Effects of Applying Biochar on Soil Cadmium Immobilisation and Cadmium Pollution Control in Lettuce (Lactuca sativa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Experimental Design
2.1.1. Materials
2.1.2. Experimental Design
2.2. Characterization Analysis of Biochar from Different Feedstocks
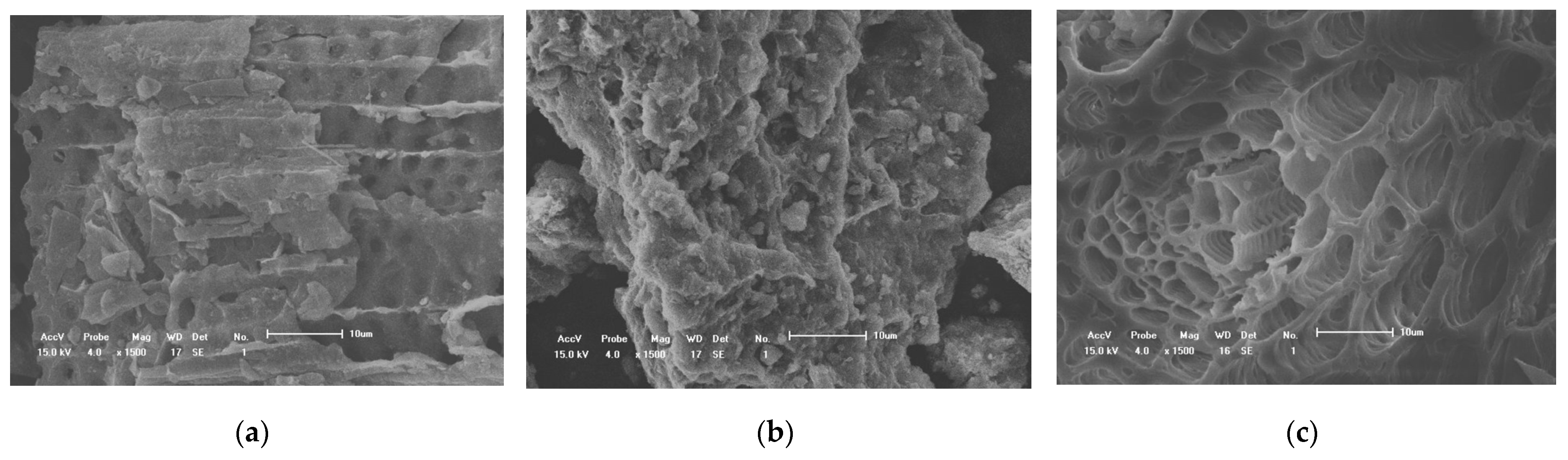
2.2.1. Scanning Electron Microscope (SEM)
2.2.2. Specific Surface Area (SSA) and Pore Size Analysis
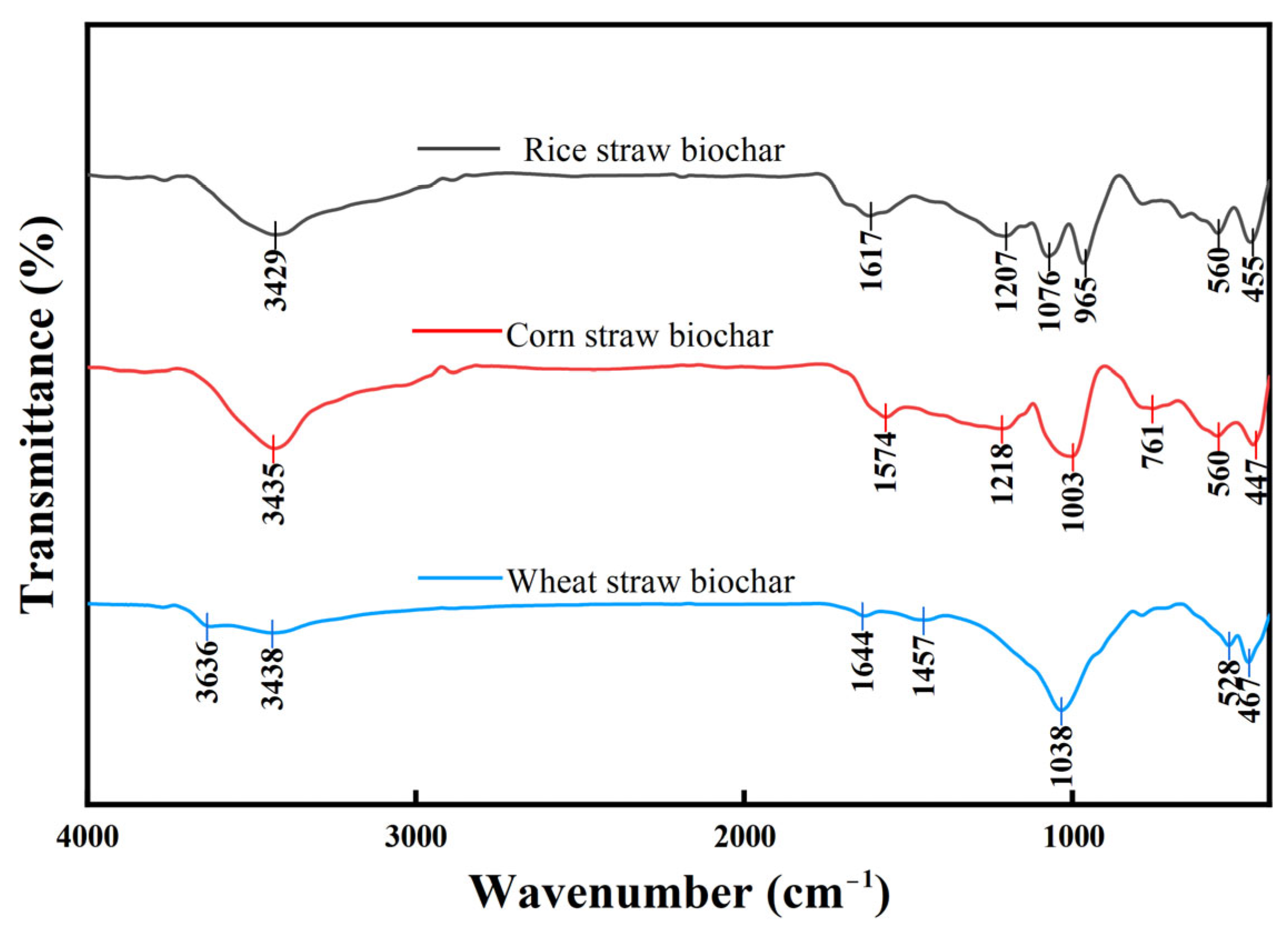
2.2.3. Fourier Transform Infrared Spectroscopy (FTIR)
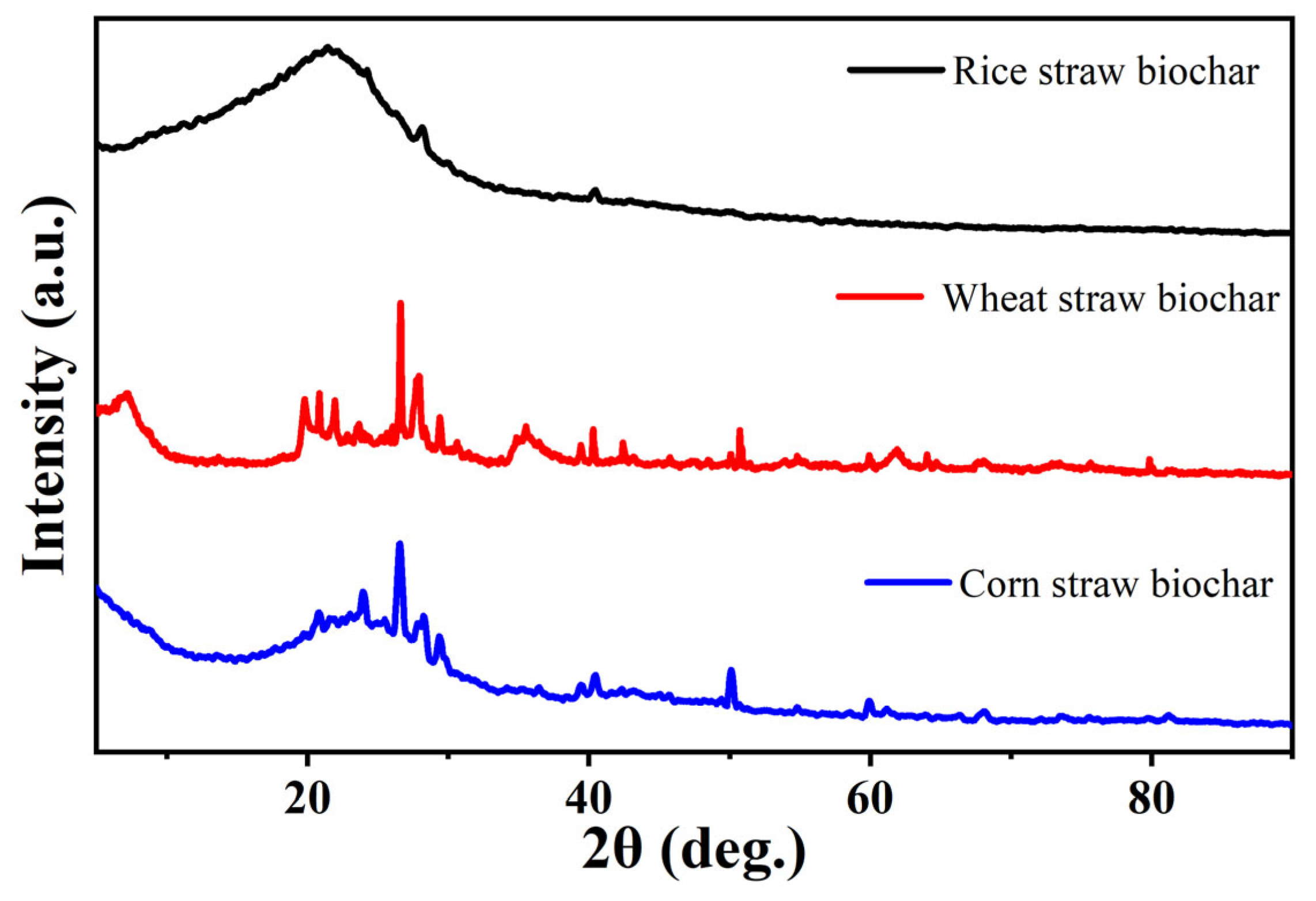
2.2.4. X-ray Diffraction Analysis (XRD)
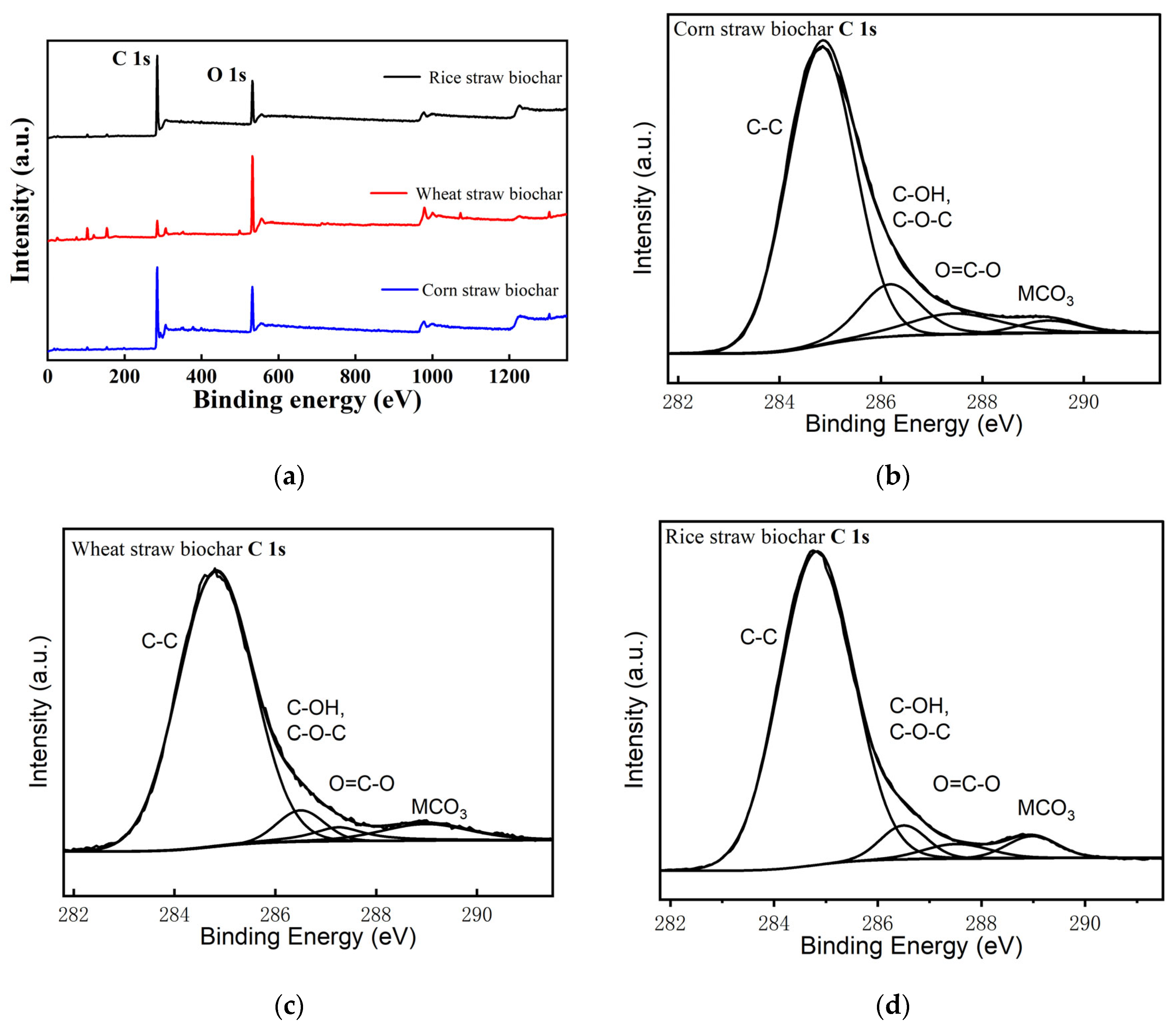
2.2.5. X-ray Photoelectron Spectroscopy Analysis (XPS)
2.3. Soil Environmental Factor Detection
2.3.1. Soil pH Determination
2.3.2. Soil Cation Exchange Capacity (CEC) Determination
2.3.3. Soil Electrical Conductivity (EC) Determination
2.3.4. Soil-Available Cd Determination
2.4. Determination of Lettuce Plant Height and Fresh Weight
2.5. Leaf Cd Content Determination
2.6. Data Processing and Analysis
3. Results and Discussion
3.1. Characterization Analysis of Different Biochar Materials
3.1.1. Scanning Electron Microscope (SEM) Analysis
3.1.2. Analysis of Pore Structure
3.1.3. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
3.1.4. X-ray Diffraction (XRD) Analysis
3.1.5. X-ray Photoelectron Spectroscopy (XPS) Analysis
3.2. Effects of Different Biochar Addition Levels on the Soil Properties
3.2.1. Effects of Different Biochar Addition Levels on the pH of Cd-Contaminated Soil
3.2.2. Effects of Different Biochar Addition Levels on the EC of Cd-Contaminated Soil
3.2.3. Effects of Different Biochar Addition Levels on the CEC of Cd-Contaminated Soil
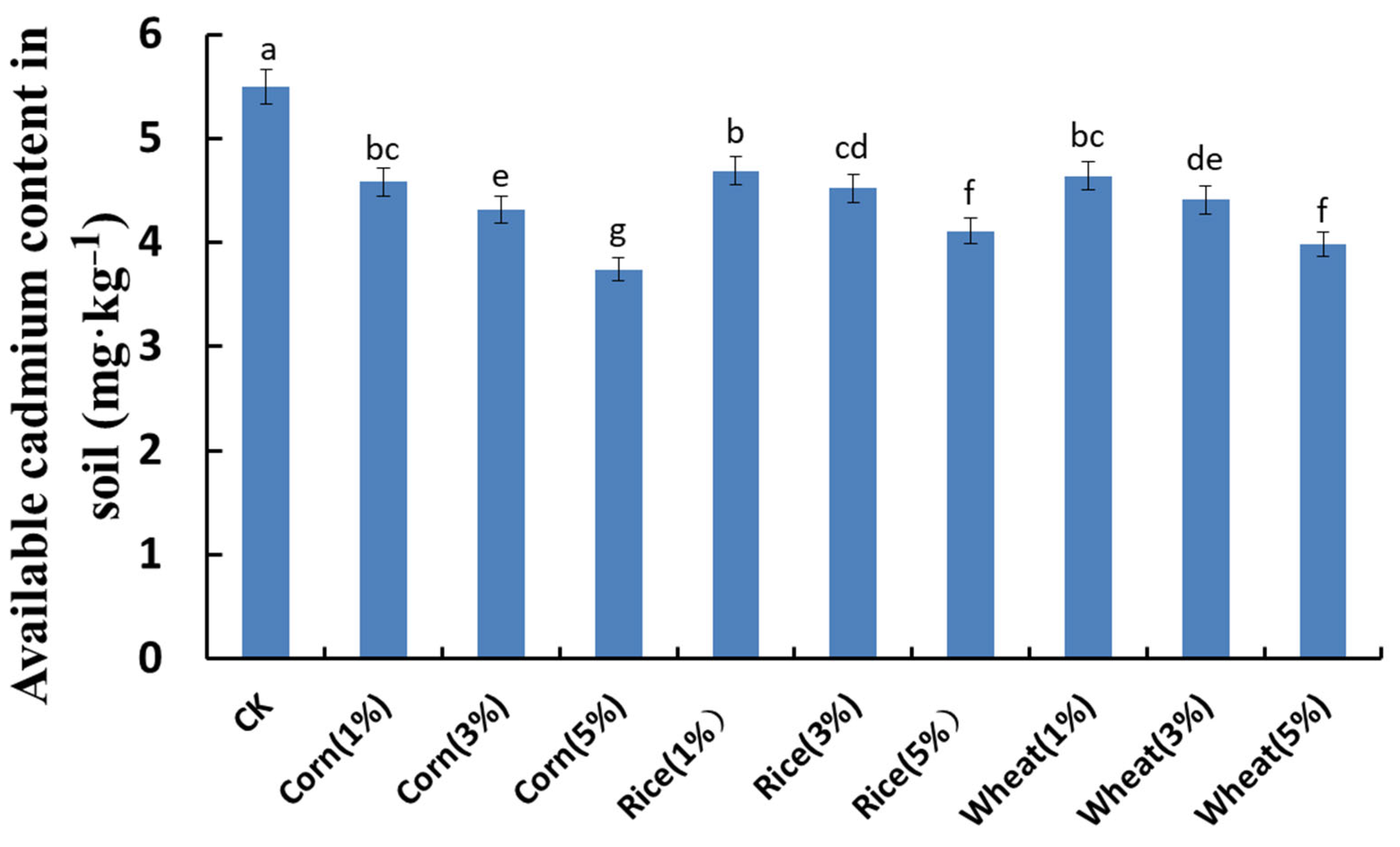
3.2.4. Effects of Different Biochar Addition Levels on the Available Cd in Cd-Contaminated Soil
3.3. Effects of Different Biochar Addition Levels on the Plant Height and Fresh Weight of Cd-Contaminated Lettuce at Maturity Stage
3.4. Effects of Different Biochar Addition Levels on the Cd Content in Mature Lettuce Leaves under Cd Contamination
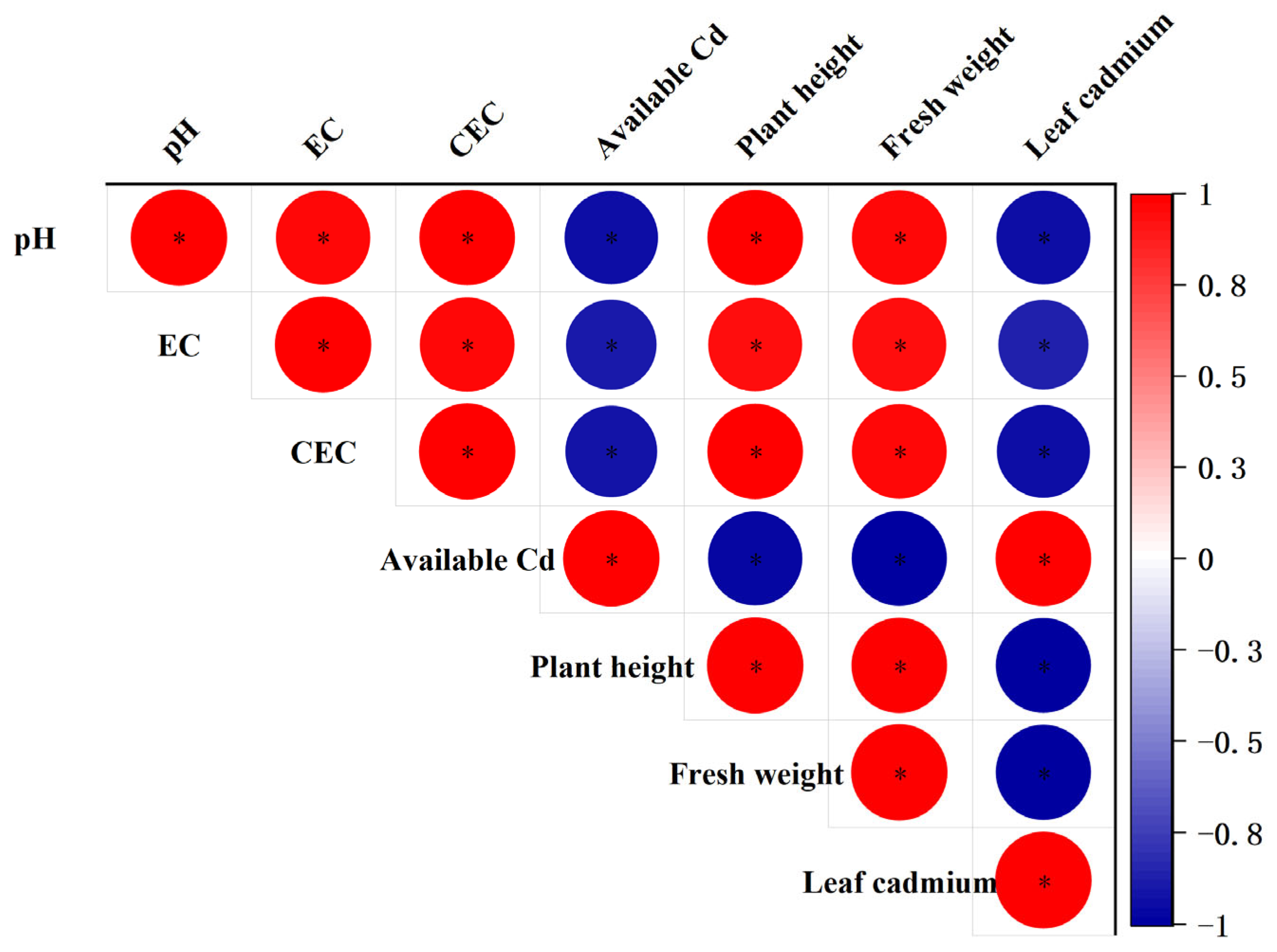
3.5. Analysis of the Correlation between Soil Properties and the Plant Height, Fresh Weight, and Leaf Cd Content of Lettuce
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Song, W.; Chen, B.M.; Liu, L. Soil Heavy Metal Pollution of Cultivated Land in China. Res. Soil Water Conserv. 2013, 20, 293–298. [Google Scholar]
- Zhao, F.J.; Ma, Y.B.; Zhu, Y.G.; Tang, Z.; McGrath, S.P. Soil Contamination in China: Current Status and Mitigation Strategies. Environ. Sci. Technol. 2014, 49, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Pandey, C.V.; Bajpai, O.; Singh, N. Energy crops in sustainable phytoremediation. Renew. Sustain. Energy Rev. 2016, 54, 58–73. [Google Scholar] [CrossRef]
- Raiesi, F.; Razmkhah, M.; Kiani, S. Salinity stress accelerates the effect of cadmium toxicity on soil N dynamics and cycling: Does joint effect of these stresses matter? Ecotoxicol. Environ. Saf. 2018, 153, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Kong, S.F.; Yao, X.C.; Zhang, J.Y.; Yao, X.D.; Zeng, H. Review of Characteristics of Biochar and Research Progress of Its Applications. Ecol. Environ. Sci. 2015, 24, 716–723. [Google Scholar]
- Yang, K.D.; Xin, Y.J.; Wu, M.; Wu, D.; Luo, Y. Effects of Three Kinds of Biochar on the Biomass and Quality of Chinese Cabbage in Cadmium Polluted Yellow Soil. Anhui Agric. Sci. Bull. 2021, 27, 145–148. [Google Scholar]
- Pu, S.Y.; Shangguan, L.X.; Liu, S.B.; Shi, Q.Q.; Wang, X.K.; Zhang, Y. A Review of the application of biochar and its composites in soil remediation. Ecol. Environ. Sci. 2019, 28, 629–635. [Google Scholar]
- Yin, D.X.; Wang, X.; Peng, B.; Tan, C.Y.; Ma, L.N.Q. Effect of biochar and Fe-biochar on Cd and As mobility and transfer in soil-rice system. Chemosphere 2017, 186, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Wang, X.B.; Wang, X.L.; Feng, K.; Su, J.C.; Dong, J.N. The mechanism of cadmium sorption by sulphur-modified wheat straw biochar and its application cadmium-contaminated soil. Sci. Total Environ. 2020, 714, 136550. [Google Scholar] [CrossRef]
- Abbas, T.; Rizwan, M.; Ali, S.; Rehman, M.Z.U.; Qayyum, M.F.; Abbas, F.; Hannan, F.; Rinklebe, J.; Ok, Y.S. Effect of biochar on cadmium bioavailability and uptake in wheat (Triticum aestivum L.) grown in a soil with aged contamination. Ecotoxicol. Environ. Saf. 2017, 140, 37–47. [Google Scholar] [CrossRef]
- Yan, C.X.; Jia, H.T.; Sun, T.; Dina, T.; Luo, W.W.; Xie, S.; Sun, Y.B. Characteristics of chicken manure biochars and its effect on Cd and Pb remediation in water and soil. Trans. CSAE 2019, 35, 225–233. [Google Scholar]
- Fang, X.B.; Zhang, Z.J.; Lai, Y.Q.; Ye, M.; Diao, Z.H. Remediation of heavy metals Cr and Cd in soil by a novel sludge-derived biochar. Ecol. Environ. Sci. 2022, 31, 1647–1656. [Google Scholar]
- Liu, Y.X.; Wang, Y.Y.; Lu, H.H.; Lonappan, L.S.; Brar, S.K.; He, L.L.; Chen, J.Y.; Yang, S.M. Biochar application as a soil amendment for decreasing cadmium availability in soil and accumulation in Brassica chinensis. J. Soils Sediments 2018, 18, 2511–2519. [Google Scholar] [CrossRef]
- Chen, C.; Li, F.M.; Yang, L.; Nie, X.X.; Liu, B.; Hong, J. Mechanism and application of different types of biochar to remediation of Cd contaminated paddy soils: A review. Environ. Chem. 2022, 41, 4165–4179. [Google Scholar]
- Yuan, S.; Zhao, L.X.; Meng, H.B.; Shen, Y.J. The main types of biochar and their properties and expectative researches. J. Plant Nutr. Fertil. 2016, 22, 1402–1417. [Google Scholar]
- Hu, S.M.; Chen, C.; Chen, X.M.; Jing, F.; Wen, X. A field study of biochar application impact on adsorption and accumulation of Cd in paddy soil and rice. Arch. Agron. Soil Sci. 2023, 69, 48–59. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, Y.; Liang, Y.; Shi, W.L. Immobilization and its mechanisms of cadmium contaminated soils by biochar. Chin. J. Environ. Eng. 2017, 11, 2528–2534. [Google Scholar]
- GB 15618-2018; Soil Environmental Quality Risk Control Standard for Agricultural Land (Trial). China Environmental Science Press: Beijing, China, 2018.
- Cao, S.Z.; Mu, Y.; Cui, J.X.; Liu, A.D.; Cheng, X.; Fu, Y.H.; Wei, S.Q.; Zhang, J.Z. Cd Pollution and Safe Planting Zoning in Paddy Soils: A Case Study in a District of Chongqing. Environ. Sci. 2021, 42, 5535–5544. [Google Scholar]
- Li, J.; Lin, Q.; Xu, S.H. Desorption and Migration Characteristics of Cu/Cd Composite Contaminated Soil Under Different pH/Ionic Strength. Acta Pedol. Sin. 2023, 60, 1026–1034. [Google Scholar]
- Tao, L.; Zhang, N.M. Growth Response of Three Leafy Vegetables to Cd Pollution and Their Cd Accumulation Characteristics. Chin. Agric. Sci. Bull. 2018, 34, 99–106. [Google Scholar]
- Sun, J.; Zhang, Y.C.; Mao, H.P.; Wu, X.H.; Chen, Y.; Weng, Q.P. Responses Analysis of Lettuce Leaf Pollution in Cadmium Stress Based on Computer Vision. Trans. Chin. Soc. Agric. Mach. 2018, 49, 166–172. [Google Scholar]
- Hu, W.; Cheng, J.Z.; Gao, W.C.; Tu, C.L.; Tang, Y. Remediation of Contaminated Soil in Pb-Zn Mining Area by Raw and Modified Tobacco Stem-derived Biochar. Earth Environ. 2024, 52, 144–153. [Google Scholar]
- Sun, Y.L.; Zhao, J.W.; Liu, X.S.; Li, S.Y.; Ma, C.H.; Wang, X.Z.; Zhang, Q.B. Effect of nitrogen application on photosynthetic daily variation, leaf morphology and dry matter yield of alfalfa at the early flowering growth stage. Acta Prataculturae Sin. 2022, 31, 63–75. [Google Scholar]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.M.; Mine, A.; et al. Balancing trade-offs between biotic and abiotic stress responses through leaf age-dependent variation in stress hormone cross-talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.T.; Wang, X.L.; Ren, Z.S.; Zheng, J.Y. Microscopic Morphology and Surface Features of Biochars Derived from Different Raw Materials. Environ. Sci. Technol. 2017, 40, 42–48. [Google Scholar]
- Hou, J.W.; Xing, C.F.; Deng, X.M.; Chen, F.; Liu, M.; Wang, Z.H.; Li, T.J.; Hu, Y.L. Physical and Chemical Properties of Different Straw Biochars. Chin. J. Soil Sci. 2020, 51, 130–135. [Google Scholar]
- Ma, K.Y.; Zhang, H.; Song, N.N.; Wang, F.L.; Lin, D.S. Mechanism of cadmium adsorption by oxidative aging corn straw biochar. J. Agro-Environ. Sci. 2022, 41, 1230–1240. [Google Scholar]
- Xin, X.; Bao, L.C.; Li, Z.Z. Transformation, morphology, and dissolution of silicon and carbon in rice straw-derived biochars under different pyrolytic temperatures. Environ. Sci. Technol. 2014, 48, 3411–3419. [Google Scholar]
- Rechberger, M.V.; Kloss, S.; Wang, S.L.; Lehmann, J.; Rennhofer, H.; Ottner, F.; Wriessnig, K.; Daudin, G.; Lichtenegger, H.; Soja, G.; et al. Enhanced Cu and Cd sorption after soil aging of woodchip-derived biochar: What were the driving factors? Chemosphere 2019, 216, 463–471. [Google Scholar] [CrossRef]
- Cao, Q.Q.; Song, M.; Meng, F.Y.; Zhao, Y.; Song, B. Remediation of cadmium contaminated soils by FeAl-LDHs/biochar and its mechanism. Ecol. Environ. Sci. 2020, 29, 834–841. [Google Scholar]
- He, J.W.; He, C.X.; Guo, H.Y.; Ming, L. Adsorption of methylene blue by five straw biochars and its performance comparison. J. Nanjing Agric. Univ. 2019, 42, 382–388. [Google Scholar]
- Yuan, J.D.; Wang, C.J.; Tang, Z.T.; Chu, T.Z.; Zheng, C.; Han, Q.R.; Chen, H.L.; Tan, Y.Z. Biochar derived from traditional Chinese medicine residues: An efficient adsorbent for heavy metal Pb(II). Arab. J. Chem. 2024, 17, 105606. [Google Scholar] [CrossRef]
- Ran, T.S.; Long, J.; Liao, H.K.; Li, J.; Yang, G.M.; Zhao, Y.X. Effects of Biochar Application on Physicochemical Properties and Bacterial Communities of Microplastic-contaminated Calcareous Soil. Environ. Sci. 2023, 44, 4507–4518. [Google Scholar]
- Muhammad, R.; Shafaqat, A.; Tahir, A.; Muhammad, A.; Muhammad, Z.R.; Muhammad, I.; Farhat, A.; Muhammad, F.Q.; Rab, N. Residual effects of biochar on growth, photosynthesis and cadmium uptake in rice (Oryza sativa L.) under Cd stress with different water conditions. J. Environ. Manag. 2018, 206, 676–683. [Google Scholar]
- Zheng, X.D.; Li, X.; Singh, B.P.; Wei, L.; Huang, L.X.; Huang, Y.F.; Huang, Q.; Chen, X.B.; Su, Y.R.; Liu, Z.Z.; et al. Biochar protects hydrophilic dissolved organic matter against mineralization and enhances its microbial carbon use efficiency. Sci. Total Environ. 2021, 795, 148793. [Google Scholar] [CrossRef]
- Zhang, C.S.; Lin, Y.; Tian, X.Y.; Xu, Q.; Chen, Z.H.; Lin, W. Tobacco bacterial wilt suppression with biochar soil addition associates to improved soil physiochemical properties and increased rhizosphere bacteria abundance. Appl. Soil Ecol. 2016, 112, 90–96. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black Carbon Increases Cation Exchange Capacity in Soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Liu, S.D.; Han, Y.G.; Zhu, X.P.; Wu, X.N. Effects of Cotton Stalk Biochar on the Structure and Function of Fungi Community in Alkaline Rhizosphere Soil of Rice Under Cadmium Pollution. Environ. Sci. 2020, 41, 3846–3854. [Google Scholar]
- Nie, X.X.; Zhang, Z.Y.; Huang, Y.H.; Feng, J.Y.; Zhang, Z.Y.; Yang, L. Effects of simultaneous biochar and nitrogen fertilizer application on the photosynthesis and Cd uptake of sorghum. J. Agro-Environ. Sci. 2019, 38, 2749–2756. [Google Scholar]
- Cheng, Z.; Cai, X.F.; Zhao, S. Effect of biochar with different particle size on remediation of soil heavy metal pollution in native zinc smelting area in northwest of Guizhou province. J. Guizhou Norm. Univ. 2023, 41, 21–28. [Google Scholar]
- Yang, W.W.; Zhang, C.L.; Cao, M.Z.; Yan, J.N.; Qin, X.; Liang, D.G. Immobilization and remediation of cadmium-contaminated soil with four kinds of biochars. J. Soil Water Conserv. 2015, 29, 239–243. [Google Scholar]
- Luo, Y.; Liang, Y.X.; Yang, K.D.; Wang, F.; Yang, X.L. Preparation of Two Different Straw Carbons and their Passivation Effect on Cd in Cd-contaminated Yellow Soil. Guizhou Agric. Sci. 2020, 48, 29–33. [Google Scholar]
- Chi, T.; Zuo, J.; Liu, F. Performance and mechanism for cadmium and lead adsorption from water and soil by corn straw biochar. Front. Environ. Sci. Eng. 2017, 11, 15. [Google Scholar] [CrossRef]
- Yu, C.; Wang, Y.Y.; Liu, Q.Z.; Wang, Y.; Shen, H.; Feng, Y. Effects of application of biochar from different raw materials combined with inorganic amendments on cadmium accumulation in pakchoi shoots and soil cadmium inactivation. Acta Agric. Zhejiangensis 2024, 36, 613–621. [Google Scholar]
- Luo, Y.; Zhang, G.L.; Wang, F.; Xiang, Y.Z.; Ren, J. Effect of Chili Straw Biochar on Chemical Characteristics of Yellow Soil and Growth of Chinese Cabbage. J. Sichuan Agric. Univ. 2022, 40, 847–852. [Google Scholar]
- Al, Y.M.; Wang, Y.; Li, J.Q.; Zhang, C.Y.; Xu, X.Y.; Feng, J.P.; Zhou, J.H. Effects of Soil Microbial Activity and Cu, Cd, Pb Enrichment of Vetiveria zizanioides to Biochar Addition in Contaminated Soil in Copper Mining Areas. J. Soil Water Conserv. 2022, 36, 402–409. [Google Scholar]
- Kang, Z.Y.; Han, M.W.; Zhou, M.W.; Li, L.; Yan, S.Y. Representative analysis of the average value of petroleum exploration and development data volume. J. Geol. 2022, 46, 136–141. [Google Scholar]
- Egene, C.E.; Poucke, R.V.; Ok, Y.S.; Meers, E.; Tack, F.M.G. Impact of organic amendments (biochar, compost and peat) on Cd and Zn mobility and solubility in contaminated soil of the Campine region after three years. Sci. Total Environ. 2018, 626, 195–202. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Zhao, L.; Mei, Y.Y.; Li, F.Y.; Cao, X.D. Release of nutrients and heavy metals from biochar-amended soil under environmentally relevant conditions. Environ. Sci. Pollut. Res. Int. 2018, 25, 2517–2527. [Google Scholar] [CrossRef]

| Biochar Materials | Specific Surface Area (m2/g) | Total Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| Corn straw biochar | 20.087 | 0.023 | 4.565 |
| Wheat straw biochar | 17.969 | 0.051 | 10.962 |
| Rice straw biochar | 1.042 | 0.005 | 19.670 |
| Treatment | Biochar | Biochar Addition Levels (%) | pH | EC (μs/cm) | CEC (cmol/kg) |
|---|---|---|---|---|---|
| CK | / | / | 6.47 ± 0.03 h | 1138.67 ± 16.86 f | 16.98 ± 0.24 i |
| 1 | Corn straw biochar | 1 | 6.57 ± 0.02 efg | 1184.33 ± 11.06 e | 18.04 ± 0.19 g |
| 2 | 3 | 6.66 ± 0.04 cd | 1244.33 ± 11.68 d | 20.23 ± 0.17 d | |
| 3 | 5 | 6.78 ± 0.05 a | 1457.00 ± 11.00 a | 22.66 ± 0.11 a | |
| 4 | Rice straw biochar | 1 | 6.52 ± 0.01 gh | 1165.00 ± 17.52 ef | 17.51 ± 0.13 h |
| 5 | 3 | 6.58 ± 0.04 ef | 1219.67 ± 16.17 d | 19.23 ± 0.07 f | |
| 6 | 5 | 6.68 ± 0.03 bc | 1341.67 ± 14.50 c | 21.24 ± 0.08 c | |
| 7 | Wheat straw biochar | 1 | 6.54 ± 0.02 fg | 1182.33 ± 10.50 e | 17.73 ± 0.11 h |
| 8 | 3 | 6.62 ± 0.03 de | 1229.67 ± 23.54 d | 19.56 ± 0.17 e | |
| 9 | 5 | 6.73 ± 0.01 ab | 1374.33 ± 17.04 b | 21.74 ± 0.10 b |
| Treatment | Biochar | Biochar Addition Levels (%) | Plant Height (cm) | Fresh Weight (g) |
|---|---|---|---|---|
| CK | / | / | 25.43 ± 0.75 g | 39.86 ± 2.36 d |
| 1 | Corn straw biochar | 1 | 27.77 ± 0.50 ef | 44.47 ± 0.75 c |
| 2 | 3 | 29.60 ± 0.26 cd | 47.50 ± 1.10 b | |
| 3 | 5 | 31.93 ± 1.15 a | 52.34 ± 0.94 a | |
| 4 | Rice straw biochar | 1 | 26.97 ± 0.40 f | 44.23 ± 1.12 c |
| 5 | 3 | 28.73 ± 0.65 de | 46.45 ± 0.74 bc | |
| 6 | 5 | 30.53 ± 0.70 bc | 50.40 ± 1.41 a | |
| 7 | Wheat straw biochar | 1 | 27.13 ± 0.70 f | 44.35 ± 0.62 c |
| 8 | 3 | 28.97 ± 0.60 d | 46.25 ± 0.88 bc | |
| 9 | 5 | 30.83 ± 0.45 ab | 50.39 ± 1.64 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Zhou, L.; Wu, H.; Li, J.; Kong, L.; Yang, H. Effects of Applying Biochar on Soil Cadmium Immobilisation and Cadmium Pollution Control in Lettuce (Lactuca sativa L.). Agriculture 2024, 14, 1068. https://doi.org/10.3390/agriculture14071068
Zhou L, Zhou L, Wu H, Li J, Kong L, Yang H. Effects of Applying Biochar on Soil Cadmium Immobilisation and Cadmium Pollution Control in Lettuce (Lactuca sativa L.). Agriculture. 2024; 14(7):1068. https://doi.org/10.3390/agriculture14071068
Chicago/Turabian StyleZhou, Lina, Leijinyu Zhou, Hongbo Wu, Jinsheng Li, Lijuan Kong, and Haoyu Yang. 2024. "Effects of Applying Biochar on Soil Cadmium Immobilisation and Cadmium Pollution Control in Lettuce (Lactuca sativa L.)" Agriculture 14, no. 7: 1068. https://doi.org/10.3390/agriculture14071068





