Abstract
In this research, our objective was to investigate the combined impact of microbial extracts and chemical fungicides on Northern corn leaf blight (NCLB), which is induced by Exserohilum turcicum, and the growth-promoting effect of the crude extracts was also determined. NCLB poses a serious threat to global maize production, necessitating sustainable and environmentally friendly solutions. Mycelial growth rate assays were used to assess the single or synergistic effects of microbial crude extracts and chemical fungicides, and the seed-soaking and root irrigation method was used to detect the growth-promoting effect of the crude extracts on maize seedlings. The results revealed an 84.60% inhibition rate of B. amyloliquefaciens gfj-4 against E. turcicum, and with an EC50 of 49.01 mg·L−1 for the crude extracts. Chemical fungicides demonstrated varying toxicity levels, with fludioxonil exhibiting the highest potency. The mixture of the crude extracts and pyraclostrobin at an 8:2 volume ratio displayed the highest toxicity ratio of 1.24, indicating a synergistic effect. The selected combinations exhibited strong synergistic effects. Soaking maize seeds with 80 mg·L−1 of the crude extracts followed by root irrigation with 40 mg·L−1 produced the most significant growth-promoting effect on maize seedlings. This study highlights the potential of microbial crude extracts to enhance the control of NCLB when combined with pyraclostrobin, along with its growth-promoting effects on maize seedlings.
1. Introduction
Northern corn leaf blight (NCLB) poses a global threat to corn crops, causing substantial damage to leaf tissues, reducing photosynthetically active areas, and significantly impacting corn yield and quality [1,2]. Exserohilum turcicum is the causative agent of NCLB [3]. In epidemic years of northern corn leaf blight, susceptible varieties can experience 30–70% yield loss [4,5]. The repercussions of NCLB include premature aging of corn, making plants susceptible to other diseases like stalk rot, ultimately hastening corn plant death, and, in severe cases, leading to complete crop failure [6,7]. The development of NCLB-resistant varieties is a pivotal strategy for disease prevention. However, the extended breeding cycle and the variability in the physiological races of E. turcicum may compromise resistance in varieties [8,9]. Agricultural practices such as straw return and reduced-tillage systems have increased the accumulation of pathogenic fungi in field soils, promoting the prevalence of NCLB in maize production globally [10]. Thus, effective control measures are essential for the sustainable management of NCLB.
At present, studies on the control of NCLB have primarily focused on screening efficient biocontrol agents, synthesizing effective and low-risk chemical fungicides, and developing synergistic combinations for disease control [11]. There have been some reports on the use of biocontrol bacteria to control NCLB. Zhang et al. [12] explored a new strain of Chaetomium globosum No.05 and found that the germination of spores on the detached leaves of maize was completely inhibited when the proportion of biocontrol strain culture medium was 20%. The control effect of strain culture medium on NCLB was 81.9% 2 h after spraying spores of E. turcicum, and chaetoglobosin A was an important active component that exerted an antifungal effect. Zhang et al. [13] found that Klebsiella jilinsis 2N3 can promote the growth and chlorophyll synthesis of maize seedlings, and the disease index decreased by 67.44% compared with that of the control after 14 days of artificial inoculation. It also demonstrated that strain 2N3 can increase the activity of various stress-resistant enzymes in maize leaves. Ma et al. [14] screened 11 Trichoderma strains with strong inhibitory effect on E. turcicum using the confrontation culture method, among which Trichoderma asperellum 576 showed the strongest inhibitory activity. Spraying the spore suspension at a concentration of 107 spores·mL−1 significantly promoted seed germination and seedling growth and had a significant inhibitory effect on NCLB. In summary, many indoor control effect tests of biocontrol bacteria have shown excellent performance, but these bacteria are easily affected by environmental conditions and other factors during field application, so it is difficult to achieve the desired effect [15]. As the identification of highly effective and new chemical inhibitors for NCLB is slow and expensive, traditional fungicides are still used to control NCLB, mainly including triazole fungicides like tebuconazole, flutriafol, and strobilurin fungicides such as pyraclostrobin and azoxystrobin [16,17]. The prolonged application of chemical fungicides has resulted in the development of dominant resistant strains in agricultural fields, leading to a persistent rise in fungicide usage [18,19] and the release of excess chemical agents into the environment, ultimately causing severe ecological damage and agricultural product quality and safety concerns [20,21]. The proportion of mixed fungicide used for chemical disease control has been steadily rising, such as the mixture of tebuconazole and trifloxystrobin, flutriafol and azoxystrobin, among others [22,23,24]. However, it is crucial to note that due to the scarcity of new fungicides, the screening for new synergistic combinations has become increasingly challenging.
At present, active antimicrobial substances from other sources such as microbial metabolites have attracted more and more attention. Luo et al. [25] isolated lasiodiplodin from the mycelium of Lasiodiplodia pseudotheobromae J-10, which had the strongest inhibitory effect on E. turcicum, with an EC50 value of 15.50 μg·mL−1. The mycelium treated with lasiodiplodin was severely distorted, nonseptate mycelia appeared, and the relative permeability of mycelium cells increased. Limdolthamand et al. [26] isolated 73 strains of endophytic Trichoderma spp. from healthy maize leaves, of which 15 strains had high antagonistic activity. The control effect of Trichoderma harzianum KUFA0710 dry spore powder on NCLB was 47.46%, and the control effect of liquid preparation was 55.70%. The isolated Trichoderma spp. showed good compatibility with azoxystrobin and the mixture of difenoconazole and azoxystrobin, and had important value in the combination of biocontrol bacteria and chemical fungicides.
In a previous experiment, a bacterial strain gfj-4 with biocontrol potential was isolated from tomato fruits in our laboratory. The strain was identified as B. amyloliquefaciens by traditional physiological and biochemical methods and 16S rDNA sequencing. Strain gfj-4 displayed marked antagonistic effects against important fungal pathogens such as E. turcicum, Botrytis cinerea, Fusarium graminearum, Rhizoctonia solani, Alternaria solani, and Coniella diplodiella. The inhibitory activity against E. turcicum was the highest in vitro. This indicated that the fermentation supernatant contained components with high inhibitory activity against the pathogen. Therefore, this study aimed to isolate crude extracts from the fermentation supernatant for the following exploration. A mixture of antifungal agents with different mechanisms is more likely to be a synergistic combination [27]. It can be speculated that the inhibition mechanism of the metabolites of B. amyloliquefaciens gfj-4 against E. turcicum may be quite different from that of commonly used chemical fungicides, so there is a high probability of developing new synergistic combinations. In addition, the growth-promoting effect of microbial metabolites on crops has application value. Cun et al. [28] isolated 174 strains of endophytic microorganisms from the roots of maize seedlings, of which 21 strains had a good antagonistic effect on E. turcicum and promoted the growth of maize. In this study, on the basis of detection of the inhibitory activity of the fermentation metabolites of biocontrol bacteria and six chemical fungicides against E. turcicum, the synergistic combination of fermentation metabolites and chemical fungicides was screened, and a synergistic combination was obtained. Finally, the growth-promoting effect of the crude extracts on maize seedlings was determined. The aim was to provide an important basis for the sustainable control of NCLB, the utilization of biocontrol bacteria, and the reduction in the use of chemical fungicides.
2. Materials and Methods
2.1. Fungicides, Plant Materials, Pathogens, and Antagonistic Bacteria
Fungicides included prochloraz (Shandong Huayang Technology Co., Ltd., Tai’an, China), propiconazole and hexaconazole (Jiangsu Fengdeng Crop Protection Co., Ltd., Changzhou, China), pyraclostrobin (Qingdao Hansheng Biotechnology Co., Ltd., Qingdao, China), trifloxystrobin (Zhejiang Yulong Biotechnology Co., Ltd., Jiaxing, China ), and fludioxonil (Jiangsu Yunnong Chemical Co., Ltd., Zhenjiang, China), culture substrate (Huai’an Zhonghe Agricultural Science and Technology Development Co., Ltd., Huai’an, China). Northern corn leaf blight pathogen (E. turcicum (Pass.) Leonard et Suggs), B. amyloliquefaciens gfj-4, and maize inbred line 21, which is susceptible to NCLB, were preserved in the Plant Soil Borne Disease Control Laboratory of Anhui Science and Technology University, China.
2.2. Inhibition Effect of B. amyloliquefaciens gfj-4 on E. turcicum
Using the confrontation culture method, 15 mL of PDA (potato dextrose agar) media was added to a culture dish [29]. Following solidification of the medium, a 4-day-old E. turcicum culture was added to the center of the dish. Filter papers were positioned in a cross shape surrounding the pathogen, spaced 5 cm apart. Then, 2 μL of strain gfj-4 culture with an OD600 = 1.0 was dispensed onto the filter paper using a pipette, while sterile water served as the control. The Petri dishes were incubated at a temperature of 26 °C for a duration of 96 h, and the percentage of colony growth inhibition was determined using the following method: the size of the E. turcicum colonies on control PDA plates (ΦA) and on PDA plates co-cultured with strain gfj-4 (ΦB) was recorded, and the inhibition percentages were calculated as follows: inhibition ratio (%) = (ΦA − ΦB)/(ΦA-diameter of fungus cake) × 100. Each treatment was performed in three repetitions for accuracy.
2.3. Inhibitory Activity of Microbial Crude Extracts and Chemical Fungicides on E. turcicum
The method of Ohno et al. [30] was used to prepare the crude extracts of B. amyloliquefaciens. The inhibitory activity was assessed by measuring the rate of mycelial growth [31]. The microbial crude extracts were diluted to different concentrations of 3200, 1600, 800, 500, 400, and 320 mg·L−1. Six fungicides were used, and the final concentrations were set as shown in Table 1. Next, 5 mL of every diluted extracts was mixed with 45 mL of PDA medium, along with 1 mL of streptomycin sulfate at a concentration of 2500 mg·L−1 to inhibit bacterial growth. After the agar plates solidified, a 4-day-old culture of the pathogen was inoculated in the center of each plate. The plates were placed in a growth chamber at 26 °C for a period of 96 h, during which the diameters of the colonies were assessed to determine a dose–response equation and the EC50 value.

Table 1.
Concentration settings for six fungicides.
2.4. Synergistic Effects of Microbial Crude Extracts and Chemical Fungicides
Based on the EC50 values of the microbial crude extracts and fungicides against E. turcicum, eleven volume ratios were prepared (10:0, 9:1, 8:2, 7:3, 6:4, 5:5, 4:6, 3:7, 2:8, 1:9, 0:10), and sterile water was used as the control. The actual inhibition rate and toxicity ratio of each ratio were determined based on the mycelial growth rate [31]. Then, the optimal volume ratio was verified according to Wadley’s method [32], and the dose–response equation, EC50, and synergistic ratio (SR) were calculated.
2.5. Control Effects of Selected Combinations on NCLB
2.5.1. Control Effects In Vitro
Uniform and intact maize leaves were rinsed with sterile water at least three times. Then, the leaves were treated with a mixture of pyraclostrobin (29.16 mg·L−1) and microbial crude extracts (157.06 mg·L−1) at the EC80 concentration. The mixture was prepared with the optimal volume ratio of 8:2 for microbial crude extracts and pyraclostrobin. The treated leaves were immersed in the solution for 30 s and then placed in a fresh-keeping box with filter papers; each box contained two leaves. A 4-day-old culture of the pathogen (Φ = 7 mm) was inoculated on leaf surfaces, and sterile water was used as the control. Following 4 days of treatment, the longest length and the widest width of the lesion were measured, and the inhibition rate was determined based on the half of the sum of the two as the size of the lesion. The lesion sizes of the control (ΦC) and the treatments (ΦD) were recorded, and the percentage of inhibition rate was calculated as follows: inhibition ratio (%) = (ΦC − ΦD)/(ΦC) × 100. Each treatment was repeated three times.
2.5.2. Control Effects In Vivo
Maize seeds were soaked in sterile water for germination. After the seeds sprouted, six seeds were transplanted into pots filled with culture substrate (top diameter 16.0 cm, height 14.0 cm). After the seedlings reached the three-leaf stage, the following experiments were conducted:
(1) Preventive effect: We prepared the EC80 concentrations of pyraclostrobin (29.16 mg·L−1) and crude extracts (157.06 mg·L−1). The combination of microbial crude extracts and pyraclostrobin (157.06 mg·L−1 and 29.16 mg·L−1, respectively, with a volume ratio of 8:2) was prepared immediately before use, and sterile water was used as the control. After spraying, the plants were placed in a growth chamber (light: 8 h, 6000 lux, 24 °C; dark: 16 h, 20 °C; RH 90%). Following 12 h of treatment, a solution containing 105 CFU·mL−1 (colony-forming units per mL) of E. turcicum was applied through spraying. The calculation of the disease index was conducted using the standards outlined in the study conducted by Liu et al. (2018) [33]. Each treatment was replicated three times.
(2) Curative effect: A suspension of 105 CFU·mL−1 of E. turcicum was sprayed on the maize seedlings. The plants were then placed in a growth chamber. In 12 h intervals, pyraclostrobin, microbial crude extracts, and the combination of microbial crude extracts and pyraclostrobin were sprayed, separately, at each of the concentrations used in the preventive group. Sterile water was used as a control. Each treatment was subjected to three repetitions. The disease index calculation method and control effect calculation method were consistent with those of the preventive group.
2.6. Effects of Maize Seeds Soaked with Crude Extracts of Fermentation Broth on the Growth of Maize Plants
Maize seeds were treated with 75% ethanol for 3 min to sterilize them. Following this, the seeds were washed thrice with sterile water. They were then exposed to various diluted concentrations of microbial crude extracts, with sterile water serving as a control. After 12 h of treatment, the seeds were cultured in boxes with 20 seeds per box and placed in a growth chamber (light: 8 h, 6000 lux, 24 °C; dark: 16 h, 20 °C; RH 90%). Ten days later, the fresh and dry weights of both the aboveground and underground components of maize seedlings were documented. Each treatment was replicated three times.
2.7. Effects of Crude Extracts on the Growth of Maize Plants
2.7.1. Soil Preparation
The soil that was prepared had organic matter totaling 13.4 g/kg, nitrogen available at 51.6 mg/kg, phosphorus available at 20.4 mg/kg, potassium available at 73.1 mg/kg, with a pH of 6.5. A mixture of soil and culture substrate was combined in a 2:1 ratio. This combined mixture was then subjected to sterilization at 121 °C for a duration of 30 min. Following sterilization, the mixture was left to dry naturally in a room before planting maize.
2.7.2. Growth Promotion Effect Determination
Maize seeds were immersed in 80 mg·L−1 of microbial crude extracts for 12 h, and then six seeds were sown in each pot (pot diameter 16.0 cm, height 14.0 cm, installing 450 g above prepared soil). These pots were placed in a growth chamber (light: 8 h, 6000 lux, 24 °C; darkness: 16 h, 20 °C; RH 90%). Following a 7-day cultivation period, a pair of robust seedlings per container was retained for the subsequent trial. The roots of the maize plants were watered with varying levels of microbial crude extracts at a concentration of 10, 20, 40, 80, 160, or 320 mg·L−1, and sterile water was used as a control. There were three pots per treatment. After 10 days of continuous culture, the fresh and dry weights of the aboveground and underground parts of maize seedlings were measured.
2.8. Data Analysis
IBM SPSS software 25.0 was employed for performing statistical analysis. Mean ± standard error from three biological replicates is presented in the dataset. Duncan’s multiple range tests were carried out using a one-way analysis of variance (ANOVA) to identify significant differences among the different experimental groups. Distinct letters are used to denote statistically significant differences, indicating a significance level of p < 0.05.
3. Results
3.1. Antagonistic Effects of B. amyloliquefaciens on E. turcicum
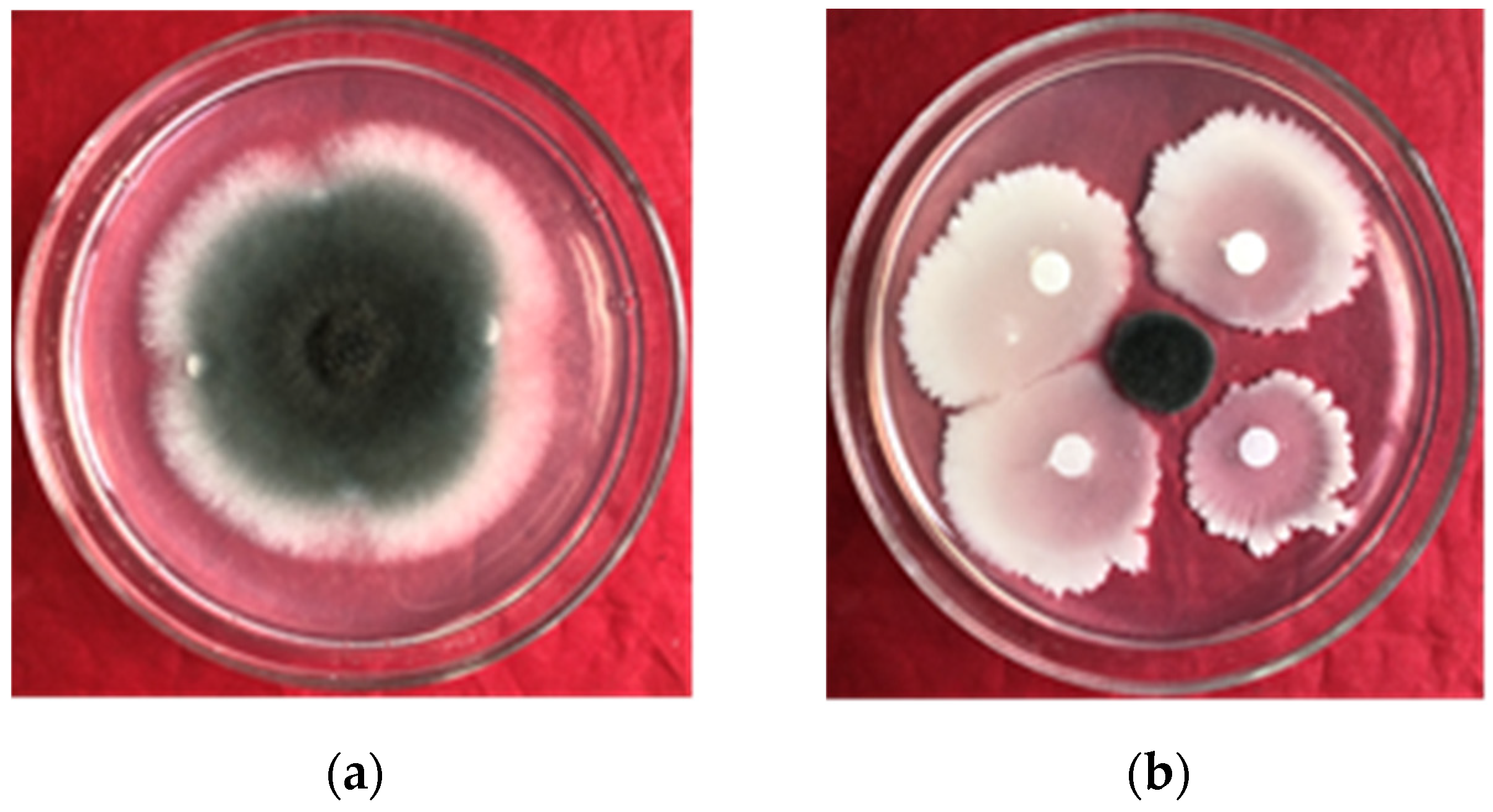
After 96 h of coculture, B. amyloliquefaciens gfj-4 exhibited stronger inhibitory effects on E. turcicum than the control (Figure 1). In the presence of gfj-4 crude extracts, the growth of E. turcicum was notably impeded, resulting in an inhibition rate of 84.60% ± 1.25%. This indicated the significant inhibitory effect of B. amyloliquefaciens gfj-4 on E. turcicum.
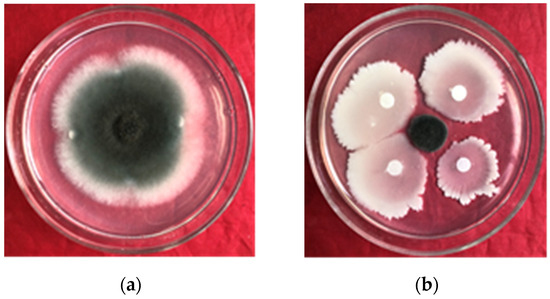
Figure 1.
Confrontation culture of B. amyloliquefaciens gfj-4 and E. turcicum after 96 h; (a) and (b) the control and the inhibition effect of strain gfj-4 of E. turcicum, respectively.
3.2. Inhibitory Effects of Crude Extracts and Chemical Fungicides on E. turcicum
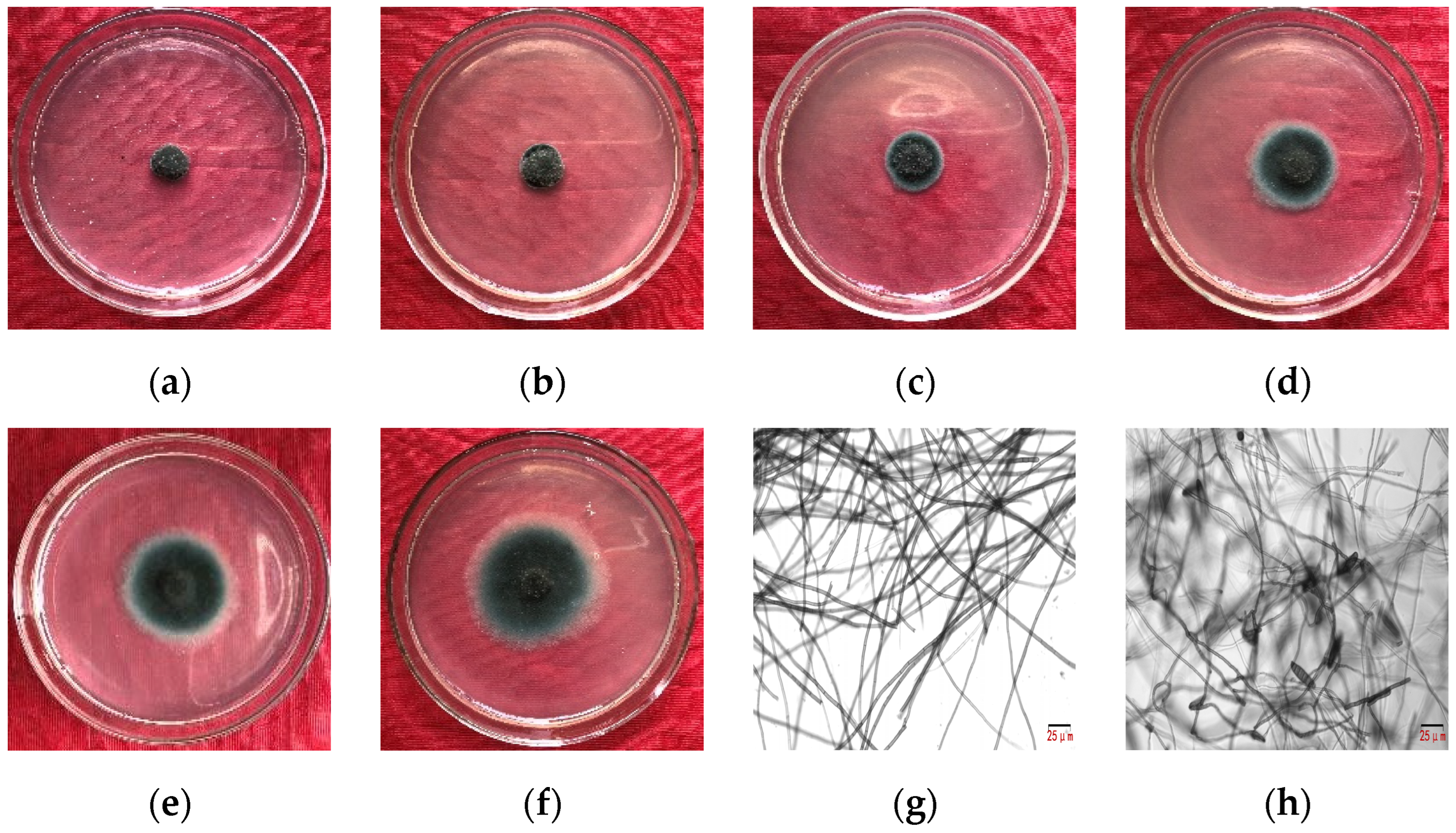
As indicated in Table 2, the concentration of microbial crude extracts from 32.0 mg·L−1 to 320.0 mg·L−1 correlated with an increased inhibition rate of E. turcicum, ranging from 35.36% to 89.86%. The EC50 of the microbial crude extracts against E. turcicum was 49.01 mg·L−1, with a toxicity regression equation of y = 1.66x + 2.22 (R2 = 0.98). Notably, even at 50.0 mg·L−1, the inhibition rate exceeded 50%, indicating a substantial inhibitory effect. Morphological observations revealed distorted hyphae, increased septa, bead-like terminal ends, and augmented spore production (Figure 2).

Table 2.
Inhibition effect of crude extracts of fermentation broth mixed with six fungicides on E. turcicum.
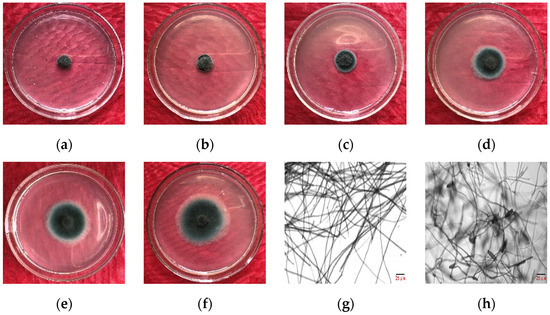
Figure 2.
Inhibition effect of crude extracts from B. amyloliquefaciens gfj-4 on E. turcicum by mycelial growth rate method. (a–f) Colony size of the treatment with 320.0, 160.0, 80.0, 50.0, 40.0, and 32.0 mg·L−1 after 96 h of culture, respectively. (g) Normal hyphae of E. turcicum and (h) deformed hyphae of E. turcicum treated with microbial crude extracts of 49.01 mg·L−1 after 96 h of culture.
The lower the EC50 value of the chemical fungicide, the better the inhibitory effect on the pathogen, and vice versa [34]. Among the fungicides tested, fludioxonil exhibited the highest toxicity, with an EC50 of 0.05 mg·L−1. Within the ergosterol biosynthesis inhibitors (EBIs), prochloraz, hexazole, and propiconazole displayed toxicity with EC50 values of 0.15, 0.26, and 0.32 mg·L−1, respectively. Strobilurin fungicides had relatively higher EC50 values: pyraclostrobin at 8.55 mg·L−1 and trifloxystrobin at 16.47 mg·L−1 (Table 3).

Table 3.
Antifungal activity of six fungicides against E. turcicum.
3.3. Antagonistic Effects of the Combination of Microbial Crude Extracts and Chemical Fungicides
The toxicity ratio of microbial crude extracts combined with triazole fungicides demonstrated an additive effect. The most significant enhancement occurred at a volume ratio of 3:7 for microbial crude extracts to hexaconazole, yielding a toxicity ratio of 1.1. The combination with pyraclostrobin primarily exhibited an enhancing or additive effect, peaking at a volume ratio of 8:2, with a toxicity ratio of 1.24 (Table 4). Overall, the combination with pyraclostrobin displayed substantial enhancement, leading to further assays on the combination of microbial crude extracts and pyraclostrobin.

Table 4.
Inhibition effect of microbial crude extracts mixed with six fungicides on E. turcicum.
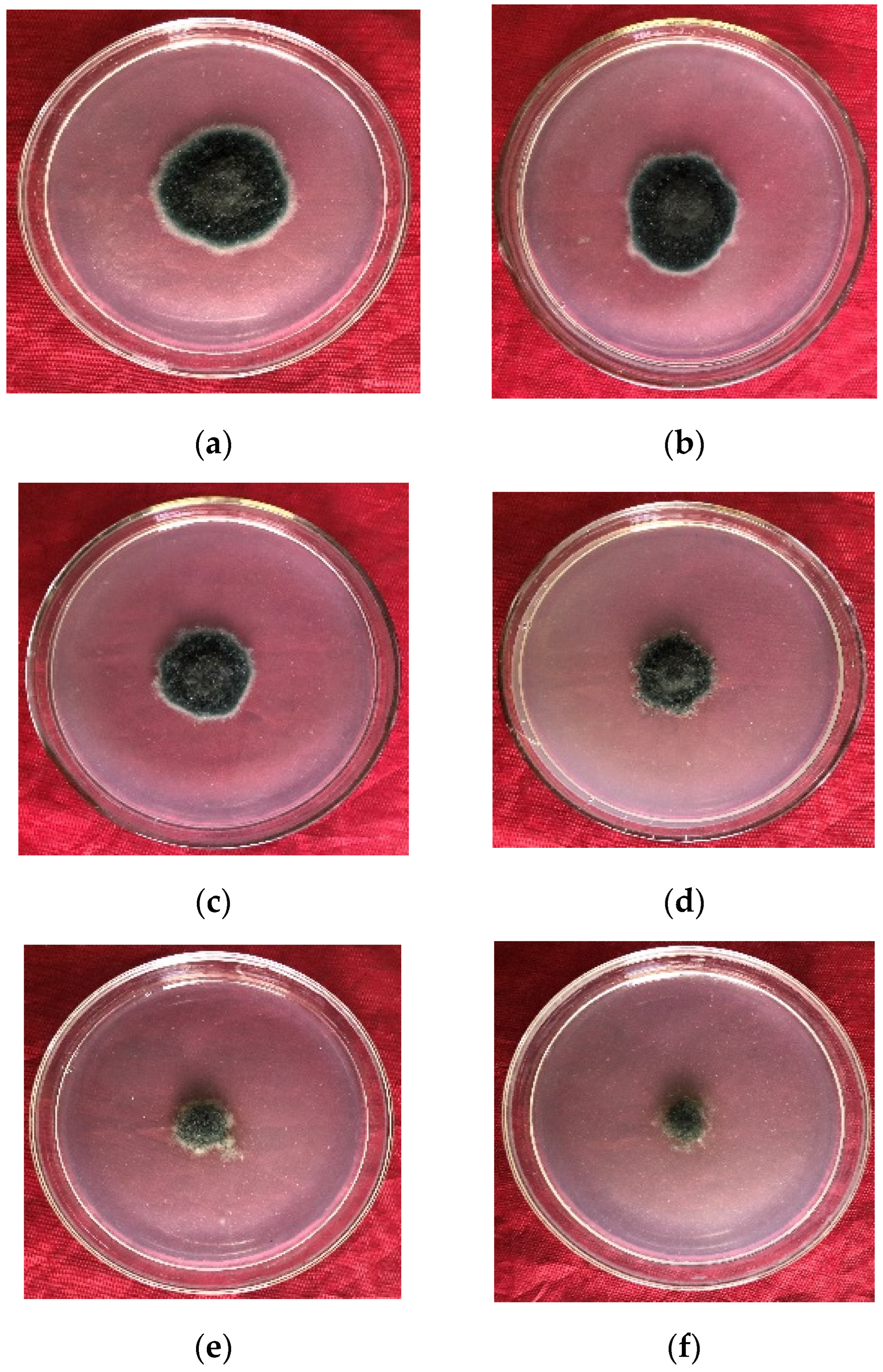
As the level of the microbial crude extracts combined with pyraclostrobin rose, so too did the level of inhibition of E. turcicum (Table 5). When the concentration reached 120 mg·L−1, the inhibition rate peaked at 81.54% ± 0.92%. The toxicity regression equation for the mixture was y = 0.92x + 3.86 (R2 = 0.95), with a theoretical EC50 for E. turcicum of 40.91 mg·L−1. The observed EC50 value was 17.29 mg·L−1, with an SR (synergistic ratio) of 2.37, confirming a synergistic effect. High concentrations of the mixture significantly inhibited mycelial growth. As the concentration increased, the colony color gradually changed from olive to light yellow, and the growth momentum weakened (Figure 3).

Table 5.
Inhibition effect of microbial crude extracts mixed with pyraclostrobin on E. turcicum.
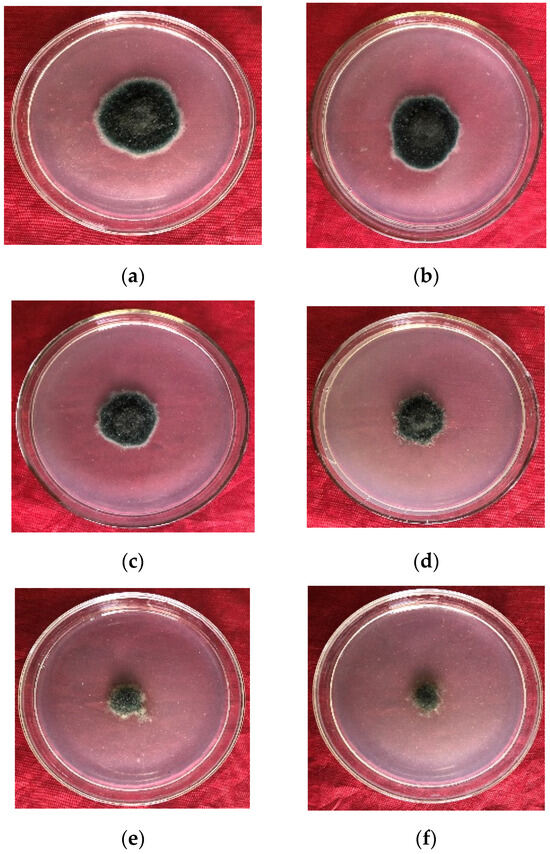
Figure 3.
Inhibition effect of mixture of microbial crude extracts and pyraclostrobin on E. turcicum by Wadley method: (a–f) 5, 10, 20, 40, 80, and 120 mg·L−1 after 96 h of culture, respectively.
3.4. Control of Pathogen Infection on Maize Leaves with Microbial Crude Extracts and Pyraclostrobin
As shown in Figure 4, E. turcicum infection produced severe symptoms on detached leaves. However, the leaves pretreated with microbial crude extracts showed a reduction in pathogen infection, with an inhibition rate of 36.36%. Treatment with pyraclostrobin inhibited the expansion of lesions, and, when compared to single treatment with microbial crude extracts, pyraclostrobin showed greater control efficacy, with an inhibition rate of 55.84%. The lesion areas were smaller with combined treatment, maintaining an overall green color compared to the controls, indicating a better control effect with an inhibition rate of 63.64%. Thus, the combined treatment showed a synergistic inhibitory effect on E. turcicum.

Figure 4.
Inhibition effect of different treatments on leaf inoculation of E. turcicum. (a–d) The control, microbial crude extracts, pyraclostrobin, and synergistic combination treatment, respectively.
3.5. Enhancing Control Efficacy of E. turcicum on Maize Plants by Combining Microbial Crude Extracts and Pyraclostrobin
The disease index of the control treatments from the preventive and curative groups was 87.30% and 77.78%, respectively (Table 6). The maize seedlings in the control treatments exhibited symptoms of leaf withering and death (Figure 5). Moreover, the combination of microbial crude extracts and pyraclostrobin further demonstrated synergistic efficacy, reaching the highest efficacy at 40.0% for the preventive group and 56.48% for the curative group (p < 0.05). The control efficacy of the combined treatments was increased by 13.18% and 9.57% compared to single treatments in the preventive group and by 19.93% and 14.96% in the curative group, respectively. The curative effect on E. turcicum was superior to the preventive effect.

Table 6.
Potted control effect of microbial crude extracts mixed with pyrazoxystrobin on NCLB.

Figure 5.
Control effect of curative methods on E. turcicum. (a–d) The CK, microbial crude extracts, pyraclostrobin, and mixture in the curative group, respectively.
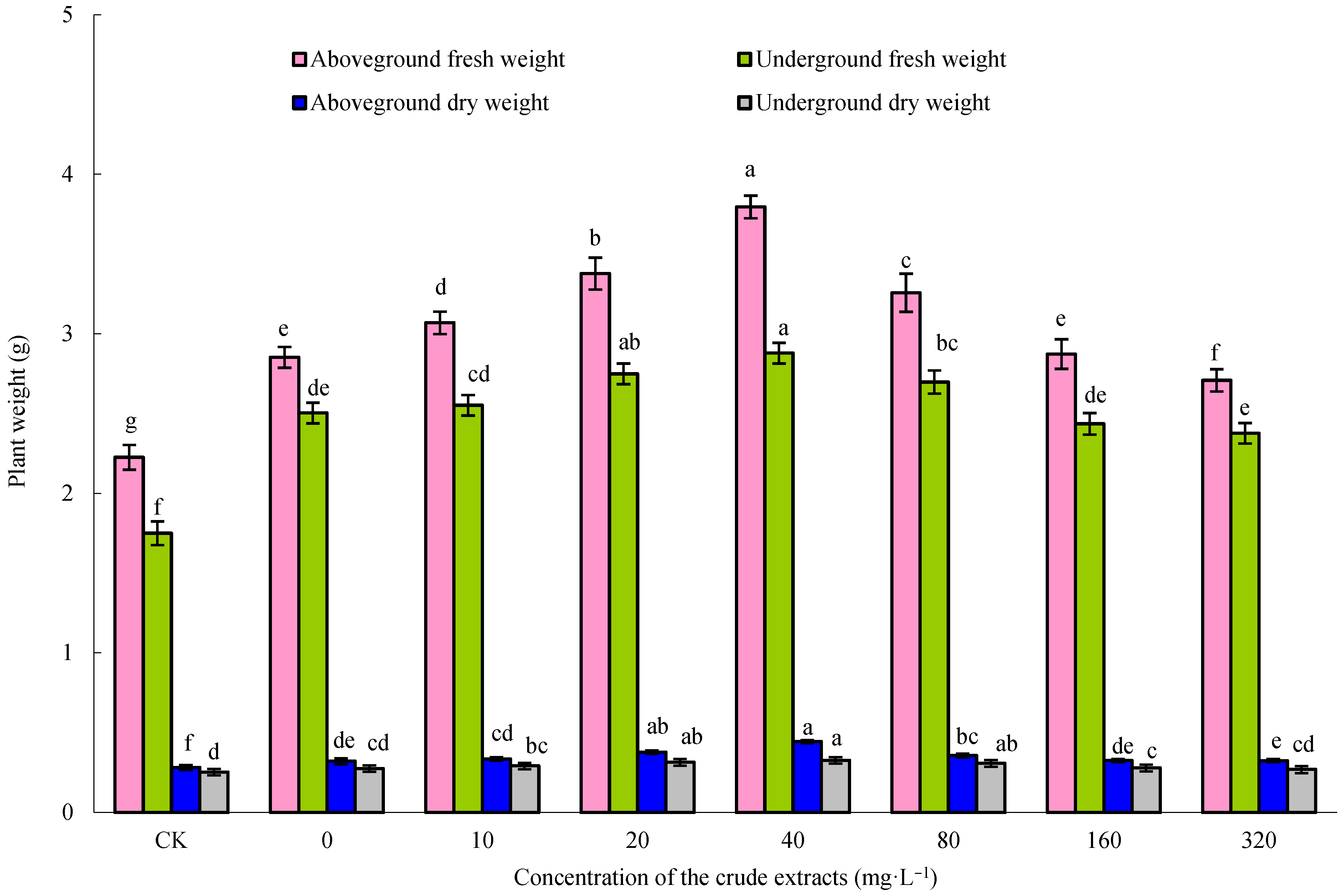
3.6. Growth-Promoting Effects of Microbial Crude Extracts on Maize Plants
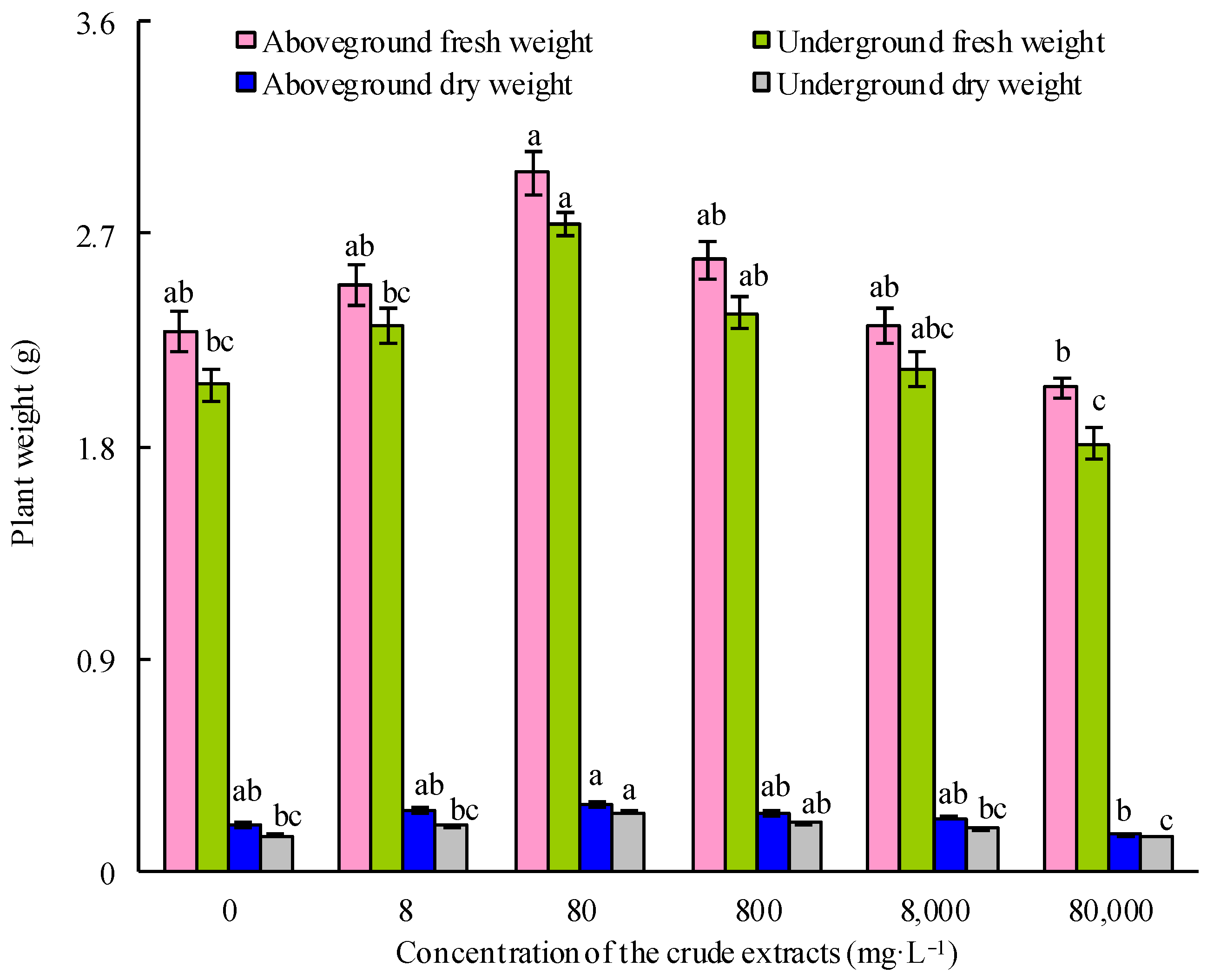
With the increase in the seed soaking concentration of the microbial crude extracts, the fresh and dry weights of both the aboveground and underground parts of the maize seedlings initially increased and then decreased (Figure 6). Upon treatment with 80 mg·L−1 crude extracts, maize seedlings exhibited the highest fresh and dry weights (p < 0.05). This optimal concentration of microbial crude extracts was employed in the subsequent experiments. As shown in Figure 7, the crude extracts, when utilized as a seedsoaking solution at a concentration of 80 mg·L−1 followed by subsequent watering with sterile water, exhibited a notable growth-promoting effect on maize plants compared to the control group. These findings suggested that an optimal concentration of microbial crude extracts for seed soaking indeed bolstered the growth of maize seedlings. Remarkably, the most significant impact on the growth of maize seedlings was observed when using a soaking concentration of 80 mg·L−1 of the extracts, followed by root irrigation with a concentration of 40 mg·L−1. These findings demonstrate the effectiveness of using crude extracts at ideal concentrations and in consecutive applications to enhance the growth of maize seedlings, as shown by the notable enhancement in growth factors.
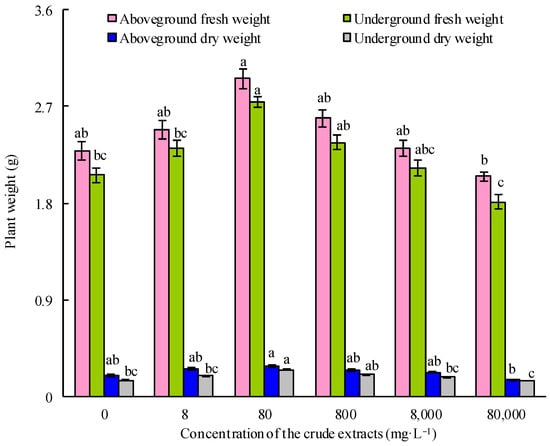
Figure 6.
Effect of soaking seeds with different concentrations of microbial crude extracts on the growth of maize seedlings. Bars above columns represent the standard error.
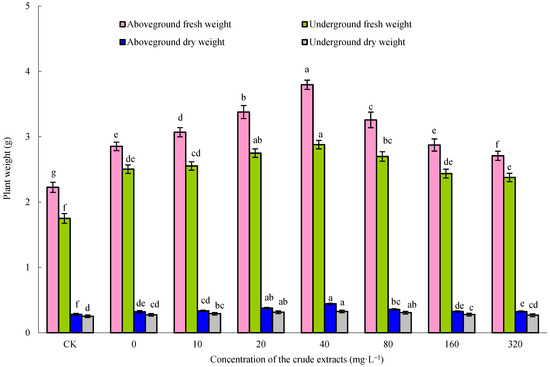
Figure 7.
Effect of microbial crude extracts on dry and fresh weight of aboveground and underground maize parts. Bars above columns represent the standard error.
4. Discussion
Maize is a crucial global crop, playing a significant role in ensuring food and feed security [35,36]. NCLB, a major threat to global maize production, is characterized by rapid spread, high outbreak frequency, and causing extensive damage [37]. Current control strategies for NCLB encompass resistance breeding, chemical treatments, and biological control [38,39]. Chemically spraying fungicides during the corn tasseling period remains a prevalent preventive measure against NCLB, with propiconazole, azoxystrobin, and pyraclostrobin being common choices [40,41]. In this study, toxicity assessments revealed that fludioxonil exhibited the highest toxicity, followed by C-14 demethylation inhibitors, including tebuconazole, hexaconazole, and propiconazole. Pyrazoxystrobin is an important class of respiratory inhibitors, mainly acting on cytochrome bc1Q0 ubiquinone oxidase. It is widely used in the prevention and cure of maize leaf spot diseases [42]. In order to avoid the rapid development of resistance to pyrazoxystrobin and prolong its lifespan, it is necessary to search for an exogenous substance that can enhance its inhibitory activity.
Sartori et al. [43] investigated the inhibitory effects of various biocontrol agents, including genera such as Bacillus, Pantoea, Corynebacterium, and Enterococcus, on NCLB. Leaf spraying with these agents reduced disease incidence by 30–78% on day 20 and 39–56% on day 39. The application of B. amyloliquefaciens crude extracts to maize leaves, particularly at the 10-leaf stage, demonstrated optimal disease control efficacy, with high stability on the leaf surface. Liu et al. [33] isolated Chaetomium globosum LB-2, which exhibited significant inhibition against the NCLB pathogen. The application of 1 mg·mL−1 n-butanol crude extract resulted in an 89.99% inhibition rate. In greenhouse trials, 1000.0 mg·L−1 n-butanol crude extract effectively reduced disease severity by 53.47%, suggesting potential antifungal applications through competition and secretion of antifungal substances. Luo et al. [44] isolated a strain from Stephania kwangsiensis and found that the ethyl acetate extract from its liquid fermentation inhibited E. turcicum with an EC50 of 10.0 mg·L−1. One component, griseofulvin, displayed an EC50 of 1.3 mg·L−1, indicating its potential value in disease control. In this study, the EC50 of B. amyloliquefaciens gfj-4 crude extract against E. turcicum was 49.01 mg·L−1, indicating the need for further purification and the identification of its active components. Chang et al. [45] demonstrated the effectiveness of Beauveria bassiana inoculation in reducing the incidence of NCLB. Additionally, Burkholderia and Pseudomonas abundance increased in maize roots, suggesting synergy between biocontrol agents and beneficial indigenous bacteria and fungi. Zahra et al. [46] explored the use of biochar derived from cow dung to enhance soil quality and induce resistance, which reduced the incidence of NCLB. This study highlights the potential of beneficial microorganisms, such as the B. amyloliquefaciens gfj-4, in controlling aboveground diseases through root application. This research examined the suppressive impact of strain gfj-4 crude extracts on NCLB. While additional investigation is needed on the active ingredients, the promise of strain gfj-4 as a biocontrol agent for managing NCLB is clear. The combination of microbial crude extracts and triazole fungicides showed an additive effect, with pyraclostrobin demonstrating significant enhancement. The application of this combination demonstrated synergistic efficacy against NCLB. Moreover, the gfj-4 crude extracts exhibited a growth-promoting effect on maize plants. These findings provide valuable insights into the potential of strain gfj-4 as a biocontrol agent and the development of novel antifungal substances. Further research is needed to identify strategies for sustainable maize production.
The inhibitors of ergosterol biosynthesis affect the structure and function of the cell membrane of fungi [47]. In this study, the synergistic effect of microbial crude extracts and fungicides on E. turcicum was not significant, which may have been caused by the similar action mechanisms of the microbial crude extracts and fungicides. There have been many reports on the research on biocontrol bacteria and their mixed microbial crude extracts and chemical fungicides in managing plant diseases. Ji et al. [48] investigated the bacteriostasis effect of crude Bacillus extracts combined with fludioxonil on tomato grey mold and showed that the bacteriostasis effect of the mixed treatment was higher than that of the single treatment. It was also found that there was a synergistic effect between the bacteria and fungicides in pot experiments. The control effect of 108 CFU·mL−1 of Bacillus TA-1 and 50 g·hm−2 of fludioxonil on grey mold could reached 70.16%, which was higher than that of the single treatment. Kim et al. [49] showed that the butanol extract of the fermentation broth of B. amyloliquefaciens JCK-12 and a mixture of iprodione, fludioxonil, benomyl, difenoconazole, and tebuconazole had obvious synergistic activity against F. graminearum, which causes scab of wheat, and it was concluded that it had the strongest synergistic activity with benomyl. Further greenhouse and field efficacy tests showed that the usage of chemical fungicides could be significantly reduced. It was speculated that the reason for the synergistic effect of the combination was that the crude extracts increased the permeability of the cell membrane by destroying its structure. Next, the dose of the chemical fungicides entering the cells increased, indicating that microbial crude extracts can enhance the sensitivity of pathogenic fungi to some fungicides. Pyraclostrobin is one of the most widely used chemical fungicides, and it has great adaptability in mixing with other types of fungicides [50]. In this study, the volume ratio of microbial crude extract and pyraclostrobin was 8:2, which exhibited synergistic effects on pathogen infection. Therefore, the crude extracts from B. amyloliquefaciens gfj-4 enhanced the antibacterial activity of pyraclostrobin. However, what components of the crude extract played the main role remains to be further explored.
It has been reported that one of the biocontrol mechanisms of Bacillus species is the induction of compensation or tolerance caused by its promotion of growth [51,52]. Nunes et al. [53] reported that both B. subtilis FMCH002 and B. licheniformis FMCH001 can significantly promote the growth of tomato plants, which can produce IAA. Cui et al. [54] found that a strain of B. amyloliquefaciens, B9601-Y2, can significantly promote the growth of maize seedlings and the activity of soil enzymes, thereby reducing disease incidence and improving the yield of maize. This study indicated that soaking seeds with 80 mg·L−1 crude extract followed by root irrigation with 40 mg·L−1 crude extract had a significant effect on the growth of maize. However, the active components in the crude extracts that play a role in promoting growth remain to be further explored.
5. Conclusions
In summary, this study underscores the inhibitory potential of B. amyloliquefaciens gfj-4 and its crude extracts against NCLB. A promising strategy for enhanced disease control was demonstrated by the synergistic effects observed when a microbial crude extract was combined with chemical fungicides, particularly pyraclostrobin, at a volume ratio of 8:2 (microbial crude extracts: pyraclostrobin at the EC50). Additionally, the dual benefits of utilizing microbial crude extracts in agricultural practices was demonstrated by the growth-promoting effects on maize plants. This was achieved through seed soaking at a concentration of 80 mg·L−1 of the crude extracts, followed by root irrigation at a concentration of 40 mg·L−1. The results lay the foundation for utilizing and implementing these synergistic combinations as efficient and long-lasting strategies for managing NCLB and enhancing maize growth.
6. Patents
A Chinese patent named “Bacillus amyloliquefaciens gfj-4 and its composition” resulted from this work. Inventor: Haiming Duan and Li Yu. Patentee: Anhui Science and Technology University.
Author Contributions
Conceptualization, H.D.; methodology, M.W.; software, X.M.; validation, H.D. and C.Z.; formal analysis, M.W.; investigation, L.Y.; resources, H.D.; data curation, H.D.; writing—original draft preparation, M.W.; writing—review and editing, H.D. and C.Z.; visualization, W.L.; supervision, H.Y.; project administration, H.D.; funding acquisition, H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Anhui Provincial Natural Science Foundation (2308085MC90, 2023AH020024), the Open Fund of National and Local Joint Engineering Laboratory for Crop Stress Resistance Breeding and Disaster Reduction (NELCOF20190102), the Science and Technology Plan Project of Fengyang County (NY2022-05, 2023TPY04), and the Science and Technology Plan Project of Shouxian County (2023SY31).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Giulio, T.; Amedeo, R.; Massimo, B. Foliar fungicide application to maize: Yield and grain hardness enhancement in different environmental conditions. Crop Sci. 2015, 55, 1782–1790. [Google Scholar]
- Liu, H.; Guo, F.F.; Chen, X.L.; Wu, B.M. Temporal progress and spatial patterns of northern corn leaf blight in corn fields in China. Phytopathology 2022, 112, 1936–1945. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Cao, Z.Y.; Cao, K.K.; Ma, S.X.; Gong, X.D.; Jia, H.; Dai, D.Q.; Dong, J.G. Identification of laccase-like multicopper oxidases from the pathogenic fungus Setosphaeria turcica and their expression pattern during growth and infection. Eur. J. Plant Pathol. 2019, 153, 1149–1163. [Google Scholar] [CrossRef]
- Ferguson, L.M.; Carson, M.L. Spatial diversity of Setosphaeria turcica sampled from the eastern United States. Phytopathology 2004, 94, 892–900. [Google Scholar] [CrossRef] [PubMed]
- Badu-Apraku, B.; Bankole, F.A.; Fakorede, M.A.B.; Ayinde, O.; Ortega-Beltran, A. Genetic analysis of grain yield and resistance of extra-early-maturing maize inbreds to northern corn leaf blight. Crop Sci. 2021, 61, 1864–1880. [Google Scholar] [CrossRef]
- Fang, Y.L.; Zhou, Y.Y.; Li, X.; Gao, Y.; Wang, D.L.; Liu, M.J.; Zhang, Z.J. Histological characterization of the early-stage infection events of Setosphaeria turcica in maize. Plant Pathol. 2022, 71, 251–261. [Google Scholar] [CrossRef]
- De Rossi, R.L.; Guerra, F.A.; Plazas, M.C.; Vuletic, E.E.; Brücher, E.; Guerra, G.D.; Reis, E.M. Crop damage, economic losses, and the economic damage threshold for Northern corn leaf blight. Crop Prot. 2022, 154, 105901. [Google Scholar] [CrossRef]
- Navarro, B.L.; Romero, L.R.; Kistner, M.B.; Iglesias, J.; Von Tiedemann, A. Assessment of physiological races of Exserohilum turcicum isolates from maize in Argentina and Brazil. Trop. Plant Pathol. 2021, 46, 371–380. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Bankole, F.A.; Ajayo, B.S.; Fakorede, M.A.B.; Akinwale, R.O.; Talabi, A.O.; Bandyopadhyay, R.; Ortega-Beltran, A. Identification of early and extra-early maturing tropical maize inbred lines resistant to Exserohilum turcicum in sub-Saharan Africa. Crop Prot. 2021, 139, 105386. [Google Scholar] [CrossRef] [PubMed]
- Montemarani, A.; Sartori, M.; Nesci, A.; Etcheverry, M.; Barros, G. Influence of crop residues, matric potential and temperature on growth of Exserohilum turcicum an emerging maize pathogen in Argentina. Lett. Appl. Microbiol. 2018, 67, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Grichar, W.J.; Janak, T.W.; McGinty, J.A.; Brewer, M.J. Using biostimulants, soil additives, and plant protectants to improve corn yield in South Texas. Agronomy 2023, 13, 1429. [Google Scholar] [CrossRef]
- Zhang, G.Z.; Wang, F.T.; Qin, J.C.; Wang, D.; Zhang, J.Y.; Zhang, Y.H.; Zhang, S.H.; Pan, H.Y. Efficacy assessment of antifungal metabolites from Chaetomium globosum No.05, a new biocontrol agent, against Setosphaeria turcica. Biol. Control 2013, 64, 90–98. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhang, S.S.; Yu, H.L.; Pan, H.Y.; Zhang, H. Klebsiella jilinsis 2N3 promotes maize growth and induces resistance to Northern corn leaf blight. Biol. Control 2021, 156, 104554. [Google Scholar] [CrossRef]
- Ma, Y.; Li, Y.; Yang, S.; Li, Y.; Zhu, Z. Biocontrol potential of Trichoderma asperellum strain 576 against Exserohilum turcicum in Zea mays. J. Fungi 2023, 9, 936. [Google Scholar] [CrossRef] [PubMed]
- Mark, M.; Shiri, F. Prospects for biological soilborne disease control: Application of indigenous versus synthetic microbiomes. Phytopathology 2017, 107, 256–263. [Google Scholar]
- Kalinina, T.A.; Balandina, V.I.; Obydennov, K.L.; Slepukhin, P.A.; Fan, Z.J.; Bakulev, V.A.; Glukhareva, T.V. Synthesis, fungicidal activity and plant protective properties of 1, 2, 3-thiadiazole and isothiazole-based N-acyl-N-arylalaninates. Molecules 2023, 28, 419. [Google Scholar] [CrossRef] [PubMed]
- Sarli, D.A.; Sánchez, L.A.; Delgado, O.D. Burkholderia gladioli MB39 an antarctic strain as a biocontrol agent. Curr. Microbiol. 2021, 78, 2332–2344. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Beer, K.D.; Kuivila, K.M.; Chiller, T.M.; Jackson, B.R. Trends in agricultural triazole fungicide use in the United States, 1992–2016 and possible implications for antifungal-resistant fungi in human disease. Environ. Health Perspect. 2021, 129, 55001. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, A.; Sautua, F.; Carmona, M.; Chulze, S.; Palazzini, J. Tan spot of wheat: Can biological control interact with actual management practices to counteract this global disease? Eur. J. Plant Pathol. 2023, 166, 27–38. [Google Scholar] [CrossRef]
- Mikaberidze, A.; Paveley, N.; Bonhoeffer, S.; Van den Bosch, F. Emergence of resistance to fungicides: The role of fungicide dose. Phytopathology 2017, 107, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Klocke, B.; Sommerfeldt, N.; Wagner, C.; Schwarz, J.; Baumecker, M.; Ellmer, F.; Jacobi, A.; Matschiner, K.; Petersen, J.; Wehling, P.; et al. Disease threshold-based fungicide applications: Potential of multi-disease resistance in winter wheat cultivars in Germany. Eur. J. Plant Pathol. 2023, 165, 363–383. [Google Scholar] [CrossRef]
- Yen, T.B.; Chang, S.T. Synergistic effects of cinnamaldehyde in combination with eugenol against wood decay fungi. Bioresour. Technol. 2008, 99, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Carpane, P.D.; Peper, A.M.; Kohn, F. Management of Northern Corn Leaf Blight using Nativo (Trifloxistrobin+Tebuconazole) fungicide applications. Crop Prot. 2020, 127, 104982. [Google Scholar] [CrossRef]
- Silva, T.S.; Da Fonseca, L.F.; Yamada, J.K.; De Carvalho Pontes, N. Flutriafol and azoxystrobin: An efficient combination to control fungal leaf diseases in corn crops. Crop Prot. 2021, 140, 105394. [Google Scholar] [CrossRef]
- Luo, H.Y.; Meng, S.Y.; Deng, Y.C.; Deng, Z.Y.; Shi, H.L. In vitro antifungal activity of lasiodiplodin, isolated from endophytic fungus Lasiodiplodia pseudotheobromae J-10 associated with Sarcandra glabra and optimization of culture conditions for lasiodiplodin production. Arch. Microbiol. 2023, 205, 140. [Google Scholar] [CrossRef] [PubMed]
- Limdolthamand, S.; Songkumarn, P.; Suwannarat, S.; Jantasorn, A.; Dethoup, T. Biocontrol efficacy of endophytic Trichoderma spp. in fresh and dry powder formulations in controlling Northern corn leaf blight in sweet corn. Biol. Control 2023, 181, 105217. [Google Scholar] [CrossRef]
- Song, J.H.; Lei, T.Y.; Hao, X.J.; Yuan, H.Z.; Sun, W.; Chen, S.N. Synergistic effects of Clonostachys rosea isolates and succinate dehydrogenase inhibitors fungicides against gray mold on tomato. Microorganisms 2022, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Cun, H.C.; Munir, S.; He, P.F.; Wu, Y.X.; He, P.B.; Ahmed, A.; Che, H.B.; Li, J.; He, Y.Q. Diversity of root endophytic bacteria from maize seedling involved in biocontrol and plant growth promotion. Egypt. J. Biol. Pest Control 2022, 32, 129. [Google Scholar] [CrossRef]
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Ohno, A.; Ano, T.; Shoda, M. Production of a lipopeptide antibiotic, surfactin, by recombinant Bacillus subtilis in solid state fermentation. Biotechnol. Bioeng. 1995, 47, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.M.; Fu, L.Y.; Hai, F.; Jiang, J.; Che, Z.P.; Tian, Y.E.; Chen, G.Q. Sensitivity to boscalid in field isolates of Sclerotinia sclerotiorum from rapeseed in Henan Province, China. J. Phytopathol. 2018, 166, 227–232. [Google Scholar] [CrossRef]
- Wadley, F.M. The Evidence Required to Show Synergistic Action of Insecticides and a Short Cut in Analysis; U.S. Government Printing Office: Washington, DC, USA, 1945.
- Liu, C.Y.; Chang, Z. Identification of the biocontrol strain LB-2 and determination of its antifungal effects on plant pathogenic fungi. J. Plant Pathol. 2018, 100, 25–32. [Google Scholar] [CrossRef]
- Lu, X.M.; He, S.; Ma, H.J.; Li, J.H.; Zhu, F.X. Hormetic effects of flusilazole preconditioning on mycelial growth and virulence of Sclerotinia sclerotiorum. Plant Dis. 2018, 102, 1165–1170. [Google Scholar] [CrossRef] [PubMed]
- Erenstein, O.; Jaleta, M.; Sonder, K.; Mottaleb, K.; Prasanna, B.M. Global maize production, consumption and trade: Trends and R&D implications. Food Secur. 2022, 14, 1295–1319. [Google Scholar]
- Jean-Paul, C.; Giorgia, R.; Salvatore, D.F.; Giovanni, D.L.; Fabian, C. Agricultural diversification, productivity, and food security across time and space. Agric. Econ. 2022, 53, 41–58. [Google Scholar]
- Haasbroek, M.P.; Craven, M.; Barnes, I.; Crampton, B.G. Microsatellite and mating type primers for the maize and sorghum pathogen, Exserohilum turcicum. Australas. Plant Pathol. 2014, 43, 577–581. [Google Scholar] [CrossRef]
- Juthaporn, K.; Kamol, L.; Weerasak, S.; Jirawat, S.; Nooduan, M.; Piyada, T. Identification of RAPD and SCAR markers linked to northern leaf blight resistance in waxy corn (Zea mays var. ceratina). Euphytica 2009, 164, 615–625. [Google Scholar]
- Madias, A.; Borrás, L.; Gambin, B.L. Foliar fungicides help maize farmers reduce yield gaps in late sown crops in a temperate region. Eur. J. Agron. 2023, 145, 126768. [Google Scholar] [CrossRef]
- Weems, J.D.; Bradley, C.A. Sensitivity of Exserohilum turcicum to demethylation inhibitor fungicides. Crop Prot. 2017, 99, 85–92. [Google Scholar] [CrossRef]
- Testa, G.; Reyneri, A.; Cardinale, F.; Blandino, M. Grain yield enhancement through fungicide application on maize hybrids with different susceptibility to Northern corn leaf blight. Cereal Res. Commun. 2015, 43, 415–425. [Google Scholar] [CrossRef]
- Nelson, K.A.; Dudenhoeffer, C.J.; Burdick, B.; Harder, D. Enhanced efficiency foliar nitrogen and pyraclostrobin applications for high yielding corn. J. Agric. Sci. 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Sartori, M.; Nesci, A.; García, J.; Passone, M.A.; Montemarani, A.; Etcheverry, M. Efficacy of epiphytic bacteria to prevent northern leaf blight caused by Exserohilum turcicum in maize. Rev. Argent. Microbiol. 2017, 49, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.Y.; Zhou, Q.Y.; Deng, Y.C.; Deng, Z.Y.; Qing, Z.; Sun, W.B. Antifungal activity of the extract and the active substances of endophytic Nigrospora sp. from the traditional Chinese medicinal plant Stephania kwangsiensis. Nat. Prod. Commun. 2017, 12, 1889–1892. [Google Scholar] [CrossRef]
- Zahra, M.B.; Fayyaz, B.; Aftab, Z.-E.; Akhter, A.; Bahar, T.; Anwar, W.; Haider, M.S. Characterization and utilization of cow manure biochar as soil amendment for the management of Northern corn leaf blight. J. Soil Sci. Plant Nutr. 2022, 22, 3348–3363. [Google Scholar] [CrossRef]
- Chang, Y.M.; Xia, X.Y.; Sui, L.; Kang, Q.; Yang, L.; Le, L.; Liu, W.D.; Li, Q.Y.; Zhang, Z.K. Endophytic colonization of entomopathogenic fungi increases plant disease resistance by changing the endophytic bacterial community. J. Basic Microbiol. 2021, 61, 1098–1112. [Google Scholar] [CrossRef] [PubMed]
- Fayed, E.A.; Ebrahim, M.A.; Fathy, U.; ElSaeed, H.S.; Khalaf, W.S. Evaluation of quinoxaline derivatives as potential ergosterol biosynthesis inhibitors: Design, synthesis, ADMET, molecular docking studies, and antifungal activities. J. Mol. Struct. 2022, 1267, 133578. [Google Scholar] [CrossRef]
- Ji, X.X.; Li, J.J.; Meng, Z.; Zhang, S.A.; Dong, B.; Qiao, K. Synergistic effect of combined application of a new fungicide fluopimomide with a biocontrol agent Bacillus methylotrophicus TA-1 for management of gray mold in tomato. Plant Dis. 2019, 103, 1991–1997. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Lee, Y.; Ha, A.; Kim, J.I.; Park, A.R.; Yu, N.H.; Son, H.; Choi, G.J.; Park, H.W.; Lee, C.W.; et al. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front. Plant Sci. 2017, 8, 2010–2016. [Google Scholar] [CrossRef]
- Li, B.X.; Wang, W.C.; Zhang, X.P.; Zhang, D.X.; Ren, Y.P.; Gao, Y.; Mu, W.; Liu, F. Using coordination assembly as the microencapsulation strategy to promote the efficacy and environmental safety of pyraclostrobin. Adv. Funct. Mater. 2017, 27, 1701841. [Google Scholar] [CrossRef]
- López-Bucio, J.; Campos-Cuevas, J.C.; Hernández-Calderón, E.; Velásquez-Becerra, C.; Farías-Rodriguez, R.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin-and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant-Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Palaniyandi, S.A.; Yang, S.H.; Zhang, L.; Suh, J.W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013, 97, 9621–9636. [Google Scholar] [CrossRef] [PubMed]
- de O. Nunes, P.S.; de Medeiros, F.H.V.; de Oliveira, T.S.; de Almeida Zago, J.R.; Bettiol, W. Bacillus subtilis and Bacillus licheniformis promote tomato growth. Braz. J. Microbiol. 2023, 54, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.Y.; He, P.J.; Shahzad, M.; He, P.B.; Li, X.Y.; Li, Y.M.; Wu, J.J.; Wu, Y.X.; Yang, L.J.; He, P.F.; et al. Efficacy of plant growth promoting bacteria Bacillus amyloliquefaciens B9601-Y2 for biocontrol of Southern corn leaf blight. Biol. Control 2019, 139, 104080. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).