Ion-Exchanged Clinoptilolite as a Substrate for Space Farming
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinoptilolite Ion Exchange
2.2. Clinoptilolite Characterization
2.3. Plant Experiments
2.4. Chlorophyll Fluorescence and Leaf Pigment Measurements
2.5. Biochemical Analyses
2.5.1. Soluble Proteins
2.5.2. Carbohydrates
2.5.3. Polyphenols
2.5.4. Statistical Analysis
3. Results and Discussion
3.1. Effects of Clinoptilolite Ion Exchange on the Structure and Chemical Composition of Zeolite
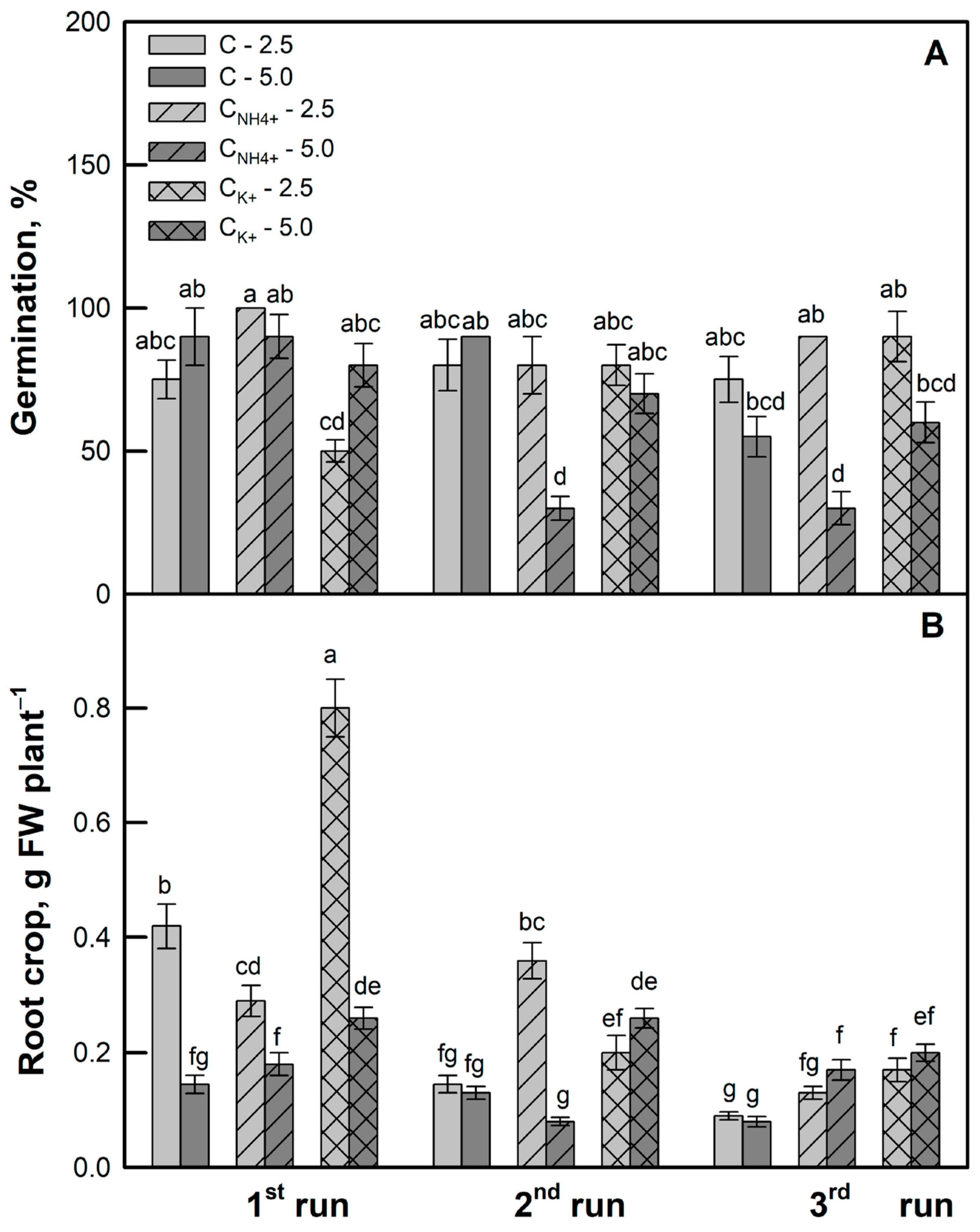
3.2. Effects of Clinoptilolite Particle Size and Ion-Exchange Modification on Seed Germination and Plant Growth
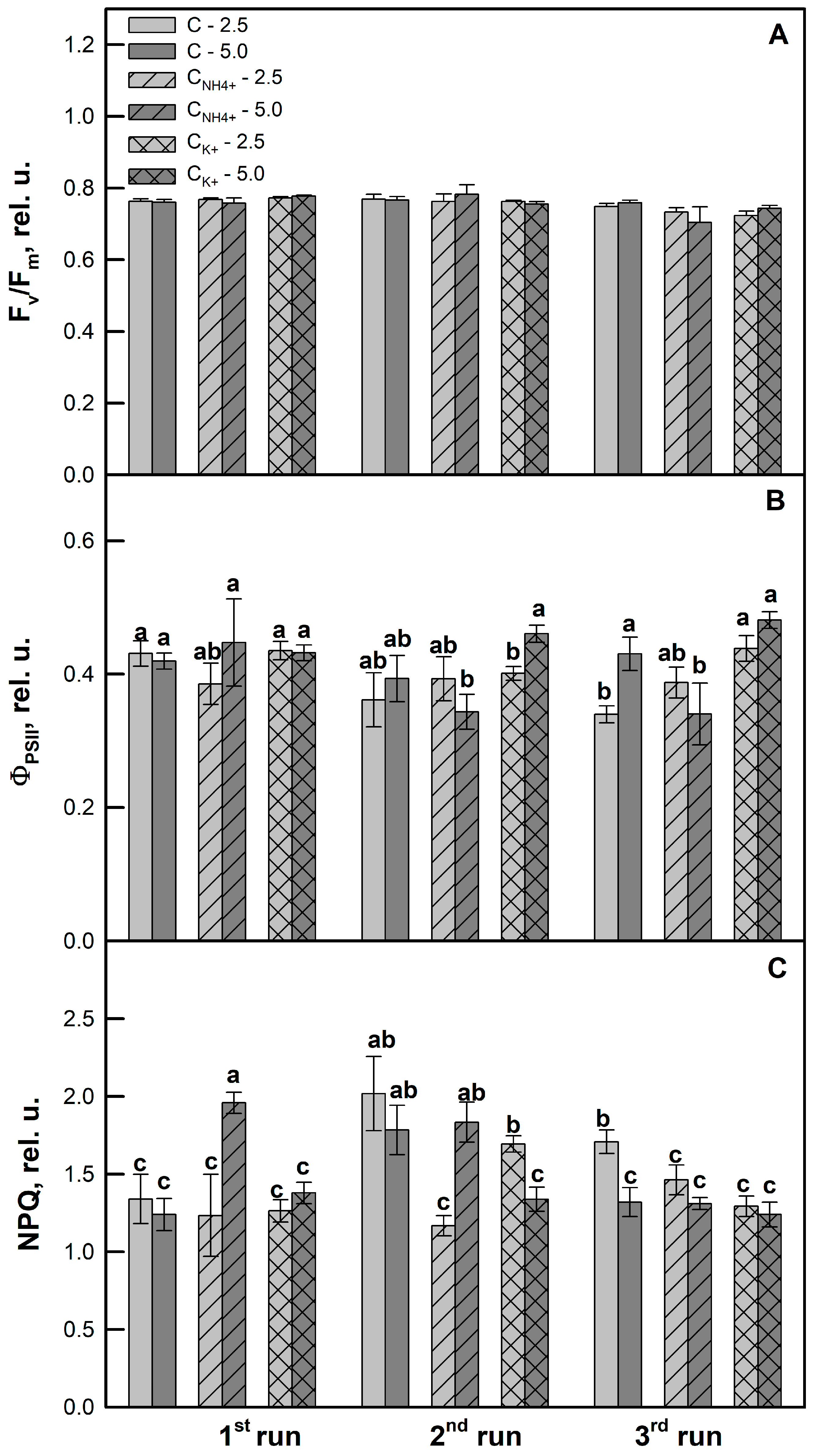
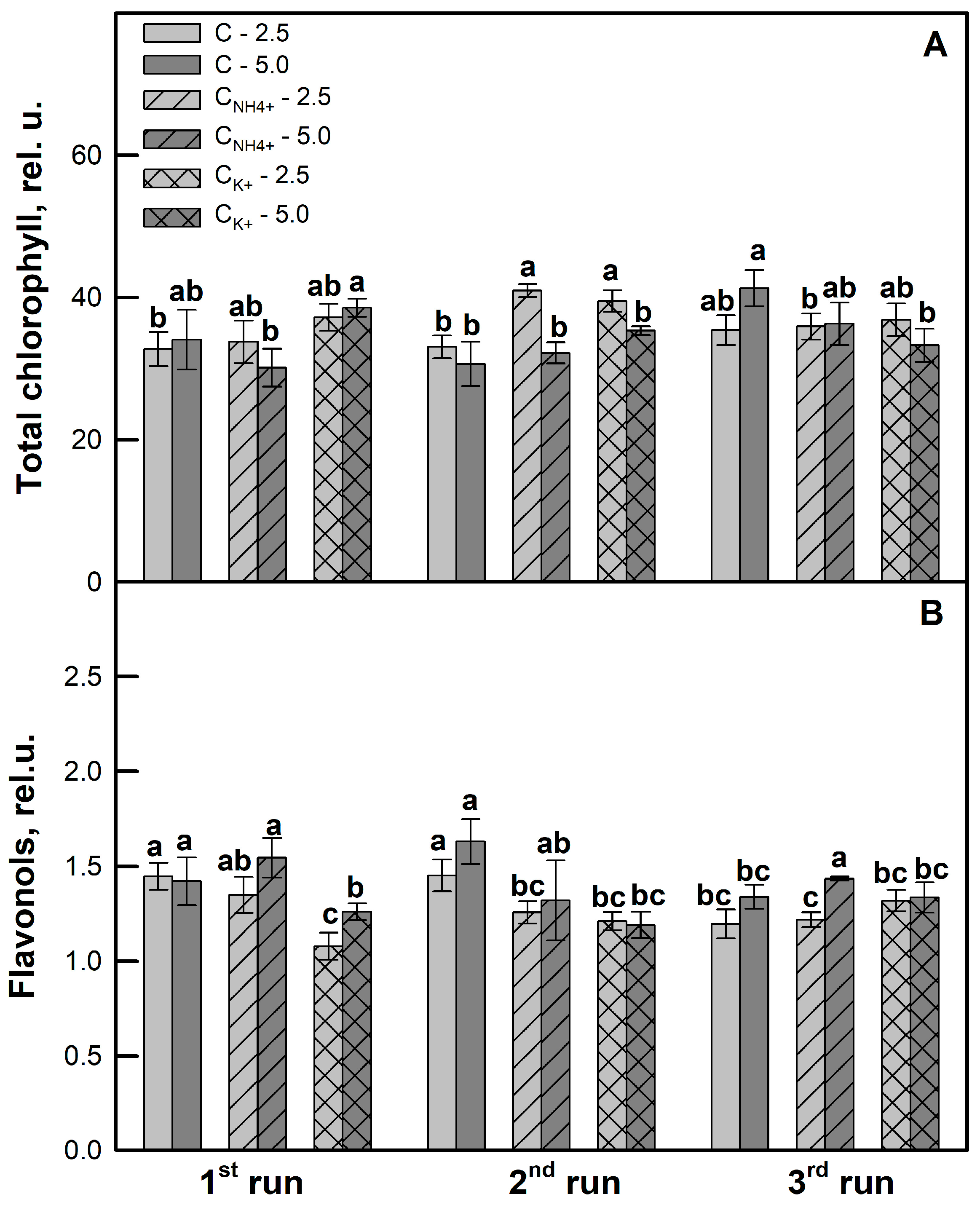
3.3. Effects of Clinoptilolite Particle Size and Ion-Exchange Modification on Photochemistry of Photosynthesis and Leaf Pigment
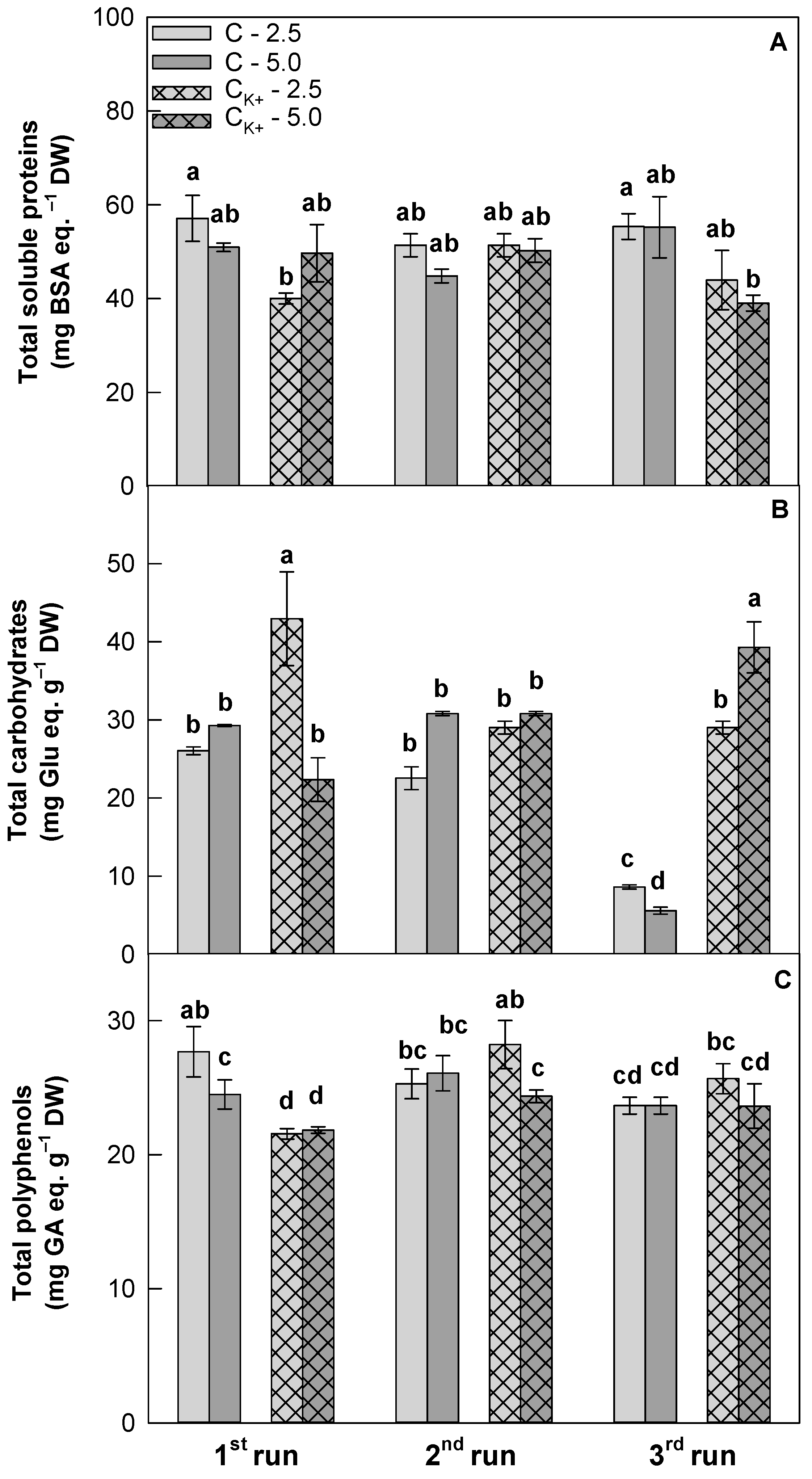
3.4. Effects of Clinoptilolite Particle Size and Ion-Exchange Modification on Plant Biochemical Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Cousins, C.R.; Cockell, C.S. An ESA roadmap for geobiology in space exploration. Acta Astronaut. 2016, 118, 286–295. [Google Scholar] [CrossRef]
- Maggi, F.; Pallud, C. Space agriculture in micro- and hypo-gravity: A comparative study of soil hydraulics and biogeochemistry in a cropping unit on Earth, Mars, the Moon and the space station. Planet. Space Sci. 2010, 58, 1996–2007. [Google Scholar] [CrossRef]
- Mondal, M.; Biswas, B.; Garai, S.; Sarkar, S.; Banerjee, H.; Brahmachari, K.; Bandyopadhyay, P.K.; Maitra, S.; Brestic, M.; Skalicky, M.; et al. Zeolites enhance soil health, crop productivity and environmental safety. Agronomy 2021, 11, 448. [Google Scholar] [CrossRef]
- Gruener, J.E.; Ming, D.W.; Henderson, K.E.; Galindo, C. Common ion effects in zeoponic substrates: Wheat plant growth experiment. Microporous Mesoporous Mater. 2003, 61, 223–230. [Google Scholar] [CrossRef]
- Gruener, J.E.; Ming, D.E.; Galindo, C., Jr.; Henderson, K.E.; Golden, D.C. Plant productivity and characterization of zeoponic substrates after three successive crops of radish (Raphanus sativus L.). Micropor. Mesopor. Mat. 2007, 105, 279–284. [Google Scholar] [CrossRef]
- Ruff, S.W. Spectral evidence for zeolite in the dust on Mars. Icarus 2004, 168, 131–143. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Mustard, J.F.; Swayze, G.A.; Clark, R.N.; Bishop, J.L.; Poulet, F.; Des Marais, D.J.; Roach, L.H.; Milliken, R.E.; Wray, J.J.; et al. Identification of hydrated silicate minerals on Mars using MRO-CRISM: Geologic context near Nili Fossae and implications for aqueous alteration. J. Geophys. Res. Planets 2009, 114, E00D08. [Google Scholar] [CrossRef]
- Carter, J.; Poulet, F.; Bibring, J.-P.; Manold, N.; Murchie, S. Hydrous minerals on Mars as seen by the CRISM and OMEGA imaging spectrometers: Updated global view. IGR Planets 2013, 118, 831. [Google Scholar] [CrossRef]
- Ehlmann, B.L.; Edwards, C.S. Mineralogy of the Martian surface. Annu. Rev. Earth Planet. Sci. 2014, 42, 291. [Google Scholar] [CrossRef]
- Mousis, O.; Simon, J.-M.; Bellat, J.-P.; Schmidt, F.; Bouley, S.; Chassefiere, E.; Sautter, V.; Quesn, Y.; Picaud, S.; Lectez, S. Martian zeolites as a source of atmospheric methane. Icarus 2016, 278, 1–6. [Google Scholar] [CrossRef]
- Barrer, R.M. Zeolites and Clay Minerals as Sorbents and Molecular Sieves; Academic Press: London, UK; New York, NY, USA, 1978. [Google Scholar] [CrossRef]
- Koyama, K.; Takeuchi, Y. Clinoptilolite: The distribution of potassium atoms and its role in thermal stability. Z. Kristallogr. 1977, 145, 216–239. [Google Scholar] [CrossRef]
- Flanigen, E. Molecular sieve zeolite technology—The first twenty-five years. Pure Appl. Chem. 1980, 52, 2191–2211. [Google Scholar] [CrossRef]
- Petrov, G.S.; Petkov, I.A.; Etropolski, H.I.; Dimitrov, D.N.; Popov, N.N.; Uzunov, A.I. Mineralagro. U.S. Patent 4337078, 29 June 1982. [Google Scholar]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Masciandaro, G.; Manzi, D.; Masini, C.M.; Mattii, G.B. Application of zeolites in agriculture and other potential uses: A Review. Agronomy 2021, 11, 1547. [Google Scholar] [CrossRef]
- Belviso, C.; Satriani, A.; Lovelli, S.; Comegna, A.; Coppola, A.; Dragonetti, G.; Cavalcante, F.; Rivelli, A.R. Impact of zeolite from coal fly ash on soil hydrophysical properties and plant growth. Agriculture 2022, 12, 356. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The use of zeolites as an addition to fertilisers—A review. CATENA 2022, 213, 106125. [Google Scholar] [CrossRef]
- Mostara, M.R.; Roy, R.N. Guide to Laboratory Establishment for Plant Nutrient Analysis; Food and Agricultural Organization of the United Nations: Rome, Italy, 2008; 411p. [Google Scholar]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Bilger, W.; Björkman, O. Temperature dependence of violaxanthin de-epoxidation and non-photochemical fluorescence quenching in intact leaves of Gossypium hirsutum L. and Malva Parviflora L. Planta 1991, 184, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Im, J.; Ko, J.; Kim, H.Y.; Ha, B.K. Biochemical responses of soybean (Glycine max L. Merr.) to proton beam irradiation. Plant Breed. Biotechnol. 2017, 5, 97–105. [Google Scholar] [CrossRef]
- Hedge, J.E.; Hofreiter, B.T. Carbohydrate chemistry. In Methods in Carbohydrate Chemistry; Whistler, R.L., Be Miller, J.N., Eds.; Academic Press: New York, NY, USA, 1962; Volume 17. [Google Scholar]
- Vitale, E.; Izzo, L.G.; Amitrano, C.; Velikova, V.; Tsonev, T.; Simoniello, P.; De Micco, V.; Arena, C. Light quality modulates photosynthesis and antioxidant properties of B. vulgaris L. plants from seeds irradiated with high-energy heavy ions: Implications for cultivation in space. Plants 2022, 11, 1816. [Google Scholar] [CrossRef] [PubMed]
- Vitale, E.; Velikova, V.; Tsonev, T.; Ferrandino, I.; Capriello, T.; Arena, C. The interplay between light quality and biostimulant application affects the antioxidant capacity and photosynthetic traits of soybean (Glycine max L. Merrill). Plants 2021, 10, 861. [Google Scholar] [CrossRef] [PubMed]
- Aydin, F.; Zhan, C.; Ritt, C.; Epsztein, R.; Elimelech, M.; Schwegler, E.; Pham, T. Similarities and differences between potassium and ammonium ions in liquid water: A first-principles study. Phys. Chem. Chem. Phys. 2020, 22, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Abou-Issa, F.; Hasenstein, K.H. Space flight cultivation for radish (Raphanus sativus) in the advanced plant habitat. Gravit. Space Res. 2021, 9, 121–132. [Google Scholar] [CrossRef]
- Huang, L.; Dong, B.C.; Xue, W.; Peng, Y.K.; Zhang, M.X.; Yu, F.H. Soil particle heterogeneity affects the growth of a rhizomatous wetland plant. PLoS ONE 2013, 8, e69836. [Google Scholar] [CrossRef] [PubMed]
- Asaduzzamana, M.; Kobayashia, Y.; Mondala, M.D.F.; Band, T.; Matsubarae, H.; Adachia, F.; Asaoa, T. Growing carrots hydroponically using perlite substrates. Sci. Hortic. 2013, 159, 113–121. [Google Scholar] [CrossRef]
- Nazari, F.; Khosh-Khui, M.; Salehi, H.; Eshghi, S. Effect of natural zeolite on vegetative and physiological characteristics of African marigold (Tagetes erecta L. ‘Queen’). Hortic. Environ. Biotechnol. 2007, 48, 241–245. [Google Scholar]
- Rahimi, E.; Nazari, F.; Javadi, T.; Samadi, S.; Teixeira da Silva, J.A. Potassium-enriched clinoptilolite zeolite mitigates the adverse impacts of salinity stress in perennial ryegrass (Lolium perenne L.) by increasing silicon absorption and improving the K/Na ratio. J. Environ. Manag. 2021, 285, 112142. [Google Scholar] [CrossRef]
- Baninasab, B. Effects of the application of natural zeolite on the growth and nutrient status of radish (Raphanus sativus L.). J. Hortic. Sci. Biotech. 2009, 84, 13–16. [Google Scholar] [CrossRef]
- Noori, M.; Zendehdel, M.; Ahmadi, A. Using natural zeolite for the improvement of soil salinity and crop yield. Toxicol. Environ. Chem. 2006, 88, 77–84. [Google Scholar] [CrossRef]
- Costamagna, G.; Chiabrando, V.; Fassone, E.; Mania, I.; Gorra, R.; Ginepro, M.; Giacalone, G. Characterization and use of absorbent materials as slow-release fertilizers for growing strawberry: Preliminary results. Sustainability 2020, 12, 6854. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. [Google Scholar] [CrossRef]
- Wang, M.; Zheng, Q.; Shen, Q.; Guo, S. The critical role of potassium in plant stress response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [PubMed]
- Mitsui, Y.; Shimomura, M.; Komatsu, K.; Namiki, N.; Shibata-Hatta, M.; Imai, M.; Katayose, Y.; Mukai, Y.; Kanamori, H.; Kurita, K.; et al. The radish genome and comprehensive gene expression profile of tuberous root formation and development. Sci. Rep. 2015, 5, 10835. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Gouveia, A.M.; Corrêa, C.V.; de Souza Silva, M.; Zocoler de Mendonça, V.; Galhardo Jorge, L.; Menezes Martins, B.N.; Evangelista, R.M.; Inácio Cardoso, A.I. Macro and micronutrients accumulation in radish (Raphanus sativus L.) subjected to potassium (K) fertilization. Aust. J. Crop Sci. 2018, 12, 1738–1742. [Google Scholar] [CrossRef]
- Tsouvaltzis, P.; Brecht, J.K. Quality and antioxidant activity of radish. J. Food Qual. 2014, 37, 157–167. [Google Scholar] [CrossRef]
- Pushkala, R.; Raghuram, P.K.; Srividya, N. Chitosan based powder coating technique to enhance phytochemicals and shelf life quality of radish shreds. Postharvest Biol. Technol. 2013, 86, 402–408. [Google Scholar] [CrossRef]
- Goyeneche, R.; Roura, S.; Ponce, A.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Uribe, E.; Di Scala, K. Chemical characterization and antioxidant capacity of red radish (Raphanus sativus L.) leaves and roots. J. Funct. Foods 2015, 16, 256–264. [Google Scholar] [CrossRef]
- Bruni, R.; Sacchetti, G. Factors affecting polyphenol biosynthesis in wild and field grown St. John’s wort (Hypericum perforatum L. Hypericaceae/Guttiferae). Molecules 2009, 14, 682–725. [Google Scholar] [CrossRef]
- Borș, M.D.; Semeniuc, C.A.; Socaci, S.; Lârva, L.; Moldovan, O.; Vlaic, R.; Tofană, M. Total phenolic content and antioxidant capacity of radish as influenced by the variety and vegetative stage. Bul. UASVM Food Sci. Tech. 2015, 72, 77–81. [Google Scholar] [CrossRef]
- De Pascale, S.; Arena, C.; Aronne, G.; De Micco, V.; Pannico, A.; Paradiso, R.; Rouphael, Y. Biology and crop production in Space environments: Challenges and opportunities. Life Sci. Space Res. 2021, 29, 30–37. [Google Scholar] [CrossRef]

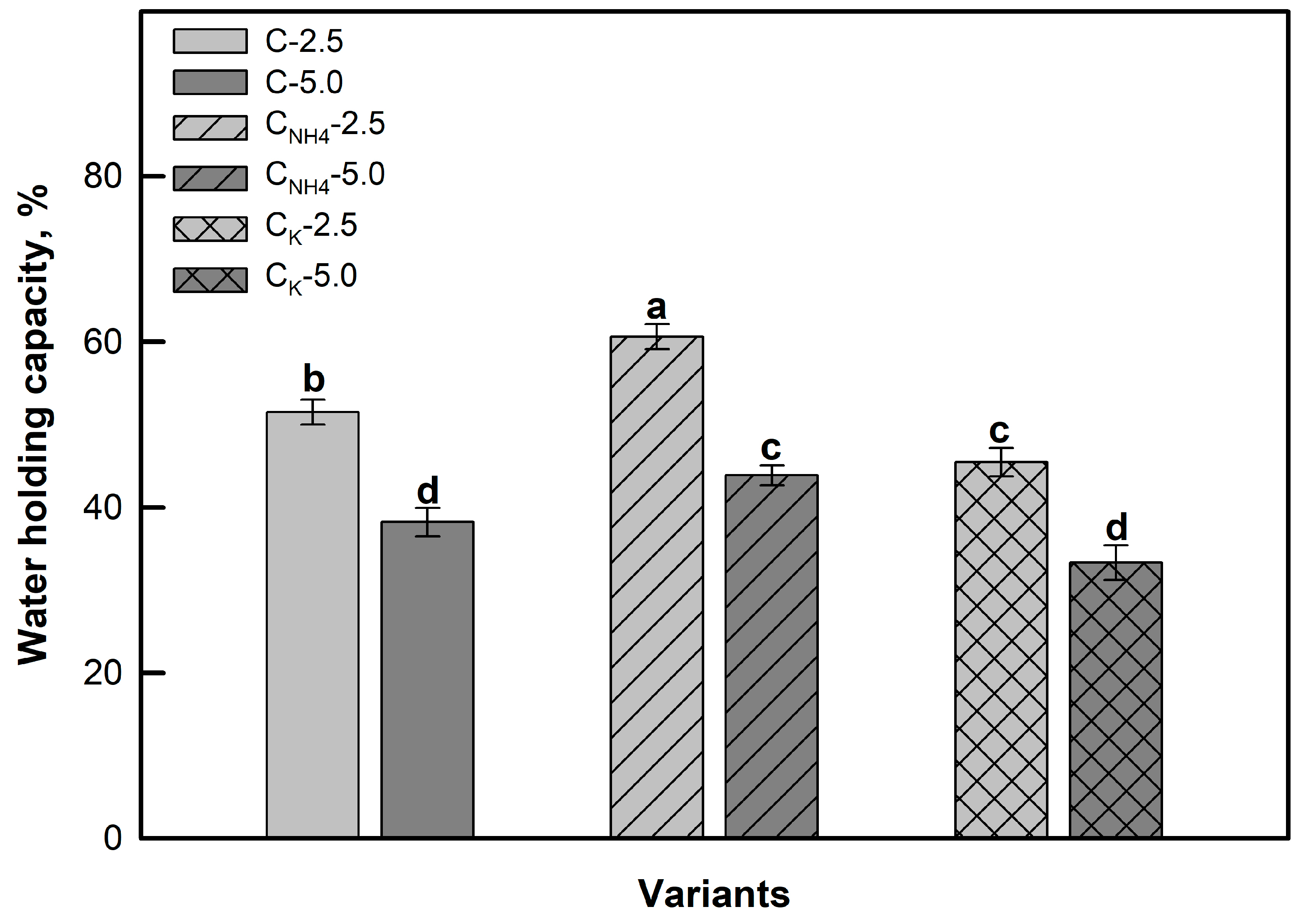
| non-ion-exchanged clinoptilolite with zeolite particle size in the range 0.8–2.5 mm | C-2.5 |
| non-ion-exchanged clinoptilolite with zeolite particle size in the range 2.5–5.0 mm | C-5.0 |
| ion-exchanged clinoptilolite with ammonium nitrate with zeolite particle size in the range 0.8–2.5 mm | CNH4-2.5 |
| ion-exchanged clinoptilolite with ammonium nitrate with zeolite particle size in the range 2.5–5.0 mm | CNH4-5.0 |
| ion-exchanged clinoptilolite with potassium nitrate with zeolite particle size in the range 0.8–2.5 mm | CK-2.5 |
| ion-exchanged clinoptilolite with potassium nitrate with zeolite particle size in the range 2.5–5.0 mm | CK-5.0 |
| Clinoptilolite (C): 0.8–2.5 mm/wt.% | |||||||
|---|---|---|---|---|---|---|---|
| Sample | Al2O3 | CaO | Fe2O3 | MgO | K2O | Na2O | SiO2 |
| C-2.5 | 11.74 | 3.49 | 0.87 | 0.89 | 2.91 | 0.55 | 71.42 |
| C-2.5 after 1st run | 12.00 | 3.42 | 0.84 | 0.90 | 2.94 | 0.46 | 71.31 |
| C-2.5 after 2nd run | 11.80 | 3.45 | 0.84 | 0.93 | 2.88 | 0.45 | 71.23 |
| C-2.5 after 3rd run | 11.85 | 3.48 | 0.85 | 0.95 | 2.82 | 0.44 | 71.48 |
| CNH4-2.5 | 11.85 | 1.61 | 0.85 | 0.77 | 2.67 | 0.22 | 71.68 |
| CNH4-2.5 after 1st run | 11.74 | 1.80 | 0.85 | 0.82 | 2.59 | 0.32 | 71.54 |
| CNH4-2.5 after 2nd run | 11.84 | 1.84 | 0.84 | 0.80 | 2.48 | 0.17 | 71.59 |
| CNH4-2.5 after 3rd run | 11.81 | 1.78 | 0.86 | 0.81 | 2.46 | 0.23 | 71.40 |
| Ck-2.5 | 11.32 | 1.38 | 0.80 | 0.70 | 7.40 | ≤0.01 | 71.38 |
| Ck-2.5 after 1st run | 11.25 | 1.44 | 0.93 | 0.74 | 7.23 | ≤0.01 | 71.35 |
| Ck-2.5 after 2nd run | 11.46 | 1.38 | 0.82 | 0.73 | 7.14 | ≤0.01 | 71.41 |
| Ck-2.5 after 3rd run | 11.57 | 1.34 | 0.85 | 0.75 | 7.08 | ≤0.01 | 71.33 |
| Clinoptilolite (C): 2.5–5.0 mm/wt.% | |||||||
| Sample | Al2O3 | CaO | Fe2O3 | MgO | K2O | Na2O | SiO2 |
| C-5.0 | 11.72 | 3.33 | 0.83 | 0.81 | 3.39 | 0.69 | 71.53 |
| C-5.0 after 1st run | 11.89 | 3.63 | 0.85 | 0.81 | 3.14 | 0.70 | 71.05 |
| C-5.0 after 2nd run | 11.72 | 3.22 | 0.81 | 0.72 | 3.03 | 0.58 | 72.08 |
| C-5.0 after 3rd run | 11.89 | 3.36 | 0.83 | 0.74 | 3.05 | 0.50 | 71.21 |
| CNH4-5.0 | 11.98 | 1.41 | 0.86 | 0.87 | 2.98 | 0.44 | 71.09 |
| CNH4-5.0 after 1st run | 11.76 | 1.51 | 0.87 | 0.77 | 2.90 | 0.35 | 71.22 |
| CNH4-5.0 after 2nd run | 11.78 | 1.73 | 0.84 | 0.75 | 2.81 | 0.36 | 71.30 |
| CNH4-5.0 after 3rd run | 11.89 | 1.55 | 0.86 | 0.72 | 2.58 | 0.25 | 71.29 |
| Ck-5.0 | 12.03 | 1.16 | 0.92 | 0.68 | 10.58 | 0.08 | 70.25 |
| Ck-5.0 after 1st run | 11.77 | 1.44 | 1.06 | 0.79 | 10.12 | 0.05 | 69.60 |
| Ck-5.0 after 2nd run | 11.70 | 1.01 | 0.94 | 0.66 | 8.14 | 0.04 | 71.18 |
| Ck-5.0 after 3rd run | 11.67 | 1.09 | 0.83 | 0.62 | 8.17 | ≤0.01 | 71.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kalvachev, Y.; Vitale, E.; Arena, C.; Todorova, T.; Ilkov, D.; Velikova, V. Ion-Exchanged Clinoptilolite as a Substrate for Space Farming. Agriculture 2024, 14, 350. https://doi.org/10.3390/agriculture14030350
Kalvachev Y, Vitale E, Arena C, Todorova T, Ilkov D, Velikova V. Ion-Exchanged Clinoptilolite as a Substrate for Space Farming. Agriculture. 2024; 14(3):350. https://doi.org/10.3390/agriculture14030350
Chicago/Turabian StyleKalvachev, Yuri, Ermenegilda Vitale, Carmen Arena, Totka Todorova, Daniel Ilkov, and Violeta Velikova. 2024. "Ion-Exchanged Clinoptilolite as a Substrate for Space Farming" Agriculture 14, no. 3: 350. https://doi.org/10.3390/agriculture14030350








