Compost-Derived Bacterial Communities Offer Promise as Biocontrol Agents against Meloidogyne javanica and Promote Plant Growth in Tomato
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of M. javanica Inoculum
2.2. Preparation of Bacterial SCs
2.3. Pot Experiment
2.4. Experimental Design
- (a)
- SC1: Plants treated with SC1
- (b)
- SC2: Plants treated with SC2
- (c)
- Pochar: Plants treated with Pochar
- (d)
- H2O: Plants treated with water
- (e)
- SC1+nem: Plants treated with SC1 and then inoculated with nematodes
- (f)
- SC2+nem: Plants treated with SC2 and then inoculated with nematodes
- (g)
- Pochar+nem: Plants treated with Pochar and then inoculated with nematodes
- (h)
- H2O+nem: Plants treated with water and then inoculated with nematodes
2.5. Plant and Nematode Variables Evaluated
- Assessment of the total number of egg masses and eggs/egg mass: The total number of egg masses per plant was counted under a stereomicroscope. Subsequently, for each plant, ten egg masses were randomly selected, placed in 1% sodium hypochlorite, and shaken for 10 min to disperse the eggs, and then, the number of eggs per egg mass was estimated under the stereomicroscope, using a nematode counting dish [21,24].
- Assessment of the nematode population in the soil: The entire volume of soil extracted from each pot was processed following the conventional Baermann funnel technique [25] to estimate the RKN soil population.
- Assessment of the total number of progeny: For each plant, the number of eggs per egg mass was multiplied by the total number of egg masses and added to the RKN soil population.
- Assessment of the fresh and dry weights of the aerial parts: The aerial parts were separated from the roots, their fresh weight was weighed on a 2-digit scale, and then, they were placed in a paper bag and left in an oven at 50 °C until completely dry, and subsequently, their dry weight was recorded.
- Assessment of plant height: Plant height, i.e., the height from the plant collar up to its top, was measured with a measuring tape in cm.
- Assessment of the fresh weight of roots: The roots of each plant were separately washed thoroughly but carefully, under running tap water, excess water was removed with a paper towel, and their fresh weight was weighed on a 2-digit scale.
2.6. Statistical Analysis
3. Results
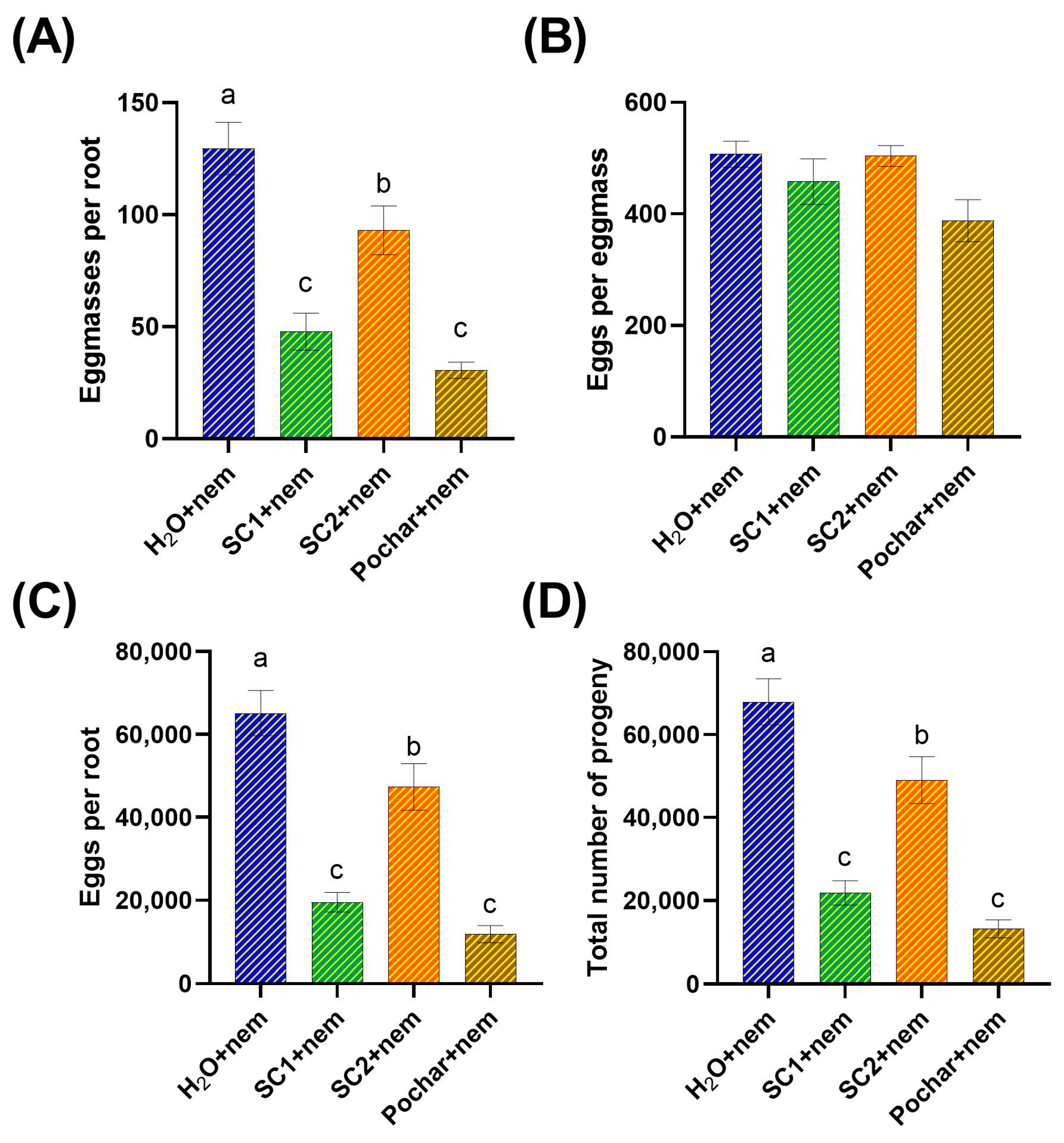
3.1. Nematode Population
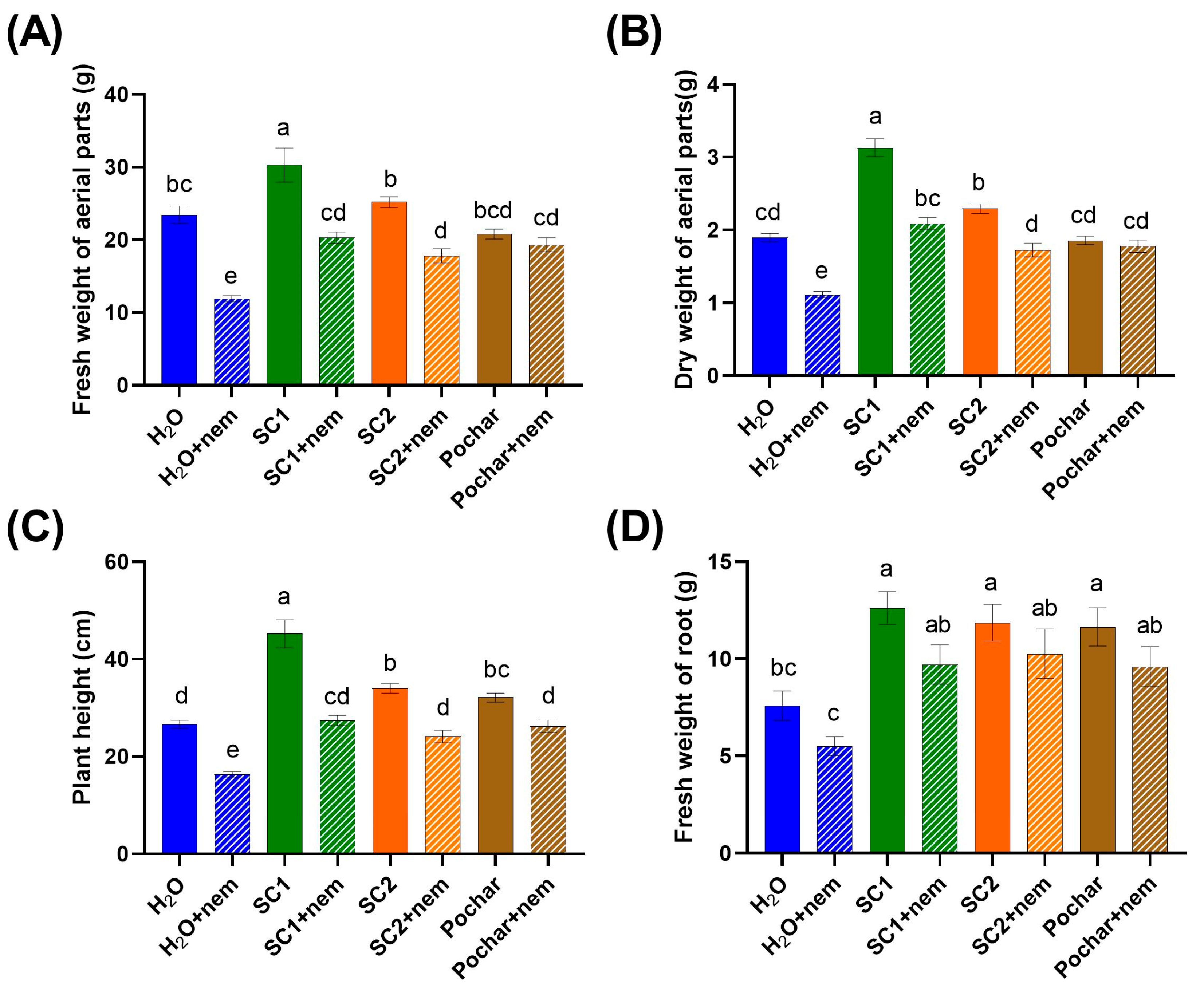
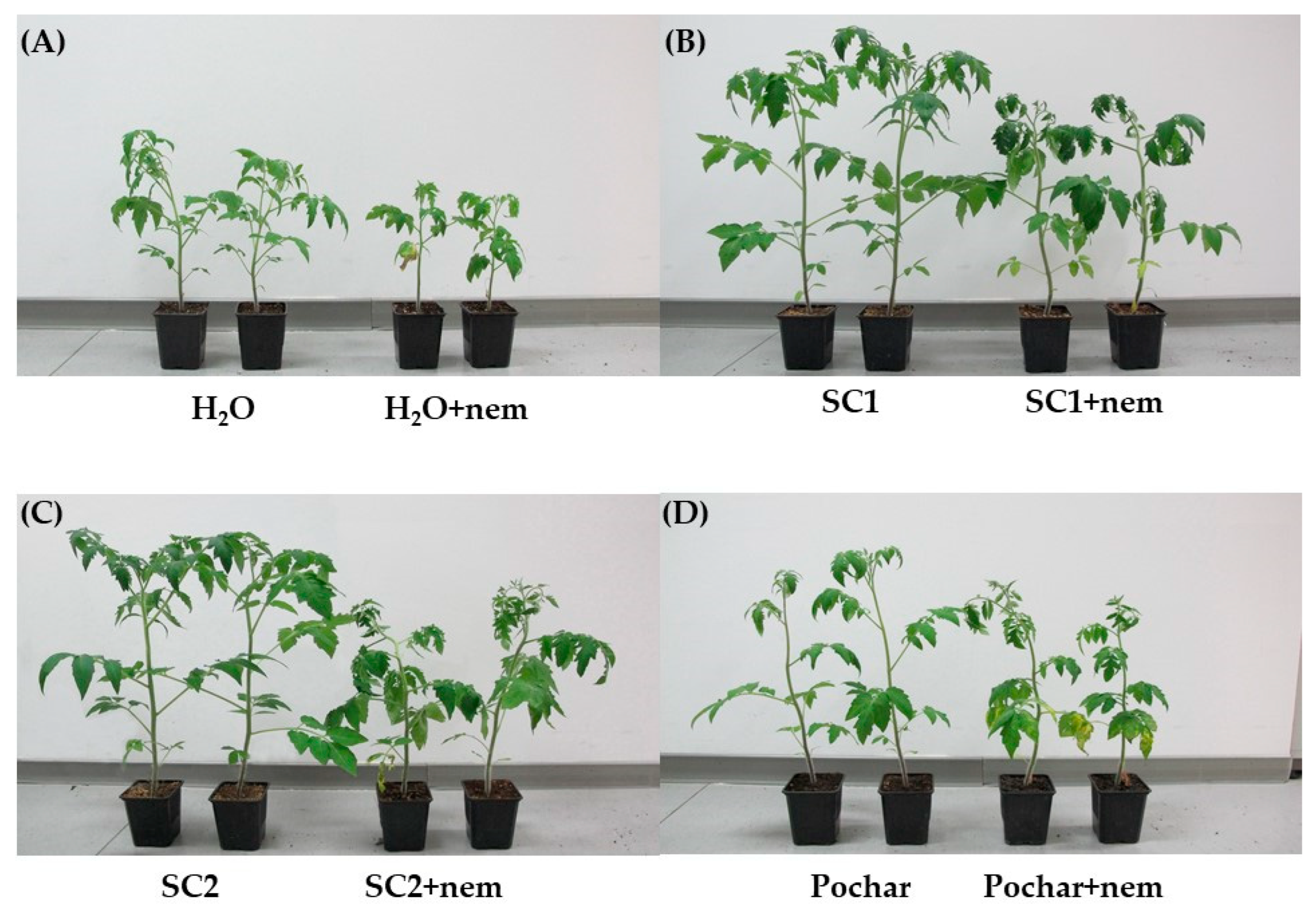
3.2. Morphological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talavera-Rubia, M.; Vela-Delgado, M.D.; Verdejo-Lucas, S. A Cost-Benefit Analysis of Soil Disinfestation Methods against Root-Knot Nematodes in Mediterranean Intensive Horticulture. Plants 2022, 11, 2774. [Google Scholar] [CrossRef]
- Gharabadiyan, F.; Jamali, S.; Komeili, R.H. Determining of root-knot nematode (Meloidogyne javanica) damage function for tomato cultivars. J. Agric. Sci. 2013, 58, 147–157. [Google Scholar] [CrossRef]
- Tzortzakakis, E.A.; da Conceicao, I.L.P.M.; dos Santos, M.C.V.; Abrantes, I.M.D.O. Root-knot nematodes (Meloidogyne spp.) in Greece. Hell. Plant Prot. J. 2011, 4, 25–30. [Google Scholar]
- Nyczepir, A.P.; Thomas, S.H. Current and future management strategies in intensive crop production systems. In Root-Knot Nematodes; CABI: Wallingford, UK, 2009; pp. 412–443. [Google Scholar]
- Riyaz, M.; Mathew, P.; Zuber, S.M.; Rather, G.A. Botanical pesticides for an eco-friendly and sustainable agriculture: New challenges and prospects. In Sustainable Agriculture; Springer: Cham, Switzerland, 2022; pp. 69–96. [Google Scholar]
- Ali, W.M.; Abdel-Mageed, M.A.; Hegazy, M.G.A.; Abou-Shlell, M.K.; Sultan, S.M.E.; Salama, E.A.A.; Yousef, A.F. Biocontrol agent of root-knot nematode Meloidogyne javanica and root-rot fungi, Fusarium solani in okra morphological, anatomical characteristics and productivity under greenhouse conditions. Sci. Rep. 2023, 13, 11103. [Google Scholar] [CrossRef]
- Khalil, M.S.E.D.H.; Allam, A.F.G.; Barakat, A.S.T. Nematicidal activity of some biopesticide agents and microorganisms against root-knot nematode on tomato plants under greenhouse conditions. J. Plant Prot. Res. 2012, 52, 47–52. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Mao, Z.; Ling, J.; Yang, Y.; Li, Y.; Xie, B. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic bacteria and root-knot nematode. Microorganisms 2020, 8, 401. [Google Scholar] [CrossRef]
- Naz, I.; Khan, R.A.A.; Masood, T.; Baig, A.; Siddique, I.; Haq, S. Biological control of root knot nematode, Meloidogyne incognita, in vitro, greenhouse and field in cucumber. Biol. Control 2021, 152, 104429. [Google Scholar] [CrossRef]
- Campos, M.A.S. Bioprotection by arbuscular mycorrhizal fungi in plants infected with Meloidogyne nematodes: A sustainable alternative. Crop Prot. 2020, 135, 105203. [Google Scholar] [CrossRef]
- Ahmad, G.; Nishat, Y.; Ansari, M.; Khan, A.; Haris, M.; Khan, A.A. Eco-friendly approaches for the alleviation of root-knot nematodes. In Plant Growth-Promoting Microbes for Sustainable Biotic and Abiotic Stress Management; Springer: Cham, Switzerland, 2021; pp. 557–575. [Google Scholar]
- Bais, H.P.; Weir, T.L.; Perry, L.G.; Gilroy, S.; Vivanco, J.M. The Role of root exudates in rhizosphere interactions with plants and other organisms. Annu. Rev. Plant Biol. 2006, 57, 233–266. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.H.M.; Pieterse, C.M.J. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef]
- Stringlis, I.A.; Proietti, S.; Hickman, R.; Van Verk, M.C.; Zamioudis, C.; Pieterse, C.M.J. Root transcriptional dynamics induced by beneficial rhizobacteria and microbial immune elicitors reveal signatures of adaptation to mutualists. Plant J. 2018, 93, 166–180. [Google Scholar] [CrossRef]
- Tsolakidou, M.-D.; Stringlis, I.A.; Fanega-Sleziak, N.; Papageorgiou, S.; Tsalakou, A.; Pantelides, I.S. Rhizosphere-enriched microbes as a pool to design synthetic communities for reproducible beneficial outputs. FEMS Microbiol. Ecol. 2019, 95, fiz138. [Google Scholar] [CrossRef]
- El-Nagdi, W.M.A.; Abd-El-Khair, H. Biological control of Meloidogyne incognita and Fusarium solani in dry common bean in the field. Arch. Phytopathol. Plant Prot. 2014, 47, 388–397. [Google Scholar] [CrossRef]
- Munawar, M.; Khan, S.A.; Javed, N.; Ul Haq, I.; Gondal, A.S. Bio-management of tomato wilt complex caused by Meloidogyne incognita and Fusarium oxysporum f. sp. lycopersici. Nematology 2015, 17, 479–485. [Google Scholar] [CrossRef]
- Patil, J.A.; Yadav, S.; Kumar, A. Management of root-knot nematode, Meloidogyne incognita and soil borne fungus, Fusarium oxysporum in cucumber using three bioagents under polyhouse conditions. Saudi J. Biol. Sci. 2021, 28, 7006–7011. [Google Scholar] [CrossRef]
- Shi, X.; Qiao, K.; Li, B.; Zhang, S. Integrated management of Meloidogyne incognita and Fusarium oxysporum in cucumber by combined application of abamectin and fludioxonil. Crop Prot. 2019, 126, 104922. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Chatzistathis, T.; Fanourakis, D.; Nikoloudakis, N.; Kotsiras, A.; Delis, C.; Tzortzakakis, E.A. Leaf antioxidant machinery stimulation by Meloidogyne javanica infestation: A case study on Cucumis melo seedlings. Plant Stress 2021, 1, 100002. [Google Scholar] [CrossRef]
- Hussey, R.S.; Barker, K.R. A Comparison of Methods of Collecting Inocula of Meloidogyne Species, Including a New Technique. Plant Dis. Report. 1973, 57, 1025–1028. [Google Scholar]
- Antoniou, A.; Tsolakidou, M.-D.; Stringlis, I.A.; Pantelides, I.S. Rhizosphere microbiome recruited from a suppressive compost improves plant fitness and increases protection against vascular wilt pathogens of tomato. Front. Plant Sci. 2017, 8, 2022. [Google Scholar] [CrossRef]
- Chialva, M.; Zouari, I.; Salvioli, A.; Novero, M.; Vrebalov, J.; Giovannoni, J.J.; Bonfante, P. Gr and hp-1 tomato mutants unveil unprecedented interactions between arbuscularmycorrhizal symbiosis and fruit ripening. Planta 2016, 244, 155–165. [Google Scholar] [CrossRef]
- Baermann, G. Eine einfache Methode zur Auffindung von Anklostomum (Nematoden) Larven in Erdproben. Geneeskd Tijdschr Ned Indie 1917, 57, 131–137. [Google Scholar]
- Zhou, D.; Feng, H.; Schuelke, T.; De Santiago, A.; Zhang, Q.; Zhang, J.; Luo, C.; Wei, L. Rhizosphere Microbiomes from Root Knot Nematode Non-infested Plants Suppress Nematode Infection. Microb. Ecol. 2019, 78, 470–481. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. The Use of Plant Growth-Promoting Bacteria to Prevent Nematode Damage to Plants. Biology 2020, 9, 381. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kamalanathan, V.; Sevugapperumal, N.; Nallusamy, S. Antagonistic Bacteria Bacillus velezensis VB7 Possess Nematicidal Action and Induce an Immune Response to Suppress the Infection of Root-Knot Nematode (RKN) in Tomato. Genes 2023, 14, 1335. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mhatre, P.H.; Karthik, C.; Kadirvelu, K.; Divya, K.; Venkatasalam, E.; Srinivasan, S.; Ramkumar, G.; Saranya, C.; Shanmuganathan, R. Plant growth promoting rhizobacteria (PGPR): A potential alternative tool for nematodes bio-control. Biocatal. Agric. Biotechnol. 2019, 17, 119–128. [Google Scholar] [CrossRef]
- Sidhu, H.S. Potential of plant growth-promoting rhizobacteria in the management of nematodes: A review. J. Entomol. Zool. Stud. 2018, 6, 1536–1545. [Google Scholar]
- Wharton, D. Nematode eggshells. Parasitology 1980, 81, 447–463. [Google Scholar] [CrossRef]
- Ray, S.; Reddigarim, S.R.; Jansma, P.L.; Allen, R.; Hussey, R.S. Immunocytochemical analysis of the stage-specific distribution of collagen in the cuticle of Meloidogyne incognita. Fundam. Appl. Nematol. 1996, 19, 71–78. [Google Scholar]
- Khanna, K.; Kohli, S.K.; Ohri, P.; Bhardwaj, R. Plants-nematodes-microbes crosstalk within soil: A trade-off among friends or foes. Microbiol. Res. 2021, 248, 126755. [Google Scholar] [CrossRef]
- Krechel, A.; Faupel, A.; Hallmann, J.; Ulrich, A.; Berg, G. Potato-associated bacteria and their antagonistic potential towards plant-pathogenic fungi and the plant-parasitic nematode Meloidogyne incognita (Kofoid & White) Chitwood. Can. J. Microbiol. 2002, 48, 772–786. [Google Scholar]
- El-Hadad, M.E.; Mustafa, M.I.; Selim, S.M.; El-Tayeb, T.S.; Mahgoob, A.E.A.; Aziz, N.H.A. The nematicidal effect of some bacterial biofertilizers on Meloidogyne incognita in sandy soil. Braz. J. Microbiol. 2011, 42, 105–113. [Google Scholar] [CrossRef]
- Siddiqui, I.A.; Shaukat, S.S. Rhizobacteria-mediated induction of systemic resistance (ISR) in tomato against Meloidogyne javanica. J. Phytopathol. 2002, 150, 469–473. [Google Scholar] [CrossRef]
- Sikora, R.A. Interrelationship between plant health promoting rhizobacteria, plant parasitic nematodes and soil microorganisms. Meded. Fac. Landbouww. Rijksuniv. Gent 1988, 53, 867–878. [Google Scholar]
- Kloepper, J.W.; Ryu, C.-M.; Zhang, S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology 2004, 94, 1259–1266. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Role of bacteria in the management of plant parasitic nematodes: A review. Bioresour. Technol. 1999, 69, 167–179. [Google Scholar] [CrossRef]
- Ramamoorthy, V.; Viswanathan, R.; Raguchander, T.; Prakasam, V.; Samiyappan, R. Induction by systemic resistance by plant growth promoting rhizobacteria in crop plants against pests and diseases. Crop Prot. 2001, 20, 1–11. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Geraats, B.P.J.; Linthorst, H.J.M. Ethylene as a modulator of disease resistance in plants. Trends Plant Sci. 2006, 11, 184–191. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; De Bruijn, I.; Nybroe, O.; Ongena, M. Natural functions of lipopeptides from Bacillus and Pseudomonas: More than surfactants and antibiotics. FEMS Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Falardeau, J.; Wise, C.; Novitsky, L.; Avis, T.J. Ecological and mechanistic insights into the direct and indirect antimicrobial properties of Bacillus subtilis lipopeptides on plant pathogens. J. Chem. Ecol. 2013, 39, 869–878. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol. Plant. 2003, 118, 10–15. [Google Scholar] [CrossRef]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M.J. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef]
- Asari, S.; Tarkowská, D.; Rolčík, J.; Novák, O.; Palmero, D.V.; Bejai, S.; Meijer, J. Analysis of plant growth-promoting properties of Bacillus amyloliquefaciens UCMB5113 using Arabidopsis thaliana as host plant. Planta 2017, 245, 15–30. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Bakker, P.A.H.M.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1988, 36, 453–483. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Vicente, C.S.; Barbosa, P.; Espada, M.; Glick, B.R.; Mota, M.; Oliveira, S. Evidence for the involvement of ACC deaminase from Pseudomonas putida UW4 in the biocontrol of pine wilt disease caused by Bursaphelenchus xylophilus. BioControl 2013, 58, 427–433. [Google Scholar] [CrossRef]
| SC1 | SC2 |
|---|---|
| Agrobacterium tumefaciens 2Ba4 | B. chandigarhensis 3Ba3 |
| Bacillus endophyticus 1Ba2 | B. endophyticus 1Ba2 |
| B. humi 3Ba30 | B. endophyticus 3Ba21 |
| B. licheniformis 3Ba17 | B. humi 1Ba28 |
| B. licheniformis 3Ba9 | B. humi 1Ba43 |
| B. firmus 2Ba55 | B. humi 3Ba30 |
| B. firmus 2Ba2 | B. licheniformis 1Ba18 |
| B. niacini 1Ba10 | B. licheniformis 3Ba16 |
| Bacillus sp. 2Ba43 | B. licheniformis 3Ba17 |
| Chryseobacterium sp. 2Ba19 | B. licheniformis 3Ba20 |
| C. wanjuense 2Ba46s | B. licheniformis 3Ba28 |
| Enterobacter cloacae 2Ba30 | B. licheniformis 3Ba44 |
| Luteimonas mephitis 1Ba33 | B. licheniformis 3Ba45 |
| Microbacterium foliorum 1Ba13 | B. licheniformis 3Ba46 |
| M. foliorum 2Ba37 | B. licheniformis 3Ba9 |
| Microbacterium sp. 1Ba51 | B. megaterium 1Ba20 |
| M. esteraromaticum 3Ba33 | B. subtilis 1Ba19 |
| Ochrobactrum sp. 2Ba42 | B. subtilis 2Ba19s |
| Ochrobactrum sp. 2Ba51 | B. subtilis CCS |
| Pseudomonas anguilliseptica 1Ba3 | B. circulans 1Ba46 |
| P. putida 3Ba4 | B. firmus 2Ba55 |
| Stenotrophomonas maltophilia 1Ba16 | B. foraminis 1Ba49 |
| S. maltophilia 2Ba27 | B. niacini 1Ba10 |
| S. maltophilia 2Ba50 | Bacillus sp. 2Ba43 |
| S. maltophilia 2Ba32 | Bacillus sp. 3Ba35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanastasi, E.; Kotsantonis, V.; Pantelides, I.S. Compost-Derived Bacterial Communities Offer Promise as Biocontrol Agents against Meloidogyne javanica and Promote Plant Growth in Tomato. Agriculture 2024, 14, 891. https://doi.org/10.3390/agriculture14060891
Karanastasi E, Kotsantonis V, Pantelides IS. Compost-Derived Bacterial Communities Offer Promise as Biocontrol Agents against Meloidogyne javanica and Promote Plant Growth in Tomato. Agriculture. 2024; 14(6):891. https://doi.org/10.3390/agriculture14060891
Chicago/Turabian StyleKaranastasi, Eirini, Vasileios Kotsantonis, and Iakovos S. Pantelides. 2024. "Compost-Derived Bacterial Communities Offer Promise as Biocontrol Agents against Meloidogyne javanica and Promote Plant Growth in Tomato" Agriculture 14, no. 6: 891. https://doi.org/10.3390/agriculture14060891
APA StyleKaranastasi, E., Kotsantonis, V., & Pantelides, I. S. (2024). Compost-Derived Bacterial Communities Offer Promise as Biocontrol Agents against Meloidogyne javanica and Promote Plant Growth in Tomato. Agriculture, 14(6), 891. https://doi.org/10.3390/agriculture14060891








