Inflorescence Yield, Essential Oil Composition and Antioxidant Activity of Cannabis sativa L. cv ‘Futura 75’ in a Multilocation and On-Farm Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plant Material, Filed Experiment and Sampling
2.3. Hydrodistillation of the Essential Oil (EO)
2.4. GC-MS Analyses and Peak Identification
2.5. Antiradical Activity Evaluation through DPPH Assay
2.6. Statistical Analyses
3. Results
3.1. Weather Conditions
3.2. Inflorescence and EO Yield
3.3. Essential Oil (EO) Compositions
3.4. Statistical Evaluation of the EO Compositions
3.5. Evaluation of the Correlation between Chemical and Agronomic Traits with the Meteorological Data
3.6. EOs’ Antioxidant Activity and Correlation with Their Compositions
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amaducci, S.; Zatta, A.; Pelatti, F.; Venturi, G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crops Res. 2008, 107, 161–169. [Google Scholar] [CrossRef]
- Angelini, L.G.; Tavarini, S.; Di Candilo, M. Performance of new and traditional fiber hemp (Cannabis sativa L.) cultivars for novel applications: Stem, bark, and core yield and chemical composition. J. Nat. Fibers 2016, 13, 238–252. [Google Scholar] [CrossRef]
- Struik, P.C.; Amaducci, S.; Bullard, M.J.; Stutterheim, N.C.; Venturi, G.; Cromack, H.T.H. Agronomy of fibre hemp (Cannabis sativa L.) in Europe. Ind. Crops Prod. 2000, 11, 107–118. [Google Scholar] [CrossRef]
- Satriani, A.; Loperte, A.; Pascucci, S. The cultivation of industrial hemp as alternative crop in a less-favoured agricultural area in southern Italy: The Pignola case study. Pollutants 2021, 1, 169–180. [Google Scholar] [CrossRef]
- Van der Werf, H.; Mathussen, E.; Haverkort, A. The potential of hemp (Cannabis sativa L.) for sustainable fibre production: A crop physiological appraisal. Ann. Appl. Biol. 1996, 129, 109–123. [Google Scholar] [CrossRef]
- Carus, M. Record Cultivation of Industrial Hemp in Europe in 2016. In Proceedings of the 14th International Conference of the European Industrial Hemp Association (EIHA), Cologne, Germany, 7–8 June 2017. [Google Scholar]
- Shahzad, A. Hemp fiber and its composites—A review. J. Compos. Mater. 2012, 46, 973–986. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Ceccarini, L.; Tavarini, S.; Flamini, G.; Angelini, L.G. Valorisation of hemp inflorescence after seed harvest: Cultivation site and harvest time influence agronomic characteristics and essential oil yield and composition. Ind. Crops Prod. 2019, 139, 111541. [Google Scholar] [CrossRef]
- Booth, J.K.; Page, J.E.; Bohlmann, J. Terpene synthases from Cannabis sativa. PLoS ONE 2017, 12, e0173911. [Google Scholar] [CrossRef]
- Kim, E.S.; Mahlberg, P.G. Secretory cavity development in glandular trichomes of Cannabis sativa L. (Cannabaceae). Am. J. Bot. 1991, 78, 220. [Google Scholar] [CrossRef]
- Mudge, E.M.; Brown, P.N.; Murch, S.J. The terroir of Cannabis: Terpene metabolomics as a tool to understand Cannabis sativa selections. Planta Med. 2019, 85, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E.; Elsohly, M.A.; Boeren, E.G. Constituents of Cannabis sativa L. XVII. A Review of the Natural Constituents. J. Nat. Prod. 1980, 43, 169–234. [Google Scholar] [CrossRef] [PubMed]
- Flores-Sanchez, I.J.; Verpoorte, R. Secondary metabolism in Cannabis. Phytochem. Rev. 2008, 7, 615–639. [Google Scholar] [CrossRef]
- ElSohly, M.A.; Gul, W. Constituents of Cannabis sativa. In Handbook of Cannabis; Pertwee, R., Ed.; Oxford University Press: Oxford, UK, 2014; pp. 3–22. [Google Scholar] [CrossRef]
- Giese, M.W.; Lewis, M.A.; Giese, L.; Smith, K.M. Method for the analysis of cannabinoids and terpenes in Cannabis. J. AOAC Int. 2015, 98, 1503–1522. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L. The origin and use of Cannabis in eastern Asia linguistic-cultural implications. J. Econ. Bot. 1973, 28, 293–301. [Google Scholar] [CrossRef]
- Small, E. Evolution and classification of Cannabis sativa (Marijuana, Hemp) in relation to human utilization. Bot. Rev. 2015, 81, 189–294. [Google Scholar] [CrossRef]
- Clarke, R.C.; Merlin, M.D. Cannabis domestication, breeding history, present-day genetic diversity, and future prospects. CRC Crit. Rev. Plant Sci. 2016, 35, 293–327. [Google Scholar] [CrossRef]
- Nissen, L.; Zatta, A.; Stefanini, I.; Grandi, S.; Sgorbati, B.; Biavati, B.; Monti, A. Characterization and antimicrobial activity of essential oils of industrial hemp varieties (Cannabis sativa L.). Fitoterapia 2010, 81, 413–419. [Google Scholar] [CrossRef]
- Nafis, A.; Kasrati, A.; Jamali, C.A.; Mezrioui, N.; Setzer, W.; Abbad, A.; Hassani, L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019, 137, 396–400. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crops Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef]
- Górski, R.; Sobieralski, K.; Siwulski, M. The effect of hemp essential oil on mortality Aulacorthum solani Kalt. and Tetranychus urticae Koch. Ecol. Chem. Eng. S 2016, 23, 505–511. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Lupidi, G.; Nabissi, M.; Petrelli, R.; Ngahang Kamte, S.L.; Cappellacci, L.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; et al. The crop-residue of fiber hemp cv. Futura 75: From a waste product to a source of botanical insecticides. Environ. Sci. Pollut. Res. 2017, 25, 10515–10525. [Google Scholar] [CrossRef]
- Benelli, G.; Pavela, R.; Petrelli, R.; Cappellacci, L.; Santini, G.; Fiorini, D.; Sut, S.; Dall’Acqua, S.; Canale, A.; Maggi, F. The essential oil from industrial hemp (Cannabis sativa L.) by-products as an effective tool for insect pest management in organic crops. Ind. Crops Prod. 2018, 122, 308–315. [Google Scholar] [CrossRef]
- Meier, C.; Mediavilla, V. Factors influencing the yield and the quality of hemp (Cannabis sativa L.) essential oil. J. Int. Hemp Assoc. 1998, 5, 16–20. [Google Scholar]
- Abdollahi, M.; Sefidkon, F.; Calagari, M.; Mousavi, A.; Mahomoodally, M.F. Impact of four hemp (Cannabis sativa L.) varieties and stage of plant growth on yield and composition of essential oils. Ind. Crops Prod. 2020, 155, 112793. [Google Scholar] [CrossRef]
- Pieracci, Y.; Ascrizzi, R.; Terreni, V.; Pistelli, L.; Flamini, G.; Bassolino, L.; Fulvio, F.; Montanari, M.; Paris, R. Essential oil of Cannabis sativa L: Comparison of yield and chemical composition of 11 hemp genotypes. Molecules 2021, 26, 4080. [Google Scholar] [CrossRef] [PubMed]
- Cosentino, S.L.; Riggi, E.; Testa, G.; Scordia, D.; Copani, V. Evaluation of European development fibre hemp genotypes (Cannabis sativa L.) in semi-arid Mediterranean environment. Ind. Crops Prod. 2013, 50, 312–324. [Google Scholar] [CrossRef]
- Duong, H.; Pearson, B.; Anderson, S.; Berthold, E.; Kjelgren, R. Variation in hydric response of two industrial hemp varieties (Cannabis sativa L.) to induced water stress. Horticulturae 2023, 9, 431. [Google Scholar] [CrossRef]
- Amaducci, S.; Colauzzi, M.; Bellocchi, G.; Venturi, G. Modelling post-emergent hemp phenology (Cannabis sativa L.): Theory and evaluation. Eur. J. Agron. 2008, 28, 90–102. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Pavlovic, R.; Giorgi, A. Influence of altitude on phytochemical composition of hemp inflorescence: A metabolomic approach. Molecules 2020, 25, 1381. [Google Scholar] [CrossRef] [PubMed]
- Galic, A.; Grab, H.; Kaczmar, N.; Maser, K.; Miller, W.B.; Smart, L.B. Effects of cold temperatures and acclimation on cold tolerance and cannabinoid profiles of Cannabis sativa L. (Hemp). Horticolturae 2022, 8, 531. [Google Scholar] [CrossRef]
- Giupponi, L.; Leoni, V.; Carrer, M.; Ceciliani, G.; Sala, S.; Panseri, S.; Pavlovic, R.; Giorgi, A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020, 15, 1552. [Google Scholar] [CrossRef]
- Elbersen, B.; van Eupen, E.; Mantel, S.; Verzandvoort, S.; Boogaard, H.; Mucher, S.; Cicarreli, T.; Elbersen, W.; Bai, Z.; Iqbal, Y.; et al. D2.1. Definition and classification of marginal lands suitable for industrial crops in Europe (Version V1). Zenodo 2017, 1–61. [Google Scholar] [CrossRef]
- McLean, E.O. Soil pH and lime requirement. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 199–224. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen total. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Thomas, G.W. Exchangeable cations. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 159–165. [Google Scholar]
- Nelson, P.W.; Sommers, C.E. Total Carbon, organic Carbon and organic matter. In Methods of Soil Analysis, Part 2: Chemical and Microbiological Properties; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; America Society of Agronomy, Inc.: Madison, WI, USA, 1982; pp. 539–579. [Google Scholar]
- Mediavilla, V.; Jonquera, M.; Schmin-Slembrouck, I.; Soldati, A. Decimal code for growth stages of hemp (Cannabis sativa L.). J. Int. Hemp Assoc. 1998, 5, 68–74. [Google Scholar]
- Kowalski, R.; Wawrzykowski, J. Effect of ultrasound-assisted maceration on the quality of oil from the leaves of thyme Thymus vulgaris L. Flavour Fragr. J. 2009, 24, 69–74. [Google Scholar] [CrossRef]
- Bantawa, P.; Da Silva, J.A.T.; Ghosh, S.K.; Mondal, T.K. Determination of essential oil contents and micropropagation of Gaultheria fragrantissima, an endangered woody aromatic plant of India. J. Hortic. Sci. Biotechnol. 2011, 86, 479–485. [Google Scholar] [CrossRef]
- Ascrizzi, R.; González-Rivera, J.; Pomelli, C.S.; Chiappe, C.; Margari, P.; Costagli, F.; Longo, I.; Tiné, M.R.; Flamini, G.; Duce, C. Ionic liquids, ultra-sounds and microwaves: An effective combination for a sustainable extraction with higher yields. The cumin essential oil case. React. Chem. Eng. 2017, 2, 577–589. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library, NIST Standard Reference Database Number 69; The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Pistelli, L.; Najara, B.; Giovanelli, S.; Lorenzini, L.; Tavarini, S.; Angelini, L.G. Agronomic and phytochemical evaluation of lavandin and lavender cultivars cultivated in the Tyrrhenian area of Tuscany (Italy). Ind. Crops Prod. 2017, 109, 37–44. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Giusiani, M.; Stefanelli, F.; Deriu, V.; Chericoni, S. VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools. Forensic Toxicol. 2018, 36, 243–260. [Google Scholar] [CrossRef]
- Petit, J.; Salentijn, E.M.J.; Paulo, M.; Denneboom, C.; Trindade, L.M. Genetic architecture of flowering time and sex determination in hemp (Cannabis sativa L.): A genome-wide association study. Front. Plant Sci. 2020, 11, 569958. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Anderson, S.L.; Brym, Z.T.; Pearson, B.J. Photoperiodic flowering response of essential oil, grain, and fiber hemp (Cannabis sativa L.) cultivars. Front. Plant Sci. 2021, 12, 694153. [Google Scholar] [CrossRef]
- Heslop-Harrison, J.; Heslop-Harrison, Y. Cannabis sativa L. In The Induction of Flowering. Some Case Studies; Evans, L.T., Ed.; MacMillan Co. Pty Ltd.: South Melbourne, Australia, 1969; pp. 205–206. [Google Scholar]
- Sunoj Valiaparambil Sebastian, J.; Dong, X.; Trostle, C.; Pham, H.; Joshi, M.V.; Jessup, R.W.; Burow, M.D.; Provin, T.L. Hemp agronomy: Current advances, questions, challenges, and opportunities. Agronomy 2023, 13, 475. [Google Scholar] [CrossRef]
- Malceva, M.; Vikmane, M.; Stramkale, V. Changes of photosynthesis-related parameters and productivity of Cannabis sativa under different nitrogen supply. Environ. Exp. Biol. 2011, 9, 61–69. [Google Scholar]
- Tang, K.; Struik, P.C.; Yin, X.; Calzolari, D.; Musio, S.; Thouminot, C.; Bjelkova, M.; Stramkale, V.; Magagnini, G.; Amaducci, S. A comprehensive study of planting density and nitrogen fertilization effect on dual-purpose hemp (Cannabis sativa L.) cultivation. Ind. Crops Prod. 2017, 107, 427–438. [Google Scholar] [CrossRef]
- Landi, S.; Berni, R.; Capasso, G.; Hausman, J.; Guerriero, G.; Esposito, S. Impact of nitrogen nutrition on Cannabis sativa: An update on the current knowledge and future prospects. Int. J. Mol. Sci. 2019, 20, 5803. [Google Scholar] [CrossRef]
- Barčauskaitė, K.; Bakšinskaitė, A.; Szumny, A.; Tilvikienė, V. Variation of secondary metabolites in Cannabis sativa L. inflorescences under applied agrotechnological measures. Ind. Crops Prod. 2022, 188, 115570. [Google Scholar] [CrossRef]
- Amaducci, S.; Scordia, D.; Liu, F.H.; Zhang, Q.; Guo, H.; Testa, G.; Cosentino, S.L. Key cultivation techniques for hemp in Europe and China. Ind. Crops Prod. 2015, 68, 2–16. [Google Scholar] [CrossRef]
- Vuerich, M.; Ferfuia, C.; Zuliani, F.; Piani, B.; Sepulcri, B.; Baldini, M. Yield and quality of essential oils in hemp varieties in different environments. Agronomy 2019, 9, 356. [Google Scholar] [CrossRef]
- Bertoli, A.; Tozzi, S.; Pistelli, L.; Angelini, L.G. Fibre hemp inflorescences: From crop-residues to essential oil production. Ind. Crops Prod. 2010, 32, 329–337. [Google Scholar] [CrossRef]
- Wu, Y.; Duan, S.; Zhao, L.; Gao, Z.; Luo, M.; Song, S.; Xu, W.; Zhang, C.; Ma, C.; Wang, S. Aroma characterization based on aromatic series analysis in table grapes. Sci. Rep. 2016, 6, 31116. [Google Scholar] [CrossRef] [PubMed]
- Mediavilla, V.; Steinemann, S. Essential oil of Cannabis sativa L. strains. J. Int. Hemp Assoc. 1997, 4, 82–84. [Google Scholar]
- Burdock, G.A. Fenaroli’s Handbook of Flavor Ingredients, 6th. ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- The Good Scents Company. Available online: http://www.thegoodscentscompany.com/data/rw1384461.html (accessed on 22 December 2023).
- Aqeel, U.; Aftab, T.; Khan, M.M.A.; Naeem, M. Regulation of essential oil in aromatic plants under changing environment. J. Appl. Res. Med. Aromat. Plants 2023, 32, 100441. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Wan Salleh, W.M.N.H.; Kammil, M.F.; Ahmad, F.; Sirat, H.M. Antioxidant and anti-inflammatory activities of essential oil and extracts of Piper miniatum. Nat. Prod. Commun. 2015, 10, 1934578X1501001. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ozer, M.S.; Calli, N.; Popović-Djordjević, J. Essential oil composition and antioxidant activity of endemic Marrubium parviflorum subsp. oligodon. Ind. Crops Prod. 2018, 119, 209–213. [Google Scholar] [CrossRef]
- Figueiredo, P.L.B.; Pinto, L.C.; da Costa, J.S.; da Silva, A.R.C.; Mourão, R.H.V.; Montenegro, R.C.; da Silva, J.K.R.; Maia, J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the Amazon. J. Ethnopharmacol. 2019, 232, 30–38. [Google Scholar] [CrossRef]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]

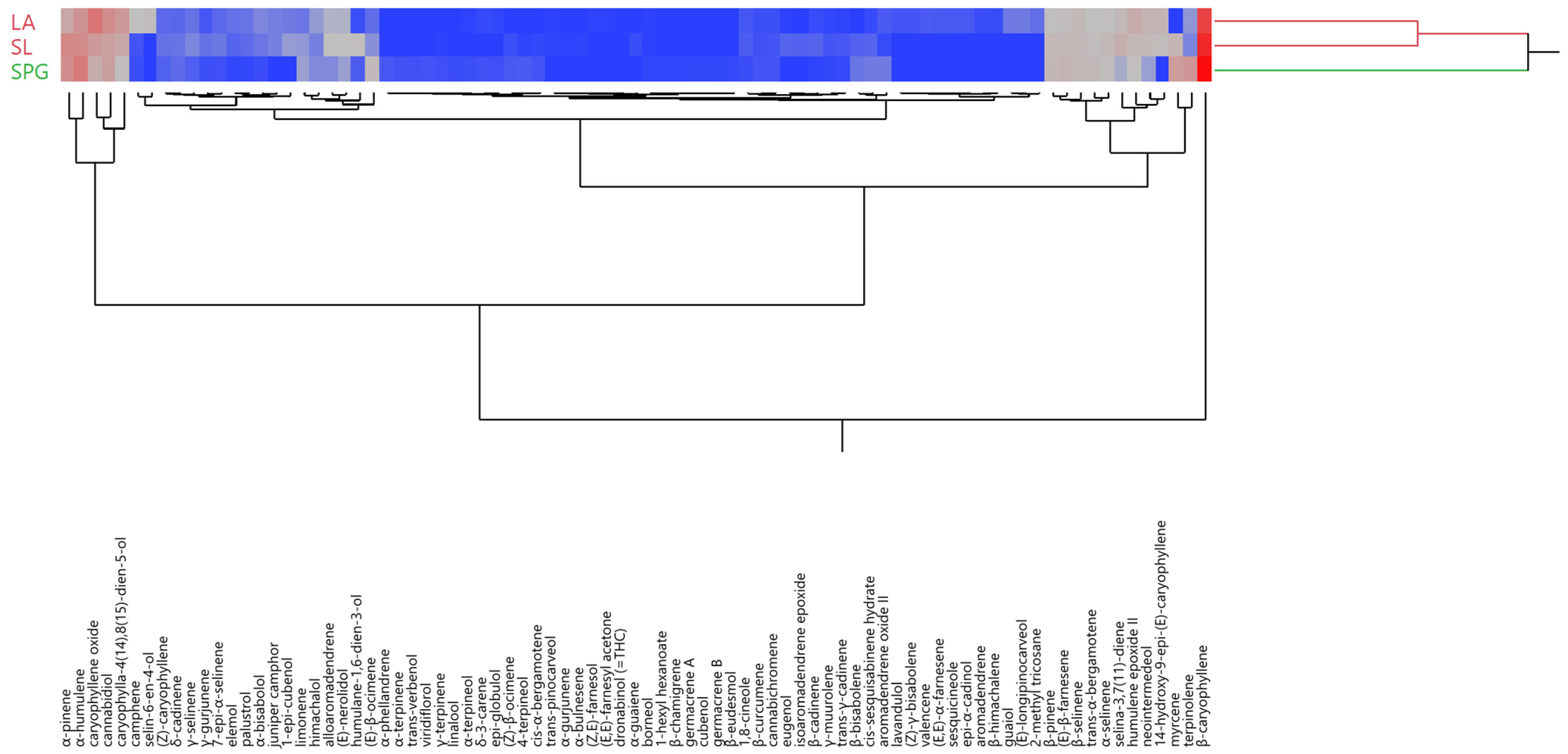
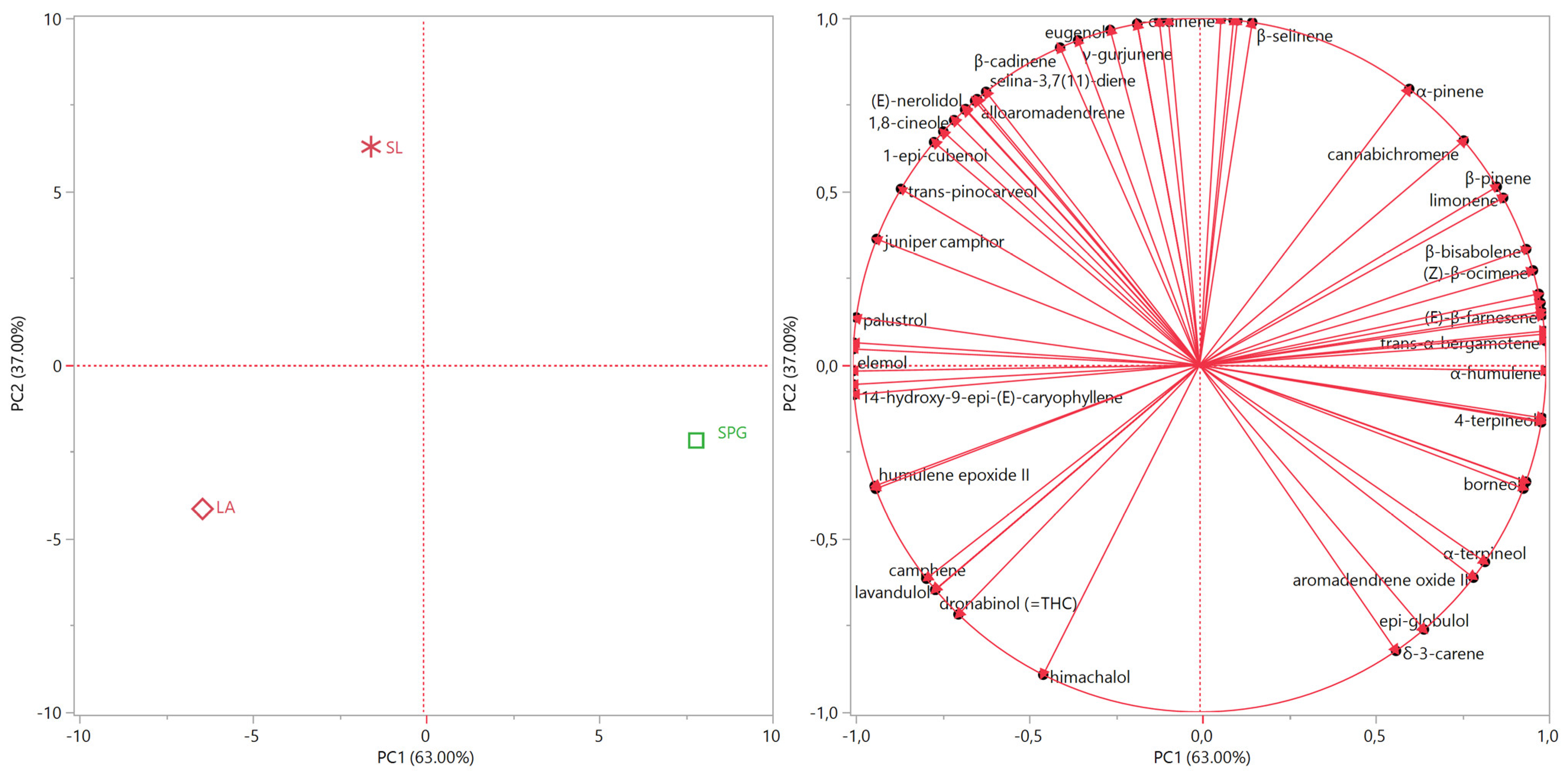

| Site | SPG (Plain Area, Pisa Province) | SL (Hilly Area, Pisa Province) | LA (Plain Area, Pistoia Province) |
|---|---|---|---|
| Primary tillage | Shallow ploughing (30 cm) at the end of August | Shallow ploughing (30 cm) at the end of September | Shallow ploughing (30 cm) associated with subsoiling at the beginning of March |
| Seedbed preparation | Disk harrow (2 passes) | Disk harrow (three passes) | Spring-tooth harrow combined with a rotary harrow |
| Sowing method | 25 kg ha−1 on 15 cm-spaced rows using a plot drill for wheat | 30 kg ha−1 on 14 cm-spaced rows using a plot drill for wheat | 25 kg ha−1 on 25 cm-spaced rows using a plot drill for wheat |
| Fertilisation | 80 kg ha−1 of P2O5 (triple superphosphate) and 80 kg ha−1 of K2O (potassium sulphate) for pre-sowing; 40 kg N ha−1 (ammonium nitrate) as topdressing distribution | 20 Mg ha−1 of digestate applied in March | 0.5 Mg ha−1 of commercial organic fertiliser (12% Norg) |
| Weed and Pest Control | No chemical applications or manual weeding Weeds are controlled by stale seedbed technique before sowing | ||
| Plant Height (cm) | Inflorescence Dry Yield (Mg ha−1) | Harvest Index | Hydrodistillation Yield (% w w−1) | EO Yield (kg ha−1) | |
|---|---|---|---|---|---|
| SPG | 207.0 ± 18.9 a | 4.9 ± 0.5 a | 42.8 ± 2.0 a | 0.15 ± 0.04 a | 7.1 ± 1.8 a |
| SL | 218.2 ± 7.3 a | 3.4 ± 0.5 b | 26.1 ± 2.8 b | 0.22 ± 0.08 a | 7.5 ± 2.5 a |
| LA | 133.2 ± 5.5 b | 1.3 ± 0.2 c | 21.1 ± 5.5 b | 0.20 ± 0.01 a | 2.6 ± 0.1 b |
| Compounds | Experimental l.r.i. 1 | Literature l.r.i. 2 | Relative Abundance ± SD | ||
|---|---|---|---|---|---|
| LA | SPG | SL | |||
| α-pinene * | 941 | 939 | 4.7 ± 1.04 B | 8.3 ± 0.79 A | 9.4 ± 1.20 A |
| camphene * | 954 | 954 | - 3 | 0.1 ± 0.02 | 0.2 ± 0.06 |
| β-pinene * | 982 | 981 | 1.2 ± 0.27 B | 2.6 ± 0.30 A | 2.4 ± 0.46 A |
| myrcene * | 993 | 992 | 1.4 ± 0.34 C | 6.4 ± 0.41 A | 2.8 ± 0.24 B |
| α-phellandrene * | 1005 | 1005 | - | 0.2 ± 0.01 | - |
| δ-3-carene * | 1011 | 1011 | 0.1 ± 0.09 | 0.2 ± 0.02 | - |
| α-terpinene * | 1018 | 1018 | - | 0.2 ± 0.01 | - |
| limonene * | 1032 | 1031 | 0.5 ± 0.10 | 0.9 ± 0.08 | 0.8 ± 0.10 |
| 1,8-cineole * | 1034 | 1033 | 0.2 ± 0.06 | 0.1 ± 0.01 | 0.3 ± 0.05 |
| (Z)-β-ocimene | 1042 | 1043 | - | 0.2 ± 0.01 | 0.1 ± 0.03 |
| (E)-β-ocimene | 1052 | 1052 | 0.4 ± 0.15 C | 2.1 ± 0.03 A | 0.7 ± 0.12 B |
| γ-terpinene * | 1062 | 1062 | - | 0.2 ± 0.02 | 0.0 ± 0.06 |
| terpinolene * | 1088 | 1088 | 0.8 ± 0.26 B | 7.3 ± 0.19 A | 0.6 ± 0.36 B |
| linalool * | 1101 | 1099 | - | 0.1 ± 0.03 | - |
| trans-pinocarveol | 1139 | 1140 | 0.0 ± 0.06 | - | 0.0 ± 0.08 |
| trans-verbenol | 1144 | 1145 | - | 0.2 ± 0.01 | - |
| borneol * | 1165 | 1168 | 0.0 ± 0.06 | 0.1 ± 0.06 | 0 ± 0.06 |
| lavandulol * | 1168 | 1170 | 0.2 ± 0.06 | - | |
| 4-terpineol * | 1179 | 1177 | 0.2 ± 0.06 | 0.3 ± 0.03 | 0.1 ± 0.09 |
| α-terpineol * | 1189 | 1189 | 0.0 ± 0.06 | 0.2 ± 0.0 | - |
| eugenol * | 1358 | 1358 | 0.0 ± 0.06 | - | 0.3 ± 0.03 |
| 1-hexyl hexanoate * | 1387 | 1386 | - | 0.1 ± 0.06 | - |
| (Z)-caryophyllene | 1405 | 1403 | 0.4 ± 0.05 | 0.3 ± 0.07 | 0.5 ± 0.08 |
| α-gurjunene | 1410 | 1410 | - | - | 0.0 ± 0.06 |
| cis-α-bergamotene | 1416 | 1415 | 0.2 ± 0.04 | 0.2 ± 0.19 | 0.1 ± 0.13 |
| β-caryophyllene * | 1420 | 1418 | 19.1 ± 0.47 B | 27.1 ± 3.49 A | 22.9 ± 1.24 A,B |
| trans-α-bergamotene | 1438 | 1438 | 1.3 ± 0.13 B | 2.3 ± 0.57 A | 1.7 ± 0.19 A,B |
| α-guaiene | 1440 | 1440 | - | - | 0.1 ± 0.13 |
| aromadendrene * | 1445 | 1443 | 0.1 ± 0.02 | - | - |
| α-humulene * | 1456 | 1455 | 7.8 ± 0.11 C | 11.3 ± 0.29 A | 9.0 ± 0.64 B |
| (E)-β-farnesene * | 1460 | 1459 | 1.6 ± 0.15 B | 3.1 ± 0.93 A | 2.3 ± 0.22 A,B |
| alloaromadendrene | 1461 | 1461 | 1.1 ± 0.02 A,B | 0.7 ± 0.34 B | 1.5 ± 0.14 A |
| β-chamigrene | 1473 | 1472 | - | 0.1 ± 0.06 | - |
| γ-gurjunene | 1474 | 1474 | 0.2 ± 0.10 | 0.2 ± 0.00 | 0.5 ± 0.05 |
| γ-muurolene | 1477 | 1477 | 0.1 ± 0.11 | 0.1 ± 0.07 | 0.2 ± 0.05 |
| γ-selinene | 1482 | 1481 | 0.5 ± 0.04 | 0.3 ± 0.29 | 0.7 ± 0.07 |
| β-selinene | 1485 | 1486 | 2.3 ± 0.07 A | 2.3 ± 0.75 A | 2.4 ± 0.43 A |
| valencene * | 1492 | 1491 | 0.3 ± 0.06 | - | - |
| α-selinene | 1494 | 1497 | 1.5 ± 0.09 A | 1.7 ± 0.71 A | 2.3 ± 0.27 A |
| germacrene A | 1503 | 1506 | - | 0.1 ± 0.07 | - |
| α-bulnesene | 1504 | 1505 | - | - | 0.1 ± 0.09 |
| β-himachalene | 1505 | 1503 | 0.1 ± 0.01 | - | - |
| (E,E)-α-farnesene | 1507 | 1508 | 0.2 ± 0.06 | - | - |
| β-bisabolene | 1509 | 1509 | - | 0.5 ± 0.10 | 0.3 ± 0.04 |
| β-curcumene | 1512 | 1510 | 0.1 ± 0.04 | 0.2 ± 0.05 | 0.2 ± 0.01 |
| trans-γ-cadinene | 1513 | 1513 | 0.1 ± 0.09 | 0.1 ± 0.10 | 0.2 ± 0.03 |
| sesquicineole | 1514 | 1513 | 0.2 ± 0.03 | - | - |
| (Z)-γ-bisabolene | 1515 | 1515 | 0.2 ± 0.18 | - | - |
| 7-epi-α-selinene | 1517 | 1517 | 0.4 ± 0.02 | 0.2 ± 0.16 | 0.5 ± 0.07 |
| β-cadinene | 1520 | 1520 | 0.1 ± 0.11 | 0.1 ± 0.05 | 0.3 ± 0.16 |
| δ-cadinene | 1524 | 1524 | 0.4 ± 0.06 | 0.4 ± 0.13 | 0.5 ± 0.05 |
| selina-3,7(11)-diene | 1542 | 1542 | 2.3 ± 0.38 A,B | 1.0 ± 0.99 B | 3.8 ± 1.57 A |
| cis-sesquisabinene hydrate | 1545 | 1544 | 0.1 ± 0.20 | 0.5 ± 0.25 | 0.3 ± 0.06 |
| elemol | 1549 | 1549 | 0.5 ± 0.04 | 0.1 ± 0.06 | 0.3 ± 0.07 |
| germacrene B | 1557 | 1556 | - | 0.1 ± 0.08 | - |
| (E)-nerolidol * | 1565 | 1566 | 1.1 ± 0.12 A,B | 0.9 ± 0.10 B | 1.2 ± 0.17 A |
| palustrol | 1568 | 1571 | 0.5 ± 0.07 | 0.1 ± 0.07 | 0.4 ± 0.07 |
| caryophyllene oxide * | 1581 | 1578 | 12.1 ± 0.57 A | 3.9 ± 0.83 C | 6.7 ± 0.87 B |
| isoaromadendrene epoxide | 1589 | 1590 | - | - | 0.3 ± 0.04 |
| epi-globulol | 1590 | 1587 | 0.1 ± 0.07 | 0.2 ± 0.04 | - |
| viridiflorol * | 1591 | 1589 | 0.5 ± 0.04 | 0.2 ± 0.19 | - |
| guaiol * | 1595 | 1597 | 0.5 ± 0.04 | - | - |
| humulene epoxide II | 1608 | 1605 | 4.3 ± 0.23 A | 1.5 ± 0.24 C | 2.4 ± 0.21 B |
| humulane-1,6-dien-3-ol | 1613 | 1619 | 0.1 ± 0.13 | 0.3 ± 0.11 | 1.4 ± 0.20 |
| selin-6-en-4-ol | 1618 | 1624 | 1.8 ± 0.13 | - | - |
| (E)-longipinocarveol | 1624 | 0.5 ± 0.10 | - | - | |
| 1-epi-cubenol | 1628 | 1629 | 0.6 ± 0.21 | - | 0.9 ± 0.38 |
| caryophylla-4(14),8(15)-dien-5-ol | 1637 | 1636 | 6.9 ± 0.39 A | 1.6 ± 0.45 C | 4.8 ± 0.80 B |
| epi-α-cadinol | 1641 | 1640 | 0.2 ± 0.03 | - | - |
| cubenol | 1643 | 1642 | - | 0.1 ± 0.07 | - |
| β-eudesmol | 1645 | 1645 | - | 0.1 ± 0.09 | - |
| himachalol | 1646 | 0.8 ± 0.07 | 0.7 ± 0.13 | 0.6 ± 0.23 | |
| neointermedeol | 1660 | 1662 | 3.0 ± 0.11 A | 0.9 ± 0.18 C | 2.2 ± 0.26 B |
| 14-hydroxy-9-epi-(E)-caryophyllene | 1664 | 1665 | 3.0 ± 0.18 A | - C | 1.8 ± 0.29 B |
| aromadendrene oxide II | 1678 | 1678 | 0.3 ± 0.02 | 0.5 ± 0.18 | 0.1 ± 0.09 |
| α-bisabolol * | 1683 | 1685 | 0.7 ± 0.03 | 0.1 ± 0.13 | 0.5 ± 0.13 |
| juniper camphor | 1692 | 1700 | 0.6 ± 0.1 | - | 0.6 ± 0.15 |
| (Z,E)-farnesol | 1697 | 1697 | 0.0 ± 0.08 | - | - |
| (E,E)-farnesyl acetone | 1920 | 1921 | 0.0 ± 0.08 | - | - |
| 2-methyl tricosane | 2365 | 2365 | 0.4 ± 0.74 | - | - |
| cannabidiol | 2419 | 8.8 ± 0.95 A | 6.1 ± 0.70 B | 5.7 ± 1.20 B | |
| cannabichromene | 2427 | 0.1 ± 0.01 | 0.2 ± 0.01 | 0.2 ± 0.01 | |
| dronabinol (= THC) | 2468 | tr 4 | tr | tr | |
| Monoterpene hydrocarbons | 9.1 ± 2.22 C | 28.8 ± 1.00 A | 17.2 ± 1.98 B | ||
| Oxygenated monoterpenes | 0.5 ± 0.30 A | 0.9 ± 0.06 A | 0.4 ± 0.28 A | ||
| Sesquiterpene hydrocarbons | 40.3 ± 0.48 B | 52.0 ± 1.50 A | 50.0 ± 2.30 A | ||
| Oxygenated sesquiterpenes | 37.8 ± 1.58 A | 11.7 ± 1.18 C | 24.6 ± 3.10 B | ||
| Cannabinoids | 9.0 ± 0.95 A | 6.2 ± 0.71 B | 5.9 ± 1.21 B | ||
| Apocarotenoids | 0.0 ± 0.08 A | - A | - A | ||
| Phenylpropanoids | 0.0 ± 0.06 A | - B | 0.3 ± 0.03 B | ||
| Other non-terpene derivatives | 0.4 ± 0.74 A | 0.1 ± 0.06 A | - A | ||
| Total identified (%): | 97.2 ± 0.76 | 99.6 ± 0.09 | 98.4 ± 0.64 | ||
| Variable | Variable by | Spearman’s ρ | Prob > |ρ| |
|---|---|---|---|
| Significant negative correlations | |||
| Tmin | OS | −0.9487 | <0.0001 |
| ΔT | Inflorescence yield | −0.9487 | <0.0001 |
| Tmean | PP | −0.866 | 0.0025 |
| ΔT | SH | −0.8433 | 0.0043 |
| ΔT | Harvest index | −0.8433 | 0.0043 |
| Inflorescence yield | OS | −0.8333 | 0.0053 |
| Harvest index | OS | −0.8167 | 0.0072 |
| Plant height | OS | −0.7833 | 0.0125 |
| Plant height | CAN | −0.75 | 0.0199 |
| Tmax | Plant height | −0.7379 | 0.0232 |
| ΔT | EO yield | −0.7379 | 0.0232 |
| Tmax | PP | −0.6928 | 0.0386 |
| Tmax | EO yield | −0.6852 | 0.0417 |
| ΔT | MH | −0.6852 | 0.0417 |
| ΔT | Plant height | −0.6852 | 0.0417 |
| Tmean | Hydrodistillation yield | −0.6644 | 0.051 |
| Tmean | Plant height | −0.0527 | 0.8929 |
| Significant positive correlations | |||
| EO yield | Hydrodistillation yield | 0.0756 | 0.8467 |
| Tmean | MH | 0.1054 | 0.7872 |
| Tmean | NT | 0.2887 | 0.4512 |
| Tmean | Inflorescence yield | 0.4743 | 0.197 |
| Plant height | SH | 0.6667 | 0.0499 |
| EO yield | Plant height | 0.6667 | 0.0499 |
| EO yield | Harvest index | 0.6667 | 0.0499 |
| Tmin | MH | 0.6852 | 0.0417 |
| Tmin | Plant height | 0.6852 | 0.0417 |
| EO yield | Inflorescence yield | 0.7333 | 0.0246 |
| Tmin | EO yield | 0.7379 | 0.0232 |
| Harvest index | SH | 0.7833 | 0.0125 |
| Inflorescence yield | SH | 0.8333 | 0.0053 |
| Harvest index | Inflorescence yield | 0.8333 | 0.0053 |
| Tmin | SH | 0.8433 | 0.0043 |
| Tmin | Harvest index | 0.8433 | 0.0043 |
| Plant height | MH | 0.85 | 0.0037 |
| Rainfall | PP | 0.866 | 0.0025 |
| Tmin | Inflorescence yield | 0.9487 | <0.0001 |
| ΔT | OS | 0.9487 | <0.0001 |
| Site | DPPH mmol TE/g | Inhibition % |
|---|---|---|
| SPG | 0.038 ± 0.0032 c | 62.24 ± 5.25 c |
| SL | 0.051 ± 0.0015 a | 85.19 ± 2.56 a |
| LA | 0.046 ± 0.0014 b | 76.62 ± 2.34 b |
| Compounds | Spearman’s ρ | Prob > |ρ| |
|---|---|---|
| Significant negative correlations | ||
| α-phellandrene | −0.69310328 | 0.038441 |
| α-terpinene | −0.69310328 | 0.038441 |
| γ-terpinene | −0.821583836 | 0.006603 |
| terpinolene | −0.85 | 0.003705 |
| linalool | −0.732709182 | 0.024739 |
| trans-verbenol | −0.677296212 | 0.045043 |
| 4-terpineol | −0.821583836 | 0.006603 |
| α-terpineol | −0.689454293 | 0.039906 |
| Significant positive correlations | ||
| 7-epi-α-selinene | 0.75 | 0.019942 |
| β-cadinene | 0.669461927 | 0.048568 |
| selina-3,7(11)-diene | 0.666666667 | 0.049867 |
| palustrol | 0.666666667 | 0.049867 |
| 1-epi-cubenol | 0.91538573 | 0.000534 |
| caryophylla-4(14),8(15)-dien-5-ol | 0.7 | 0.03577 |
| 14-hydroxy-9-epi-(E)-caryophyllene | 0.678063504 | 0.044707 |
| α-bisabolol | 0.677830201 | 0.044809 |
| juniper camphor | 0.876794227 | 0.001912 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ascrizzi, R.; Flamini, G.; Rossi, A.; Santini, A.; Angelini, L.G.; Tavarini, S. Inflorescence Yield, Essential Oil Composition and Antioxidant Activity of Cannabis sativa L. cv ‘Futura 75’ in a Multilocation and On-Farm Study. Agriculture 2024, 14, 225. https://doi.org/10.3390/agriculture14020225
Ascrizzi R, Flamini G, Rossi A, Santini A, Angelini LG, Tavarini S. Inflorescence Yield, Essential Oil Composition and Antioxidant Activity of Cannabis sativa L. cv ‘Futura 75’ in a Multilocation and On-Farm Study. Agriculture. 2024; 14(2):225. https://doi.org/10.3390/agriculture14020225
Chicago/Turabian StyleAscrizzi, Roberta, Guido Flamini, Alessandro Rossi, Andrea Santini, Luciana G. Angelini, and Silvia Tavarini. 2024. "Inflorescence Yield, Essential Oil Composition and Antioxidant Activity of Cannabis sativa L. cv ‘Futura 75’ in a Multilocation and On-Farm Study" Agriculture 14, no. 2: 225. https://doi.org/10.3390/agriculture14020225
APA StyleAscrizzi, R., Flamini, G., Rossi, A., Santini, A., Angelini, L. G., & Tavarini, S. (2024). Inflorescence Yield, Essential Oil Composition and Antioxidant Activity of Cannabis sativa L. cv ‘Futura 75’ in a Multilocation and On-Farm Study. Agriculture, 14(2), 225. https://doi.org/10.3390/agriculture14020225







