Selected Carbon and Nitrogen Compounds in a Maize Agroecosystem under the Use of Nitrogen Mineral Fertilizer, Farmyard Manure, Urease, and Nitrification Inhibitors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Design
2.2. Soil Sampling and Analysis
2.3. CO2 and N2O Measurements
2.4. Statistical Analysis
3. Results and Discussion
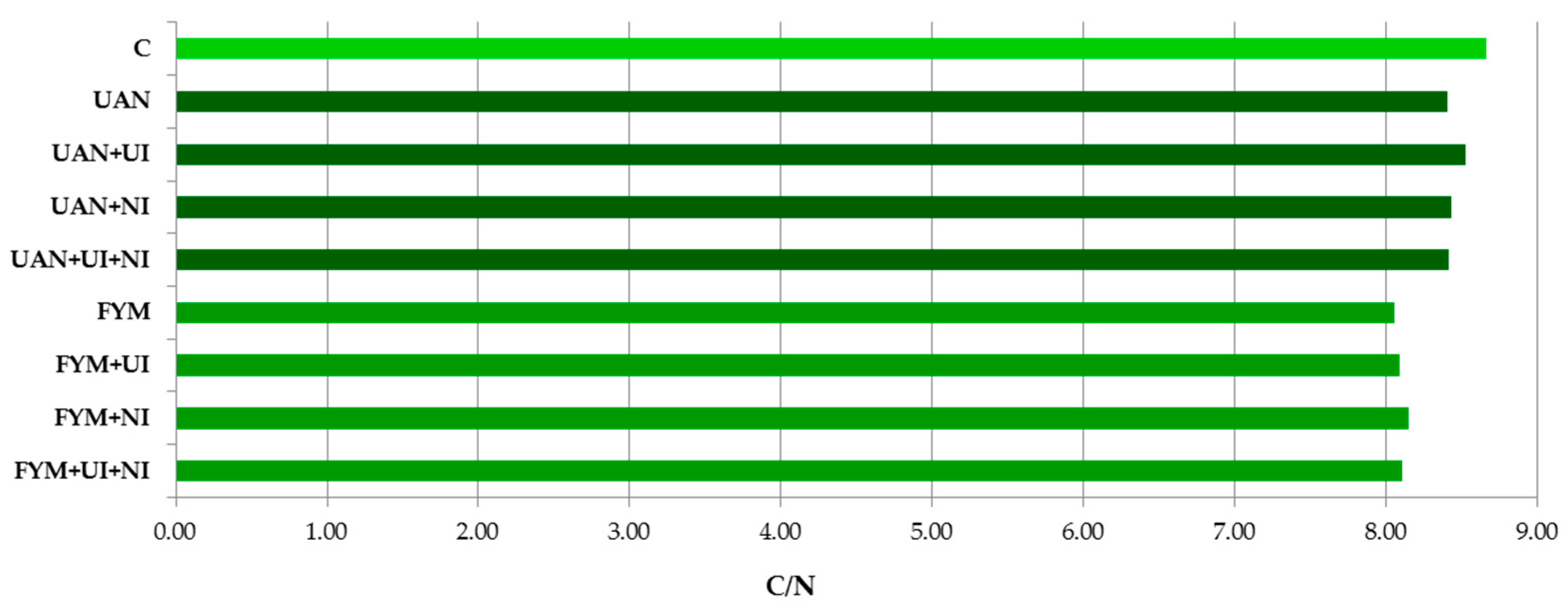
3.1. Soil Organic Carbon and Total Nitrogen in the Soil
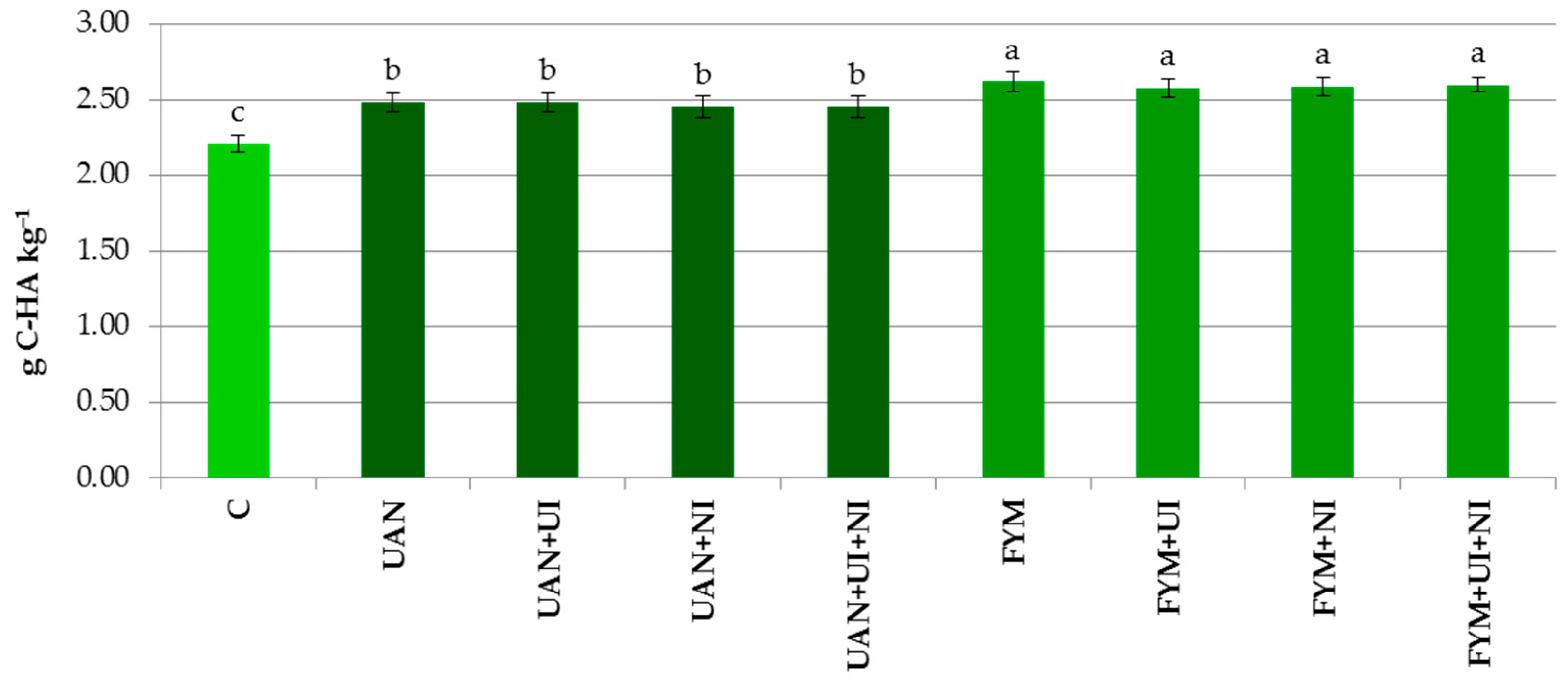
3.2. Humus Fractions in the Soil
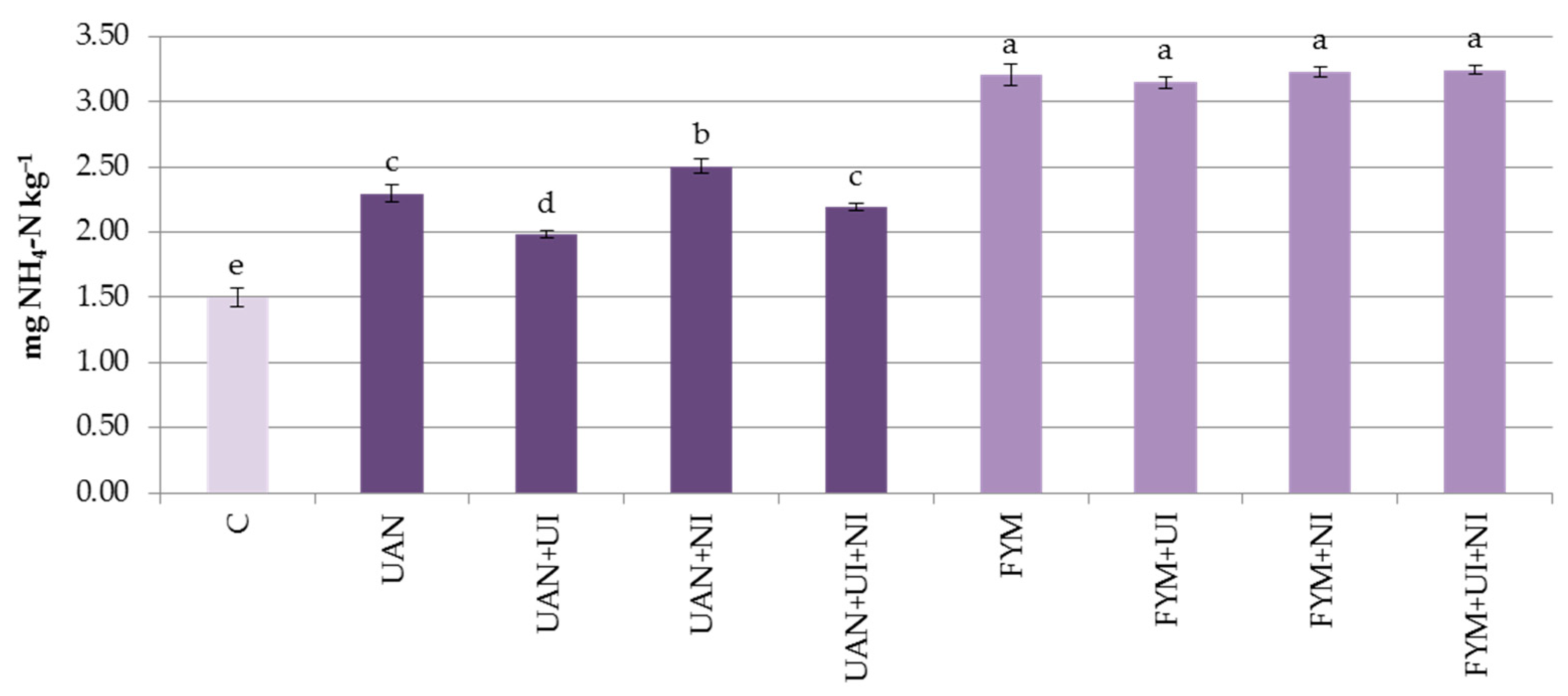
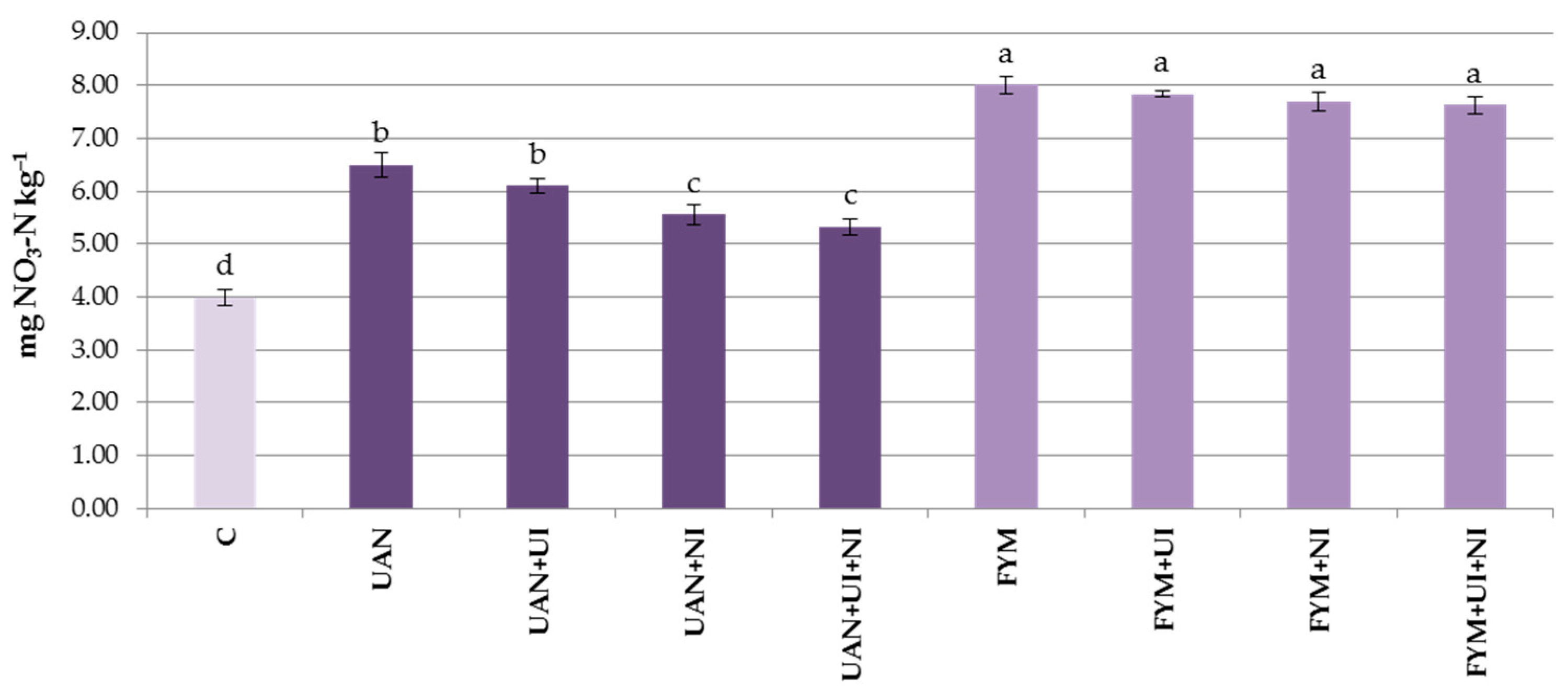
3.3. Mineral Nitrogen in the Soil
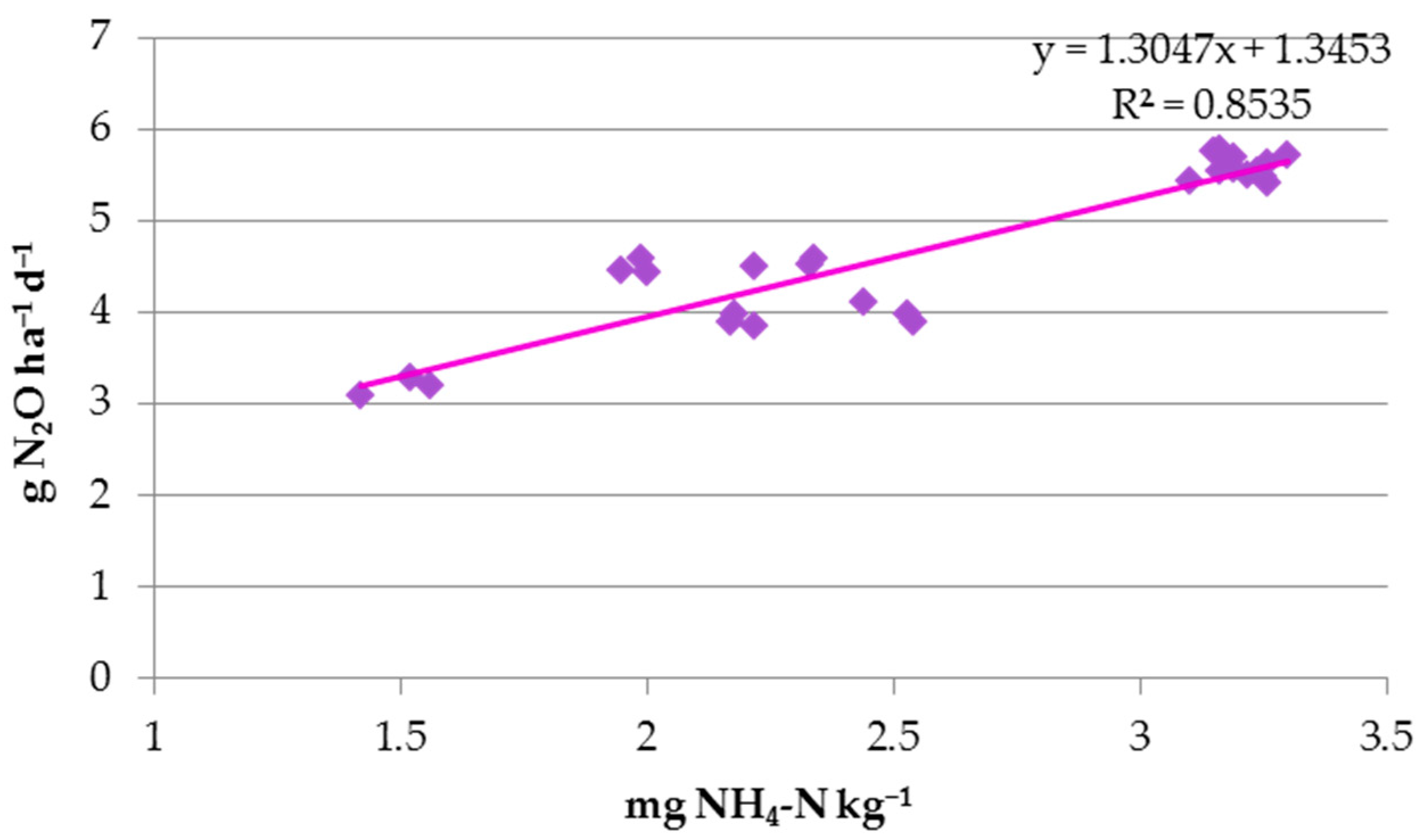
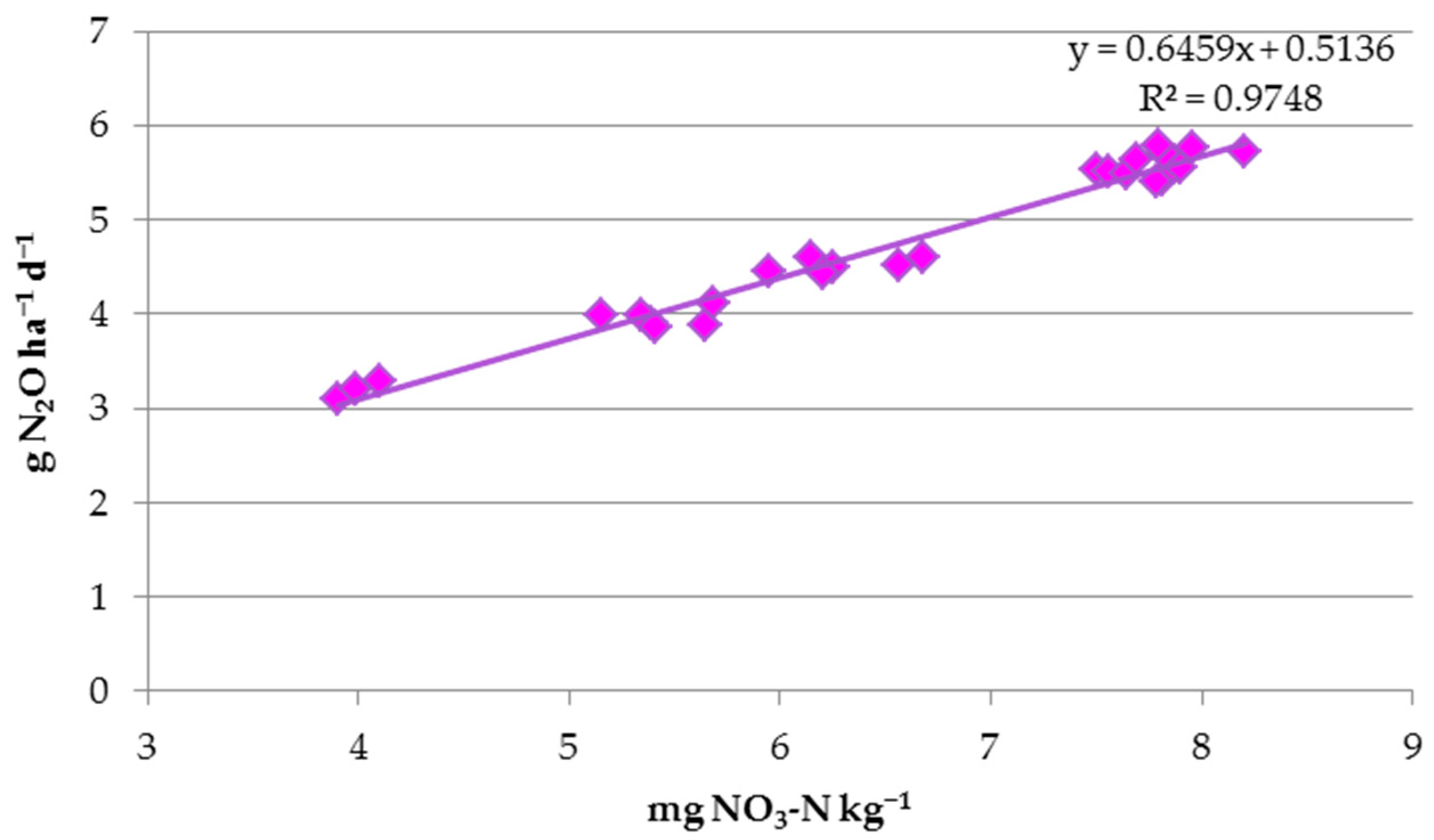
3.4. CO2 and N2O Emissions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kuśmierz, S.; Skowrońska, M.; Tkaczyk, P.; Lipiński, W.; Mielniczuk, J. Soil organic carbon and mineral nitrogen contents in soils as affected by their ph, texture and fertilization. Agronomy 2023, 13, 267. [Google Scholar] [CrossRef]
- Pham, D.M.; Kasai, T.; Yamaura, M.; Katayama, A. Humin: No longer inactive natural organic matter. Chemosphere 2021, 269, 128697. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, W.; Wei, W.; He, Z.; Kuzyakov, Y.; Bol, R.; Hu, R. Soil organic matter priming and carbon balance after straw addition is regulated by long-term fertilization. Soil Biol. Biochem. 2019, 135, 383–391. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, X.; Liang, X.Z.; Jin, V.L.; Birru, G.; Schmer, M.R.; Moglen, G.E. Simulating agroecosystem soil inorganic nitrogen dynamics under long-term management with an improved SWAT-C model. Sci. Total Environ. 2023, 879, 162906. [Google Scholar] [CrossRef]
- Valenzuela, H. Ecological Management of the Nitrogen Cycle in Organic Farms. Nitrogen 2023, 4, 58–84. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Zhang, Y.; Rees, R.; Wang, D.; Rees, R.M.; Ju, X. Using nitrification inhibitors and deep placement to tackle the trade-offs between NH3 and N2O emissions in global croplands. Glob Chang. Biol. 2022, 28, 4409–4422. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Li, D.; Wu, Z.; Xue, Y.; Xiao, F.; Gong, P.; Zhang, L.; Song, Y.; Yu, C.; Du, Y.; et al. Effects of combined nitrification inhibitors on soil nitrification, maize yield and nitrogen use efficiency in three agricultural soils. PLoS ONE 2022, 17, e0272935. [Google Scholar] [CrossRef]
- Mishram, P.K.; Sharma, N.K.; Mandal, D.; Kumar, G.; Kaushal, R.; Das, A.; Singh, R.J. (Eds.) Sustainable Land Management: Issues, Problems and Prospects; Satish Serial Publishing House: Azadpur, Delhi, India, 2019. [Google Scholar]
- Hu, X.; Gu, H.; Liu, J.; Wei, D.; Zhu, P.; Zhou, B.; Wang, G. Metagenomics reveals divergent functional profiles of soil carbon and nitrogen cycling under long-term addition of chemical and organic fertilizers in the black soil region. Geoderma 2022, 418, 115846. [Google Scholar] [CrossRef]
- Harindintwali, J.D.; Zhou, J.; Muhoza, B.; Wang, F.; Herzberger, A.; Yu, X. Integrated eco-strategies towards sustainable carbon and nitrogen cycling in agriculture. J. Environ. Manag. 2021, 293, 112856. [Google Scholar] [CrossRef]
- Skowrońska, M. Influence of waste application on selected soil quality parameters. Chem. Ecol. Eng. 2007, 14, 1073–1082. [Google Scholar]
- Murawska, B.; Spychaj-Fabisiak, E.; Janowiak, J. The influence of minerał fertilization on chosen indicators of soil fertility. Zesz. Probl. Post. Nauk Rol. 2006, 512, 439–447. [Google Scholar]
- Berthrong, S.T.; Buckely, D.H.; Drinkwater, L.E. Agricultural management and labile carbon additions affect soil microbial community structure and interact with carbon and nitrogen cycling. Soil Microbiol. 2013, 66, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.; Glaser, B. Meta-analysis on how manure application changes soil organic carbon storage. Sci. Rep. 2021, 11, 5516. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Tang, J.; Wang, W.; Ma, J.; Shi, J.; Ren, W. Organic amendment effects on cropland soil organic carbon and its implications: A global synthesis. Catena 2023, 231, 107343. [Google Scholar] [CrossRef]
- Li, M.; Hu, H.; He, X.; Jia, J.; Drosos, M.; Wang, G.; Liu, F.; Hu, Z.; Xi, B. Organic carbon sequestration in soil humic substances as affected by application of different nitrogen fertilizers in a vegetable-rotation cropping system. J. Agric. Food Chem. 2019, 67, 3106–3113. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, N.A.; Madramootoo, C.A.; Zhang, T.; Tan, C.S. Soil nutrients and plant uptake parameters as related to greenhouse gas emissions. J. Environ. Qual. 2022, 51, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Chataut, G.; Bhatta, B.; Joshi, D.; Subedi, K.; Kafle, K. Greenhouse gases emission from agricultural soil: A review. J. Agric. Food Res. 2023, 11, 100533. [Google Scholar] [CrossRef]
- Lal, R. Soil management for carbon sequestration. S. Afr. J. Plant Soil. 2021, 38, 231–237. [Google Scholar] [CrossRef]
- Ni, K.; Vietinghoff, M.; Pacholski, A. Targeting yield and reducing nitrous oxide emission by use of single and double inhibitor treated urea during winter wheat season in Northern Germany. Agric. Ecosyst. Environ. 2023, 347, 108391. [Google Scholar] [CrossRef]
- Liu, C.; Liu, H.; Liu, X.; Zhang, Y.; Zang, H.; Li, G.; Pan, B.; Zhang, M.; Li, Z. Nitrogen stabilizers mitigate nitrous oxide emissions across maize production areas of China: A multi-agroecosystems evaluation. Eur. J. Agron. 2023, 143, 126692. [Google Scholar] [CrossRef]
- Poland’s National Inventory Report. Greenhouse Gas Inventory for 1988–2021 Submission under the UN Framework Convention on Climate Change; National Centre for Emission Management (KOBiZE) at the Institute of Environmental Protection–National Research Institute: Warsaw, Poland, 2023. [Google Scholar]
- Akiyama, H.; Yan, X.; Yagi, K. Evaluation of effectiveness of enhanced-efficiency fertilizers as mitigation options for N2O and NO emissions from agricultural soils: Meta-analysis. Glob. Chang. Biol. 2009, 16, 1837–1846. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Wang, S.; Hou, W.; Yan, L. The combined use of liquid fertilizer and urease/nitrification inhibitors on maize yield, nitrogen loss and utilization in the Mollisol region. Plants 2023, 12, 1486. [Google Scholar] [CrossRef]
- Gupta, S.; Yildirim, S.; Andrikopoulos, B.; Wille, U.; Roessner, U. Deciphering the interactions in the root–soil nexus caused by urease and nitrification inhibitors: A review. Agronomy 2023, 13, 1603. [Google Scholar] [CrossRef]
- Zaman, M.; Nguyen, M.L. How application timings of urease and nitrification inhibitors affect N losses from urine patches in pastoral system. Agric. Ecosyst. Environ. 2012, 156, 37–48. [Google Scholar] [CrossRef]
- Chen, M.; Schievano, A.; Bosco, S.; Montero-Castano, A.; Tamburini, G.; Pérez-Soba, M.; Makowski, D. Evidence map of the benefits of enhanced-efficiency fertilisers for the environment, nutrient use efficiency, soil fertility, and crop production. Environ. Res. Lett. 2023, 18, 043005. [Google Scholar] [CrossRef]
- Jones, J.B., Jr.; Case, V.V. Sampling, handling, and analyzing plant tissue samples. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; SSSA Book Series 3; Soil Science Society of America: Madison, WI, USA, 1990. [Google Scholar]
- Schnitzer, M.; Shuppli, P. Method for sequential exctraction of organic matter from soils and soil fractions. Soil. Sci. Am. J. 1989, 53, 1418–1424. [Google Scholar] [CrossRef]
- Abdalla, K.; Sun, Y.; Zarebanadkouki, M.; Gaiser, T.; Seidel, S.; Pausch, J. Long-term continuous farmyard manure application increases soil carbon when combined with mineral fertilizers due to lower priming effects. Geoderma 2022, 428, 116216. [Google Scholar] [CrossRef]
- Singh, P.; Bijay-Singh; Farmaha, B.S. Nutrient management impacts on organic carbon pool in soils under different cropping systems in the Indo-Gangetic Plains in South Asia. Proc. Indian Natl. Sci. Acad. 2023, 89, 520–559. [Google Scholar] [CrossRef]
- Mohan Meena, H.; Sharma, R.P. Long-term effect of fertilizers and amendments on different fractions of organic matter in an Acid Alfisol. Commun. Soil Sci. Plant Anal. 2016, 47, 1430–1440. [Google Scholar] [CrossRef]
- Šimanský, V.; Juriga, M.; Jonczak, J.; Uzarowicz, Ł.; Stępień, W. How relationships between soil organic matter parameters and soil structure characteristics are affected by the long-term fertilization of a sandy soil. Geoderma 2019, 342, 75–84. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Klimkowicz-Pawlas, A.; Smreczak, B. Characterization of organic matter fractions in the top layer of soils under different land uses in Central-Eastern Europe. Soil Use Manag. 2019, 35, 595–606. [Google Scholar] [CrossRef]
- Dou, S.; Zhang, J.; Li, K. Effect of organic matter applications on 13C-NMR spectra of humic acids of soil. Eur. J. Soil Sci. 2008, 59, 532–539. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Bejger, R.; Debaene, G.; Smreczak, B. Characterization of soil organic matter individual fractions (fulvic acids, humic acids, and humins) by spectroscopic and electrochemical techniques in agricultural soils. Agronomy 2021, 11, 1067. [Google Scholar] [CrossRef]
- Loura, D.; Kumar, S.; Kumar, P.; Bedwal, S.A. Comparative effect of organic and inorganic sources of nutrients on yield, soil properties, and economics of wheat under rice-wheat cropping system. Indian J. Tradit. Knowl. 2022, 21, 685–694. [Google Scholar]
- Yang, Q.; Liu, P.; Dong, S.; Zhang, J.; Zhao, B. Combined application of organic and inorganic fertilizers mitigates ammonia and nitrous oxide emissions in a maize field. Nutr. Cycl. Agroecosyst. 2020, 117, 13–27. [Google Scholar] [CrossRef]
- Zaman Khan, H.; Iqbal, S.; Akbar, N.; Farrukh Saleem, M.; Iqbal, A. Integrated management of different nitrogen sources for maize production. Pak. J. Agric. Sci. 2013, 50, 55–61. [Google Scholar]
- Shamsuzzaman, S.M.; Hanafi, M.M.H.; Samsuri, A.W.; Halimi, S.M.; Begum, M.; Maisarah, J.N. Impact of nitrification inhibitor with organic manure and urea on nitrogen dynamics and N2O emission in acid sulphate soil. Bragantia 2015, 75, 108–117. [Google Scholar] [CrossRef]
- Ma, H.; Jia, X.; Yang, J.; Liu, J.; Shangguan, Z.; Yan, W. Inhibitors mitigate N2O emissions more effectively than biochar: A global perspective. Sci. Total Environ. 2023, 859, 160416. [Google Scholar] [CrossRef]
- Guardia, G.; Marsden, K.A.; Vallejo, A.; Jones, D.L.; Chadwick, D.R. Determining the influence of environmental and edaphic factors on the fate of the nitrification inhibitors DCD and DMPP in soil. Sci. Total Environ. 2018, 624, 1202–1212. [Google Scholar] [CrossRef]
- Hao, X.; Sun, L.; Zhou, B.; Ma, X.; Wang, S.; Liu, S.; Ji, J.; Kuang, E.; Qiu, S. Change in maize yield, N use efficiencies and climatic warming potential after urea combined with Nitrapyrin and NBPT during the growing season in a black soil. Soil Tillage Res. 2023, 231, 105721. [Google Scholar] [CrossRef]
- Abalos, D.; Jeffery, S.; Sanz-Cobena, A.; Guardia, G.; Vallejo, A. Meta-analysis of the effect of urease and nitrification inhibitors on crop productivity and nitrogen use efficiency. Agric. Ecosyst. Environ. 2014, 189, 136–144. [Google Scholar] [CrossRef]
- Cai, Z.; Gao, S.; Hendratna, A.; Duan, Y.; Xu, M.; Hanson, B.D. Key factors, soil nitrogen processes, and nitrite accumulation affecting nitrous oxide emissions. Soil Sci. Soc. Am. J. 2016, 80, 1560–1571. [Google Scholar] [CrossRef]
- Huérfano, X.; Fuertes-Mendizábal, T.; Duñabeitia, M.K.; González-Murua, C.; Estavillo, J.M.; Menéndez, S. Splitting the application of 3,4-dimethylpyrazole phosphate (DMPP): Influence on greenhouse gases emissions and wheat yield and quality under humid Mediterranean conditions. Eur. J. Agron. 2015, 64, 47–57. [Google Scholar] [CrossRef]
- Sistani, K.R.; Jn-Baptiste, M.; Lovanh, N.; Cook, K.L. Atmospheric emissions of nitrous oxide, methane, and carbon dioxide from different nitrogen fertilizers. J. Environ. Qual. 2011, 40, 1797–1805. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, L.; Tao, R.; Hu, J.; Chu, G. Nitrapyrin addition mitigated CO2 emission from a calcareous soil was closely associated with its effect on decreasing cellulolytic. J. Agric. Food Chem. 2022, 70, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Köbke, S.; Dittert, K. Use of urease and nitrification inhibitors to reduce gaseous nitrogen emissions from fertilizers containing ammonium nitrate and urea. Glob. Ecol. Conserv. 2020, 22, e00933. [Google Scholar] [CrossRef]
- Zheng, J.; Wang, H.; Fan, J.; Zhang, F.; Guo, J.; Liao, Z.; Zhuang, Q. Wheat straw mulching with nitrification inhibitor application improves grain yield and economic benefit while mitigating gaseous emissions from a dryland maize field in northwest China. Field Crops Res. 2021, 265, 108125. [Google Scholar] [CrossRef]
- Dong, D.; Yang, W.; Sun, H.; Kong, S.; Xu, H. Effects of animal manure and nitrification inhibitor on N2O emissions and soil carbon stocks of a maize cropping system in Northeast China. Sci. Rep. 2022, 12, 15202. [Google Scholar] [CrossRef]
- Liu, M.; Song, F.; Yin, Z.; Chen, P.; Zhang, Z.; Qi, Z.; Wang, B.; Zheng, E. Organic fertilizer substitutions maintain maize yield and mitigate ammonia emissions but increase nitrous oxide emissions. Environ. Sci. Pollut. Res. 2023, 30, 53115–53127. [Google Scholar] [CrossRef]
- Halvorson, A.D.; Del Grosso, S.J.; Pozzi Jantalia, C. Nitrogen source effects on soil nitrous oxide emissions from strip-till corn. J. Environ. Qual. 2011, 40, 1775–1786. [Google Scholar] [CrossRef]
- Lasisi, A.A.; Akinremi, O.O.; Kumaragamage, D. Nitrification inhibitor reduces the inhibitory effect of N-(n-butyl) thiophosphoric triamide (NBPT) on the hydrolysis of urea. Soil Sci. Soc. Am. J. 2020, 84, 1782–1794. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Stępień, W.; Szymańska, M. Nitrous oxide emissions from the soil under different fertilization systems on a long-term experiment. Plant Soil Environ. 2014, 60, 481–488. [Google Scholar] [CrossRef]







| Treatment | Mineral N Fertilization | Organic Fertilization | Inhibitor |
|---|---|---|---|
| C | – | – | – |
| UAN | UAN (150 kg N ha−1) | – | – |
| UAN+UI | UAN (150 kg N ha−1 ) | – | NBPT * |
| UAN+NI | UAN (150 kg N ha−1 ) | – | DMPP * |
| UAN+UI+NI | UAN (150 kg N ha−1 ) | – | NBPT+DMPP * |
| FYM | UAN (150 kg N ha−1) | Cattle FYM (129 kg N ha−1) | – |
| FYM+UI | UAN (150 kg N ha−1) | Cattle FYM (129 kg N ha−1) | NBPT ** |
| FYM+NI | UAN (150 kg N ha−1) | Cattle FYM (129 kg N ha−1) | DMPP ** |
| FYM+UI+NI | UAN (150 kg N ha−1) | Cattle FYM (129 kg N ha−1) | NBPT+DMPP ** |
| Parameter | SOC | TN | C-HA | C-FA | C-H | NH4-N | NO3-N | CO2 | N2O |
|---|---|---|---|---|---|---|---|---|---|
| SOC | 0.930 *** | 0.935 *** | 0.750 *** | 0.914 *** | 0.790 *** | 0.818 *** | 0.622 *** | 0.786 *** | |
| TN | 0.930 *** | 0.948 *** | 0.529 *** | 0.942 *** | 0.913 *** | 0.908 *** | 0.794 *** | 0.884 *** | |
| C-HA | 0.935 *** | 0.948 *** | 0.585 *** | 0.965 *** | 0.904 *** | 0.947 *** | 0.833 *** | 0.931 *** | |
| C-FA | 0.750 *** | 0.529 *** | 0.585 *** | ns | ns | ns | ns | ns | |
| C-H | 0.914 *** | 0.942 *** | 0.965 *** | ns | 0.930 *** | 0.918 *** | 0.844 *** | 0.907 *** | |
| NH4-N | 0.790 *** | 0.913 *** | 0.904 *** | ns | 0.930 *** | 0.929 *** | 0.921 *** | 0.924 *** | |
| NO3-N | 0.818 *** | 0.908 *** | 0.947 *** | ns | 0.918 *** | 0.929 *** | 0.932 *** | 0.987 *** | |
| CO2 | 0.622 *** | 0.794 *** | 0.833 *** | ns | 0.84 ***4 | 0.921 *** | 0.932 *** | 0.936 *** | |
| N2O | 0.786 *** | 0.884 *** | 0.931 *** | ns | 0.907 *** | 0.924 *** | 0.987 *** | 0.936 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skowrońska, M.; Kuśmierz, S.; Walczak, J. Selected Carbon and Nitrogen Compounds in a Maize Agroecosystem under the Use of Nitrogen Mineral Fertilizer, Farmyard Manure, Urease, and Nitrification Inhibitors. Agriculture 2024, 14, 274. https://doi.org/10.3390/agriculture14020274
Skowrońska M, Kuśmierz S, Walczak J. Selected Carbon and Nitrogen Compounds in a Maize Agroecosystem under the Use of Nitrogen Mineral Fertilizer, Farmyard Manure, Urease, and Nitrification Inhibitors. Agriculture. 2024; 14(2):274. https://doi.org/10.3390/agriculture14020274
Chicago/Turabian StyleSkowrońska, Monika, Sebastian Kuśmierz, and Jacek Walczak. 2024. "Selected Carbon and Nitrogen Compounds in a Maize Agroecosystem under the Use of Nitrogen Mineral Fertilizer, Farmyard Manure, Urease, and Nitrification Inhibitors" Agriculture 14, no. 2: 274. https://doi.org/10.3390/agriculture14020274





