Diversity Distribution Analysis of Guava (Psidium guajava L.) Populations in Cultivated and Wild Habitats in the Mid-Hills of Uttarakhand, India
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Socio-Economic Data Recorded
2.3. Ecological Sampling and Quantitative Data Analysis
2.4. Fruit Morphological Parameters Recorded
3. Results
3.1. Guava Cultivation and Socio-Economic Status of the Local People
3.2. Variability Analysis in Fruit Characteristics
3.3. Analysis of Phytosociological Characteristics and Population Structure
3.4. Diversity Indices among the Sites
3.5. Participatory Approach in Guava Conservation and Management Planning
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Candolle, A.P. Origin of Cultivated Plants; Kegal Paul: London, UK, 1904. [Google Scholar]
- Purseglove, J.W. Tropical Crops: Dicotyledons; John Wiley and Sons, Inc.: New York, NY, USA, 1968. [Google Scholar]
- Hayes, W.B. Fruit Growing in India; Kitabistan: Allahabad, India, 1953. [Google Scholar]
- Pontikis, C.A. Psidium guajava L. (Guava). In Trees IV. Biotechnology in Agriculture and Forestry; Bajaj, Y.P.S., Ed.; Springer: Berlin/Heidelberg, Germany, 1996; Volume 35. [Google Scholar]
- Baloda, S.; Sharma, S.K.; Sehrawat, V.P.; Ahlawat, S.; Bhatia, K.; Dahiya, D.S. Present status of Guava Research JR and Future Thrust in India. Haryana J. Hortic. Sci. 2011, 40, 105–116. [Google Scholar]
- Yadav, I.S. Germplasm Collection of Mango, Grape, Guava, and Litchi in India. Report of All India Coordinated Research Project on Sub-tropical Fruits; CIHNP: Lucknow, India, 1990. [Google Scholar]
- Gangappa, N.D.; Singh, C.; Verma, M.K.; Thakre, M.; Sevanthi, A.M.; Singh, R.; Srivastava, M.; Raghunandan, K.; Anusha, C.; Yadav, V.; et al. Assessing the genetic diversity of guava germplasm characterized by morpho-biochemical traits. Front. Nutr. 2022, 9, 1017680. [Google Scholar] [CrossRef]
- Alves, J.E.; Freitas, B.M. Pollination requirements of guava. Cienc. Rural 2007, 37, 1281–1286. [Google Scholar] [CrossRef]
- Sarkar, T.; Sarkar, S.K. Pollination characteristics and intervarietal hybridization of Psidium guajava. J. Crop Weed 2022, 18, 96–103. [Google Scholar] [CrossRef]
- Naga Chaithanya, M.V.; Dinesh, M.R.; Vasugi, C.; Lakshmana Reddy, D.C.; Sailaja, D.; Aswath, C. Assessment of genetic diversity in guava (Psidium guajava) germplasm using microsatellites. J. Hortic. Sci. 2014, 9, 117–125. [Google Scholar] [CrossRef]
- Naseer, S.M.; Rahman, M.; Pervaiz, N.; Naeem, N.; Hussain, S. The phytochemistry and medicinal value of Psidium guajava L. (guava). Clin. Phytosci. 2018, 4, 8. [Google Scholar] [CrossRef]
- Gill, K.S. Guavas. In Encyclopedia of Food and Health; Caballero, B.C., Paul, M.F., Fidel, T., Eds.; Academic Press: London, UK, 2016; pp. 270–277. [Google Scholar]
- Angulo-Lopez, J.E.; Flores-Gallegos, A.; Torres-Leon, C.; Ramirez-Guzman, K.N.; Martinez, G.A.; Aguilar, C.N. Guava (Psidium guajava L.) Fruit and Valorization of Industrialization By-Products. Processes 2021, 9, 1075. [Google Scholar] [CrossRef]
- National Horticulture Board (NHB). Indian Production of Guava: 1st Advance Estimate 2021-22. 2022. Available online: https://agriexchange.apeda.gov.in/India%20Production/India_Productions.aspx?cat=fruit&hscode=1046 (accessed on 28 December 2023).
- Government of India. Horticultural Statistics at a Glance—2018; Horticulture Statistics Division Department of Agriculture, Cooperation & Farmers’ Welfare Ministry of Agriculture & Farmers’ Welfare, Government of India: New Delhi, India, 2018; 460p.
- Dhar, U.; Rawal, R.S.; Samant, S.S. Structural diversity and representativeness of forest vegetation in a protected area of Kumaon Himalaya, India, Implications for conservation. Biodiv. Conser. 1997, 6, 1045–1062. [Google Scholar] [CrossRef]
- Singh, J.S.; Singh, S.P. Forests of Himalaya: Structure, Functioning, and Impact of Man; Gyanodaya Prakashan: Nainital, India, 1992. [Google Scholar]
- Menzel, C.M. Guava: An exotic fruit with potential in Queensland. Queensl. Agric. J. 1985, 111, 93–98. [Google Scholar]
- Howard, R.A. Flora of the Lesser Antilles, Leeward and Windward Islands. Volume 5. Arnold Arboretum; Harvard University: Jamaica Plain, MA, USA, 1989; 604p. [Google Scholar]
- Walsh, S.J.; McCleary, A.L.; Mena, C.F.; Shao, Y.; Tuttle, J.P.; González, A.; Atkinson, R. Quick Bird and Hyperion data analysis of an invasive plant species in the Galapagos Islands of Ecuador: Implications for control and land use management. Remote Sens. Environ. 2008, 112, 1927–1941. [Google Scholar] [CrossRef]
- Mauchamp, A. Threats from Alien plant species in the Galápagos Islands. Conserv. Biol. 1911, 11, 260–263. [Google Scholar] [CrossRef]
- Tye, A.; Atkinson, R.; Carrion, V. Increase in the number of introduced plant species in Galapagos (No. 2006–2007; pp. 133–135). DPNG, GCREG, FCD, GC. DPNG, GCREG, FCD, GC. 2007. Available online: http://www.galapagos.org/wp-content/uploads/2012/04/biodiv7-introduced-plants-increase.pdf (accessed on 21 November 2023).
- Urquia, D.; Gutierrez, B.; Pozo, G.; Pozo, M.J.; Espin, A.; Torres, M. Psidium guajava in the Galapagos Islands: Population genetics and history of an invasive species. PLoS ONE 2019, 14, e0203737. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, C.S.; Burns, B.R.; Stanley, M.C. Predicting plant invasions under climate change: Are species distribution models validated by field trials? Glob. Chang. Biol. 2014, 20, 2800–2814. [Google Scholar] [CrossRef]
- Witt, A.; Luke, Q. (Eds.) Guide to the Naturalized and Invasive Plants of Eastern Africa; CABI: Wallingford, UK, 2017; 601p, Available online: http://www.cabi.org/cabebooks/ebook/20173158959 (accessed on 21 November 2023).
- Compendium on Soils. Compendium of Soil Resources for Sustainable Land Management of Uttarakhand; Soil and Land Use Survey of India (Department of Agriculture, Co-operation & Farmers Welfare) Ministry of Agriculture & Farmers Welfare Government of India: New Delhi, India, 2018.
- Bisht, I.S.; Rao, K.S.; Bhandari, D.C.; Nautiyal, S.; Maikhuri, R.K.; Dhillon, B.S. A suitable site for in situ (on-farm) management of plant diversity in traditional agro-ecosystems of western Himalaya in Uttaranchal state: A case study. Genet Resour. Crop Evol. 2006, 53, 1333–1350. [Google Scholar] [CrossRef]
- Kershaw, K.A. Quantitative and Dynamic Plant Ecology; American Elsevier Publishing Company: London, UK, 1974; 308p. [Google Scholar]
- Misra, R. Ecology Work Book; Oxford and IBH Publishing Company: New Delhi, India, 1968; p. 244. [Google Scholar]
- Polunin, O.; Stainton, A. Flowers of the Himalaya; Oxford Press: New Delhi, India, 1984. [Google Scholar]
- Naithani, B.D. Flora of Chamoli; Botanical Survey of India: Howrah, India, 1984.
- Curtis, J.T.; Cottam, G. The use of distance measures in phytosociological sampling. Ecology 1956, 37, 151–160. [Google Scholar]
- Curtis, J.T.; McIntosh, R.P. The interrelations of certain analytic and synthetic phytosociological characters. Ecology 1950, 31, 434–455. [Google Scholar] [CrossRef]
- Whitford, P.B. Distribution of woodland plants in relation to succession and colonal growth. Ecology 1949, 30, 199–208. [Google Scholar] [CrossRef]
- Magurran, A.E. Ecological Diversity and Its Measurement; Croom Helm: London, UK, 1988. [Google Scholar]
- Simpson, E.H. Measurement of diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Sinha, M.; Sinha, A.M.P. Value addition of guava cheese cv. Allahabad safeda by medicinal herbs. J. Pharmacogn. Phytochem. 2017, 6, 856–859. [Google Scholar]
- Otuoma, J.; Nyongesah, J.M.; Owino, J.; Onyango, A.A.; Okello, V.S. Ecological manipulation of Psidium guajava to facilitate secondary forest succession in tropical forests. J. Ecol. Eng. 2020, 21, 210–221. [Google Scholar] [CrossRef]
- Li, S.P.; Cadotte, M.W.; Meiners, S.J.; Hua, Z.S.; Shu, H.Y.; Li, J.T.; Shu, W.S. The effects of phylogenetic relatedness on invasion success and impact: Deconstructing Darwin’s naturalization conundrum. Ecol. Lett. 2015, 18, 1285–1292. [Google Scholar] [CrossRef]
- Park, D.S.; Feng, X.; Meitner, B.S.; Ernst, K.C.; EnQuest, B.J. Darwin’s naturalization conundrum can be explained by spatial scale. Proc. Natl. Acad. Sci. USA 2020, 117, 10904–10910. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Sandel, B. Darwin’s pre-adaptation hypothesis and the phylogenetic structure of native and alien regional plant assemblages across North America. Glob. Ecol. Biogeogr. 2021, 31, 531–545. [Google Scholar] [CrossRef]
- Cadotte, M.W.; Campbell, S.E.; Li, S.P.; Sodhi, D.S.; Mandrake, N.E. Preadaptation and naturalization of non-native species: Darwin’s two fundamental insights into species invasion. Annu. Rev. Plant Biol. 2018, 89, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Mohapatra. Development of agroforestry system for mid-hills of north east region. Ann. Ag. Res. 2022, 43, 1–5. [Google Scholar]
- Rathore, A.C.; Abhishek, K.; Toman, J.; Jayaprakash, J.; Mehta, H.; Kaushal, R.; Alam, N.M.; Gupta, A.K.; Raizada, A.; Chaturvedi, O.P. Predictive models for biomass and carbon stock estimation in Psidium guajava on bouldery riverbed landsin North-Western Himalayas, India. Agrofor. Syst. 2018, 92, 171–182. [Google Scholar] [CrossRef]
- Dhyani, S.; Thummarukuddy, M. Ecological engineering for disaster risk reduction and climate change adaptation. Environ. Sci. Pollut. Res. 2016, 23, 20049–20052. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.P.S.; Ramakrishnan, P.S.; Tripathi, R.S. Population Dynamics of Eupatorium odoratum in Successional Environments Following Slash and Burn Agriculture. J. Appl. Ecol. 1981, 18, 529–535. [Google Scholar] [CrossRef]
- Reddy, S.; Bagayanarayana, G.; Reddy, K.N.; Raju, V.S. Invasive Alien Flora of India; US Geological Survey: Reston, VA, USA, 2008; pp. 1–28.
- NBA. Invasive Alien Species of India—Technical Report; NBA: Chennai, India, 2018. [Google Scholar]



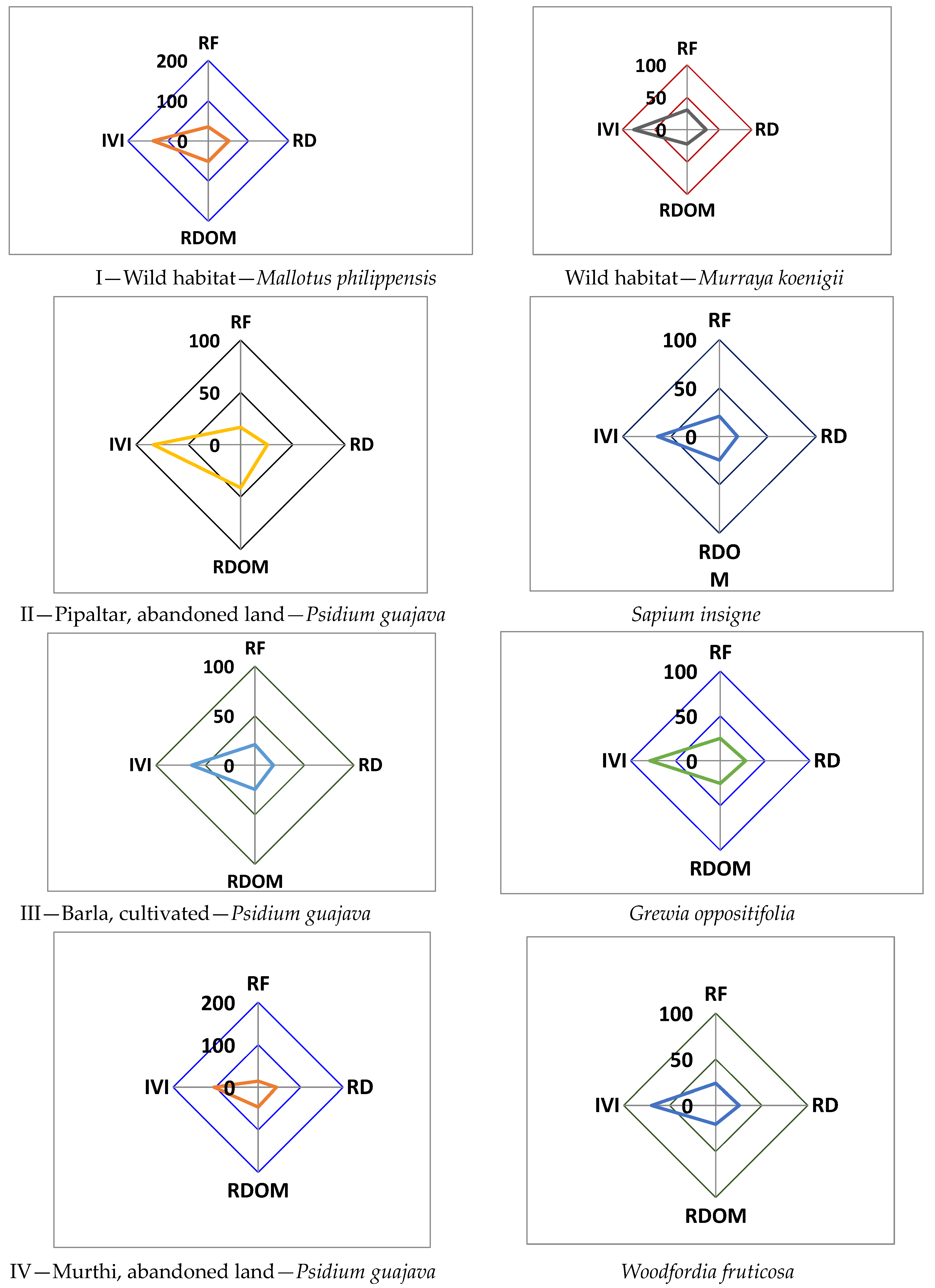
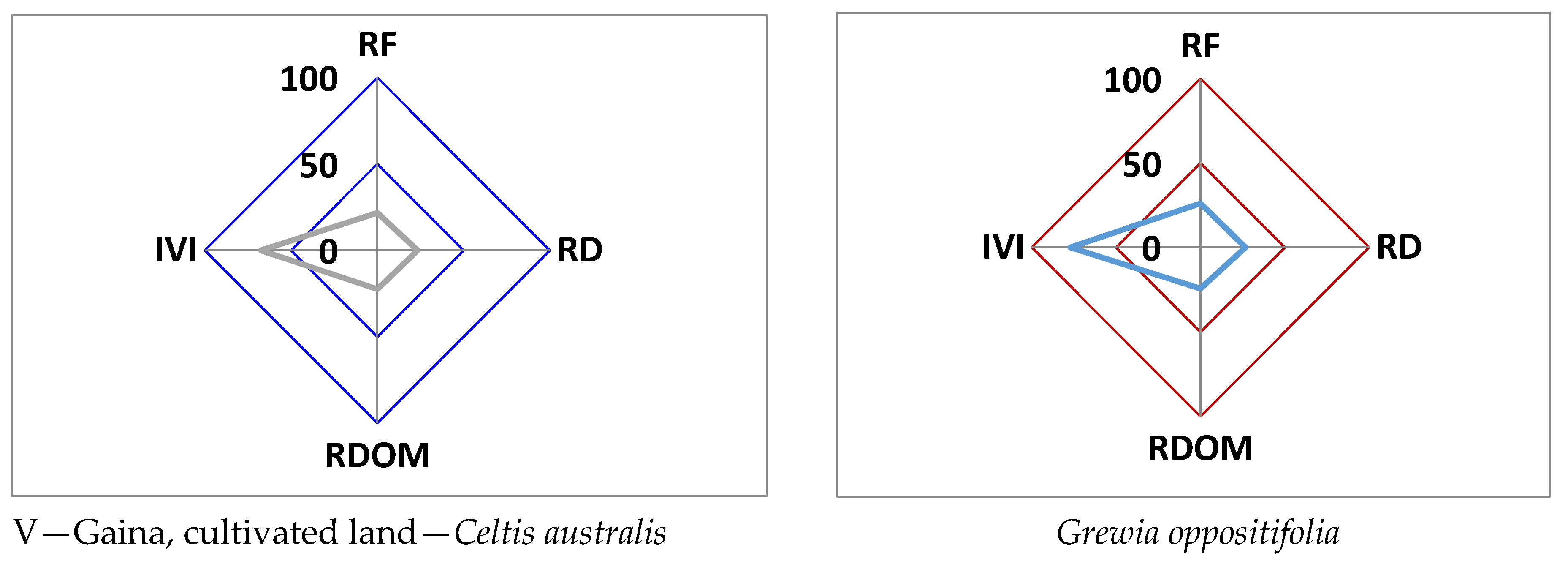
| Study Sites | Vegetation (Tree/Shrub/ Seedling/Sapling) | Common Name | Frequency (%) | Density (Trees/100 m2) | Distribution Pattern (A/F Ratio) | IVI |
|---|---|---|---|---|---|---|
| I. Barla (Wild habitat) | Trees | |||||
| Psidium guajava L. | Guava | 30 | 0.8 | 0.089 | 33.64 | |
| Mallotus philippensis (Lam.) Muell. Arg. | Kamela dye | 80 | 4.5 | 0.070 | 137.07 | |
| Murraya koenigii (L.) Spreng. | Curry leaf | 70 | 2.6 | 0.053 | 82.13 | |
| Sapium insigne (Royle) Benth. Hook.f. | Khinna, Khirun | 50 | 0.9 | 0.036 | 47.17 | |
| Shrubs | ||||||
| Colebrookia oppositifolia | Pansre, Binda | 60 | 2.2 | 0.061 | 126.45 | |
| Woodfordia fruticosa | Dhavari | 50 | 1.2 | 0.048 | 87.33 | |
| Murraya koenigii | Curry leaf | 60 | 1.0 | 0.028 | 86.22 | |
| II. Pipaltar (Abandoned land) | Trees | |||||
| Psidium guajava L. | Guava | 40 | 1.1 | 0.069 | 83.40 | |
| Jatropha curcus L. | Ratanjot | 20 | 0.5 | 0.125 | 29.47 | |
| Murraya koenigii (L.) Spreng. | Curry leaf | 40 | 0.7 | 0.044 | 34.07 | |
| Mallotus philippensis (Lam.) Muell. Arg. | Kamela dye tree | 40 | 0.7 | 0.044 | 42.23 | |
| Sapium insigne (Royle) Benth. Hook.f. | Khinna, Khirun | 50 | 0.8 | 0.032 | 63.87 | |
| Pyrus pashia Buch.-Ham. ex D. Don. | Mahul, wild pear | 40 | 0.4 | 0.023 | 34.82 | |
| Aegle marmelos (L.) Correa | Bael, stone apple | 10 | 0.1 | 0.100 | 12.14 | |
| Shrubs | ||||||
| Agave americana | Gwarpatha | 20 | 0.3 | 0.075 | 300.00 | |
| III. Barla village (Cultivated) | Trees | |||||
| Psidium guajava L. | Guava | 40 | 0.7 | 0.044 | 52.17 | |
| Mangifera indica L. | Aam, mango | 30 | 0.3 | 0.033 | 26.02 | |
| Emblica officinalis L. | Aonla | 20 | 0.2 | 0.050 | 22.36 | |
| Celtis australis L. | Kharik | 40 | 0.6 | 0.038 | 44.42 | |
| Grewia optiva J.R. Drumn. Ex Burret | Bhimal | 70 | 1.1 | 0.022 | 78.50 | |
| Toona ciliata M.Roem. | Toon | 30 | 0.3 | 0.033 | 24.41 | |
| Litsea monopetala (Roxb. Ex Baker) Pers. | Katmarra | 20 | 0.3 | 0.075 | 21.20 | |
| Ficus semicordata Buch.Ham.ex Roxb. | Timla, Timul | 30 | 0.4 | 0.044 | 30.91 | |
| Seedling | ||||||
| Psidium guajava L. | Guava | 30 | 0.3 | 0.033 | 300.00 | |
| IV Murti (Abandoned land) | Trees | |||||
| Psidium guajava L. | Guava | 30 | 2.2 | 0.244 | 103.59 | |
| Woodfordia fruticosa (L.) Kurz | Dhavari | 50 | 1.3 | 0.052 | 69.79 | |
| Madhuca longifolia (J.Koenig ex (L.) J.F.Macbr. | Mahua | 50 | 0.7 | 0.028 | 51.15 | |
| Syzygium cumini (L.) | Jamun, black plum | 40 | 0.4 | 0.024 | 35.07 | |
| Erythrina indica Lam. | Indian coral tree | 40 | 0.5 | 0.031 | 40.39 | |
| Shrubs/Saplings | ||||||
| Psidium guajava L. | Guava | 50 | 2.9 | 0.116 | 204.37 | |
| Woodfordia fruticosa (L.) Kurz | Dhavari | 70 | 1.3 | 0.026 | 95.63 | |
| Seedlings | ||||||
| Psidium guajava L. | Guava | 30 | 1.1 | 0.122 | 300.00 | |
| Site V. Gaina (Cultivated) | Trees | |||||
| Psidium guajava L. | Guava | 40 | 0.6 | 0.038 | 57.87 | |
| Ficus semicordata Buch.Ham.ex Roxb. | Timla, Timul | 20 | 0.2 | 0.050 | 22.92 | |
| Celtis australis L. | Kharik | 50 | 0.7 | 0.028 | 67.51 | |
| Grewia optiva J.R. Drumn. Ex Burret | Bhimal | 60 | 0.8 | 0.022 | 77.10 | |
| Toona ciliata M.Roem. | Toon | 20 | 0.2 | 0.050 | 24.41 | |
| Litsea polyantha Juss. | Katmarra | 40 | 0.5 | 0.031 | 50.19 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semwal, D.P.; Longkumar, S.; Chandra, P.; Rathi, R.S.; Rai, K.M.; Arya, M.; Ahlawat, S.P.; Singh, P.K. Diversity Distribution Analysis of Guava (Psidium guajava L.) Populations in Cultivated and Wild Habitats in the Mid-Hills of Uttarakhand, India. Agriculture 2024, 14, 575. https://doi.org/10.3390/agriculture14040575
Semwal DP, Longkumar S, Chandra P, Rathi RS, Rai KM, Arya M, Ahlawat SP, Singh PK. Diversity Distribution Analysis of Guava (Psidium guajava L.) Populations in Cultivated and Wild Habitats in the Mid-Hills of Uttarakhand, India. Agriculture. 2024; 14(4):575. https://doi.org/10.3390/agriculture14040575
Chicago/Turabian StyleSemwal, Dinesh P., Soyimchiten Longkumar, Puran Chandra, Ranbir S. Rathi, Krishna M. Rai, Mamta Arya, Sudhir P. Ahlawat, and Praveen K. Singh. 2024. "Diversity Distribution Analysis of Guava (Psidium guajava L.) Populations in Cultivated and Wild Habitats in the Mid-Hills of Uttarakhand, India" Agriculture 14, no. 4: 575. https://doi.org/10.3390/agriculture14040575
APA StyleSemwal, D. P., Longkumar, S., Chandra, P., Rathi, R. S., Rai, K. M., Arya, M., Ahlawat, S. P., & Singh, P. K. (2024). Diversity Distribution Analysis of Guava (Psidium guajava L.) Populations in Cultivated and Wild Habitats in the Mid-Hills of Uttarakhand, India. Agriculture, 14(4), 575. https://doi.org/10.3390/agriculture14040575






