Green Manuring with Oilseed Rape (Brassica napus L.) Mitigates Methane (CH4) and Nitrous Oxide (N2O) Emissions in a Rice-Ratooning System in Central China
Abstract
1. Introduction
2. Materials and Methods
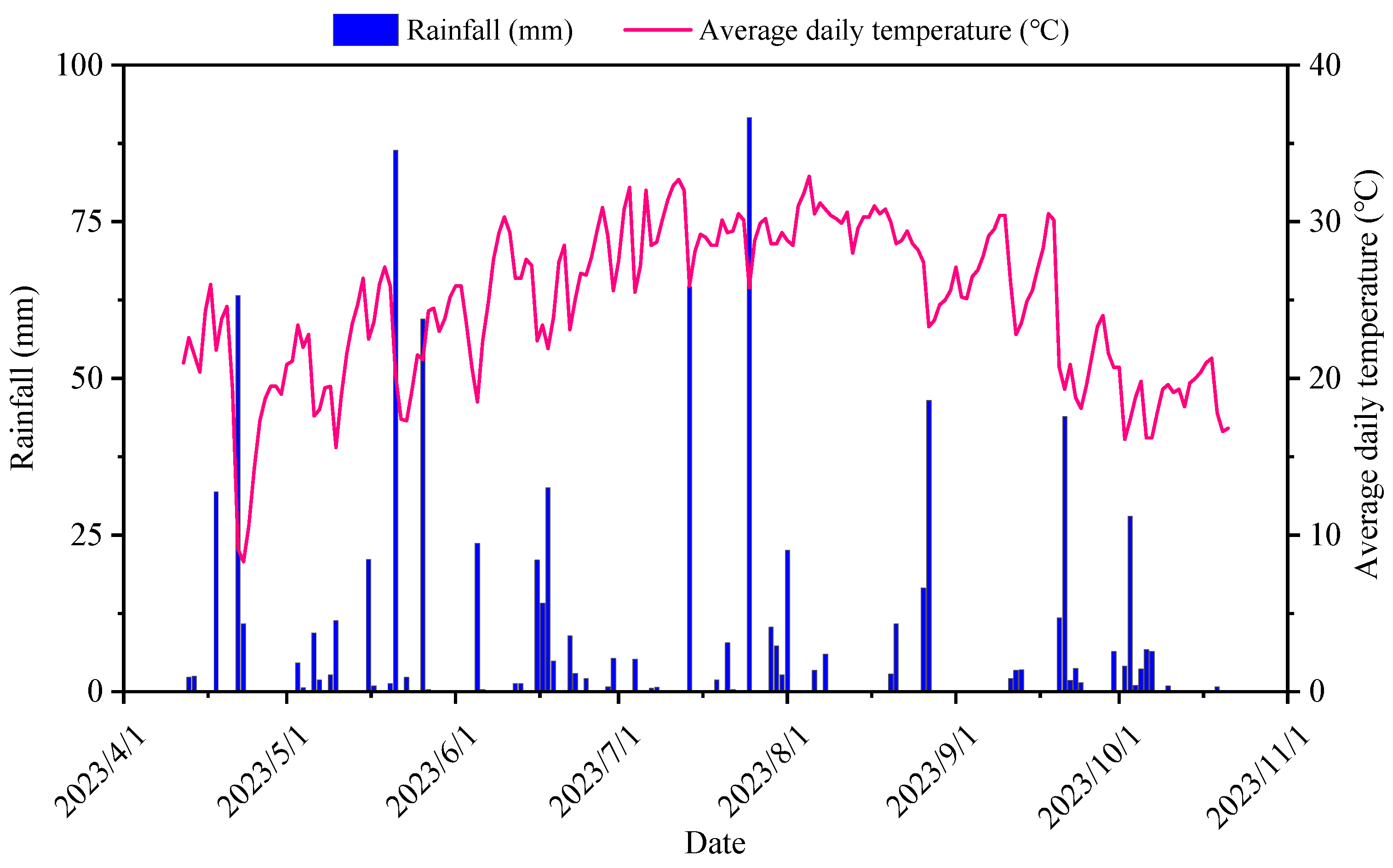
2.1. Study Area
2.2. Experiment 1: CH4 and N2O Emissions with OS-Urea Substitution
2.3. Experiment 2: Release of C and N during OS Humification
2.4. Sample Collection and Measurement
2.5. Data Calculation Formula
2.6. Statistical Analysis
3. Results
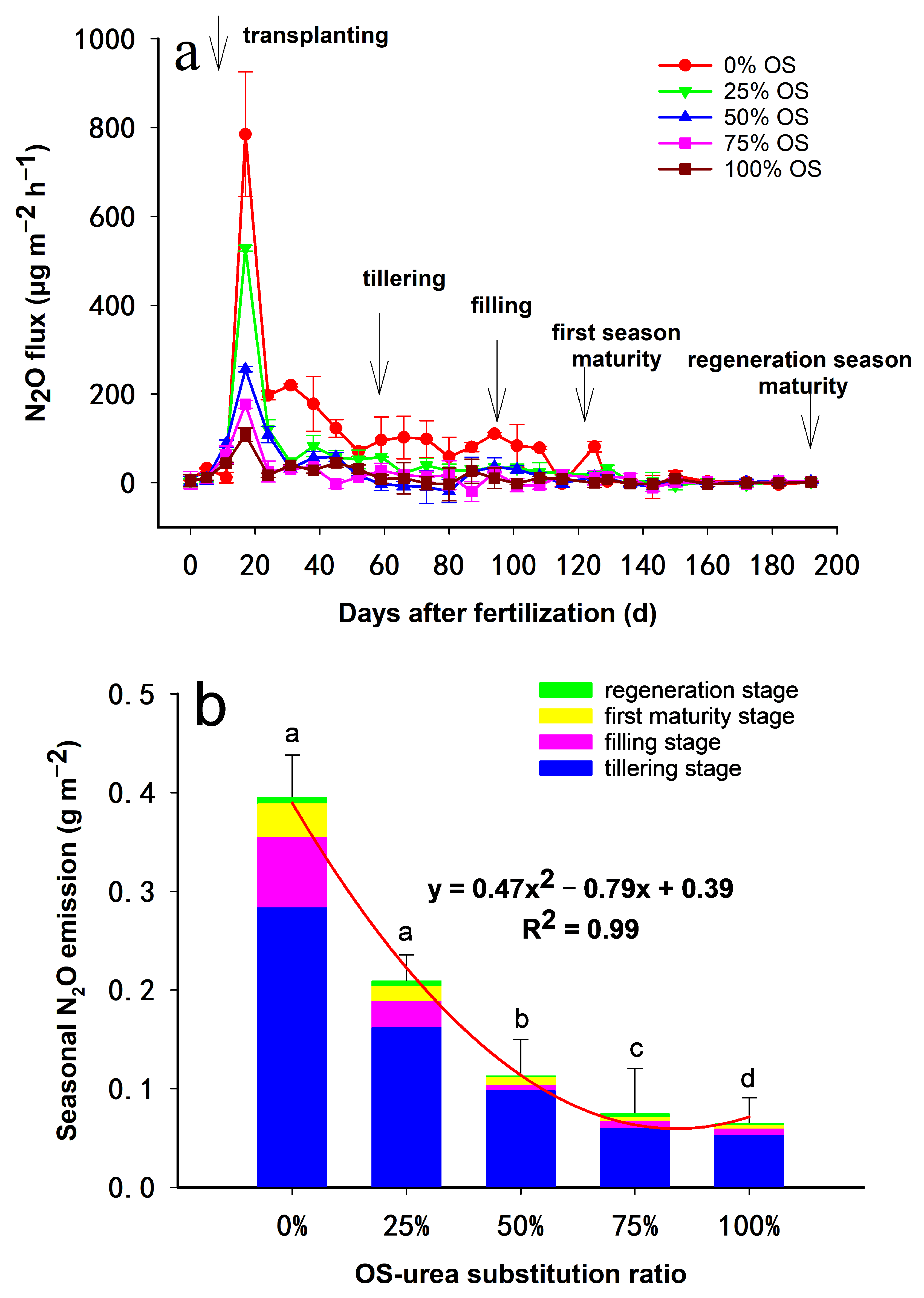
3.1. Fluxes of CH4 and N2O
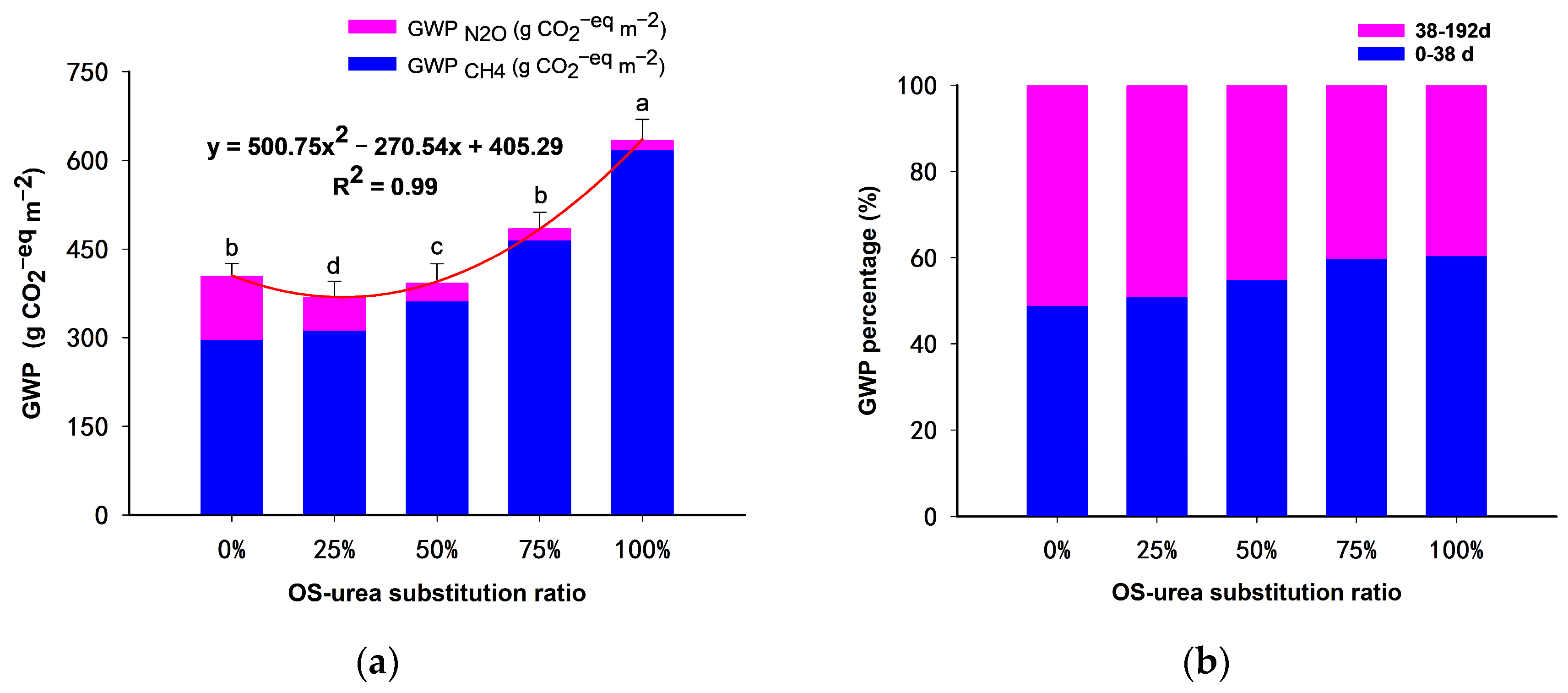
3.2. Yield, GWP and GHGI
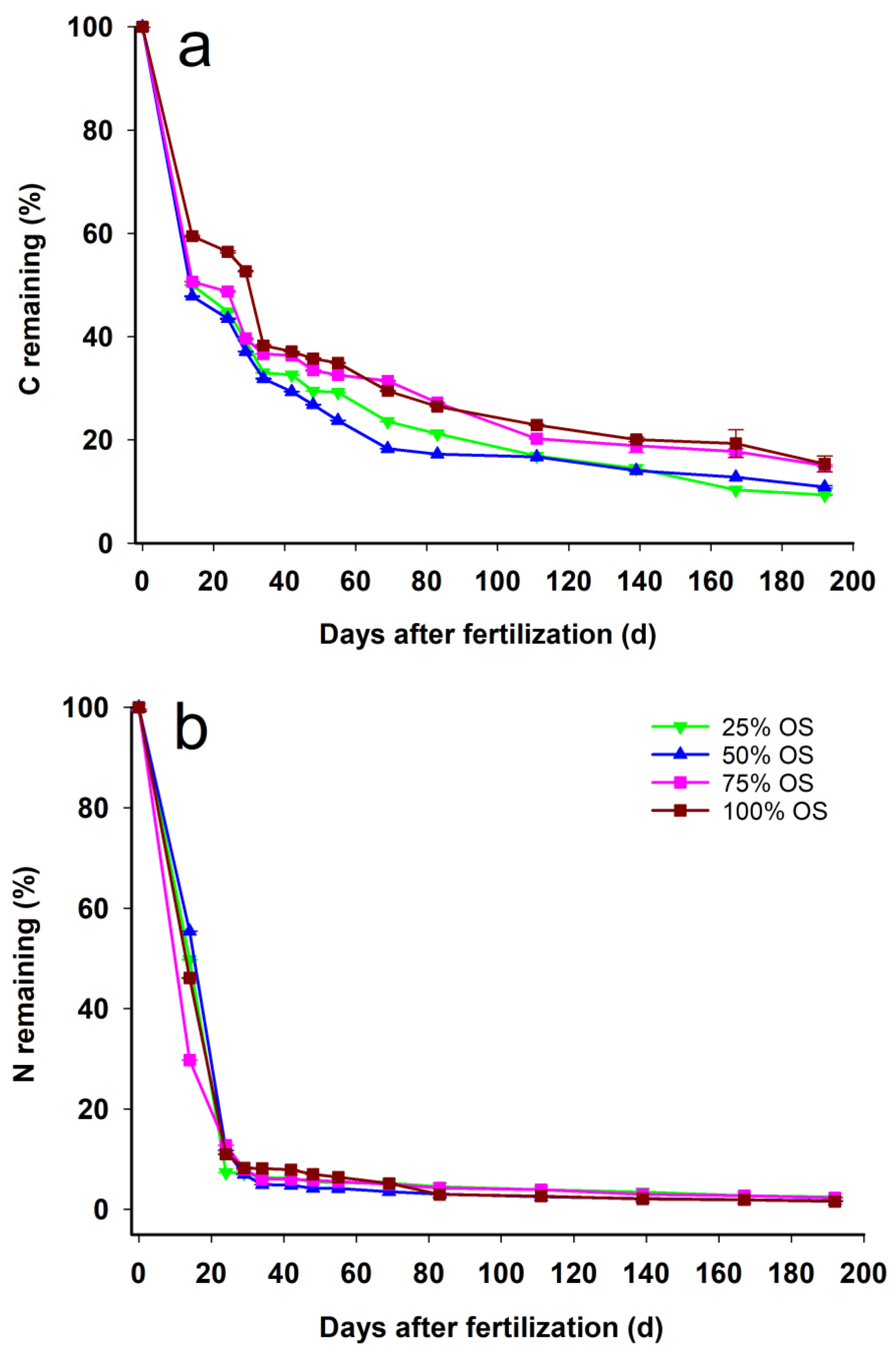
3.3. C and N Released during Decomposition of OS
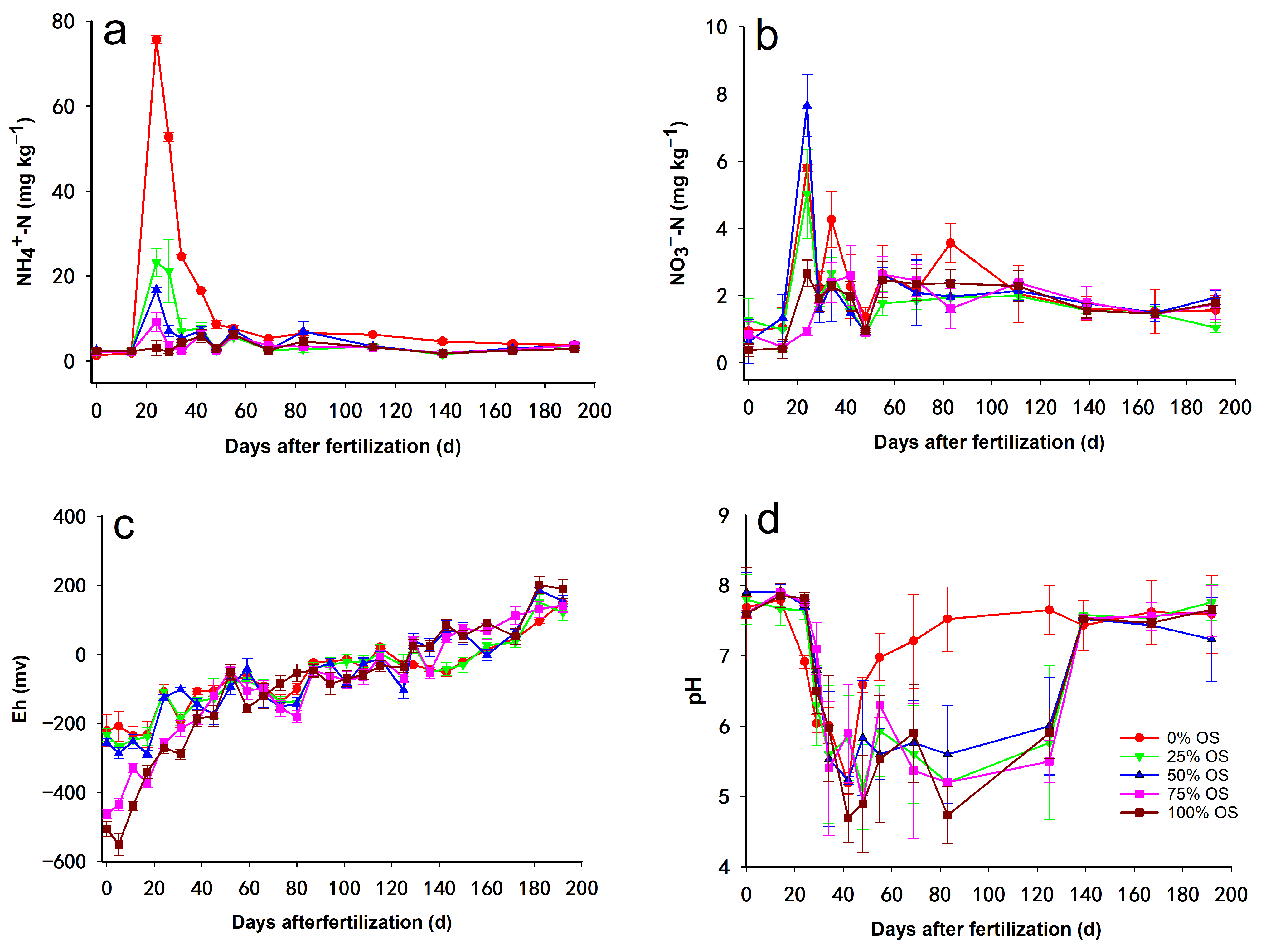
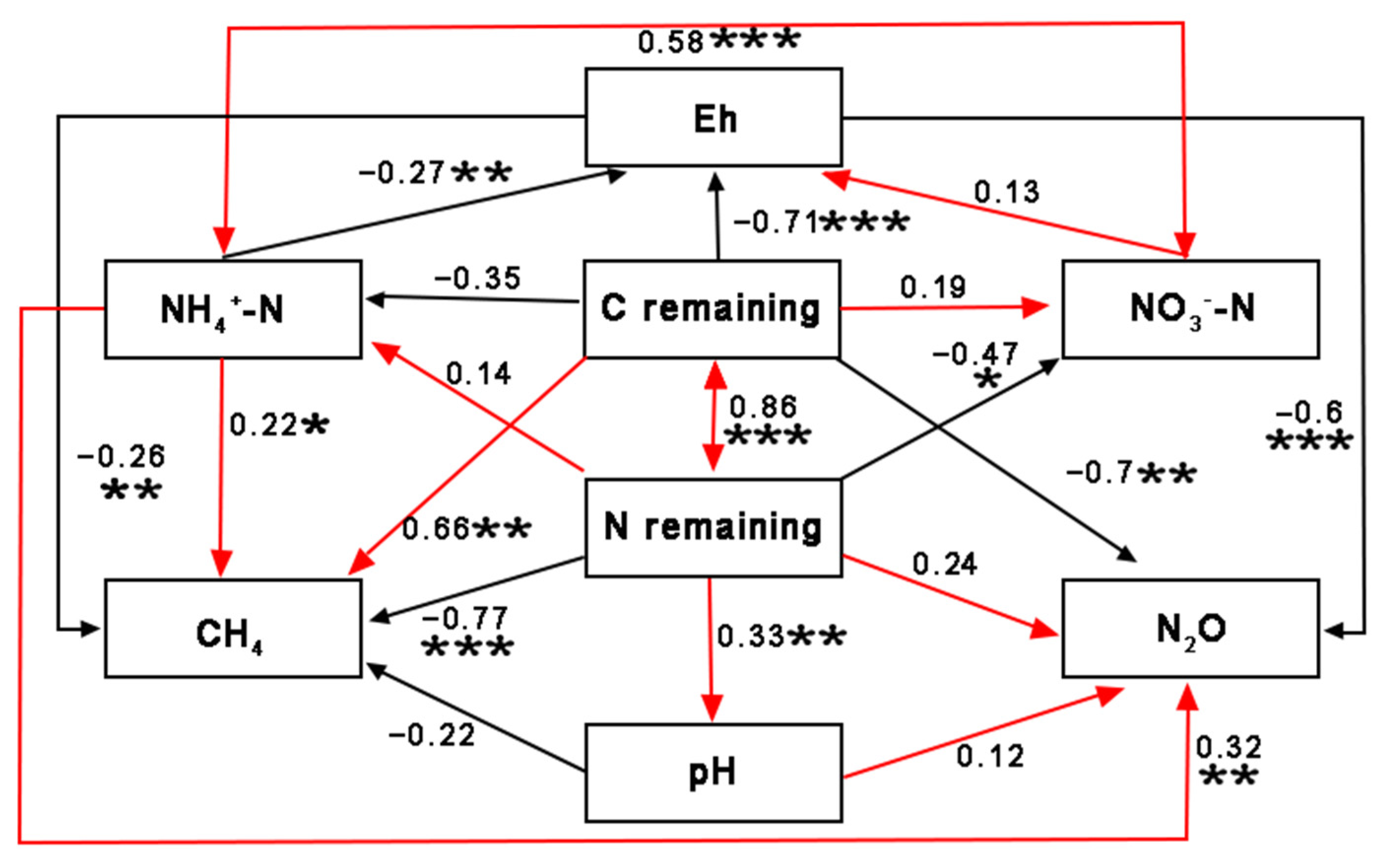
3.4. Effect of OS Decomposition on Soil Properties and the Relationship between the Concentrations of Different N and C Forms Produced
4. Discussion
4.1. Influence of OS-Urea Substitution Ratio on CH4 Emission
4.2. Influence of OS-Urea Substitution Ratio on N2O Emission
4.3. GWP and GHGI
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ye, S.; Song, C.; Shen, S.; Gao, P.; Cheng, C.; Cheng, F.; Zhu, D. Spatial pattern of arable land-use intensity in China. Land Use Policy 2020, 99, 104845. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Production: Crops and Livestock Products; FAO: Rome, Itlay, 2023; Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 6 March 2024).
- Hasukawa, H.; Inoda, Y.; Toritsuka, S.; Sudo, S.; Oura, N.; Sano, T.; Shirato, Y.; Yanai, J. Effect of Paddy-Upland Rotation System on the Net Greenhouse Gas Balance as the Sum of Methane and Nitrous Oxide Emissions and Soil Carbon Storage: A Case in Western Japan. Agriculture 2021, 11, 52. [Google Scholar] [CrossRef]
- Saunois, M.; Stavert, A.R.; Poulter, B.; Bousquet, P.; Canadell, J.G.; Jackson, R.B.; Raymond, P.A.; Dlugokencky, E.J.; Houweling, S.; Patra, P.K.; et al. The global methane budget 2000–2017. Earth Syst. Sci. Data 2020, 12, 1561–1623. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2022: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2022; Available online: https://www.ipcc.ch/report/ar6/wg3/ (accessed on 14 March 2023).
- Heffer, P.; Gruere, A.; Roberts, T. Assessment of Fertilizer Use by Crop at the Global Level 2014-2014/15; International Fertilizer Association, International Plant Nutrition Institute: Paris, France, 2017. [Google Scholar]
- Shaukat, M.; Samoy-Pascual, K.; Maas, E.D.; Ahmad, A. Simultaneous effects of biochar and nitrogen fertilization on nitrous oxide and methane emissions from paddy rice. J. Environ. Manag. 2019, 248, 109242. [Google Scholar] [CrossRef] [PubMed]
- Iboko, M.P.; Dossou-Yovo, E.R.; Obalum, S.E.; Oraegbunam, C.J.; Diedhiou, S.; Brümmer, C.; Témé, N. Paddy rice yield and greenhouse gas emissions: Any trade-off due to co-application of biochar and nitrogen fertilizer? A systematic review. Heliyon 2023, 9, e22132. [Google Scholar] [CrossRef] [PubMed]
- Bonginkosi, S.V.; Rebecca, Z.; Paramu, M.; Napo, N.; James, T. Seasonal Efuxes of Greenhouse Gases Under Diferent Tillage and N Fertilizer Management in a Dryland Maize Mono-crop. J. Soil Sci. Plant Nutr. 2021, 21, 2873–2883. [Google Scholar] [CrossRef]
- Zeng, Y.; Li, F. Impacts of Nitrogen Fertilizer Substitution on Greenhouse Gas Emission in a Paddy Field of South China Under Ridge Irrigation. J. Soil Sci. Plant Nutr. 2022, 22, 837–847. [Google Scholar] [CrossRef]
- Alpana, S.; Vishwakarma, P.; Adhya, T.K.; Inubushi, K.; Dubey, S.K. Molecular ecological perspective of methanogenic archaeal community in rice agroecosystem. Sci. Total Environ. 2017, 596–597, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.M.M.; Gaihre, Y.K.; Islam, M.R.; Ahmed, M.N.; Akter, M.; Singh, U.; Sander, B.O. Mitigating greenhouse gas emissions from irrigated rice cultivation through improved fertilizer and water management. J. Environ. Manag. 2022, 307, 114520. [Google Scholar] [CrossRef]
- Senthilraja, K.; Venkatesan, S.; Udhaya Nandhini, D.; Dhasarathan, M.; Prabha, B.; Boomiraj, K.; Geethalakshmi, V. Mitigating Methane Emission from the Rice Ecosystem through Organic Amendments. Agriculture 2023, 13, 1037. [Google Scholar] [CrossRef]
- Popa, D.C.; Laurent, Y.; Popa, R.A.; Pasat, A.; Bălănescu, M.; Svertoka, E.; Marin, M.P. A Platform for GHG Emissions Management in Mixed Farms. Agriculture 2023, 14, 78. [Google Scholar] [CrossRef]
- Kim, G.W.; Gwon, H.S.; Jeong, S.T.; Hwang, H.Y.; Kim, P.J. Different responses of nitrogen fertilization on methane emission in rice plant included and excluded soils during cropping season. Agric. Ecosyst. Environ. 2016, 230, 162–168. [Google Scholar] [CrossRef]
- Braker, G.; Conrad, R. Diversity, structure, and size of N2O-producing microbial communities in soils—What matters for their functioning? Adv. Appl. Microbiol. 2011, 75, 33–70. [Google Scholar] [CrossRef] [PubMed]
- Nyamadzawo, G.; Wuta, M.; Nyamangara, J.; Smith, J.L.; Rees, R.M. Nitrous oxide and methane emissions from cultivated seasonal wetland (dambo) soils with inorganic, organic and integrated nutrient management. Nutr. Cycl. Agroecosyst. 2014, 100, 161–175. [Google Scholar] [CrossRef]
- Yuan, J.; Yuan, Y.; Zhu, Y.; Cao, L. Effects of different fertilizers on methane emissions and methanogenic community structures in paddy rhizosphere soil. Sci. Total Environ. 2018, 627, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Hodge, A.; Stewart, J.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Competition between roots and soil micro-organisms for nutrients from nitrogen-rich patches of varying complexity. J. Ecol. 2000, 88, 150–164. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Xu, X. Competition between roots and microorganisms for nitrogen: Mechanisms and ecological relevance. New Phytol. 2013, 198, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Lashermes, G.; Nicolardot, B.; Parnaudeau, V.; Thuriès, L.; Chaussod, R.; Guillotin, M.L.; Linères, M.; Mary, B.; Metzger, L.; Morvan, T.; et al. Typology of exogenous organic matters based on chemical and biochemical composition to predict potential nitrogen mineralization. Bioresour. Technol. 2010, 101, 157–164. [Google Scholar] [CrossRef]
- Guo, T.; Zhang, Q.; Ai, C.; Liang, G.; He, P.; Zhou, W. Nitrogen enrichment regulates straw decomposition and its associated microbial community in a double-rice cropping system. Sci. Rep. 2018, 8, 1812–1847. [Google Scholar] [CrossRef]
- Zhu, B.; Yi, L.; Hu, Y.; Zeng, Z.; Lin, C.; Tang, H.; Yang, G.; Xiao, X. Nitrogen release from incorporated ¹⁵N-labelled Chinese milk vetch (Astragalus sinicus L.) residue and its dynamics in a double rice cropping system. Plant Soil 2014, 374, 331–344. [Google Scholar] [CrossRef]
- Bhattacharyya, P.; Roy, K.S.; Neogi, S.; Adhya, T.K.; Rao, K.S.; Manna, M.C. Effects of rice straw and nitrogen fertilization on greenhouse gas emissions and carbon storage in tropical flooded soil planted with rice. Soil. Tillage Res. 2012, 124, 119–130. [Google Scholar] [CrossRef]
- Linquist, B.A.; Marcos, M.; Adviento-Borbe, M.A.; Anders, M.; Harrell, D.; Linscombe, S.; Reba, M.L.; Runkle, B.R.K.; Tarpley, L.; Thomson, A. Greenhouse Gas Emissions and Management Practices that Affect Emissions in US Rice Systems. J. Environ. Manag. 2018, 47, 395–409. [Google Scholar] [CrossRef]
- Sun, H.F.; Zhou, S.; Zhang, J.N.; Zhang, X.X.; Wang, C. Effects of controlled-release fertilizer on rice grain yield, nitrogen use efficiency, and greenhouse gas emissions in a paddy field with straw incorporation. Field Crops Res. 2020, 253, 107814. [Google Scholar] [CrossRef]
- Song, K.; Zhang, G.; Ma, J.; Peng, S.; Lv, S.; Xu, H. Greenhouse gas emissions from ratoon rice fields among different varieties. Field Crops Res. 2022, 277, 108423. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2021: The Physical Science Basis: Working Group I Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2021; Available online: https://www.ipcc.ch/report/ar6/wg1/?__cf_chl_jschl_tk__=pmd_817adc6b26470b2060154bd1c68bd1ea450c9a50-1628507325-0-gqNtZGzNAjijcnBszQfO (accessed on 25 May 2023).
- Hu, Q.Y.; Liu, T.Q.; Jiang, S.S.; Cao, C.G.; Li, C.F.; Chen, B.; Liu, J.B. Combined Effects of Straw Returning and Chemical N Fertilization on Greenhouse Gas Emissions and Yield from Paddy Fields in Northwest Hubei Province, China. J. Soil Sci. Plant Nutr. 2020, 20, 392–406. [Google Scholar] [CrossRef]
- Wu, J.Q.; Wang, M.; Li, P.; Shen, L.Y.; Ma, M.Y.; Xu, B.Y.; Zhang, S.Y.; Sha, C.Y.; Ye, C.M.; Xiong, L.J.; et al. Effects of pig manure and its organic fertilizer application on archaea and methane emission in paddy fields. Land 2022, 4, 499. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, H.; Muhammad, A.; Zhong, C.; Li, P.; Zhang, P.; Yang, B.; Huang, G. Rice yield and greenhouse gas emissions affected by Chinese milk vetch and rice straw retention with reduced nitrogen fertilization. Agron. J. 2019, 111, 3028–3038. [Google Scholar] [CrossRef]
- Watanabe, T.; Kimura, M.; Asakawa, S. Dynamics of methanogenic archaeal communities based on rRNA analysis and their relation to methanogenic activity in Japanese paddy field soils. Soil. Biol. Biochem. 2007, 39, 2877–2887. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, B.; Liu, J.; Li, D.; Yang, Y.; Zhang, K.; Jiang, Y.; Hu, Y.; Zeng, Z. Azolla planting reduces methane emission and nitrogen fertilizer application in double rice cropping system in southern China. Agron. Sustain. Dev. 2017, 37, 29. [Google Scholar] [CrossRef]
- Muhammad, Q.; Huang, J.; Waqas, A.; Li, D.C.; Liu, S.J.; Zhang, L.; Cai, A.D.; Liu, L.S.; Xu, Y.M.; Gao, J.S.; et al. Yield sustainability, soil organic carbon sequestration and nutrients balance under long-term combined application of manure and inorganic fertilizers in acidic paddy soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Pereira-Mora, L.; Terra, J.A.; Fernández-Scavino, A. Methanogenic community linked to organic acids fermentation from root exudates are affected by rice intensification in rotational soil systems. Appl. Soil. Ecol. 2022, 176, 104498. [Google Scholar] [CrossRef]
- Baumann, K.; Marschner, P.; Smernik, R.J.; Baldock, J.A. Residue chemistry and microbial community structure during decomposition of eucalypt, wheat and vetch residues. Soil Biol. Biochem. 2009, 41, 1966–1975. [Google Scholar] [CrossRef]
- Fan, L.; Dippold, M.A.; Ge, T.; Wu, J.; Thiel, V.; Kuzyakov, Y.; Dorodnikov, M. Anaerobic oxidation of methane in paddy soil: Role of electron acceptors and fertilization in mitigating CH4 fluxes. Soil Biol. Biochem. 2020, 141, 107685. [Google Scholar] [CrossRef]
- Sun, G.L.; Zhang, Z.G.; Xiong, S.W.; Guo, X.Y.; Han, Y.C.; Wang, G.P.; Feng, L.; Lei, Y.Q.; Li, X.F.; Yang, B.F.; et al. Mitigating greenhouse gas emissions and ammonia volatilization from cotton fields by integrating cover crops with reduced use of nitrogen fertilizer. Agric. Ecosyst. Environ. 2022, 332, 107946. [Google Scholar] [CrossRef]
- Xu, P.S.; Li, Z.T.; Wang, J.Y.; Zou, J.W. Fertilizer-induced nitrous oxide emissions from global orchards and its estimate of China. Agric. Ecosyst. Environ. 2022, 328, 107854. [Google Scholar] [CrossRef]
- Yang, W.; Yao, L.; Zhu, M.Z.; Li, C.W.; Li, S.Q.; Wang, B.; Dijkstra, P.; Liu, Z.Y.; Zhu, B. Replacing urea-N with Chinese milk vetch (Astragalus sinicus L.) mitigates CH4 and N2O emissions in rice paddy. Agric. Ecosyst. Environ. 2022, 336, 108033. [Google Scholar] [CrossRef]
- Zhou, G.P.; Cao, W.D.; Bai, J.S.; Xu, C.X.; Zeng, N.H.; Gao, S.J.; Rees, R.M.; Dou, F.G. Co-incorporation of rice straw and leguminous green manure can increase soil available nitrogen (N) and reduce carbon and N losses: An incubation study. Pedosphere 2020, 30, 661–670. [Google Scholar] [CrossRef]
- Bi, R.Y.; Xu, X.T.; Zhan, L.P.; Chen, A.F.; Zhang, Q.Q.; Xiong, Z.Q. Proper organic substitution attenuated both N2O and NO emissions derived from AOB in vegetable soils by enhancing the proportion of Nitrosomonas. Sci. Total Environ. 2023, 866, 161231. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, W.; Huang, R.; Yan, X.Y.; Wang, S.Q. Extremely high N2O but unexpectedly low NO emissions from a highly organic and chemical fertilized peach orchard system in China. Agric. Ecosyst. Environ. 2017, 246, 202–209. [Google Scholar] [CrossRef]
- Ariani, M.; Haryono, E.; Hanudin, E. Greenhouse gas emission from rice field in Indonesia: Challenge for future research and development. Indones. J. Geogr. 2021, 53, 30–44. [Google Scholar] [CrossRef]
- Wang, W.; Lai, D.Y.F.; Wang, C.; Tong, C.; Zeng, C. Effects of inorganic amendments, rice cultivars and cultivation methods on greenhouse gas emissions and rice productivity in a subtropical paddy field. Ecol. Eng. 2016, 95, 770–778. [Google Scholar] [CrossRef]
- Zhou, W.; Ma, Q.X.; Wu, L.; Hu, R.G.; Jones, D.L.; Chadwick, D.R.; Jiang, Y.B.; Wu, Y.P.; Xia, X.G.; Yang, L.; et al. The effect of organic manure or green manure incorporation with reductions in chemical fertilizer on yield-scaled N2O emissions in a citrus orchard. Agric. Ecosyst. Environ. 2022, 326, 107806. [Google Scholar] [CrossRef]
- Latifmanesh, H.; Deng, A.; Li, L.; Chen, Z.J.; Zheng, Y.T.; Bao, X.T.; Zheng, C.Y.; Zhang, W.J. How incorporation depth of corn straw affects straw decomposition rate and C&N release in the wheat-corn cropping system. Agric. Ecosyst. Environ. 2020, 300, 107000. [Google Scholar] [CrossRef]

| Treatment | N Applied from Urea (g pot−1) | N Applied from OS (g pot−1) | C Applied from OS (g pot−1) |
|---|---|---|---|
| 0% OS | 0.3 | 0 | 0 |
| 25% OS | 0.225 | 0.075 | 0.67 |
| 50% OS | 0.15 | 0.15 | 1.35 |
| 75% OS | 0.075 | 0.225 | 2.03 |
| 100% OS | 0 | 0.3 | 2.71 |
| Treatment | CH4-Induced GWP | N2O-Induced GWP | Total GWP (g CO2-eq m−2) | Grain Yield (g m−2) | GHGI (kg CO2-eq kg−1 Yield) | ||
|---|---|---|---|---|---|---|---|
| GWP (g CO2−eq m−2) | Account for Total GWP (%) | GWP (g CO2−eq m−2) | Account for Total GWP (%) | ||||
| 0% OS | 297.1 e | 73.3 | 108.1 a | 26.7 | 405.2 b | 785.7 a | 0.52 b |
| 25% OS | 312.3 d | 84.5 | 57.3 a | 15.5 | 369.7 d | 755.7 a | 0.49 c |
| 50% OS | 362.4 c | 92.1 | 31.2 b | 7.9 | 393.5 c | 648.0 b | 0.61 a |
| 75% OS | 465.0 b | 95.8 | 20.6 c | 4.2 | 485.6 b | 526.1 bc | 0.92 a |
| 100% OS | 617.2 a | 97.2 | 17.8 d | 2.8 | 635.0 a | 444.3 c | 1.43 a |
| Treatment | KC | r2 | KN | r2 |
|---|---|---|---|---|
| 25% OS | 0.0272 | 0.8313 | 0.0706 | 0.9574 |
| 50% OS | 0.0303 | 0.8380 | 0.0667 | 0.9563 |
| 75% OS | 0.0232 | 0.6834 | 0.0832 | 0.9855 |
| 100% OS | 0.0202 | 0.7946 | 0.0689 | 0.9747 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Zhu, J.; Yang, W.; Zhao, D.; Zhou, Y.; Li, S.; Nie, J.; Yi, L.; Liu, Z.; Zhu, B. Green Manuring with Oilseed Rape (Brassica napus L.) Mitigates Methane (CH4) and Nitrous Oxide (N2O) Emissions in a Rice-Ratooning System in Central China. Agriculture 2024, 14, 839. https://doi.org/10.3390/agriculture14060839
Yao L, Zhu J, Yang W, Zhao D, Zhou Y, Li S, Nie J, Yi L, Liu Z, Zhu B. Green Manuring with Oilseed Rape (Brassica napus L.) Mitigates Methane (CH4) and Nitrous Oxide (N2O) Emissions in a Rice-Ratooning System in Central China. Agriculture. 2024; 14(6):839. https://doi.org/10.3390/agriculture14060839
Chicago/Turabian StyleYao, Lai, Jie Zhu, Wei Yang, Dongzhu Zhao, Yong Zhou, Shaoqiu Li, Jiangwen Nie, Lixia Yi, Zhangyong Liu, and Bo Zhu. 2024. "Green Manuring with Oilseed Rape (Brassica napus L.) Mitigates Methane (CH4) and Nitrous Oxide (N2O) Emissions in a Rice-Ratooning System in Central China" Agriculture 14, no. 6: 839. https://doi.org/10.3390/agriculture14060839
APA StyleYao, L., Zhu, J., Yang, W., Zhao, D., Zhou, Y., Li, S., Nie, J., Yi, L., Liu, Z., & Zhu, B. (2024). Green Manuring with Oilseed Rape (Brassica napus L.) Mitigates Methane (CH4) and Nitrous Oxide (N2O) Emissions in a Rice-Ratooning System in Central China. Agriculture, 14(6), 839. https://doi.org/10.3390/agriculture14060839







