Abstract
Drought at the flower and pod stage, which is the most moisture-sensitive stage of soybean development, is the main cause of yield loss in soybean. Nitrogen is a vital nutrient for soybeans. The objective of this study was to assess the potential of post-drought nitrogen fertilization at the soybean (Heihe 45) pod stage to (1) reduce pod shedding and increase yield, and (2) elucidate the mechanisms by which nitrogen fertilization regulates soybean growth under drought stress. The pot experiment was designed with two moisture levels and three nitrogen levels, resulting in a total of six treatments. The results show that nitrogen reduces cellular oxidation by regulating key enzymes of sucrose metabolism, such as sucrose synthase and sucrose phosphate synthase; and regulates cellulase to reduce shedding and mitigate drought. Comparison of low and high nitrogen conditions under drought conditions showed that the number of flowers and pods in soybean increased by 30% and 32.94%, respectively, malondialdehyde content decreased by 24%, cellulase activity in flowers and pods decreased by 15.07% and 12.31%, respectively, and yields increased by 29.98% under high nitrogen conditions. The high nitrogen treatment performed optimally and the differences between treatments reached the significant level.
1. Introduction
Drought stress is a serious threat to agriculture [1], being one of the main environmental stresses that reduce crop productivity and quality [2]. China has limited water resources, with each person having only a quarter of the world’s per capita freshwater resources. The drought situation has been worsened by rapid climate change [3], resulting in a dramatic reduction in crop yield and quality. China has achieved self-sufficiency in major crops. However, soybean production is heavily reliant on imports [4], which account for over 60% of all crop imports. Therefore, it is urgent to increase domestic soybean production to ensure food security. Xinjiang, a major agricultural province in China, boasts abundant light and heat resources [5], providing ideal conditions for biannual cropping, such as when replanting after wheat. To meet the growing domestic demand for soybeans, the cultivation of replanting soybeans after wheat is expanding. However, water resources are limited in Xinjiang, and soybeans are highly susceptible to water deficits during the flowering and podding stage [6]. During July and August each year, Xinjiang experiences a summer drought. This is also the period when soybeans are transitioning from nutritive growth to reproductive growth. However, the high temperatures and drought during this time cause a significant number of flowers and young pods to abort or fall off, resulting in yield losses. Therefore, it is important to study how to mitigate drought stress on soybean production during the pod stage.
Nitrogen (N) is a core mineral nutrient that is involved in the synthesis of active substances such as proteins, chlorophyll, hormones and metabolic enzyme [7]. It plays a vital role in plant growth and yield. Nitrogen affects photosynthesis, carbohydrates, and processes in plants [8], and participates in the metabolism of matter and energy in plant cells. Additionally, it can act as a stress ameliorator for plants [9]. Nitrogen application can improve sugar metabolism [10] and antioxidant capacity [11], while reducing cellulase activity and MDA content [12]. This can lead to a reduction in the abscission of reproductive organs and an improvement in the drought tolerance of plants. Nitrogen is involved in a variety of interrelated physiological and biochemical processes during nutrient uptake and transport and in response to drought stress [13]. Research indicates that nitrogen (N) can enhance plant tolerance to water limitation by increasing the activity of photosynthetic mechanisms and antioxidant enzyme [7]. Improvement of nitrogen levels increases the synthesis and metabolic use of sugars in Begonia shoot tips to meet the needs of shoot growth [8]. Nitrogen and phosphorus can alleviate the negative impacts of drought stress on plants [13]. This is achieved by increasing the levels of osmoprotectants and antioxidant enzyme.
Soybean is a significant crop in China, serving as a valuable source of grain, oil, and forage, as well as plant protein [14]. In the process of soybean production, pod shedding is a common phenomenon, and the pod shedding rate of summer soybean is generally 40–70%, and, in severe cases, it can be more than 80%. Research has demonstrated that drought stress triggers the immune system of crop reproductive organs, causing a shift in growth status from development to defense. This interferes with endogenous hormones and related enzyme activities [15,16], causing organ abscission and severe yield loss, as well as hindering sugar nutrient transport. The plant’s water deficit is indicated by an increase in the concentration of cell sap and a blockage of nutrient delivery to flowers and pods [17], which can result in pod abscission. Reproductive development is more sensitive to drought stress than the nutritional stage [18], particularly during the seed and fruit-sitting stages before and after fertilization [19]. Plants typically inhibit leaf extension in a reversible manner. However, this can lead to significant flower and young seed abortion [20]. During the reproductive stage, soybeans exhibit heightened sensitivity to water deficits, which is linked to the disruption of sucrose metabolism, a critical process for the allocation of carbon resources to the developing seeds and pods [21]. Sucrose signals derived from sucrose metabolism can interact with other signals to regulate plant development and stress response [22], including hormonal and redox-mediated processes. Drought stress inhibited sucrose input and sucrose metabolic enzyme activity [23]. Lower glucose levels may disrupt the energized state of the respiratory electron transport chain, leading to the overproduction of reactive oxygen species (ROS) [24]. This, in turn, leads to oxidative damage, programmed cell death, and seed failures. Malondialdehyde (MDA) is the major end product of plant membrane lipids damaged by ROS and is highly cytotoxic, binding to intracellular enzymes and causing severe damage to a wide range of enzymes and membrane systems [25], thereby destroying the structure and function of the biofilm itself. Peroxidase (POD) is a crucial protective enzyme that safeguards cells against oxidative damage, thereby enabling plants to maintain normal growth and development [26]. Cellulase is an important cellular hydrolase in organ abscission [27] and is closely related to the process of off-zone abscission. Drought stress regulates the production of hormones, such as abscisic acid and ethylene, which promote enhanced cellulase activity, leading to organ abscission.
This study aimed to determine the effects of drought stress on re-sowed soybeans in Xinjiang. Six treatments were set up, including drought, normal irrigation control, and three nitrogen application levels. The study measured flower and pod abscission, sucrose metabolism-related enzyme activity, POD activity, MDA content, flower and pod cellulase activity, yield, and harvest index. The results provide insight into the minimum rate of flower and pod abscission and yield stabilization under drought stress. This study was conducted based on the hypothesis that nitrogen has a mitigating effect on drought stress at the soybean flower pod stage. We hypothesize that (1) under drought stress, nitrogen fertilizer could alleviate the shedding of flowers and pod, regulate the activities of sugar metabolism, protective enzyme and cellulase, and finally increase the yield of soybean. Additionally, we seek to clarify the mechanism of nitrogen fertilizer regulating soybean growth under drought stress. However, further research is needed on the mitigation effect of higher nitrogen application levels on drought stress in soybean pods, as well as the mitigation effect of different forms and different morphologies of nitrogen.
2. Materials and Method
2.1. Biological Material and Field Experiment
The experiment was conducted in July 2019 at the Experimental Station of the College of Agriculture, Shihezi University (86°03′ E, 45°19′ N). The soil used in the experiment was irrigated grey desert soil with a pH of 7.84 and contained 27.2 mg/kg of alkaline dissolved nitrogen (ADN), 13.8 mg/kg of fast-acting phosphorus (FAP), 72.9 mg/kg of fast-acting potassium (FAP), and 8350 mg/kg of organic matter (OM). The soybean variety used was “Heihe 45”. The test pots were 40 cm high, and 36 cm in diameter, with each bucket filled with 25 kg of dry soil (sieved through a 5 mm sieve) and mixed with vermiculite, at the bottom of the bucket was a paved layer of stones, and gauze separating the soil and stones in order to prevent stagnant water from forming at the bottom of the bucket and to ensure air permeability. The edge of the bucket was placed vertically at equal intervals along three 2 cm diameter PVC pipes for watering.
The pot experiment was designed with two soil moisture levels and three nitrogen application levels. The soil moisture treatments were as follows: drought stress (DS), where pots were irrigated to 45–55% of field holding capacity and well-watered (WW), where pots were irrigated to 75–85% of field holding capacity. The nitrogen application treatments were as follows: low nitrogen application (N1), medium nitrogen application (N2) and high nitrogen application (N3). DSN1 represents the lowest level of nitrogen applied under drought stress, DSN2 represents the medium level of nitrogen applied under drought treatment, DSN3 represents the highest level of nitrogen applied under drought treatment, WWN1 represents low nitrogen applied under well-watered conditions, WWN2 represents medium nitrogen applied under well-watered conditions, and WWN3 represents high nitrogen applied under well-watered supply conditions. There were 6 treatments in total, with 12 pots per treatment. The fertilizers used in the experiment were all chemical fertilizers, nitrogen fertilizer was urea (N 46%), phosphorus fertilizer was diammonium phosphate (P2O5 46%), and potash fertilizer was potassium chloride (K2O 60%). Before sowing, 2.8 g (N1), 4.2 g (N2) and 5.6 g (N3) of urea, and 2.4 g of diammonium phosphate and 2 g of potassium chloride were applied as a basal fertilizer mixed evenly with the soil in the pots before sowing. The seeds were sown on 5 July 2019, with 10 seeds evenly sown in each pot, and 5 seedlings were retained at the N1 leaf stage of soybean. Other management measures were consistent with local practices for high-yielding cultivation.
All potted plants were grown outdoors under natural conditions, and soil moisture content was maintained at 70–80% of field water holding capacity before R1. Flowering stage (R1) to early stage of bulging (R5) were represented by the drought-treated potted plants using the weighing method to adjust the soil moisture control gradually to 45–55% of the field water holding capacity range. During the water stress treatment period, each treatment potted plant was weighed daily from 18:00–20:00, and when the soil moisture content fell to or near the lower limit of water for that treatment, the plants were immediately irrigated to the upper limit, in order to ensure that the drought treatment water was controlled within the design range on the day of the treatment.
2.2. Sampling and Measurement
2.2.1. A Survey of Soybean Flower Pod Shedding
At the beginning of the flowering stage (R1) of soybeans, three plants with uniform growth were selected for each treatment, and the plants were completely covered with nylon mesh bags. The number of flowers and pods dropped was recorded every 2 d before the bulging stage (R6), and the number of flowers and pods shed per plant was counted before the harvest of soybeans and investigated for the number of pods formed on a single plant.
The number of pods per plant (pcs/plant) is equal to the number of pods per plant plus the number of pods dropped per plant, the number of flowers per plant (flowers/plant) is equal to the number of pods per plant plus the number of flowers dropped per plant. The flowering rate of an individual plant, expressed as a percentage, is calculated by dividing the number of flowers that have fallen from that plant by the total number of flowers that have bloomed on the same plant, and then multiplying the result by one hundred.
The pod drop rate for a single plant, calculated as a percentage, is determined by the number of pods that have fallen from the plant t by the total number of pods present on the plant and then multiplied by 100. Additionally, the pod shedding rate, expressed as a percentage, is found by summing the number of flowers and pods that have fallen from the plant, dividing this sum by the number of flowers that have fallen, and then multiplying by 100.
2.2.2. Enzyme Extraction and MDA Content of Leaves
At the flowering stage (R2) and podding stage (R4), three soybean plants with the same growth were randomly selected from each treatment, and the middle leaves of the inverted trifoliate leaves were taken, cleaned to remove the leaf veins, treated with liquid nitrogen, and then placed in an ultra-low temperature of –80 °C for storage. For extracting sucrose synthase and sucrose-6-phosphate synthase, the required tissues (0.5–1.0 g) were homogenized in cold (3–4° C) 100 mM HEPES buffer (pH 8.2) containing 10 mM EDTA, 15 mM KCl, 5 mM MgCl2, 2 mM sodium diethyl dithiocarbamate and 5 mM β-mercaptoethanol. Insoluble polyvinylpolypyrrolidone (Sigma P2806 (St. Louis, MO, USA), 100 mg g−1 tissue) was also added while extracting the enzyme. The supernatant after centrifuging at 10,000× g for 15 min was passed through a sephadex G-25 column using 10 mM HEPES buffer (pH 7.0) to remove small molecular weight impurities. For extracting invertase, 0.02 M sodium phosphate buffer (pH 7.0) was used. Sucrose synthetase (SS), sucrose phosphate synthase (SPS), and invertase (Inv) activities were determined according to the method of Chopra et al. [28].
Samples of flower organs were collected at the R2 stage and pod organs at the R4 stage of soybean, and the samples were quick-frozen with liquid nitrogen and stored in a refrigerator at a low temperature of −80 °C. Determination of cellulase activity using the FPA method was undertaken. The international unit (IU) of filter paper activity (FPase) (FPU) is defined as the micromole of glucose equivalent liberated per minute of culture filtrate under assay conditions, where assay conditions refer to conditions, such as pH and temperature, in which the enzymes are held during the assay and which depend largely on the properties of the enzyme. For detailed steps, please refer to Mehdi et al. [29].
Malondialdehyde (MDA) was spectrophotometrically measured as an end product of lipid peroxidation using 2-thiobarbituric acid (TBA) reaction. Leaf tissues (0.5 g) were homogenized in 50 mM potassium phosphate buffer (pH 7.8) and centrifuged at 12,000× g for 20 min. Afterward, 4 mL 0.65% TBA in 20% trichloroacetic acid solution was added and the mixture was incubated at 95 °C for 30 min. The tubes were immediately placed in an ice bucket to stop the reaction. Samples were centrifuged at 10,000× g for 5 min and absorbance of the red adduct was recorded at 440, 532 and 600 nm. Leaf tissue (0.3 g) was homogenized with 3 mL ice-cold 25 mM HEPES buffer (pH 7.8) containing 0.2 mM EDTA and 2% polyvinylpyrrolidone (w/v), 2 mM ascorbic acid was added in the buffer. Malondialdehyde (MDA) content and peroxidase (POD) enzyme activities were measured as described by Golam et al. [30].
2.3. Statistical Analysis
Data were processed using Microsoft Excel 2010 software, ANOVA was performed using SPSS 17.0 software (p < 0.05), and correlation analysis and plotting were performed using Origin 2021 software.
3. Results and Analysis
3.1. Flower Pod Abortion
Under drought stress conditions (DS), soybean plants show a significant decrease in the number of flowers bloomed, flower dropped, pod set, and pod dropped compared with those under well-watered conditions (WW). At the same level of nitrogen application, the number of flowers bloomed decreased by 10.61 to 29.18% and the number of flowers dropped decreased by 6.57 to 26.85% under drought stress conditions compared with those under well-watered conditions (Table 1). Additionally, the number of pods set and dropped under drought conditions decreased by 15.07 to 31.9% and 4.75 to 20.83%, respectively, when compared with those under well-watered conditions (Table 1). The phenomenon of soybean pod shedding was exacerbated under drought stress.

Table 1.
Effects of different nitrogen application rates on flower and pod shedding numbers of soybean under drought and well-watered conditions. (DSN1 represents the lowest level of nitrogen applied under drought stress, DSN2 represents the medium level of nitrogen applied under drought treatment, DSN3 represents the highest level of nitrogen applied under drought treatment, WWN1 represents low nitrogen applied under well-watered conditions, WWN2 represents medium nitrogen applied under well-watered conditions, and WWN3 represents high nitrogen applied under well-watered supply conditions, there are six treatments).
Compared with low nitrogen, the flowering rates of medium nitrogen and high nitrogen increased by 22.36% and 30%, respectively (Table 1). Similarly, when compared with low nitrogen, the proportion of flowering and florescence increased by 22.45% and 27.63% in medium nitrogen and high nitrogen, respectively. Similarly, when compared with low N, the number of pod-setting and pod-dropping in medium N and high N were 22.19%, 32.94% and 12.28% higher, respectively (Table 1). Under the drought treatment, the peak performance was achieved at the high nitrogen level, resulting in a 3.81% reduction in pod abscission compared with the low nitrogen level. Under the drought treatment, the highest performance was achieved at the high nitrogen level, resulting in a 3.81% reduction in pod abscission when compared with the low nitrogen level.
3.2. SS Activity, Inv Activity and SPS Activity in Leaves
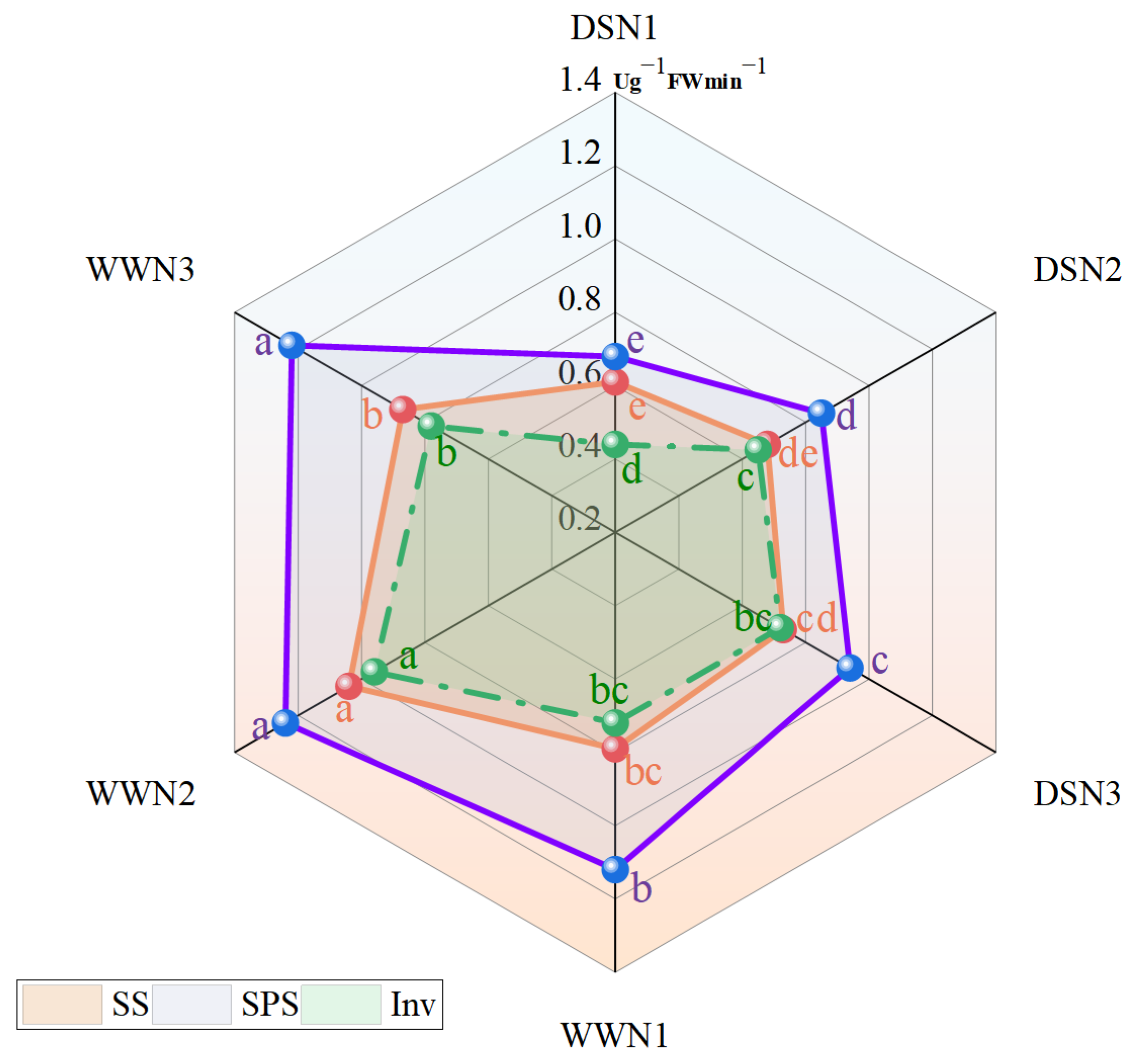
The drought treatment resulted in a reduction of sucrose synthase (SS), sucrose phosphate synthase (SPS), and invertase (Inv) activities in soybeans compared with those treated under well-watered treatment (Figure 1). However, SS, SPS and Inv enzyme activities in soybean leaves increased with nitrogen fertilizer application. In high and medium N, the SS enzyme activity increased by 19.67% and 11.48%, respectively, compared with low N. There was a significant difference between DSN3 and DSN1. The SS enzyme activity of DSN3 reached 92.41% of that of WWN1 (Figure 1).
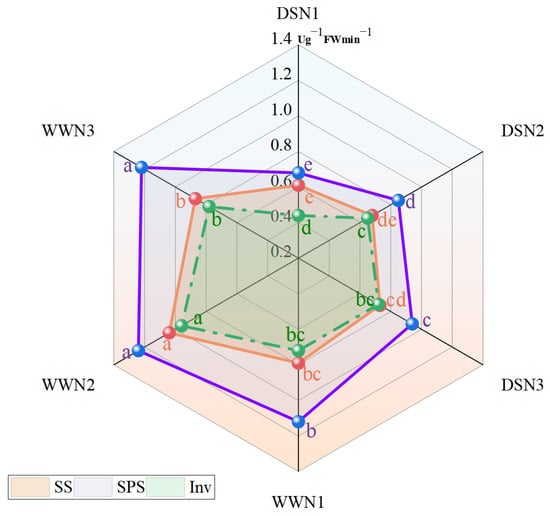
Figure 1.
Comparison of sucrose synthase (SS) activity, sucrose phosphate synthase (SPS) activity and invertase (Inv) activity in soybean leaves under different applied amounts of N and water. (DSN1 represents the lowest level of nitrogen applied under drought stress, DSN2 represents the medium level of nitrogen applied under drought treatment, DSN3 represents the highest level of nitrogen applied under drought treatment, WWN1 represents low nitrogen applied under well-watered conditions, WWN2 represents medium nitrogen applied under well-watered conditions, and WWN3 represents high nitrogen applied under well-watered supply conditions, there are six treatments). Different lowercase letters within the same type and column data indicate significant differences between treatments at the 0.05 level.
In the DS condition, the activity of the SPS enzyme decreased from 39.29% to 22.95% compared with the WW condition (Figure 1). SPS enzyme activity increased by 38.24% and 25% under drought conditions in high nitrogen and medium nitrogen, respectively, compared with low nitrogen. Significant differences were identified among DSN3, DSN2, and DSN1. The SPS enzyme activity of DSN3 increased significantly up to WWN1, reaching 83.93%.
The enzyme activity in DS decreased by 38.89%, 32.29%, and 7.69% when compared with WW (Figure 1). Meanwhile, the enzyme activity of DSN3 and DSN2 increased by 63.64% and 47.73%, respectively, compared with DSN1. Additionally, the INV enzyme activity of DSN3 increased by 10.77% when compared with DSN2.
3.3. MDA Content and POD Activity
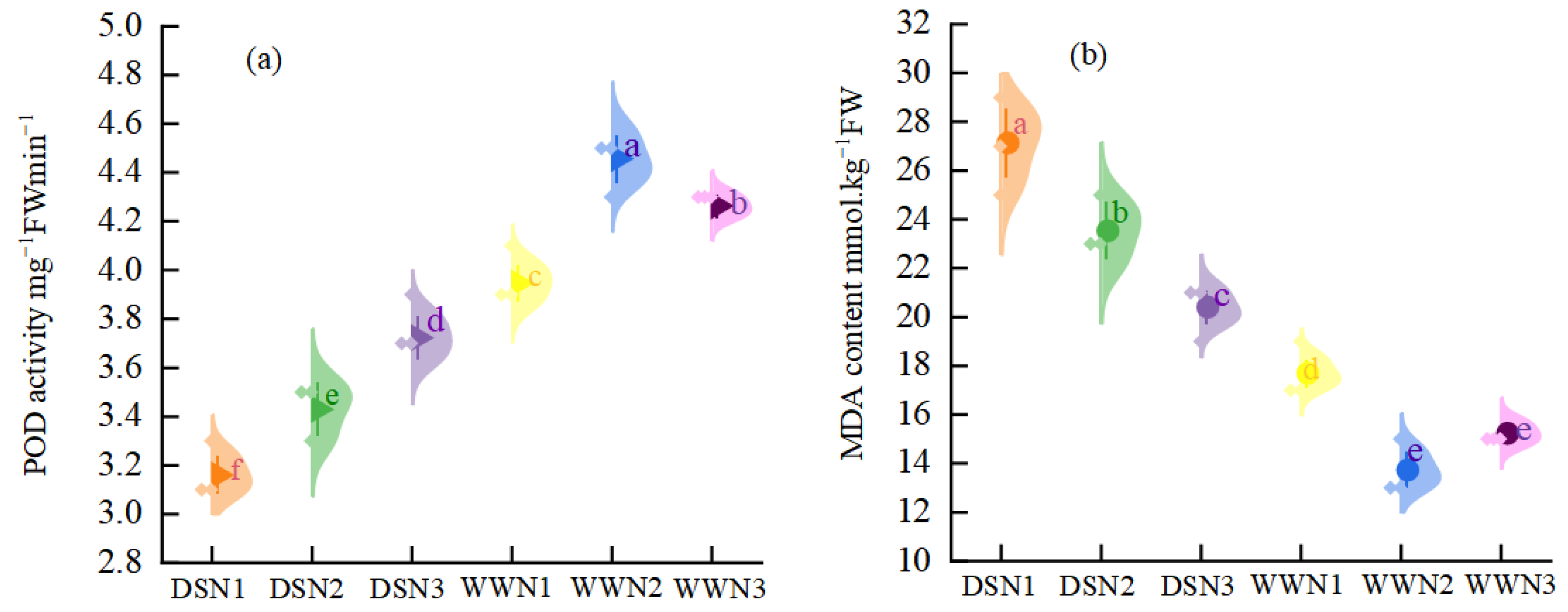
Under DS, the peroxidase enzyme (POD) activity was 20%, 23.09%, and 12.68% lower than WW (Figure 2a). However, with increased nitrogen application, there was an enhancement in POD activity. The POD activity of DSN2 and DSN3 increased by 8.54% and 17.72%, respectively, compared with that of DSN1. Under drought conditions, the highest level of POD activity was reached at N3, which was significantly different from DSN1 and DSN2. In well-watered treatment, the activity of POD showed a tendency to increase and then decrease with the application of nitrogen, reaching its peak at WWN2.
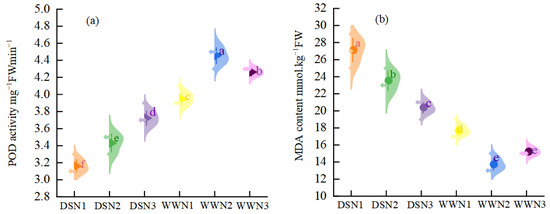
Figure 2.
Comparison of peroxidase enzyme (POD) activity (a) and malondialdehyde (MDA) content (b) in soybean leaves under different nitrogen application rates and moisture. (DSN1 represents the lowest level of nitrogen applied under drought stress, DSN2 represents the medium level of nitrogen applied under drought treatment, DSN3 represents the highest level of nitrogen applied under drought treatment, WWN1 represents low nitrogen applied under well-watered conditions, WWN2 represents medium nitrogen applied under well-watered conditions, and WWN3 represents high nitrogen applied under well-watered supply conditions, there are six treatments). Different lowercase letters within the same type and column data indicate significant differences between treatments at the 0.05 level.
In the drought treatment, leaf malondialdehyde (MDA) content was higher than in the well-watered treatment, and MDA content gradually decreased with increasing nitrogen fertilizer (Figure 2b). Under well-watered conditions, the content of MDA decreased and then increased with increasing fertilizer application. There were significant differences among treatments. The MDA content was 22.43%, 13.95%, and 10.92% higher than that of WW under DS. The MDA content of medium and high nitrogen was 13.27% and 24.81% lower than that of low nitrogen, respectively, with high nitrogen reaching the lowest level of MDA. The MDA content of DSN3 was 1.15 times higher than that of WWN1, which was closer to the MDA content of soybean leaves treated with low nitrogen under well-watered conditions.
3.4. Cellulase Activity of Flowers and Pods
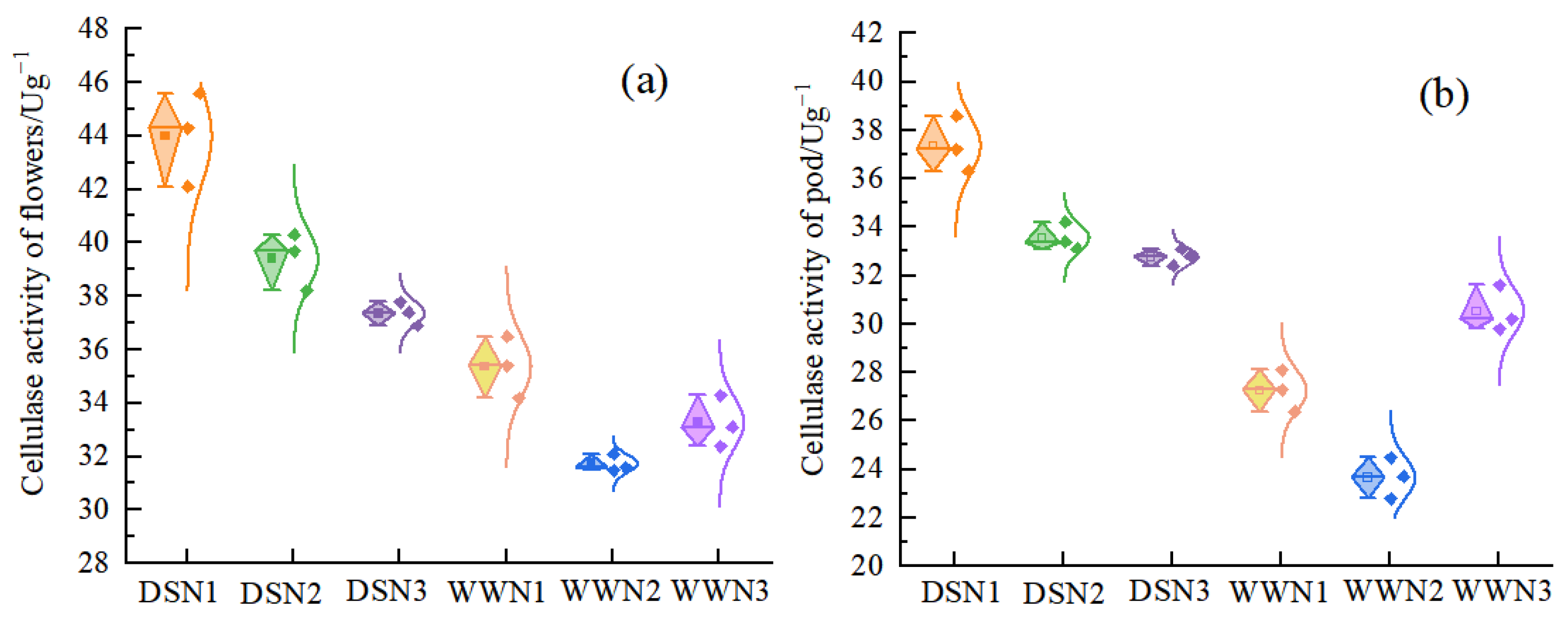
Under drought stress, the cellulase activities of flowers were lower compared with those under well-watered conditions. Additionally, the cellulase activities of flowers and pods were 24.4–12.32% and 41.83–7.34% higher under DS conditions than under WW conditions, respectively (Figure 3a,b). Under drought treatment, the cellulase activity of flowers and pods decreased with increasing nitrogen application. The activity was 10.45% and 15.07%, and 10.17% and 12.31% lower than that of DSN1 in the flowers and pods of DSN2 and DSN3, respectively. The lowest activity was observed under DSN3 treatment and was significantly different from DSN1. Under the well-watered supply, the cellulase activities of the flower and pod of WW showed an increasing and then decreasing tendency. The lowest activity was observed in the WWN2 treatment, which was 10.29% lower than WWN1 and significantly different from WWN1.
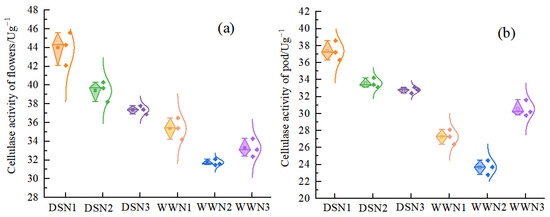
Figure 3.
Comparison of cellulase activity in soybean flowers (a) and pods (b) at different levels of nitrogen application and water. (DSN1 represents the lowest level of nitrogen applied under drought stress, DSN2 represents the medium level of nitrogen applied under drought treatment, DSN3 represents the highest level of nitrogen applied under drought treatment, WWN1 represents low nitrogen applied under well-watered conditions, WWN2 represents medium nitrogen applied under well-watered conditions, and WWN3 represents high nitrogen applied under well-watered supply conditions, there are six treatments).
3.5. Yield Per Plant and Harvest Index
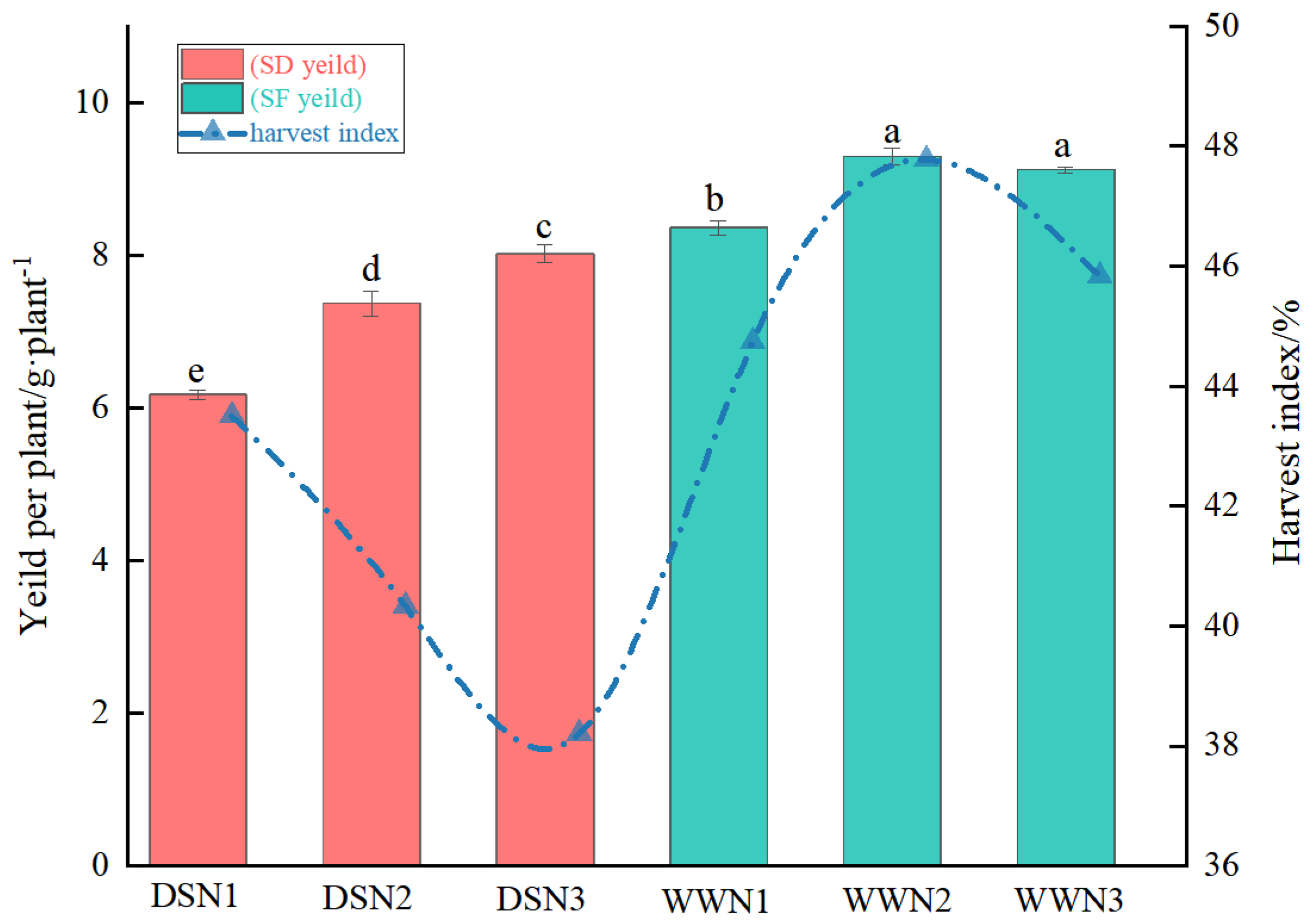
Under well-watered conditions, soybean yield per plant tended to increase with increasing nitrogen application, but then decreased. Under drought stress, soybean yield per plant increased with increasing nitrogen application, but was still lower than in normal water supply treatments (Figure 4). Compared with WW treatment, the yield per plant of DS treatment was 26.2%, 20.67%, and 11.96% lower. Soybean grain weight per plant increased by 19.45% and 29.98% in the medium and high N treatments, respectively, compared with the low N treatment. The highest yield was achieved in the high N treatment, reaching 95.93% of WWN1.
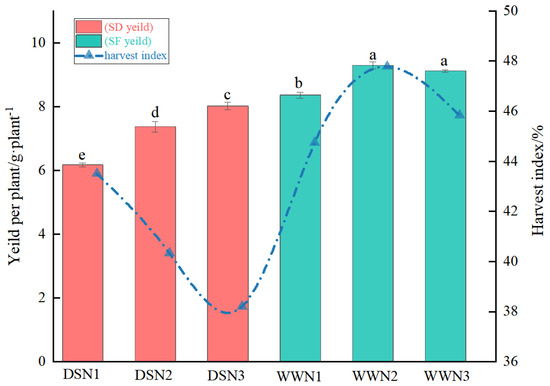
Figure 4.
Comparison of yield and harvest indices under drought and well-watered treatments. (DSN1 represents the lowest level of nitrogen applied under drought stress, DSN2 represents the medium level of nitrogen applied under drought treatment, DSN3 represents the highest level of nitrogen applied under drought treatment, WWN1 represents low nitrogen applied under well-watered conditions, WWN2 represents medium nitrogen applied under well-watered conditions, and WWN3 represents high nitrogen applied under well-watered supply conditions, there are six treatments). Different lowercase letters within the same type and column data indicate significant differences between treatments at the 0. 05 level.
Harvest indexes tended to decrease under drought treatments and were generally lower than under normal water supply (Figure 4). Conversely, harvest indexes increased and then decreased under normal water supply. Harvest indexes for DS were 2.75 to 16.65 percent lower than WW. Additionally, harvest indexes for medium and high N were 7.29 and 12.18 percent lower than those for low N, respectively. Furthermore, harvest indexes for high N were 5.28 percent lower than those for medium N. Finally, harvest indexes for DSN3 reached 85.4 percent of those for WWN1.
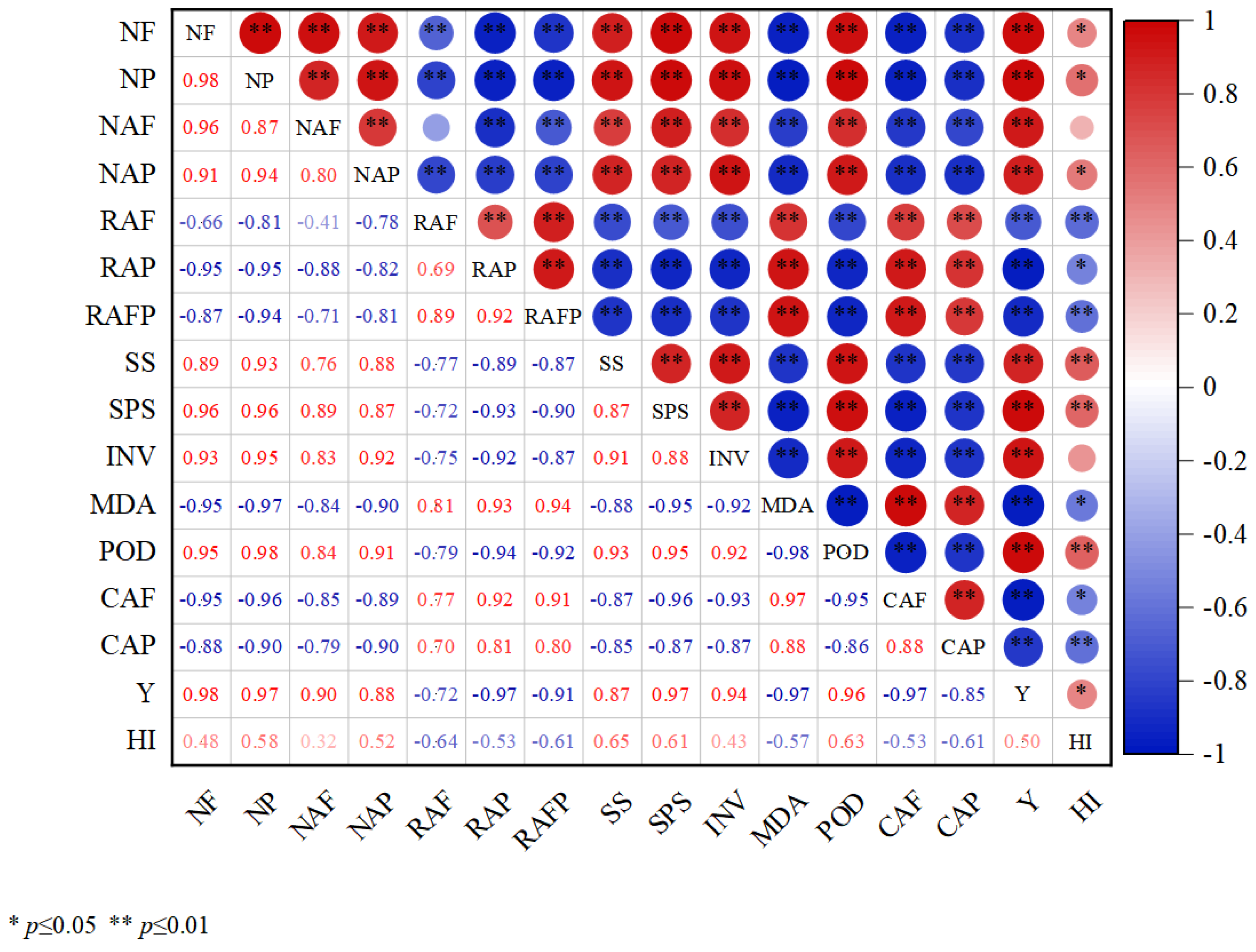
3.6. Correlation Analysis
There was a highly significant positive correlation (p < 0.01) between the number of flowers (NF), number of pods (NP), number of abscission flowers (NAF), number of abscission pods (NAP), SS, SPS, INV, POD, and yield (Y); (Figure 5) a highly significant positive correlation (p < 0.01) between flowers abscission rate (RAF), pods abscission rate (RAP), flowers and pods abscission rate (RAFP), MDA, cellulase activity of flowers (CAF), and cellulase activity of pods (CAP) were highly significant positively correlated (p < 0.01); Y and harvest index (HI) were significantly positively correlated (p < 0.05); there were highly significant negative correlations (p < −0.01) between RAP, RAFP, MDA, CAF, CAP, NF, NP, NAP, and HI and RAF, RAFP (Figure 5).
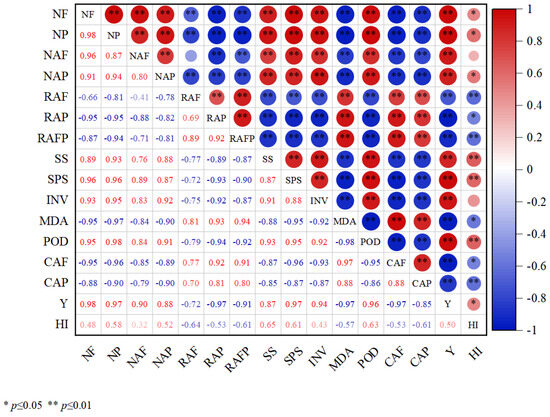
Figure 5.
Correlation analysis between flowering and podding of soybean and each indicator. (NF: number of flowers; NP: number of pods; NAF: number of abscission flowers; NAP: number of abscission pods; RAF: flowers abscission rate; RAP: pods abscission rate; RAFP: flowers and pods abscission rate; SS: sucrose synthase activities; SPS: sucrose phosphate synthase activities; INV: invertase activities; MDA: malondialdehyde; POD: peroxidase enzyme activities; CAF: cellulase activity of flowers; CAP: cellulase activity of pods; Y: yield; HI: harvest index).
4. Discussion
4.1. Effect of Nitrogen on Flower and Pod Abscission in Replanted Soybean under Drought Stress during Flowering and Podding Stages
Soybean flower pod abscission is typically between 30% and 80% with greater severity observed after experiencing stress. The hypothesis that yields can be increased by reducing flower and pod abscission has been confirmed [31]. The study results indicate that drought stress decreased the number of flowers and pods and increased the rate of pod shedding during the podding stage of soybean compared with well-watered conditions. However, the application of additional nitrogen fertilizer was able to alleviate drought stress, with a more pronounced effect at high nitrogen application [8] compared with medium and low nitrogen applications. Bhatia and Jumrani discovered that a water deficit during the reproductive stage led to a higher rate of flower failure, a reduced number of developed pods and seeds, and irreversible damage after re-watering, ultimately resulting in a loss of yield [32]. Several reports indicate that externally applied nitrogen can alleviate the effects of stress on plants [33]. Under normal water supply, high nitrogen reduced the number of flowers and pods per plant, plant height, and seed yield compared with low nitrogen. However, under drought stress, high nitrogen improved most of the traits in soybeans. This finding is consistent with the results of the present study. Additionally, Purcell and King [34] found that the application of nitrogen fertilizer can increase the number of flowers in a plant by reducing the rate of flower failure. This may be related to the fact that N metabolism-related transcripts are significantly expressed upon drought recovery in tolerant genotypes [13]. However, excessive application of nitrogen fertilizer can cause the plant to grow excessively, which weakens photosynthesis [35] and affects the synthesis and transport of organic matter, resulting in the shedding of flower pods.
4.2. Effect of Nitrogen on SS, SPS, INV, POD Activity and MDA under Drought Stress at Flowering and Podding Stage
The study results indicate that, under drought stress at the flowering and podding stages of soybean, the activities of SS, SPS, INV, and POD are lower than those of the normal irrigated treatments. However, the MDA contents were higher than those of the control in all treatments with the same level of nitrogen application. The activities of related enzymes showed an increasing trend with the increase of nitrogen fertilization, and MDA showed a decreasing trend. Under drought stress, the accumulation of reactive oxygen species (ROS) in the cell membrane resulted in an increase in the level of cellular plasma peroxidation, a decrease in POD activity, and the production of excess MDA. According to Du et al., sensitivity to abiotic stress during reproductive developmental stages is associated with disruption of sucrose metabolism [21]. Nitrogen regulates sugar metabolism. Zhang discovered that efficient nitrogen nutrition can also help alleviate the damage caused by drought stress by maintaining metabolic activity even at low levels of organizational water potential [36]. Zhang Lihua have also found that sucrose and related enzyme activities are enhanced and expression levels increased in Begonia under high nitrogen conditions [8]. This enhances sucrose metabolism, uptake intensity, and transport, allowing plants to maintain growth under adverse conditions. Nitrogen fertilization increases INV, SPS and SS activities [37]; SPS plays an important role in carbon metabolism and reservoir organs such as flowers and fruits [38]; and SS and INV are the main enzymes involved in sucrose degradation. Inv activity increases with increased nitrogen application and will catalyze the cleavage of sucrose to glucose and fructose, which plays a key role in sucrose metabolism, cellulose biosynthesis, the scavenging of reactive oxygen species and osmotic stress acclimatization to improve the drought tolerance of crops.
POD is an antioxidant enzyme that scavenges excess ROS in plants, reducing injury caused by adversity. In a previous study, the increased application of nitrogen fertilizer was seen to regulate the activity of POD, and reduce the MDA content of rice under high nitrogen conditions [11], which is consistent with the results of this paper. Gelaw discovered that nitrogen can enhance plant tolerance to water scarcity by increasing the activity of photosynthetic mechanisms and antioxidant enzymes [13]. Both high nitrogen [26] and low nitrogen concentrations regulated the activities of plant antioxidant enzymes and slowed down damage caused by stress.
4.3. Effect of Nitrogen on Cellulase Activity, Yield and Harvest Index under Drought Stress at Flowering and Podding Stage
Cellulase is a crucial cell wall hydrolase in plant abscission, capable of breaking down pectin, cellulose, and hemicellulose, which are the primary components of the cell wall [39]. The study results indicate that cellulase activity in soybean flowers and pods increases after drought, but gradually decreases with increasing nitrogen application. This reduction in cellulase activity leads to increased shedding of flowers and pods, ultimately reducing the yield. Drought signaling stimulates the phytohormones, such as abscisic acid and ethylene, which promote cellulase activity expression, resulting in organ abscission. Changes in nitrogen or nitrogen forms can alter ethylene biosynthesis [40] and signal transduction under stress conditions, affecting cellulase activity through hormonal changes to reduce abscission. External nitrogen supply affects the biosynthesis and translocation of plant hormones [41] in response to external environmental changes. Nitrogen fertilizer can significantly enhance photosynthesis and increase dry matter accumulation for higher yields in crops [42] under water stress, with an inverse relationship between yield and harvest index. Nitrogen application promotes sucrose metabolism, and plants will preferentially use the synthesized carbohydrates for survival rather than reproduction. Nitrogen is closely related to photosynthesis, and, under drought conditions, plants may use the extra nitrogen to promote nutrient growth so as to increase leaf area, improve the capacity for photosynthesis, and better resist droughts. This will lead to better development of nutrient organs and a decrease in the harvest index.
5. Conclusions
The results of this study show that nitrogen application could mitigate a range of negative effects caused by drought, such as flower and pod abscission, reduction in sucrose metabolizing enzyme activity, pod activity, yield and harvest index, and significant increase in flower and pod cellulase activity and MDA content. In comparison with the low and medium nitrogen treatments, the high nitrogen treatment was the most effective in mitigating drought stress, exhibiting a significant difference from the other treatments. The sucrose metabolism in soybean leaves was further regulated with the uptake of a high nitrogen application. Sucrose phosphate synthase promotes carbon metabolism, leading to an increase in the number of flowering pods. Sucrose synthase and invertase catalyze the cleavage of sucrose, enhancing the plant’s antioxidant capacity and resistance to osmotic stress. Concurrently, elevated nitrogen levels influence the biosynthesis and translocation of phytohormones, resulting in a reduction in cellulase activity in flowers and pods and a decline in flower and pod shedding, which ultimately impacts yield. In soybean seeds affected by drought, high nitrogen levels can promote bioaccumulation while simultaneously reducing transit accumulation of dry matter within the seed, which can result in a reduced harvest index. Insufficient application of fertilizer did not have a significant slow-release effect on drought stress, which resulted in a futile application of fertilizer, which in turn increased the cost of crop production in dryland areas. Under normal water supply, the impact of water and nitrogen was more significant. Further research is needed on the mitigation effect of higher nitrogen application levels on drought stress in soybean pods, as well as on the mitigation effect of different forms and different morphologies of nitrogen.
Author Contributions
Conceptualization, M.L., K.Z. and J.L.; methodology, M.L. and K.Z.; validation, M.L.; formal analysis, M.L.; resources, M.L. and J.L.; data curation, M.L.; writing—original draft, M.L.; writing—review and editing, M.L., G.N.u.D. and J.L.; visualization, M.L.; supervision, M.L., G.N.u.D. and J.L.; project administration, J.L.; funding acquisition, J.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program (No.2016YFC0501406).and NO.7GROUP-Shihezi University Science and Technology Innovation Special Project (No. QS2023013).
Data Availability Statement
Data presented in this study are available upon reasonable request to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, H.; Wang, S.; Wang, J.; Lu, H.; Guo, A.; Zhu, Z.; Myneni, R.B.; Shugart, H.H. Assessing spatiotemporal variation of drought in China and its impact on agriculture during 1982-2011 by using PDSI indices and agriculture drought survey data. J. Geophys. Res.-Atmos. 2016, 121, 2283–2298. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagon, D.; Arbona, V.; Gomez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Ahanger, M.A.; Qi, M.; Huang, Z.; Xu, X.; Begum, N.; Qin, C.; Zhang, C.; Ahmad, N.; Mustafa, N.S.; Ashraf, M.; et al. Improving growth and photosynthetic performance of drought stressed tomato by application of nano-organic fertilizer involves up-regulation of nitrogen, antioxidant and osmolyte metabolism. Ecotox. Environ. Safe. 2021, 216, 112195. [Google Scholar] [CrossRef] [PubMed]
- Ali, T.; Zhou, B.; Cleary, D.; Xie, W. The Impact of Climate Change on China and Brazil’s Soybean Trade. Land 2022, 11, 2286. [Google Scholar] [CrossRef]
- Fei, C.; Dong, Y.Q.; An, S.Z. Factors driving the biomass and species richness of desert plants in northern Xinjiang China. PLoS ONE 2022, 17, e271575. [Google Scholar] [CrossRef] [PubMed]
- Zeleke, K.; Nendel, C. Yield response and water productivity of soybean (Glycine max L.) to deficit irrigation and sowing time in south-eastern Australia. Agric. Water Manag. 2024, 296, 108815. [Google Scholar] [CrossRef]
- Zhong, C.; Cao, X.; Hu, J.; Zhu, L.; Zhang, J.; Huang, J.; Jin, Q. Nitrogen Metabolism in Adaptation of Photosynthesis to Water Stress in Rice Grown under Different Nitrogen Levels. Front. Plant Sci. 2017, 8, 1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Sun, S.; Liang, Y.; Li, B.; Ma, S.; Wang, Z.; Ma, B.; Li, M. Nitrogen Levels Regulate Sugar Metabolism and Transport in the Shoot Tips of Crabapple Plants. Front. Plant Sci. 2021, 12, 626149. [Google Scholar] [CrossRef] [PubMed]
- Shabbir, R.N.; Waraich, E.A.; Ali, H.; Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Awan, M.I.; Ahmad, S.; Irfan, M.; Hussain, S.; et al. Supplemental exogenous NPK application alters biochemical processes to improve yield and drought tolerance in wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2016, 23, 2651–2662. [Google Scholar] [CrossRef]
- He, F.; Zhao, Q.; Huang, J.; Niu, M.; Feng, H.; Shi, Y.; Zhao, K.; Cui, X.; Wu, X.; Mi, J.; et al. External application of nitrogen alleviates toxicity of cadmium on poplars via starch and sucrose metabolism. Tree Physiol. 2021, 41, 2126–2141. [Google Scholar] [CrossRef]
- Liao, G.; Yang, Y.; Xiao, W.; Mo, Z. Nitrogen Modulates Grain Yield, Nitrogen Metabolism, and Antioxidant Response in Different Rice Genotypes. J. Plant Growth Regul. 2023, 42, 2103–2114. [Google Scholar] [CrossRef]
- Wu, Z.Z.; Ying, Y.Q.; Zhang, Y.B.; Bi, Y.F.; Wang, A.K.; Du, X.H. Alleviation of drought stress in Phyllostachys edulis by N and P application. Sci. Rep. 2018, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Gelaw, T.A.; Goswami, K.; Sanan-Mishra, N. Individual and Interactive Effects of Nitrogen and Phosphorus on Drought Stress Response and Recovery in Maize Seedlings. Agriculture 2023, 13, 654. [Google Scholar] [CrossRef]
- Coleman, K.; Whitmore, A.P.; Hassall, K.L.; Shield, I.; Semenov, M.A.; Dobermann, A.; Bourhis, Y.; Eskandary, A.; Milne, A.E. The potential for soybean to diversify the production of plant-based protein in the UK. Sci. Total Environ. 2021, 767, 144903. [Google Scholar] [CrossRef] [PubMed]
- Nemati, F.; Ghanati, F.; Ahmadi Gavlighi, H.; Sharifi, M. Comparison of sucrose metabolism in wheat seedlings during drought stress and subsequent recovery. Biol. Plant. 2018, 62, 595–599. [Google Scholar] [CrossRef]
- Wang, W.; Kim, Y.; Lee, H.; Kim, K.; Deng, X.; Kwak, S. Analysis of antioxidant enzyme activity during germination of alfalfa under salt and drought stresses. Plant Physiol. Biochem. 2009, 47, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Schonbeck, L.; Li, M.; Lehmann, M.M.; Rigling, A.; Schaub, M.; Hoch, G.; Kahmen, A.; Gessler, A. Soil nutrient availability alters tree carbon allocation dynamics during drought. Tree Physiol. 2021, 41, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Hu, M.; Xu, W.; Wang, Y.; Huang, K.; Zhang, C.; Wen, J. Understanding the molecular mechanism of anther development under abiotic stresses. Plant Mol.Biol. 2021, 105, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Patrick, J.W.; Bouzayen, M.; Osorio, S.; Fernie, A.R. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012, 17, 656–665. [Google Scholar] [CrossRef]
- Batth, R.; Singh, K.; Kumari, S.; Mustafiz, A. Transcript Profiling Reveals the Presence of Abiotic Stress and Developmental Stage Specific Ascorbate Oxidase Genes in Plants. Front. Plant Sci. 2017, 8, 198. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, H.P.; Batish, D.R.; Kaur, S.; Kohli, R.K. EMF radiations (1800 MHz)-inhibited early seedling growth of maize (Zea mays) involves alterations in starch and sucrose metabolism. Protoplasma 2016, 253, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jin, Y.; Yang, Y.; Li, G.; Boyer, J.S. Sugar Input, Metabolism, and Signaling Mediated by Invertase: Roles in Development, Yield Potential, and Response to Drought and Heat. Mol. Plant. 2010, 3, 942–955. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhao, Q.; Chen, L.; Yao, X.; Zhang, H.; Wu, J.; Xie, F. Effect of Drought Stress during Soybean R2-R6 Growth Stages on Sucrose Metabolism in Leaf and Seed. Int. J. Mol. Sci. 2020, 21, 618. [Google Scholar] [CrossRef] [PubMed]
- Chenna, S.; Koopman, W.J.H.; Prehn, J.H.M.; Connolly, N.M.C. Mechanisms and mathematical modeling of ROS production by the mitochondrial electron transport chain. Am. J. Physiol.-Cell Physiol. 2022, 323, C69–C83. [Google Scholar] [CrossRef] [PubMed]
- Ghassemi, S.; Ghassemi-Golezani, K.; Salmasi, S.Z. Changes in antioxidant enzymes activities and physiological traits of ajowan in response to water stress and hormonal application. Sci. Hortic. 2019, 246, 957–964. [Google Scholar] [CrossRef]
- Chang, Z.; Liu, Y.; Dong, H.; Teng, K.; Han, L.; Zhang, X. Effects of Cytokinin and Nitrogen on Drought Tolerance of Creeping Bentgrass. PLoS ONE 2016, 11, e154005. [Google Scholar] [CrossRef]
- Del Campillo, E.U.O.M.; Bennett, A.B. Pedicel breakstrength and cellulase gene expression during tomato flower abscission. Plant Physiol. 1996, 111, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Chopra, J.; Kaur, N.; Gupta, A.K. Ontogenic changes in enzymes of carbon metabolism in relation to carbohydrate status in developing mungbean reproductive structures. Phytochemistry 2000, 53, 539–548. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Maki, M.; Leung, K.T.; Mao, C.; Qin, W. Cellulase activities in biomass conversion: Measurement methods and comparison. Crit. Rev. Biotechnol. 2010, 30, 302–309. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. Endogenous melatonin deficiency aggravates high temperature-induced oxidative stress in Solanum lycopersicum L. Environ. Exp. Bot. 2019, 161, 303–311. [Google Scholar] [CrossRef]
- Zhu, R.; Wang, X.; Yan, Z.; Qiao, Y.; Tian, H.; Hu, Z.; Zhang, Z.; Li, Y.; Zhao, H.; Xin, D.; et al. Exploring Soybean Flower and Pod Variation Patterns During Reproductive Period Based on Fusion Deep Learning. Front. Plant Sci. 2022, 13, 922030. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Sedri, M.H.; Roohi, E.; Niazian, M.; Niedbała, G. Interactive Effects of Nitrogen and Potassium Fertilizers on Quantitative-Qualitative Traits and Drought Tolerance Indices of Rainfed Wheat Cultivar. Agronomy 2022, 12, 30. [Google Scholar] [CrossRef]
- Purcell, L.C.; King, C.A. Drought and nitrogen source effects on nitrogen nutrition, seed growth, and yield in soybean. J. Plant Nutr. 1996, 19, 969–993. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, R.; Liu, Z.; Hu, Y.; Xia, Z.; Hu, B.; Rennenberg, H. Consequences of excess urea application on photosynthetic characteristics and nitrogen metabolism of Robinia pseudoacacia seedlings. Chemosphere 2024, 346, 140619. [Google Scholar] [CrossRef] [PubMed]
- Agami, R.A.; Alamri, S.A.M.; Abd El-Mageed, T.A.; Abousekken, M.S.M.; Hashem, M. Role of exogenous nitrogen supply in alleviating the deficit irrigation stress in wheat plants. Agric. Water Manag. 2018, 210, 261–270. [Google Scholar] [CrossRef]
- Yue, K.; Li, L.; Xie, J.; Liu, Y.; Xie, J.; Anwar, S.; Fudjoe, S.K. Nitrogen Supply Affects Yield and Grain Filling of Maize by Regulating Starch Metabolizing Enzyme Activities and Endogenous Hormone Contents. Front. Plant Sci. 2022, 12, 798119. [Google Scholar] [CrossRef]
- Zanor, M.I.; Osorio, S.; Nunes-Nesi, A.; Carrari, F.; Lohse, M.; Usadel, B.; Kuehn, C.; Bleiss, W.; Giavalisco, P.; Willmitzer, L.; et al. RNA Interference of LIN5 in Tomato Confirms Its Role in Controlling Brix Content, Uncovers the Influence of Sugars on the Levels of Fruit Hormones, and Demonstrates the Importance of Sucrose Cleavage for Normal Fruit Development and Fertility. Plant Physiol. 2009, 150, 1204–1218. [Google Scholar] [CrossRef] [PubMed]
- González-Carranza, Z.H.; Whitelaw, C.A.; Swarup, R.; Roberts, J.A. Temporal and Spatial Expression of a Polygalacturonase during Leaf and Flower Abscission in Oilseed Rape and Arabidopsis. Plant Physiol. 2002, 128, 534–543. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A. Nitrogen availability regulates proline and ethylene production and alleviates salinity stress in mustard (Brassica juncea). J. Plant Physiol. 2015, 178, 84–91. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Dong, Q.; Wang, X.; Gui, H.; Zhang, H.; Zhang, X.; Song, M. High Nitrogen Enhance Drought Tolerance in Cotton through Antioxidant Enzymatic Activities, Nitrogen Metabolism and Osmotic Adjustment. Plants 2020, 9, 178. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).