Internal Fruit Quality Is Maintained in Eggplant under Mild Long-Term Salt Treatment
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Seed Germination
2.2. Plant Growth and Salt Treatment
2.3. Substrate Electrical Conductivity
2.4. Fruit Weight
2.5. Mineral Content Quantification
2.6. Phenolic Compounds Quantification in Fruit
2.7. Chlorogenic Acid and Sugar Contents in Fruit
2.8. Data Analysis
3. Results
3.1. Substrate Electrical Conductivity
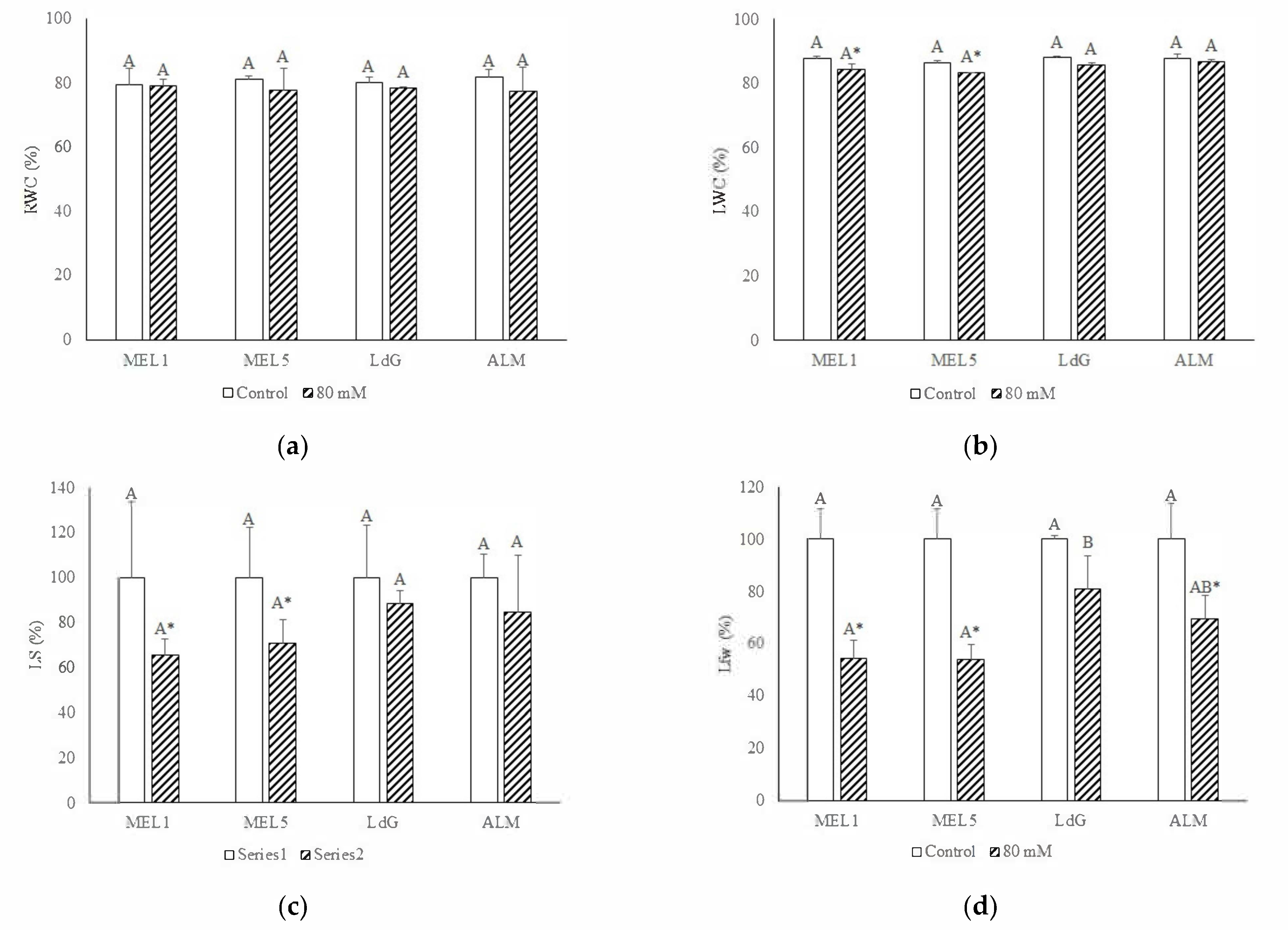
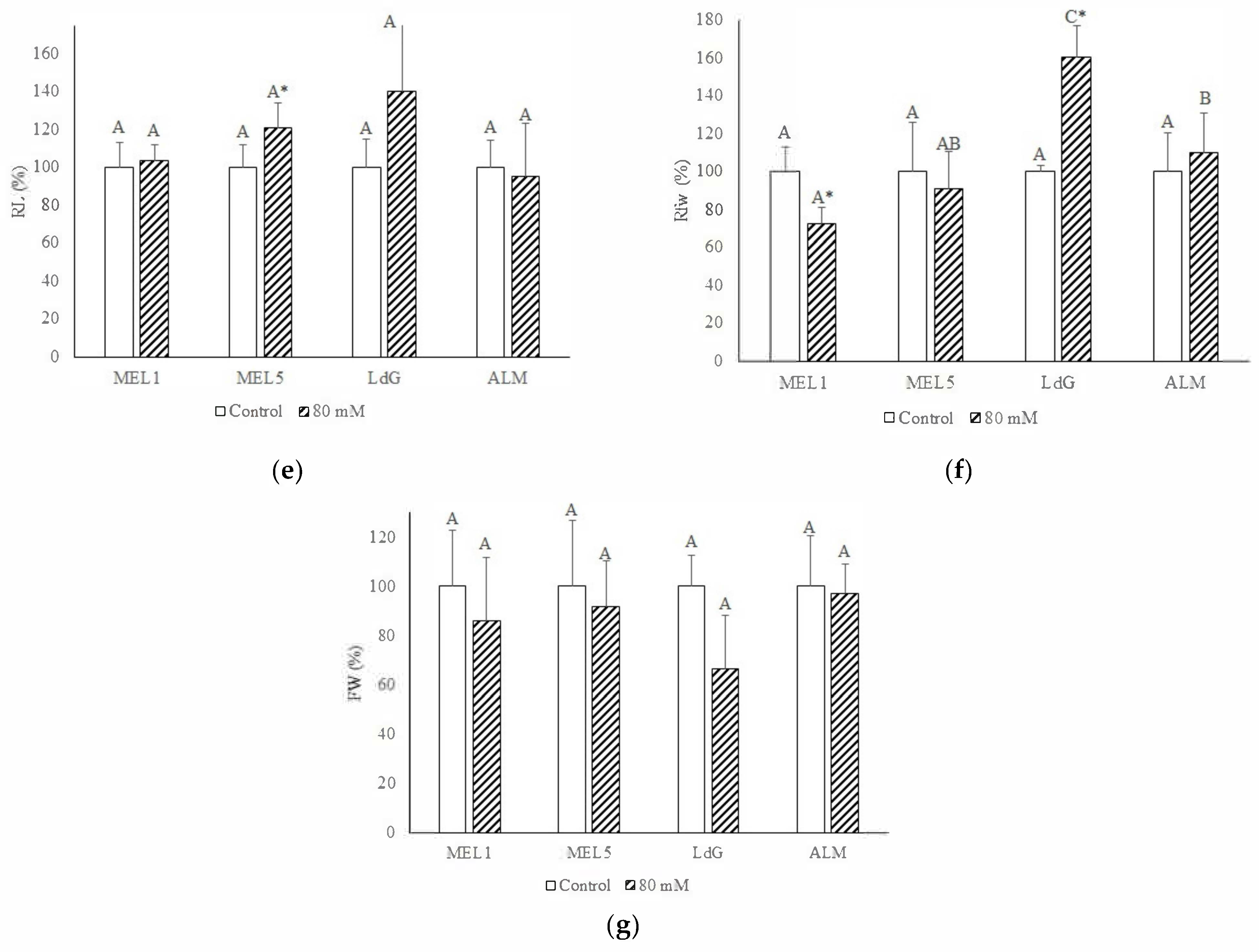
3.2. Plant Growth Parameters
3.3. Mineral Composition in Leaves, Roots and Fruits
3.4. Fruit Antioxidant Accumulation
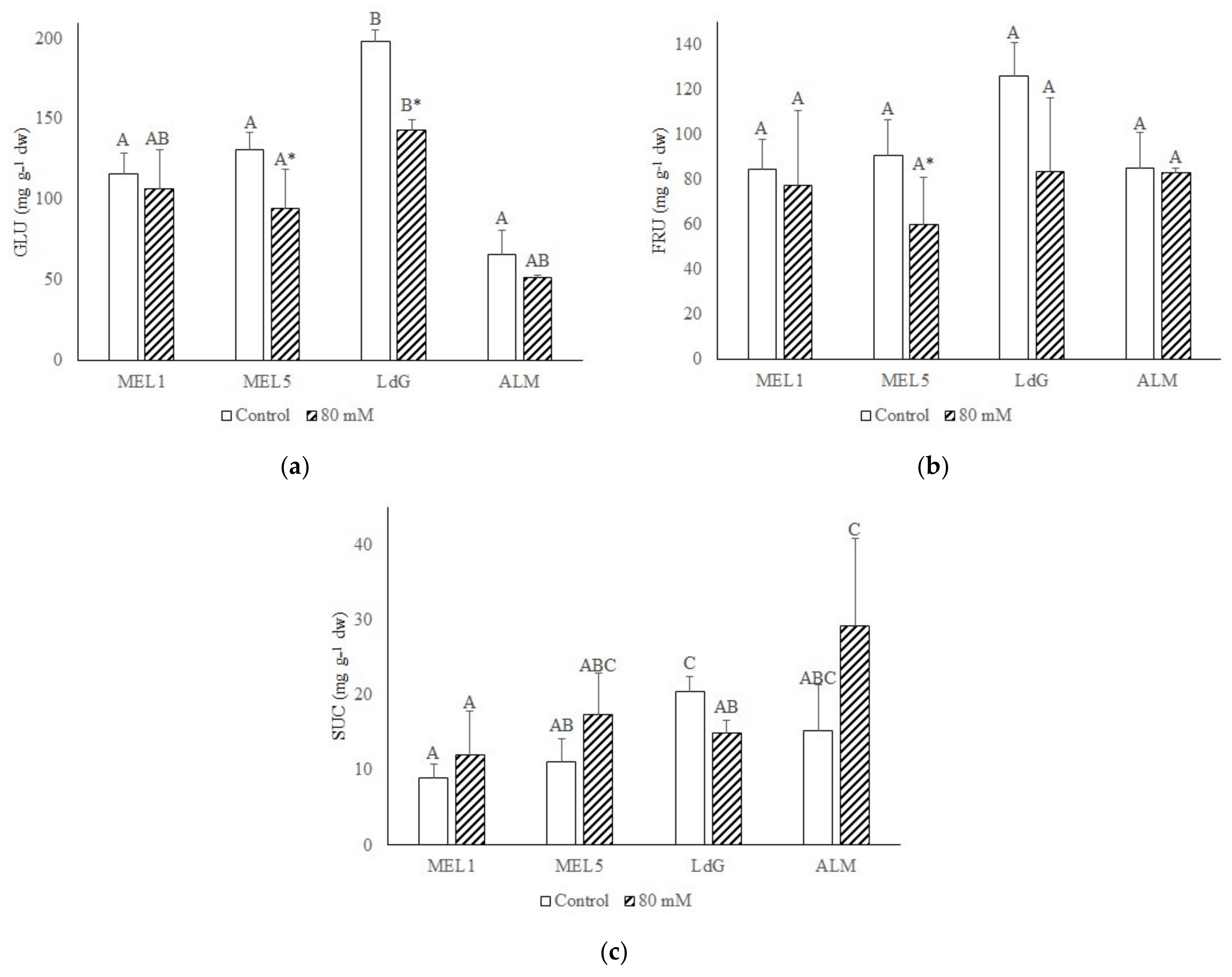
3.5. Sugar Quantification
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. Food and Agriculture Organization Corporate Statistical Database. Available online: https://www.fao.org/statistics/es/ (accessed on 10 October 2023).
- Docimo, T.; Francese, G.; Ruggiero, A.; Batelli, G.; De Palma, M.; Bassolino, L.; Toppino, L.; Rotino, G.L.; Mennella, G.; Tucci, M. Phenylpropanoids Accumulation in Eggplant Fruit: Characterization of Biosynthetic Genes and Regulation by a MYB Transcription Factor. Front. Plant Sci. 2016, 6, 1233. [Google Scholar] [CrossRef]
- Raigón, M.D.; Rodríguez-Burruezo, A.; Prohens, J. Effects of Organic and Conventional Cultivation Methods on Composition of Eggplant Fruits. J. Agric. Food Chem. 2010, 58, 6833–6840. [Google Scholar] [CrossRef] [PubMed]
- Prohens, J.; Rodríguez-Burruezo, A.; Raigón, M.D.; Nuez, F. Total Phenolic Concentration and Browning Susceptibility in a Collection of Different Varietal Types and Hybrids of Eggplant: Implications for Breeding for Higher Nutritional Quality and Reduced Browning. J. Am. Soc. Hortic. Sci. 2007, 132, 638–646. [Google Scholar] [CrossRef]
- Mennella, G.; Lo Scalzo, R.; Fibiani, M.; DAlessandro, A.; Francese, G.; Toppino, L.; Acciarri, N.; De Almeida, A.E.; Rotino, G.L. Chemical and Bioactive Quality Traits during Fruit Ripening in Eggplant (S. melongena L.) and Allied Species. J. Agric. Food Chem. 2012, 60, 11821–11831. [Google Scholar] [CrossRef] [PubMed]
- Plazas, M.; Andújar, I.; Vilanova, S.; Hurtado, M.; Gramazio, P.; Herraiz, F.J.; Prohens, J. Breeding for Chlorogenic Acid Content in Eggplant: Interest and Prospects. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 26–35. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, H.; Song, C.; Zhu, J.K.; Shabala, S. Mechanisms of Plant Responses and Adaptation to Soil Salinity. Innovation 2020, 1, 100017. [Google Scholar] [CrossRef] [PubMed]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Blumwald, E. Sodium Transport and Salt Tolerance in Plants. Curr. Opin. Cell Biol. 2000, 12, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Murata, N. Revised Scheme for the Mechanism of Photoinhibition and Its Application to Enhance the Abiotic Stress Tolerance of the Photosynthetic Machinery. Appl. Microbiol. Biotechnol. 2014, 98, 8777–8796. [Google Scholar] [CrossRef]
- Mady, E.; Abd El-Wahed, A.H.M.; Awad, A.H.; Asar, T.O.; Al-Farga, A.; Abd El-Raouf, H.S.; Randhir, R.; Alnuzaili, E.S.; El-Taher, A.M.; Randhir, T.O.; et al. Evaluation of Salicylic Acid Effects on Growth, Biochemical, Yield, and Anatomical Characteristics of Eggplant (Solanum melongena L.) Plants under Salt Stress Conditions. Agronomy 2023, 13, 2213. [Google Scholar] [CrossRef]
- Agius, C.; von Tucher, S.; Rozhon, W. The Effect of Salinity on Fruit Quality and Yield of Cherry Tomatoes. Horticulturae 2022, 8, 59. [Google Scholar] [CrossRef]
- Meza, S.L.R.; Egea, I.; Massaretto, I.L.; Morales, B.; Purgatto, E.; Egea-Fernández, J.M.; Bolarin, M.C.; Flores, F.B. Traditional Tomato Varieties Improve Fruit Quality without Affecting Fruit Yield under Moderate Salt Stress. Front. Plant Sci. 2020, 11, 587754. [Google Scholar] [CrossRef] [PubMed]
- Galli, V.; da Silva Messias, R.; Perin, E.C.; Borowski, J.M.; Bamberg, A.L.; Rombaldi, C.V. Mild Salt Stress Improves Strawberry Fruit Quality. LWT-Food Sci. Technol. 2016, 73, 693–699. [Google Scholar] [CrossRef]
- Massaretto, I.L.; Albaladejo, I.; Purgatto, E.; Flores, F.B.; Plasencia, F.; Egea-Fernández, J.M.; Bolarin, M.C.; Egea, I. Recovering Tomato Landraces to Simultaneously Improve Fruit Yield and Nutritional Quality against Salt Stress. Front. Plant Sci. 2018, 9, 1778. [Google Scholar] [CrossRef]
- Ünlükara, A.; Kurunç, A.; Kesmez, G.D.; Yurtseven, E.; Suarez, D.L. Effects of Salinity on Eggplant (Solanum melongena L.) Growth and Evapotranspiration. Irrig. Drain. 2010, 59, 203–214. [Google Scholar] [CrossRef]
- Ortega-Albero, N.; González-Orenga, S.; Vicente, O.; Rodríguez-Burruezo, A.; Fita, A. Responses to Salt Stress of the Interspecific Hybrid Solanum insanum × Solanum melongena and Its Parental Species. Plants 2023, 12, 295. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; Pérez, J.; González-Orenga, S.; Solana, A.; Boscaiu, M.; Prohens, J.; Plazas, M.; Fita, A.; Vicente, O. Comparative Studies on the Physiological and Biochemical Responses to Salt Stress of Eggplant (Solanum melongena) and Its Rootstock S. torvum. Agriculture 2020, 10, 328. [Google Scholar] [CrossRef]
- Sanwal, S.K.; Mann, A.; Kumar, A.; Kesh, H.; Kaur, G.; Rai, A.K.; Kumar, R.; Sharma, P.C.; Kumar, A.; Bahadur, A.; et al. Salt Tolerant Eggplant Rootstocks Modulate Sodium Partitioning in Tomato Scion and Improve Performance under Saline Conditions. Agriculture 2022, 12, 183. [Google Scholar] [CrossRef]
- Plazas, M.; Vilanova, S.; Gramazio, P.; Rodríguez-Burruezo, A.; Fita, A.; Herraiz, F.J.; Ranil, R.; Fonseka, R.; Niran, L.; Fonseka, H.; et al. Interspecific Hybridization between Eggplant and Wild Relatives from Different Genepools. J. Am. Soc. Hortic. Sci. 2016, 141, 34–44. [Google Scholar] [CrossRef]
- Gramazio, P.; Prohens, J.; Plazas, M.; Mangino, G.; Herraiz, F.J.; Vilanova, S. Development and Genetic Characterization of Advanced Backcross Materials and an Introgression Line Population of Solanum incanum in a S. melongena Background. Front. Plant Sci. 2017, 8, 1477. [Google Scholar] [CrossRef]
- Ranil, R.H.G.; Niran, H.M.L.; Plazas, M.; Fonseka, R.M.; Fonseka, H.H.; Vilanova, S.; Andújar, I.; Gramazio, P.; Fita, A.; Prohens, J. Improving Seed Germination of the Eggplant Rootstock Solanum torvum by Testing Multiple Factors Using an Orthogonal Array Design. Sci. Hortic. 2015, 193, 174–181. [Google Scholar] [CrossRef]
- De Paz, J.M.; Visconti, F.; Zapata, R.; Sánchez, J. Integration of Two Simple Models in a Geographical Information System to Evaluate Salinization Risk in Irrigated Land of the Valencian Community, Spain. Soil Use Manag. 2004, 20, 333–342. [Google Scholar] [CrossRef]
- De Paz, J.M.; Visconti, F.; Rubio, J.L. Spatial Evaluation of Soil Salinity Using the WET Sensor in the Irrigated Area of the Segura River Lowland. J. Plant Nutr. Soil Sci. 2011, 174, 103–112. [Google Scholar] [CrossRef]
- Kumari, R.; Akhtar, S.; Kumar, R.; Verma, R.B.; Kumari, R. Influence of Seasonal Variation on Phenolic Composition and Antioxidant Capacity of Eggplant (Solanum melongena L.) Hybrids. Sci. Hortic. 2022, 295, 110865. [Google Scholar] [CrossRef]
- Guijarro-Real, C.; Adalid-Martínez, A.M.; Pires, C.K.; Ribes-Moya, A.M.; Fita, A.; Rodríguez-Burruezo, A. The Effect of the Varietal Type, Ripening Stage, and Growing Conditions on the Content and Profile of Sugars and Capsaicinoids in Capsicum Peppers. Plants 2023, 12, 231. [Google Scholar] [CrossRef]
- Plazas, M.; Prohens, J.; Cuñat, A.N.; Vilanova, S.; Gramazio, P.; Herraiz, F.J.; Andújar, I. Reducing Capacity, Chlorogenic Acid Content and Biological Activity in a Collection of Scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) Eggplants. Int. J. Mol. Sci. 2014, 15, 17221–17241. [Google Scholar] [CrossRef]
- Brenes, M.; Solana, A.; Boscaiu, M.; Fita, A.; Vicente, O.; Calatayud, Á.; Prohens, J.; Plazas, M. Physiological and Biochemical Responses to Salt Stress in Cultivated Eggplant (Solanum melongena L.) and in S. insanum L., a Close Wild Relative. Agronomy 2020, 10, 651. [Google Scholar] [CrossRef]
- Plazas, M.; González-Orenga, S.; Nguyen, H.T.; Morar, I.M.; Fita, A.; Boscaiu, M.; Prohens, J.; Vicente, O. Growth and Antioxidant Responses Triggered by Water Stress in Wild Relatives of Eggplant. Sci. Hortic. 2022, 293, 110685. [Google Scholar] [CrossRef]
- Jaramillo Roman, V.; van de Zedde, R.; Peller, J.; Visser, R.G.F.; van der Linden, C.G.; van Loo, E.N. High-Resolution Analysis of Growth and Transpiration of Quinoa Under Saline Conditions. Front. Plant Sci. 2021, 12, 634311. [Google Scholar] [CrossRef]
- Derbali, W.; Goussi, R.; Koyro, H.W.; Abdelly, C.; Manaa, A. Physiological and Biochemical Markers for Screening Salt Tolerant Quinoa Genotypes at Early Seedling Stage. J. Plant Interact. 2020, 15, 27–38. [Google Scholar] [CrossRef]
- Quinet, M.; Angosto, T.; Yuste-Lisbona, F.J.; Blanchard-Gros, R.; Bigot, S.; Martinez, J.P.; Lutts, S. Tomato Fruit Development and Metabolism. Front. Plant Sci. 2019, 10, 1554. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Mu, X.; Shao, H.; Wang, H.; Brestic, M. Global Plant-Responding Mechanisms to Salt Stress: Physiological and Molecular Levels and Implications in Biotechnology. Crit. Rev. Biotechnol. 2015, 35, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Singh, A. Soil Salinity: A Global Threat to Sustainable Development. Soil Use Manag. 2022, 38, 39–67. [Google Scholar] [CrossRef]
- Nilsson, L.; Müller, R.; Nielsen, T.H. Dissecting the Plant Transcriptome and the Regulatory Responses to Phosphate Deprivation. Physiol. Plant. 2010, 139, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Amtmann, A.; Armengaud, P. Effects of N, P, K and S on Metabolism: New Knowledge Gained from Multi-Level Analysis. Curr. Opin. Plant Biol. 2009, 12, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.A.; Borges, B.M.M.N.; Almeida, H.J.; Prado, R.D.M. Growth and Nutritional Disorders of Eggplant Cultivated in Nutrients Solutions with Suppressed Macronutrients. J. Plant Nutr. 2015, 38, 1097–1109. [Google Scholar] [CrossRef]
- Blom-Zandstra, M.; Vogelzang, S.A.; Veen, B.W. Sodium Fluxes in Sweet Pepper Exposed to Varying Sodium Concentrations. J. Exp. Bot. 1998, 49, 1863–1868. [Google Scholar] [CrossRef]
- Baghour, M.; Gálvez, F.J.; Sánchez, M.E.; Aranda, M.N.; Venema, K.; Rodríguez-Rosales, M.P. Overexpression of LeNHX2 and SlSOS2 Increases Salt Tolerance and Fruit Production in Double Transgenic Tomato Plants. Plant Physiol. Biochem. 2019, 135, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Guo, S.; Zhang, C.; Zhang, B.; Ma, R.; Korir, N.K.; Yu, M. KT/HAK/KUP Potassium Transporter Genes Differentially Expressed during Fruit Development, Ripening, and Postharvest Shelf-Life of ‘Xiahui6’ Peaches. Acta Physiol. Plant. 2015, 37, 131. [Google Scholar] [CrossRef]
- Martínez-Ispizua, E.; Calatayud, Á.; Marsal, J.I.; Mateos-Fernández, R.; Díez, M.J.; Soler, S.; Valcárcel, J.V.; Martínez-Cuenca, M.R. Phenotyping Local Eggplant Varieties: Commitment to Biodiversity and Nutritional Quality Preservation. Front. Plant Sci. 2021, 12, 696272. [Google Scholar] [CrossRef]
- Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value. Agronomy 2020, 10, 500. [Google Scholar] [CrossRef]
- Ozgur, R.; Uzilday, B.; Sekmen, A.H.; Turkan, I. Reactive Oxygen Species Regulation and Antioxidant Defence in Halophytes. Funct. Plant Biol. 2013, 40, 832–847. [Google Scholar] [CrossRef] [PubMed]
- Stommel, J.R.; Whitaker, B.D.; Haynes, K.G.; Prohens, J. Genotype × Environment Interactions in Eggplant for Fruit Phenolic Acid Content. Euphytica 2015, 205, 823–836. [Google Scholar] [CrossRef]
- Raigón, M.D.; Prohens, J.; Muñoz-Falcón, J.E.; Nuez, F. Comparison of Eggplant Landraces and Commercial Varieties for Fruit Content of Phenolics, Minerals, Dry Matter and Protein. J. Food Compos. Anal. 2008, 21, 370–376. [Google Scholar] [CrossRef]
- San José, R.; Sánchez, M.C.; Cámara, M.M.; Prohens, J. Composition of Eggplant Cultivars of the Occidental Type and Implications for the Improvement of Nutritional and Functional Quality. Int. J. Food Sci. Technol. 2013, 48, 2490–2499. [Google Scholar] [CrossRef]
- Boo, H.; Kim, H.; Lee, H. Changes in Sugar Content and Sucrose Synthase Enzymes during Fruit Growth in Eggplant (Solanum melongena L.) Grown on Different Polyethylene Mulches. HortScience 2010, 45, 775–777. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, X.; Gong, B.; Yan, Y.; Shi, Q. Proteomics and Metabolomics Analysis of Tomato Fruit at Different Maturity Stages and under Salt Treatment. Food Chem. 2020, 311, 126009. [Google Scholar] [CrossRef] [PubMed]
- Mascellani, A.; Natali, L.; Cavallini, A.; Mascagni, F.; Caruso, G.; Gucci, R.; Havlik, J.; Bernardi, R. Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. Cv. Dottato). Plants 2021, 10, 1861. [Google Scholar] [CrossRef] [PubMed]
- Suarez, D.L.; Celis, N.; Ferreira, J.F.S.; Reynolds, T.; Sandhu, D. Linking Genetic Determinants with Salinity Tolerance and Ion Relationships in Eggplant, Tomato and Pepper. Sci. Rep. 2021, 11, 16298. [Google Scholar] [CrossRef] [PubMed]
- Darko, R.O.; Yuan, S.; Sekyere, J.D.O.; Liu, J. Effect of Deficit Irrigation on Yield and Quality of Eggplant. Int. J. Environ. Agric. Biotechnol. 2019, 4, 1325–1333. [Google Scholar] [CrossRef]
| Genotype | Control | Salt Treatment | |
|---|---|---|---|
| EC1:5 | MEL1 | 1.86 ± 1.1 A | 4.36 ± 0.4 A,* |
| MEL5 | 1.2 ± 0.1 AB | 5.61 ± 0.8 A,* | |
| LdG | 1.78 ± 0.2 AB | 5.3 ± 0.3 A,* | |
| ALM | 2.32 ± 0.2 B | 4.02 ± 2.1 A |
| NaCl Treatment (mM) | Fold-Change Salt vs. Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Root | Leaf | Fruit | Root | Leaf | Fruit | |||||
| 0 | 80 | 0 | 80 | 0 | 80 | |||||
| Na | MEL1 | 4.22 ± 1.6 A | 10.29 ± 0.7 A | 0.17 ± 0.1 AB | 2.88 ± 0.7 B | 0.34 ± 0.2 A | 4.45 ± 0.6 A | 2.44 * | 17.44 * | 13.19 * |
| MEL5 | 3.25 ± 0.8 A | 12.65 ± 1.4 A | 0.13 ± 0.1 AB | 1.09 ± 0.4 A | 0.45 ± 0.2 A | 2.36 ± 1.1 A | 3.89 * | 8.66 * | 5.29 * | |
| LdG | 4.12 ± 1.7 A | 13.47 ± 0.5 A | 0.09 ± 0 A | 1.52 ± 0.1 AB | 0.76 ± 0.4 A | 4.02 ± 0.3 A | 3.27 * | 17.64 * | 5.31 * | |
| ALM | 5.7 ± 1.2 A | 12.25 ± 2.2 A | 0.22 ± 0.1 AB | 0.9 ± 0.6 A | 0.38 ± 0.2 A | 2.54 ± 0.9 A | 2.15 * | 4.13 * | 6.69 * | |
| K | MEL1 | 7.79 ± 0.8 A | 7.07 ± 2.1 A | 11.12 ± 0.6 A | 10.26 ± 0.5 AB | 9.66 ± 0.3 A | 9.01 ± 0.4 A | 0.91 | 0.92 * | 0.93 * |
| MEL5 | 7.09 ± 1.2 A | 6.37 ± 0.7 A | 10.54 ± 0.2 A | 9.82 ± 0.4 A | 9.52 ± 0.4 A | 9.71 ± 0.5 A | 0.90 | 0.93 * | 1.02 | |
| LdG | 7.13 ± 0.5 A | 5.24 ± 1.2 A | 10.46 ± 0.3 A | 10.75 ± 0.7 B | 9.82 ± 0.1 A | 9.13 ± 0.5 A | 0.74 | 1.03 | 0.93 | |
| ALM | 7.69 ± 0.7 A | 6.75 ± 1.9 A | 10.37 ± 0.3 A | 10.49 ± 0.5 AB | 9.41 ± 0.2 A | 8.75 ± 0.4 A | 0.88 | 1.01 | 0.93 * | |
| Ca | MEL1 | 11.52 ± 1.1 A | 8.8 ± 1.5 A | 20.55 ± 2.2 AB | 18.78 ± 2.6 AB | 3.25 ± 0.9 B | 2.96 ± 0.8 A | 0.76 * | 0.91 | 0.91 |
| MEL5 | 13.27 ± 1.2 A | 13.17 ± 2.9 B | 21.58 ± 2.8 AB | 21.7 ± 2.7 BC | 2.63 ± 0.2 AB | 2.4 ± 0.3 A | 0 99 | 1.01 | 0.91 | |
| LdG | 13.3 ± 1.4 A | 12.68 ± 3.6 B | 25.76 ± 3.9 BC | 22.86 ± 2.8 BC | 2.85 ± 0.6 AB | 3.23 ± 0.4 A | 0.95 | 0.89 | 1.13 | |
| ALM | 12.44 ± 0.9 A | 14.05 ± 1.6 B | 28.33 ± 2.5 C | 25.25 ± 1.5 C | 2.2 ± 0.4 AB | 2.09 ± 0.2 A | 1.13 | 0.89 | 0.95 | |
| P | MEL1 | 3.91 ± 1 B | 2.42 ± 0.3 A | 7.6 ± 0.3 B | 5.87 ± 1.5 A | 4.11 ± 0.4 A | 4.49 ± 0.3 A | 0.62 * | 0.77 * | 1.09 |
| MEL5 | 2.51 ± 0.4 A | 2.39 ± 0.4 A | 5.8 ± 0.2 A | 5.72 ± 0.2 A | 4.78 ± 0.3 AB | 4.88 ± 0.4 AB | 0.95 | 0.99 | 1.02 | |
| LdG | 2.8 ± 0.1 A | 2.51 ± 0.2 A | 6.76 ± 0 B | 7.39 ± 0.8 A | 4.84 ± 0.2 AB | 5.12 ± 0.6 AB | 0.89 | 1.09 | 1.06 | |
| ALM | 2.63 ± 0.4 A | 2.56 ± 0.5 A | 6.73 ± 0.5 B | 6.69 ± 0.8 A | 5.36 ± 0.4 B | 5.5 ± 0.2 B | 0.97 | 0.99 | 1.03 | |
| Mg | MEL1 | 4.25 ± 0.3 AB | 3.41 ± 0.5 A | 5.54 ± 0.3 A | 5.17 ± 0.4 A | 2.94 ± 0.2 A | 2.79 ± 0.3 AB | 0.80 * | 0.93 | 0.95 |
| MEL5 | 4.09 ± 0.4 AB | 3.54 ± 0.4 A | 6.22 ± 0.5 A | 6.43 ± 0.4 BC | 2.75 ± 0.1 A | 2.94 ± 0.2 AB | 0.87 * | 1.03 | 1.07 | |
| LdG | 4.63 ± 0.2 B | 3.93 ± 0.4 A | 6.21 ± 0.2 A | 5.76 ± 0.4 AB | 3.11 ± 0.2 A | 3.22 ± 0.2 B | 0.85 | 0.93 | 1.03 | |
| ALM | 4.11 ± 0.3 AB | 3.79 ± 0.7 A | 7.5 ± 0.4 B | 7.01 ± 0.4 C | 2.72 ± 0.2 A | 2.57 ± 0.1 AB | 0.92 | 0.93 | 0.95 | |
| Fe | MEL1 | 0.71 ± 0.1 A | 0.67 ± 0.1 A | 0.09 ± 0 A | 0.08 ± 0 A | 0.03 ± 0 B | 0.04 ± 0 A | 0.94 * | 0.85 | 1.23 |
| MEL5 | 0.98 ± 0.3 A | 0.91 ± 0.2 A | 0.09 ± 0 A | 0.08 ± 0 A | 0.03 ± 0 AB | 0.02 ± 0 A | 0.93 | 0.82 * | 0.96 | |
| LdG | 0.73 ± 0.1 A | 0.74 ± 0.4 A | 0.09 ± 0 A | 0.09 ± 0 B | 0.03 ± 0 AB | 0.03 ± 0 A | 1.01 | 0.94 | 1.12 | |
| ALM | 0.53 ± 0.3 A | 0.72 ± 0.3 A | 0.08 ± 0 A | 0.1 ± 0 C | 0.02 ± 0 A | 0.02 ± 0 A | 1.35 | 1.39 * | 1.00 | |
| B | MEL1 | 0.01 ± 0 A | 0.012 ± 0 A | 0.05 ± 0 AB | 0.05 ± 0 B | 0.02 ± 0 B | 0.02 ± 0 A | 0.79 * | 1.00 | 0.76 |
| MEL5 | 0.011 ± 0 A | 0.011 ± 0 A | 0.06 ± 0 B | 0.06 ± 0 C | 0.02 ± 0 AB | 0.02 ± 0 A | 0.92 | 1.03 | 1.06 | |
| LdG | 0.011 ± 0 A | 0.01 ± 0 A | 0.05 ± 0 AB | 0.04 ± 0 A | 0.02 ± 0 AB | 0.02 ± 0 A | 0.91 | 0.73 * | 1.00 | |
| ALM | 0.014 ± 0 B | 0.011 ± 0 A | 0.06 ± 0 B | 0.05 ± 0 B | 0.02 ± 0 AB | 0.01 ± 0 A | 1.20 * | 0.84 * | 0.88 | |
| Mn | MEL1 | 0.06 ± 0 AB | 0.05 ± 0 A | 0.07 ± 0 A | 0.06 ± 0 A | 0.02 ± 0 B | 0.02 ± 0 A | 0.88 | 0.84 | 0.91 |
| MEL5 | 0.07 ± 0 B | 0.07 ± 0 A | 0.11 ± 0 B | 0.07 ± 0 A | 0.02 ± 0 AB | 0.02 ± 0 A | 1.01 | 0.65 * | 0.89 * | |
| LdG | 0.05 ± 0 AB | 0.05 ± 0 A | 0.12 ± 0 BC | 0.08 ± 0 A | 0.02 ± 0 B | 0.02 ± 0 A | 1.11 | 0.64 * | 0.96 | |
| ALM | 0.03 ± 0 A | 0.05 ± 0 A | 0.14 ± 0 C | 0.11 ± 0 B | 0.02 ± 0 AB | 0.02 ± 0 A | 1.50 * | 0.77 * | 0.95 | |
| Zn | MEL1 | 0.02 ± 0 A | 0.01 ± 0 A | 0.09 ± 0.1 A | 0.03 ± 0 A | 0.04 ± 0 A | 0.05 ± 0 B | 0.64 * | 0.35 * | 1.18 |
| MEL5 | 0.02 ± 0 A | 0.01 ± 0 A | 0.08 ± 0 A | 0.11 ± 0.1 A | 0.04 ± 0 A | 0.03 ± 0 AB | 0.82 | 1.36 | 0.76 | |
| LdG | 0.01 ± 0 A | 0.02 ± 0 A | 0.12 ± 0.1 A | 0.08 ± 0 A | 0.03 ± 0 A | 0.03 ± 0 A | 1.42 | 0.64 | 0.76 | |
| ALM | 0.02 ± 0 A | 0.04 ± 0 A | 0.05 ± 0 A | 0.11 ± 0 A | 0.03 ± 0 A | 0.03 ± 0 AB | 1.86 | 2.15* | 1.28 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Albero, N.; Adalid-Martínez, A.M.; Castell-Zeising, V.; Raigón, M.D.; Rodríguez-Burruezo, A.; Fita, A. Internal Fruit Quality Is Maintained in Eggplant under Mild Long-Term Salt Treatment. Agriculture 2024, 14, 871. https://doi.org/10.3390/agriculture14060871
Ortega-Albero N, Adalid-Martínez AM, Castell-Zeising V, Raigón MD, Rodríguez-Burruezo A, Fita A. Internal Fruit Quality Is Maintained in Eggplant under Mild Long-Term Salt Treatment. Agriculture. 2024; 14(6):871. https://doi.org/10.3390/agriculture14060871
Chicago/Turabian StyleOrtega-Albero, Neus, Ana María Adalid-Martínez, Vicente Castell-Zeising, María Dolores Raigón, Adrián Rodríguez-Burruezo, and Ana Fita. 2024. "Internal Fruit Quality Is Maintained in Eggplant under Mild Long-Term Salt Treatment" Agriculture 14, no. 6: 871. https://doi.org/10.3390/agriculture14060871
APA StyleOrtega-Albero, N., Adalid-Martínez, A. M., Castell-Zeising, V., Raigón, M. D., Rodríguez-Burruezo, A., & Fita, A. (2024). Internal Fruit Quality Is Maintained in Eggplant under Mild Long-Term Salt Treatment. Agriculture, 14(6), 871. https://doi.org/10.3390/agriculture14060871








