Physical Traits and Phenolic Compound Diversity in Maize Accessions with Blue-Purple Grain (Zea mays L.) of Mexican Races
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Reagents and Standards Used
2.3. Physical Grain Analysis
2.4. Phenolic Composition of Maize Grain
2.5. Anthocyanin Profiling of Grain Accessions
2.6. Analysis of the Data
3. Results and Discussion
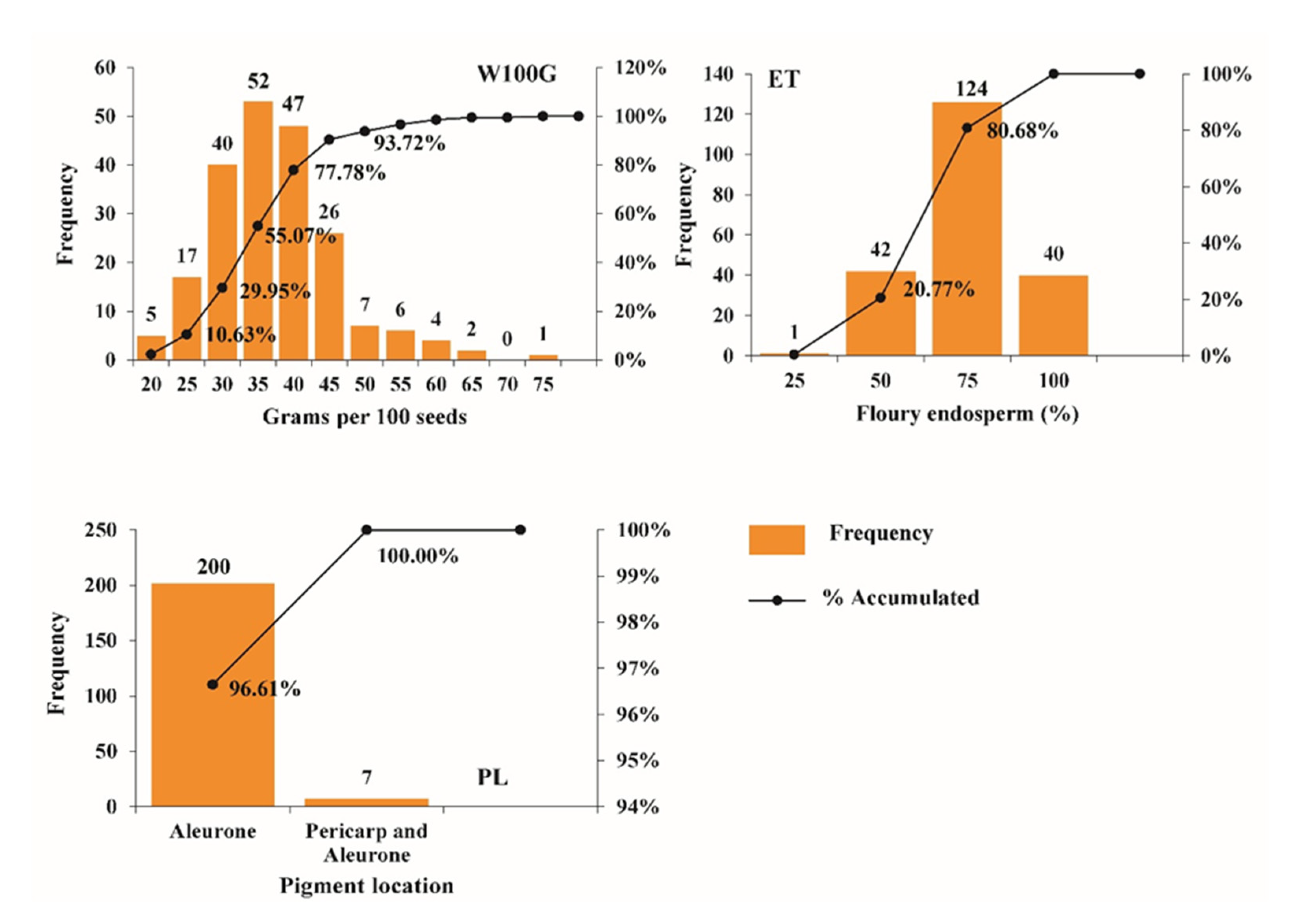
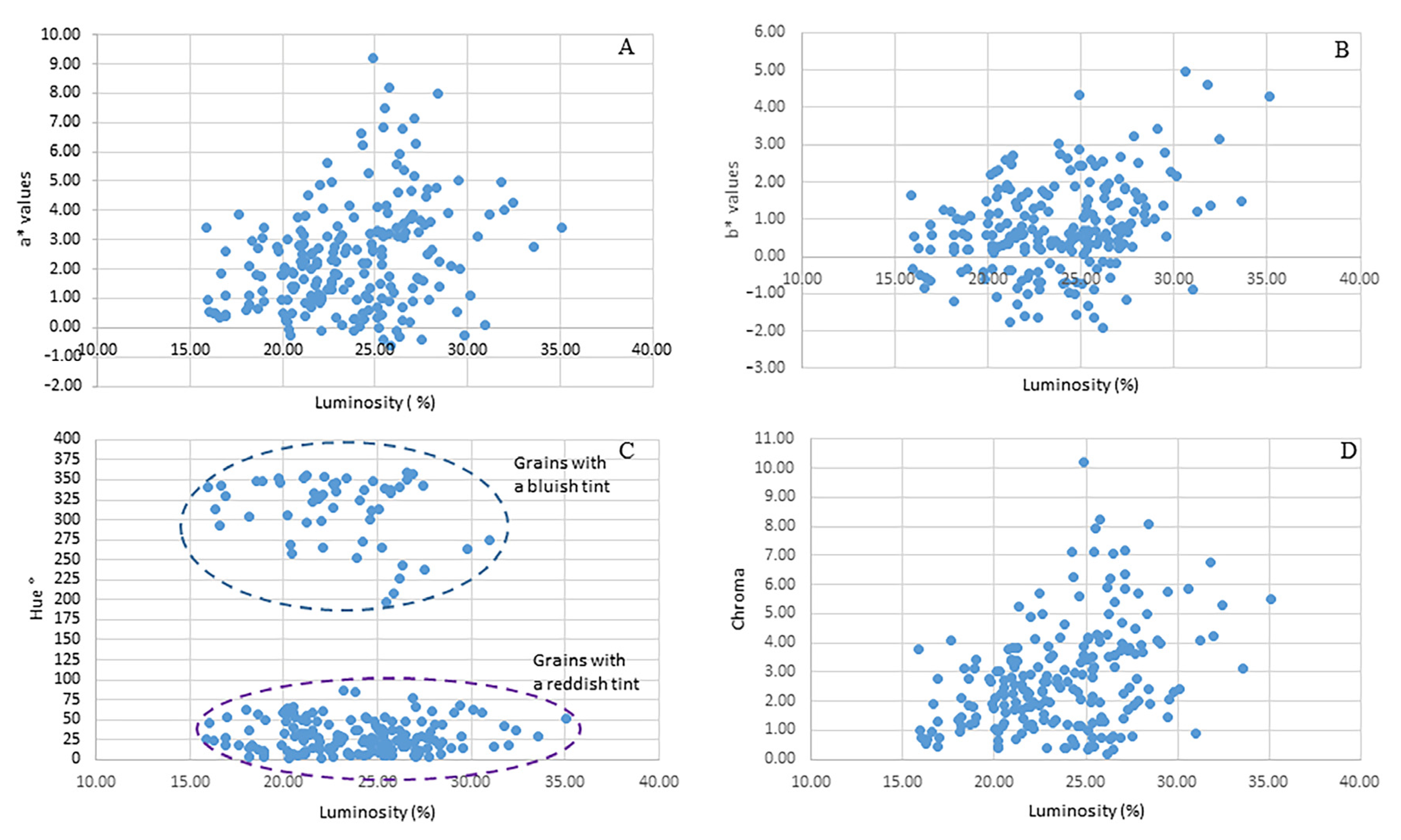
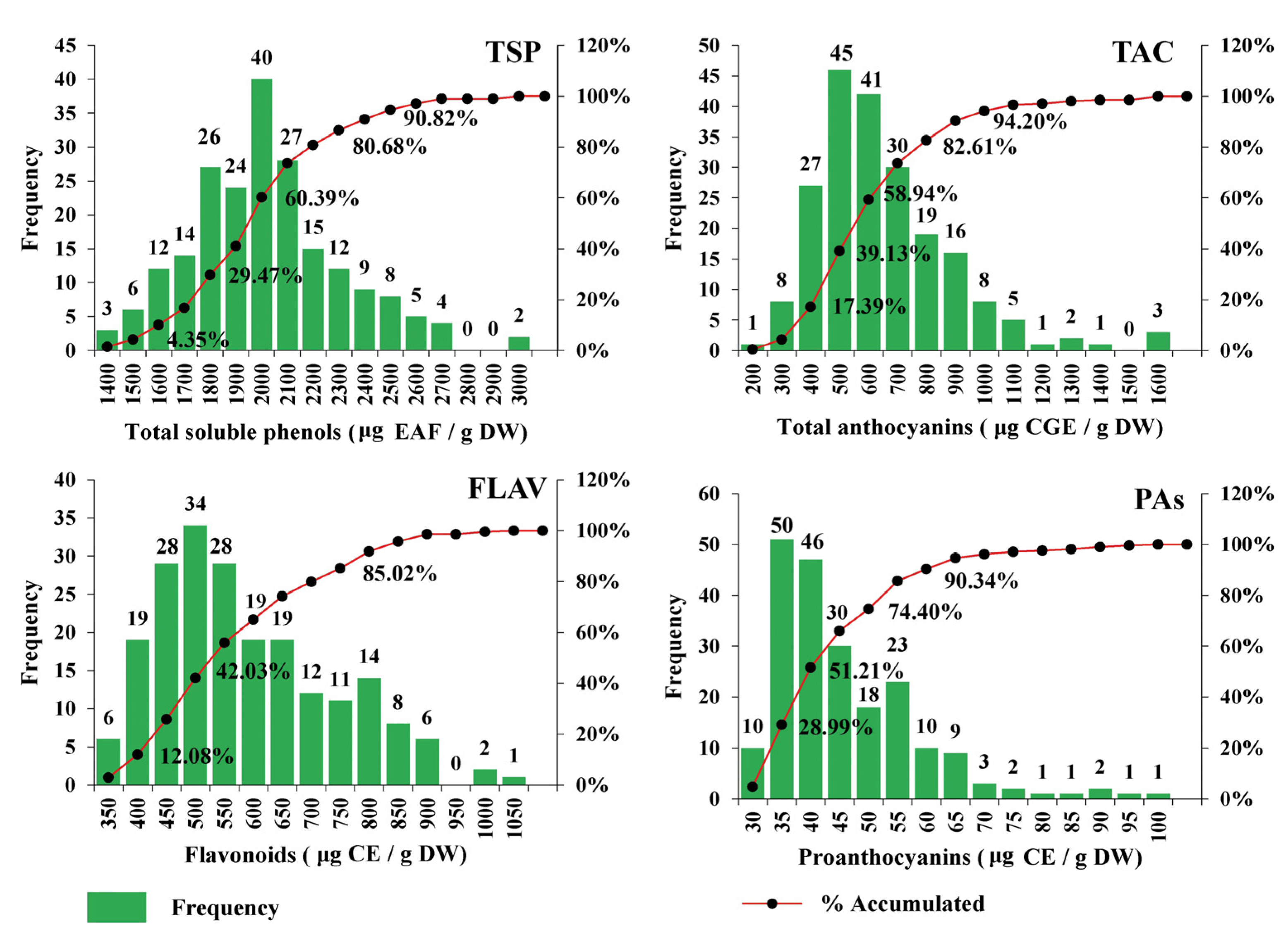
3.1. Physical Traits of Maize Grain and Phenolic Composition of the Accessions
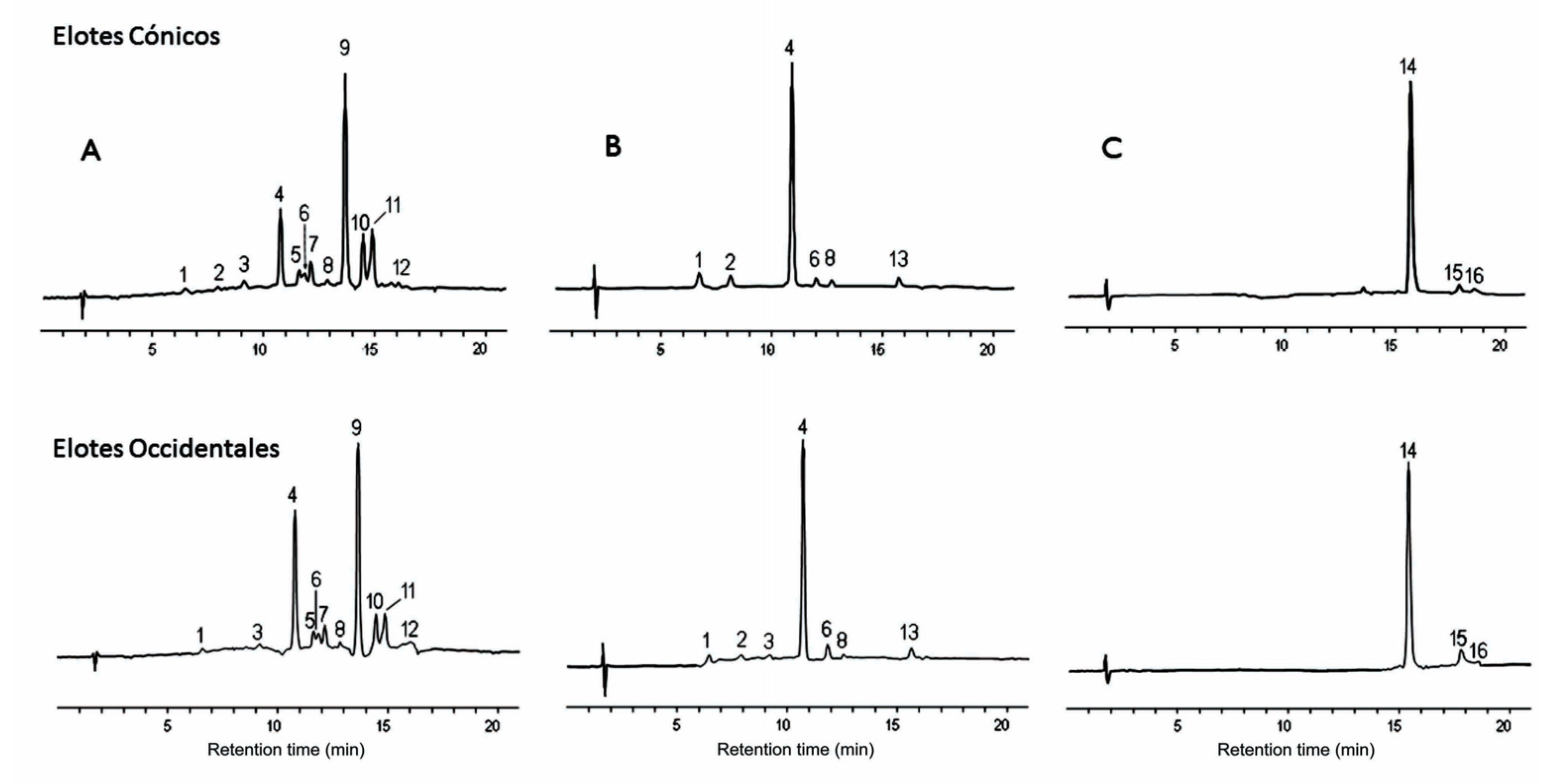
3.2. Anthocyanin Profiling of Maize Grain Accessions in Mexican Varieties
3.3. Hierarchical Analysis and Principal Component Analysis of Blue-Purple Maize Grain Races in Mexico
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilkes, H.G. Maize: Domestication, racial evolution and spread. In Foraging and Farming: The Evolution of Plant Exploration; Harris, D.R., Hillman, G.C., Eds.; Unwin-Hyman: London, UK, 1989; pp. 440–455. [Google Scholar]
- Zizumbo-Villarreal, D.; Colunga-GarcíaMarín, P. Origin of agriculture and plant domestication in West Mesoamerica. Genet. Resour. Crop. Evol. 2010, 57, 813–825. [Google Scholar] [CrossRef]
- Kato, T.Á.; Mapes, C.; Mera, L.M.; Serratos, J.A.; Bye, R.A. Origen y Diversificación del Maíz: Una Revisión Analítica; UNAM-CONABIO: Mexico City, Mexico, 2009; D.F., 116. [Google Scholar]
- Rubín de la Borbolla, S. Mexican Cuisine: An Entire Cultural System; Universidad Nacional Autónoma de México, Coordinación de Humanidades, Centro de Investigaciones sobre América del Norte: Ciudad de Mexico, Mexico, 2018. [Google Scholar]
- CONABIO. Comisión Nacional para el Conocimiento y Uso de la Biodiversidad. Portal de Geoinformación [en línea]. Available online: http://www.conabio.gob.mx/informacion/gis/ (accessed on 4 July 2019).
- Alam, M.A.; Islam, P.; Subhan, N.; Rahman, M.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential health benefits of anthocyanins in oxidative stress related disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Ranilla, L.G.; Zolla, G.; Afaray-Carazas, A.; Vera-Vega, M.; Huanuqueño, H.; Begazo-Gutiérrez, H.; Chirinos, R.; Pedreschi, R.; Shetty, K. Integrated metabolite analysis and health-relevant in vitro functionality of white, red, and orange maize (Zea mays L.) from the Peruvian Andean race Cabanita at different maturity stages. Front. Nutr. 2023, 10, 1132228. [Google Scholar] [CrossRef] [PubMed]
- Langyan, S.; Bhardwaj, R.; Kumari, J.; Jacob, S.R.; Bisht, I.S.; Pandravada, S.R.; Singh, A.; Singh, P.B.; Dar, Z.A.; Kumar, A.; et al. Nutritional Diversity in Native Germplasm of Maize Collected from Three Different Fragile Ecosystems of India. Front. Nutr. 2022, 9, 812599. [Google Scholar] [CrossRef] [PubMed]
- Lezzi, A.; Stagnati, L.; Madormo, F.; Chabloz, D.; Lanubile, A.; Letey, M.; Marocco, A.; Bassignana, M.; Busconi, M. Characterization and Valorization of Maize Landraces from Aosta Valley. Plants 2023, 12, 2674. [Google Scholar] [CrossRef] [PubMed]
- Bessa-Pereira, C.; Dias, R.; Brandão, E.; Mateus, N.; Freitas, V.d.; Soares, S.; Pérez-Gregorio, R. Eat Tasty and Healthy: Role of Polyphenols in Functional Foods. In Functional Foods; Muhammad Sajid, A., Muhammad Haseeb, A., Eds.; IntechOpen: London, UK, 2021; pp. 1–32. [Google Scholar]
- Lopez-Martinez, L.X.; Oliart-Ros, R.M.; Valerio-Alfaro, G.; Chen-Hsien, L.; Parkin, K.L.; Garcia, H.S. Antioxidant activity, phenolic compounds and anthocyanins content of eighteen strains of Mexican maize. LWT—Food Sci. Technol. 2009, 42, 1187–1192. [Google Scholar] [CrossRef]
- Rodríguez-Salinas, P.A.; Zavala-García, F.; Urías-Orona, V.; Muy-Rangel, D.; Heredia, J.B.; Niño-Medina, G. Chromatic, Nutritional and Nutraceutical Properties of Pigmented Native Maize (Zea mays L.) Genotypes from the Northeast of Mexico. Arab. J. Sci. Eng. 2020, 45, 95–112. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Pérez-Alonso, J.J.; Vázquez-Carrillo, G.; Aragón-Cuevas, F.; Velázquez-Cardelas, G.A. Antocianinas y actividad antioxidante en maíces (Zea mays L.) de las razas Chalqueño, Elotes Cónicos y Bolita. Agrociencia 2012, 46, 693–706. [Google Scholar]
- Bedolla, S.; Rooney, L. Cooking maize for masa production. J. Cereal Foods World 1982, 27, 219–221. [Google Scholar]
- McGuire, R.G. Reporting of objective color measurements. HortScience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- AACC. Aproved Methods of the American Association of Cereal Chemists, 9th ed.; International: St. Paul, MN, USA, 1995. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1999; Volume 299, pp. 152–178. [Google Scholar]
- Abdel-Aal, E.-S.M.s.; Hucl, P. A Rapid Method for Quantifying Total Anthocyanins in Blue Aleurone and Purple Pericarp Wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Sánchez, G.S.; Hernández, D.R.; Lobato, N.R. Characterization of anthocyanin extracts from maize kernels. J. Chromatogr. Sci. 2005, 43, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Sumczynski, D.; Bubelova, Z.; Sneyd, J.; Erb-Weber, S.; Mlcek, J. Total phenolics, flavonoids, antioxidant activity, crude fibre and digestibility in non-traditional wheat flakes and muesli. Food Chem. 2015, 174, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Giusti, M.M. Evaluation of Parameters that Affect the 4-Dimethylaminocinnamaldehyde Assay for Flavanols and Proanthocyanidins. J. Food Sci. 2010, 75, C619–C625. [Google Scholar] [CrossRef] [PubMed]
- Fossen, T.; Slimestad, R.; Andersen, Ø.M. Anthocyanins from Maize (Zea mays) and Reed Canarygrass (Phalaris arundinacea). J. Agric. Food Chem. 2001, 49, 2318–2321. [Google Scholar] [CrossRef] [PubMed]
- de Pascual-Teresa, S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. LC–MS analysis of anthocyanins from purple corn cob. J. Sci. Food Agric. 2002, 82, 1003–1006. [Google Scholar] [CrossRef]
- Paulsmeyer, M.N.; Vermillion, K.E.; Juvik, J.A. Assessing the diversity of anthocyanin composition in various tissues of purple corn (Zea mays L.). Phytochemistry 2022, 201, 113263. [Google Scholar] [CrossRef]
- Kôhn, H.F.; Hubert, L.J. Hierarchical Cluster Analysis; Wiley Online: Hoboken, NJ, USA, 2014; pp. 1–13. [Google Scholar] [CrossRef]
- Everitt, B.; Hothom, T. An Introduction to Applied Multivariate Analysis with R; Springer: New York, NY, USA, 2011. [Google Scholar]
- Uriarte-Aceves, P.M.; Cuevas-Rodríguez, E.O.; Gutiérrez-Dorado, R.; Mora-Rochín, S.; Reyes-Moreno, C.; Puangpraphant, S.; Milán-Carrillo, J. Physical, Compositional, and Wet-Milling Characteristics of Mexican Blue Maize (Zea mays L.) Landrace. Cereal Chem. 2015, 92, 491–496. [Google Scholar] [CrossRef]
- Nankar, A.; Holguin, F.O.; Scott, M.P.; Pratt, R.C. Grain and Nutritional Quality Traits of Southwestern U.S. Blue Maize Landraces. Cereal Chem. 2017, 94, 950–955. [Google Scholar] [CrossRef]
- Mahan, A.L.; Murray, S.C.; Rooney, L.W.; Crosby, K.M. Combining Ability for Total Phenols and Secondary Traits in a Diverse Set of Colored (Red, Blue, and Purple) Maize. Crop. Sci. 2013, 53, 1248–1255. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; García-Salinas, C.; Ramírez-Díaz, J.L.; Alemán-de la Torre, I. Phenolic Compounds in Maize Grains and Its Nixtamalized Products. In Phenolic Compounds; Soto-Hernandez, M., Palma-Tenango, M., Garcia-Mateos, M.d.R., Eds.; IntechOpen: Rijeka, Croatia, 2017; Ch. 8.; pp. 215–232. [Google Scholar]
- Salinas-Moreno, Y.; Cruz Chávez, F.J.; Díaz Ortiz, S.A.; Castillo González, F. Granos de maíces pigmentados de Chiapas, características físicas, contenido de antocianinas y valor nutracéutico. Rev. Fitotec. Mex. 2012, 35, 33–41. [Google Scholar] [CrossRef]
- Espinosa-Trujillo, E.; Mendoza-Castillo, M.d.C.; Castillo-González, F. Diversidad fenotípica entre poblaciones de maíz con diferentes grados de pigmentación. Rev. Fitotec. Mex. 2006, 29, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bernal, Ó.A.; Torres-Aguirre, G.A.; Núñez-Gastélum, J.A.; Rosa, L.A.; Rodrigo-García, J.; Ayala-Zavala, J.F.; Álvarez-Parrilla, E. New approach to the interaction between Folin-Ciocalteu reactive and sugars during the quantification of total phenols. TIP. Rev. Espec. En Cienc. Químico-Biológicas 2017, 20, 23–28. [Google Scholar] [CrossRef]
- Bernardi, J.; Stagnati, L.; Lucini, L.; Rocchetti, G.; Lanubile, A.; Cortellini, C.; De Poli, G.; Busconi, M.; Marocco, A. Phenolic Profile and Susceptibility to Fusarium Infection of Pigmented Maize Cultivars. Front. Plant Sci. 2018, 9, 1189. [Google Scholar] [CrossRef] [PubMed]
- Urias-Peraldí, M.; Gutiérrez-Uribe, J.A.; Preciado-Ortiz, R.E.; Cruz-Morales, A.S.; Serna-Saldívar, S.O.; García-Lara, S. Nutraceutical profiles of improved blue maize (Zea mays) hybrids for subtropical regions. Field Crops. Res. 2013, 141, 69–76. [Google Scholar] [CrossRef]
- Mora-Rochín, S.; Gaxiola-Cuevas, N.; Gutiérrez-Uribe, J.A.; Milán-Carrillo, J.; Milán-Noris, E.M.; Reyes-Moreno, C.; Serna-Saldivar, S.O.; Cuevas-Rodríguez, E.O. Effect of traditional nixtamalization on anthocyanin content and profile in Mexican blue maize (Zea mays L.) landraces. LWT—Food Sci. Technol. 2016, 68, 563–569. [Google Scholar] [CrossRef]
- Ramos-Escudero, F.; Muñoz, A.M.; Alvarado-Ortíz, C.; Alvarado, Á.; Yáñez, J.A. Purple Corn (Zea mays L.) Phenolic Compounds Profile and Its Assessment as an Agent Against Oxidative Stress in Isolated Mouse Organs. J. Med. Food 2011, 15, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Luna-Ramírez, K.Y. Variabilidad en el Contenido de Antociaininas y Proantocianidinas en Granos de Maíz azul y Rojo. Bachelor Thesis, Universidad Autónoma Chapingo, Texcoco, México, 2011. [Google Scholar]
- Chen, C.; Somavat, P.; Singh, V.; Gonzalez de Mejia, E. Chemical characterization of proanthocyanidins in purple, blue, and red maize coproducts from different milling processes and their anti-inflammatory properties. Ind. Crop. Prod. 2017, 109, 464–475. [Google Scholar] [CrossRef]
- Salinas-Moreno, Y.; Esquivel-Esquivel, G.; Ramírez-Díaz, J.L.; Alemán de la Torre, I.; Bautista-Ramírez, E.; Santillán-Fernández, A. Selecciòn de germoplasma de maíz morado (Zea mays L.) con potencial para extracción de pigmentos. Rev. Fitotec. Mex. 2021, 44, 309–321. [Google Scholar] [CrossRef]
- Li, Q.; Somavat, P.; Singh, V.; Chatham, L.; Gonzalez de Mejia, E. A comparative study of anthocyanin distribution in purple and blue corn coproducts from three conventional fractionation processes. Food Chem. 2017, 231, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Paulsmeyer, M.; Chatham, L.; Becker, T.; West, M.; West, L.; Juvik, J. Survey of Anthocyanin Composition and Concentration in Diverse Maize Germplasms. J. Agric. Food Chem. 2017, 65, 4341–4350. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, J.; Li, W.; Wen, T.; Li, T.; Guo, X.-B.; Liu, R.H. Anthocyanin accumulation, biosynthesis and antioxidant capacity of black sweet corn (Zea mays L.) during kernel development over two growing seasons. J. Cereal Sci. 2020, 95, 103065. [Google Scholar] [CrossRef]
- González-Manzano, S.; Pérez-Alonso, J.J.; Salinas-Moreno, Y.; Mateus, N.; Silva, A.M.S.; de Freitas, V.; Santos-Buelga, C. Flavanol–anthocyanin pigments in corn: NMR characterisation and presence in different purple corn varieties. J. Food Compos. Anal. 2008, 21, 521–526. [Google Scholar] [CrossRef]
- Gonzalez-Martinez, J.; Rocandio-Rodríguez, M.; Contreras-Toledo, A.R.; Joaquin-Cancino, S.; Vanoye-Eligio, V.; Chacon-Hernandez, J.C.; Hernandez-Bautista, A. Morphological and agronomic diversity of maize native to the high plateau of Tamaulipas, Mexico. Rev. Fitotec. Mex. 2020, 43, 361–370. [Google Scholar]
- Chávez-Servia, J.L.; Torres-Escamilla, F.; Diego-Flores, P.; Carrillo-Rodríguez, J.C. Variabilidad agromorfológica entre poblaciones de maíz azul y rojo de la Mixteca oaxaqueña, México. e-CUCBA 2020, 6, 29–48. [Google Scholar] [CrossRef]
- Gómez-Orea, D. Ordenación del Territorio: Una Aproximación Desde el Medio Físico; Editorial Agrícola Española: Madrid, Spain, 1994. [Google Scholar]
- Ruiz-Corral, J.A.; Durán Puga, N.; Sanchez Gonzalez, J.d.J.; Ron Parra, J.; Gonzalez Eguiarte, D.R.; Holland, J.; Medina Garcia, G. Climatic adaptation and ecological descriptors of 42 Mexican maize races. Crop. Sci. 2008, 48, 1502–1512. [Google Scholar] [CrossRef]
- Ureta, C.; González-Salazar, C.; González, E.J.; Álvarez-Buylla, E.R.; Martínez-Meyer, E. Environmental and social factors account for Mexican maize richness and distribution: A data mining approach. Agric. Ecosyst. Environ. 2013, 179, 25–34. [Google Scholar] [CrossRef]


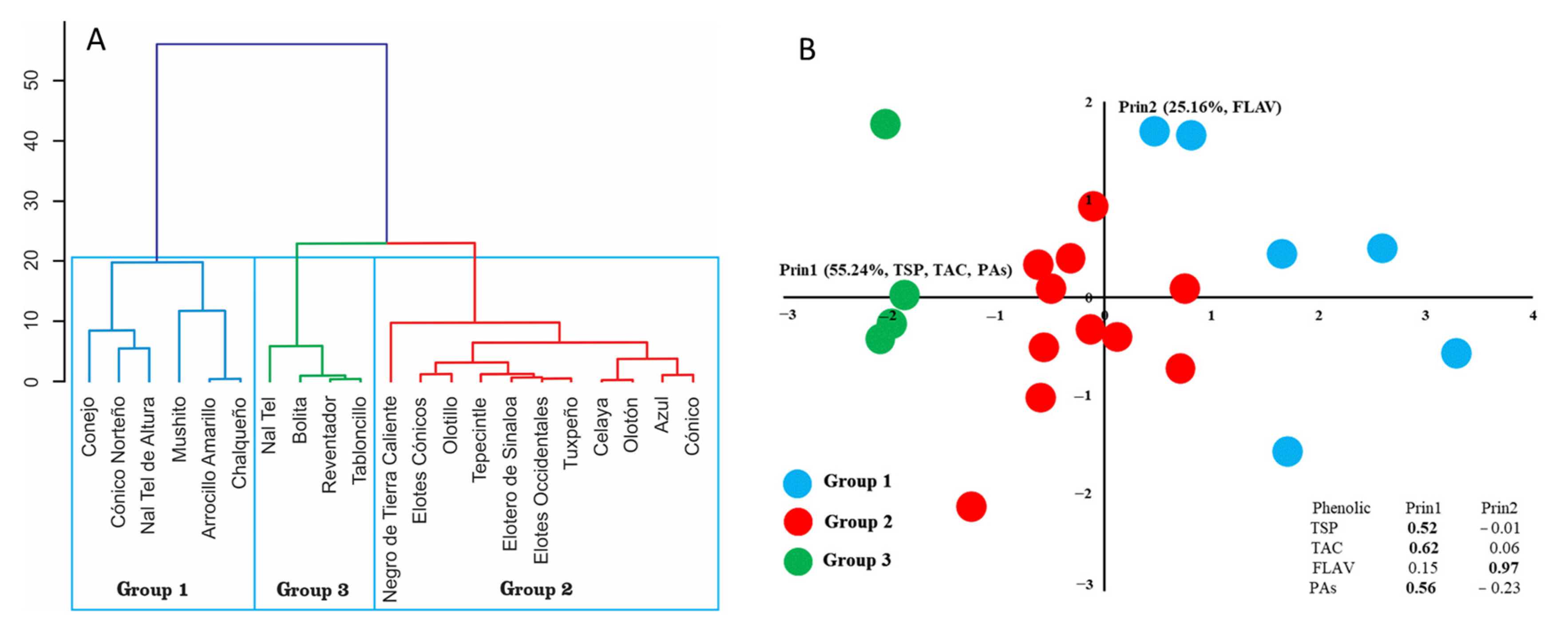
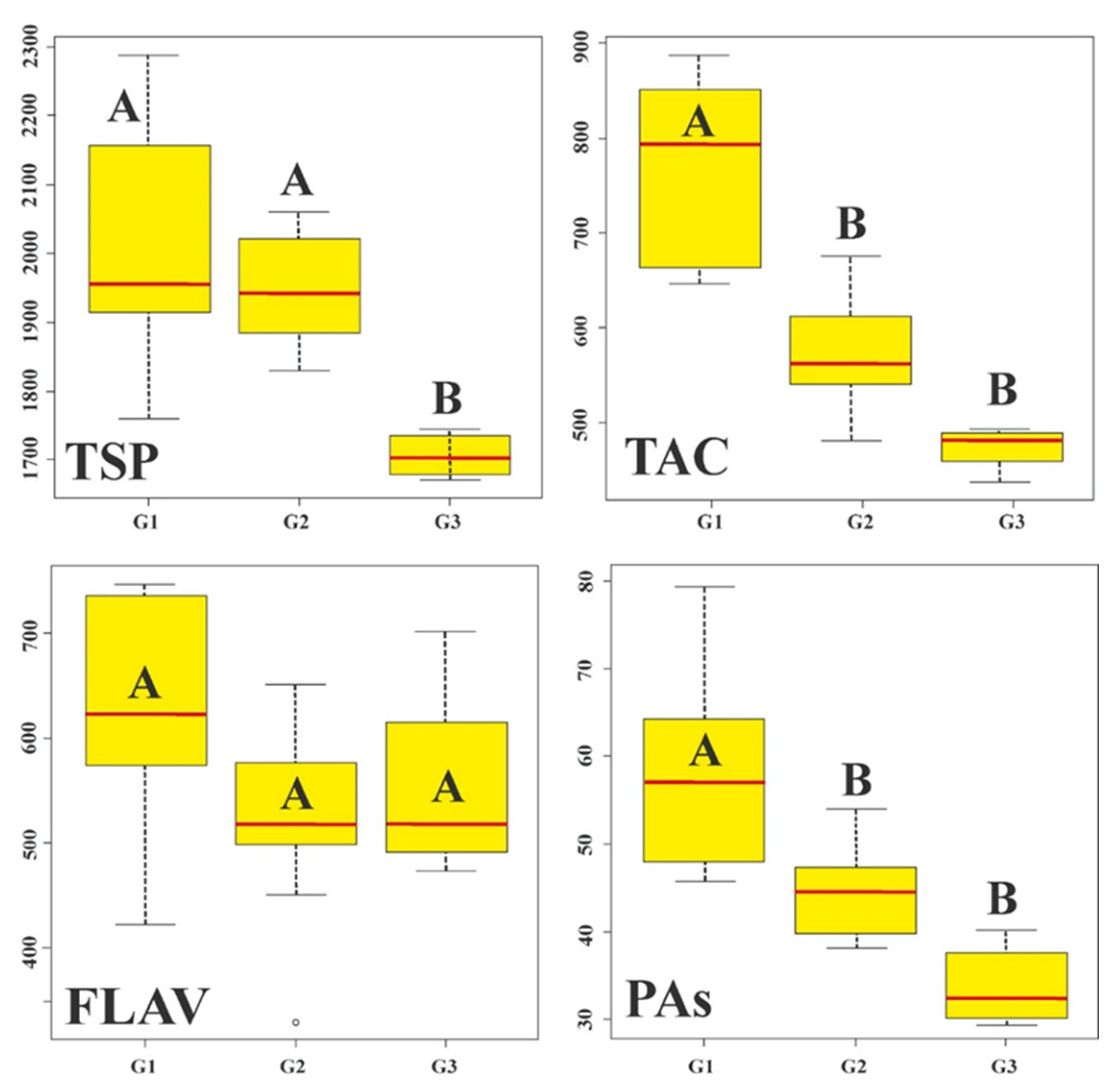
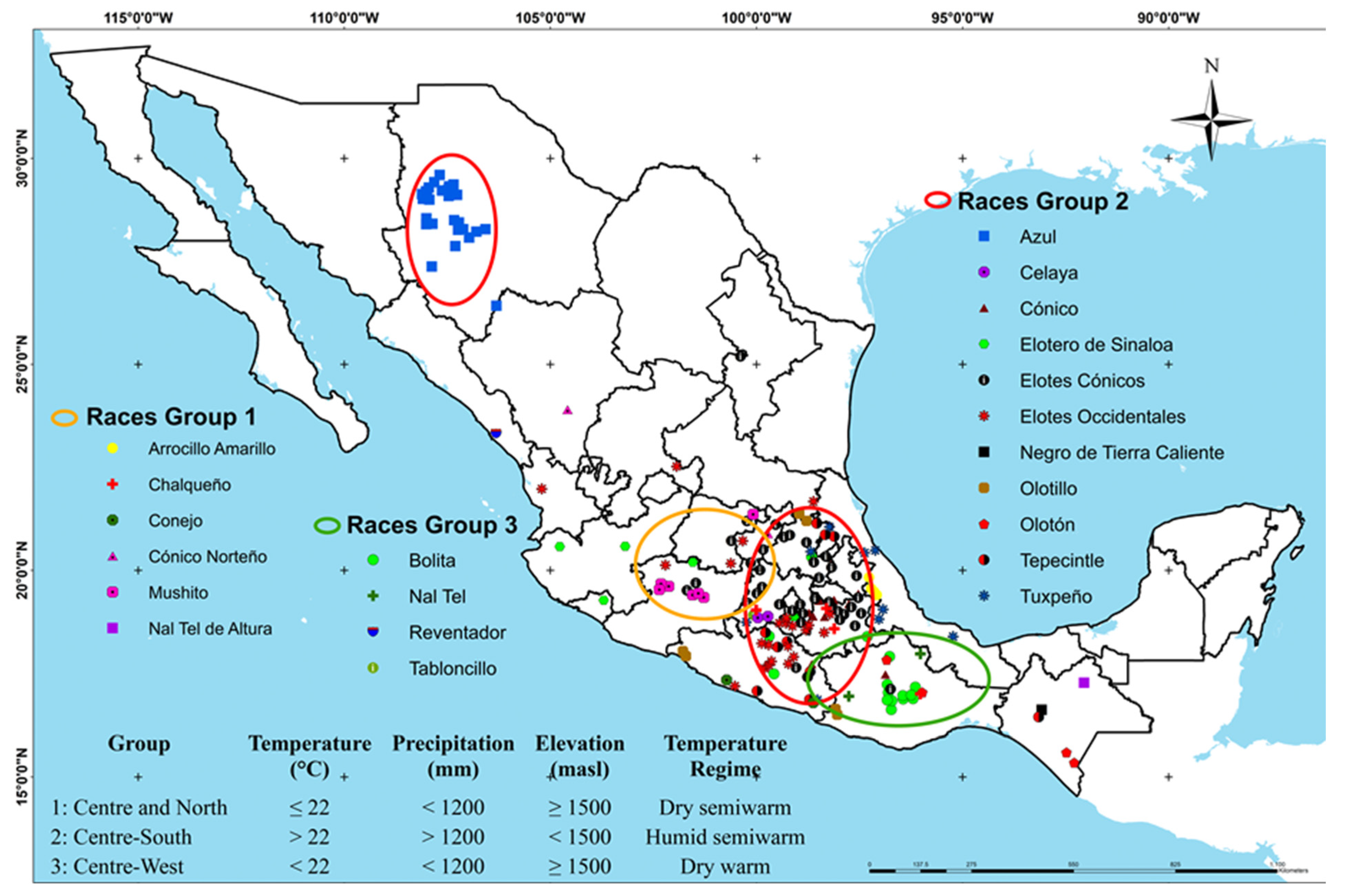
| Maize Races | Number of Accessions Analyzed per Race | Elevation (masl) | Precipitation (mm) | Temperature (°C) |
|---|---|---|---|---|
| Arrocillo Amarillo | 4 | 1900 to 2100 | 700 to 1200 | 12 to 20 |
| Azul | 28 | 2000 to 2600 | 600 to 1000 | 10 to 18 |
| Bolita | 17 | 1400 to 1800 | 800 to 1200 | 18 to 23 |
| Celaya | 2 | 1600 to 1800 | 1100 to 2000 | 16 to 28 |
| Chalqueño | 4 | 1600 to 1800 | 600 to 1000 | 19 to 25 |
| Conejo | 2 | 2000 to 2500 | 1000 to 1200 | 21 to 28 |
| Cónico | 15 | 2000 to 2500 | 800 to 1200 | 12 to 24 |
| Cónico Norteño | 2 | 1600 to 1900 | 500 to 700 | 20 to 24 |
| Elotero de Sinaloa | 4 | 600 to 1500 | 800 to 1300 | 18 to 26 |
| Elotes Cónicos | 51 | 2000 to 2500 | 800 to 1200 | 16 to 22 |
| Elotes Occidentales | 33 | 800 to 1600 | 700 to 1200 | 18 to 26 |
| Mushito | 8 | 2200 to 2500 | 1100 to 1500 | 10 to 22 |
| Nal-Tel | 2 | 200 to 500 | 500 to 800 | 25 to 29 |
| Nal-Tel de Altura | 1 | 1000 to 1200 | 1000 to 1200 | 28 to 32 |
| Negro de Tierra Caliente | 1 | 800 to 1000 | 800 to 1200 | 24 to 28 |
| Olotillo | 6 | 400 to 1000 | 600 to 1300 | 18 to 30 |
| Olotón | 4 | 2000 to 2000 | 1200 to 1800 | 14 to 24 |
| Reventador | 1 | 100 to 200 | 400 to 800 | 25 to 28 |
| Tabloncillo | 1 | 1000 to 1200 | 1200 to 1800 | 15 to 28 |
| Tepecintle | 11 | 400 to 1200 | 1200 to 2000 | 20 to 28 |
| Tuxpeño | 10 | 200 to 1500 | 1200 to 1400 | 14 to 28 |
| Indicator | Physical Characteristics of Grain 1 | Phenolic Composition 2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Type | Name | W100G | TE | PL | TSP | TAC | FLAV | PAs |
| Central | Mean | 34.91 | 74.52 | 2.03 | 1965.93 | 601.84 | 562.51 | 43.11 |
| Median | 33.90 | 75.00 | 2.00 | 1960.99 | 558.85 | 529.58 | 39.59 | |
| Mode | 28.05 | 75.00 | 2.00 | * | * | 516.93 | 39.10 | |
| Dispersion | Standard deviation | 8.69 | 16.07 | 0.18 | 291.26 | 237.40 | 149.88 | 12.63 |
| Variance | 75.44 | 258.18 | 0.03 | 84,830.17 | 56,356.60 | 22,463.99 | 159.47 | |
| Coefficient of variation | 24.88 | 21.56 | 8.87 | 14.82 | 39.44 | 26.64 | 29.29 | |
| Range | 54.39 | 75.00 | 1.00 | 1621.75 | 1375.09 | 680.56 | 73.62 | |
| Minimum | 16.09 | 25.00 | 2.00 | 1369.15 | 180.21 | 319.91 | 25.63 | |
| Maximum | 70.47 | 100.00 | 3.00 3 | 2990.91 | 1555.30 | 1000.47 | 99.26 | |
| Symmetric | Kurtosis | 1.73 | −0.22 | 25.53 | 0.53 | 2.79 | −0.24 | 3.20 |
| Asymmetric coefficient | 0.94 | −0.09 | 5.22 | 0.55 | 1.36 | 0.69 | 1.58 | |
| Peak | Rt (min) | Identity | Anthocyanin Type | References |
|---|---|---|---|---|
| 1 | 6.52 | NI | No acylated | |
| 2 | 7.97 | NI | No acylated | |
| 3 | 9.14 | NI | Acylated | |
| 4 | 10.80 | Cyanidin 3-glucoside | No acylated | Standard |
| 5 | 11.65 | Acylated derivative | Acylated | |
| 6 | 11.89 | Pelargonidin 3-glucoside | No acylated | Standard |
| 7 | 12.17 | Acylated derivative | Acylated | |
| 8 | 12.90 | Peonidin 3-glucoside | No acylated | Standard |
| 9 | 13.71 | Cyanidin 3-malonyl glucoside | Acylated | Salinas-Moreno et al. [13]; Paulsmeyer et al. [42] |
| 10 | 14.52 | Pelargonidin 3-malonyl-glucoside | Acylated | Salinas-Moreno et al. [13]; Paulsmeyer et al. [42] |
| 11 | 14.94 | Cyanidin 3 dimalonyl-glucoside | Acylated | Salinas-Moreno et al. [13]; Paulsmeyer et al. [42] |
| 12 | 16.15 | NI | Acylated | |
| 13 | 15.71 | NI | Acylated | |
| 14 | 15.78 | Cyanidine | Aglycone | Standard |
| 15 | 18.00 | Pelargonidine | Aglycone | Standard |
| 16 | 18.67 | Peonidine | Aglycone | Standard |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salinas-Moreno, Y.; Santillán-Fernández, A.; de la Torre, I.A.; Ramírez-Díaz, J.L.; Ledesma-Miramontes, A.; Martínez-Ortiz, M.Á. Physical Traits and Phenolic Compound Diversity in Maize Accessions with Blue-Purple Grain (Zea mays L.) of Mexican Races. Agriculture 2024, 14, 564. https://doi.org/10.3390/agriculture14040564
Salinas-Moreno Y, Santillán-Fernández A, de la Torre IA, Ramírez-Díaz JL, Ledesma-Miramontes A, Martínez-Ortiz MÁ. Physical Traits and Phenolic Compound Diversity in Maize Accessions with Blue-Purple Grain (Zea mays L.) of Mexican Races. Agriculture. 2024; 14(4):564. https://doi.org/10.3390/agriculture14040564
Chicago/Turabian StyleSalinas-Moreno, Yolanda, Alberto Santillán-Fernández, Ivone Alemán de la Torre, José Luis Ramírez-Díaz, Alejandro Ledesma-Miramontes, and Miguel Ángel Martínez-Ortiz. 2024. "Physical Traits and Phenolic Compound Diversity in Maize Accessions with Blue-Purple Grain (Zea mays L.) of Mexican Races" Agriculture 14, no. 4: 564. https://doi.org/10.3390/agriculture14040564





