Combining UAV Multispectral and Thermal Infrared Data for Maize Growth Parameter Estimation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Experimental Design
2.2. Data Acquisition
2.2.1. Acquisition and Processing of UAV Images
2.2.2. Field and Experimental Data Acquisition
2.2.3. Theoretical Framework for Constructing N Curves Based on the LAI and Biomass
2.3. Information Extraction
2.3.1. Canopy Spectral Information Extraction
2.3.2. Canopy Temperature Information
2.3.3. Canopy Textural Information
2.3.4. Model Construction and Validation
3. Results and Analysis
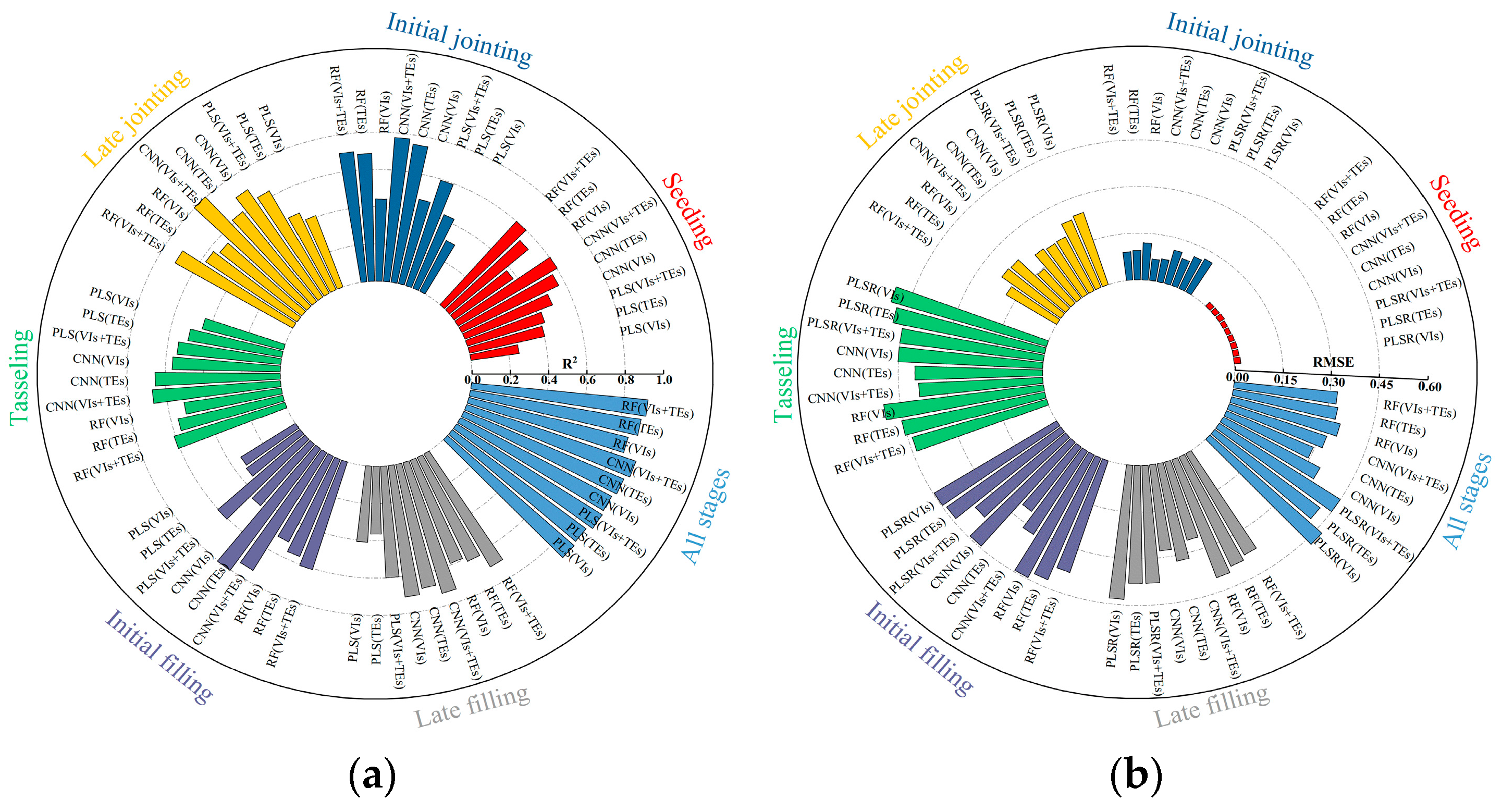
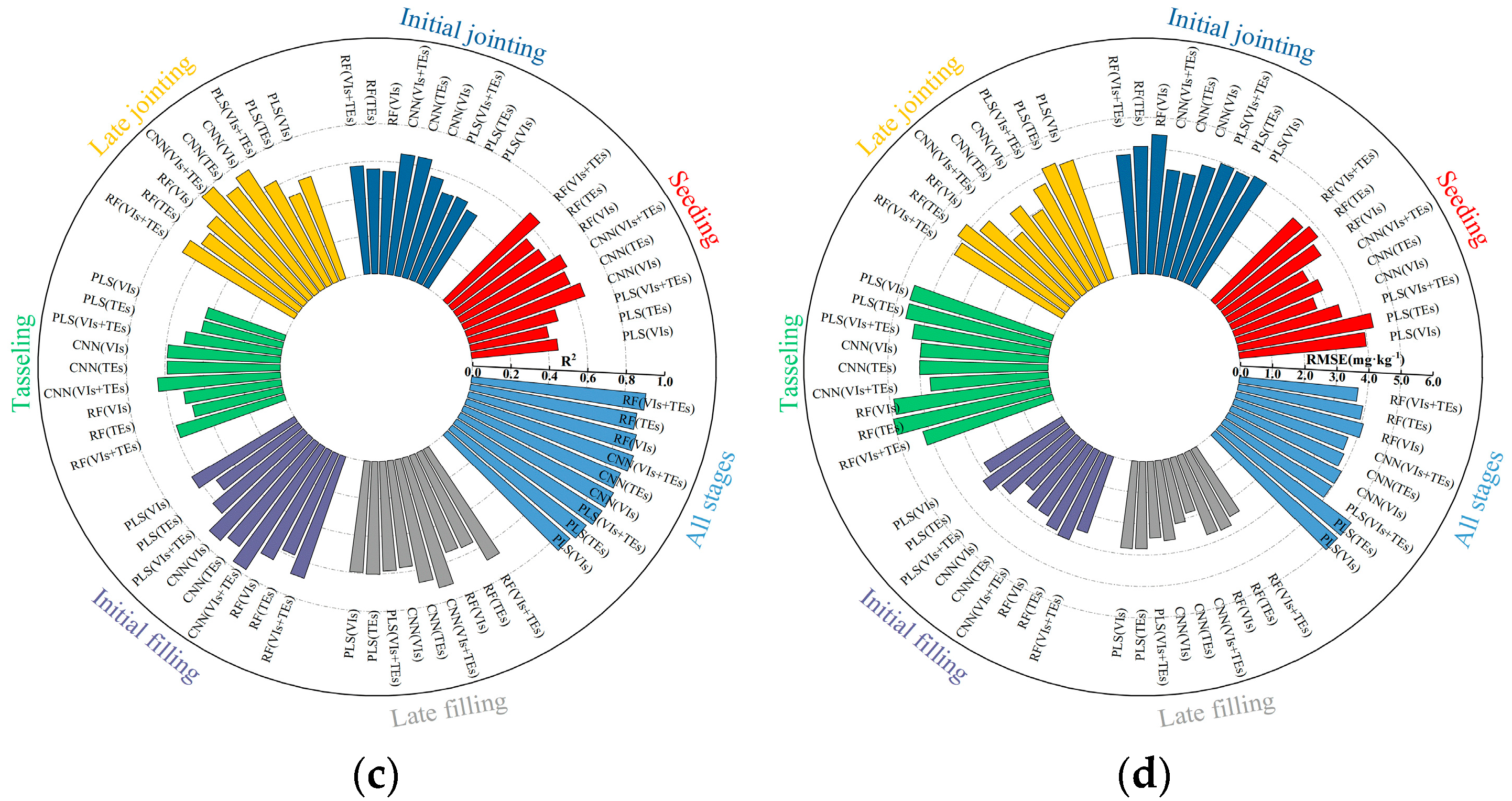
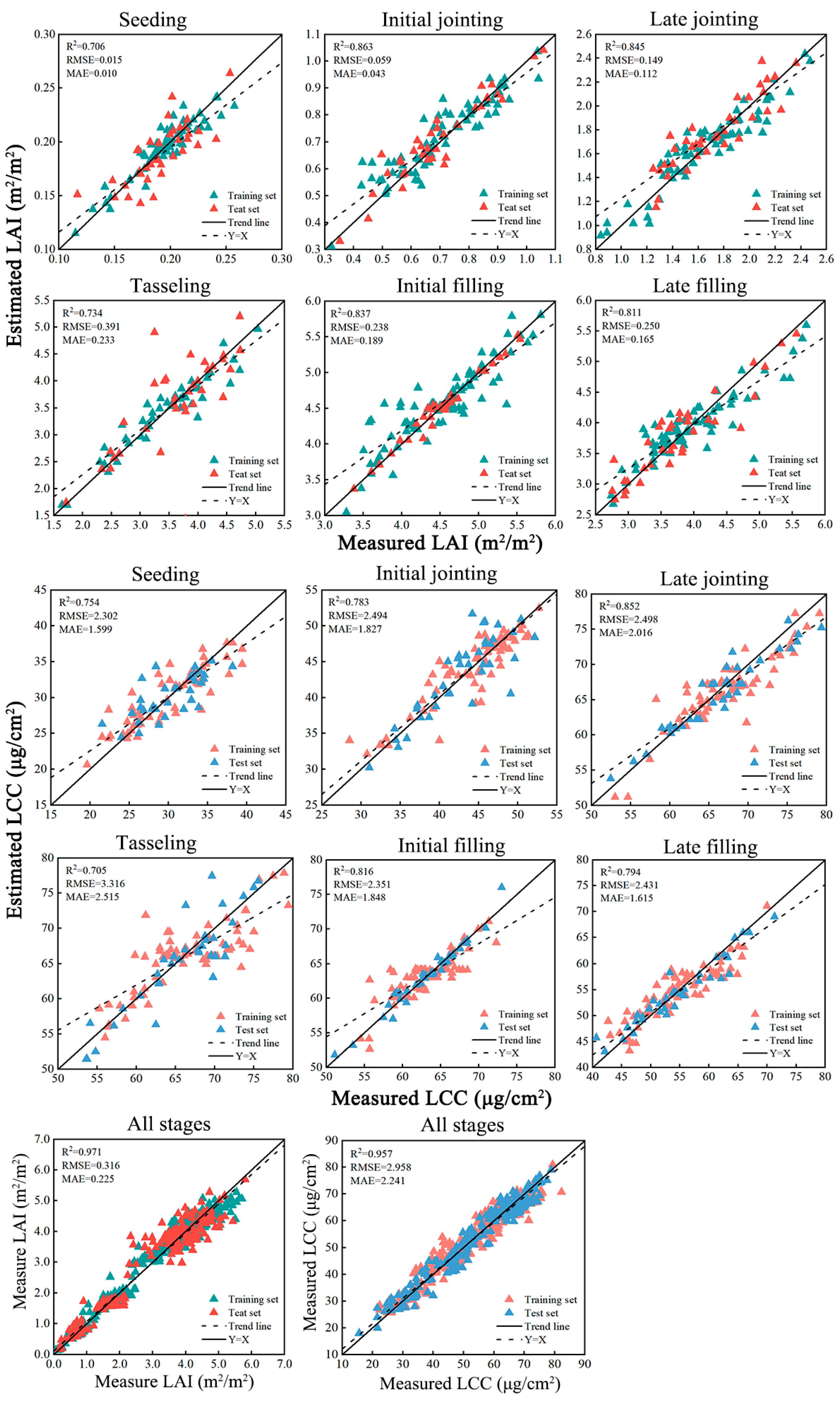
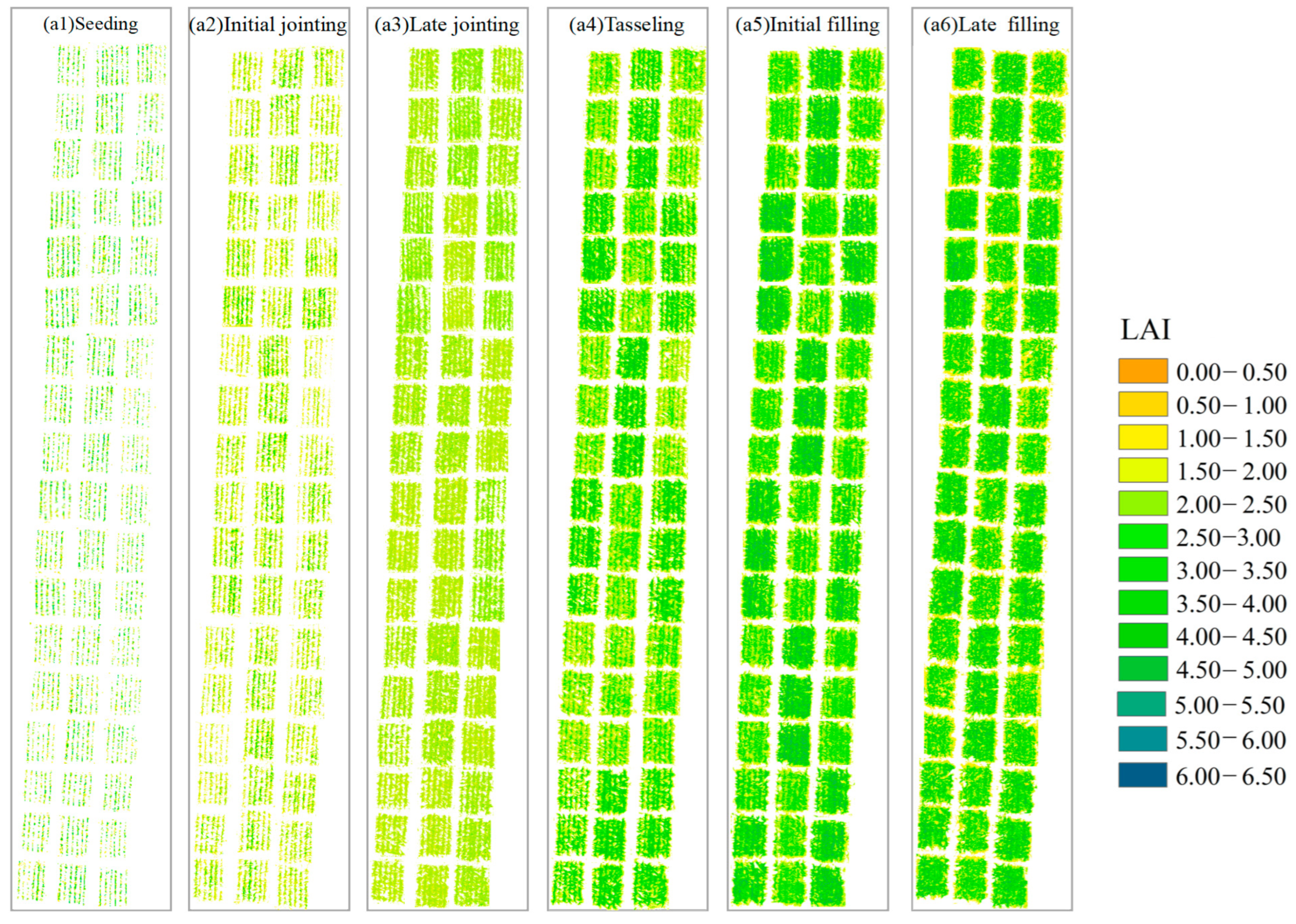
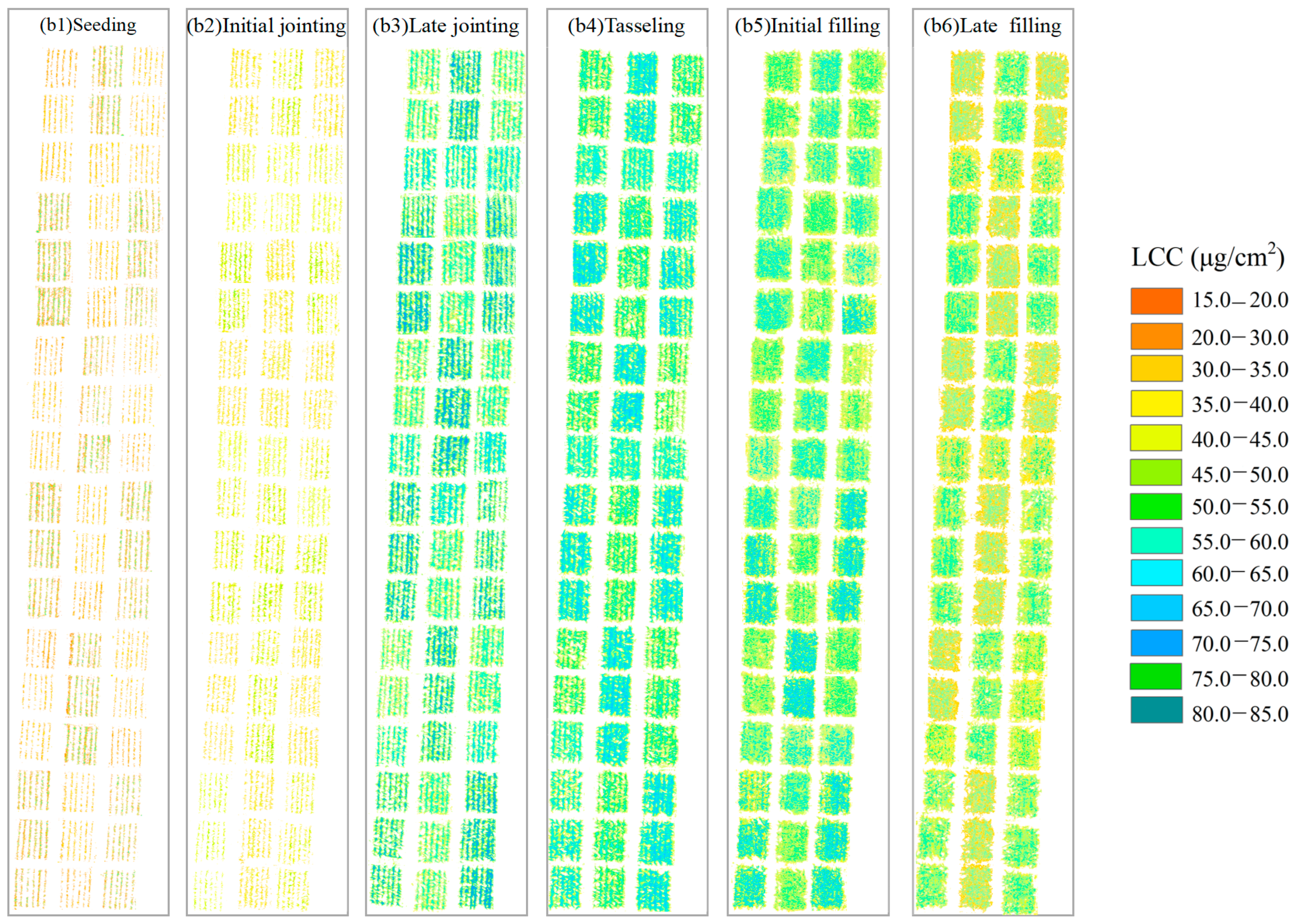
3.1. Estimation of the Maize LAI and LCC Based on UAV MS Data
3.2. Estimation of the Maize LAI and LCC Based on UAV TIR Data
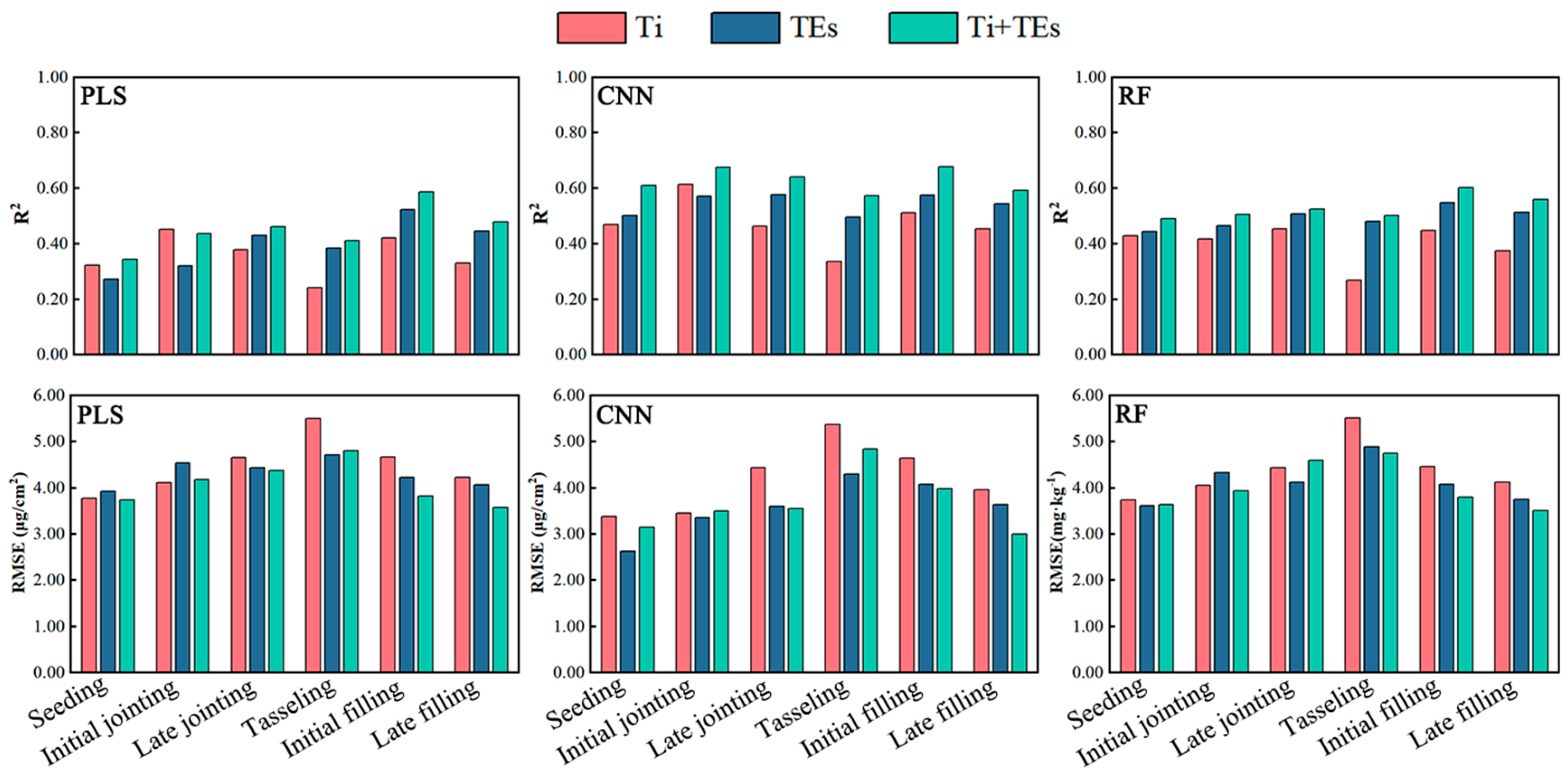
3.3. Estimation of LAI and LCC in Maize Based on Fusion of MS and TIR Data
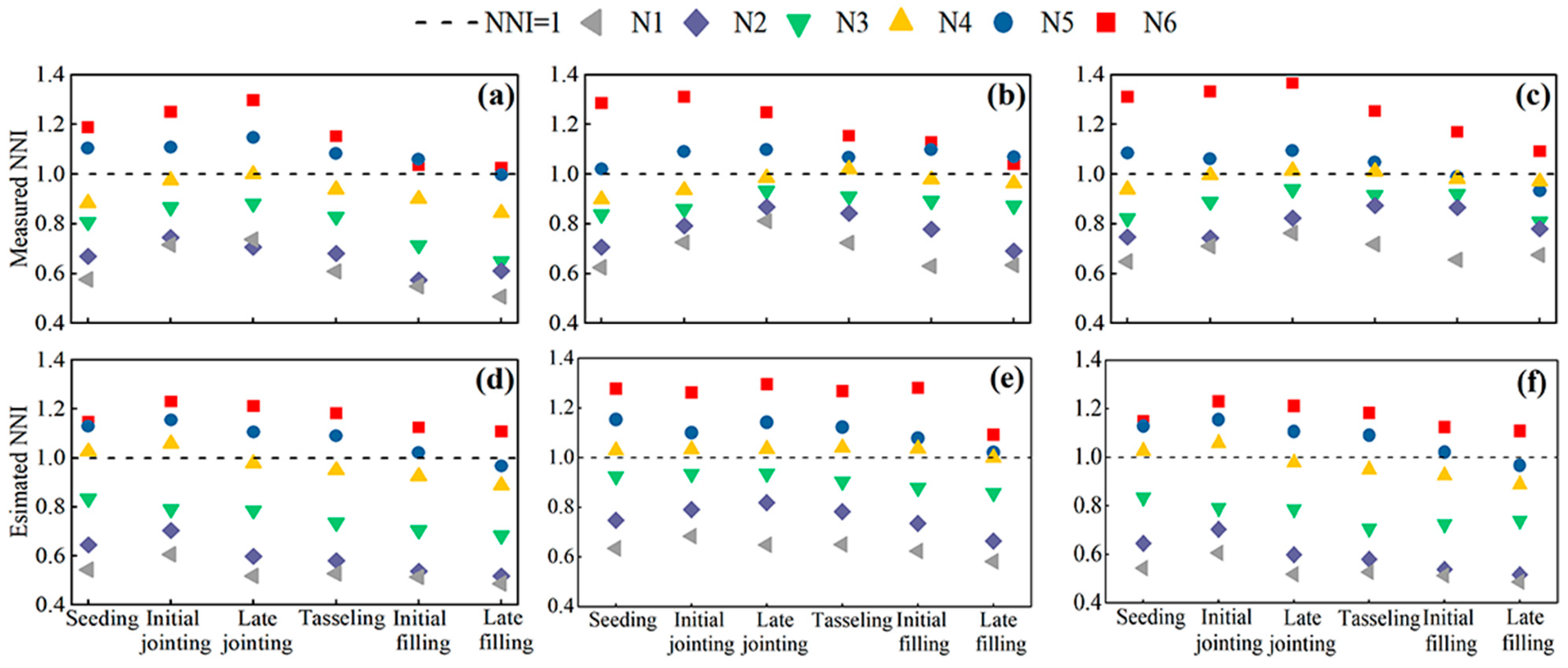
3.4. Remote Sensing Monitoring of the LAI, LCC, and Nitrogen Diagnosis
4. Discussion
4.1. Advantages of MS and TIR Information in the LAI and LCC Dynamic Monitoring
4.2. Application of Remote Sensing Technology in the Crop Nitrogen Status Diagnosis
4.3. Comparison of the Performance of the Different Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mueller, N.D.; Gerber, J.S.; Johnston, M.; Ray, D.K.; Ramankutty, N.; Foley, J.A. Closing yield gaps through nutrient and water management. Nature 2012, 490, 254–257. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef] [PubMed]
- Justes, E.; Mary, B.; Meynard, H.M.; Machet, J.M.; Thelier-Huche, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Zhu, Z.L.; Chen, D.L. Nitrogen fertilizer use in China—Contributions to food production, impacts on the environment and best management strategies. Nutr. Cycl. Agroecosyst. 2002, 63, 117–127. [Google Scholar] [CrossRef]
- Zhao, B.; Ata-Ul-Karim, S.T.; Duan, A.; Liu, Z.; Wang, X.; Xiao, J.; Liu, Z.; Qin, A.; Ning, D.; Zhang, W.; et al. Determination of critical nitrogen concentration and dilution curve based on leaf area index for summer maize. Field Crops Res. 2018, 228, 195–203. [Google Scholar] [CrossRef]
- Du, R.; Chen, J.; Xiang, Y.; Zhang, Z.; Yang, N.; Yang, X.; Tang, Z.; Wang, H.; Wang, X.; Shi, H.; et al. Incremental learning for crop growth parameters estimation and nitrogen diagnosis from hyperspectral data. Comput. Electron. Agric. 2023, 215, 108356. [Google Scholar] [CrossRef]
- Hedley, C. The role of precision agriculture for improved nutrient management on farms. J. Sci. Food Agric. 2015, 95, 12–19. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Ji, R.; Min, J.; Shi, W.; Wang, D. Development of a model using the nitrogen nutrition index to estimate in-season rice nitrogen requirement. Field Crops Res. 2020, 245, 107664. [Google Scholar] [CrossRef]
- Lemaire, G.; Salette, J.; Sigogne, M.; Terrasson, J. Relation entre dynamique de croissance et dynamique de prélèvement d’azote pour un peuplement de graminées fourragères. I.—Etude de l’effet du milieu. Agronomie 1984, 4, 423–430. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.L.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Sheehy, J.E.; Dionora, M.; Mitchell, P.L.; Peng, S.; Cassman, K.G.; Lemaire, G.; Williams, R. Critical nitrogen concentrations: Implications for high-yielding rice (Oryza sativa L.) cultivars in the tropics. Field Crops Res. 1998, 59, 31–41. [Google Scholar] [CrossRef]
- Shi, P.; Wang, Y.; Xu, J.; Zhao, Y.; Yang, B.; Yuan, Z.; Sun, Q. Rice nitrogen nutrition estimation with RGB images and machine learning methods. Comput. Electron. Agric. 2020, 180, 105860. [Google Scholar] [CrossRef]
- Plénet, D.; Lemaire, G. Relationships between dynamics of nitrogen uptake and dry matter accumulation in maize crops. Determination of critical N concentration. Plant Soil. 2000, 216, 65–82. [Google Scholar] [CrossRef]
- Zhao, B.; Tahir, A.; Yao, X.; Tian, Y.C.; Cao, W.X.; Zhu, Y.; Liu, X.; Manuel, R. A new curve of critical nitrogen concentration based on spike dry matter for winter wheat in eastern china. PLoS ONE 2016, 11, 0164545. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.M.; Yan, Y.; Zheng, H.; Yao, X.; Zhu, Y.; Cao, W.; Cheng, T. Estimating leaf nitrogen content by coupling a nitrogen allocation model with canopy reflectance. Rem. Sens. Environ. 2022, 283, 113314. [Google Scholar] [CrossRef]
- Li, R.; Wang, D.; Zhu, B.; Liu, T.; Sun, C.; Zhang, Z. Estimation of nitrogen content in wheat using indices derived from RGB and thermal infrared imaging. Field Crops Res. 2022, 289, 108735. [Google Scholar] [CrossRef]
- Fitzgerald, G.J.; Rodríguez, D.; O’Leary, G. Measuring and predicting canopy nitrogen nutrition in wheat using a spectral index—The canopy chlorophyll content index (CCCI). Field Crops Res. 2010, 116, 318–324. [Google Scholar] [CrossRef]
- Niinemets, Ü. Variation in leaf photosynthetic capacity within plant canopies: Optimization, structural, and physiological constraints and inefficiencies. Photosynth Res. 2023, 158, 131–149. [Google Scholar] [CrossRef]
- Parker, G.G. Tamm review: Leaf Area Index (LAI) is both a determinant and a consequence of important processes in vegetation canopies. Forest Ecol. Manag. 2020, 31, 118–128. [Google Scholar] [CrossRef]
- Wei, H.; Geng, X.; Xiang, Z.; Wang, Z.; Xubin, Z.; Chen, Y.; Huo, Z.; Zhou, G.; Meng, T.; Qigen, D. Grain Yield, Biomass Accumulation, and Leaf Photosynthetic Characteristics of Rice under Combined Salinity-Drought Stress. Rice Sci. 2023, 1, 118–128. [Google Scholar]
- Jeuffroy, M.H.; Ney, B.; and Oury, A. Integrated physiological and agronomic modeling of N capture and use within the plant. J. Exp. Bot. 2002, 53, 809–823. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, G.; van Oosterom, E.; Sheehy, J.; Jeuffroy, M.H.; Massignam, A.; Rossato, L. Is crop demand more closely related to dry matter accumulation or leaf area expansion during vegetative growth? Field Crops Res. 2007, 100, 91–106. [Google Scholar] [CrossRef]
- Xu, H.; He, H.; Yang, K.; Ren, H.; Zhu, T.; Ke, J.; You, C.; Guo, S.; Wu, L. Application of the nitrogen nutrition index to estimate the yield of indica hybrid rice grown from machine-transplanted bowl seedlings. Agronomy 2022, 12, 742. [Google Scholar] [CrossRef]
- Eickhout, B.; Bouwman, A.F.; and Zeijts, H.V. The role of nitrogen in world food production and environmental sustainability. Agric. Ecosys. Environ. 2006, 116, 4–14. [Google Scholar] [CrossRef]
- Moharana, S.; Dutta, S. Spatial variability of chlorophyll and nitrogen content of rice from hyperspectral imagery. ISPRS J. Photogramm. Rem. Sens. 2016, 122, 17–29. [Google Scholar] [CrossRef]
- Fourty, T.; Baret, F.; Jacquemoud, S.; Schmuck, G.; Verdebout, J. Leaf optical properties with explicit description of its biochemical composition: Direct and inverse problems. Remote Sens. Environ. 1996, 56, 104–117. [Google Scholar] [CrossRef]
- Gianquinto, G.; Orsini, F.; Fecondini, M.; Mezzetti, M.; Sambo, P.; Bona, S. A methodological approach for defining spectral indices for assessing tomato nitrogen status and yield. Eur. J. Agron. 2011, 35, 135–143. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Lee, B. Assessment of rice leaf growth and nitrogen status by hyperspectral canopy reflectance and partial least square regression. Eur. J. Agron. 2006, 24, 349–356. [Google Scholar] [CrossRef]
- Cui, H.; Luo, Y.; Li, C.; Chang, Y.; Jin, M.; Li, Y.; Wang, Z. Effects of nitrogen forms on nitrogen utilization, yield, and quality of two wheat varieties with different gluten characteristics. Eur. J. Agron. 2023, 149, 126919. [Google Scholar] [CrossRef]
- Field, C.B.; Mooney, H.A. The photosynthesisp–nitrogen relationship in wild plants. In On the eEconomy of Plant Form and Function; Givnish, T.J., Ed.; Cambridge University Press: Cambridge, UK, 1986; pp. 25–55. [Google Scholar]
- Gitelson, A.A.; Keydan, G.P.; Merzlyak, M.N. Three-band model for noninvasive estimation of chlorophyll, carotenoids, and anthocyanin contents in higher plant leaves. Geophys. Res. Lett. 2006, 33, L11402. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Viña, A.; Arkebauer, T.J.; Rundquist, D.C.; Keydan, G.; Leavitt, B. Estimation of Leaf Area Index and Green Leaf Biomass in Maize Canopies. Geophys. Res. Lett. 2003, 30, 1248. [Google Scholar] [CrossRef]
- Berger, K.; Verrelst, J.; Féret, J.; Wang, Z.; Wocher, M.; Strathmann, M.; Danner, M.; Mauser, W.; Hank, T.B. Crop nitrogen monitoring: Recent progress and principal developments in the context of imaging spectroscopy missions. Remote Sens. Environ. 2020, 242, 111758. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Nitrogen and photosynthesis in the flag leaf of wheat (Triticum aestivum L.). Plant Physiol. 1983, 72, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.R. Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, X.; Cui, Y.; Chao, Y.; Song, G.; Nie, C.; Wang, L. Response of different varieties of maize to nitrogen stress and diagnosis of leaf nitrogen using hyperspectral data. Sci. Rep. 2023, 13, 5890. [Google Scholar] [CrossRef]
- Homolová, L.; Malenovský, Z.; Clevers, J.G.P.W.; García-Santos, G.; Schaepman, M.E. Review of optical-based remote sensing for plant trait mapping. Ecol. Complex. 2013, 15, 1–16. [Google Scholar] [CrossRef]
- Féret, J.-B.; Gitelson, A.A.; Noble, S.D.; Jacquemoud, S. PROSPECT-D: Towards modeling leaf optical properties through a complete lifecycle. Remote Sens. Environ. 2017, 193, 204–215. [Google Scholar] [CrossRef]
- Daughtry, C.S.T.; Walthall, C.L.; Kim, M.S.; de Colstoun, E.B.; McMurtrey, J.E. Estimating corn leaf chlorophyll concentration from leaf and canopy reflectance. Remote Sens. Environ. 2000, 74, 229–239. [Google Scholar] [CrossRef]
- Zhu, W.; Sun, Z.; Yang, T.; Li, J.; Peng, J.; Zhu, K.; Li, S.; Gong, H.; Lyu, Y.; Li, B.; et al. Estimating leaf chlorophyll content of crops via optimal unmanned aerial vehicle hyperspectral data at multi-scales. Comput. Electron. Agric. 2020, 178, 105786. [Google Scholar] [CrossRef]
- Wang, X.; Yan, S.; Wang, W.; Yin, L.; Li, M.; Yu, Z.; Chang, S.; Hou, F. Monitoring leaf area index of the sown mixture pasture through UAV multispectral image and texture characteristics. Comput. Electron. Agric. 2023, 214, 108333. [Google Scholar] [CrossRef]
- Zhang, J.; Qiu, X.; Wu, Y.; Zhu, Y.; Cao, Q.; Liu, X.; Cao, W. Combining texture, color, and vegetation indices from fixed-wing UAS imagery to estimate wheat growth parameters using multivariate regression methods. Comput. Electron. Agric. 2021, 185, 106138. [Google Scholar] [CrossRef]
- Maimaitijiang, M.; Sagan, V.; Sidike, P.; Hartling, S.; Esposito, F.; Fritschi, F.B. Soybean yield prediction from UAV using multimodal data fusion and deep learning. Remote Sens. Environ. 2020, 237, 111599. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Rouget, M.; Kumar, L. Estimating Biomass of Native Grass Grown under Complex Management Treatments Using WorldView-3 Spectral Derivatives. Remote Sens. 2017, 9, 55. [Google Scholar] [CrossRef]
- Yang, M.-D.; Huang, K.-S.; Kuo, Y.-H.; Tsai, H.; Lin, L.-M. Spatial and Spectral Hybrid Image Classification for Rice Lodging Assessment through UAV Imagery. Remote Sens. 2017, 9, 583. [Google Scholar] [CrossRef]
- Qiao, L.; Zhao, R.; Tang, W.; An, L.; Sun, H.; Li, M.; Wang, N.; Liu, Y.; Liu, G. Estimating maize LAI by exploring deep features of vegetation index map from UAV multispectral images. Field Crops Res. 2022, 289, 108739. [Google Scholar] [CrossRef]
- Zhang, C.; Yi, Y.; Wang, L.; Zhang, X.; Chen, S.; Su, Z.; Zhang, S.; Xue, Y. Estimation of the Bio-Parameters of Winter Wheat by Combining Feature Selection with Machine Learning Using Multi-Temporal Unmanned Aerial Vehicle Multispectral Images. Remote. Sens. 2024, 16, 469. [Google Scholar] [CrossRef]
- Chen, L.; Lin, L.; Cai, G.; Sun, Y.; Huang, T.; Wang, K.; Deng, J. Identification of nitrogen, phosphorus, and potassium deficiencies in rice based on static scanning technology and hierarchical identification method. PLoS ONE 2014, 9, e113200. [Google Scholar] [CrossRef]
- Rischbeck, P.; Elsayed, S.; Mistele, B.; Barmeier, G.; Heil, K.; Schmidhalter, U. Data fusion of spectral, thermal and canopy height parameters for improved yield prediction of drought stressed spring barley. Eur. J. Agron. 2016, 78, 44–59. [Google Scholar] [CrossRef]
- Guo, J.X.; Tian, G.L.; Zhou, Y.; Wang, M.; Ling, N.; Shen, Q.R.; Guo, S.W. Evaluation of the grain yield and nitrogen nutrient status of wheat (Triticum aestivum L.) using thermal imaging. Field Crop Res. 2016, 196, 463–472. [Google Scholar] [CrossRef]
- Makino, A.; Osmond, B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 1991, 96, 355–362. [Google Scholar] [CrossRef]
- Lemaire, G.; Jeuffroy, M.-H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Li, F.; Miao, Y.; Feng, G.; Yuan, F.; Yue, S.; Gao, X.; Liu, Y.; Liu, B.; Ustin, S.L.; Chen, X. Improving estimation of summer maize nitrogen status with red edge-based spectral vegetation indices. Field Crops Res. 2014, 157, 111–123. [Google Scholar] [CrossRef]
- Yang, N.; Zhang, Z.; Zhang, J.; Guo, Y.; Yang, X.; Yu, G.; Bai, X.; Chen, J.; Chen, Y.; Shi, L.; et al. Improving estimation of maize leaf area index by combining of UAV-based multispectral and thermal infrared data: The potential of new texture index. Comput. Electron. Agric. 2023, 214, 108294. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Baret, F. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Haboudane, D.; Miller, J.R.; Tremblay, N.; Zarco-Tejada, P.J.; Dextraze, L. Integrated narrow-band vegetation indices for prediction of crop chlorophyll content for application to precision agriculture. Remote Sens. Environ. 2002, 81, 416–426. [Google Scholar] [CrossRef]
- Bendig, J.; Yu, K.; Aasen, H.; Bolten, A.; Bennertz, S.; Broscheit, J.; Gnyp, M.L.; Bareth, G. Combining UAV-based plant height from crop surface models, visible, and near infrared vegetation indices for biomass monitoring in barley. Int. J. Appl. Earth Observ. Geoinformation. 2015, 39, 79–87. [Google Scholar] [CrossRef]
- Louhaichi, M.; Borman, M.M.; Johnson, D.E. Spatially located platform and aerial photography for documentation of grazing impacts on wheat. Geocarto Int. 2008, 16, 65–70. [Google Scholar] [CrossRef]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crop Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.S.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef]
- Lang, Q.; Dehua, G.; Junyi, Z.; Minzan, L.; Hong, S.; Junyong, M. Dynamic influence elimination and chlorophyll content diagnosis of maize using UAV spectral imagery. Remote Sens. 2020, 12, 162650. [Google Scholar] [CrossRef]
- Ali, A.; Imran, M.M. Evaluating the potential of red edge position (REP) of hyperspectral remote sensing data for real time estimation of LAI and chlorophyll content of kinnow mandarin (Citrus reticulata) fruit orchards. Sci. Hortic. 2020, 267, 109326. [Google Scholar] [CrossRef]
- Kipp, S.; Mistele, B.; Schmidhalter, U. The performance of active spectral reflectance sensors as influenced by measuring distance, device temperature and light intensity. Comput. Electron. Agric. 2014, 100, 24–33. [Google Scholar] [CrossRef]
- Huang, S.; Miao, Y.; Zhao, G.; Yuan, F.; Ma, X.; Tan, C.; Yu, W.; Gnyp, M.; Lenz Wiedemann, V.; Rascher, U.; et al. Satellite remote sensing-based inseason diagnosis of rice nitrogen status in Northeast China. Rem. Sens. 2015, 7, 10646–10667. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L. Using hyperspectral remote sensing data for retrieving canopy chlorophyll and nitrogen content. IEEE JSTARS 2012, 5, 574–583. [Google Scholar] [CrossRef]
- Mulero, G.; Bacher, H.; Kleiner, U.; Peleg, Z.; Herrmann, I. Spectral estimation of in vivo wheat chlorophyll a/b ratio under contrasting water availabilities. Rem. Sens. 2022, 14, 2585. [Google Scholar] [CrossRef]
- Li, X.; Ata-UI-Karim, S.T.; Li, Y.; Yuan, F.; Miao, Y.; Yoichiro, K.; Cheng, T.; Tang, L.; Tian, X.; Liu, X.; et al. Advances in the estimations and applications of critical nitrogen dilution curve and nitrogen nutrition index of major cereal crops. A review. Comput. Electron. Agric. 2022, 197, 106998. [Google Scholar] [CrossRef]


| Images | Features | Formulation | References |
|---|---|---|---|
| MS (spectral information) | Green, Red, Blue, Red-edge, Near-infrared | Raw reflectance of each band | / |
| Normalized difference vegetation index | NDVI = (NIR − R)/(NIR + R) | [49] | |
| Normalized difference red-edge | NDRE = (NIR − RE)/(NIR + RE) | [32] | |
| Optimized soil-adjusted vegetation index | OSAVI = (NIR − R)/(NIR − R + L) (L = 0.16) | [55] | |
| Modified chlorophyll absorption in the reflectance index | MCARI = [(RE − R) − 0.2(RE − G)](RE/R) | [39] | |
| Red edge chlorophyll index | CIred edge = (NIR/Red Edge) − 1 | [32] | |
| Transformed chlorophyll absorption in the reflectance index | TCARI = 3[(RE − R) − 0.2(RE − G)(RE/R)] | [56] | |
| Green chlorophyll index | CIgreen = (NIR/G) − 1 | [32] | |
| Red green blue vegetation index | RGBVI = (G2 − B × R2)/(G2 + B × R2) | [57] | |
| Green leaf index | GLI = (2G − R + B)/(2G + R + B) | [58] | |
| Green leaf algorithm | GLA = (2G − R − B)/(2G + R + B) | [58] | |
| TIR information) | Normalized relative canopy temperature | NRCT = (Ti − Tmin)/(Ti − Tmax) | [59] |
| MS and TIR | Gray-level co-occurrence matrix | CON, ENT, VAR, MEA, HOM, DIS, SEM, COR | [60] |
| Stages | Seeding | Initial Jointing | Late Jointing | Tasseling | Initial Filling | Late Filling | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VIs | LAI | LCC | LAI | LCC | LAI | LCC | LAI | LCC | LAI | LCC | LAI | LCC |
| NDVI | 0.23 | 0.43 ** | 0.42 ** | 0.53 *** | 0.64 *** | 0.57 *** | 0.45 *** | 0.49 *** | 0.45 *** | 0.36 ** | 0.29 * | 0.34 * |
| NDRE | 0.35 * | 0.44 *** | 0.47 *** | 0.61 *** | 0.60 *** | 0.59 *** | 0.50 *** | 0.57 *** | 0.50 *** | 0.44 *** | 0.23 | 0.45 *** |
| OSAVI | 0.23 | 0.42 ** | 0.42 ** | 0.53 *** | 0.63 *** | 0.57 *** | 0.43 ** | 0.51 *** | 0.46 *** | 0.37 ** | 0.26 | 0.35 ** |
| MCRAI | 0.19 | 0.31 * | 0.37 ** | 0.46 *** | 0.59 *** | 0.52 *** | 0.27 * | 0.31 * | 0.38 ** | 0.29 * | 0.24 | 0.28 * |
| CIred-edge | 0.32 * | 0.49 *** | 0.51 *** | 0.60 *** | 0.59 *** | 0.58 *** | 0.49 *** | 0.54 ** | 0.48 *** | 0.46 *** | 0.24 | 0.46 *** |
| TCARI | 0.18 | 0.30 * | 0.38 ** | 0.47 *** | 0.53 *** | 0.45 *** | 0.12 | 0.23 | 0.39 ** | 0.24 | 0.32 * | 0.21 |
| CIgreen | 0.29 * | 0.48 *** | 0.46 *** | 0.57 *** | 0.63 *** | 0.59 *** | 0.48 *** | 0.47 *** | 0.51 *** | 0.41 ** | 0.26 | 0.38 ** |
| RGBVI | 0.17 | 0.4 ** | 0.38 ** | 0.47 *** | 0.64 *** | 0.49 *** | 0.39 ** | 0.53 *** | 0.34 * | 0.28 * | 0.24 | 0.16 |
| GLI | 0.11 | 0.33 * | 0.30 * | 0.35 * | 0.56 *** | 0.34 * | 0.17 | 0.29 ** | 0.27 | 0.19 | 0.18 | 0.11 |
| GI | 0.12 | 0.31 * | 0.31 * | 0.39 ** | 0.58 *** | 0.46 *** | 0.36 ** | 0.51 *** | 0.35 ** | 0.27 | 0.28 * | 0.21 |
| Number | Texture | Correlation Coefficient | Number | Texture | Correlation Coefficient | ||
|---|---|---|---|---|---|---|---|
| LAI | LCC | LAI | LCC | ||||
| 1 | MEAB | −0.60 *** | −0.57 *** | 25 | DISB | −0.57 *** | −0.44 *** |
| 2 | MEAG | −0.58 *** | −0.55 *** | 26 | DISG | −0.47 *** | −0.31 *** |
| 3 | MEAR | −0.61 *** | −0.57 *** | 27 | DISR | −0.66 *** | −0.53 *** |
| 4 | MEARE | −0.52 *** | −0.53 *** | 28 | DISRE | 0.51 *** | 0.50 *** |
| 5 | MEANIR | 0.41 *** | 0.35 *** | 29 | DISNIR | 0.62 *** | 0.63 *** |
| 6 | MEATIR | −0.56 *** | −0.52 *** | 30 | DISTIR | −0.72 *** | −0.62 *** |
| 7 | VARB | −0.53 *** | −0.36 *** | 31 | ENTB | −0.73 *** | −0.63 *** |
| 8 | VARG | −0.34 *** | −0.18 ** | 32 | ENTG | −0.65 *** | −0.53 *** |
| 9 | VARR | −0.67 *** | −0.48 *** | 33 | ENTR | −0.76 *** | −0.68 *** |
| 10 | VARRE | 0.58 *** | 0.55 *** | 34 | ENTRE | 0.10 | 0.14 * |
| 11 | VARNIR | 0.65 *** | 0.61 *** | 35 | ENTNIR | 0.53 *** | 0.62 *** |
| 12 | VARTIR | −0.68 *** | −0.55 *** | 36 | ENTTIR | −0.60 *** | −0.58 *** |
| 13 | HOMB | 0.69 *** | 0.57 *** | 37 | SECB | 0.74 *** | 0.64 *** |
| 14 | HOMG | 0.56 *** | 0.44 *** | 38 | SECG | 0.68 *** | 0.55 *** |
| 15 | HOMR | 0.74 *** | 0.64 *** | 39 | SECR | 0.75 *** | 0.68 *** |
| 16 | HOMRE | −0.34 *** | −0.37 *** | 40 | SECRE | 0.04 | 0.07 |
| 17 | HOMNIR | −0.54 *** | −0.60 *** | 41 | SECNIR | −0.50 *** | −0.59 *** |
| 18 | HOMTIR | 0.69 *** | 0.64 *** | 42 | SECTIR | 0.68 | 0.66 |
| 19 | CONB | −0.48 *** | −0.31 *** | 43 | CORB | −0.30 *** | −0.09 |
| 20 | CONG | −0.27 *** | −0.13 * | 44 | CORG | −0.17 ** | 0.07 |
| 21 | CONR | −0.53 *** | −0.37 *** | 45 | CORR | −0.58 *** | −0.36 *** |
| 22 | CONRE | 0.51 *** | 0.47 *** | 46 | CORRE | 0.12 | 0.20 *** |
| 23 | CONNIR | 0.66 *** | 0.59 *** | 47 | CORNIR | 0.09 | 0.30 *** |
| 24 | CONTIR | −0.69 *** | −0.58 *** | 48 | CORTIR | −0.61 *** | −0.43 *** |
| Growth Stages | Seeding | Initial Jointing | Late Jointing | Tasseling | Initial Filling | Late Filling | All Stages | ||
|---|---|---|---|---|---|---|---|---|---|
| LAI | PLS | R2 | 0.578 | 0.665 | 0.699 | 0.632 | 0.693 | 0.684 | 0.918 |
| RMSE | 0.019 | 0.091 | 0.204 | 0.450 | 0.326 | 0.319 | 0.489 | ||
| CNN | R2 | 0.706 | 0.863 | 0.845 | 0.734 | 0.837 | 0.811 | 0.971 | |
| RMSE | 0.015 | 0.059 | 0.149 | 0.391 | 0.238 | 0.250 | 0.316 | ||
| RF | R2 | 0.665 | 0.748 | 0.726 | 0.715 | 0.732 | 0.733 | 0.922 | |
| RMSE | 0.017 | 0.078 | 0.206 | 0.406 | 0.310 | 0.294 | 0.483 | ||
| LCC (μg/cm2) | PLS | R2 | 0.543 | 0.613 | 0.689 | 0.605 | 0.670 | 0.729 | 0.893 |
| RMSE | 3.136 | 3.382 | 3.571 | 3.779 | 2.923 | 2.975 | 4.713 | ||
| CNN | R2 | 0.754 | 0.783 | 0.852 | 0.705 | 0.816 | 0.794 | 0.957 | |
| RMSE | 2.302 | 2.494 | 2.498 | 3.316 | 2.351 | 2.431 | 2.958 | ||
| RF | R2 | 0.681 | 0.704 | 0.775 | 0.665 | 0.748 | 0.774 | 0.935 | |
| RMSE | 2.803 | 3.264 | 3.045 | 3.503 | 2.538 | 2.763 | 3.659 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Huo, X.; Qian, L.; Du, Y.; Liu, D.; Cao, Q.; Wang, W.; Hu, X.; Yang, X.; Fan, S. Combining UAV Multispectral and Thermal Infrared Data for Maize Growth Parameter Estimation. Agriculture 2024, 14, 2004. https://doi.org/10.3390/agriculture14112004
Yu X, Huo X, Qian L, Du Y, Liu D, Cao Q, Wang W, Hu X, Yang X, Fan S. Combining UAV Multispectral and Thermal Infrared Data for Maize Growth Parameter Estimation. Agriculture. 2024; 14(11):2004. https://doi.org/10.3390/agriculture14112004
Chicago/Turabian StyleYu, Xingjiao, Xuefei Huo, Long Qian, Yiying Du, Dukun Liu, Qi Cao, Wen’e Wang, Xiaotao Hu, Xiaofei Yang, and Shaoshuai Fan. 2024. "Combining UAV Multispectral and Thermal Infrared Data for Maize Growth Parameter Estimation" Agriculture 14, no. 11: 2004. https://doi.org/10.3390/agriculture14112004
APA StyleYu, X., Huo, X., Qian, L., Du, Y., Liu, D., Cao, Q., Wang, W., Hu, X., Yang, X., & Fan, S. (2024). Combining UAV Multispectral and Thermal Infrared Data for Maize Growth Parameter Estimation. Agriculture, 14(11), 2004. https://doi.org/10.3390/agriculture14112004







