Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses
Abstract
:1. Introduction
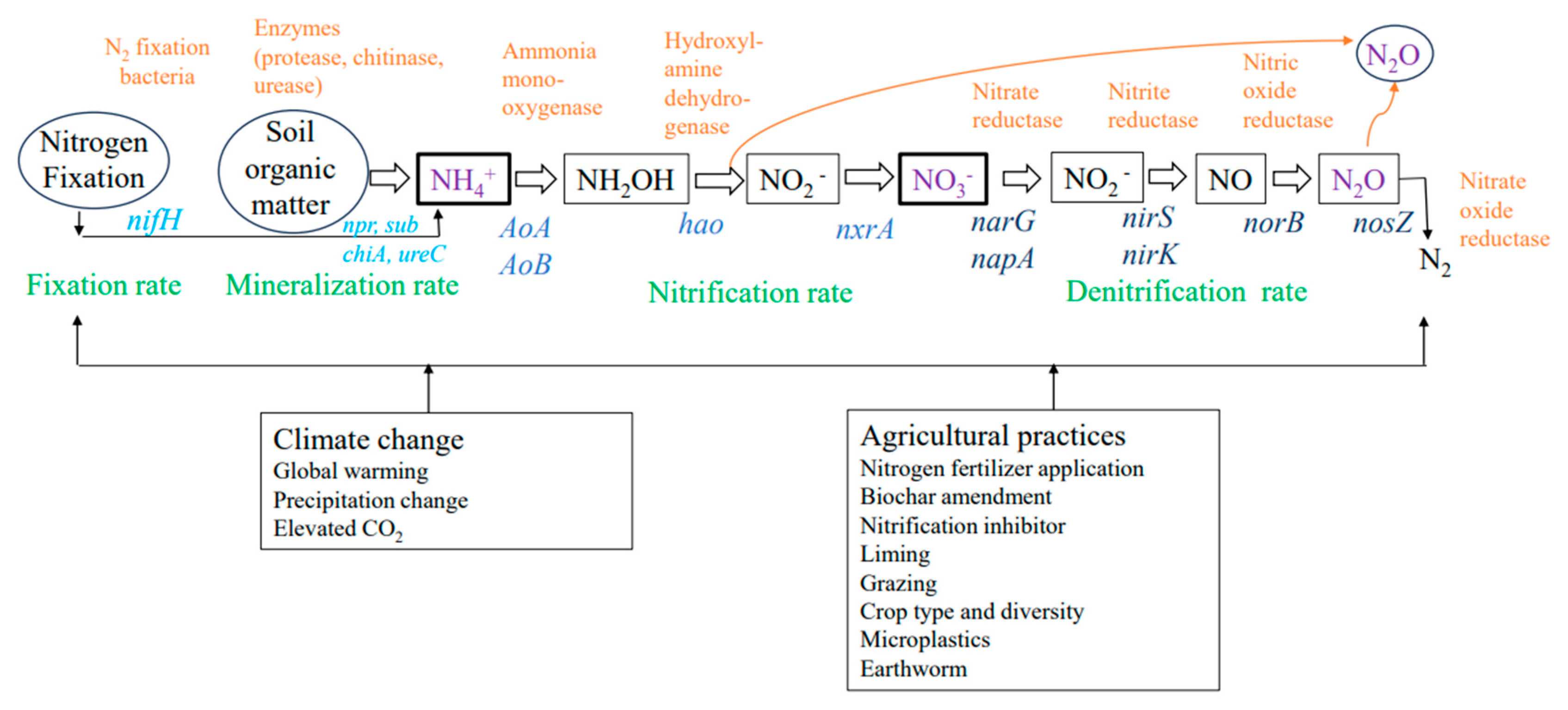
2. Nitrogen Processes, Enzymes, Genes, and Soil N2O Emissions
3. Methods of Study: Experimental Study, Meta-Analysis, and Mega-Analysis
4. Impacts of Climate Change on Gene, Enzyme, Nitrification and Denitrification Processes, and Soil N2O Emissions
4.1. Impacts of Global Warming
4.2. Impacts of Elevated CO2
4.3. Impacts of Precipitation
5. Impacts of Agricultural Practices on Gene, Enzyme, Nitrification and Denitrification, and Soil N2O Emissions
5.1. Impacts of Nitrogen Application
5.2. Impacts of Biochar Applications
5.3. Impacts of Nitrification Inhibitor Usage
5.4. Effects of Liming
5.5. Impacts of Microplastics
5.6. Impacts of Crop Diversity
5.7. Impacts of Grazing
5.8. Impacts of Earthworms
6. Regulating Factors on Soil N2O Emissions
7. Conclusions and Future Research Directions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Foley, J.; Ramankutty, N.; Brauman, K.; Cassidy, E.; Gerber, J.; Johnston, M.; Mueller, N.; O’Connell, C.; Ray, D.; West, P.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef]
- Tian, H.; Lu, C.; Ciais, P.; Michalak, A.; Canadell, J.; Saikawa, E.; Huntzinger, D.; Gurney, K.; Sitch, S.; Zhang, B.; et al. The terrestrial biosphere as a net source of greenhouse gases to the atmosphere. Nature 2016, 531, 225–228. [Google Scholar] [CrossRef]
- Cassman, K.; Grassini, P. A global perspective on sustainable intensification research. Nat. Sustain. 2020, 3, 262–268. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Li, L.; Zheng, J.; Qu, J.; Zheng, J.; Zhang, X.; Pan, G. Consistent increase in abundance and diversity but variable change in community composition of bacteria in topsoil of rice paddy under short term biochar treatment across three sites from South China. Appl. Soil Ecol. 2015, 91, 68–79. [Google Scholar] [CrossRef]
- Guo, T.; Bai, S.; Omidvar, N.; Wang, Y.; Chen, F.; Zhang, M. Insight into the functional mechanisms of nitrogen-cycling inhibitors in decreasing yield-scaled ammonia volatilization and nitrous oxide emission: A global meta-analysis. Chemosphere 2023, 338, 139611. [Google Scholar] [CrossRef]
- Kroeze, C.; Mosier, A.; Bouwman, L. Closing the global N2O budget: A retrospective analysis 1500–1994. Glob. Biogeochem. Cycles 1999, 13, 1–8. [Google Scholar] [CrossRef]
- Tian, H.; Xu, R.; Canadell, J.; Thompson, R.; Winiwarter, W.; Suntharalingam, P.; Davidson, E.; Ciais, P.; Jackson, R.; Janssens-Maenhout, G.; et al. A comprehensive quantification of global nitrous oxide sources and sinks. Nature 2020, 586, 248–256. [Google Scholar] [CrossRef]
- Yin, M.; Gao, X.; Kuang, W.; Tenuta, M. Soil N2O emissions and functional genes in response to grazing grassland with livestock: A meta-analysis. Geoderma 2023, 436, 116538. [Google Scholar] [CrossRef]
- You, L.; Ros, G.; Chen, Y.-L.; Yang, X.; Cui, Z.; Liu, X.; Jiang, R.; Zhang, F.; Vries, W. Global meta-analysis of terrestrial nitrous oxide emissions and associated functional genes under nitrogen addition. Soil Biol. Biochem. 2021, 165, 108523. [Google Scholar] [CrossRef]
- Ravishankara, A.; Daniel, J.; Portmann, R. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science 2009, 326, 123–125. [Google Scholar] [CrossRef] [PubMed]
- Ollivier, Q.; Maher, D.; Pitfield, C.; Macreadie, P. Winter emissions of CO2, CH4, and N2O from temperate agricultural dams: Fluxes, sources, and processes. Ecosphere 2019, 10, e02914. [Google Scholar] [CrossRef]
- Du, Y.; Guo, X.; Li, J.; Liu, Y.; Luo, J.; Liang, Y.; Li, T. Elevated carbon dioxide stimulates nitrous oxide emission in agricultural soils: A global meta-analysis. Pedosphere 2022, 32, 3–14. [Google Scholar] [CrossRef]
- Hui, D.; Deng, Q.; Tian, H.; Luo, Y. Global climate change and greenhouse gases emissions in terrestrial ecosystems. In Handbook of Climate Change Mitigation and Adaptation; Lackner, M., Sajjadi, B., Chen, W.-Y., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 23–76. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, Y.; Chen, C.; Xiang, Y.; Rashti, M.; Li, Y.; Deng, Q.; Zhang, R. Effects of biochar application on soil nitrogen transformation, microbial functional genes, enzyme activity, and plant nitrogen uptake: A meta-analysis of field studies. GCB Bioenergy 2021, 13, 1859–1873. [Google Scholar] [CrossRef]
- Soussana, J.; Allard, V.; Pilegaard, K.; Ambus, P.; Amman, C.; Campbell, C.; Ceschia, E.; Clifton-Brown, J.; Czobel, S.; Domingues, R.; et al. Full accounting of the greenhouse gas (CO2, N2O, CH4) budget of nine European grassland sites. Agric. Ecosyst. Environ. 2007, 121, 121–134. [Google Scholar] [CrossRef]
- Guenet, B.; Gabrielle, B.; Chenu, C.; Arrouays, D.; Balesdent, J.; Bernoux, M.; Bruni, E.; Caliman, J.; Cardinael, R.; Chen, S.; et al. Can N2O emissions offset the benefits from soil organic carbon storage? Glob. Chang. Biol. 2021, 27, 237–256. [Google Scholar] [CrossRef] [PubMed]
- Garvin, J.; Buick, R.; Anbar, A.; Arnold, G.; Kaufman, A. Isotopic evidence for an aerobic nitrogen cycle in the latest Archean. Science 2009, 323, 1045–1048. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Xu, J.; Allen, S.D.; Khan, S.; Nadir, S.; Arif, M.S.; Yasmeen, T. Unraveling consequences of soil micro- and nano-plastic pollution on soil-plant system: Implications for nitrogen (N) cycling and soil microbial activity. Chemosphere 2020, 260, 127578. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Zhang, Q.; Xi, Z.; Xiong, Z. Mechanisms of mitigating nitrous oxide emissions from vegetable soil varied with manure, biochar and nitrification inhibitors. Agric. For. Meteorol. 2019, 278, 107672. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, C.; Liu, M.; Yu, Y. Integrated reclamation of saline soil nitrogen transformation in the hyphosphere by earthworms and arbuscular mycorrhizal fungus. Appl. Soil Ecol. 2019, 135, 137–146. [Google Scholar] [CrossRef]
- Tang, S.; Rao, Y.; Huang, S.; Xu, Y.; Zeng, K.; Liang, X.; Ling, Q.; Liu, K.; Ma, J.; Yu, F.; et al. Impact of environmental factors on the ammonia-oxidizing and denitrifying microbial community and functional genes along soil profiles from different ecologically degraded areas in the Siding mine. J. Environ. Manag. 2023, 326, 116641. [Google Scholar] [CrossRef]
- Gineyts, R.; Niboyet, A. Nitrification, denitrification, and related functional genes under elevated CO2: A meta-analysis in terrestrial ecosystems. Glob. Chang. Biol. 2023, 29, 1839–1853. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Wang, J.; Iwuozo, S.; Yu, C.; Jima, T.; Smart, D.; Reddy, C.; Dennis, S. Corn yield and soil nitrous oxide emission under different fertilizer and soil management: A three-year field experiment in middle Tennessee. PLoS ONE 2015, 10, e0125406. [Google Scholar] [CrossRef]
- Hassan, M.; Aamer, M.; Mahmood, A.; Awan, M.; Barbanti, L.; Seleiman, M.; Bakhsh, G.; Alkharabsheh, H.; Babur, E.; Shao, J.; et al. Management strategies to mitigate N2O emissions in agriculture. Life 2022, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Kieffer, C.; Ren, W.; Hui, D. How much is soil nitrous oxide emission reduced with biochar application? An evaluation of meta-analyses. GCB Bioenergy 2023, 15, 24–37. [Google Scholar] [CrossRef]
- Borchard, N.; Schirrmann, M.; Cayuela, M.L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Sigua, G.; Spokas, K.; Ippolito, J.A.; et al. Biochar, soil and land-use interactions that reduce nitrate leaching and N2O emissions: A meta-analysis. Sci. Total Environ. 2019, 651, 2354–2364. [Google Scholar] [CrossRef]
- Ouyang, Y.; Evans, S.; Friesen, M.; Tiemann, L. Effect of nitrogen fertilization on the abundance of nitrogen cycling genes in agricultural soils: A meta-analysis of field studies. Soil Biol. Biochem. 2018, 127, 71–78. [Google Scholar] [CrossRef]
- Dai, Z.; Yu, M.; Chen, H.; Zhao, H.; Huang, Y.; Su, W.; Xia, F.; Chang, S.; Brookes, P.; Dahlgren, R.; et al. Elevated temperature shifts soil N cycling from microbial immobilization to enhanced mineralization, nitrification and denitrification across global terrestrial ecosystems. Glob. Chang. Biol. 2020, 26, 5267–5276. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Wang, J.; Wan, Q.; Liao, L.; Liu, G.; Zhang, C. Grazing exclusion reduces soil N2O emissions by regulating nirK− and nosZ− type denitrifiers in alpine meadows. J. Soils Sediments 2021, 21, 3753–3769. [Google Scholar] [CrossRef]
- Yin, M.; Gao, X.; Kuang, W.; Zhang, Y. Meta-analysis of the effect of nitrification inhibitors on the abundance and community structure of N2O-related functional genes in agricultural soils. Sci. Total Environ. 2023, 865, 161215. [Google Scholar] [CrossRef]
- Hao, J.; Feng, Y.; Wang, X.; Yu, Q.; Zhang, F.; Yang, G.; Ren, G.; Han, X.; Wang, X.; Ren, C. Soil microbial nitrogen-cycling gene abundances in response to crop diversification: A meta-analysis. Sci. Total Environ. 2022, 838, 156621. [Google Scholar] [CrossRef]
- Yang, Y.; Feng, X.; Hu, Y.; Ren, C.; Zeng, Z. Effects of legume-oat intercropping on abundance and community structure of soil N2-fixing bacteria. Chin. J. Appl. Ecol. 2017, 28, 957–965. [Google Scholar] [CrossRef]
- Vadakattu, G.; Roper, M.; Roget, D. Potential for non-symbiotic N2-fixation in different agroecological zones of southern Australia. Aust. J. Soil Res. 2006, 44, 343–354. [Google Scholar] [CrossRef]
- Collavino, M.; Tripp, H.; Frank, I.; Vidoz, M.; Calderoli, P.; Donato, M.; Zehr, J.; Aguilar, O. nifH pyrosequencing reveals the potential for location-specific soil chemistry to influence N2-fixing community dynamics. Environ. Microbiol. 2014, 16, 3211–3223. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, J.; Liu, Y.; Jing, H. How climate and soil properties affect the abundances of nitrogen-cycling genes in nitrogen-treated ecosystems: A meta-analysis. Plant Soil 2022, 477, 389–404. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J. Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Ouyang, Y.; Norton, J. Short-term nitrogen fertilization affects microbial community composition and nitrogen mineralization functions in an agricultural soil. Appl. Environ. Microbiol. 2020, 86, e02278-19. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, S.; Wilcke, W.; Eisenhauer, N.; Oelmann, Y. Net ammonification as influenced by plant diversity in experimental grasslands. Soil Biol. Biochem. 2012, 48, 78–87. [Google Scholar] [CrossRef]
- Pester, M.; Maixner, F.; Berry, D.; Rattei, T.; Koch, H.; Lücker, S.; Nowka, B.; Richter, A.; Spieck, E.; Lebedeva, E.; et al. NxrB encoding the beta subunit of nitrite oxidoreductase as functional and phylogenetic marker for nitrite-oxidizing Nitrospira. Environ. Microbiol. 2014, 16, 3055–3071. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Rasmann, S.; Yue, L.; Lian, F.; Zou, H.; Wang, Z. The effect of biochar amendment on N-cycling genes in soils: A meta-analysis. Sci. Total Environ. 2019, 696, 133984. [Google Scholar] [CrossRef]
- Martínez-Espinosa, C.; Sauvage, S.; Al Bitar, A.; Green, P.; Vörösmarty, C.; Sánchez-Pérez, J. Denitrification in wetlands: A review towards a quantification at global scale. Sci. Total Environ. 2021, 754, 142398. [Google Scholar] [CrossRef]
- Canfield, D.; Glazer, A.; Falkowski, P. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [CrossRef]
- Kandeler, E.; Deiglmayr, K.; Tscherko, D.; Bru, D.; Philippot, L. Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl. Environ. Microbiol. 2006, 72, 5957–5962. [Google Scholar] [CrossRef] [PubMed]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Henry, S.; Bru, D.; Stres, B.; Hallet, S.; Philippot, L. Quantitative detection of the nosZ gene, encoding nitrous oxide reductase, and comparison of the abundances of 16S rRNA, narG, nirK, and nosZ genes in soils. Appl. Environ. Microbiol. 2006, 72, 5181–5189. [Google Scholar] [CrossRef] [PubMed]
- Morales, S.E.; Cosart, T.; Holben, W.E. Bacterial gene abundances as indicators of greenhouse gas emission in soils. ISME J. 2010, 4, 799–808. [Google Scholar] [CrossRef] [PubMed]
- McCarty, G. Modes of action of nitrification inhibitors. Biol. Fertil. Soils 1999, 29, 1–9. [Google Scholar] [CrossRef]
- Deng, Q.; Hui, D.; Wang, J.; Yu, C.; Li, C.; Reddy, C.; Dennis, S. Assessing the impacts of tillage and fertilization management on nitrous oxide emissions in a cornfield using the DNDC model. J. Geophys. Res. Biogeosci. 2016, 121, 337–349. [Google Scholar] [CrossRef]
- Salazar, A.; Rousk, K.; Jónsdóttir, I.; Bellenger, J.-P.; Andrésson, Ó. Faster nitrogen cycling and more fungal and root biomass in cold ecosystems under experimental warming: A meta-analysis. Ecology 2020, 101, e02938. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Ran, Y.; Li, Y.; Zhang, Q.; Liu, Y.; Pan, H.; Guan, X.; Li, J.; Shi, J.; Dong, L.; et al. Warmer and drier conditions alter the nitrifier and denitrifier communities and reduce N2O emissions in fertilized vegetable soils. Agric. Ecosyst. Environ. 2016, 231, 133–142. [Google Scholar] [CrossRef]
- Huang, X.; Zou, Y.; Qiao, C.; Liu, Q.; Liu, J.; Kang, R.; Ren, L.; Wu, W. Effects of biological nitrification inhibitor on nitrous oxide and nosZ, nirK, nirS denitrifying bacteria in Paddy soils. Sustainability 2023, 15, 5348. [Google Scholar] [CrossRef]
- Curtis, P. A meta-analysis of leaf gas exchange and nitrogen in trees grown under elevated carbon dioxide. Plant Cell Environ. 1996, 19, 127–137. [Google Scholar] [CrossRef]
- Luo, Y.; Hui, D.; Zhang, D. Elevated CO2 stimulates net accumulations of carbon and nitrogen in land ecosystems: A meta-analysis. Ecology 2006, 87, 53–63. [Google Scholar] [CrossRef]
- Fox, J. How much does the typical ecological meta-analysis overestimate the true mean effect size? Ecol. Evol. 2022, 12, e9521. [Google Scholar] [CrossRef]
- Makowski, D.; Catarino, R.; Chen, M.; Bosco, S.; Montero-Castaño, A.; Pérez-Soba, M.; Schievano, A.; Tamburini, G. Synthesising results of meta-analyses to inform policy: A comparison of fast-track methods. Environ. Evid. 2023, 12, 16. [Google Scholar] [CrossRef]
- Hui, D.; Kaur, N.; Ray, A.; Kasrija, L.; Li, Q. Literature Review, Meta-Analysis, and Mega-Analysis in Ecological and Agricultural Sciences. Agric. Sci. 2023, 14, 474–484. [Google Scholar] [CrossRef]
- Cui, P.; Fan, F.; Yin, C.; Song, A.; Huang, P.; Tang, Y.; Zhu, P.; Peng, C.; Li, T.; Wakelin, S.; et al. Long-term organic and inorganic fertilization alters temperature sensitivity of potential N2O emissions and associated microbes. Soil Biol. Biochem. 2015, 93, 131–141. [Google Scholar] [CrossRef]
- Li, L.; Zheng, Z.; Wang, W.; Biederman, J.; Xu, X.; Ran, Q.; Qian, R.; Xu, C.; Zhang, B.; Wang, F.; et al. Terrestrial N2O emissions and related functional genes under climate change: A global meta-analysis. Glob. Chang. Biol. 2020, 26, 931–943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, J.; Yu, Y.; Li, Y.; Shen, X.; Huo, S.; Xia, X. Effects of multiple global change factors on soil microbial richness, diversity and functional gene abundances: A meta-analysis. Sci. Total Environ. 2022, 815, 152737. [Google Scholar] [CrossRef] [PubMed]
- Niu, S.; Sherry, R.; Zhou, X.; Wan, S.; Luo, Y. Nitrogen regulation of the climate–carbon feedback: Evidence from a long-term global change experiment. Ecology 2010, 91, 3261–3273. [Google Scholar] [CrossRef]
- Weedon, J.; Kowalchuk, G.; Aerts, R.; van Hal, J.; van Logtestijn, R.; Taş, N.; Röling, W.; van Bodegom, P. Summer warming accelerates sub-arctic peatland nitrogen cycling without changing enzyme pools or microbial community structure. Glob. Chang. Biol. 2012, 18, 138–150. [Google Scholar] [CrossRef]
- Mooshammer, M.; Wanek, W.; Hämmerle, I.; Fuchslueger, L.; Hofhansl, F.; Knoltsch, A.; Schnecker, J.; Takriti, M.; Watzka, M.; Wild, B.; et al. Adjustment of microbial nitrogen use efficiency to carbon:nitrogen imbalances regulates soil nitrogen cycling. Nat. Commun. 2014, 5, 3694. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Xie, J.; Zhou, A.; Liu, F.; Li, D.; Wu, L.; Deng, Y.; He, Z.; Van Nostrand, J.; Luo, Y.; et al. Warming alters expressions of microbial functional genes important to ecosystem functioning. Front. Microbiol. 2016, 7, 668. [Google Scholar] [CrossRef] [PubMed]
- Xue, K.; Yuan, M.; Shi, Z.; Qin, Y.; Deng, Y.; Cheng, L.; Wu, L.; He, Z.; Van Nostrand, J.; Bracho, R.; et al. Tundra soil carbon is vulnerable to rapid microbial decomposition under climate warming. Nat. Clim. Chang. 2016, 6, 595–600. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, H.; Wang, J.; Liu, X.; Cheng, K.; Li, L.; Zheng, J.; Zhang, X.; Pan, G.-X. Short-term response of nitrifier communities and potential nitrification activity to elevated CO2 and temperature interaction in a Chinese paddy field. Appl. Soil Ecol. 2015, 96, 88–98. [Google Scholar] [CrossRef]
- Waghmode, T.; Chen, S.; Li, J.; Sun, R.; Liu, B.; Hu, C. Response of nitrifier and denitrifier abundance and microbial community structure to experimental warming in an agricultural ecosystem. Front. Microbiol. 2018, 9, 474. [Google Scholar] [CrossRef]
- Bai, E.; Li, S.; Xu, W.; Li, W.; Dai, W.; Jiang, P. A meta-analysis of experimental warming effects on terrestrial nitrogen pools and dynamics. New Phytol. 2013, 199, 441–451. [Google Scholar] [CrossRef]
- Liu, S.; Zheng, Y.; Ma, R.; Yu, K.; Han, Z.; Xiao, S.; Li, Z.; Wu, S.; Li, S.; Wang, J.; et al. Increased soil release of greenhouse gases shrinks terrestrial carbon uptake enhancement under warming. Glob. Chang. Biol. 2020, 26, 4601–4613. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.; Dove, N.; Beman, J.; Hart, S.; Aronson, E. Meta-analysis reveals ammonia-oxidizing bacteria respond more strongly to nitrogen addition than ammonia-oxidizing archaea. Soil Biol. Biochem. 2016, 99, 158–166. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Li, J.; Roland, B.; Du, Z.; Wu, D. Potential denitrification activity response to long-term nitrogen fertilization—A global meta-analysis. J. Clean. Prod. 2022, 336, 130451. [Google Scholar] [CrossRef]
- Gao, Y.; Tan, Z.; Wang, H.; Zhu, Y. Nitrogen fertilization and the rhizosphere effect on nitrogen cycling: A meta-analysis. Appl. Soil Ecol. 2023, 186, 104788. [Google Scholar] [CrossRef]
- Meng, X.; Yu, H.; Zhang, X.; Li, Y.; Zamanien, K.; Yao, H. Fertilization regimes and the nitrification process in paddy soils: Lessons for agricultural sustainability from a meta-analysis. Appl. Soil Ecol. 2023, 186, 104844. [Google Scholar] [CrossRef]
- Zhang, H.-M.; Liang, Z.; Li, Y.; Chen, Z.-X.; Zhang, J.-B.; Cai, Z.-C.; Elsgaard, L.; Cheng, Y.; van Groenigen, K.; Abalos, D. Liming modifies greenhouse gas fluxes from soils: A meta-analysis of biological drivers. Agric. Ecosyst. Environ. 2022, 340, 108182. [Google Scholar] [CrossRef]
- Su, P.; Gao, C.; Zhang, X.; Zhang, D.; Liu, X.; Xiang, T.; Luo, Y.; Chu, K.; Zhang, G.; Bu, N.; et al. Microplastics stimulated nitrous oxide emissions primarily through denitrification: A meta-analysis. J. Hazard. Mater. 2023, 445, 130500. [Google Scholar] [CrossRef]
- Xue, R.; Wang, C.; Liu, X.; Liu, M. Earthworm regulation of nitrogen pools and dynamics and marker genes of nitrogen cycling: A meta-analysis. Pedosphere 2022, 32, 131–139. [Google Scholar] [CrossRef]
- Jansson, J.; Hofmockel, K. Soil microbiomes and climate change. Nat. Rev. Microbiol. 2020, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Yang, Y.; Liu, J.; Hu, Z.; Liu, X.; Tian, M.; Yang, W.; Jin, J.; Wang, H.; Wang, Y.; et al. Different responses of ammonia-oxidizing archaea and bacteria in paddy soils to elevated CO2 concentration. Environ. Pollut. 2021, 286, 117558. [Google Scholar] [CrossRef] [PubMed]
- Simonin, M.; Le Roux, X.; Poly, F.; Lerondelle, C.; Hungate, B.; Nunan, N.; Niboyet, A. Coupling between and among ammonia oxidizers and nitrite oxidizers in grassland mesocosms submitted to elevated CO2 and nitrogen supply. Microb. Ecol. 2015, 70, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, Q.; Yuan, M.; Shi, Z.; Chiariello, N.; Docherty, K.; Dong, S.; Field, C.; Gu, Y.; Gutknecht, J.; et al. Long-term elevated CO2 shifts composition of soil microbial communities in a Californian annual grassland, reducing growth and N utilization potentials. Sci. Total Environ. 2019, 652, 1474–1481. [Google Scholar] [CrossRef] [PubMed]
- Barnard, R.; Leadley, P.; Hungate, B. Global change, nitrification, and denitrification: A review. Glob. Biogeochem. Cycles 2005, 19, GB1007. [Google Scholar] [CrossRef]
- Šťovíček, A.; Kim, M.; Or, D.; Gillor, O. Microbial community response to hydration-desiccation cycles in desert soil. Sci. Rep. 2017, 7, 45735. [Google Scholar] [CrossRef] [PubMed]
- Homyak, P.; Allison, S.; Huxman, T.; Goulden, M.; Treseder, K. Effects of drought manipulation on soil nitrogen cycling: A meta-analysis. J. Geophys. Res. Biogeosci. 2017, 122, 3260–3272. [Google Scholar] [CrossRef]
- Yan, G.; Mu, C.; Xing, Y.; Wang, Q. Responses and mechanisms of soil greenhouse gas fluxes to changes in precipitation intensity and duration: A meta-analysis for a global perspective. Can. J. Soil Sci. 2018, 98, 591–603. [Google Scholar] [CrossRef]
- Vitousek, P.; Aber, J.; Howarth, R.; Likens, G.; Matson, P.; Schindler, D.; Schlesinger, W.; Tilman, D. Human alteration of the global nitrogen cycle: Sources and consequences. Ecol. Appl. 1997, 7, 737–750. [Google Scholar] [CrossRef]
- Lebauer, D.; Treseder, K. Nitrogen limitation of net primary productivity in terrestrial ecosystems is globally distributed. Ecology 2008, 89, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Domeignoz-Horta, L.; Philippot, L.; Peyrard, C.; Bru, D.; Breuil, M.; Bizouard, F.; Justes, E.; Mary, B.; Léonard, J.; Spor, A. Peaks of in situ N2O emissions are influenced by N2O-producing and reducing microbial communities across arable soils. Glob. Chang. Biol. 2018, 24, 360–370. [Google Scholar] [CrossRef]
- Kelly, J.; Policht, K.; Grancharova, T.; Hundal, L. Distinct responses in ammonia-oxidizing archaea and bacteria after addition of biosolids to an agricultural soil. Appl. Environ. Microbiolgy 2011, 77, 6551–6558. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Lauber, C.; Ramirez, K.; Zaneveld, J.; Bradford, M.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef]
- Soares, J.; Cassman, N.; Kielak, A.; Pijl, A.; Carmo, J.; Lourenço, K.; Laanbroek, H.; Cantarella, H.; Kuramae, E. Nitrous oxide emission related to ammonia-oxidizing bacteria and mitigation options from N fertilization in a tropical soil. Sci. Rep. 2016, 6, 30349. [Google Scholar] [CrossRef]
- Hallin, S.; Jones, C.; Schloter, M.; Philippot, L. Relationship between N-cycling communities and ecosystem functioning in a 50-year-old fertilization experiment. ISME J. 2009, 3, 597–605. [Google Scholar] [CrossRef]
- Liang, Y.; Ning, D.; Lu, Z.; Zhang, N.; Hale, L.; Wu, L.; Clark, I.; McGrath, S.; Storkey, J.; Hirsch, P.; et al. Century long fertilization reduces stochasticity controlling grassland microbial community succession. Soil Biol. Biochem. 2020, 151, 108023. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.; Thies, J.; Masiello, C.; Hockaday, W.; Crowley, D. Biochar effects on soil biota—A review. Soil Biol. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H.; et al. Biochar application as a tool to decrease soil nitrogen losses (NH3 volatilization, N2O emissions, and N leaching) from croplands: Options and mitigation strength in a global perspective. Glob. Chang. Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef] [PubMed]
- Hui, D. Effects of biochar application on soil properties, plant biomass production, and soil greenhouse gas emissions: A mini-review. Agric. Sci. 2021, 12, 213–236. [Google Scholar] [CrossRef]
- Ducey, T.; Ippolito, J.A.; Cantrell, K.; Novak, J.; Lentz, R. Addition of activated switchgrass biochar to an aridic subsoil increases microbial nitrogen cycling gene abundances. Appl. Soil Ecol. 2013, 65, 65–72. [Google Scholar] [CrossRef]
- Yu, M.; Meng, J.; Yu, L.; Su, W.; Afzal, M.; Li, Y.; Brookes, P.; Redmile-Gordon, M.; Luo, Y.; Xu, J. Changes in nitrogen related functional genes along soil pH, C and nutrient gradients in the charosphere. Sci. Total Environ. 2019, 650, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Liu, B.; Zhang, Y.; Lin, Z.; Zhu, T.; Sun, R.; Wang, X.; Ma, J.; Bei, Q.; Liu, G.; et al. Can biochar alleviate soil compaction stress on wheat growth and mitigate soil N2O emissions? Soil Biol. Biochem. 2017, 104, 8–17. [Google Scholar] [CrossRef]
- Edwards, J.; Pittelkow, C.; Kent, A.; Yang, W. Dynamic biochar effects on soil nitrous oxide emissions and underlying microbial processes during the maize growing season. Soil Biol. Biochem. 2018, 122, 81–90. [Google Scholar] [CrossRef]
- Bai, S.; Reverchon, F.; Xu, C.; Xu, Z.; Blumfield, T.; Zhao, H.; Van Zwieten, L.; Wallace, H. Wood biochar increases nitrogen retention in field settings mainly through abiotic processes. Soil Biol. Biochem. 2015, 90, 232–240. [Google Scholar] [CrossRef]
- Liang, F.; Li, G.-T.; Lin, Q.-M.; Zhao, X.-R. Crop yield and soil properties in the first 3 years after biochar application to a Calcareous soil. J. Integr. Agric. 2014, 13, 525–532. [Google Scholar] [CrossRef]
- Van Zwieten, L.; Singh, B.; Kimber, S.; Murphy, D.; Macdonald, L.; Rust, J.; Morris, S. An incubation study investigating the mechanisms that impact N2O flux from soil following biochar application. Agric. Ecosyst. Environ. 2014, 191, 53–62. [Google Scholar] [CrossRef]
- Cayuela, M.; van Zwieten, L.; Singh, B.; Jeffery, S.; Roig, A.; Sánchez-Monedero, M. Biochar’s role in mitigating soil nitrous oxide emissions: A review and meta-analysis. Agric. Ecosyst. Environ. 2014, 191, 5–16. [Google Scholar] [CrossRef]
- He, Y.; Zhou, X.; Jiang, L.; Li, M.; Du, Z.; Zhou, G.; Shao, J.; Wang, X.; Xu, Z.; Bai, S.; et al. Effects of biochar application on soil greenhouse gas fluxes: A meta-analysis. GCB Bioenergy 2017, 9, 743–755. [Google Scholar] [CrossRef]
- Nguyen, T.; Xu, C.; Tahmasbian, I.; Che, R.; Xu, Z.; Zhou, X.; Wallace, H.; Bai, S. Effects of biochar on soil available inorganic nitrogen: A review and meta-analysis. Geoderma 2017, 288, 79–96. [Google Scholar] [CrossRef]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2010, 16, 1837–1846. [Google Scholar] [CrossRef]
- Lei, J.; Fan, Q.; Yu, J.; Ma, Y.; Yin, J.; Liu, R. A meta-analysis to examine whether nitrification inhibitors work through selectively inhibiting ammonia-oxidizing bacteria. Front. Microbiol. 2022, 13, 962146. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.; Lehmann, A. Microplastic in terrestrial ecosystems. Science 2020, 368, 1430–1431. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Li, X.; He, D. Interaction of microplastics and soil animals in agricultural ecosystems. Curr. Opin. Environ. Sci. Health 2022, 26, 100327. [Google Scholar] [CrossRef]
- Zhu, F.; Yan, Y.; Doyle, E.; Zhu, C.; Jin, X.; Chen, Z.; Wang, C.; He, H.; Zhou, D.; Gu, C. Microplastics altered soil microbiome and nitrogen cycling: The role of phthalate plasticizer. J. Hazard. Mater. 2022, 427, 127944. [Google Scholar] [CrossRef]
- Li, H.; Liu, L.; Xu, Y.; Zhang, J. Microplastic effects on soil system parameters: A meta-analysis study. Environ. Sci. Pollut. Res. 2022, 29, 11027–11038. [Google Scholar] [CrossRef]
- Song, L.; Niu, S. Increased soil microbial AOB amoA and narG abundances sustain long-term positive responses of nitrification and denitrification to N deposition. Soil Biol. Biochem. 2022, 166, 108539. [Google Scholar] [CrossRef]
- Gao, H.; Liu, Q.; Yan, C.; Mancl, K.; Gong, D.; He, J.; Mei, X. Macro-and/or microplastics as an emerging threat effect crop growth and soil health. Resour. Conserv. Recycl. 2022, 186, 106549. [Google Scholar] [CrossRef]
- Yu, Y.; Li, X.; Feng, Z.; Xiao, M.; Ge, T.; Li, Y.; Yao, H. Polyethylene microplastics alter the microbial functional gene abundances and increase nitrous oxide emissions from Paddy soils. J. Hazard. Mater. 2022, 432, 128721. [Google Scholar] [CrossRef] [PubMed]
- Seeley, M.; Song, B.; Passie, R.; Hale, R. Microplastics affect sedimentary microbial communities and nitrogen cycling. Nat. Commun. 2020, 11, 2372. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, J.; Zhu, X.; Liang, X.; Lei, Y.; He, C. N-fixing trees in wetland restoration plantings: Effects on nitrogensupply and soil microbial communities. Environ. Sci. Pollut. Res. 2016, 23, 24749–24757. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Zhang, H.; Zhu, H.; Xu, S.; Zhang, D.; Lv, W. Rice rotation system affects the spatial dynamics of the diazotrophic community in Paddy soil of the Yangtze Delta, China. Eurasian Soil Sci. 2019, 52, 696–706. [Google Scholar] [CrossRef]
- Zou, J.; Yao, Q.; Liu, J.; Li, Y.; Song, F.; Liu, X.; Wang, G. Changes of diazotrophic communities in response to cropping systems in a Mollisol of Northeast China. PeerJ 2020, 8, e9550. [Google Scholar] [CrossRef] [PubMed]
- Barros, F.; Fracetto, F.; Lira Junior, M.; Bertini, S.; Fracetto, G. Spatial and seasonal responses of diazotrophs and ammonium-oxidizing bacteria to legume-based silvopastoral systems. Appl. Soil Ecol. 2021, 158, 103797. [Google Scholar] [CrossRef]
- Chen, J.; Arafat, Y.; Wu, L.; Xiao, Z.; Li, Q.; Khan, M.; Khan, M.; Lin, S.; Lin, W. Shifts in soil microbial community, soil enzymes and crop yield under peanut/maize intercropping with reduced nitrogen levels. Appl. Soil Ecol. 2018, 124, 327–334. [Google Scholar] [CrossRef]
- Reverchon, F.; Bai, S.; Liu, X.; Blumfield, T. Tree plantation systems influence nitrogen retention and the abundance of nitrogen functional genes in the Solomon Islands. Front. Microbiol. 2015, 6, 1439. [Google Scholar] [CrossRef]
- Chu, H.; Morimoto, S.; Fujii, T.; Yagi, K.; Nishimura, S. Soil ammonia-oxidizing bacterial communities in paddy rice fields as affected by upland conversion history. Soil Sci. Soc. Am. J. 2009, 73, 2026–2031. [Google Scholar] [CrossRef]
- Pan, H.; Xie, K.; Zhang, Q.; Jia, Z.; Xu, J.; Di, H.; Li, Y. Archaea and bacteria respectively dominate nitrification in lightly and heavily grazed soil in a grassland system. Biol. Fertil. Soils 2018, 54, 41–54. [Google Scholar] [CrossRef]
- Le Roux, X.; Poly, F.; Currey, P.; Commeaux, C.; Hai, B.; Nicol, G.; Prosser, J.; Schloter, M.; Attard, E.; Klumpp, K. Effects of aboveground grazing on coupling among nitrifier activity, abundance and community structure. ISME J. 2008, 2, 221–232. [Google Scholar] [CrossRef]
- Zhong, L.; Zhou, X.; Wang, Y.; Li, F.; Zhou, S.; Bai, Y.; Rui, Y. Mixed grazing and clipping is beneficial to ecosystem recovery but may increase potential N2O emissions in a semi-arid grassland. Soil Biol. Biochem. 2017, 114, 42–51. [Google Scholar] [CrossRef]
- Ding, K.; Zhong, L.; Xin, X.; Xu, Z.; Kang, X.; Liu, W.; Rui, Y.; Jiang, L.; Tang, L.; Wang, Y. Effect of grazing on the abundance of functional genes associated with N cycling in three types of grassland in Inner Mongolia. J. Soils Sediments 2015, 15, 683–693. [Google Scholar] [CrossRef]
- Willems, J.; Marinissen, J.; Blair, J. Effects of earthworms on nitrogen mineralization. Biol. Fertil. Soils 1996, 23, 57–63. [Google Scholar] [CrossRef]
- Blouin, M.; Hodson, M.; Delgado, E.; Baker, G.; Brussaard, L.; Butt, K.; Dai, J.; Dendooven, L.; Peres, G.; Tondoh, J.; et al. A review of earthworm impact on soil function and ecosystem services. Eur. J. Soil Sci. 2013, 64, 161–182. [Google Scholar] [CrossRef]
- Hoang, D.; Pausch, J.; Razavi, B.; Kuzyakova, I.; Banfield, C.; Kuzyakov, Y. Hotspots of microbial activity induced by earthworm burrows, old root channels, and their combination in subsoil. Biol. Fertil. Soils 2016, 52, 1105–1119. [Google Scholar] [CrossRef]
- Lubbers, I.; van Groenigen, K.; Fonte, S.; Six, J.; Brussaard, L.; Van Groenigen, J. Greenhouse gas emissions from soils increased by earthworms. Nat. Clim. Chang. 2013, 3, 187–194. [Google Scholar] [CrossRef]
| Treatment | Nitrogen Fixation | Mineralization | Nitrification | Denitrification | MBC | MBN | Soil N2O Emission | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rate | Bacteria | Rate | Protease | Urease | NH4+-N | Rate | Enzyme | NO3−-N | Rate | Enzyme | |||||
| Climate change | |||||||||||||||
| Warming | −9.1% [−34.7%, 26.5%] | 153.0% [106.9%, 209.4%] | 38.7% [6.4%, 80.7%] | 216.5% [59.5%, 528.0%] | −0.6% [−20.8%, 24.7%] | 62.0% [33.2%, 97.1%] | 8.6% [−14.0%, 37.4%] | 159.7% [127.1%, 196.9%] | −15.1% [−27.4%, −0.7%] | 147.9% [92.2%, 219.7%] | [28,49,58,59] | ||||
| Elevated CO2 | −0.3% [−7.1%, 7.0%] | 32.7% [7.4%, 63.9%] | −18.4% [−32.7%, −1.0%] | 13.1% [4.7%, 22.1%] | 5.3% [−7.7%, 20.1%] | −20.8% [−34.5%, −4.2%] | 1.6% [−20.1%, 29.1%] | 27.8% [16.4%, 40.3%] | 40.6% [25.3%, 57.8%] | [12,22,59] | |||||
| PPT+ | 54.2% [29.5%, 83.7%] | [58,59] | |||||||||||||
| PPT− | −45.9% [−55.9%, −33.6%] | [58,59] | |||||||||||||
| Agricultural practices | |||||||||||||||
| Nitrogen fertilization | −3.0% [−11.3%, 6.2%] | 27.1% [15.0%, 40.5%] | 81.1% [67.3%, 96.2%] | 33.9% [17.4%, 52.9%] | 198.0% [98.4%, 347.8%] 216.0% [99.5%, 400.5%] | 94.2% [68.4%, 123.9%] | 42.0% [9.6%, 84.0%] | 28.5% [2.4%, 61.5%] | 113.7% [−87.2%, 3463%] | 28.4% [7.4%, 53.6%] | 153.2% [109.6%, 205.9%] | [9,27,35,69,70,71,72] | |||
| Biochar addition | 13.2% [2.9%, 24.6%] | 44.4% [29.4%, 61.1%] | 14.4% [1.2%, 29.3%] | 4.0% [−18.2, 32.3%] | 6.0% [1.0%, 11.3%] | 40.8% [1.8%, 94.8%] | 3.4% [0.4%, 6.5%] | 13.3% [−2.9%, 32.2%] | 27.3% [−0.4%, 62.7%] | 13.2% [8.3%, 18.3%] | −15.8% [−20.5%, −10.8%] | [14,40,72] | |||
| Nitrification inhibitor | 87.8% [68.2%, 109.6%] | −21.3% [−26.6%, −15.7%] | −32.8% [−38.8%, −26.4%] | −37.7% [−41.9%, −33.3%] | −25.3% [−34.1, −15.2%] | −56.1% [−64.8%, −45.0%] | [5,30,72] | ||||||||
| Liming | −20.8% [−39.4%, 3.6%] | −3.1% [−24.3%, 23.8%] | 62.7% [27.6%, 107.4%] | 55.8% [35.2%, 79.6%] | 201.1% [94.7%, 365.7%] | −37.9% [−48.2%, −25.5%] | [73] | ||||||||
| Microplastics | 4.9% [−9.9%, 22.1] | 149.2% [112.0%, 192.8%] | 6.8% [2.1%, 11.7%] | −6.8% [−12.8%, −0.4%] | 0.9% [−2.5%, 4.5%] | −22.3% −28.3%, −16.0%] | 17.8% [1.4%, 36.8%] | 27.5% [4.4%, 55.8%] | 140.4% [73.7%, 232.7%] | [74] | |||||
| Crop diversity | −6.6% [−21.1%, 10.5%] | −11.4% [−23.4%, 2.4%] | −24.4% [−35.1%, −12.0%] | −6.8% [−24.6%, 15.1%] | −24.6% [−49.1%, 11.7%] | −19.9% [−32.0%, −5.6%] | [31] | ||||||||
| Grazing | −6.6% [−21.1%, 10.5%] | −11.4% [−23.4%, 2.4%] | −24.4% [−35.1%, −12.0%] | −6.9% [−24.6%, 15.1%] | −24.6% [−49.1%, 11.7%] | −19.9% [−32.0%, −5.6%] | [8] | ||||||||
| Earthworm | 217.8% [45.5%, 594.1%] | 71.2% [31.6%, 122.6%] | 58.5% [−40.9%, 324.7%] | 17.9% [0.4%, 38.6%] | 38.6% [17.8%, 63.0%] 0.3 [0.2,0.5] | 13.7% [0.3%, 28.8%] | 28.9% [0, 68.0%] | [75] | |||||||
| Treatment | Nitrogen Fixation | Nitrification | Denitrification | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| nifH | AOA | AOB | narG | nirS | nirK | norB | norZ | ||
| Climate change | |||||||||
| Warming | −5.1% [−11.3%, 6.7%] | 0.7% [−15.5%, 20.0%] | −1.34% [−22.0%, 24.7%] | 12.7% [−2.3%, 30.0%] | −9.7% [−23.5%, 6.7%] | 50.9% [−6.9%, 144.6%] | [28,49,58,59] | ||
| Elevated CO2 | 5.7% [−9.8%, 23.9%] | 12.8% [−9.5%, 40.6%] | 18.0% [0.4%, 38.6%] | 18.6% [−2.8%, 43.3%] | 2.6% [−16.9%, 26.7%] | [12,22,59] | |||
| PPT+ | −5.1% [−29.9%, 28.4%] | −33.2% [−44.7%, 9.4%] | 31.2% [−9.0%, 89.1%] | −5.0% [−20.9%, 14.2%] | 4.2% [−15.6%, 28.5%] | [58,59] | |||
| PPT− | 28.5% [−20.0%, 106.6%] | 23.4% [−4.1%, 58.8%] | 173.6% [39.5%, 436.5%] | 79.6% [−8.0%, 250.6%] | 48.7% [−19.6%, 175.0%] | [58,59] | |||
| Agricultural practices | |||||||||
| Nitrogen fertilization | −5.9% [−23.5%, 15.6%] | 15.0% [−0.8%, 33.3%] | 146.3% [108.9%, 190.4%] | 31.8% [6.5%, 63.0%] | 43.2% [12.2%, 82.8%] | 38.2% [−6.9%, 105.1%] | [9,27,35,69,70,71,72] | ||
| Biochar addition | 4.7% [−9.8%, 21.6%] | 23.0% [10.1%, 37.4%] | 11.0% [−4.3%, 28.7%] | 17.2% [0.2%, 37.1%] | 19.1% [6.2%, 33.7%] | 28.3% [13.1%, 45.4%] | 17.1% [7.5%, 27.6%] | [14,40,72] | |
| Nitrification inhibitor | −6.3% [−14.0%, 2.1%] | −51.9% [−61.5%, −40.0%] | −4.3% [−52.5%, 93.1%] | −22.7% [−37.9%, −3.9%] | −20.0% [−28.7%, −10.3%] | −0.04% [−16.3%, 19.7%] | [5,30,72] | ||
| Liming | 70.2% [16.2%, 149.2%] | 132.6% [35.2%, 299.9%] | 37.5% [9.9%, 72.0%] | 142.0% [54.3%, 279.6%] | 16.0% [−1.5%, 36.5%] | [73] | |||
| Microplastics | 0.4% [−11.2%, 14.2%] | −5.2% [−8.7%, −1.5%] | 18.6% [5.1%, 34.0%] | −36.8% [−55.5%, −10.1%] | 10.6% [2.7%, 19.1%] | [74] | |||
| Crop diversity | 33.8% [17.3%, 52.2%] | 18.2% [−1.4%, 41.6%] | −2.8% [−12.7%, 8.3%] | 17.8% [5.1%, 32.2%] | 39.4% [18.5%, 63.9%] | 14.0% [0.5%, 29.3%] | 3.8% [−8.4%, 17.5%] | [31] | |
| Grazing | −34.9% [−57.5%, −1.99%] | −28.5% [−57.3, 19.5%] | −28.3% [−40.5%, −14.0%] | −35.3% [−55.3%, −6.1%] | −3.4% [−61.8%, 142.7%] | −22.1% [−42.2%, 4.9%] | [8] | ||
| Earthworm | 27.3% [−7.2%, 74.8%] | 269.6% [−34.1%, 1974.4%] | 10.8% [1.3%, 21.1%] | −12.3% [−32.6%, 14.2%] | 5.1% [−8.0%, 21.0%] | [75] | |||
| Elevated CO2 | Nitrogen Fertilization | Biochar Addition | Nitrification Inhibitor | Liming | Grazing | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Du et al. [12] | You et al. [9] | Zhang et al. [14] | Meng et al. [72] | Zhang et al. [14] | Xiao et al. [40] | Guo et al. [5] | Lei et al. [106] | Yin et al. [30] | Zhang et al. [73] | Yin et al. [8] | |
| Climate | |||||||||||
| MAT | Linear decrease | 32.7 * | |||||||||
| MAP | Concave | 28.8 * | Nonlinear increase | ||||||||
| Soil | |||||||||||
| Soil moisture | Quadratic equation | 5.35% * | |||||||||
| Soil C:N ratio | Linear increase | ||||||||||
| NH4+-N | Linear decrease | −0.33 | 0.214 | ||||||||
| NO3−-N | Quadratic increase | 0.17 | |||||||||
| Available N | 21.0 * | 2.62% | |||||||||
| SOM | Linear decrease | ||||||||||
| pH | Liner decrease | Linear increase | −0.63 | ||||||||
| Soil texture | 0.63% | ||||||||||
| Plant | |||||||||||
| Yield | −0.99 * | ||||||||||
| Vegetation type | 8.6 | ||||||||||
| N functional gene | |||||||||||
| AOA | 11.6 * | −1.623 * | −0.070 | −0.28 * | −0.37 | −0.06 | 0.29 | −0.90 * | |||
| AOB | 10.7 * | −0.653 * | 0.849 * | −0.001 | 0.07 | 0.42 ** | 0.31 * | 0.99 * | |||
| nosZ | 30.7 * | 0.363 | −0.12 | 0.29 | −1.43 * | ||||||
| nirK | 0.965 * | 0.20 | 0.50 * | ||||||||
| nirS | 0.885 * | 0.41 * | 0.13 | ||||||||
| narG | 0.808 * | 0.69 | |||||||||
| Nitrification | Nonlinear * | ||||||||||
| Denitrification | 0.24 | −0.25 * | |||||||||
| Yield-scaled NH3 | 0.48 | ||||||||||
| Treatment | |||||||||||
| N application rate | 12.1 * | Linear increase | |||||||||
| Nitrogen form | 8.2 | ||||||||||
| Grazing intensity | 13.26% * | ||||||||||
| Grazing duration | 0.58% | ||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hui, D.; Ray, A.; Kasrija, L.; Christian, J. Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses. Agriculture 2024, 14, 240. https://doi.org/10.3390/agriculture14020240
Hui D, Ray A, Kasrija L, Christian J. Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses. Agriculture. 2024; 14(2):240. https://doi.org/10.3390/agriculture14020240
Chicago/Turabian StyleHui, Dafeng, Avedananda Ray, Lovish Kasrija, and Jaekedah Christian. 2024. "Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses" Agriculture 14, no. 2: 240. https://doi.org/10.3390/agriculture14020240
APA StyleHui, D., Ray, A., Kasrija, L., & Christian, J. (2024). Impacts of Climate Change and Agricultural Practices on Nitrogen Processes, Genes, and Soil Nitrous Oxide Emissions: A Quantitative Review of Meta-Analyses. Agriculture, 14(2), 240. https://doi.org/10.3390/agriculture14020240







