alonso, R. Foliar nitrogen uptake in broadleaf evergreen Mediterranean forests: Fertilisation experiment with labelled nitrogen. Sci. Total Environ. 2024, 926, 171865. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M. Foliar Fertilization: A Potential Strategy for Improving Plant Salt Tolerance. CRC Crit. Rev. Plant Sci. 2024, 43, 94–115. [Google Scholar] [CrossRef]
- Trivelin, P.O.C.; Carvalho, J.G.d.; Silva, A.Q.; Promavesi, A.C.P.A.; Camacho, E.; EImori, M.R.; Guilherme, M.R. Adubação foliar de Cana-de-açúcar (Sacchrum spp): Absorção e translocação de Uréia 15-N. Energ. Nucl. Agric. 1988, 9, 52–65. [Google Scholar]
- Sangplung, N.; Rosário, E.L. Response of sugarcane to foliar application of urea. Philipp. J. Crop Sci. 1978, 3, 103–109. [Google Scholar]
- Leite, J.M.; Pitumpe Arachchige, P.S.; Ciampitti, I.A.; Hettiarachchi, G.M.; Maurmann, L.; Trivelin, P.C.O.; Prasad, P.V.V.; Sunoj, S.V.J. Co-addition of humic substances and humic acids with urea enhances foliar nitrogen use efficiency in sugarcane (Saccharum officinarum L.). Heliyon 2020, 6, e05100. [Google Scholar] [CrossRef] [PubMed]
- Joris, H.A.W.; Vitti, A.C.; Ferraz-Almeida, R.; Otto, R.; Cantarella, H. Long-term N fertilization reduces uptake of N from fertilizer and increases the uptake of N from soil. Sci. Rep. 2020, 10, 18834. [Google Scholar] [CrossRef]
- Phillips, S.B.; Mullins, G.L. Foliar burn and wheat grain yield responses following topdress-applied nitrogen and sulfur fertilizers. J. Plant Nutr. 2004, 27, 921–930. [Google Scholar] [CrossRef]
- Shilpha, J.; Song, J.; Jeong, B.R. Ammonium Phytotoxicity and Tolerance: An Insight into Ammonium Nutrition to Improve Crop Productivity. Agronomy 2023, 13, 1487. [Google Scholar] [CrossRef]
- Betiol, R.A.B.; Ferraz-Almeida, R.; Otto, R.; Cesar Vitti, G. Borated Fertilizations via Foliar and Soil for Peanut Production during the Sugarcane Reform. Agriculture 2023, 13, 347. [Google Scholar] [CrossRef]
- FAO. World Reference Base for Soil Resources 2014 Maps, International Soil Classification System for Naming Soils and Creating Legends for Soil; FAO: Rome, Italy, 2015. [Google Scholar] [CrossRef]
- Crusciol, C.A.C.; Rossato, O.B.; Foltran, R.; Martello, J.M.; do Nascimento, C.A.C. Soil Fertility, Sugarcane Yield Affected by Limestone, Silicate, and Gypsum Application. Commun. Soil Sci. Plant Anal. 2017, 48, 2314–2323. [Google Scholar] [CrossRef]
- Neto, D.E.S.; de Oliveira, A.C.; Freire, F.J.; dos Santos Freire, M.B.G.; de Oliveira, E.C.A.; da Rocha, A.T. Adubação fosfatada para cana-de-açúcar em solos representativos para o cultivo da espécie no Nordeste Brasileiro. Pesqui. Agropecu. Bras. 2015, 50, 73–81. [Google Scholar] [CrossRef]
- Da Rocha, I.T.M.; Freire, F.J.; de Oliveira, E.C.A.; de Souza, E.R.; Freire, M.B.G.d.S.; Neto, D.E.S.; da Silva, A.V. Salt effect of potassium fertilizer on productivity and technological quality of sugarcane. Aust. J. Crop Sci. 2019, 13, 1552–1560. [Google Scholar] [CrossRef]
- Benett, C.G.S.; Buzetti, S.; Silva, K.S.; Teixeira Filho, M.C.M.; Garcia, C.M.d.P.; Maestrelo, P.R. Produtividade e desenvolvimento da cana-planta e soca em função de doses e fontes de manganês. Rev. Bras. Ciênc. Solo 2011, 35, 1661–1667. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Freire, F.J.; de Oliveira, E.C.A.; Neto, D.E.S.; de Medeiros, M.R.F.A.; Bezerra, P.d.C.; de Moura, M.J.A.; Barbosa, J.d.A.; Lopes, N.R.d.C.; Santos, N.d.L. Productivity and technological quality of sugarcane under fertilization of nitrogen and molybdenum. J. Soil Sci. Plant Nutr. 2018, 18, 1002–1020. [Google Scholar] [CrossRef]
- Marangoni, F.F.; Otto, R.; de Almeida, R.F.; Casarin, V.; Vitti, G.C.; Tiritan, C.S. Soluble Sources of Zinc and Boron on Sugarcane Yield in Southeast Brazil. Sugar Tech 2019, 21, 917–924. [Google Scholar] [CrossRef]
- Simões-Neto, D.E. Variedades de Cana-de-açúcar no Estado de Pernambuco Contribuição do Melhoramento Clássico da RIDESA-UFRPE. An. Acad. Pernamb. Ciênc. Agron. 2009, 5, 125–146. [Google Scholar]
- De Oliveira, E.C.A.; Freire, F.J.; de Oliveira, R.I.; Freire, M.B.G.d.S.; Neto, D.E.S.; da Silva, S.A.M. Extração e Exportação de Nutrientes por Variedades de Cana-de-Açúcar Cultivadas Sob Irrigação Plena. Rev. Bras. Cienc. Solo 2010, 34, 1343–1352. [Google Scholar] [CrossRef]
- Santana, A.C.A.; de Oliveira, E.C.A.; da Silva, V.S.G.; Dos Santos, R.L.; da Silva, M.A.; Freire, F.J. Critical nitrogen dilution curves and productivity assessments for plant cane|Curvas de diluição do nitrogênio crítico e produtividade da cana planta. Rev. Bras. Eng. Agric. Ambient. 2020, 24, 244–251. [Google Scholar] [CrossRef]
- Marin, F.R.; Pellegrino, G.Q.; Pinto, H.S.; Zullo Junior, J. Agrometerelogia dos Cultivos, 1st ed.; Monteiro, J.E.B.A., Ed.; Instituto Nacional de Meteorologia—INMET: Brasília, Brazil, 2009; p. 530. [Google Scholar]
- Embrapa. Manual de Análise Quimicas de Solo, Plantas e Fertilizantes, 2nd ed.; Embrapa: Brasília, Brazil, 2009. [Google Scholar]
- Trivelin, P.C.O.; Salati, E.; Matsui, E. Boletim Técnico; Escola Superior de Agricultura “Luiz de Queiroz”-USP/CNEN: São Paulo, Brazil, 1973; p. 44. [Google Scholar]
- Trivelin, P.C.O.; Lara Cabezas, W.A.R.; Victoria, R.L.; Reichardt, K. Evaluation of a 15N plot design for estimating plant recovery of fertilizer nitrogen applied to sugar cane. Sci. Agric. 1994, 51, 226–234. [Google Scholar] [CrossRef]
- Yang, Y.; Gao, S.; Jiang, Y.; Lin, Z.; Luo, J.; Li, M.; Guo, J.; Su, Y.; Xu, L.; Que, Y. The physiological and agronomic responses to nitrogen dosage in different sugarcane varieties. Front. Plant Sci. 2019, 10, 406. [Google Scholar] [CrossRef]
- Zeng, X.; Zhu, K.; Lu, J.; Jiang, Y.; Yang, L.; Xing, Y. Long-Term Effects of Different Nitrogen Levels on Growth, Yield, and Quality in Sugarcane. Agronomy 2020, 10, 353. [Google Scholar] [CrossRef]
- Zhang, Y.; Ding, H.; Zheng, X.; Cai, Z.; Misselbrook, T.; Carswell, A.; Müller, C.; Zhang, J. Soil N transformation mechanisms can effectively conserve N in soil under saturated conditions compared to unsaturated conditions in subtropical China. Biol. Fertil. Soils 2018, 54, 495–507. [Google Scholar] [CrossRef]
- Wang, W.; Xu, W.; Wen, Z.; Wang, D.; Wang, S.; Zhang, Z.; Zhao, Y.; Liu, X. Characteristics of Atmospheric Reactive Nitrogen Deposition in Nyingchi City. Sci. Rep. 2019, 9, 4645. [Google Scholar] [CrossRef] [PubMed]
- Pereira, W.; Oliveira, R.P.; Pereira, A.; Sousa, J.S.; Schultz, N.; Urquiaga, S.; Reis, V.M. Nitrogen acquisition and 15N-fertiliser recovery efficiency of sugarcane cultivar RB92579 inoculated with five diazotrophs. Nutr. Cycl. Agroecosyst. 2021, 119, 37–50. [Google Scholar] [CrossRef]
- Zhu, J.; He, N.; Wang, Q.; Yuan, G.; Wen, D.; Yu, G.; Jia, Y. Science of the Total Environment The composition, spatial patterns, and in fl uencing factors of atmospheric wet nitrogen deposition in Chinese terrestrial ecosystems. Sci. Total Environ. 2015, 511, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.E.L.; De Freitas, A.D.S.; Oliveira, L.M.S.; De Lyra, M.D.C.C.P.; Fonseca, M.A.C.; Santos, C.E.R.S.; De Oliveira, J.P.; De Araújo, A.S.F.; Figueiredo, M.V.B. Sugarcane inoculated with endophytic diazotrophic bacteria: Effects on yield, biological nitrogen fixation and industrial characteristics. An. Acad. Bras. Cienc. 2019, 91, e20180990. [Google Scholar] [CrossRef]
- Daubresse, C.M.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Freire, F.J.; de Oliveira, E.C.A.; Freire, M.B.G.D.S.; West, J.B.; Barbosa, J.d.A.; de Moura, M.J.A.; Bezerra, P.d.C. Nitrate reductase activity and nitrogen and biomass accumulation in sugarcane under molybdenum and nitrogen fertilization. Rev. Bras. Cienc. Solo 2019, 43, e0180171. [Google Scholar] [CrossRef]
- Yoneyama, T.; Suzuki, A. Light-independent nitrogen assimilation in plant leaves: Nitrate incorporation into glutamine, glutamate, aspartate, and asparagine traced by15n. Plants 2020, 9, 1303. [Google Scholar] [CrossRef]
- Fernández, V.; Gil-Pelegrín, E.; Eichert, T. Foliar water and solute absorption: An update. Plant J. 2021, 105, 870–883. [Google Scholar] [CrossRef]
- Schreiber, L. Polar paths of diffusion across plant cuticles: New evidence for an old hypothesis. Ann. Bot. 2005, 95, 1069–1073. [Google Scholar] [CrossRef]
- Tredenick, E.C.; Farrell, T.W.; Forstes, E.A.; Psaltis, S.T.P. Nonlinear Porous Diffusion Modeling of Hydrophilic Ionic Agrochemicals in Astomatous Plant Cuticle Aqueous Pores: A Mechanistic Approach. Front. Plant Sci. 2017, 8, 746. [Google Scholar] [CrossRef]
- Wang, M.; Ding, L.; Gao, L.; Li, Y.; Shen, Q.; Guo, S. The interactions of aquaporins and mineral nutrients in higher plants. Int. J. Mol. Sci. 2016, 17, 1229. [Google Scholar] [CrossRef] [PubMed]
- Witte, C.P. Urea metabolism in plants. Plant Sci. 2011, 180, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Ali, A. Nitrate assimilation pathway in higher plants: Critical role in nitrogen signalling and utilization. Plant Sci. Today 2020, 7, 157–163. [Google Scholar] [CrossRef]
- Xiao, C.; Fang, Y.; Wang, S.; He, K. The alleviation of ammonium toxicity in plants. J. Integr. Plant Biol. 2023, 65, 1362–1368. [Google Scholar] [CrossRef]
- Viciedo, D.O.; Prado, R.d.M.; Toledo, R.L.; Aguilar, D.S.; dos Santos, L.C.N.; Hurtado, A.C.; Calzada, K.P.; Aguilar, C.B. Physiological role of silicon in radish seedlings under ammonium toxicity. J. Sci. Food Agric. 2020, 100, 5637–5644. [Google Scholar] [CrossRef]
- Hachiya, T.; Inaba, J.; Wakazaki, M.; Sato, M.; Toyooka, K.; Miyagi, A.; Kawai-Yamada, M.; Sugiura, D.; Nakagawa, T.; Kiba, T.; et al. Excessive ammonium assimilation by plastidic glutamine synthetase causes ammonium toxicity in Arabidopsis thaliana. Nat. Commun. 2021, 12, 4944. [Google Scholar] [CrossRef]
- Liu, Y.; Von Wirén, N. Ammonium as a signal for physiological and morphological responses in plants. J. Exp. Bot. 2017, 68, 2581–2592. [Google Scholar] [CrossRef]
- De Castro, S.A.Q.; Kichey, T.; Persson, D.P.; Schjoerring, J.K. Leaf Scorching following Foliar Fertilization of Wheat with Urea or Urea–Ammonium Nitrate Is Caused by Ammonium Toxicity. Agronomy 2022, 12, 1405. [Google Scholar] [CrossRef]
- De Castro, S.G.Q.; Neto, J.R.; Kölln, O.T.; Borges, B.M.M.N.; Franco, H.C.J. Decision-making on the optimum timing for nitrogen fertilization on sugarcane ratoon. Sci. Agric. 2019, 76, 237–242. [Google Scholar] [CrossRef]
- Wanderley, L.R.d.S.; de Oliveira, E.C.A.; Freire, F.J.; Simões Neto, D.E.; dos Santos, R.L. Nutritional Requirement by Irrigated Brazilian Sugarcane Varieties. Sugar Tech 2021, 23, 762–775. [Google Scholar] [CrossRef]
- Frungillo, L. Mapping the genomic basis of developmental and metabolic responses to nitrogen. Plant Cell 2022, 34, 4663–4664. [Google Scholar] [CrossRef] [PubMed]
- Fredes, I.; Moreno, S.; Díaz, F.P.; Gutiérrez, R.A. Nitrate signaling and the control of Arabidopsis growth and development. Curr. Opin. Plant Biol. 2019, 47, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Salam, U.; Ullah, S.; Tang, Z.H.; Elateeq, A.A.; Khan, Y.; Khan, J.; Khan, A.; Ali, S. Plant Metabolomics: An Overview of the Role of Primary and Secondary Metabolites against Different Environmental Stress Factors. Life 2023, 13, 706. [Google Scholar] [CrossRef] [PubMed]

| Chemical Attributes | |
|---|---|
| pH (CaCl2) | 4.70 |
| O.M (g.dm−3) | 11.16 |
| P (m g.dm−3) | 5.38 |
| Ca+2 (cmolc.dm−1) | 1.12 |
| Mg+2 (cmolc.dm−1) | 0.52 |
| K+ (cmolc.dm−1) | 0.043 |
| Al+3 (cmolc.dm−1) | 0.04 |
| H + Al(cmolc.dm−1) | 1.17 |
| S.B (cmolc.dm−1) | 1.68 |
| CTC(total) (cmolc.dm−1) | 3.39 |
| Fe+2 (mg.dm−3) | 34.00 |
| Co+2 (mg.dm−3) | 0.51 |
| Zn+2 (mg.dm−3) | 1.90 |
| Mn (mg.dm−3) | 2.10 |
| SB (mg.dm−3) | 0.26 |
| V (%) | 49.55 |
| m (%) | 2.33 |
| Physical attributes | |
| Sandy (g.kg−1) | 970.60 |
| Silt (g.kg−1) | 22.60 |
| Clay (g.kg−1) | 1.20 |
| Ds (g.cm−3) | 1.32 |
| Dp (g.cm−3) | 2.92 |
| αtotal (%) | 31.58 |
| αmacro(%) | 26.51 |
| αmicro(%) | 5.06 |
| θCC (m3 m−3) | 0.21 |
| θPWP (m3 m−3) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrade, J.J.d.; Oliveira, E.C.A.d.; Lima, A.M.d.S.; Amorim, G.P.S.; Oliveira, E.S.; Freire, F.J.; Adelino, W.S.d.M.; Oliveira Filho, E.C.A.d. Foliar Fertilization Improves the Nitrogen Nutrition of Sugarcane. Agriculture 2024, 14, 1984. https://doi.org/10.3390/agriculture14111984
Andrade JJd, Oliveira ECAd, Lima AMdS, Amorim GPS, Oliveira ES, Freire FJ, Adelino WSdM, Oliveira Filho ECAd. Foliar Fertilization Improves the Nitrogen Nutrition of Sugarcane. Agriculture. 2024; 14(11):1984. https://doi.org/10.3390/agriculture14111984
Chicago/Turabian StyleAndrade, Joel José de, Emídio Cantídio Almeida de Oliveira, Amanda Michele dos Santos Lima, Gabriela Priscila Sena Amorim, Ester Souza Oliveira, Fernando José Freire, Wagner Sandro de Moura Adelino, and Emídio Cantídio Almeida de Oliveira Filho. 2024. "Foliar Fertilization Improves the Nitrogen Nutrition of Sugarcane" Agriculture 14, no. 11: 1984. https://doi.org/10.3390/agriculture14111984
APA StyleAndrade, J. J. d., Oliveira, E. C. A. d., Lima, A. M. d. S., Amorim, G. P. S., Oliveira, E. S., Freire, F. J., Adelino, W. S. d. M., & Oliveira Filho, E. C. A. d. (2024). Foliar Fertilization Improves the Nitrogen Nutrition of Sugarcane. Agriculture, 14(11), 1984. https://doi.org/10.3390/agriculture14111984









