Identification and Functional Prediction of Salt/Alkali-Responsive lncRNAs during Alfalfa Germination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. RNA Extraction, Library Preparation, and Sequencing
2.3. LncRNA Identification
2.4. Differentially Expressed lncRNAs and mRNAs Analyses and qPCR Validation
2.5. LncRNA Target Gene Predication
3. Results
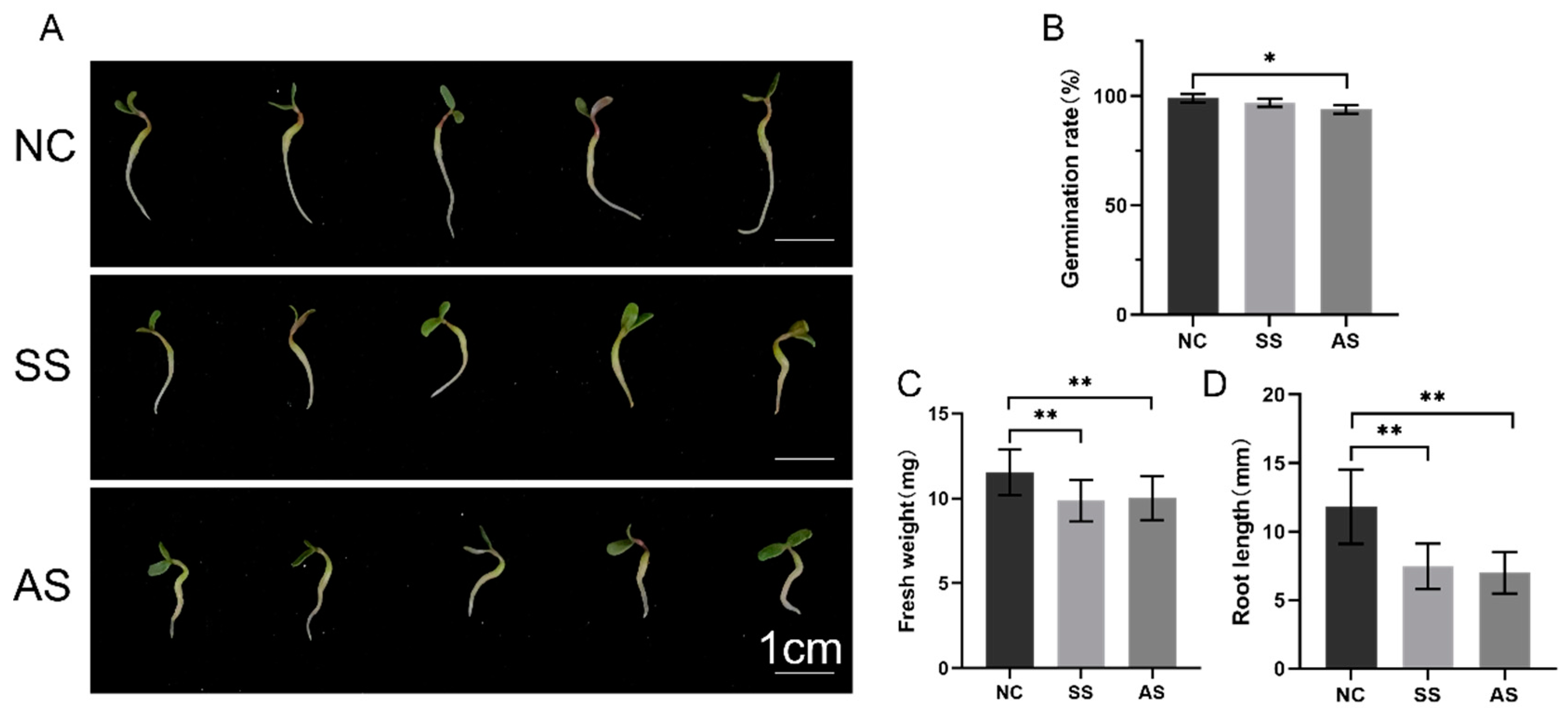
3.1. Stress Response of Alfalfa during Germination
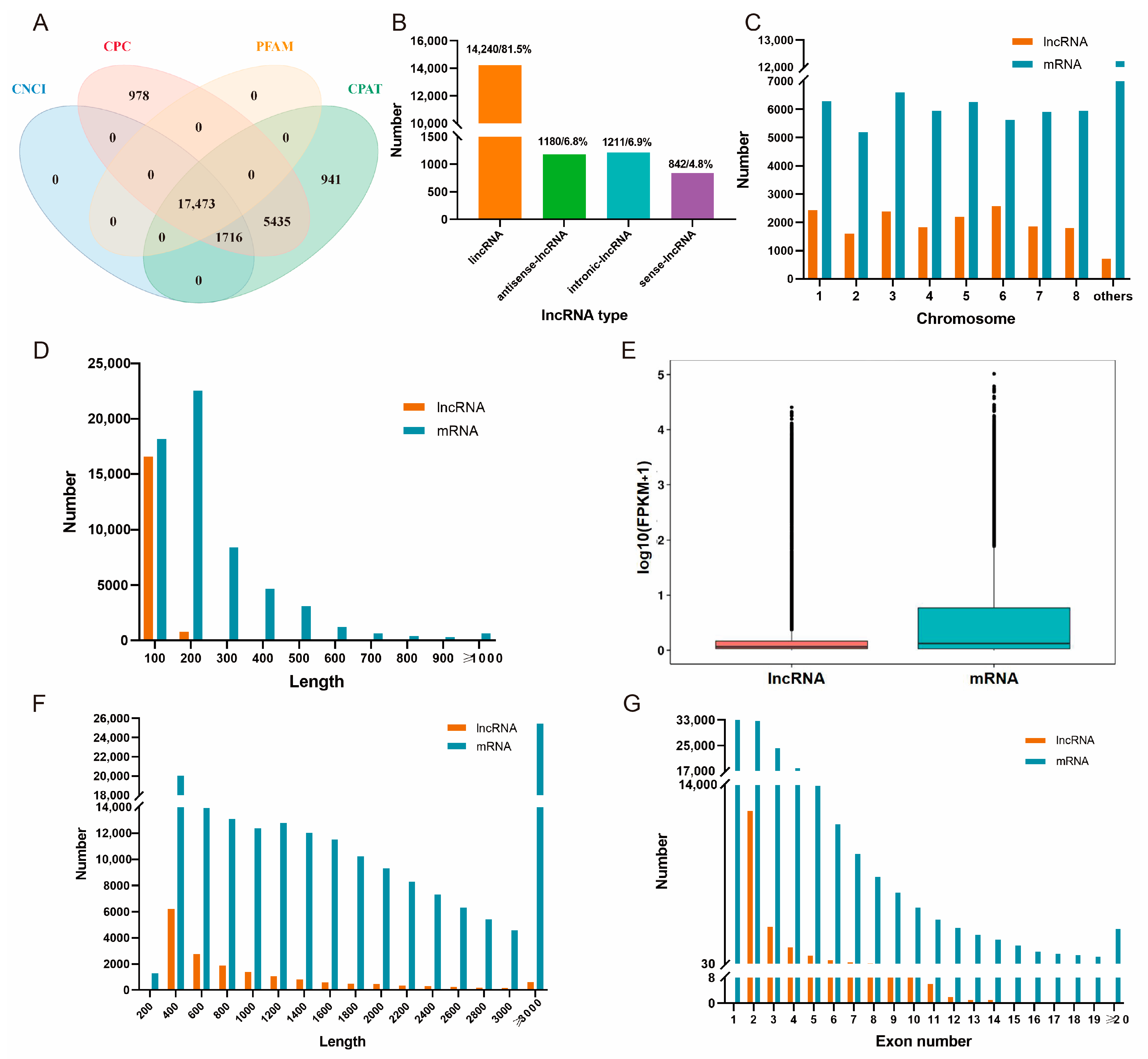
3.2. Alfalfa lncRNAs’ Identification and Characterization
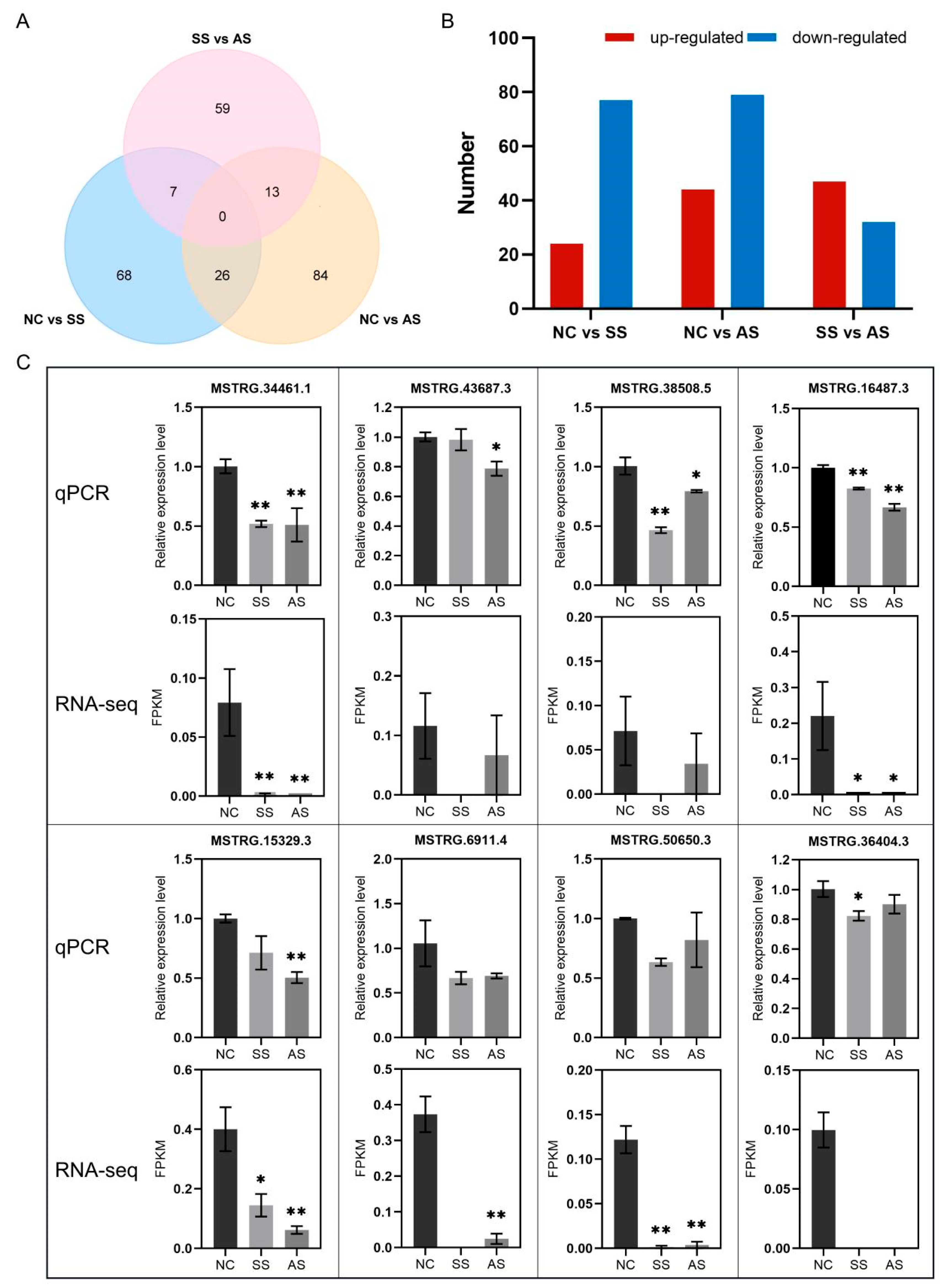
3.3. DElncRNAs’ Analysis and qPCR Validation
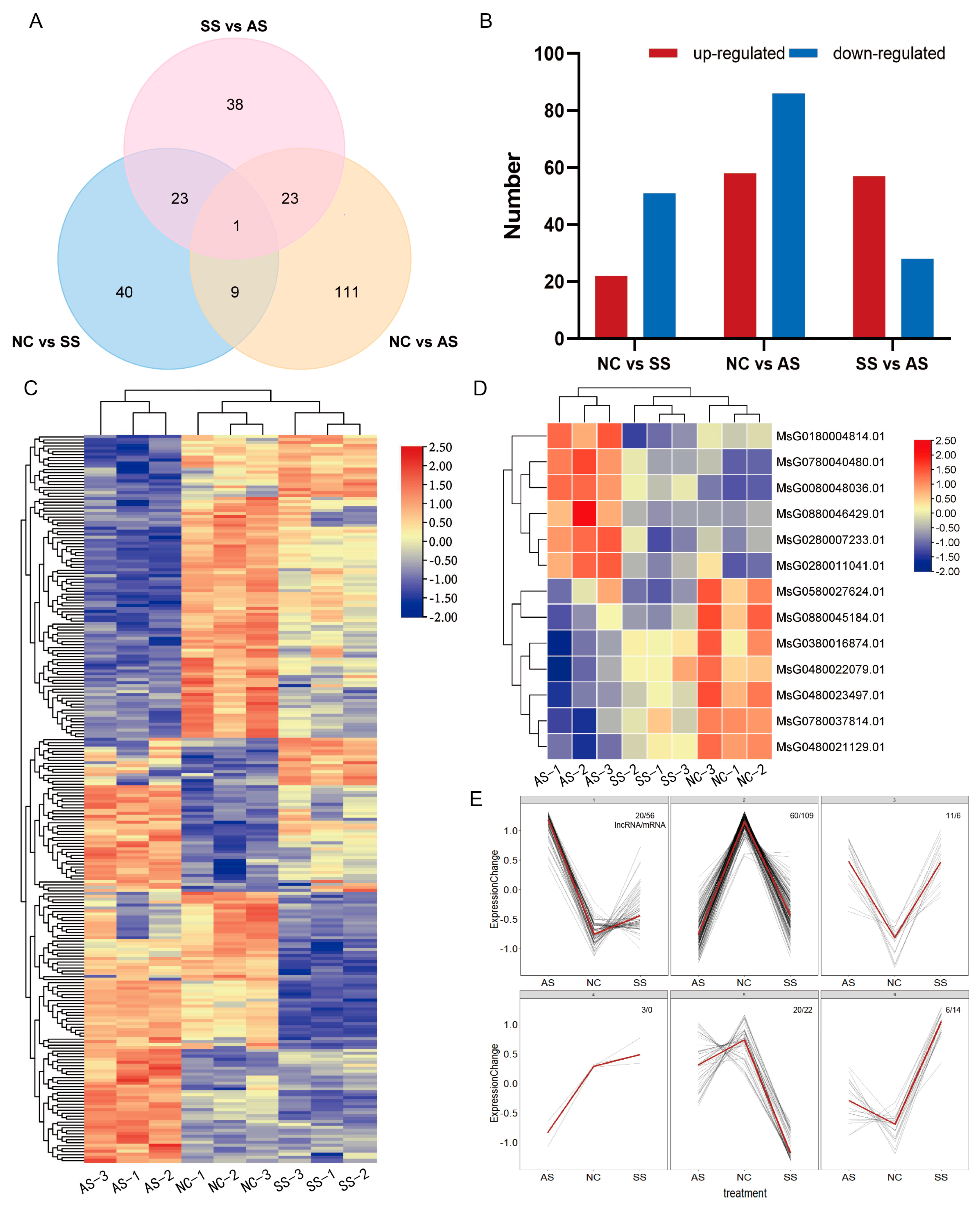
3.4. DEmRNAs’ Analysis and Clustering of DEmRNAs and DElncRNAs
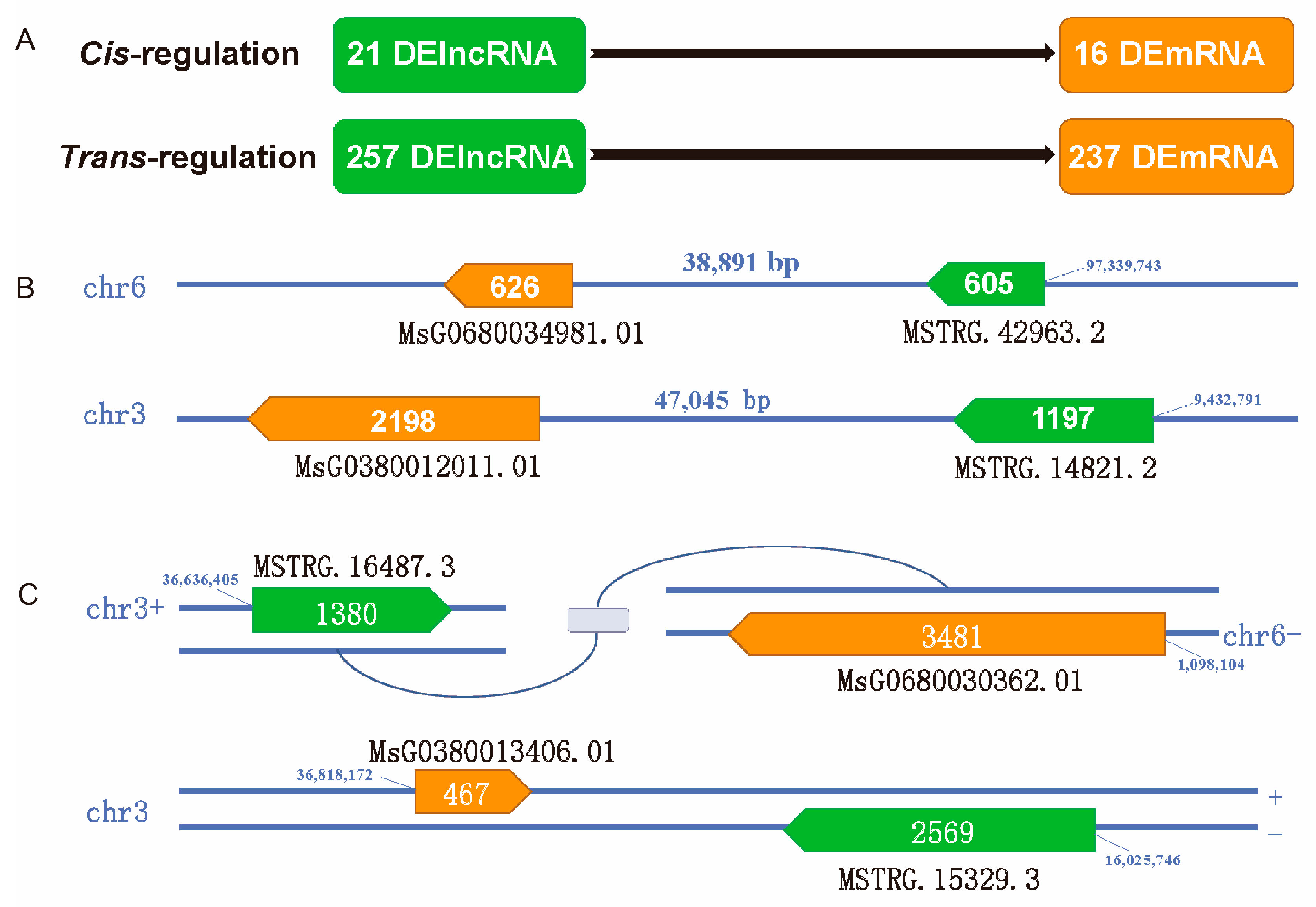
3.5. Cis- and Trans-Target Genes of DElncRNAs
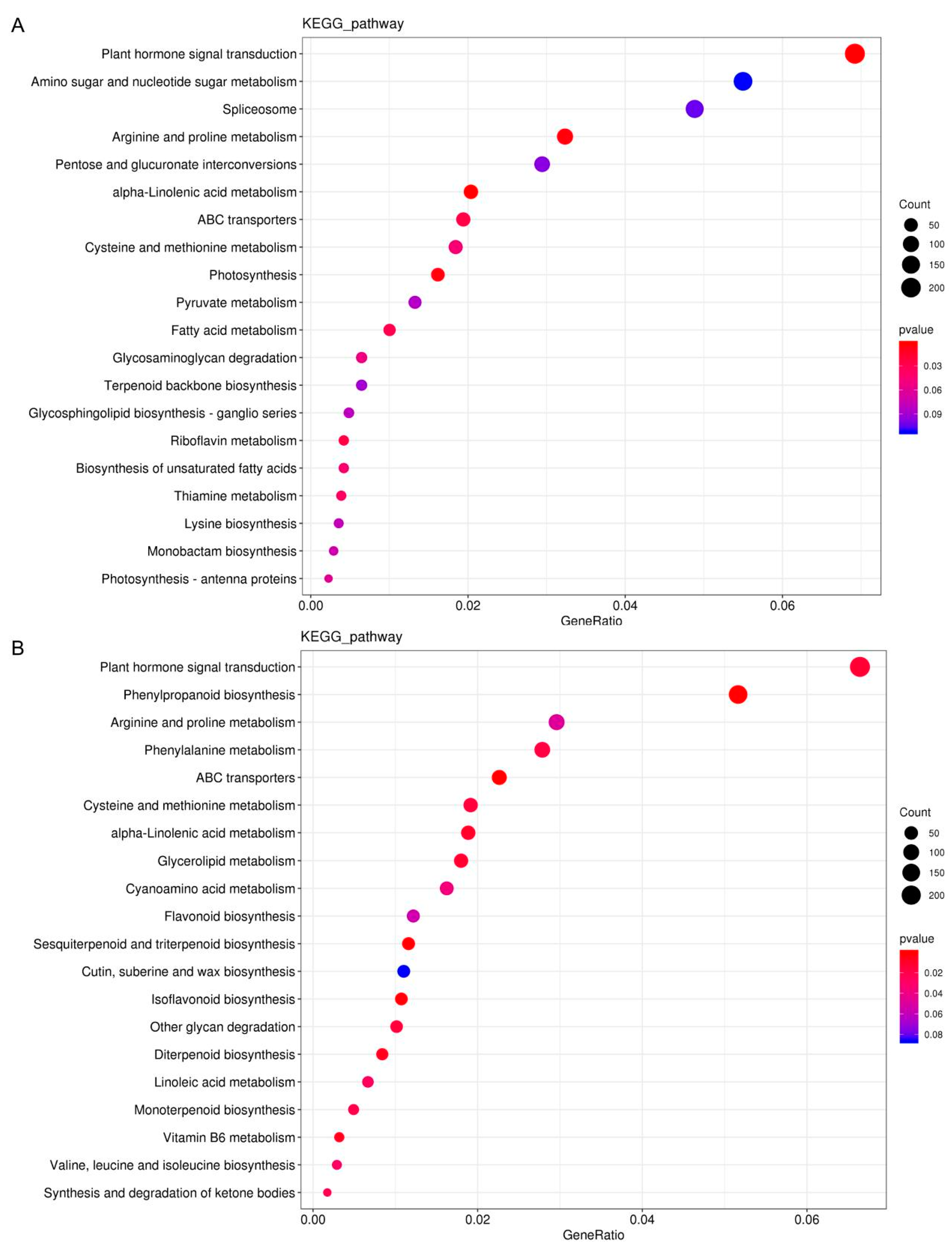
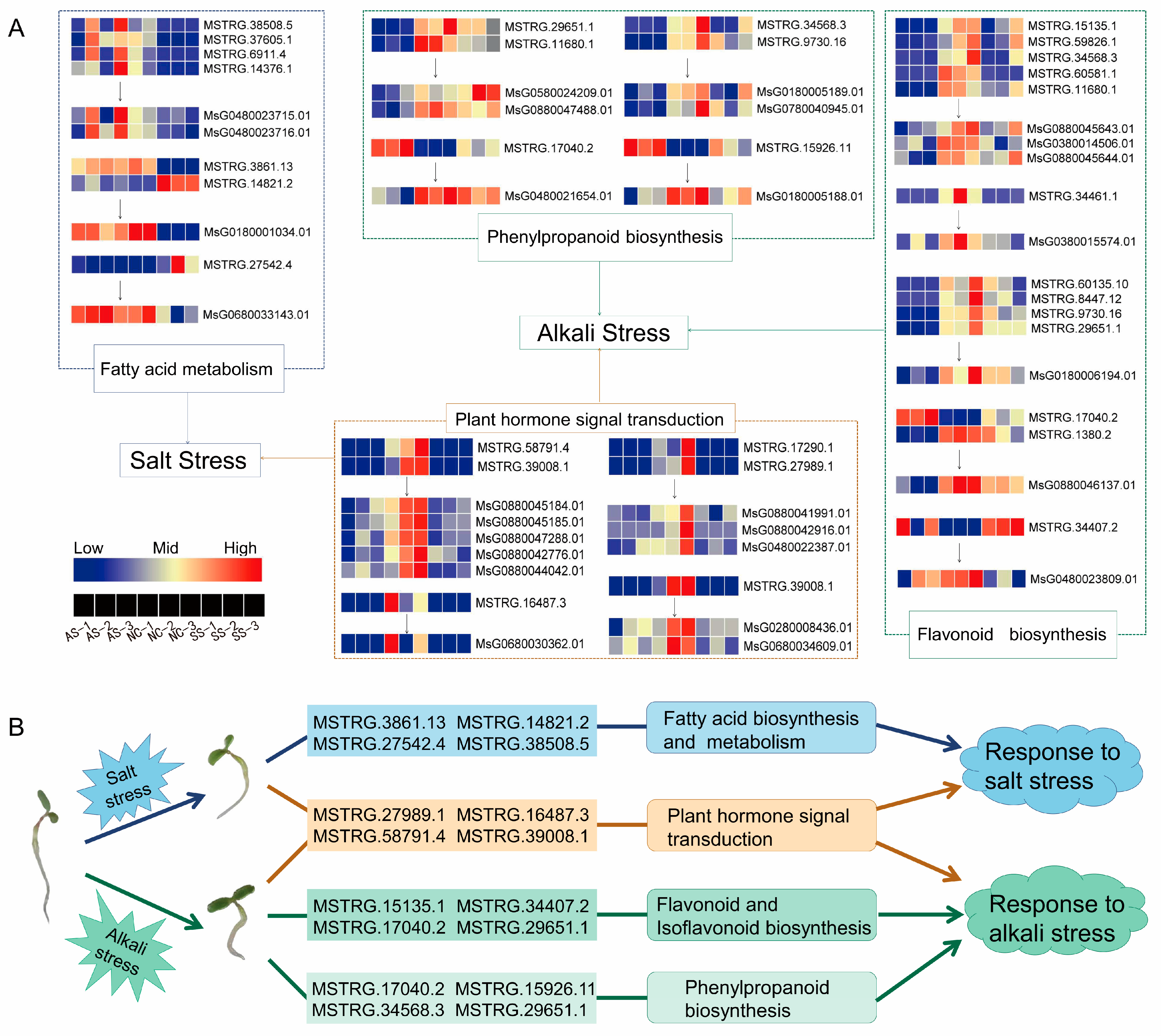
3.6. Functional Prediction of DElncRNA Target Genes during Alfalfa Germination under Salt/Alkali Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hasegawa, P.M. Sodium (Na+) Homeostasis and Salt Tolerance of Plants. Environ. Exp. Bot. 2013, 92, 19–31. [Google Scholar] [CrossRef]
- Watad, A.E.; Reuveni, M.; Bressan, R.A.; Hasegawa, P.M. Enhanced Net K Uptake Capacity of NaCl-adapted Cells. Plant Physiol. 1991, 95, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Leng, B.; Wang, B. Progress in Studying Salt Secretion from the Salt Glands in Recretohalophytes: How Do Plants Secrete Salt? Front. Plant Sci. 2016, 7, 173278. [Google Scholar] [CrossRef]
- Cheng, Y.; Xie, X.; Wang, X.; Zhu, L.; Qiu, Q.; Xu, X. Effects of the Salt-tolerant Gramineous Forage Echinochloa frumentacea on Biological Improvement and Crop Productivity in Saline-alkali Land on the Hetao Ningxia Plain in China. Sustainability 2023, 15, 5319. [Google Scholar] [CrossRef]
- Zhao, Z.; Zang, S.; Zou, W.; Pan, Y.; Yao, W.; You, C.; Que, Y. Long Non-coding RNAs: New Players in Plants. Int. J. Mol. Sci. 2022, 23, 9301. [Google Scholar] [CrossRef] [PubMed]
- Lagarde, J.; Uszczynska-Ratajczak, B.; Carbonell, S.; Perez-Lluch, S.; Abad, A.; Davis, C.; Gingeras, T.R.; Frankish, A.; Harrow, J.; Guigo, R.; et al. High-throughput Annotation of Full-length Long Noncoding RNAs with Capture Long-read Sequencing. Nat. Genet. 2017, 49, 1731–1740. [Google Scholar] [CrossRef] [PubMed]
- Karapetyan, A.R.; Buiting, C.; Kuiper, R.A.; Coolen, M.W. Regulatory Roles for Long ncRNA and mRNA. Cancers 2013, 5, 462–490. [Google Scholar] [CrossRef] [PubMed]
- Ariel, F.; Romero-Barrios, N.; Jegu, T.; Benhamed, M.; Crespi, M. Battles and Hijacks: Noncoding Transcription in Plants. Trends Plant Sci. 2015, 20, 362–371. [Google Scholar] [CrossRef]
- Palos, K.; Yu, L.; Railey, C.E.; Dittrich, A.C.N.; Nelson, A.D.L. Linking Discoveries, Mechanisms, and Technologies to Develop a Clearer Perspective on Plant Long Noncoding RNAs. Plant Cell 2023, 35, 1762–1786. [Google Scholar] [CrossRef]
- Ben, A.B.; Wirth, S.; Merchan, F.; Laporte, P.; D’Aubenton-Carafa, Y.; Hirsch, J.; Maizel, A.; Mallory, A.; Lucas, A.; Deragon, J.M.; et al. Novel Long Non-protein Coding RNAs Involved in Arabidopsis Differentiation and Stress Responses. Genome Res. 2009, 19, 57–69. [Google Scholar]
- Qin, T.; Zhao, H.; Cui, P.; Albesher, N.; Xiong, L. A Nucleus-localized Long Non-coding RNA Enhances Drought and Salt Stress Tolerance. Plant Physiol. 2017, 175, 1321–1336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Dong, J.; Deng, F.; Wang, W.; Cheng, Y.; Song, L.; Hu, M.; Shen, J.; Xu, Q.; Shen, F. The Long Non-coding RNA lncRNA973 is Involved in Cotton Response to Salt Stress. BMC Plant Biol. 2019, 19, 459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shen, J.; Xu, Q.; Dong, J.; Song, L.; Wang, W.; Shen, F. Long Noncoding RNA lncRNA354 Functions as a Competing Endogenous RNA of miR160b to Regulate ARF Genes in Response to Salt Stress in Upland Cotton. Plant Cell Environ. 2021, 44, 3302–3321. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.; You, J.; Zhang, X.; Liu, Y.; Zhao, F.; Cui, X.; Zhang, Y. Genome-wide Identification and Functional Analysis of Long Non-coding RNAs in Sesame Response to Salt Stress. J. Plant Biol. 2021, 64, 555–565. [Google Scholar] [CrossRef]
- Huanca-Mamani, W.; Arias-Carrasco, R.; Cardenas-Ninasivincha, S.; Rojas-Herrera, M.; Sepulveda-Hermosilla, G.; Carlos, C.J.; Bastias, E.; Maracaja-Coutinho, V. Long Non-coding RNAs Responsive to Salt and Boron Stress in the Hyper-arid Lluteno Maize from Atacama Desert. Genes 2018, 9, 170. [Google Scholar] [CrossRef] [PubMed]
- Fei, X.; Shi, Q.; Liu, Y.; Yang, T.; Wei, A. RNA Sequencing and Functional Analyses Reveal Regulation of Novel Drought-responsive, Long-non-coding RNA in Zanthoxylum bungeanum Maxim. Plant Growth Regul. 2020, 90, 425–440. [Google Scholar] [CrossRef]
- Pecetti, L.; Tlahig, S.; Confalonieri, M.; Cornacchione, M.V.; Hayek, T.; Angueira, S.P.; Annicchiarico, P. A Comparison of Procedures for Evaluating and Selecting Alfalfa Landrace Germplasm for Tolerance to Salinity. Crop Sci. 2024, 1–15. [Google Scholar] [CrossRef]
- Medina, C.A.; Hawkins, C.; Liu, X.; Peel, M.; Yu, L. Genome-wide Association and Prediction of Traits Related to Salt Tolerance in Autotetraploid Alfalfa (Medicago sativa L.). Int. J. Mol. Sci. 2020, 21, 3361. [Google Scholar] [CrossRef]
- Shi, S.; Nan, L.; Smith, K.F. The Current Status, Problems, and Prospects of Alfalfa (Medicago sativa L.) Breeding in China. Agronomy-basel 2017, 7, 1. [Google Scholar] [CrossRef]
- Lorenzo, C.D.; Garcia-Gagliardi, P.; Antonietti, M.S.; Sanchez-Lamas, M.; Mancini, E.; Dezar, C.A.; Vazquez, M.; Watson, G.; Yanovsky, M.J.; Cerdan, P.D. Improvement of Alfalfa Forage Quality and Management through the Down-regulation of MsFTa1. Plant Biotechnol. J. 2020, 18, 944–954. [Google Scholar] [CrossRef] [PubMed]
- Boe, A.; Kephart, K.D.; Berdahl, J.D.; Peel, M.D.; Brummer, E.C.; Xu, L.; Wu, Y. Breeding Alfalfa for Semiarid Regions in the Northern Great Plains: History and Additional Genetic Evaluations of Novel Germplasm. Agronomy 2020, 10, 1686. [Google Scholar] [CrossRef]
- Li, X.; Hou, Y.; Li, M.; Zhang, F.; Yi, F.; Kang, J.; Yang, Q.; Long, R. Overexpression of an ABA-inducible Homeodomain-leucine Zipper I Gene MsHB7 Confers Salt Stress Sensitivity to Alfalfa. Ind. Crops Prod. 2022, 177, 114463. [Google Scholar] [CrossRef]
- Long, R.; Gao, Y.; Sun, H.; Zhang, T.; Li, X.; Li, M.; Sun, Y.; Kang, J.; Wang, Z.; Ding, W.; et al. Quantitative Proteomic Analysis Using iTRAQ to Identify Salt-responsive Proteins during the Germination Stage of Two Medicago Species. Sci. Rep. 2018, 8, 9553. [Google Scholar] [CrossRef]
- Long, R.; Sun, H.; Cao, C.; Zhang, T.; Kang, J.; Wang, Z.; Li, M.; Gao, Y.; Li, X.; Yang, Q. Identification of Alkali-responsive Proteins from Early Seedling Stage of Two Contrasting Medicago Species by iTRAQ-based Quantitative Proteomic Analysis. Environ. Exp. Bot. 2019, 157, 26–34. [Google Scholar] [CrossRef]
- Marini, R.P. Approaches to Analyzing Experiments with Factorial Arrangements of Treatments Plus Other Treatments. HortScience 2003, 38, 117–120. [Google Scholar] [CrossRef]
- Li, J.; Ma, W.; Zeng, P.; Wang, J.; Geng, B.; Yang, J.; Cui, Q. LncTar: A Tool for Predicting the RNA Targets of Long Noncoding RNAs. Brief Bioinform. 2015, 16, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Du, H.; Chen, Z.; Lu, H.; Zhu, F.; Chen, H.; Meng, X.; Liu, Q.; Liu, P.; Zheng, L.; et al. The Chromosome-level Genome Sequence of the Autotetraploid Alfalfa and Resequencing of Core Germplasms Provide Genomic Resources for Alfalfa Research. Mol. Plant 2020, 13, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript Assembly and Quantification by RNA-seq Reveals Unannotated Transcripts and Isoform Switching during Cell Differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Lv, J.; Cui, W.; Liu, H.; He, H.; Xiu, Y.; Guo, J.; Liu, H.; Liu, Q.; Zeng, T.; Chen, Y.; et al. Identification and Characterization of Long Non-coding RNAs Related to Mouse Embryonic Brain Development from Available Transcriptomic Data. PLoS ONE 2013, 8, e71152. [Google Scholar] [CrossRef]
- Kelley, D.; Rinn, J. Transposable Elements Reveal a Stem Cell-specific Class of Long Noncoding RNAs. Genome Biol. 2012, 13, R107. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.; Zhang, Y.; Ye, Z.; Liu, X.; Zhao, S.; Wei, L.; Gao, G. CPC: Assess the Protein-coding Potential of Transcripts Using Sequence Features and Support Vector Machine. Nucleic Acids Res. 2007, 35, W345–W349. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Luo, H.; Bu, D.; Zhao, G.; Yu, K.; Zhang, C.; Liu, Y.; Chen, R.; Zhao, Y. Utilizing Sequence Intrinsic Composition to Classify Protein-coding and Long Non-coding Transcripts. Nucleic Acids Res. 2013, 41, e166. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Park, H.J.; Dasari, S.; Wang, S.; Kocher, J.; Li, W. CPAT: Coding-potential Assessment Tool Using an Alignment-free Logistic Regression Model. Nucleic Acids Res. 2013, 41, e74. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J.; et al. Pfam: The Protein Families Database. Nucleic Acids Res. 2014, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An Integrative Toolkit Developed for Interactive Analyses of Big Biological Data. Mol. Plant 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Araki, M.; Goto, S.; Hattori, M.; Hirakawa, M.; Itoh, M.; Katayama, T.; Kawashima, S.; Okuda, S.; Tokimatsu, T.; et al. KEGG for Linking Genomes to Life and the Environment. Nucleic Acids Res. 2008, 36, D480–D484. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Wang, L.; Han, Y.; He, Q. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Guo, D.; Zhang, H.; Che, Y.; Li, Y.; Tian, B.; Wang, Z.; Sun, G.; Zhang, H. Physiological and Comparative Transcriptome Analysis of Leaf Response and Physiological Adaption to Saline Alkali Stress across pH Values in Alfalfa (Medicago sativa). Plant Physiol. Bioch. 2021, 167, 140–152. [Google Scholar] [CrossRef]
- Medina, C.A.; Samac, D.A.; Yu, L. Pan-transcriptome Identifying Master Genes and Regulation Network in Response to Drought and Salt Stresses in Alfalfa (Medicago sativa L.). Sci. Rep. 2021, 11, 17203. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Su, H.; Niu, Q.; Yin, S. Integrated Analysis of Coding and Non-coding RNAs Reveals the Molecular Mechanism Underlying Salt Stress Response in Medicago truncatula. Front. Plant Sci. 2022, 13, 891361. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Zhang, R.; Zhang, M.; Xia, G.; Liu, S. Functional Analysis of Long Non-coding RNAs Involved in Alkaline Stress Responses in Wheat. J. Exp. Bot. 2022, 73, 5698–5714. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, H.; Wang, Q.; Guo, R.; Wei, L.; Song, H.; Kuang, W.; Liao, J.; Huang, Y.; Wang, Z. Genome-wide Identification and Characterization of Long Non-coding RNAs Involved in Flag Leaf Senescence of Rice. Plant Mol. Biol. 2021, 105, 655–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, M.; Zhao, M.; Chen, R.; Zhang, W. Identification and Characterization of Long Non-coding RNAs Involved in Osmotic and Salt Stress in Medicago truncatula Using Genome-wide High-throughput Sequencing. BMC Plant Biol. 2015, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Fu, Y.; Coulman, B.; Tanino, K.; Karunakaran, C.; Biligetu, B. Transcriptomic Analysis of Differentially Expressed Genes in Leaves and Roots of Two Alfalfa (Medicago sativa L.) Cultivars with Different Salt Tolerance. BMC Plant Biol. 2021, 21, 446. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Song, L.; Liu, Y.; Shu, Y.; Guo, C. De Novo Transcriptional Analysis of Alfalfa in Response to Saline-alkaline Stress. Front. Plant Sci. 2016, 7, 931. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Li, Z.; Rong, M.; Yang, G.; Xu, X.; Wang, G.; Xu, Z.; Du, X.; Zhang, Q. Comparative Physiology and Transcriptome Analysis to Identify the Important Coding and Non-Coding RNAs Imparting Tolerance to Salinity Stress in Alfalfa (Medicago sativa L.). Res. Sq. 2023, 1–16. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; He, F.; Li, M.; Zi, Y.; Long, R.; Zhao, G.; Zhu, L.; Hong, L.; Wang, S.; et al. Multi-omics Integrative Analysis Provided New Insights into Alkaline Stress in Alfalfa. Soc. Sci. Res. Netw. 2024, 1–33. [Google Scholar]
- Yu, C.; Wang, H.; Yang, S.; Tang, X.; Duan, M.; Meng, Q. Overexpression of Endoplasmic Reticulum Omega-3 Fatty Acid Desaturase Gene Improves Chilling Tolerance in Tomato. Plant Physiol. Bioch. 2009, 47, 1102–1112. [Google Scholar] [CrossRef]
- Zhang, M.; Barg, R.; Yin, M.G.; Gueta-Dahan, Y.; Leikin-Frenkel, A.; Salts, Y.; Shabtai, S.; Ben-Hayyim, G. Modulated Fatty Acid Desaturation via Overexpression of Two Distinct Omega-3 Desaturases Differentially Alters Tolerance to Various Abiotic Stresses in Transgenic Tobacco Cells and Plants. Plant J. 2005, 44, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Klinkenberg, J.; Faist, H.; Saupe, S.; Lambertz, S.; Krischke, M.; Stingl, N.; Fekete, A.; Mueller, M.J.; Feussner, I.; Hedrich, R.; et al. Two Fatty Acid Desaturases, Stearoyl-Acyl Carrier Protein Delta(9)-desaturase6 and Fatty Acid Desaturase3, Are Involved in Drought and Hypoxia Stress Signaling in Arabidopsis Crown Galls. Plant Physiol. 2014, 164, 570–583. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Fang, Z.; Wu, T.; Li, H.; Yang, Z.; Zhao, Y. PreliminaryAnalysis of Alfalfa Leaf Proteome under Salt-alkali Stress. Acta Agrestia Sin. 2019, 27, 574–580. [Google Scholar]
- Wu, J.; Lv, S.; Zhao, L.; Gao, T.; Yu, C.; Hu, J.; Ma, F. Advances in the Study of the Function and Mechanism of the Action of Flavonoids in Plants under Environmental Stresses. Planta 2023, 257, 108. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, K.; Raman, V.K.; Sevanthi, A.M.; Sivakumar, S.R.; Gayatri; Viswanathan, C.; Mohapatra, T.; Mandal, P.K. Stress-inducible Expression of Chalcone Isomerase2 Gene Improves Accumulation of Flavonoids and Imparts Enhanced Abiotic Stress Tolerance to Rice. Environ. Exp. Bot. 2021, 190, 104582. [Google Scholar] [CrossRef]
- Ozfidan-Konakci, C.; Yildiztugay, E.; Alp, F.N.; Kucukoduk, M.; Turkan, I. Naringenin Induces Tolerance to Salt/Osmotic Stress through the Regulation of Nitrogen Metabolism, Cellular Redox and ROS Scavenging Capacity in Bean Plants. Plant Physiol. Bioch. 2020, 157, 264–275. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Huang, Q.; Zhang, R.; Li, J.; Luo, K.; Chen, Y. Molecular Characterisation of PAL Gene Family Reveals Their Role in Abiotic Stress Response in Lucerne (Medicago sativa). Crop Pasture Sci. 2022, 73, 300–311. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Liu, P.; Guo, P.; Li, M.; Jiang, L.; Luo, L. Cloning of Cassava Phenylalanine Ammonia Lyase Genes and Their Responses to Low Temperature Stress. Chin. J. Trop. Crops 2019, 40, 483–489. [Google Scholar]
- Wasternack, C. Jasmonates: An Update on Biosynthesis, Signal Transduction and Action in Plant Stress Response, Growth and Development. Ann. Bot. 2007, 100, 681–697. [Google Scholar] [CrossRef]
- Chini, A.; Ben-Romdhane, W.; Hassairi, A.; Aboul-Soud, M.A.M. Identification of TIFY/JAZ Family Genes in Solanum lycopersicum and Their Regulation in Response to Abiotic Stresses. PLoS ONE 2017, 12, e0177381. [Google Scholar] [CrossRef]
- Monte, I.; Franco-Zorrilla, J.M.; Garcia-Casado, G.; Zamarreno, A.M.; Garcia-Mina, J.M.; Nishihama, R.; Kohchi, T.; Solano, R. A Single JAZ Repressor Controls the Jasmonate Pathway in Marchantia polymorpha. Mol. Plant 2019, 12, 185–198. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Du, M.; Deng, L.; Shen, J.; Fang, M.; Chen, Q.; Lu, Y.; Wang, Q.; Li, C.; Zhai, Q. MYC2 Regulates the Termination of Jasmonate Signaling via an Autoregulatory Negative Feedback Loop. Plant Cell 2019, 31, 106–127. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Xu, L.; Zhang, T.; Sod, B.; Xu, Y.; Li, M.; Kang, J.; Yang, Q.; Li, X.; Long, R. Identification and Functional Prediction of Salt/Alkali-Responsive lncRNAs during Alfalfa Germination. Agriculture 2024, 14, 930. https://doi.org/10.3390/agriculture14060930
Liu Y, Xu L, Zhang T, Sod B, Xu Y, Li M, Kang J, Yang Q, Li X, Long R. Identification and Functional Prediction of Salt/Alkali-Responsive lncRNAs during Alfalfa Germination. Agriculture. 2024; 14(6):930. https://doi.org/10.3390/agriculture14060930
Chicago/Turabian StyleLiu, Yajiao, Lei Xu, Tiejun Zhang, Bilig Sod, Yanchao Xu, Mingna Li, Junmei Kang, Qingchuan Yang, Xiao Li, and Ruicai Long. 2024. "Identification and Functional Prediction of Salt/Alkali-Responsive lncRNAs during Alfalfa Germination" Agriculture 14, no. 6: 930. https://doi.org/10.3390/agriculture14060930







