Enhancing Phenolic Profiles in ‘Cabernet Franc’ Grapes Through Chitooligosaccharide Treatments: Impacts on Phenolic Compounds Accumulation Across Developmental Stages
Abstract
1. Introduction
2. Materials and Methods
2.1. Grape Samples Plant Material and Open-Field Treatments
2.2. Physicochemical Parameters Measurements
2.3. Colour Indexes Measurements
2.4. UV–Vis Analysis of Phenolic Compounds
2.5. UHPLC-QqQ-MS/MS Analysis of Phenolic Compounds
2.5.1. Extraction of Phenolic Compounds
2.5.2. UHPLC-QqQ-MS/MS Analysis of Anthocyanins
2.5.3. UHPLC-QqQ-MS/MS Analysis of Non-Anthocyanins
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effects of COS Treatment on the Physicochemical Parameters
3.2. Effects of COS Treatment on the Color Indexes
3.3. Effects of COS Treatment on the Total Phenolic, Tannin, Flavonoid, Flavonol, and Anthocyanin
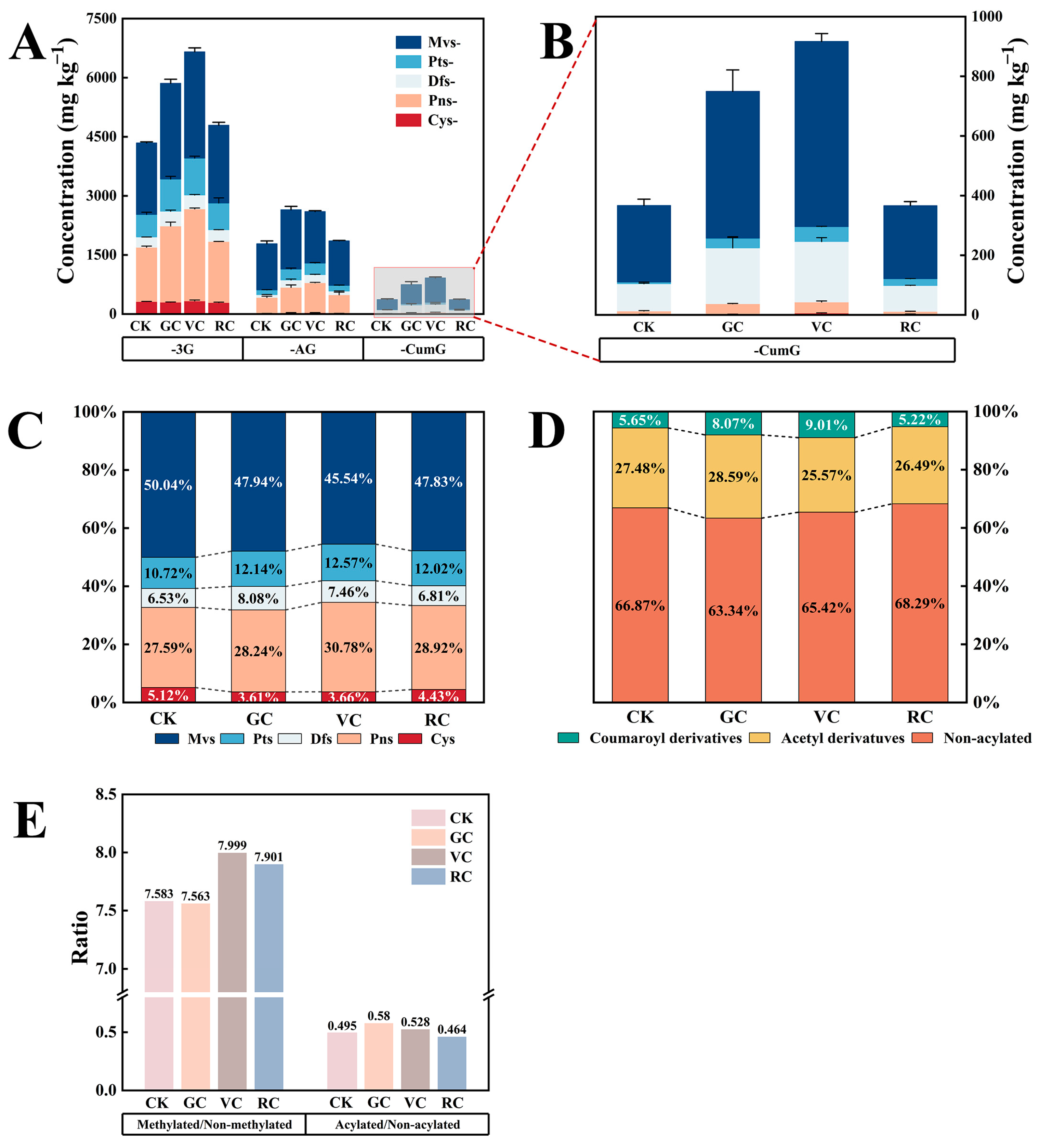
3.4. Effects of COS Treatment on Anthocyanins and Non-Anthocyanins
3.4.1. Effects of COS Treatment on Anthocyanins
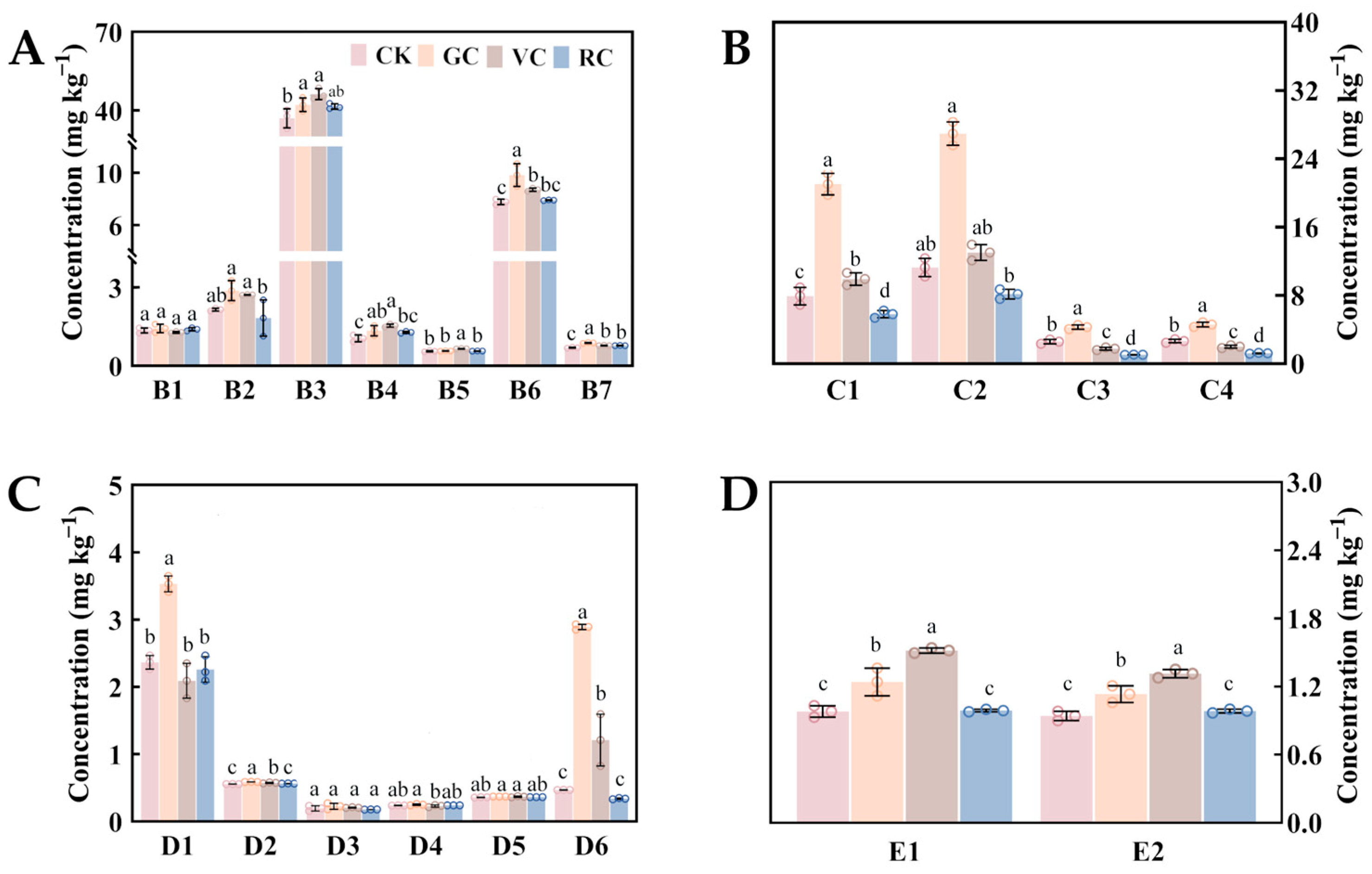
3.4.2. Effects of COS Treatment on Non-Anthocyanins
3.4.3. Non-Anthocyanins
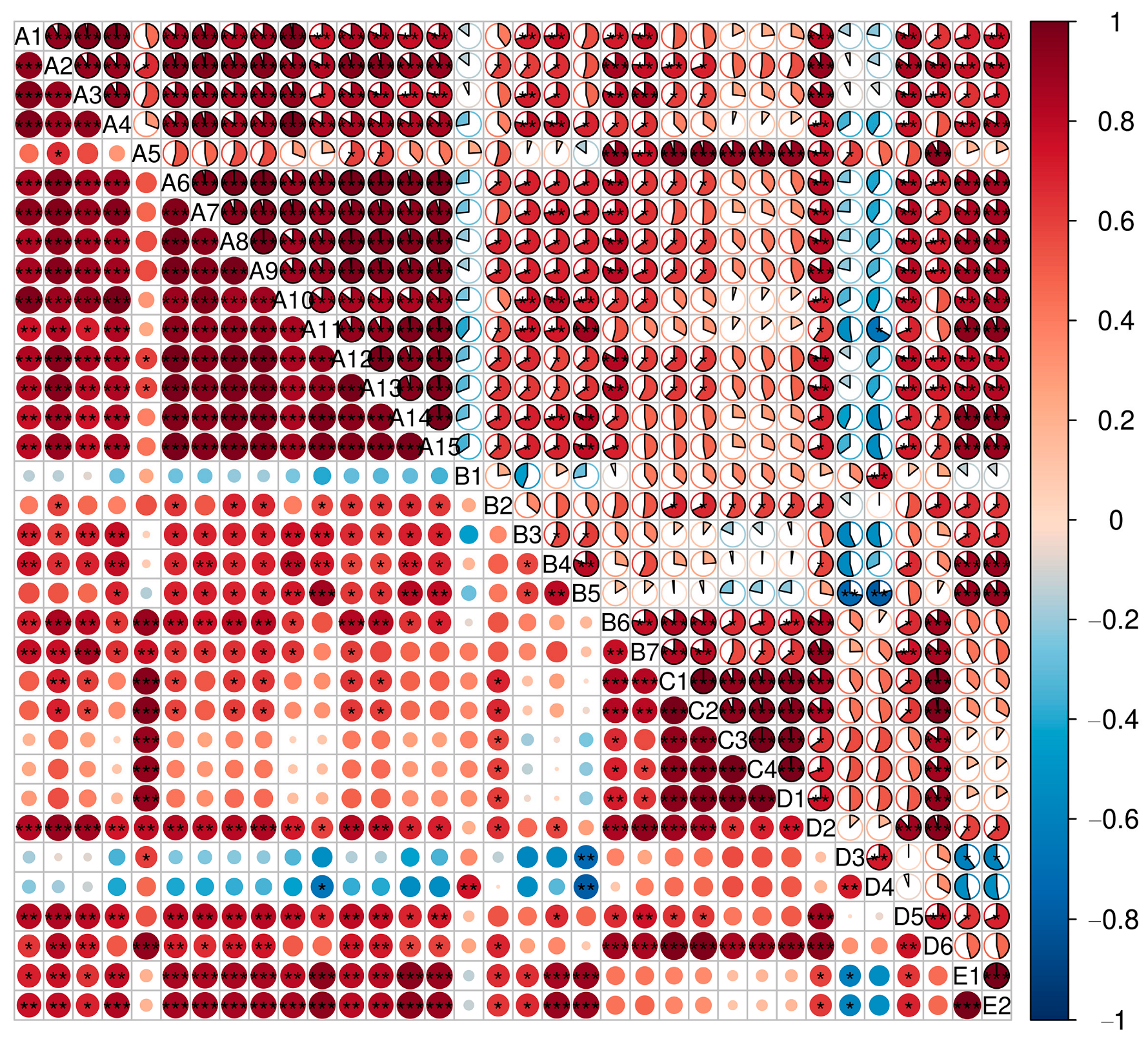
3.4.4. Correlation Analysis of Anthocyanins and Non-Anthocyanins
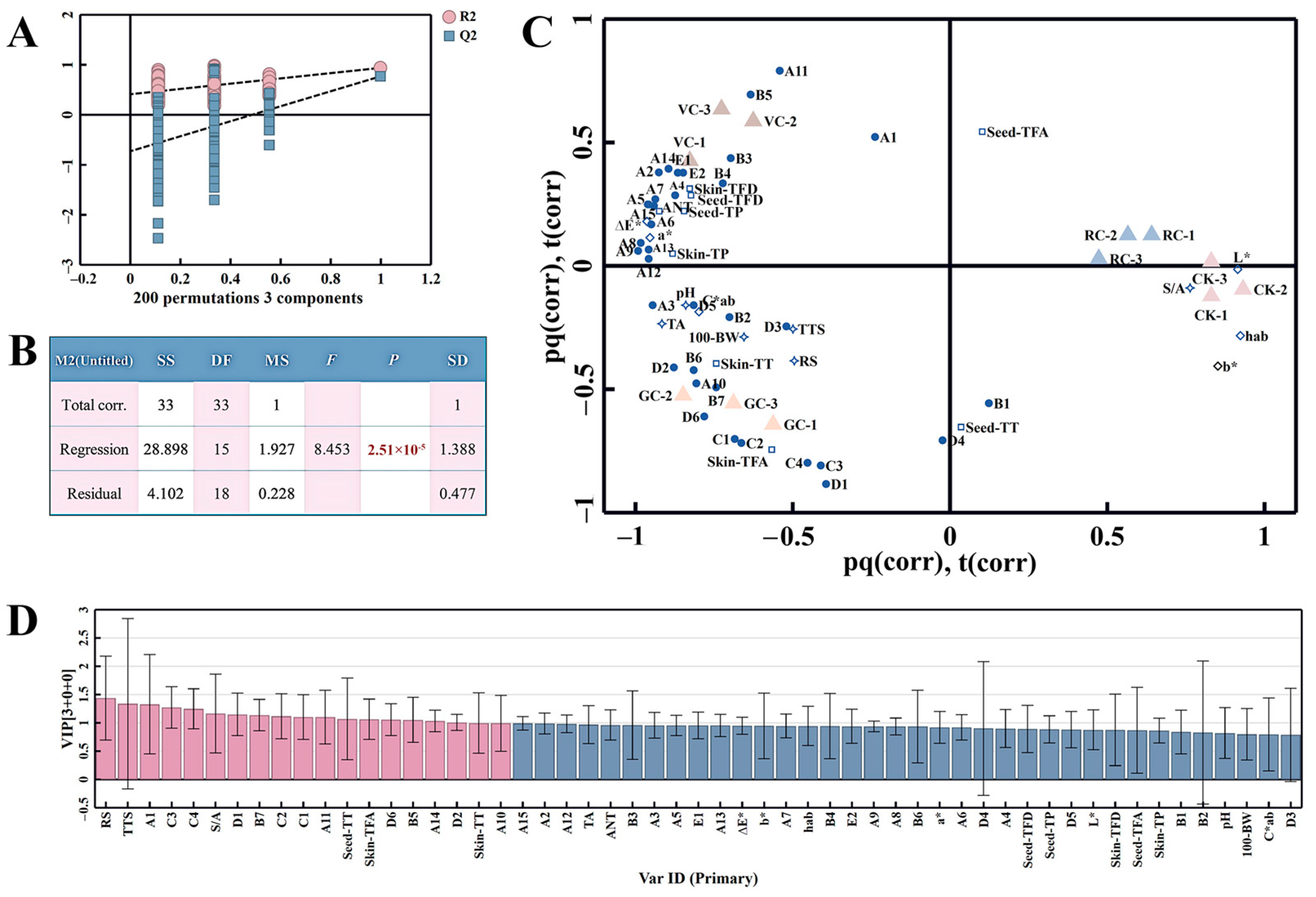
3.5. Multivariate Data Analysis of Grape Specimen Dataset
3.5.1. OPLS-DA Analysis of the Whole-Sample Dataset
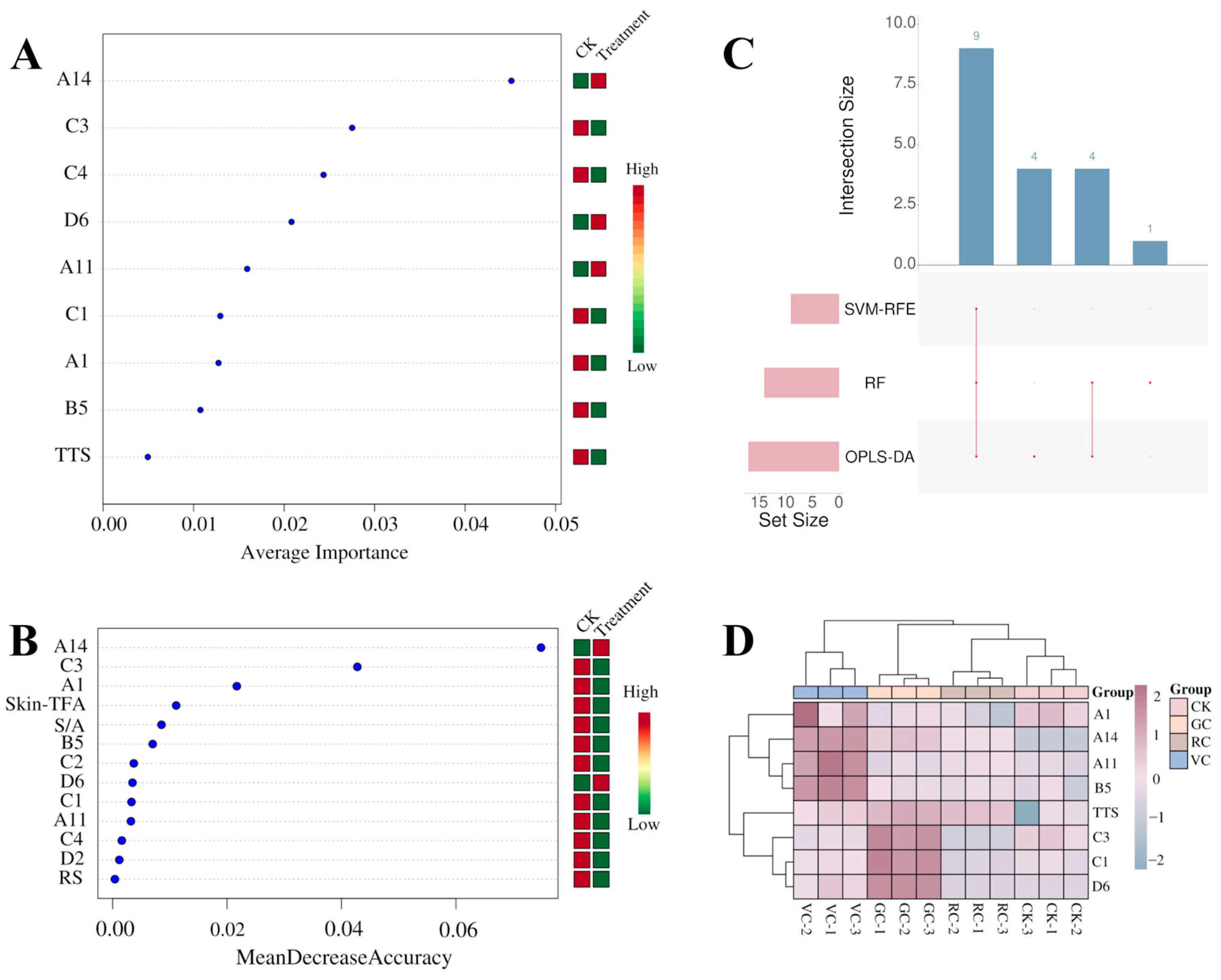
3.5.2. Uses of Machine Learning Method in Discriminating Key Variables
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Nuwer, R. Changing Old Viticulture for All the Right Rieslings. Nature 2023. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Urcan, D.E.; Lung, M.-L.; Giacosa, S.; Torchio, F.; Ferrandino, A.; Vincenzi, S.; Río Segade, S.; Pop, N.; Rolle, L. Phenolic Substances, Flavor Compounds, and Textural Properties of Three Native Romanian Wine Grape Varieties. Int. J. Food Prop. 2016, 19, 76–98. [Google Scholar] [CrossRef]
- Sun, R.-Z.; Cheng, G.; Li, Q.; Zhu, Y.-R.; Zhang, X.; Wang, Y.; He, Y.-N.; Li, S.-Y.; He, L.; Chen, W.; et al. Comparative Physiological, Metabolomic, and Transcriptomic Analyses Reveal Developmental Stage-Dependent Effects of Cluster Bagging on Phenolic Metabolism in Cabernet Sauvignon Grape Berries. BMC Plant Biol. 2019, 19, 583. [Google Scholar] [CrossRef]
- Sivilotti, P.; Falchi, R.; Vanderweide, J.; Sabbatini, P.; Bubola, M.; Vanzo, A.; Lisjak, K.; Peterlunger, E.; Herrera, J.C. Yield Reduction through Cluster or Selective Berry Thinning Similarly Modulates Anthocyanins and Proanthocyanidins Composition in Refosco Dal Peduncolo Rosso (Vitis vinifera L.) Grapes. Sci. Hortic. 2020, 264, 109166. [Google Scholar] [CrossRef]
- Hochberg, U.; Degu, A.; Cramer, G.R.; Rachmilevitch, S.; Fait, A. Cultivar Specific Metabolic Changes in Grapevines Berry Skins in Relation to Deficit Irrigation and Hydraulic Behavior. Plant Physiol. Biochem. 2015, 88, 42–52. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Romanazzi, G.; Garde-Cerdán, T.; Pérez-Álvarez, E.P. A Review of the Use of Biostimulants in the Vineyard for Improved Grape and Wine Quality: Effects on Prevention of Grapevine Diseases. J. Sci. Food Agric. 2019, 99, 1001–1009. [Google Scholar] [CrossRef]
- Świeca, M.; Sęczyk, Ł.; Gawlik-Dziki, U. Elicitation and Precursor Feeding as Tools for the Improvement of the Phenolic Content and Antioxidant Activity of Lentil Sprouts. Food Chem. 2014, 161, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.R.; Mulinacci, N.; Valletta, A.; Innocenti, M.; Pasqua, G. Effects of Elicitors on the Production of Resveratrol and Viniferins in Cell Cultures of Vitis vinifera L. cv Italia. J. Agric. Food Chem. 2011, 59, 9094–9101. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Santamaría, P.; Garde-Cerdán, T. Methyl Jasmonate Treatment to Increase Grape and Wine Phenolic Content in Tempranillo and Graciano Varieties During Two Growing Seasons. Sci. Hortic. 2018, 240, 378–386. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Fernández-Fernández, J.I.; Moreno-Olivares, J.D.; Bleda-Sánchez, J.A.; Gómez-Martínez, J.C.; Martínez-Jiménez, J.A.; Gil-Muñoz, R. Application of Elicitors in Two Ripening Periods of Vitis vinifera L. cv Monastrell: Influence on Anthocyanin Concentration of Grapes and Wines. Molecules 2021, 26, 1689. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.-L.; Liu, M.; Zhao, H.; Meng, J.-F.; Fang, Y.-L. Effect of Exogenous Abscisic Acid and Methyl Jasmonate on Anthocyanin Composition, Fatty Acids, and Volatile Compounds of Cabernet Sauvignon (Vitis vinifera L.) Grape Berries. Molecules 2016, 21, 1354. [Google Scholar] [CrossRef] [PubMed]
- Portu, J.; Santamaría, P.; López-Alfaro, I.; López, R.; Garde-Cerdán, T. Methyl Jasmonate Foliar Application to Tempranillo Vineyard Improved Grape and Wine Phenolic Content. J. Agric. Food Chem. 2015, 63, 2328–2337. [Google Scholar] [CrossRef] [PubMed]
- Tessarin, P.; Chinnici, F.; Donnini, S.; Liquori, E.; Riponi, C.; Rombolà, A.D. Influence of Canopy-Applied Chitosan on the Composition of Organic Cv. Sangiovese and Cabernet Sauvignon Berries and Wines. Food Chem. 2016, 210, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Xue, H.; Sekozawa, Y.; Sugaya, S. Effects of Gibberellin and Abscisic Acid on Fruit Quality and Aromatic Compound Formation During Fruit Development in ‘Kyoho’ Grapes (Vitis vinifera L. × Vitis labrusca L.). Environ. Control Biol. 2022, 60, 67–78. [Google Scholar] [CrossRef]
- Sáenz de Urturi, I.; Ribeiro-Gomes, F.M.; Marín-San Román, S.; Murillo-Peña, R.; Torres-Díaz, L.; González-Lázaro, M.; Pérez-Álvarez, E.P.; Garde-Cerdán, T. Vine Foliar Treatments at Veraison and Post-Veraison with Methyl Jasmonate Enhanced Aromatic, Phenolic and Nitrogen Composition of Tempranillo Blanco Grapes. Foods 2023, 12, 1142. [Google Scholar] [CrossRef]
- Miliordos, D.E.; Alatzas, A.; Kontoudakis, N.; Kouki, A.; Unlubayir, M.; Gémin, M.-P.; Tako, A.; Hatzopoulos, P.; Lanoue, A.; Kotseridis, Y. Abscisic Acid and Chitosan Modulate Polyphenol Metabolism and Berry Qualities in the Domestic White-Colored Cultivar Savvatiano. Plants 2022, 11, 1648. [Google Scholar] [CrossRef]
- Gomes, E.P.; Vanz Borges, C.; Monteiro, G.C.; Filiol Belin, M.A.; Minatel, I.O.; Pimentel Junior, A.; Tecchio, M.A.; Lima, G.P.P. Preharvest Salicylic Acid Treatments Improve Phenolic Compounds and Biogenic Amines in ‘Niagara Rosada’ Table Grape. Postharvest Biol. Technol. 2021, 176, 111505. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, Y.; Luo, W.; Deng, L.; Yao, S.; Zeng, K. Primary Metabolites Analysis of Induced Citrus Fruit Disease Resistance Upon Treatment with Oligochitosan, Salicylic Acid and Pichia Membranaefaciens. Biol. Control 2020, 148, 104289. [Google Scholar] [CrossRef]
- He, Y.; Bose, S.K.; Wang, W.; Jia, X.; Lu, H.; Yin, H. Pre-Harvest Treatment of Chitosan Oligosaccharides Improved Strawberry Fruit Quality. Int. J. Mol. Sci. 2018, 19, 2194. [Google Scholar] [CrossRef]
- Cui, K.; Shu, C.; Zhao, H.; Fan, X.; Cao, J.; Jiang, W. Preharvest Chitosan Oligochitosan and Salicylic Acid Treatments Enhance Phenol Metabolism and Maintain the Postharvest Quality of Apricots (Prunus armeniaca L.). Sci. Hortic. 2020, 267, 109334. [Google Scholar] [CrossRef]
- Yu, L.; Zong, Y.; Han, Y.; Zhang, X.; Zhu, Y.; Oyom, W.; Gong, D.; Prusky, D.; Bi, Y. Both Chitosan and Chitooligosaccharide Treatments Accelerate Wound Healing of Pear Fruit by Activating Phenylpropanoid Metabolism. Int. J. Biol. Macromol. 2022, 205, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Coombe, B.G. Growth Stages of the Grapevine: Adoption of a System for Identifying Grapevine Growth Stages. Aust. J. Grape Wine Res. 2008, 1, 104–110. [Google Scholar] [CrossRef]
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. Standards Press of China: Beijing, China, 2008; pp. 56–58.
- Liu, C.; Wu, L.; Fan, S.; Tao, Y.; Li, Y. The Protective Effect of Cyclodextrin on the Color Quality and Stability of Cabernet Sauvignon Red Wine. J. Integr. Agric. 2024, 23, 310–323. [Google Scholar] [CrossRef]
- Mhetre, V.B.; Patel, V.B.; Singh, S.K.; Mishra, G.P.; Verma, M.K.; Kumar, C.; Dahuja, A.; Kumar, S.; Singh, R.; Wasim Siddiqui, M. Unraveling the Pathways Influencing the Berry Color and Firmness of Grapevine Cv. Flame Seedless Treated with Bioregulators Using Biochemical and RNA-Seq Analysis Under Semi-Arid Subtropics. Food Chem. Mol. Sci. 2022, 5, 100116. [Google Scholar] [CrossRef]
- Asghari, M. 24-Epibrassinolide Enhanced the Quality Parameters and Phytochemical Contents of Table Grape. J. Appl. Bot. Food Qual. 2018, 91, 226–231. [Google Scholar] [CrossRef]
- Lv, X.; Li, L.; Lu, X.; Wang, W.; Sun, J.; Liu, Y.; Mu, J.; Ma, Q.; Wang, J. Effects of Organic Acids on Color Intensification, Thermodynamics, and Copigmentation Interactions with Anthocyanins. Food Chem. 2022, 396, 133691. [Google Scholar] [CrossRef]
- Tian, M.-B.; Ma, W.-H.; Xia, N.-Y.; Peng, J.; Hu, R.-Q.; Duan, C.-Q.; He, F. Soil Variables and Reflected Light Revealed the Plasticity of Grape and Wine Composition: Regulation of the Flavoromics under Inner Row Gravel Covering. Food Chem. 2023, 414, 135659. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, X.-T.; Li, H.-Q.; Lu, H.-C.; He, L.; Peng, W.-T.; Chen, W.; Li, S.-D.; Li, S.-P.; Duan, C.-Q.; et al. Microclimate Changes Caused by Black Inter-Row Mulch Decrease Flavonoids Concentrations in Grapes and Wines under Semi-Arid Climate. Food Chem. 2021, 361, 130064. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, G.; Jiang, S.; Liu, Y.X. Wekemo Bioincloud: A User-Friendly Platform for Meta-Omics Data Analyses. Imeta 2024, 3, e175. [Google Scholar] [CrossRef]
- Bredun, M.A.; Gomes, T.M.; Assumpção, T.I.; Brighenti, A.F.; Chaves, E.S.; Panceri, C.P.; Burin, V.M. Statement of Boron Application Impact on Yield, Composition and Structural Properties in Merlot Grapes. Sci. Hortic. 2021, 288, 110364. [Google Scholar] [CrossRef]
- Yue, X.; Liu, S.; Wei, S.; Fang, Y.; Zhang, Z.; Ju, Y. Transcriptomic and Metabolic Analyses Provide New Insights into the Effects of Exogenous Sucrose on Monoterpene Synthesis in “Muscat Hamburg” Grapes. J. Agric. Food Chem. 2021, 69, 4164–4176. [Google Scholar] [CrossRef] [PubMed]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An Update on Sugar Transport and Signalling in Grapevine. J. Exp. Bot. 2013, 65, 821–832. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Y.; Wang, H.; Yang, X.; Zhu, X. Effects of exogenous brassinolide on flavor quality of Chardonnay and Pinot Noir grape. J. Fruit. Sci. 2023, 40, 2574–2590. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Heo, J.-Y. Combined Treatment with Gibberellic Acid and Thidiazuron Improves Fruit Quality of ‘Red Dream’ Grape Cultivar. Not. Sci. Biol. 2023, 15, 11499. [Google Scholar] [CrossRef]
- İşçi, B.; Kacar, E.; Altındişli, A. The Effects of Some Exogenous Applications on Quality in ‘Crimson Seedless’ Grape. Erwerbs-Obstbau 2020, 62, 87–100. [Google Scholar] [CrossRef]
- Shahab, M.; Roberto, S.R.; Ahmed, S.; Colombo, R.C.; Silvestre, J.P.; Koyama, R.; de Souza, R.T. Anthocyanin Accumulation and Color Development of ‘Benitaka’ Table Grape Subjected to Exogenous Abscisic Acid Application at Different Timings of Ripening. Agronomy 2019, 9, 164. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Pérez-Mestre, C.; Ferreras-Charro, R.; Rivero, F.J.; Heredia, F.J.; Escribano-Bailón, M.T. Addition of Mannoproteins and/or Seeds During Winemaking and Their Effects on Pigment Composition and Color Stability. J. Agric. Food Chem. 2019, 67, 4031–4042. [Google Scholar] [CrossRef] [PubMed]
- Koyama, R.; Colombo, R.C.; Silva Borges, W.F.; Silvestre, J.P.; Hussain, I.; Shahab, M.; Ahmed, S.; Prudencio, S.H.; Teodoro de Souza, R.; Roberto, S.R. Abscisic Acid Application Affects Color and Acceptance of the New Hybrid ‘Brs Melodia’ Seedless Grape Grown in a Subtropical Region. HortSci. Horts 2019, 54, 1055–1060. [Google Scholar] [CrossRef]
- Silva, V.; Singh, R.K.; Gomes, N.; Soares, B.G.; Silva, A.; Falco, V.; Capita, R.; Alonso-Calleja, C.; Pereira, J.E.; Amaral, J.S.; et al. Comparative Insight Upon Chitosan Solution and Chitosan Nanoparticles Application on the Phenolic Content, Antioxidant and Antimicrobial Activities of Individual Grape Components of Sousão Variety. Antioxidants 2020, 9, 178. [Google Scholar] [CrossRef]
- Jeong, Y.J.; Park, S.H.; Park, S.-C.; Kim, S.; Kim, T.H.; Lee, J.; Kim, S.W.; Ryu, Y.B.; Jeong, J.C.; Kim, C.Y. Induced Extracellular Production of Stilbenes in Grapevine Cell Culture Medium by Elicitation with Methyl Jasmonate and Stevioside. Bioresour. Bioprocess. 2020, 7, 38. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Gao, X.; Xi, Z.-M.; Zhang, H.; Peng, X.-Q.; Wang, Z.-Z.; Wang, T.-M.; Meng, Y. Application of Exogenous 24-Epibrassinolide Enhances Proanthocyanidin Biosynthesis in Vitis vinifera ‘Cabernet Sauvignon’ Berry Skin. Plant Growth Regul. 2015, 75, 741–750. [Google Scholar] [CrossRef]
- Ju, Y.; Wang, W.; Yue, X.; Xue, W.; Zhang, Y.; Fang, Y. Integrated Metabolomic and Transcriptomic Analysis Reveals the Mechanism Underlying the Accumulation of Anthocyanins and Other Flavonoids in the Flesh and Skin of Teinturier Grapes. Plant Physiol. Biochem. 2023, 197, 107667. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.; Han, Y.; Wang, B.; Lei, C.; Sam, F.E.; Li, J.; Ma, T.; Zhang, B.; Feng, L. Transcriptome and Metabolite Profiles Reveal the Role of Benzothiadiazole in Controlling Isoprenoid Synthesis and Berry Ripening in Chardonnay Grapes. Pestic. Biochem. Physiol. 2024, 204, 106041. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, B.; Ma, L.; Zheng, X.; Gong, D.; Xue, H.; Bi, Y.; Wang, Y.; Zhang, Z.; Prusky, D. Benzo-(1, 2, 3)-Thiadiazole-7-Carbothioic Acid S-Methyl Ester (Bth) Promotes Tuber Wound Healing of Potato by Elevation of Phenylpropanoid Metabolism. Postharvest Biol. Technol. 2019, 153, 125–132. [Google Scholar] [CrossRef]
- Wang, J.; Yao, X.; Xia, N.; Sun, Q.; Duan, C.; Pan, Q. Evolution of Seed-Soluble and Insoluble Tannins During Grape Berry Maturation. Molecules 2023, 28, 3050. [Google Scholar] [CrossRef] [PubMed]
- Deluc, L.; Bogs, J.; Walker, A.R.; Ferrier, T.; Decendit, A.; Merillon, J.M.; Robinson, S.P.; Barrieu, F. The Transcription Factor Vvmyb5b Contributes to the Regulation of Anthocyanin and Proanthocyanidin Biosynthesis in Developing Grape Berries. Plant Physiol. 2008, 147, 2041–2053. [Google Scholar] [CrossRef]
- Zerbib, M.; Cazals, G.; Enjalbal, C.; Saucier, C. Identification and Quantification of Flavanol Glycosides in Vitis vinifera Grape Seeds and Skins During Ripening. Molecules 2018, 23, 2745. [Google Scholar] [CrossRef]
- Conde, A.; Badim, H.; Dinis, L.-T.; Moutinho-Pereira, J.; Ferrier, M.; Unlubayir, M.; Lanoue, A.; Gerós, H. Stimulation of Secondary Metabolism in Grape Berry Exocarps by a Nature-Based Strategy of Foliar Application of Polyols. OENO One 2024, 58, 7537. [Google Scholar] [CrossRef]
- Sun, H.-L.; Wang, X.-Y.; Shang, Y.; Wang, X.-Q.; Du, G.-D.; LÜ, D.-G. Preharvest Application of Melatonin Induces Anthocyanin Accumulation and Related Gene Upregulation in Red Pear (Pyrus ussuriensis). J. Integr. Agric. 2021, 20, 2126–2137. [Google Scholar] [CrossRef]
- Gil, R. Improving Phenolic and Chromatic Characteristics of Monastrell, Merlot and Syrah Wines by Using Methyl Jasmonate and Benzothiadiazole. OENO One 2017, 51, 17–27. [Google Scholar] [CrossRef]
- Dimitrovska, M.; Bocevska, M.; Dimitrovski, D.; Murkovic, M. Anthocyanin Composition of Vranec, Cabernet Sauvignon, Merlot and Pinot Noir Grapes as Indicator of Their Varietal Differentiation. Eur. Food Res. Technol. 2011, 232, 591–600. [Google Scholar] [CrossRef]
- Fang, J.; Jogaiah, S.; Guan, L.; Sun, X.; Abdelrahman, M. Coloring Biology in Grape Skin: A Prospective Strategy for Molecular Farming. Physiol. Plant. 2018, 164, 429–441. [Google Scholar] [CrossRef]
- Azuma, A. Genetic and Environmental Impacts on the Biosynthesis of Anthocyanins in Grapes. Hortic. J. 2018, 87, 1–17. [Google Scholar] [CrossRef]
- Sun, L.; Fan, X.; Zhang, Y.; Jiang, J.; Sun, H.; Liu, C. Transcriptome Analysis of Genes Involved in Anthocyanins Biosynthesis and Transport in Berries of Black and White Spine Grapes (Vitis davidii). Hereditas 2016, 153, 17. [Google Scholar] [CrossRef]
- Portu, J.; López, R.; Baroja, E.; Santamaría, P.; Garde-Cerdán, T. Improvement of Grape and Wine Phenolic Content by Foliar Application to Grapevine of Three Different Elicitors: Methyl Jasmonate, Chitosan, and Yeast Extract. Food Chem. 2016, 201, 213–221. [Google Scholar] [CrossRef]
- Gómez-Plaza, E.; Bautista-Ortín, A.B.; Ruiz-García, Y.; Fernández-Fernández, J.I.; Gil-Muñoz, R. Effect of Elicitors on the Evolution of Grape Phenolic Compounds During the Ripening Period. J. Sci. Food Agric. 2017, 97, 977–983. [Google Scholar] [CrossRef]
- Paladines-Quezada, D.F.; Moreno-Olivares, J.D.; Fernández-Fernández, J.I.; Bleda-Sánchez, J.A.; Martínez-Moreno, A.; Gil-Muñoz, R. Elicitors and Pre-Fermentative Cold Maceration: Effects on Polyphenol Concentration in Monastrell Grapes and Wines. Biomolecules 2019, 9, 671. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, C.; Ruan, S.; Zhang, Z.; Meng, J.; Xi, Z. Exogenous 24-Epibrassinolide Interacts with Light to Regulate Anthocyanin and Proanthocyanidin Biosynthesis in Cabernet Sauvignon (Vitis vinifera L.). Molecules 2018, 23, 93. [Google Scholar] [CrossRef]
- Lucini, L.; Baccolo, G.; Rouphael, Y.; Colla, G.; Bavaresco, L.; Trevisan, M. Chitosan Treatment Elicited Defence Mechanisms, Pentacyclic Triterpenoids and Stilbene Accumulation in Grape (Vitis vinifera L.) Bunches. Phytochemistry 2018, 156, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant Flavonoids: Classification, Distribution, Biosynthesis, and Antioxidant Activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Dixon, R.A.; Duan, C. A Role for Ascorbate Conjugates of (+)-Catechin in Proanthocyanidin Polymerization. Nat. Commun. 2022, 13, 3425. [Google Scholar] [CrossRef]
- Yu, K.; Song, Y.; Lin, J.; Dixon, R.A. The Complexities of Proanthocyanidin Biosynthesis and Its Regulation in Plants. Plant Commun. 2023, 4, 100498. [Google Scholar] [CrossRef]
- Li, J.; Han, A.; Zhang, L.; Meng, Y.; Xu, L.; Ma, F.; Liu, R. Chitosan Oligosaccharide Alleviates the Growth Inhibition Caused by Physcion and Synergistically Enhances Resilience in Maize Seedlings. Sci. Rep. 2022, 12, 162. [Google Scholar] [CrossRef]
- Li, J.; Tian, Z.; Li, J.; Askari, K.; Han, A.; Ma, J.; Liu, R. Physcion and Chitosan-Oligosaccharide (Cos) Synergistically Improve the Yield by Enhancing Photosynthetic Efficiency and Resilience in Wheat (Triticum aestivum L.). Plant Physiol. Biochem. 2023, 203, 107993. [Google Scholar] [CrossRef] [PubMed]
- Mukarram, M.; Khan, M.M.A.; Kurjak, D.; Corpas, F.J. Chitosan Oligomers (Cos) Trigger a Coordinated Biochemical Response of Lemongrass (Cymbopogon flexuosus) Plants to Palliate Salinity-Induced Oxidative Stress. Sci. Rep. 2023, 13, 8636. [Google Scholar] [CrossRef]
- Berli, F.J.; Fanzone, M.; Piccoli, P.; Bottini, R. Solar UV-B and ABA Are Involved in Phenol Metabolism of Vitis vinifera L. Increasing Biosynthesis of Berry Skin Polyphenols. J. Agric. Food Chem. 2011, 59, 4874–4884. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Lu, X.; Shi, Q.; Xu, X.-Q.; Leung, H.-C.E.; Harris, L.N.; Iglehart, J.D.; Miron, A.; Liu, J.S.; Wong, W.H. Recursive SVM Feature Selection and Sample Classification for Mass-Spectrometry and Microarray Data. BMC Bioinform. 2006, 7, 197. [Google Scholar] [CrossRef]
- Gong, Q.; Yu, J.; Guo, Z.; Fu, K.; Xu, Y.; Zou, H.; Li, C.; Si, J.; Cai, S.; Chen, D.; et al. Accumulation Mechanism of Metabolite Markers Identified by Machine Learning between Qingyuan and Xiushui Counties in Polygonatum Cyrtonema Hua. BMC Plant Biol. 2024, 24, 173. [Google Scholar] [CrossRef]
- Wang, H.; Yang, F.; Luo, Z. An Experimental Study of the Intrinsic Stability of Random Forest Variable Importance Measures. BMC Bioinform. 2016, 17, 60. [Google Scholar] [CrossRef] [PubMed]
- Statnikov, A.; Wang, L.; Aliferis, C.F. A Comprehensive Comparison of Random Forests and Support Vector Machines for Microarray-Based Cancer Classification. BMC Bioinform. 2008, 9, 319. [Google Scholar] [CrossRef] [PubMed]
- Zagajewski, B.; Kluczek, M.; Raczko, E.; Njegovec, A.; Anca, D.; Kycko, M. Comparison of Random Forest, Support Vector Machines, and Neural Networks for Post-Disaster Forest Species Mapping of the Krkonoše/Karkonosze Transboundary Biosphere Reserve. Remote Sens. 2021, 13, 2581. [Google Scholar] [CrossRef]
- Singh, R.K.; Martins, V.; Soares, B.; Castro, I.; Falco, V. Chitosan Application in Vineyards (Vitis vinifera L. Cv. Tinto Cão) Induces Accumulation of Anthocyanins and Other Phenolics in Berries, Mediated by Modifications in the Transcription of Secondary Metabolism Genes. Int. J. Mol. Sci. 2020, 21, 306. [Google Scholar] [CrossRef]
- Shi, J.; Simal-Gandara, J.; Mei, J.; Ma, W.; Peng, Q.; Shi, Y.; Xu, Q.; Lin, Z.; Lv, H. Insight into the Pigmented Anthocyanins and the Major Potential Co-Pigmented Flavonoids in Purple-Coloured Leaf Teas. Food Chem. 2021, 363, 130278. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Huang, W.; Xu, H. Effect of Salicylic Acid on the Gene Transcript and Protein Accumulation of Flavonoid Biosynthesis-related Enzymes in Vitis vinifera Cell Suspension Cultures. HortScience 2017, 52, 1772–1779. [Google Scholar] [CrossRef]

| Sample | 100-BW (g) | TSS (°Brix) | RS (g/L) | TA (g/L) | pH | S/A |
|---|---|---|---|---|---|---|
| CK | 108.99 ± 1.98 b | 21.57 ± 1.79 b | 227.80 ± 10.17 c | 4.53 ± 0.15 c | 3.78 ± 0.04 a | 50.26 ± 1.94 ab |
| GC | 120.80 ± 3.16 a | 24.27 ± 0.25 a | 256.83 ± 3.48 a | 5.37 ± 0.15 a | 3.67 ± 0.04 c | 47.87 ± 0.73 bc |
| VC | 116.43 ± 3.75 ab | 23.20 ± 0.26 ab | 241.73 ± 9.76 bc | 5.13 ± 0.06 a | 3.70 ± 0.01 bc | 47.08 ± 1.35 c |
| RC | 112.93 ± 8.65 ab | 23.80 ± 0.10 a | 251.00 ± 1.44 ab | 4.80 ± 0.10 b | 3.75 ± 0.03 ab | 52.31 ± 1.20 a |
| Sample | L* | a* | b* | C*ab | hab | ΔE*ab |
|---|---|---|---|---|---|---|
| CK | 81.57 ± 0.78 a | 16.61 ± 0.95 c | 15.54 ± 1.11 a | 22.76 ± 0.99 c | 0.75 ± 0.05 a | - |
| GC | 79.15 ± 0.77 b | 20.93 ± 0.73 a | 12.96 ± 0.20 b | 24.62 ± 0.52 a | 0.55 ± 0.02 c | 5.64 ± 0.85 a |
| VC | 79.14 ± 0.28 b | 21.63 ± 0.47 a | 10.73 ± 1.19 c | 24.16 ± 0.10 ab | 0.46 ± 0.05 d | 6.82 ± 0.16 a |
| RC | 81.19 ± 0.65 a | 18.16 ± 0.50 b | 14.27 ± 0.72 ab | 23.10 ± 0.48 bc | 0.67 ± 0.03 b | 1.94 ± 0.53 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, W.; Wang, H.; Ma, T.; Li, K.; Wang, B.; Ma, T.; Jiang, Y.; Zhang, B. Enhancing Phenolic Profiles in ‘Cabernet Franc’ Grapes Through Chitooligosaccharide Treatments: Impacts on Phenolic Compounds Accumulation Across Developmental Stages. Agriculture 2024, 14, 2039. https://doi.org/10.3390/agriculture14112039
Qiang W, Wang H, Ma T, Li K, Wang B, Ma T, Jiang Y, Zhang B. Enhancing Phenolic Profiles in ‘Cabernet Franc’ Grapes Through Chitooligosaccharide Treatments: Impacts on Phenolic Compounds Accumulation Across Developmental Stages. Agriculture. 2024; 14(11):2039. https://doi.org/10.3390/agriculture14112039
Chicago/Turabian StyleQiang, Wenle, Hongjuan Wang, Tongwei Ma, Kaian Li, Bo Wang, Tengzhen Ma, Yumei Jiang, and Bo Zhang. 2024. "Enhancing Phenolic Profiles in ‘Cabernet Franc’ Grapes Through Chitooligosaccharide Treatments: Impacts on Phenolic Compounds Accumulation Across Developmental Stages" Agriculture 14, no. 11: 2039. https://doi.org/10.3390/agriculture14112039
APA StyleQiang, W., Wang, H., Ma, T., Li, K., Wang, B., Ma, T., Jiang, Y., & Zhang, B. (2024). Enhancing Phenolic Profiles in ‘Cabernet Franc’ Grapes Through Chitooligosaccharide Treatments: Impacts on Phenolic Compounds Accumulation Across Developmental Stages. Agriculture, 14(11), 2039. https://doi.org/10.3390/agriculture14112039






