Assessing the Efficacy of Sodium Alginate and Polyacrylamide as Spray Adjuvants Combined with Bifenthrin and Imidacloprid against Lygus lineolaris and Piezodorus guildinii
Abstract
1. Introduction
2. Materials and Methods
2.1. Insect Populations
2.2. Insecticides and Adjuvant Sources
2.3. Laboratory Spray Tower Bioassays
2.4. Data Analysis
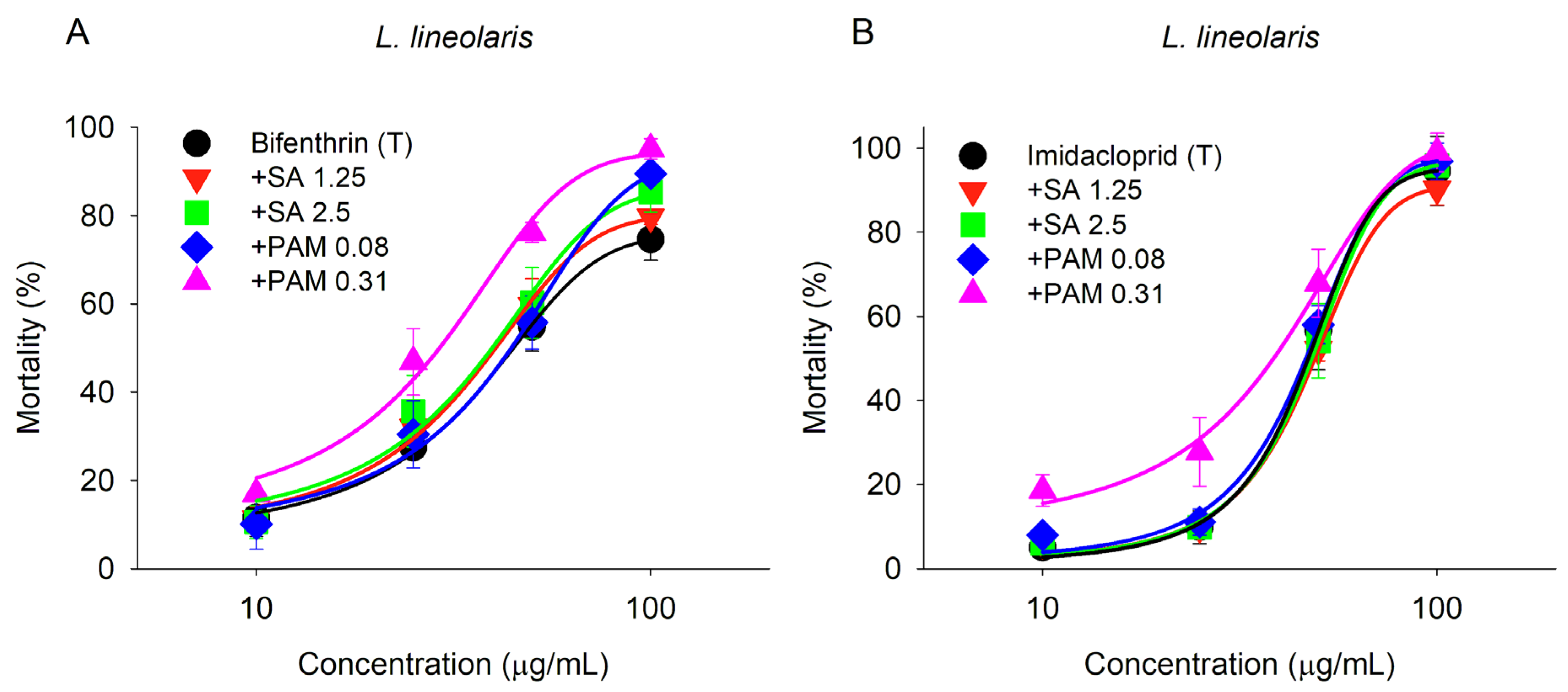
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Layton, M. Biology and damage of the tarnished plant bug, Lygus lineolaris, in cotton. Southwest. Entomol. 2000, 12 (Suppl. 23), 7–20. [Google Scholar]
- George, J.; Glover, J.P.; Gore, J.; Crow, W.D.; Reddy, G.V.P. Biology, ecology, and pest management of the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) in southern row crops. Insects 2021, 12, 807. [Google Scholar] [CrossRef] [PubMed]
- Snodgrass, G.L.; Gore, J.; Abel, C.A.; Jackson, R. Acephate resistance in populations of the tarnished plant bug (Heteroptera: Miridae) from the Mississippi River Delta. J. Econ. Entomol. 2009, 102, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Zucchi, M.I.; Cordeiro, E.M.G.; Allen, C.; Novello, M.; Viana, J.P.G.; Brown, P.J.; Manjunatha, S.; Omoto, C.; Pinheiro, J.B.; Clough, S.J. Patterns of genome-wide variation, population differentiation and SNP discovery of the red banded stink bug (Piezodorus guildinii). Sci. Rep. 2019, 9, 14480. [Google Scholar] [CrossRef] [PubMed]
- Musser, F.; Lorenz, G.; Stewart, S.; Catchot, A., Jr. Soybean insect losses for Mississippi, Tennessee, and Arkansas. Midsouth. Entomol. 2010, 4, 22–28. [Google Scholar]
- Snodgrass, G.L.; Scott, W.P. Seasonal changes in pyrethroid resistance in tarnished plant bug (Heteroptera: Miridae) populations during a three-year period in the delta area of Arkansas, Louisiana, and Mississippi. J. Econ. Entomol. 2000, 93, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.C.; Luttrell, R. Altered gene regulation and potential association with metabolic resistance development to imidacloprid in the tarnished plant bug, Lygus lineolaris. Pest Manag. Sci. 2015, 71, 40–57. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D. Amounts of pesticides reaching target pests: Environmental impacts and ethics. J. Agric. Environ. Ethics. 1995, 8, 17–29. [Google Scholar] [CrossRef]
- Dereumeaux, C.; Fillol, C.; Quenel, P.; Denys, S. Pesticide exposures for residents living close to agricultural lands: A review. Environ. Int. 2020, 134, 105210. [Google Scholar] [CrossRef]
- Zhao, X.; Li, J.; Feng, Y.; Yu, G.; Zhou, Q.; He, F.; Xiao, D.; Chen, K.; Zhang, L. Self-aggregation behavior of hydrophobic sodium alginate derivatives in aqueous solution and their application in the nanoencapsulation of acetamiprid. Int. J. Biol. Macromol. 2018, 106, 418–424. [Google Scholar] [CrossRef]
- Preftakes, C.J.; Schleier, J.J., 3rd; Kruger, G.R.; Weaver, D.K.; Peterson, R.K.D. Effect of insecticide formulation and adjuvant combination on agricultural spray drift. PeerJ 2019, 7, e7136. [Google Scholar] [CrossRef]
- EPA. Introduction to Pesticide Drift. Available online: https://www.epa.gov/reducing-pesticide-drift/introduction-pesticide-drift (accessed on 28 February 2024).
- Oliveira, R.B.d.; Antuniassi, U.R.; Mota, A.A.; Chechetto, R.G. Potential of adjuvants to reduce drift in agricultural spraying. Eng. Agric. 2013, 33, 986–992. [Google Scholar] [CrossRef]
- Shannon, B.; Jeon, H.; Johnson, R.M. The risks of spray adjuvants to honey bees. J. Insect Sci. 2023, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Gaskin, R.; Murray, R.; Krishna, H.; Carpenter, A. Effect of adjuvants on the retention of insecticide spray on cucumber and pea foliage. N. Z. Plant Prot. 2000, 53, 355–359. [Google Scholar] [CrossRef]
- Young, R.; Thacker, J.; Curtis, D. The effects of three adjuvants on the retention of insecticide formulations by cabbage leaves. J. Environ. Sci. Health. B 1996, 31, 165–1778. [Google Scholar] [CrossRef]
- Thacker, J.R.M.; Young, R.D. The effects of six adjuvants on the rainfastness of chlorpyrifos formulated as an emulsifiable concentrate. Pestic. Sci. 1999, 55, 198–200. [Google Scholar] [CrossRef]
- Adjuvant Products with CPDA Certification. Available online: https://cpda.com/cpda-certified-product/ (accessed on 20 December 2023).
- Chen, L.; Yan, Q.; Zhang, J.; Yuan, S.; Liu, X. Joint toxicity of acetamiprid and co-applied pesticide adjuvants on honeybees under semifield and laboratory conditions. Environ. Toxicol. Chem. 2019, 38, 1940–1946. [Google Scholar] [CrossRef]
- Mullin, C.A.; Chen, J.; Fine, J.D.; Frazier, M.T.; Frazier, J.L. The formulation makes the honey bee poison. Pestic. Biochem. Phys. 2015, 120, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Van de Merwe, J.P.; Neale, P.A.; Melvin, S.D.; Leusch, F.D. In vitro bioassays reveal that additives are significant contributors to the toxicity of commercial household pesticides. Aquat. Toxicol. 2018, 199, 263–268. [Google Scholar] [CrossRef]
- Wang, P. Natural and synthetic saponins as vaccine adjuvants. Vaccines 2021, 9, 222. [Google Scholar] [CrossRef]
- Wernecke, A.; Eckert, J.H.; Forster, R.; Kurlemann, N.; Odemer, R. Inert agricultural spray adjuvants may increase the adverse effects of selected insecticides on honey bees (Apis mellifera L.) under laboratory conditions. J. Plant Dis. Protect. 2022, 129, 93–105. [Google Scholar] [CrossRef]
- Zhu, W.; Schmehl, D.R.; Mullin, C.A.; Frazier, J.L. Four common pesticides, their mixtures and a formulation solvent in the hive environment have high oral toxicity to honey bee larvae. PLoS ONE 2014, 9, e77547. [Google Scholar] [CrossRef] [PubMed]
- Abbott, J.R.; Zhu, H.; Jeon, H. Retention and spread capability of impacted droplets with surfactant and hydrocolloid based adjuvants. Trans. ASABE 2021, 64, 1883–1894. [Google Scholar] [CrossRef]
- Chaturvedi, K.; Ganguly, K.; More, U.A.; Reddy, K.R.; Dugge, T.; Naik, B.; Aminabhavi, T.M.; Noolvi, M.N. Sodium alginate in drug delivery and biomedical areas. In Natural Polysaccharides in Drug Delivery and Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 59–100. [Google Scholar]
- Kim, D.; Joung, Y.S. Sodium alginate based artificial biofilms polymerized by electrophoretic deposition for microbial hydrogen generation. Int. J. Biol. Macromol. 2023, 248, 125887. [Google Scholar] [CrossRef] [PubMed]
- Administration U.F.D. CFR—Code of Federal Regulations Title 21. Volume 3. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1724 (accessed on 28 February 2024).
- Bampidis, V.; Azimonti, G.; Bastos, M.d.L.; Christensen, H.; Dusemund, B.; Fašmon Durjava, M.; Kouba, M.; López-Alonso, M.; López Puente, S. Safety and efficacy of a feed additive consisting of sodium alginate for all animal species (ALGAIA). EFSA J. 2022, 20, e07164. [Google Scholar] [PubMed]
- United States Environmental Protection Agency (USEPA), S.R.S.S. Sodium Alginate, CAS Number: 9005-38-3. Available online: https://cdxapps.epa.gov/oms-substance-registry-services/substance-details/162305 (accessed on 21 December 2023).
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan–sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimization and in vitro characterization. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y. Alginate fibres: An overview of the production processes and applications in wound management. Polym. Int. 2008, 57, 171–180. [Google Scholar] [CrossRef]
- Timmons, J. ActivHeal AquaFiber: A new soft, conformable highly-absorbent dressing for use with chronic wounds. Wounds UK 2008, 4, 88–91. [Google Scholar]
- (USFDA), U.S.F.a.D.A. Guidance for Industry Acrylamide in Foods. Office of Food Safety, HFS-300, Center for Food Safety and Applied Nutrition, Food and Drug Administration, College Park, MD 20740 (docket number: FDA-2013-D-0715). 2016. Available online: https://www.fda.gov/media/87150/download (accessed on 20 December 2023).
- Ma, J.; Fu, K.; Fu, X.; Guan, Q.; Ding, L.; Shi, J.; Zhu, G.; Zhang, X.; Zhang, S.; Jiang, L. Flocculation properties and kinetic investigation of polyacrylamide with different cationic monomer content for high turbid water purification. Sep. Purif. Technol. 2017, 182, 134–143. [Google Scholar] [CrossRef]
- Zheng, H.; Sun, Y.; Tang, X.; Tan, M.; Ma, J.; Chen, W.; Liao, Y. UV-Initiated polymerization of cationic polyacrylamide: Synthesis, characterization, and sludge dewatering performance. Sci. World J. 2013, 2013, 937937. [Google Scholar] [CrossRef]
- Dos Santos, L.A.; Carrodeguas, R.G.; Boschi, A.O.; De Arruda, A.C. Dual-setting calcium phosphate cement modified with ammonium polyacrylate. Artif. Organs 2003, 27, 412–418. [Google Scholar] [CrossRef]
- Sepaskhah, A.; Shahabizad, V. Effects of water quality and PAM application rate on the control of soil erosion, water infiltration and runoff for different soil textures measured in a rainfall simulator. Biosyst. Eng. 2010, 106, 513–520. [Google Scholar] [CrossRef]
- Sojka, R.; Bjorneberg, D.; Entry, J.; Lentz, R.; Orts, W. Polyacrylamide in agriculture and environmental land management. Adv. Agron. 2007, 92, 75–162. [Google Scholar]
- Xiong, B.; Loss, R.D.; Shields, D.; Pawlik, T.; Hochreiter, R.; Zydney, A.L.; Kumar, M. Polyacrylamide degradation and its implications in environmental systems. NPJ Clean Water 2018, 1, 17. [Google Scholar] [CrossRef]
- NTP (National Toxicology Program). Report on Carcinogens, Fifteenth Edition. Research Triangle Park, NC: U.S. Department of Health and Human Services, Public Health Service. 2021. Available online: https://ntp.niehs.nih.gov/whatwestudy/assessments/cancer/roc (accessed on 28 February 2024).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Evaluation of Certain Contaminants in Food: Seventy-Second Report of the Joint FAO/WHO Expert Committee on Food Additives. World Health Organization. Available online: https://www.who.int/publications/i/item/9789241209595 (accessed on 28 February 2024).
- Cohen, A.C. New oligidic production diet for Lygus hesperus Knight and L. lineolaris (Palisot de Beauvois). J. Entomol. Sci. 2000, 35, 301–310. [Google Scholar] [CrossRef]
- Portilla, M.; Snodgrass, G.; Streett, D. Effect of modification of the NI artificial diet on the biological fitness parameters of mass reared western tarnished plant bug, Lygus hesperus. J. Insect Sci. 2011, 11, 149. [Google Scholar] [CrossRef]
- Du, Y.; Zhu, Y.C.; Portilla, M.; Zhang, M.; Reddy, G.V.P. The mechanisms of metabolic resistance to pyrethroids and neonicotinoids fade away without selection pressure in the tarnished plant bug Lygus lineolaris. Pest Manag. Sci. 2023, 79, 3892–3902. [Google Scholar] [CrossRef]
- Yin, Y.; Yang, M.; Xi, J.; Cai, W.; Yi, Y.; He, G.; Dai, Y.; Zhou, T.; Jiang, M. A sodium alginate-based nano-pesticide delivery system for enhanced in vitro photostability and insecticidal efficacy of phloxine B. Carbohydr. Polym. 2020, 247, 116677. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhou, Q.; Wang, L.; Yu, G.; Li, J.; Feng, Y. Fabrication of a sustained release delivery system for pesticides using interpenetrating polyacrylamide/alginate/montmorillonite nanocomposite hydrogels. Appl. Clay Sci. 2019, 183, 105347. [Google Scholar] [CrossRef]
- Rust, M.K.; Soeprono, A.; Wright, S.; Greenberg, L.; Choe, D.H.; Boser, C.L.; Cory, C.; Hanna, C. Laboratory and Field Evaluations of Polyacrylamide Hydrogel Baits Against Argentine Ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2015, 108, 1228–1236. [Google Scholar] [CrossRef]
- Silapanuntakul, S.; Keanjoom, R.; Pandii, W.; Boonchuen, S.; Sombatsiri, K. Efficacy of thai need oil against Aedes Aegypti (L.) larvae. Southeast Asian J. Trop. Med. Public Health 2016, 47, 410–415. [Google Scholar]
- Tay, J.W.; Hoddle, M.S.; Mulchandani, A.; Choe, D.H. Development of an alginate hydrogel to deliver aqueous bait for pest ant management. Pest Manag. Sci. 2017, 73, 2028–2038. [Google Scholar] [CrossRef] [PubMed]
- Valladares, G.A.; Gonzalez Audino, P.; Strumia, M.C. Preparation and evaluation of alginate/chitosan microspheres containing pheromones for pest control of Megaplatypus mutatus Chapuis (Platypodinae: Platypodidae). Polym. Int. 2016, 65, 216–223. [Google Scholar] [CrossRef]
- Cedergreen, N. Quantifying synergy: A systematic review of mixture toxicity studies within environmental toxicology. PLoS ONE 2014, 9, e96580. [Google Scholar] [CrossRef] [PubMed]
- Ayub, N.M.; Kassim, N.F.A.; Sabar, S.; Webb, C.E.; Xiang, K.Z.; Hashim, N.A. An efficient and biodegradable alginate-gelatin hydrogel beads as bait against Aedes aegypti and Aedes albopictus. Int. J. Biol. Macromol. 2023, 224, 1460–1470. [Google Scholar] [CrossRef] [PubMed]
- Ishtiaq, F.; Bhatti, H.N.; Khan, A.; Iqbal, M.; Kausar, A. Polypyrole, polyaniline and sodium alginate biocomposites and adsorption-desorption efficiency for imidacloprid insecticide. Int. J. Biol. Macromol. 2020, 147, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Sreenivasan, K. Conjugation of curcumin onto alginate enhances aqueous solubility and stability of curcumin. Carbohydr. Polym. 2014, 99, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, V.; Jayakrishnan, A. Synthesis and evaluation of anti-fungal activities of sodium alginate-amphotericin B conjugates. Int. J. Bio. Macromol. 2018, 108, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Guan, H.; Chi, D.; Yu, J.; Li, X. A novel photodegradable insecticide: Preparation, characterization and properties evaluation of nano-Imidacloprid. Pestic. Biochem. Phys. 2008, 92, 83–91. [Google Scholar] [CrossRef]
- He, Q.; Rezai, T. Measuring the passive cuticular membrane permeability of potassium with a parallel artificial membrane permeability assay and the implications for foliar nutrient formulations. Chem. Biol. Technol. Agric. 2020, 7, 1–5. [Google Scholar] [CrossRef]
- Farajollahi, A.; Williams, G.M. An open-field efficacy trial using AquaDuet via an ultra-low volume cold aerosol sprayer against caged Aedes albopictus. J. Am. Mosq. Control Assoc. 2013, 29, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Bhanjana, G.; Sharma, A.; Sidhu, M.C.; Dilbaghi, N. Synthesis, characterization and on field evaluation of pesticide loaded sodium alginate nanoparticles. Carbohydr. Polym. 2014, 101, 1061–1067. [Google Scholar] [CrossRef] [PubMed]

| Compounds | Population | Slope | LC50 (μg/mL) | 95% Confidence Limits (μg/mL) | χ2 | p | ER |
|---|---|---|---|---|---|---|---|
| Bifenthrin (F) | alone | 4.298 ± 0.538 | 21.78 | 19.28–24.78 | 0.58 | 0.45 | -- |
| +SA 1.25 | 2.714 ± 0.341 | 22.75 | 19.33–27.94 | 1.93 | 0.38 | 0.96 | |
| +SA 2.5 | 2.384 ± 0.254 | 30.16 | 25.71–35.92 | 0.99 | 0.80 | 0.72 | |
| +PAM 0.08 | 2.108 ± 0.254 | 20.59 | 16.70–25.47 | 0.07 | 0.80 | 1.06 | |
| +PAM 0.31 | 2.573 ± 0.299 | 17.48 | 14.88–21.12 | 1.29 | 0.27 | 1.25 | |
| Imidacloprid (F) | alone | 1.806 ± 0.202 | 21.72 | 17.74–27.03 | 0.33 | 0.85 | -- |
| +SA 1.25 | 1.596 ± 0.188 | 21.26 | 16.90–27.25 | 2.93 | 0.23 | 0.85 | |
| +SA 2.5 | 2.597 ± 0.306 | 29.81 | 25.11–35.10 | 1.04 | 0.60 | 0.73 | |
| +PAM 0.08 | 3.712 ± 0.467 | 31.09 | 27.46–35.26 | 1.38 | 0.24 | 0.70 | |
| +PAM 0.31 | 3.721 ± 0.405 | 27.54 | 24.16–31.31 | 1.62 | 0.45 | 0.79 |
| Compounds | Population | Slope | LC50 (μg/mL) | 95% Confidence Limits (μg/mL) | χ2 | p | ER |
|---|---|---|---|---|---|---|---|
| Bifenthrin (T) | alone | 2.740 ± 0.256 | 39.81 | 37.47–46.35 | 1.56 | 0.46 | -- |
| +SA 1.25 | 4.494 ± 0.498 | 46.94 | 42.31–52.18 | 0.48 | 0.49 | 0.85 | |
| +SA 2.5 | 4.897 ± 0.511 | 46.75 | 42.40–51.42 | 0.07 | 0.80 | 0.85 | |
| +PAM 0.08 | 3.041 ± 0.254 | 32.30 | 26.70–35.47 | 0.07 | 0.80 | 1.23 | |
| +PAM 0.31 | 2.879 ± 0.290 | 26.47 | 24.33–29.13 | 1.64 | 0.44 | 1.50 | |
| Imidacloprid (T) | alone | 1.474 ± 0.212 | 39.70 | 31.24–51.28 | 0.53 | 0.77 | -- |
| +SA 1.25 | 2.127 ± 0.218 | 35.56 | 30.30–41.83 | 1.48 | 0.48 | 1.12 | |
| +SA 2.5 | 2.205 ± 0.412 | 31.75 | 26.86–37.53 | 0.35 | 0.84 | 1.25 | |
| +PAM 0.08 | 3.681 ± 0.462 | 32.79 | 29.19–37.19 | 0.10 | 0.80 | 1.21 | |
| +PAM 0.31 | 3.801 ± 0.472 | 27.40 | 24.06–31.32 | 1.53 | 0.22 | 1.45 |
| Compounds | Population | Slope | LC50 (μg/mL) | 95% Confidence Limits (μg/mL) | χ2 | p | ER |
|---|---|---|---|---|---|---|---|
| Bifenthrin (F) | alone | 2.321 ± 0.413 | 26.36 | 21.28–33.71 | 0.07 | 0.79 | -- |
| +SA 1.25 | 2.560 ± 0.392 | 32.15 | 26.84–40.66 | 0.86 | 0.35 | 0.82 | |
| +SA 2.5 | 2.369 ± 0.401 | 40.53 | 32.64–56.87 | 1.43 | 0.23 | 0.65 | |
| +PAM 0.08 | 2.361 ± 0.369 | 22.54 | 18.79–27.56 | 0.10 | 0.75 | 1.17 | |
| +PAM 0.31 | 1.552 ± 0.355 | 19.65 | 15.73–35.65 | 1.39 | 0.24 | 1.34 | |
| Imidacloprid (F) | alone | 1.871 ± 0.356 | 25.54 | 18.76–38.70 | 0.38 | 0.84 | -- |
| +SA1.25 | 2.450 ± 0.452 | 20.63 | 15.81–25.88 | 0.38 | 0.54 | 1.24 | |
| +SA 2.5 | 2.481 ± 0.441 | 30.59 | 24.60–39.70 | 0.90 | 0.34 | 0.80 | |
| +PAM 0.08 | 1.996 ± 0.411 | 20.61 | 15.19–26.95 | 0.04 | 0.85 | 1.23 | |
| +PAM 0.31 | 2.463 ± 0.434 | 22.31 | 17.63–28.09 | 1.04 | 0.31 | 1.14 |
| Compounds | Population | Slope | LC50 (μg/mL) | 95% Confidence Limits (μg/mL) | χ2 | p | ER |
|---|---|---|---|---|---|---|---|
| Bifenthrin (T) | alone | 3.488 ± 0.442 | 48.55 | 42.53–56.72 | 0.02 | 0.89 | -- |
| +SA1.25 | 3.164 ± 0.470 | 43.63 | 37.12–51.63 | 0.43 | 0.51 | 1.11 | |
| +SA 2.5 | 3.206 ± 0.441 | 43.93 | 38.07–51.68 | 0.36 | 0.55 | 1.10 | |
| +PAM 0.08 | 2.642 ± 0.412 | 47.45 | 39.54–57.08 | 0.24 | 0.63 | 1.03 | |
| +PAM 0.31 | 2.278 ± 0.341 | 28.60 | 22.35–35.29 | 2.91 | 0.23 | 1.70 | |
| Imidacloprid (T) | alone | 1.474 ± 0.212 | 39.70 | 31.24–51.28 | 0.53 | 0.77 | -- |
| +SA 1.25 | 1.274 ± 0.388 | 26.86 | 17.45–44.66 | 1.00 | 0.32 | 1.48 | |
| +SA 2.5 | 1.670 ± 0.396 | 14.78 | 8.63–20.16 | 0.19 | 0.66 | 2.68 | |
| +PAM 0.08 | 3.092 ± 0.449 | 22.04 | 18.19–26.35 | 0.95 | 0.33 | 1.80 | |
| +PAM 0.31 | 2.032 ± 0.421 | 14.51 | 9.57–19.00 | 0.48 | 0.49 | 2.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, Y.; Scheibener, S.; George, J.; Kannan, N.; Portilla, M. Assessing the Efficacy of Sodium Alginate and Polyacrylamide as Spray Adjuvants Combined with Bifenthrin and Imidacloprid against Lygus lineolaris and Piezodorus guildinii. Agriculture 2024, 14, 535. https://doi.org/10.3390/agriculture14040535
Du Y, Scheibener S, George J, Kannan N, Portilla M. Assessing the Efficacy of Sodium Alginate and Polyacrylamide as Spray Adjuvants Combined with Bifenthrin and Imidacloprid against Lygus lineolaris and Piezodorus guildinii. Agriculture. 2024; 14(4):535. https://doi.org/10.3390/agriculture14040535
Chicago/Turabian StyleDu, Yuzhe, Shane Scheibener, Justin George, Narayanan Kannan, and Maribel Portilla. 2024. "Assessing the Efficacy of Sodium Alginate and Polyacrylamide as Spray Adjuvants Combined with Bifenthrin and Imidacloprid against Lygus lineolaris and Piezodorus guildinii" Agriculture 14, no. 4: 535. https://doi.org/10.3390/agriculture14040535
APA StyleDu, Y., Scheibener, S., George, J., Kannan, N., & Portilla, M. (2024). Assessing the Efficacy of Sodium Alginate and Polyacrylamide as Spray Adjuvants Combined with Bifenthrin and Imidacloprid against Lygus lineolaris and Piezodorus guildinii. Agriculture, 14(4), 535. https://doi.org/10.3390/agriculture14040535








