Habitat Diversity Increases Chrysoperla carnea s.l. (Stephens, 1836) (Neuroptera, Chrysopidae) Abundance in Olive Landscapes
Abstract
1. Introduction
2. Materials and Methods
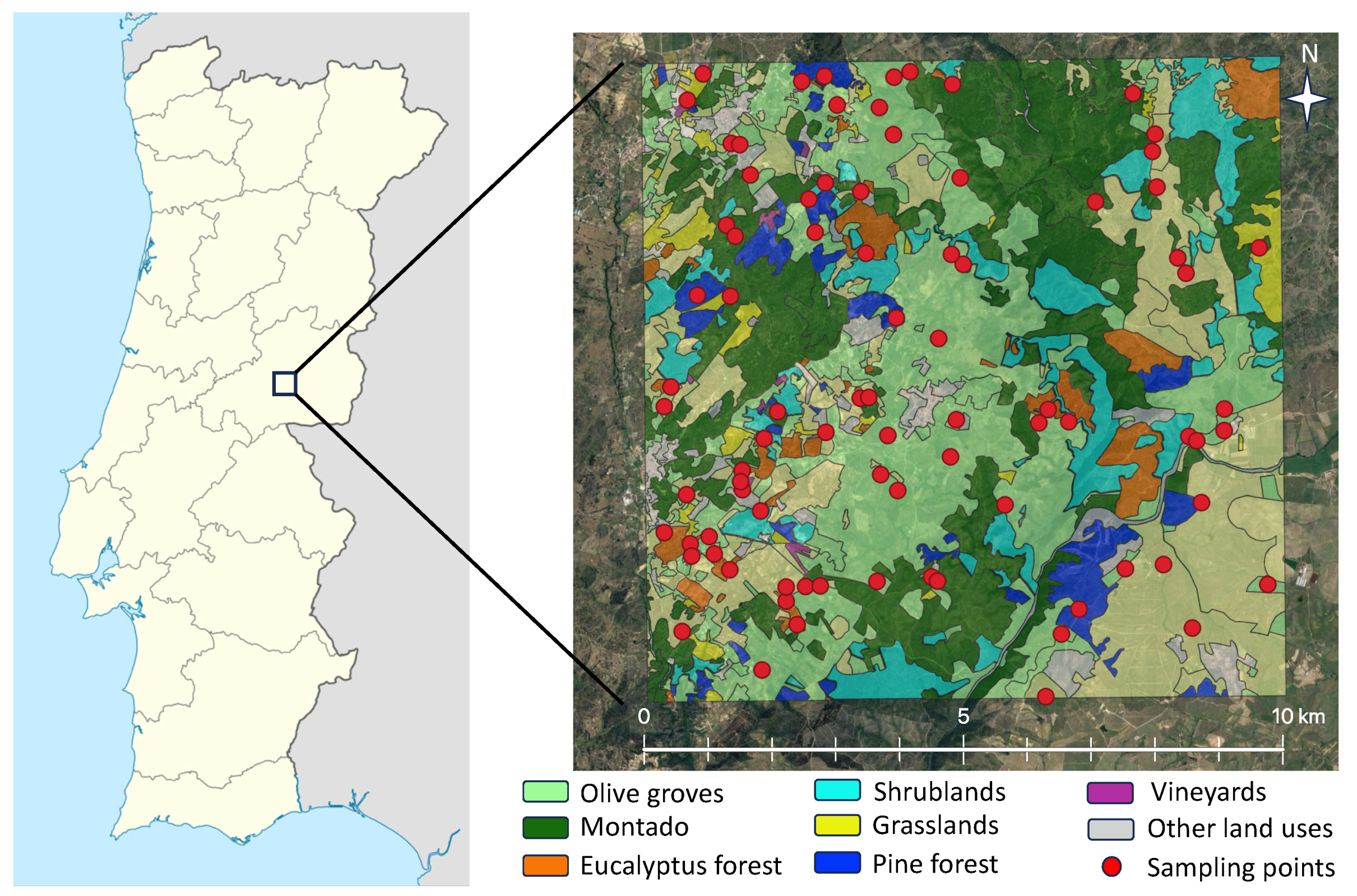
2.1. Study Locations and Insect Sampling
2.2. Landscape Analysis
2.3. Statistical Modelling
3. Results
3.1. Abundance of C. carnea s.l. in Different Habitats
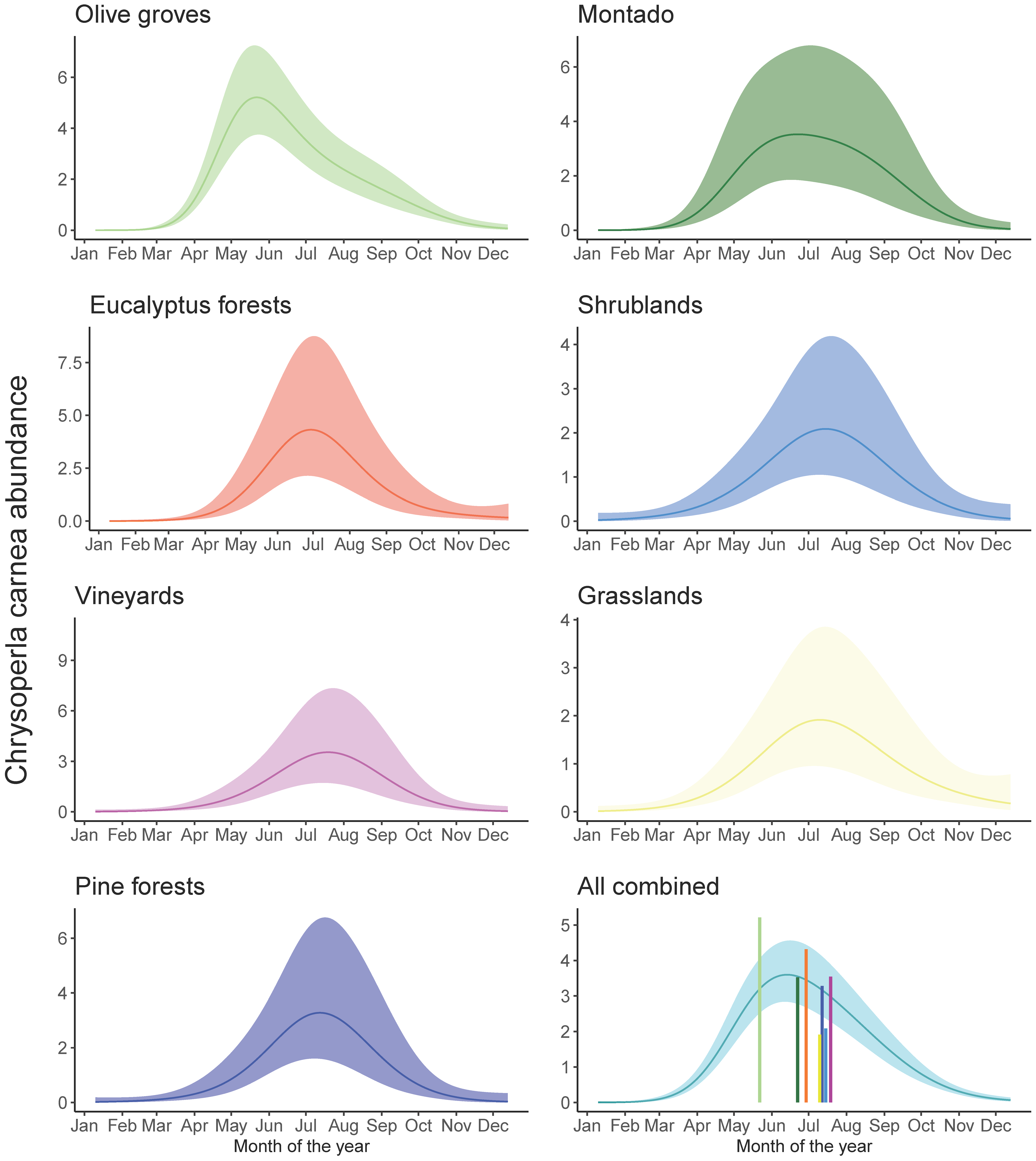
3.2. Temporal Dynamics of C. carnea s.l. in Different Habitats
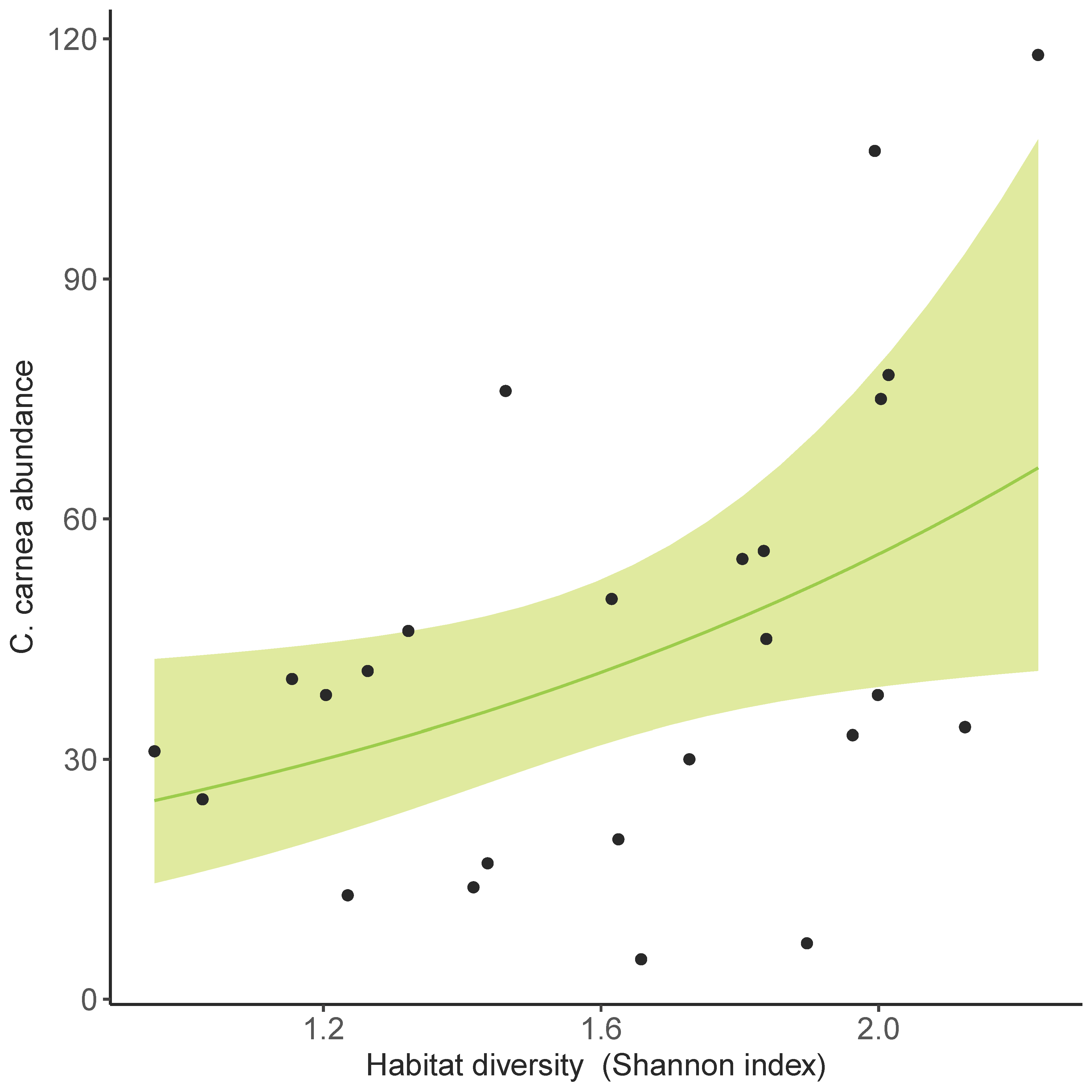
3.3. Land Use Effect on C. carnea s.l. Abundance in Olive Groves
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Foley, J.A.; DeFries, R.; Asner, G.P.; Barford, C.; Bonan, G.; Carpenter, S.R.; Chapin, F.S.; Coe, M.T.; Daily, G.C.; Gibbs, H.K.; et al. Global Consequences of Land Use. Science 2005, 309, 570–574. [Google Scholar] [CrossRef]
- Dirzo, R.; Young, H.S.; Galetti, M.; Ceballos, G.; Isaac, N.J.B.; Collen, B. Defaunation in the Anthropocene. Science 2014, 345, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Paredes, D.; Rosenheim, J.A.; Chaplin-Kramer, R.; Winter, S.; Karp, D.S. Landscape Simplification Increases Vineyard Pest Outbreaks and Insecticide Use. Ecol. Lett. 2021, 24, 73–83. [Google Scholar] [CrossRef]
- Gould, F.; Brown, Z.S.; Kuzma, J. Wicked Evolution: Can We Address the Sociobiological Dilemma of Pesticide Resistance? Science 2018, 360, 728–732. [Google Scholar] [CrossRef]
- European Commission. A Farm to Fork Strategy for a Fair, Healthy and Enviromental-Friendly System; European Commission: Brussels, Belgium, 2020; Volume 21, pp. 1–9. [Google Scholar]
- Thies, C.; Tscharntke, T. Landscape Structure and Biological Control in Agroecosystems. Science 1999, 285, 893–895. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Booij, C.J.H.; Tscharntke, T. Sustainable Pest Regulation in Agricultural Landscapes: A Review on Landscape Composition, Biodiversity and Natural Pest Control. Proc. Royal Soc. B-Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Russell, E.P. Enemies Hypothesis: A Review of the Effect of Vegetational Diversity on Predatory Insects and Parasitoids; The University of Kansas: Lawrence, KS, USA, 1989; Volume 18. [Google Scholar]
- Chaplin-Kramer, R.; O’Rourke, M.E.; Blitzer, E.J.; Kremen, C. A Meta-Analysis of Crop Pest and Natural Enemy Response to Landscape Complexity. Ecol. Lett. 2011, 14, 922–932. [Google Scholar] [CrossRef] [PubMed]
- Landis, D.A.; Wratten, S.D.; Gurr, G.M. Habitat Management to Conserve Natural Enemies of Arthropod Pests in Agriculture. Annu. Rev. Entomol. 2000, 45, 175–201. [Google Scholar] [CrossRef] [PubMed]
- Gurr, G.M.; Wratten, S.D.; Landis, D.A.; You, M. Habitat Management to Suppress Pest Populations: Progress and Prospects. Annu. Rev. Entomol. 2017, 62, 91–109. [Google Scholar] [CrossRef]
- Ferrante, M.; González, E.; Lövei, G.L. Predators Do Not Spill over from Forest Fragments to Maize Fields in a Landscape Mosaic in Central Argentina. Ecol. Evol. 2017, 7, 7699–7707. [Google Scholar] [CrossRef]
- Blitzer, E.J.; Dormann, C.F.; Holzschuh, A.; Klein, A.M.; Rand, T.A.; Tscharntke, T. Spillover of Functionally Important Organisms between Managed and Natural Habitats. Agric. Ecosyst. Environ. 2012, 146, 34–43. [Google Scholar] [CrossRef]
- Thomine, E.; Rusch, A.; Supplisson, C.; Monticelli, L.S.; Amiens-Desneux, E.; Lavoir, A.-V.; Desneux, N. Highly Diversified Crop Systems Can Promote the Dispersal and Foraging Activity of the Generalist Predator Harmonia Axyridis. Entomol. Gen. 2020, 40, 133–145. [Google Scholar] [CrossRef]
- Symondson, W.O.C.; Sunderland, K.D.; Greenstone, M.H. Can Generalist Predators Be Effective Biocontrol Agents? Annu Rev. Entomol. 2002, 47, 561–594. [Google Scholar] [CrossRef] [PubMed]
- Snyder, W.E.; Ives, A.R. Generalist Predators Disrupt Biological Control by a Specialist Parasitoid. Ecology 2001, 82, 705. [Google Scholar] [CrossRef]
- Snyder, W.E. Give Predators a Complement: Conserving Natural Enemy Biodiversity to Improve Biocontrol. Biol. Control. 2019, 135, 73–82. [Google Scholar] [CrossRef]
- Welch, K.D.; Harwood, J.D. Temporal Dynamics of Natural Enemy–Pest Interactions in a Changing Environment. Biol. Control. 2014, 75, 18–27. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Limburg, D.D.; Colfer, R.G. Impact of generalist predators on a biological control agent, Chrysoperla carnea: Direct observations. Ecol. Appl. 1999, 9, 409–417. [Google Scholar] [CrossRef]
- Atlihan, R.; Kaydan, B.; Özgökçe, M.S. Feeding Activity and Life History Characteristics of the Generalist Predator, Chrysoperla Carnea (Neuroptera: Chrysopidae) at Different Prey Densities. J. Pest. Sci. 2004, 77, 17–21. [Google Scholar] [CrossRef]
- Duelli, P. Dispersal and Opposition Strategies in Chrysoperla Carnea. In Progress in World´s Neuroptera; Johann Gepp: Graz, Austria, 1984. [Google Scholar]
- Alves, J.F.; Mendes, S.; da Silva, A.A.; Sousa, J.P.; Paredes, D. Land-Use Effect on Olive Groves Pest Prays Oleae and on Its Potential Biocontrol Agent Chrysoperla Carnea. Insects 2021, 12, 46. [Google Scholar] [CrossRef]
- Paredes, D.; Cayuela, L.; Gurr, G.M.; Campos, M. Effect of Non-Crop Vegetation Types on Conservation Biological Control of Pests in Olive Groves. PeerJ 2013, 2013, e116. [Google Scholar] [CrossRef]
- Paredes, D.; Cayuela, L.; Gurr, G.M.; Campos, M. Single Best Species or Natural Enemy Assemblages? A Correlational Approach to Investigating Ecosystem Function. BioControl 2015, 60, 37–45. [Google Scholar] [CrossRef]
- Kubiak, K.L.; Pereira, J.A.; Tessaro, D.; Santos, S.A.P.; Benhadi-Marín, J. The Assemblage of Beetles in the Olive Grove and Surrounding Mediterranean Shrublands in Portugal. Agriculture 2022, 12, 771. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Cluff, E.; Lippey, M.K.; Cass, B.N.; Paredes, D.; Parsa, S.; Karp, D.S.; Chaplin-Kramer, R. Increasing Crop Field Size Does Not Consistently Exacerbate Insect Pest Problems. Proc. Natl. Acad. Sci. USA 2022, 119, e2208813119. [Google Scholar] [CrossRef] [PubMed]
- Porcel, M.; Ruano, F.; Sanllorente, O.; Caballero, J.A.; Campos, M. Comunicación Corta. Efecto del Trampeo Masivo Tipo OLIPE Sobre Los Artrópodos No Objetivo del Olivar. SJAR 2009, 7, 660–664. [Google Scholar] [CrossRef]
- Bozsik, A.; González-Ruíz, R.; Hurtado-Lara, B. Distribution of the Chrysoperla Carnea Complex in Southern Spain (Neuroptera: Chrysopidae); University of Oradea Publishing House: Oradea, Romania, 2009. [Google Scholar]
- Villa, M.; Somavilla, I.; Santos, S.A.P.; López-Sáez, J.A.; Pereira, J.A. Pollen Feeding Habits of Chrysoperla Carnea s.l. Adults in the Olive Grove Agroecosystem. Agric. Ecosyst. Environ. 2019, 283, 106573. [Google Scholar] [CrossRef]
- Villa, M.; Santos, S.A.P.; Benhadi-Marín, J.; Mexia, A.; Bento, A.; Pereira, J.A. Life-History Parameters of Chrysoperla Carnea s.l. Fed on Spontaneous Plant Species and Insect Honeydews: Importance for Conservation Biological Control. BioControl 2016, 61, 533–543. [Google Scholar] [CrossRef]
- QGIS.org. QGIS Geographic Information System. Open Source Geospatial Foundation Project. 2019. Available online: http://qgis.osgeo.org (accessed on 16 January 2024).
- Benjamini, J.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Series B Stat. Methodol. 1995, 57, 89–300. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference; Springer: New York, NY, USA, 2004. [Google Scholar] [CrossRef]
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. Series B Stat. Methodol. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.M.; Walker, S.C. Fitting Linear Mixed-Effects Models Using Lme4. J. Stat. Softw. 2015, 67, 48. [Google Scholar] [CrossRef]
- Harting F DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 6 November 2023).
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Thomine, E.; Mumford, J.; Rusch, A.; Desneux, N. Using Crop Diversity to Lower Pesticide Use: Socio-Ecological Approaches. Sci. Total Environ. 2022, 804, 150156. [Google Scholar] [CrossRef]
- Gurr, G.M.; Lu, Z.; Zheng, X.; Xu, H.; Zhu, P.; Chen, G.; Yao, X.; Cheng, J.; Zhu, Z.; Catindig, J.L.; et al. Multi-Country Evidence That Crop Diversification Promotes Ecological Intensification of Agriculture. Nat. Plants 2016, 2, 1–4. [Google Scholar] [CrossRef]
- Thiéry, D.; Moreau, J. Relative Performance of European Grapevine Moth (Lobesia botrana) on Grapes and Other Hosts. Oecologia 2005, 143, 548–557. [Google Scholar] [CrossRef]
- Serée, L.; Rouzes, R.; Thiéry, D.; Rusch, A. Temporal Variation of the Effects of Landscape Composition on Lacewings (Chrysopidae: Neuroptera) in Vineyards. Agric. For. Entomol. 2020, 22, 274–283. [Google Scholar] [CrossRef]
- Duelli, P. Lacewings in Field Crops. In Lacewings in the Crop Environment; Cambridge University Press: Cambridge, UK, 2001; pp. 158–171. [Google Scholar]
- Gómez-Guzmán, J.A.; Herrera, J.M.; Rivera, V.; Barreiro, S.; Muñoz-Rojas, J.; García-Ruiz, R.; González-Ruiz, R. A Comparison of IPM and Organic Farming Systems Based on the Efficiency of Oophagous Predation on the Olive Moth (Prays Oleae Bernard) in Olive Groves of Southern Iberia. Horticulturae 2022, 8, 977. [Google Scholar] [CrossRef]
- Alcalá Herrera, R.; Ruano, F. Impact of Woody Semi-Natural Habitats on the Abundance and Diversity of Green Lacewings in Olive Orchards. Biol. Control 2022, 174, 105003. [Google Scholar] [CrossRef]
- Paredes, D.; Karp, D.S.; Chaplin-Kramer, R.; Benítez, E.; Campos, M. Natural Habitat Increases Natural Pest Control in Olive Groves: Economic Implications. J. Pest. Sci. 2019, 92, 1111–1121. [Google Scholar] [CrossRef]
- Dainese, M.; Martin, E.A.; Aizen, M.A.; Albrecht, M.; Bartomeus, I.; Bommarco, R.; Carvalheiro, L.G.; Chaplin-Kramer, R.; Gagic, V.; Garibaldi, L.A.; et al. A Global Synthesis Reveals Biodiversity-Mediated Benefits for Crop Production. Sci. Adv. 2019, 5, eaax0121. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Eckerter, P.W.; Ammann, L.; Entling, M.H.; Gobet, E.; Herzog, F.; Mestre, L.; Tinner, W.; Albrecht, M. Seasonal Shifts and Complementary Use of Pollen Sources by Two Bees, a Lacewing and a Ladybeetle Species in European Agricultural Landscapes. J. App. Ecol. 2019, 56, 2431–2442. [Google Scholar] [CrossRef]
- Fountain, M.T. Impacts of Wildflower Interventions on Beneficial Insects in Fruit Crops: A Review. Insects 2022, 13, 304. [Google Scholar] [CrossRef] [PubMed]
- Karamaouna, F.; Kati, V.; Volakakis, N.; Varikou, K.; Garantonakis, N.; Economou, L.; Birouraki, A.; Markellou, E.; Liberopoulou, S.; Edwards, M. Ground Cover Management with Mixtures of Flowering Plants to Enhance Insect Pollinators and Natural Enemies of Pests in Olive Groves. Agric. Ecosyst. Environ. 2019, 274, 76–89. [Google Scholar] [CrossRef]
- Batáry, P.; Dicks, L.V.; Kleijn, D.; Sutherland, W.J. The Role of Agri-Environment Schemes in Conservation and Environmental Management. Conserv. Biol. 2015, 29, 1006–1016. [Google Scholar] [CrossRef] [PubMed]

| Eucalyptus Forests | Shrublands | Montado | Olive groves | Grasslands | Pine Forests | |
|---|---|---|---|---|---|---|
| Shrublands | 0.465 | |||||
| Montado | 0.178 | 0.031 | ||||
| Olive groves | 0.011 | <0.001 | 0.816 | |||
| Grasslands | 0.297 | 0.557 | 0.014 | <0.001 | ||
| Pine forests | 0.557 | 1.000 | 0.031 | <0.001 | 0.557 | |
| Vineyards | 0.625 | 0.557 | 0.071 | 0.004 | 0.465 | 0.625 |
| Habitat | AICc |
|---|---|
| Null | 236.00 |
| Montado | 238.56 |
| Eucalyptus forests | 237.29 |
| Grasslands | 236.54 |
| Pine forests | 237.44 |
| Shrublands | 237.41 |
| Vineyards | 238.29 |
| Olive groves | 238.59 |
| Habitat diversity (Shannon index) | 233.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paredes, D.; Mendes, S.; Sousa, J.P. Habitat Diversity Increases Chrysoperla carnea s.l. (Stephens, 1836) (Neuroptera, Chrysopidae) Abundance in Olive Landscapes. Agriculture 2024, 14, 298. https://doi.org/10.3390/agriculture14020298
Paredes D, Mendes S, Sousa JP. Habitat Diversity Increases Chrysoperla carnea s.l. (Stephens, 1836) (Neuroptera, Chrysopidae) Abundance in Olive Landscapes. Agriculture. 2024; 14(2):298. https://doi.org/10.3390/agriculture14020298
Chicago/Turabian StyleParedes, Daniel, Sara Mendes, and José Paulo Sousa. 2024. "Habitat Diversity Increases Chrysoperla carnea s.l. (Stephens, 1836) (Neuroptera, Chrysopidae) Abundance in Olive Landscapes" Agriculture 14, no. 2: 298. https://doi.org/10.3390/agriculture14020298
APA StyleParedes, D., Mendes, S., & Sousa, J. P. (2024). Habitat Diversity Increases Chrysoperla carnea s.l. (Stephens, 1836) (Neuroptera, Chrysopidae) Abundance in Olive Landscapes. Agriculture, 14(2), 298. https://doi.org/10.3390/agriculture14020298







