Effects of Polyethylene Microplastics in Agricultural Soil on Eisenia fetida (Annelida: Oligochaeta) Behavior, Biomass, and Mortality
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil and Microplastics
2.2. Earthworms
2.3. Avoidance Bioassays
2.4. Biomass and Mortality Bioassays
2.5. Statistical Analyses
3. Results and Discussion
3.1. Soil Properties and Microplastic Characteristics
3.2. E. fetida Avoidance Bioassays
3.3. E. fetida Biomass and Mortality Bioassays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- d’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. 2019, 12–21. Available online: http://journals.openedition.org/factsreports/5102 (accessed on 21 December 2023).
- Sarda, P.; Hanan, J.C.; Lawrence, J.G.; Allahkarami, M. Sustainability performance of polyethylene terephthalate, clarifying challenges and opportunities. J. Polym. Sci. 2022, 60, 7–31. [Google Scholar] [CrossRef]
- Hartmann, N.B.; Hüffer, T.; Thompson, R.C.; Hassellöv, M.; Verschoor, A.; Daugaard, A.E.; Rist, S.; Karlsson, T.; Brennholt, N.; Cole, M.; et al. Are We Speaking the Same Language? Recommendations for a Definition and Categorization Framework for Plastic Debris. Environ. Sci. Technol. 2019, 53, 1039–1047. [Google Scholar] [CrossRef] [PubMed]
- Baloš, M.; Petrović, A.; Tubić, A.; Gvozdenac, S.; Prvulović, D.; Bursić, V. Significance of Microplastics in Agricultural Soil. JATEM 2023, 6, 910–918. [Google Scholar] [CrossRef]
- Bläsing, M.; Amelung, W. Plastics in soil: Analytical methods and possible sources. Sci. Total Environ. 2018, 612, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Khalid, N.; Aqeel, M.; Noman, A. Microplastics could be a threat to plants in terrestrial systems directly or indirectly. Environ. Pollut. 2020, 267, 115653. [Google Scholar] [CrossRef] [PubMed]
- FAO (Food and Agriculture Organization of the United Nations). The State of the World’s Land and Water Resources for Food and Agriculture-Systems at Breaking Point (Main Report); FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Espi, E.; Salmerón, A.; Fontecha, A.; García, Y.; Real, A.I. Plastic films for agricultural applications. J. Plast. Film Sheeting 2006, 22, 85–102. [Google Scholar] [CrossRef]
- Zeremski, T.; Vasin, J.; Milić, S.; Sekulić, P.; Hansman, S.; Bursić, V. Occurrence and distribution of the cyclodiene-type organochlorine pesticides in soils of Vojvodina Province, Serbia. J. Serb. Chem. Soc. 2016, 81, 707–716. [Google Scholar] [CrossRef]
- Đurović-Pejčev, R.; Radmanović, S.; Tomić, Z.; Kaluđerović, L.; Bursić, V.; Šantić, L. Adsorption-desorption behaviour of clomazone in Regosol and Chernozem agricultural soils. J. Serb. Chem. Soc. 2019, 85, 809–819. [Google Scholar] [CrossRef]
- Baensch-Baltruschat, B.; Kocher, B.; Kochleus, C.; Stock, F.; Reifferscheid, G. Tyre and road wear particles—A calculation of generation, transport and release to water and soil with special regard to German roads. Sci. Total Environ. 2021, 752, 141939. [Google Scholar] [CrossRef]
- Corradini, F.; Casado, F.; Leiva, V.; Huerta-Lwanga, E.; Geissen, V. Microplastics occurrence and frequency in soils under different land uses on a regional scale. Sci. Total Environ. 2021, 752, 141917. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, Q.; Jia, W.; Yan, C.; Wang, J. Agricultural plastic mulching as a source of microplastics in the terrestrial environment. Environ. Pollut. 2020, 260, 114096. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Chang, Y.; Zhang, T.; Qiao, Y.; Klobučar, G.; Li, M. Toxicological effects of polystyrene microplastics on earthworm (Eisenia fetida). Environ. Pollut. 2020, 259, 113896. [Google Scholar] [CrossRef] [PubMed]
- Ren, A.T.; Zhou, R.; Mo, F.; Liu, S.T.; Li, J.Y.; Chen, Y.; Zhao, L.; Xiong, Y.C. Soil water balance dynamics under plastic mulching in dryland rainfed agroecosystem across the Loess Plateau. Agric. Ecosyst. Environ. 2021, 312, 107354. [Google Scholar] [CrossRef]
- Horton, A.A.; Walton, A.; Spurgeon, D.J.; Lahive, E.; Svendsen, C. Microplastics in freshwater and terrestrial environments: Evaluating the current understanding to identify the knowledge gaps and future research priorities. Sci. Total Environ. 2017, 586, 127–141. [Google Scholar] [CrossRef]
- Rillig, M.C. Microplastic in terrestrial ecosystems and the soil? Environ. Sci. Technol. 2012, 46, 6453–6454. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, X.; Wang, J. Characterization of microplastics and the association of heavy metals with microplastics in suburban soil of central China. Sci. Total Environ. 2019, 694, 133798. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, M.; Huang, Q.; Khan, S.; Khan, M.A.; Liu, Y.; Wang, J.; Lian, F.; Wang, Q.; Guo, G. Microplastics in the soil environment: A critical review. Environ. Technol. Innov. 2022, 27, 102408. [Google Scholar] [CrossRef]
- Ding, W.; Li, Z.; Qi, R.; Jones, D.L.; Liu, Q.; Liu, Q.; Yan, C. Effect thresholds for the earthworm Eisenia fetida: Toxicity comparison between conventional and biodegradable microplastics. Sci. Total Environ. 2021, 781, 146884. [Google Scholar] [CrossRef]
- Calisi, A.; Zaccarelli, N.; Lionetto, M.G.; Schettino, T. Integrated biomarker analysis in the earthworm Lumbricus terrestris: Application to the monitoring of soil heavy metal pollution. Chemosphere 2013, 90, 2637–2644. [Google Scholar] [CrossRef]
- Paoletti, M.G. The role of earthworms for assessment of sustainability and as bioindicators. Agric. Ecosyst. Environ. 1999, 74, 137–155. [Google Scholar] [CrossRef]
- Ahmed, N.; Al-Mutairim, K.A. Earthworms Effect on Microbial Population and Soil Fertility as Well as Their Interaction with Agriculture Practices. Sustainability 2022, 14, 7803. [Google Scholar] [CrossRef]
- Büks, F.; Schaik, N.; Kaupenjohann, M. What do we know about how the terrestrial multicellular soil fauna reacts to microplastic? Soil 2020, 6, 245–267. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, X.; Wang, J. Ecotoxicological effects of microplastics and cadmium on the earthworm Eisenia foetida. J. Hazard. Mater. 2020, 392, 122273. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, X.; Leng, Y.; Wang, J. Defense responses in earthworms (Eisenia fetida) exposed to low-density polyethylene microplastics in soils. Ecotoxicol. Environ. Saf. 2020, 187, 109788. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, Y.; Song, X.; Li, M.; Yu, Y. Size effects of microplastics on accumulation and elimination of phenanthrene in earthworms. J. Hazard. Mater. 2021, 403, 123966. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Coffin, S.; Schlenk, D.; Gan, J.J. Accumulation of HOCs via pre-contaminated microplastics by earthworm Eisenia fetida in soil. Environ. Sci. Technol. 2020, 54, 11220–11229. [Google Scholar] [CrossRef] [PubMed]
- Haixiao, L.; Xueqiang, L.; Shiyu, W.; Boyang, Z.; Yan, X. Vertical migration of microplastics along soil profile under different crop root systems. Environ. Pollut. 2021, 278, 116833. [Google Scholar] [CrossRef]
- Boots, B.; Russell, C.W.; Green, D.S. Effects of Microplastics in Soil Ecosystems: Above and Below Ground. Environ. Sci. Technol. 2019, 53, 11496–11506. [Google Scholar] [CrossRef]
- Kim, S.W.; Liang, Y.; Zhao, T.; Rillig, M.C. Indirect Effects of Microplastic-Contaminated Soils on Adjacent Soil Layers: Vertical Changes in Soil Physical Structure and Water Flow. Front. Environ. Sci. 2021, 9, 681934. [Google Scholar] [CrossRef]
- de Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics Can Change Soil Properties and Affect Plant Performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef]
- Li, X.; Wang, Q.; Jiang, N.; Lv, H.; Liang, C.; Yang, H.; Yao, X.; Wang, J. Occurrence, source, ecological risk, and mitigation of phthalates (PAEs) in agricultural soils and the environment: A review. Environ. Res. 2023, 220, 115196. [Google Scholar] [CrossRef] [PubMed]
- Mai, H.; Thien, N.D.; Dung, N.T.; Valentin, C. Impacts of microplastics and heavy metals on the earthworm Eisenia fetida and on soil organic carbon, nitrogen, and phosphorus. Environ. Sci. Pollut. Res. 2023, 30, 64576–64588. [Google Scholar] [CrossRef]
- Zimmermann, L.; Dierkes, G.; Ternes, T.A.; Völker, C.; Wagner, M. Benchmarking the in vitro toxicity andchemical composition of plastic consumer products. Environ. Sci. Technol. 2019, 3, 11467–11477. [Google Scholar] [CrossRef] [PubMed]
- ISO 11464: 2004; Soil Quality—Pretreatment of Samples for Physico-Chemical Analyses. International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 10390: 1994; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 1994.
- ISO 14235: 1998; Soil Quality—Determination of Organic Carbon by Sulfochromic Oxidation. International Organization for Standardization: Geneva, Switzerland, 1998.
- ISO 10693: 1995; Soil Quality—Determination of Carbonate Content—Volumetric Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- Van Reeuwijk, L.P. Procedures for Soil Analysis, 6th ed.; Technical Paper No. 9; ISRIC: Wageningen, The Netherlands, 2002. [Google Scholar]
- A.O.A.C. AOAC Official Method 972.43. In Microchemical Determination of Carbon, Hydrogen, and Nitrogen, Automated Method; AOAC International, Ed.; Official Methods of Analysis of AOAC International; AOAC International: Gaithersburg, MD, USA, 2006. [Google Scholar]
- Egner, H.; Riehm, H.; Domingo, W.R. Untersuchungen über die chemische Bodenanalyse als Grundlage für die Beurteilung des Nahrstoffzustandes der Boden, II: Chemische Extractionsmetoden zu Phosphorund Kaliumbestimmung. Kungliga Lantbrukshügskolans Annaler 1960, 26, 199–215. [Google Scholar]
- Murphy, J.; Riley, J.P. A modified single solution method for determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- EN ISO 17512-1: 2020; Soil Quality—Avoidance Test for Determining the Quality of Soils and Effects of Chemicals on behaviour—Part 1: Test with Earthworms (Eisenia fetida and Eisenia andrei). International Organization for Standardization: Geneva, Switzerland, 2020.
- Sobhani, Z.; Panneerselvan, L.; Fang, C.; Naidu, R.; Megharaj, M. Chronic and transgenerational effects of polyethylene microplastics at environmentally relevant concentrations in earthworms. Environ. Toxicol. Innov. 2022, 25, 102226. [Google Scholar] [CrossRef]
- He, W.; Megharaj, M.; Naidu, R. Toxicity of perfluorooctanoic acid towards earthworm and enzymatic activities in soil. Environ. Monit. Assess. 2016, 188, 424. [Google Scholar] [CrossRef] [PubMed]
- Püntener, W. Manual for field trials in plant protection. In Agricultural Division, 2nd ed.; Ciba-Geigy Limited: Basel, Switzerland, 1981. [Google Scholar]
- Gebremeskel Weldmichael, T.; Szegi, T.; Denish, L.; Kumar Gangwar, R.; Michéli, E.; Simon, B. The patterns of soil microbial respiration and earthworm communities as influenced by soil and land-use type in selected soils of Hungary. Soil Sci. Ann. 2020, 71, 43–52, 139–148. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Ronca, S. Chapter 10: Polyethylene. In Brydson’s Plastics Materials, 8th ed.; Butterworth-Heinemann: Oxford, UK, 2017; pp. 247–278. [Google Scholar] [CrossRef]
- Hakkarainen, M.; Albertsson, A.C. Environmental Degradation of Polyethylene. In Long Term Properties of Polyolefins; Albertsson, A.C., Ed.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2004; Volume 169. [Google Scholar] [CrossRef]
- Qiang, L.; Hu, H.; Li, G.; Xu, J.; Cheng, J.; Wang, J.; Zhang, R. Plastic mulching, and occurrence, incorporation, degradation, and impacts of polyethylene microplastics in agroecosystems. Ecotoxicol. Environ. Saf. 2023, 263, 115274. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Ziersch, L.; Hempel, S. Microplastic transport in soil by earthworms. Sci. Rep. 2017, 7, 1362. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Seijo, A.; Lourenço, J.; Rocha-Santos, T.A.P.; Da Costa, J.; Duarte, A.C.; Vala, H.; Pereira, R. Histopathological and molecular effects of microplastics in Eisenia andrei Bouché. Environ. Pollut. 2017, 220, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Tilley, R.M.; Tyler, C.R.; Ormerod, S.J. Microplastic ingestion by riverine macroinvertebrates. Sci. Total Environ. 2019, 646, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Green, D.S.; Boots, B.; Sigwart, J.; Jiang, S.; Rocha, C. Effects of conventional and biodegradable microplastics on a marine ecosystem engineer (Arenicola marina) and sediment nutrient cycling. Environ. Pollut. 2016, 208, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Medyńska-Juraszek, A.; Szczepańska, A. Microplastic Pollution in EU Farmland Soils: Preliminary Findings from Agricultural Soils (Southwestern Poland). Agriculture 2023, 13, 1733. [Google Scholar] [CrossRef]
- Cao, D.; Wang, X.; Luo, X.; Liu, G. Effects of polystyrene microplastics on the fitness of earthworms in an agricultural soil. In Proceedings of the IOP Conference Series: Earth and Environmental Science. Third International Conference on Energy Materials and Environment Engineering, Bangkok, Thailand, 10–12 March 2017; Volume 61, p. 012148. [Google Scholar] [CrossRef]
- Wang, J.; Scott, C.; Chengliang, S.; Daniel, S.; Jay, G. Negligible effects of microplastics on animal fitness and HOC bioaccumulation in earthworm Eisenia fetida in soil. Environ. Pollut. 2019, 249, 776–784. [Google Scholar] [CrossRef]

| Sample | Coarse Sand (%) 2–0.2 mm | Fine Sand (%) 0.2–0.02 mm | Silt (%) 0.02–0.002 mm | Clay (%) <0.002 mm | Soil Texture Class * |
|---|---|---|---|---|---|
| Banat 1 | 1.26 | 35.50 | 30.20 | 33.04 | Loamy clay |
| Banat 2 | 0.43 | 22.45 | 32.92 | 44.20 | Loamy clay |
| Bačka | 36.43 | 56.25 | 4.84 | 2.48 | Loamy fine sand |
| Sample | pH | CaCO3 (%) | Organic Matter (%) | Total N (%) | AL-P2O5 mg/100 g | AL-K2O mg/100 g | |
|---|---|---|---|---|---|---|---|
| in KCl | in H2O | ||||||
| Banat 1 | 7.32 | 8.33 | 10.66 | 3.11 | 0.213 | 18.66 | 42.69 |
| Banat 2 | 6.85 | 7.83 | 1.11 | 3.27 | 0.224 | 57.08 | 72.02 |
| Bačka | 6.86 | 7.27 | 2.27 | 5.17 | 0.332 | 18.25 | 31.74 |
| Sample | MP Conc. (%) | Average Number of E. fetida | Mortality (%) | A (%) | Average Avoidance (%) | |
|---|---|---|---|---|---|---|
| Left Section (MP) | Right Section (Control) | |||||
| Banat 1 | 0.10 | 4.70 | 5.30 | 0.00 | 6.00 | 18.67 |
| 0.20 | 4.10 | 5.90 | 0.00 | 18.00 | ||
| 0.30 | 3.40 | 6.60 | 0.00 | 32.00 | ||
| Banat 2 | 0.10 | 4.50 | 5.50 | 0.00 | 10.00 | 23.70 |
| 0.20 | 3.60 | 6.40 | 0.00 | 28.00 | ||
| 0.30 | 3.30 | 6.50 | 2.00 | 33.11 | ||
| Bačka | 0.10 | 4.30 | 5.50 | 2.00 | 12.20 | 27.40 |
| 0.20 | 3.20 | 6.60 | 2.00 | 34.67 | ||
| 0.30 | 3.10 | 6.50 | 4.00 | 35.33 | ||
| Sample | Valid N | T | Z | p-Value |
|---|---|---|---|---|
| Banat 1 | 12 | 0.00 | 3.059412 | 0.002218 * |
| Banat 2 | 13 | 0.00 | 3.179797 | 0.001474 * |
| Bačka | 14 | 0.00 | 3.295765 | 0.000982 * |
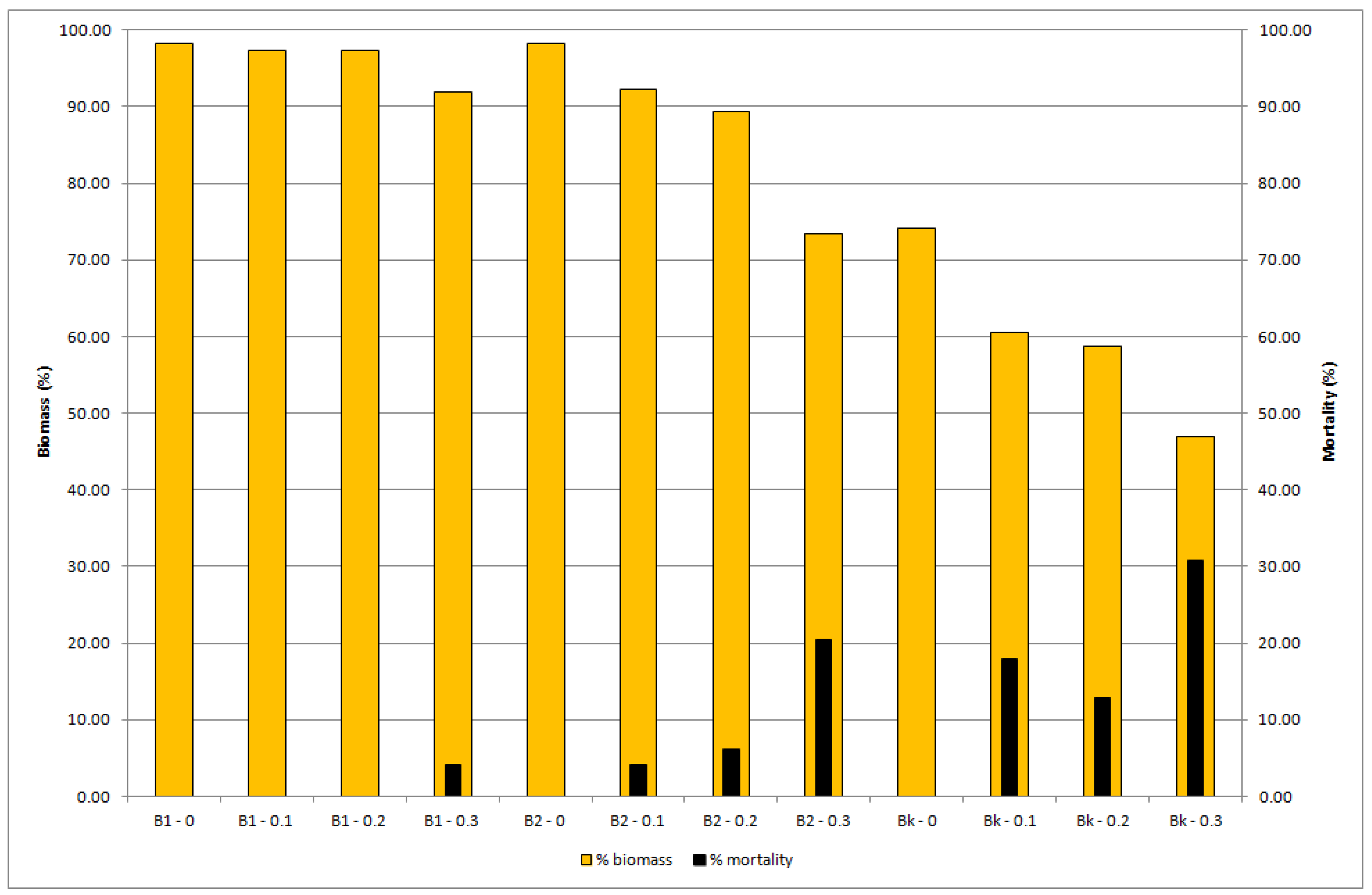
| Sample | MP Conc. (%) | Pre-Test Number | Pre-Test Biomass (g) | SD | Post-Test Number | Post-Test Biomass (g) | SD | Biomass (%) | SD | Biomass Change (of 100%) | M (%) | Mcorr. (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Banat 1 | - | 10 | 4.274 | 0.284 | 9.8 | 4.187 | 0.263 | 98.20 | 6.747 | −1.80 | 2.00 | |
| 0.10 | 10 | 4.099 | 0.203 | 9.8 | 3.989 | 0.255 | 97.31 | 3.778 | −2.69 | 2.00 | 0.00 | |
| 0.20 | 10 | 4.099 | 0.166 | 9.8 | 3.992 | 0.355 | 97.29 | 6.007 | −2.71 | 2.00 | 0.00 | |
| 0.30 | 10 | 4.100 | 0.103 | 9.4 | 3.772 | 0.241 | 91.97 | 4.668 | −8.03 | 6.00 | 4.08 | |
| Banat 2 | - | 10 | 3.999 | 0.293 | 9.8 | 3.923 | 0.425 | 98.16 | 8.395 | −1.84 | 2.00 | |
| 0.10 | 10 | 4.265 | 0.213 | 9.4 | 3.930 | 0.326 | 92.27 | 8.147 | −7.73 | 6.00 | 4.08 | |
| 0.20 | 10 | 4.070 | 0.188 | 9.2 | 3.638 | 0.438 | 89.41 | 10.388 | −10.59 | 8.00 | 6.12 | |
| 0.30 | 10 | 4.189 | 0.324 | 7.8 | 3.054 | 0.458 | 73.34 | 12.503 | −26.66 | 22.00 | 20.41 | |
| Bačka | - | 10 | 4.243 | 0.278 | 7.8 | 3.142 | 0.394 | 74.07 | 8.274 | −25.93 | 22.00 | |
| 0.10 | 10 | 4.427 | 0.382 | 6.4 | 2.673 | 0.257 | 60.56 | 6.078 | −39.44 | 36.00 | 17.95 | |
| 0.20 | 10 | 4.320 | 0.293 | 6.8 | 2.692 | 0.482 | 58.68 | 8.918 | −41.32 | 32.00 | 12.82 | |
| 0.30 | 10 | 4.290 | 0.459 | 5.4 | 1.805 | 0.617 | 46.95 | 11.836 | −53.05 | 46.00 | 30.77 |
| Dependent Variable | Categorical Variable | p Values | Significance: * for p < 0.05; ** for p < 0.01 |
|---|---|---|---|
| Post-test number of earthworms (ptn) | Soil sample | 0.000000 | ** |
| MP concentrations | 0.057028 | - | |
| Replicates | 0.931137 | - | |
| Post-test biomass (g) (ptb) | Soil sample | 0.000000 | ** |
| MP concentrations | 0.023188 | * | |
| Replicates | 0.700555 | - | |
| Biomass change (of 100%) (bc) | Soil sample | 0.000000 | ** |
| MP concentrations | 0.034925 | * | |
| Replicates | 0.893958 | - | |
| Mortality (%) (m) | Soil sample | 0.000000 | ** |
| MP concentrations | 0.057028 | - | |
| Replicates | 0.931137 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baloš, M.; Petrović, A.; Tubić, A.; Zeremski, T.; Gvozdenac, S.; Supić, D.; Bursić, V. Effects of Polyethylene Microplastics in Agricultural Soil on Eisenia fetida (Annelida: Oligochaeta) Behavior, Biomass, and Mortality. Agriculture 2024, 14, 578. https://doi.org/10.3390/agriculture14040578
Baloš M, Petrović A, Tubić A, Zeremski T, Gvozdenac S, Supić D, Bursić V. Effects of Polyethylene Microplastics in Agricultural Soil on Eisenia fetida (Annelida: Oligochaeta) Behavior, Biomass, and Mortality. Agriculture. 2024; 14(4):578. https://doi.org/10.3390/agriculture14040578
Chicago/Turabian StyleBaloš, Milica, Aleksandra Petrović, Aleksandra Tubić, Tijana Zeremski, Sonja Gvozdenac, Dejan Supić, and Vojislava Bursić. 2024. "Effects of Polyethylene Microplastics in Agricultural Soil on Eisenia fetida (Annelida: Oligochaeta) Behavior, Biomass, and Mortality" Agriculture 14, no. 4: 578. https://doi.org/10.3390/agriculture14040578





