Phenotyping the Anthocyanin Content of Various Organs in Purple Corn Using a Digital Camera
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Sampling
2.2. Digital Image Acquisition and Preprocessing
2.3. Image Analysis and Processing of the Color Data
2.4. Extraction and Quantification of Anthocyanin
2.5. Establishment and Validation of the Model
2.6. Statistical Analysis
3. Results
3.1. Anthocyanin Content of Various Organs in Purple Corn
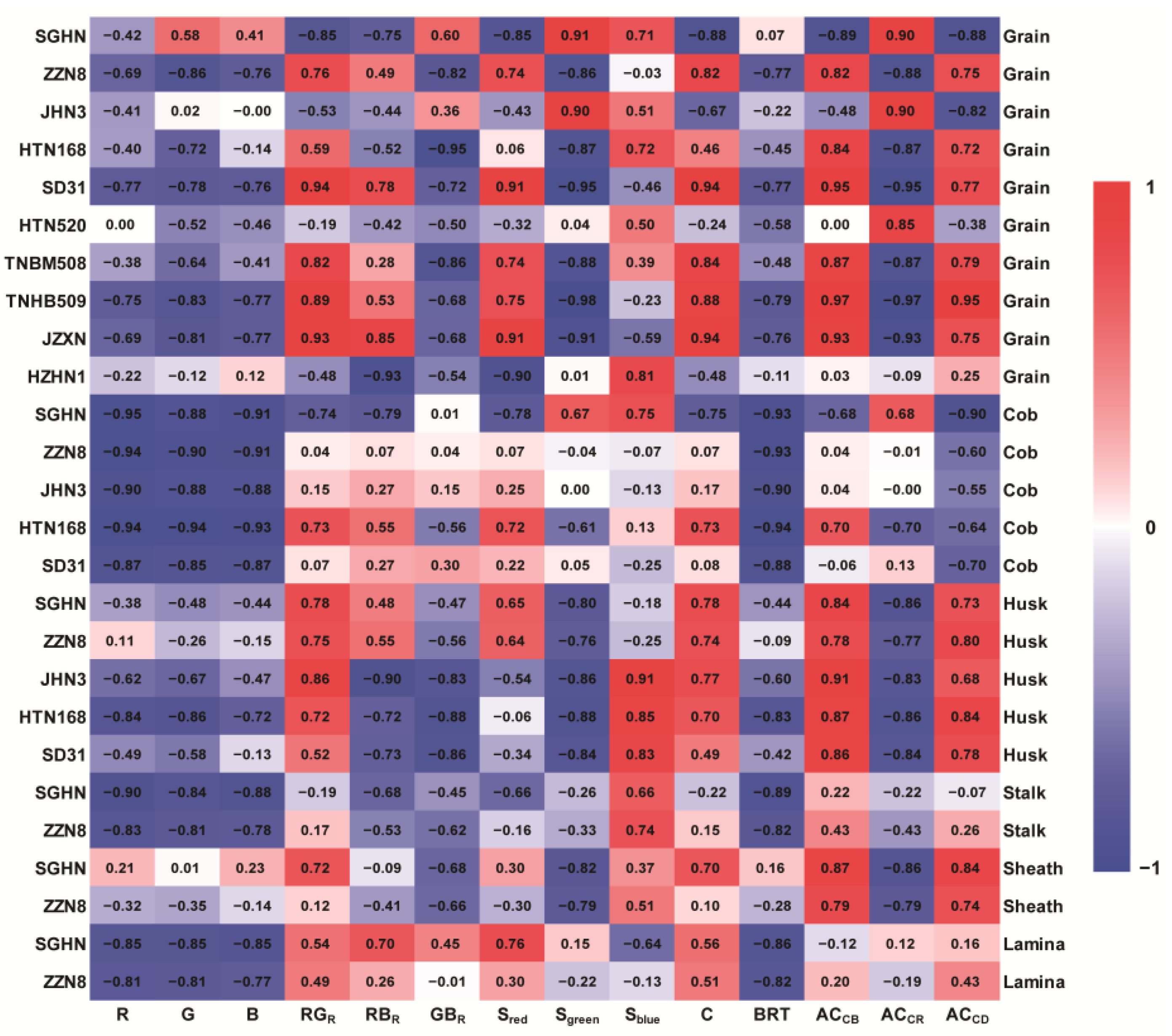
3.2. Correlation Analysis of Anthocyanin Content and Color Indices in Purple Corn
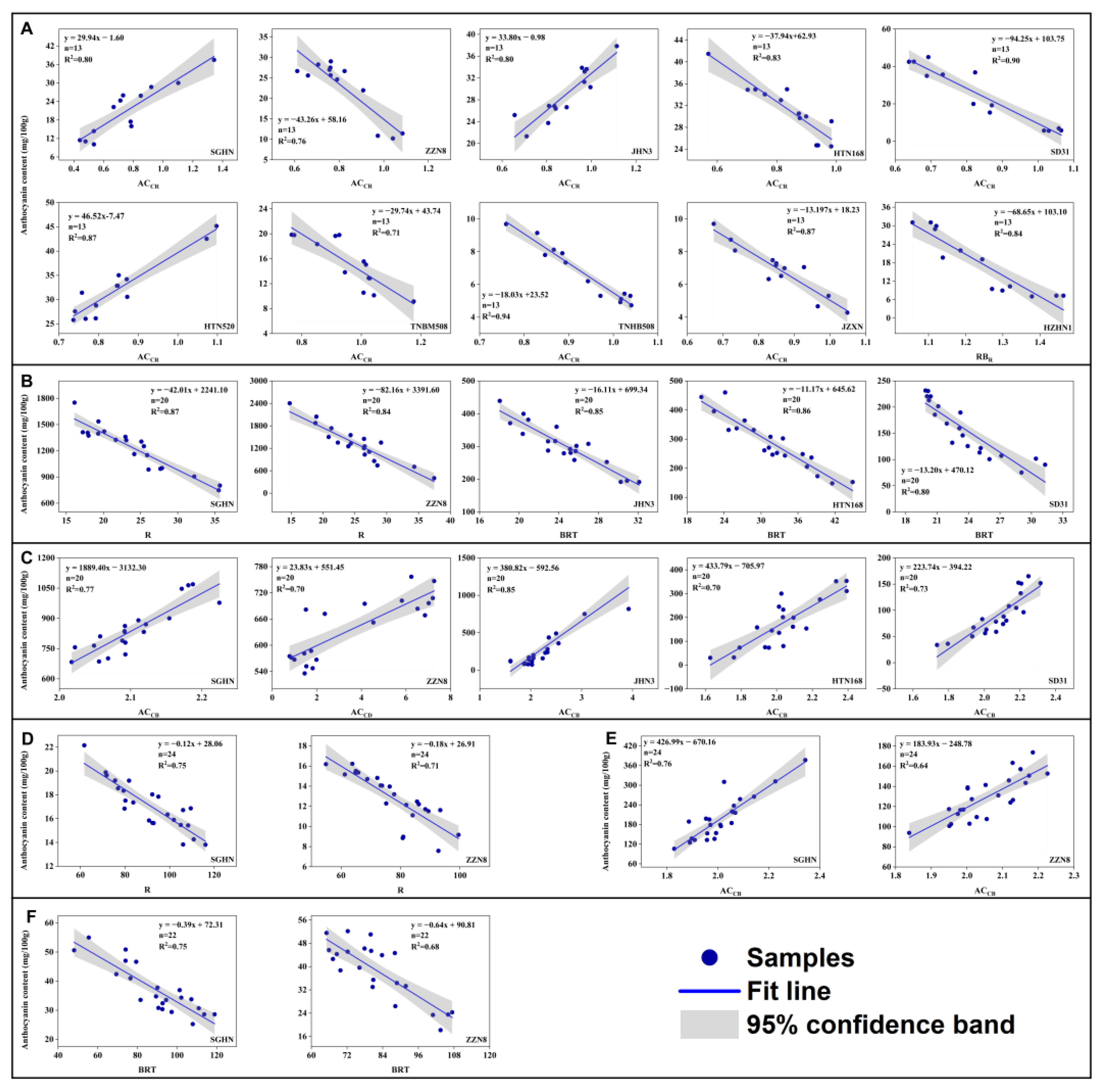
3.3. Fitting Robustness of the Relationships between Anthocyanin Content and the Color Indices
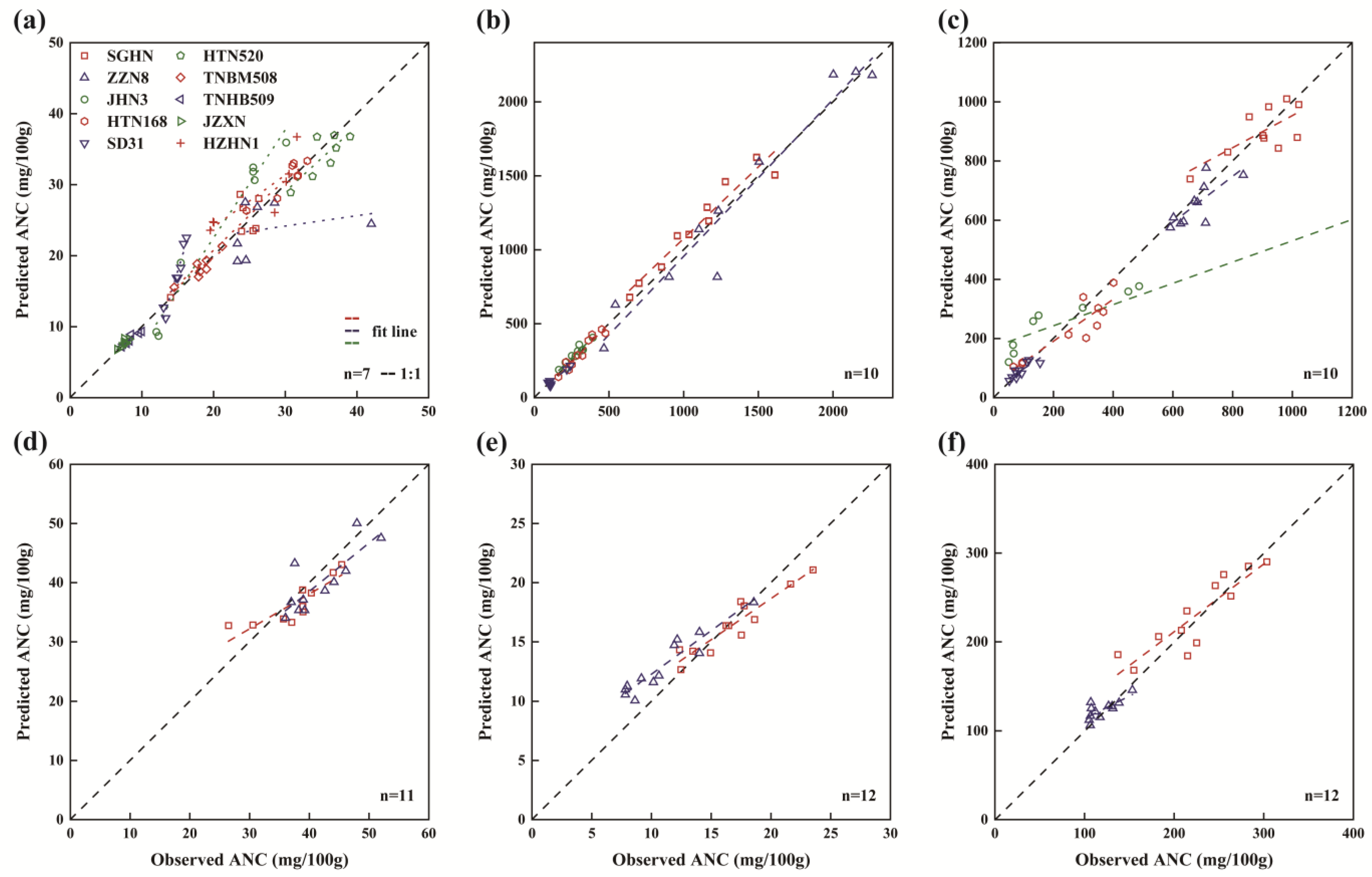
3.4. Model Validation with an Independent Dataset
4. Discussion
4.1. Anthocyanins for Industry Use
4.2. Modeling Robustness
4.3. Model Application
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Custodio-Mendoza, J.A.; Aktaş, H.; Zalewska, M.; Wyrwisz, J.; Kurek, M.A. A Review of Quantitative and Topical Analysis of Anthocyanins in Food. Molecules 2024, 29, 1735. [Google Scholar] [CrossRef] [PubMed]
- Cheaib, A.; Mahmoud, L.M.; Vincent, C.; Killiny, N.; Dutt, M. Influence of Anthocyanin Expression on the Performance of Photosynthesis in Sweet Orange, Citrus sinensis (L.) Osbeck. Plants 2023, 12, 3965. [Google Scholar] [CrossRef] [PubMed]
- Landi, M.; Tattini, M.; Gould, K.S. Multiple functional roles of anthocyanins in plant-environment interactions. Environ. Exp. Bot. 2015, 119, 4–17. [Google Scholar] [CrossRef]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized metabolites: Physiological and biochemical role in stress resistance, strategies to improve their accumulation, and new applications in crop breeding and management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liu, Z.; Wu, Y.; Zheng, L.; Zhang, G. Regulatory Mechanisms of Anthocyanin Biosynthesis in Apple and Pear. Int. J. Mol. Sci. 2021, 22, 8441. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Xiang, S.; Cui, Z.; Li, K.; Zhang, Z. Molecular Dynamic Regulation of Na and Mg Ions on Lithium Carbonate Crystallisation in Salt Lakes. J. Wuhan Univ. Technol.-Mat. Sci. Edit. 2021, 36, 22–28. [Google Scholar] [CrossRef]
- Marino, M.; Gardana, C.; Rendine, M.; Klimis-Zacas, D.; Riso, P.; Porrini, M.; Del Bo’, C. Nutritional and Phytochemical Characterization of Freeze-Dried Raspberry (Rubus idaeus): A Comprehensive Analysis. Foods 2024, 13, 1051. [Google Scholar] [CrossRef]
- Cozzolino, D. Phenolics and spectroscopy: Challenges and successful stories in the grape and wine industry. J. Sci. Food Agric. 2023. [Google Scholar] [CrossRef]
- Chachar, Z.; Lai, R.; Ahmed, N.; Lingling, M.; Chachar, S.; Paker, N.P.; Qi, Y. Cloned genes and genetic regulation of anthocyanin biosynthesis in maize, a comparative review. Front. Plant Sci. 2024, 15, 1310634. [Google Scholar] [CrossRef]
- García-Villegas, A.; Fernández-Ochoa, Á.; Alañón, M.E.; Rojas-García, A.; Arráez-Román, D.; Cádiz-Gurrea, M.d.l.L.; Segura-Carretero, A. Bioactive Compounds and Potential Health Benefits through Cosmetic Applications of Cherry Stem Extract. Int. J. Mol. Sci. 2024, 25, 3723. [Google Scholar] [CrossRef]
- Global Flavonoids Market Size & Share Report, 2025. Available online: https://www.grandviewresearch.com/industry-analysis/flavonoids-market (accessed on 13 November 2023).
- Romani, A.; Campo, M.; Urciuoli, S.; Marrone, G.; Noce, A.; Bernini, R. An Industrial and Sustainable Platform for the Production of Bioactive Micronized Powders and Extracts Enriched in Polyphenols from Olea europaea L. and Vitis vinifera L. Wastes. Front. Nutr. 2020, 7, 120. [Google Scholar] [CrossRef]
- Leonarski, E.; Kuasnei, M.; Cesca, K.; de Oliveira, D.; Zielinski, A.A.F. Black rice and its by-products: Anthocyanin-rich extracts and their biological potential. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.E. Anthocyanin extraction from plant tissues: A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 3072–3083. [Google Scholar] [CrossRef]
- Somavat, P.; Kumar, D.; Singh, V. Techno-economic feasibility analysis of blue and purple corn processing for anthocyanin extraction and ethanol production using modified dry grind process. Ind. Crops Prod. 2018, 115, 78–87. [Google Scholar] [CrossRef]
- Jasińska, K.; Fabiszewska, A.; Białecka-Florjańczyk, E.; Zieniuk, B. Mini-Review on the Enzymatic Lipophilization of Phenolics Present in Plant Extracts with the Special Emphasis on Anthocyanins. Antioxidants 2022, 11, 1528. [Google Scholar] [CrossRef] [PubMed]
- Carrillo, C.; Nieto, G.; Martínez-Zamora, L.; Ros, G.; Kamiloglu, S.; Munekata, P.E.S.; Pateiro, M.; Lorenzo, J.M.; Fernández-López, J.; Viuda-Martos, M.; et al. Novel Approaches for the Recovery of Natural Pigments with Potential Health Effects. J. Agric. Food Chem. 2022, 70, 6864–6883. [Google Scholar] [CrossRef]
- Veloso, M.I.; Coelho, E.; Trabulo, O.; Coimbra, M.A. Elderberry Concentrate Juice Industrial By-Products Characterization and Valorisation. Appl. Sci. 2022, 12, 9463. [Google Scholar] [CrossRef]
- Li, C.-Y.; Kim, H.-W.; Won, S.; Min, H.-K.; Park, K.-J.; Park, J.-Y.; Ahn, M.-S.; Rhee, H.-I. Corn Husk as a Potential Source of Anthocyanins. J. Agric. Food Chem. 2008, 56, 11413–11416. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Aulis, F.; Hernandez-Vazquez, L.; Aguilar-Osorio, G.; Arrieta-Baez, D.; Navarro-Ocana, A. Extraction and Identification of Anthocyanins in Corn Cob and Corn Husk from Cacahuacintle Maize. J. Food Sci. 2019, 84, 954–962. [Google Scholar] [CrossRef]
- Hong, H.T.; Netzel, M.E.; O’Hare, T.J. Optimisation of extraction procedure and development of LC–DAD–MS methodology for anthocyanin analysis in anthocyanin-pigmented corn kernels. Food Chem. 2020, 319, 126515. [Google Scholar] [CrossRef]
- Ata-Ul-Karim, S.T.; Cao, Q.; Zhu, Y.; Tang, L.; Rehmani, M.I.A.; Cao, W. Non-destructive Assessment of Plant Nitrogen Parameters Using Leaf Chlorophyll Measurements in Rice. Front. Plant Sci. 2016, 7, 1829. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Chen, Z.; Cheng, Q.; Liu, R.; Li, M.; Yan, X.; Li, G.; Wang, D.; Fu, L.; Ma, Y.; et al. Estimation of plant height and yield based on UAV imagery in faba bean (Vicia faba L.). Plant Methods 2022, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Deng, J.; Lan, Y.; Yang, A.; Zhang, L.; Wen, S.; Zhang, H.; Zhang, Y.; Deng, Y. Detection of Helminthosporium Leaf Blotch Disease Based on UAV Imagery. Appl. Sci. 2019, 9, 558. [Google Scholar] [CrossRef]
- Heidarian Dehkordi, R.; El Jarroudi, M.; Kouadio, L.; Meersmans, J.; Beyer, M. Monitoring Wheat Leaf Rust and Stripe Rust in Winter Wheat Using High-Resolution UAV-Based Red-Green-Blue Imagery. Remote Sens. 2020, 12, 3696. [Google Scholar] [CrossRef]
- Wan, L.; Zhang, J.; Dong, X.; Du, X.; Cen, H. Unmanned aerial vehicle-based field phenotyping of crop biomass using growth traits retrieved from PROSAIL model. Comput. Electron. Agric. 2021, 187, 106304. [Google Scholar] [CrossRef]
- Makanza, R.; Zaman-Allah, M.; Cairns, J.E.; Magorokosho, C.; Tarekegne, A.; Olsen, M.; Prasanna, B.M. High-Throughput Phenotyping of Canopy Cover and Senescence in Maize Field Trials Using Aerial Digital Canopy Imaging. Remote Sens. 2018, 10, 330. [Google Scholar] [CrossRef] [PubMed]
- del Valle, J.C.; Gallardo-López, A.; Buide, M.L.; Whittall, J.B.; Narbona, E. Digital photography provides a fast, reliable, and noninvasive method to estimate anthocyanin pigment concentration in reproductive and vegetative plant tissues. Ecol. Evol. 2018, 8, 3064–3076. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhu, C.; Luo, Y.; Fu, X. Spectral Reflectance Reconstruction from Red-Green-Blue (RGB) Images for Chlorophyll Content Detection. Appl. Spectrosc. 2023, 77, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Riccardi, M.; Mele, G.; Pulvento, C.; Lavini, A.; d’Andria, R.; Jacobsen, S.-E. Non-destructive evaluation of chlorophyll content in quinoa and amaranth leaves by simple and multiple regression analysis of RGB image components. Photosynth. Res. 2014, 120, 263–272. [Google Scholar] [CrossRef]
- Wood, N.J.; Baker, A.; Quinnell, R.J.; Camargo-Valero, M.A. A Simple and Non-destructive Method for Chlorophyll Quantification of Chlamydomonas Cultures Using Digital Image Analysis. Front. Bioeng. Biotechnol. 2020, 8, 746. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Shi, P.; Omasa, K. Estimating rice chlorophyll content and leaf nitrogen concentration with a digital still color camera under natural light. Plant Methods 2014, 10, 36. [Google Scholar] [CrossRef] [PubMed]
- Faragó, D.; Sass, L.; Valkai, I.; Andrási, N.; Szabados, L. PlantSize Offers an Affordable, Non-destructive Method to Measure Plant Size and Color in Vitro. Front. Plant Sci. 2018, 9, 219. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ge, Y.; Xie, X.; Atefi, A.; Wijewardane, N.K.; Thapa, S. High throughput analysis of leaf chlorophyll content in sorghum using RGB, hyperspectral, and fluorescence imaging and sensor fusion. Plant Methods 2022, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.; Sookwong, P.; Jakmunee, J.; Mahatheeranont, S. Smartphone digital image colorimetric determination of the total monomeric anthocyanin content in black rice via the pH differential method. Anal. Methods 2021, 13, 3348–3358. [Google Scholar] [CrossRef] [PubMed]
- Junker, L.V.; Ensminger, I. Relationship between leaf optical properties, chlorophyll fluorescence and pigment changes in senescing Acer saccharum leaves. Tree Physiol. 2016, 36, 694–711. [Google Scholar] [CrossRef]
- Wang, Z.; Sun, W.; Liu, X.; Li, Y.; Collins, B.; Ullah, N.; Song, Y. Analysis on Heat Characteristics for Summer Maize Cropping in a Semi-Arid Region. Agronomy 2022, 12, 1435. [Google Scholar] [CrossRef]
- Andersen, Ø.M.; Jordheim, M. Anthocyanins. In Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2010; ISBN 978-0-470-01590-2. [Google Scholar]
- Alappat, B.; Alappat, J. Anthocyanin Pigments: Beyond Aesthetics. Molecules 2020, 25, 5500. [Google Scholar] [CrossRef]
- White, T.E.; Dalrymple, R.L.; Noble, D.W.A.; O’Hanlon, J.C.; Zurek, D.B.; Umbers, K.D.L. Reproducible research in the study of biological coloration. Anim. Behav. 2015, 106, 51–57. [Google Scholar] [CrossRef]
- Stevens, M.; Párraga, C.A.; Cuthill, I.C.; Partridge, J.C.; Troscianko, T.S. Using digital photography to study animal coloration. Biol. J. Linn. Soc. 2007, 90, 211–237. [Google Scholar] [CrossRef]
- Akkaynak, D.; Treibitz, T.; Xiao, B.; Gürkan, U.A.; Allen, J.J.; Demirci, U.; Hanlon, R.T. Use of commercial off-the-shelf digital cameras for scientific data acquisition and scene-specific color calibration. J. Opt. Soc. Am. A JOSAA 2014, 31, 312–321. [Google Scholar] [CrossRef]
- Tedla, S.; Wang, Y.; Patel, M.; Brown, M.S. Analyzing color imaging failure on consumer-grade cameras. J. Opt. Soc. Am. A JOSAA 2022, 39, B21–B27. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Urano, D.; Liao, K.-L.; Hedrick, T.L.; Gao, Y.; Jones, A.M. A nondestructive method to estimate the chlorophyll content of Arabidopsis seedlings. Plant Methods 2017, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, R.; Pouget, M.; Cervelle, B.; Escadafal, R. Relationships between Satellite-Based Radiometric Indices Simulated Using Laboratory Reflectance Data and Typic Soil Color of an Arid Environment. Remote Sens. Environ. 1998, 66, 17–28. [Google Scholar] [CrossRef]
- Mizunuma, T.; Mencuccini, M.; Wingate, L.; Ogee, J.; Nichol, C.; Grace, J. Sensitivity of colour indices for discriminating leaf colours from digital photographs. Methods Ecol. Evol. 2014, 5, 1078–1085. [Google Scholar] [CrossRef]
- Tan, J.; Han, Y.; Han, B.; Qi, X.; Cai, X.; Ge, S.; Xue, H. Extraction and purification of anthocyanins: A review. J. Agric. Food Res. 2022, 8, 100306. [Google Scholar] [CrossRef]
- Bai, X.; Zhou, L.; Zhou, L.; Cang, S.; Liu, Y.; Liu, R.; Liu, J.; Feng, X.; Fan, R. The Research Progress of Extraction, Purification and Analysis Methods of Phenolic Compounds from Blueberry: A Comprehensive Review. Molecules 2023, 28, 3610. [Google Scholar] [CrossRef]
- Warton, D.I.; Wright, I.J.; Falster, D.S.; Westoby, M. Bivariate line-fitting methods for allometry. Biol. Rev. Camb. Philos. Soc. 2006, 81, 259–291. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, X.; Song, P.X.K.; Zhang, J.Y.; So, W.F.K.; Team, T.M.S.; Sugar, C.A.; James, G.M.; Serban, N.; Wasserman, L. The R Book; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012; pp. 449–497. ISBN 978-1-118-44890-8. [Google Scholar]
- Loague, K.; Green, R.E. Statistical and graphical methods for evaluating solute transport models: Overview and application. J. Contam. Hydrol. 1991, 7, 51–73. [Google Scholar] [CrossRef]
- Qiao, L.; Tang, W.; Gao, D.; Zhao, R.; An, L.; Li, M.; Sun, H.; Song, D. UAV-based chlorophyll content estimation by evaluating vegetation index responses under different crop coverages. Comput. Electron. Agric. 2022, 196, 106775. [Google Scholar] [CrossRef]
- Kim, C.; van Iersel, M.W. Image-based phenotyping to estimate anthocyanin concentrations in lettuce. Front. Plant Sci. 2023, 14, 1155722. [Google Scholar] [CrossRef]
- He, L.; Sun, W.; Chen, X.; Han, L.; Li, J.; Ma, Y.; Song, Y. Modeling Maize Canopy Morphology in Response to Increased Plant Density. Front. Plant Sci. 2021, 11, 533514. [Google Scholar] [CrossRef] [PubMed]
- Cuevas Montilla, E.; Hillebrand, S.; Antezana, A.; Winterhalter, P. Soluble and Bound Phenolic Compounds in Different Bolivian Purple Corn (Zea mays L.) Cultivars. J. Agric. Food Chem. 2011, 59, 7068–7074. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Ge-Zhang, S.; Song, M. Anthocyanins in metabolites of purple corn. Front. Plant Sci. 2023, 14, 1154535. [Google Scholar] [CrossRef] [PubMed]
- Gullón, P.; Eibes, G.; Lorenzo, J.M.; Pérez-Rodríguez, N.; Lú-Chau, T.A.; Gullón, B. Green sustainable process to revalorize purple corn cobs within a biorefinery frame: Co-production of bioactive extracts. Sci. Total Environ. 2020, 709, 136236. [Google Scholar] [CrossRef] [PubMed]
- Yadav, S.P.; Ibaraki, Y.; Gupta, S.D. Estimation of the chlorophyll content of micropropagated potato plants using RGB based image analysis. Plant Cell Tissue Organ. Cult. 2010, 100, 183–188. [Google Scholar] [CrossRef]
- Lou, H.; Hu, Y.; Zhang, L.; Sun, P.; Lu, H. Nondestructive evaluation of the changes of total flavonoid, total phenols, ABTS and DPPH radical scavenging activities, and sugars during mulberry (Morus alba L.) fruits development by chlorophyll fluorescence and RGB intensity values. LWT-Food Sci. Technol. 2012, 47, 19–24. [Google Scholar] [CrossRef]
- Qin, Y.; Liu, Y.; Yong, H.; Liu, J.; Zhang, X.; Liu, J. Preparation and characterization of active and intelligent packaging films based on cassava starch and anthocyanins from Lycium ruthenicum Murr. Int. J. Biol. Macromol. 2019, 134, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ghareaghajlou, N.; Hallaj-Nezhadi, S.; Ghasempour, Z. Red cabbage anthocyanins: Stability, extraction, biological activities and applications in food systems. Food Chem. 2021, 365, 130482. [Google Scholar] [CrossRef]
- Brudzyńska, P.; Sionkowska, A.; Grisel, M. Plant-Derived Colorants for Food, Cosmetic and Textile Industries: A Review. Materials 2021, 14, 3484. [Google Scholar] [CrossRef]
- Cai, D.; Li, X.; Chen, J.; Jiang, X.; Ma, X.; Sun, J.; Tian, L.; Vidyarthi, S.K.; Xu, J.; Pan, Z.; et al. A comprehensive review on innovative and advanced stabilization approaches of anthocyanin by modifying structure and controlling environmental factors. Food Chem. 2022, 366, 130611. [Google Scholar] [CrossRef]
- Calderón-Reyes, C.; Pezoa, R.S.; Leal, P.; Ribera-Fonseca, A.; Cáceres, C.; Riquelme, I.; Zambrano, T.; Peña, D.; Alberdi, M.; Reyes-Díaz, M. Anthocyanin-Rich Extracts of Calafate (Berberis microphylla G. Forst.) Fruits Decrease In Vitro Viability and Migration of Human Gastric and Gallbladder Cancer Cell Lines. J. Soil. Sci. Plant Nutr. 2020, 20, 1891–1903. [Google Scholar] [CrossRef]
- Gašić, U.; Ćirić, I.; Pejčić, T.; Radenković, D.; Djordjević, V.; Radulović, S.; Tešić, Ž. Polyphenols as Possible Agents for Pancreatic Diseases. Antioxidants 2020, 9, 547. [Google Scholar] [CrossRef] [PubMed]
- Matboli, M.; Hasanin, A.H.; Hussein, R.; El-Nakeep, S.; Habib, E.K.; Ellackany, R.; Saleh, L.A. Cyanidin 3-glucoside modulated cell cycle progression in liver precancerous lesion, in vivo study. World J. Gastroenterol. 2021, 27, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, M.; Lee, M. Effects of Anthocyanin Supplementation on Reduction of Obesity Criteria: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Kan, J.; Sun, R.; Cai, H.; Hong, J.; Jin, C.; Zong, S. Anthocyanins from purple sweet potato alleviate doxorubicin-induced cardiotoxicity in vitro and in vivo. J. Food Biochem. 2021, 45, e13869. [Google Scholar] [CrossRef]
- Bechtold, T.; Mahmud-Ali, A.; Mussak, R. Anthocyanin dyes extracted from grape pomace for the purpose of textile dyeing. J. Sci. Food Agric. 2007, 87, 2589–2595. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.S.; Nguyen, H.P.; Shen, S.; Schug, K.A. General method for extraction of blueberry anthocyanins and identification using high performance liquid chromatography–electrospray ionization-ion trap-time of flight-mass spectrometry. J. Chromatogr. A 2009, 1216, 4728–4735. [Google Scholar] [CrossRef] [PubMed]
- Assous, M.T.M.; Abdel-Hady, M.M.; Medany, G.M. Evaluation of red pigment extracted from purple carrots and its utilization as antioxidant and natural food colorants. Ann. Agric. Sci. 2014, 59, 1–7. [Google Scholar] [CrossRef]
- Tanner, F.; Tonn, S.; de Wit, J.; Van den Ackerveken, G.; Berger, B.; Plett, D. Sensor-based phenotyping of above-ground plant-pathogen interactions. Plant Methods 2022, 18, 35. [Google Scholar] [CrossRef]
- Choe, E.; Rocheford, T.R. Genetic and QTL analysis of pericarp thickness and ear architecture traits of Korean waxy corn germplasm. Euphytica 2012, 183, 243–260. [Google Scholar] [CrossRef]
- Lim, S.; Yi, G. Investigating seed mineral composition in Korean landrace maize (Zea mays L.) and its kernel texture specificity. J. Integr. Agric. 2019, 18, 1996–2005. [Google Scholar] [CrossRef]
- Salvador-Reyes, R.; Clerici, M.T.P.S. Peruvian Andean maize: General characteristics, nutritional properties, bioactive compounds, and culinary uses. Food Res. Int. 2020, 130, 108934. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-T.; Yi, G.; Chung, I.-M.; Son, B.-Y.; Bae, H.-H.; Go, Y.S.; Ha, J.Y.; Baek, S.-B.; Kim, S.-L. Timing and Pattern of Anthocyanin Accumulation during Grain Filling in Purple Waxy Corn (Zea mays L.) Suggest Optimal Harvest Dates. ACS Omega 2020, 5, 15702–15708. [Google Scholar] [CrossRef] [PubMed]
- Chatham, L.A.; Paulsmeyer, M.; Juvik, J.A. Prospects for economical natural colorants: Insights from maize. Theor. Appl. Genet. 2019, 132, 2927–2946. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, C.; Calvini, R.; Nigro, G.; Tessarin, P.; Bossio, D.; Calderisi, M.; Ferrari, V.; Foca, G.; Ulrici, A. Design and application of a smartphone-based device for in vineyard determination of anthocyanins content in red grapes. Microchem. J. 2023, 191, 108811. [Google Scholar] [CrossRef]
- Ingrouille, M. Understanding flowers and flowering: An integrated approach. Ann. Bot. 2009, 103, vi–vii. [Google Scholar] [CrossRef][Green Version]
- Kay, Q.O.N.; Daoud, H.S.; Stirton, C.H. Pigment distribution, light reflection and cell structure in petals. Bot. J. Linn. Soc. 1981, 83, 57–83. [Google Scholar] [CrossRef]
- van der Kooi, C.J.; Elzenga, J.T.M.; Staal, M.; Stavenga, D.G. How to colour a flower: On the optical principles of flower coloration. Proc. Biol. Sci. 2016, 283, 20160429. [Google Scholar] [CrossRef] [PubMed]
- Gómez, J.; Lián-Cembrano, G. SpotEgg: An image-processing tool for automatised analysis of colouration and spottiness. J. Avian Biol. 2017, 48, 502–512. [Google Scholar] [CrossRef]
- Garcia, J.E.; Greentree, A.D.; Shrestha, M.; Dorin, A.; Dyer, A.G. Flower colours through the lens: Quantitative measurement with visible and ultraviolet digital photography. PLoS ONE 2014, 9, e96646. [Google Scholar] [CrossRef]
- Berger, K.; Machwitz, M.; Kycko, M.; Kefauver, S.C.; Van Wittenberghe, S.; Gerhards, M.; Verrelst, J.; Atzberger, C.; van der Tol, C.; Damm, A.; et al. Multi-sensor spectral synergies for crop stress detection and monitoring in the optical domain: A review. Remote Sens. Environ. 2022, 280, 113198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zhou, Y.; Feng, X. Anthocyanins in Different Food Matrices: Recent Updates on Extraction, Purification and Analysis Techniques. Crit. Rev. Anal. Chem. 2022, 1–32. [Google Scholar] [CrossRef]
- Petroni, K.; Pilu, R.; Tonelli, C. Anthocyanins in corn: A wealth of genes for human health. Planta 2014, 240, 901–911. [Google Scholar] [CrossRef]
- Lao, F.; Sigurdson, G.T.; Giusti, M.M. Health Benefits of Purple Corn (Zea mays L.) Phenolic Compounds. Compr. Rev. Food Sci. Food Saf. 2017, 16, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Elisa, D.-H.; Marcela, G.-M.; Janet Alejandra, G.-U.; Martha Elena, D.-H. The nutraceutical value of maize (Zea mays L.) landraces and the determinants of its variability: A review. J. Cereal Sci. 2022, 103, 103399. [Google Scholar] [CrossRef]
- Robles-Plata, V.T.; Serna Saldivar, S.; de Dios Figueroa-Cárdenas, J.; Rooney, W.L.; Dávila-Vega, J.P.; Chuck-Hernández, C.; Escalante-Aburto, A. Biophysical, Nutraceutical, and Technofunctional Features of Specialty Cereals: Pigmented Popcorn and Sorghum. Foods 2023, 12, 2301. [Google Scholar] [CrossRef]
- Loladze, A.; Rodrigues, F.A.; Toledo, F.; San Vicente, F.; Gérard, B.; Boddupalli, M.P. Application of Remote Sensing for Phenotyping Tar Spot Complex Resistance in Maize. Front. Plant Sci. 2019, 10, 552. [Google Scholar] [CrossRef]
- Watt, M.; Fiorani, F.; Usadel, B.; Rascher, U.; Muller, O.; Schurr, U. Phenotyping: New Windows into the Plant for Breeders. Annu. Rev. Plant Biol. 2020, 71, 689–712. [Google Scholar] [CrossRef]
- Lobos, G.A.; Estrada, F.; Del Pozo, A.; Romero-Bravo, S.; Astudillo, C.A.; Mora-Poblete, F. Challenges for a Massive Implementation of Phenomics in Plant Breeding Programs. Methods Mol. Biol. 2022, 2539, 135–157. [Google Scholar] [CrossRef]
- Hu, Y.; Schmidhalter, U. Opportunity and challenges of phenotyping plant salt tolerance. Trends Plant Sci. 2023, 28, 552–566. [Google Scholar] [CrossRef]
- Rößle, D.; Prey, L.; Ramgraber, L.; Hanemann, A.; Cremers, D.; Noack, P.O.; Schön, T. Efficient Noninvasive FHB Estimation using RGB Images from a Novel Multiyear, Multirater Dataset. Plant Phenomics 2023, 5, 0068. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Tu, H.; Bai, B.; Yang, C.; Zhao, B.; Guo, Z.; Liu, Q.; Zhao, H.; Yang, W.; Xiong, L.; et al. Combining UAV-RGB high-throughput field phenotyping and genome-wide association study to reveal genetic variation of rice germplasms in dynamic response to drought stress. New Phytol. 2021, 232, 440–455. [Google Scholar] [CrossRef] [PubMed]
- Shu, M.; Fei, S.; Zhang, B.; Yang, X.; Guo, Y.; Li, B.; Ma, Y. Application of UAV Multisensor Data and Ensemble Approach for High-Throughput Estimation of Maize Phenotyping Traits. Plant Phenomics 2022, 2022, 9802585. [Google Scholar] [CrossRef] [PubMed]
- Fei, S.; Hassan, M.A.; Xiao, Y.; Su, X.; Chen, Z.; Cheng, Q.; Duan, F.; Chen, R.; Ma, Y. UAV-based multi-sensor data fusion and machine learning algorithm for yield prediction in wheat. Precis. Agric. 2023, 24, 187–212. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Zhou, J.; Scaboo, A.; Zhou, J.; Aloysius, N.; Lim, T.T. Assessment of Soybean Lodging Using UAV Imagery and Machine Learning. Plants 2023, 12, 2893. [Google Scholar] [CrossRef] [PubMed]
- Patrignani, A.; Ochsner, T.E. Canopeo: A Powerful New Tool for Measuring Fractional Green Canopy Cover. Agron. J. 2015, 107, 2312–2320. [Google Scholar] [CrossRef]
- Müller-Linow, M.; Wilhelm, J.; Briese, C.; Wojciechowski, T.; Schurr, U.; Fiorani, F. Plant Screen Mobile: An open-source mobile device app for plant trait analysis. Plant Methods 2019, 15, 2. [Google Scholar] [CrossRef]
- Röckel, F.; Schreiber, T.; Schüler, D.; Braun, U.; Krukenberg, I.; Schwander, F.; Peil, A.; Brandt, C.; Willner, E.; Gransow, D.; et al. PhenoApp: A mobile tool for plant phenotyping to record field and greenhouse observations. F1000Research 2022, 11, 12. [Google Scholar] [CrossRef]

| Variety | Organ with Anthocyanins | Growth Period |
|---|---|---|
| SGHN | Grain, Cob, Husk, Stem, Sheath, Lamina | 85 days |
| ZZN8 | Grain, Cob, Husk, Stem, Sheath, Lamina | 85 days |
| JHN3 | Grain, Cob, Husk | 85 days |
| HTN168 | Grain, Cob, Husk | 85 days |
| SD31 | Grain, Cob, Husk | 86 days |
| HTN520 | Grain | 80–85 days |
| TNBM508 | Grain | 85 days |
| TNHB509 | Grain | 85 days |
| JZXN | Grain | 80–85 days |
| HZHN1 | Grain | 90 days |
| Color Indices | Formula Used for Digital Images | References |
|---|---|---|
| Red:green ratio | RGR = Nred/Ngreen | [45] |
| Red:blue ratio | RBR = Nred/Nblue | [45] |
| Green:blue ratio | GBR = Ngreen/Nblue | [45] |
| Strength of red | Sred = Ngreen/(Nred + Ngreen + Nblue) | [46] |
| Strength of green | Sgreen = Ngreen/(Nred + Ngreen + Nblue) | [46] |
| Strength of blue | Sblue = Ngreen/(Nred + Ngreen + Nblue) | [46] |
| Brightness | [28] | |
| Chroma | C = (Nred − Ngreen)/[(Nred + Ngreen + Nblue)/3] | [28] |
| Anthocyanin content, chroma basic | ACCB = (Nblue + Nred)/Ngreen | [28] |
| Anthocyanin content, chroma ratio | ACCR = Ngreen/[(Nblue + Nred)/2] | [28] |
| Anthocyanin content, chroma difference | ACCD = (Nblue + Nred)/2 − Ngreen | [28] |
| Variety | Anthocyanin Content of Specific Organ Type (mg/100 g) | |||||
|---|---|---|---|---|---|---|
| Grain | Cob | Husk | Sheath | Lamina | Stem | |
| SGHN | 22.22 de | 1183.12 d | 862.33 a | 201.23 a | 37.40 a | 17.02 a |
| ZZN8 | 24.39 cd | 1334.10 a | 650.48 b | 126.10 b | 39.45 a | 12.25 a |
| JHN3 | 27.65 bc | 292.74 c | 336.46 c | |||
| HTN168 | 30.84 ab | 295.22 c | 205.73 d | |||
| SD31 | 19.94 ef | 143.84 d | 91.27 e | |||
| HTN520 | 33.26 a | |||||
| TNBM508 | 16.24 f | |||||
| TNHB509 | 7.29 g | |||||
| JZXN | 7.11 g | |||||
| HZHN1 | 20.60 de | |||||
| Indices | R | G | B | RGR | RBR | GBR | Sred | Sgreen | Sblue | C | BRT | ACCB | ACCR | ACCD | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGHN | Grain | 0.10 | 0.27 * | 0.09 | 0.70 ** | 0.52 ** | 0.30 * | 0.71 ** | 0.80 ** | 0.45 ** | 0.76 ** | <0.01 | 0.77 ** | 0.80 ** | 0.76 ** |
| Cob | 0.87 ** | 0.66 ** | 0.76 ** | 0.42 ** | 0.49 ** | <0.01 | 0.47 ** | 0.34 ** | 0.42 ** | 0.43 ** | 0.82 ** | 0.34 | 0.34 | 0.73 ** | |
| Husk | 0.05 | 0.15 | 0.10 | 0.57 ** | 0.18 | 0.30 | 0.38 * | 0.76 ** | <0.01 | 0.56 ** | 0.10 | 0.77 ** | 0.76 ** | 0.61 ** | |
| Stem | 0.75 ** | 0.54 * | 0.71 ** | 0.01 | 0.24 | 0.02 | 0.26 | <0.01 | 0.15 | 0.02 | 0.71 ** | <0.01 | <0.01 | <0.01 | |
| Sheath | 0.14 | <0.01 | 0.25 | 0.51 ** | <0.01 | 0.58 ** | 0.10 | 0.75 ** | 0.19 | 0.48 ** | 0.10 | 0.76 ** | 0.75 ** | 0.73 ** | |
| Lamina | 0.71 ** | 0.75 ** | 0.72 ** | 0.28 | 0.47 * | 0.16 | 0.62 ** | <0.01 | 0.31 * | 0.27 | 0.75 ** | <0.01 | <0.01 | <0.01 | |
| ZZN8 | Grain | 0.43 ** | 0.72 ** | 0.54 ** | 0.55 ** | 0.18 | 0.64 ** | 0.51 ** | 0.73 ** | <0.01 | 0.64 ** | 0.55 ** | 0.63 ** | 0.76 ** | 0.52 ** |
| Cob | 0.84 ** | 0.67 ** | 0.70 ** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.79 ** | <0.01 | <0.01 | 0.17 | |
| Husk | <0.01 | 0.10 | 0.03 | 0.61 ** | 0.36 ** | 0.34 ** | 0.51 ** | 0.67 ** | 0.08 | 0.60 ** | 0.00 | 0.67 ** | 0.67 ** | 0.70 ** | |
| Stem | 0.70 ** | 0.64 ** | 0.54 ** | <0.01 | 0.18 | 0.32 * | <0.01 | 0.11 | 0.40 * | <0.01 | 0.67 ** | 0.11 | 0.11 | 0.03 | |
| Sheath | 0.02 | 0.05 | <0.01 | 0.02 | 0.22 * | 0.48 ** | 0.06 | 0.63 ** | 0.33 ** | 0.01 | 0.00 | 0.64 ** | 0.62 ** | 0.55 ** | |
| Lamina | 0.64 ** | 0.67 ** | 0.57 ** | 0.19 | 0.03 | <0.01 | 0.21 | <0.01 | <0.01 | 0.19 | 0.68 ** | <0.01 | <0.01 | 0.12 | |
| JHN3 | Grain | 0.09 | <0.01 | <0.01 | 0.22 | 0.12 | 0.05 | 0.12 | 0.79 ** | 0.21 | 0.40 * | <0.01 | 0.16 | 0.79 ** | 0.65 ** |
| Cob | 0.84 ** | 0.69 ** | 0.47 ** | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | <0.01 | 0.85 ** | <0.01 | <0.01 | 0.23 | |
| Husk | 0.77 ** | 0.81 ** | 0.68 ** | 0.80 ** | 0.81 ** | 0.72 ** | 0.07 | 0.79 ** | 0.84 ** | 0.67 ** | 0.78 ** | 0.85 ** | 0.73 ** | 0.32 * | |
| HTN168 | Grain | 0.02 | 0.36 * | <0.01 | 0.42 ** | 0.12 | 0.82 ** | <0.01 | 0.82 ** | 0.37 * | 0.26 | 0.06 | 0.79 ** | 0.83 ** | 0.58 ** |
| Cob | 0.86 ** | 0.85 ** | 0.84 ** | 0.35 ** | 0.10 | 0.12 | 0.29 * | 0.32 * | <0.01 | 0.35 ** | 0.86 ** | 0.32 * | 0.31 * | 0.30 * | |
| Husk | 0.51 ** | 0.57 ** | 0.25 * | 0.37 * | 0.34 * | 0.70 ** | <0.01 | 0.67 ** | 0.57 ** | 0.34 * | 0.49 ** | 0.70 ** | 0.66 ** | 0.60 ** | |
| SD31 | Grain | 0.55 ** | 0.58 ** | 0.54 ** | 0.87 ** | 0.57 ** | 0.48 ** | 0.80 ** | 0.90 ** | 0.14 | 0.87 ** | 0.56 ** | 0.89 ** | 0.90 ** | 0.55 ** |
| Cob | 0.80 ** | 0.75 ** | 0.76 ** | <0.01 | 0.04 | <0.01 | <0.01 | <0.01 | 0.08 | <0.01 | 0.80 ** | <0.01 | <0.01 | 0.30 * | |
| Husk | 0.20 | 0.29 * | <0.01 | 0.23 * | 0.51 ** | 0.73 ** | 0.10 | 0.70 ** | 0.66 ** | 0.20 * | 0.13 | 0.73 ** | 0.69 ** | 0.58 ** | |
| HTN520 | Grain | 0.14 | <0.01 | <0.01 | 0.88 ** | 0.71 ** | <0.01 | 0.85 ** | 0.88 ** | 0.60 ** | 0.70 ** | <0.01 | 0.86 ** | 0.88 ** | 0.76 ** |
| TNBM508 | Grain | 0.01 | 0.35 ** | 0.02 | 0.56 ** | <0.01 | 0.70 ** | 0.34 ** | 0.70 ** | 0.34 ** | 0.58 ** | 0.11 | 0.67 ** | 0.71 ** | 0.60 ** |
| TNHB509 | Grain | 0.65 ** | 0.71 ** | 0.56 ** | 0.69 ** | 0.08 | 0.46 ** | 0.38 ** | 0.94 ** | <0.01 | 0.68 ** | 0.65 ** | 0.94 ** | 0.94 ** | 0.90 ** |
| JZXN | Grain | 0.40 ** | 0.64 ** | 0.55 ** | 0.86 ** | 0.71 ** | 0.42 ** | 0.84 ** | 0.86 ** | 0.33 ** | 0.87 ** | 0.53 ** | 0.87 ** | 0.86 ** | 0.54 ** |
| HZHN1 | Grain | <0.01 | <0.01 | <0.01 | 0.15 | 0.84 ** | 0.22 | 0.77 ** | <0.01 | 0.61 ** | 0.18 | <0.01 | <0.01 | <0.01 | <0.01 |
| Variety | Organ | Color Index | Validation | |
|---|---|---|---|---|
| NRMSE (%) | RMSE (mg/100 g) | |||
| SGHN | Grain | ACCR | 15.05 | 3.51 |
| ZZN8 | Grain | ACCR | 16.19 | 4.44 |
| JHN3 | Grain | ACCR | 27.49 | 5.75 |
| HTN168 | Grain | ACCR | 4.01 | 1.22 |
| SD31 | Grain | ACCR | 20.65 | 6.85 |
| HTN520 | Grain | ACCR | 6.35 | 2.25 |
| TNBM508 | Grain | ACCR | 4.25 | 0.77 |
| TNHB509 | Grain | ACCR | 5.04 | 0.42 |
| JZXN | Grain | ACCB | 4.14 | 0.31 |
| HZHN1 | Grain | RBR | 13.60 | 3.50 |
| SGHN | Cob | R | 3.03 | 33.00 |
| ZZN8 | Cob | R | 2.73 | 36.59 |
| JHN3 | Cob | BRT | 6.04 | 16.55 |
| HTN168 | Cob | BRT | 5.64 | 17.73 |
| SD31 | Cob | BRT | 23.55 | 18.68 |
| SGHN | Husk | ACCB | 3.33 | 29.99 |
| ZZN8 | Husk | ACCD | 3.84 | 26.02 |
| JHN3 | Husk | ACCB | 4.48 | 22.31 |
| HTN168 | Husk | ACCB | 6.22 | 16.08 |
| SD31 | Husk | ACCB | 26.62 | 24.13 |
| SGHN | Stalk | R | 24.35 | 4.11 |
| ZZN8 | Stalk | R | 30.06 | 3.33 |
| SGHN | Sheath | ACCB | 6.68 | 14.96 |
| ZZN8 | Sheath | ACCB | 9.11 | 10.98 |
| SGHN | Lamina | BRT | 16.18 | 6.18 |
| ZZN8 | Lamina | BRT | 15.48 | 6.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Liu, Y.; Wang, K.; Wang, Y.; Wang, X.; Liu, J.; Xu, C.; Song, Y. Phenotyping the Anthocyanin Content of Various Organs in Purple Corn Using a Digital Camera. Agriculture 2024, 14, 744. https://doi.org/10.3390/agriculture14050744
Wang Z, Liu Y, Wang K, Wang Y, Wang X, Liu J, Xu C, Song Y. Phenotyping the Anthocyanin Content of Various Organs in Purple Corn Using a Digital Camera. Agriculture. 2024; 14(5):744. https://doi.org/10.3390/agriculture14050744
Chicago/Turabian StyleWang, Zhengxin, Ye Liu, Ke Wang, Yusong Wang, Xue Wang, Jiaming Liu, Cheng Xu, and Youhong Song. 2024. "Phenotyping the Anthocyanin Content of Various Organs in Purple Corn Using a Digital Camera" Agriculture 14, no. 5: 744. https://doi.org/10.3390/agriculture14050744
APA StyleWang, Z., Liu, Y., Wang, K., Wang, Y., Wang, X., Liu, J., Xu, C., & Song, Y. (2024). Phenotyping the Anthocyanin Content of Various Organs in Purple Corn Using a Digital Camera. Agriculture, 14(5), 744. https://doi.org/10.3390/agriculture14050744







