Abstract
Climate change is affecting all regions of the world, and the Mediterranean region is one of the most affected. Plants accumulate secondary metabolites as an adaptive response to stress circumstances. The present study investigated the effect of different abiotic factor conditions (drought, moderate heat, severe heat, salinity, and UV-B radiation) on the essential oil (EO) yield, composition (volatile profile), and biological activity (enzyme inhibition and antioxidant activity) of Lavandula viridis L’Hér. In general, the environmental conditions increased the extraction yield of EO. Eighty-two compounds were identified in the EO and environmental factors induced some quantitative changes in EO composition. Severe heat and salinity conditions increased the concentration of the two most abundant compounds, 1,8-cineole and camphor. Severe heat also increased the potential of EO to inhibit the enzymes butyrylcholinesterase and tyrosinase. Drought, salinity, and UV-B radiation promoted the ability of EO to inhibit acetylcholinesterase. In addition, heat and drought enhanced the antioxidant activity of EO. These results are relevant for exploring the potential of this EO for industrial applications, although future studies combining the factors studied are important to understand the influence of synergistic effects on the composition and bioactivity of the plant products obtained.
1. Introduction
Climate change is the most significant global environmental challenge of this century, with certain regions being more vulnerable to its impact. According to the Intergovernmental Panel on Climate Change (IPCC), a rise in average temperatures and a shift in precipitation patterns are expected, leading to a decrease in soil water availability and an increase in drought and soil salinity [1]. These environmental conditions can have negative effects on plant growth and development, and plants must acquire strategies to cope with these factors. One of the main defense adaptation strategies is the change in the accumulation of secondary metabolites [2], with terpenes, phenolic compounds, and nitrogen-containing compounds being the three main groups [3].
Due to the geographical location of the Mediterranean region, namely between North Africa (with an arid climate) and Central Europe (with a temperate and rainy climate), this basin is particularly vulnerable to changes in climate. Mediterranean-type regions, characterized by warm or hot summers with little or no rainfall (drought summers) and mild or cold winters [4], represent ideal ecosystems for the production of lavender species, which are essentially characterized by their antiseptic and relaxing properties [5]. In addition, Lavandula species have other biological properties, such as antimicrobial, neurological, anti-diabetic, anti-parasitic, analgesic, among others [6]. Specifically, Lavandula viridis L’Hér is a Mediterranean aromatic species endemic to the Iberian Peninsula [7], present mainly in natural populations, whose existence has been affected in recent years due to climatic changes [8]. This plant produces high-value bioactive phytochemicals such as volatile (essential oil, EO) and phenolic compounds with antioxidant, antifungal, antibacterial, nematocidal, anti-protozoal, and enzyme inhibitory properties [7,9,10,11,12,13,14,15,16,17,18,19].
Lavander EO is traditionally and industrially used in aromatherapy and is a valuable raw material for the food industry (as flavors), cosmetics, and perfumery sectors, especially the EO from Lavandula angustifolia Mill. [20]. Due to the growing market demand and widespread use of L. angustifolia EO (species distributed worldwide), regulations and standards have been established to maintain the highest quality and safety [21,22]. Lavenders from the Iberian Peninsula are somewhat underexplored, resulting in low recognition in global markets. L. viridis EO is very different from those of other lavender species due to its lemon scent [20]. In recent years, its industrial exploitation has increased and it is currently sold in different countries such as Portugal, Spain, and Brazil [23,24,25,26,27]. The main constituent of L. viridis EO is the monoterpene 1,8-cineole, also known as eucalyptol [20,28]. This volatile component is often added to cosmetic or fragrance products or used as a food flavoring agent due to its pleasant taste and aroma and its bioactivity. In addition, 1,8-cineole has several pharmacological properties, such as antioxidant and anti-inflammatory, mainly through the regulation of the nuclear factor erythroid-2-related factor 2 (Nrf2) and nuclear factor-kappa B (NF-jB) pathways, respectively, and has entered clinical trials for the treatment of several diseases, such as cardiovascular, digestive, respiratory, pulmonary, and neurological diseases [29].
The applications of EOs are highly dependent on their quantity (yield) and quality, which are not constant as they are affected by various factors, including environmental conditions. For this reason, it is essential to monitor the changes in EO components in order to conclude the potential applications expected depending on the prevailing factors of the climate change scenarios where the plant is growing. This study was carried out to evaluate the effect of drought, heat, salinity, and UV-B radiation on the yield and chemical profile of EO, as well as on its antioxidant capacity and capability to inhibit enzymes (acetylcholinesterase, AChE; butyrylcholinesterase, BChE; and tyrosinase, Tyr) associated with neurodegenerative disorders.
2. Materials and Methods
2.1. Plant Material and Environmental Tests
In order to obtain uniform plants, micropropagated plants of Lavandula viridis L’Hér, produced according to Mansinhos et al. [7] and fully acclimatized to ex vitro conditions, were used in the experiments. The environmental experiments were carried out in a growth chamber (750 E, Aralab, Lisbon, Portugal) supplied with four Osram L 18 W/840 lamps (16/8 h, light/dark). Control plants were watered every two days (100 mL/plot) for two weeks and maintained at 25/18 °C (16/8 h, light/dark). Plants exposed to drought (25/18 °C) were watered every two days for one week and one week without watering. For heat experiments, plants were kept at 30/23 °C (moderate heat) or 35/28 °C (severe heat) for two weeks and watered as in the control treatment. Plants exposed to salt conditions (25/18 °C) were watered with 50 mM of NaCl every two days for two weeks. Finally, for the UV-B experiment, the four lamps were replaced by UV-B lamps (Philips TL 20 W/12 RS SLV/25) and the plants were kept at 25/18 °C for two weeks.
2.2. Essential Oils
2.2.1. Extraction of EOs
Dried plant material of L. viridis (50 g) from the different environmental experiments was subjected to hydrodistillation (3 h) with distilled water using a Clevenger-type apparatus. The EO obtained was dried with anhydrous sodium, transferred to a vial, and stored at 4 °C in a sealed tube until it was used for chemical and biological analysis. Yields were calculated based on the dry weight of the plant material.
2.2.2. Gas Chromatography–Mass Spectrometry (GC-MS) Analysis
The EO was analyzed using a CP-3800 gas chromatograph (Varian, Inc., Walnut Creek, CA, USA) equipped with an automated sampler (COMBI PAL) and a 4000 MS mass spectrometer (Varian, Inc., Walnut Creek, CA, USA). Gas chromatography–mass spectrometry analyses were performed on a non-polar DB-5MS fused-silica capillary column (60 m × 0.25 mm × 1 μm). The carrier gas was helium, supplied at a constant flow rate of 1 mL/min, and the samples were injected (1 μL) at a split ratio of 1:50. The oven temperature was set at 40 °C for 2 min, increased at 3 °C /min to 250 °C, and then 5 °C/min to 300 °C [11]. Data were acquired using MS Data Review (Varian, Inc., Walnut Creek, CA, USA). The chromatograms were then analyzed using Xcalibur™ software, version 3.0 (Thermo Fisher Scientific; Waltham, MA, USA). Retention indices were determined experimentally using n-alkanes (C8-C20 and C21-C40). The compounds in the samples were identified by matching their retention indices and mass spectra with those in the National Institute of Standards and Technology (NIST) mass spectra library and with the literature [11,16,20,28,30,31].
2.2.3. Antioxidant Capacity
The antioxidant capacity of the EOs was evaluated following the Mansinhos et al. study [32], using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) and 2,2-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) methods, with slight modifications. In both methods, the EOs were dissolved in 86% ethanol. For DPPH scavenging, 30 μL of diluted EO was added to 300 μL of DPPH solution (90 μM) and mixed with 570 μL of 80% methanol. After 30 min at room temperature, the absorbance was read at 515 nm. For the ABTS method, the stock solution of ABTS (7 mM) was prepared 12–16 h before use, using potassium persulfate (2.45 mM). After obtaining an absorbance of 0.700 ± 0.02 (734 nm) with the reagent test, 10 μL of diluted EO was added to 190 μL of the reagent test and the absorbance was measured at 734 nm. The results of both antioxidant tests were expressed as micrograms of Trolox equivalents per gram of EO (μgTE/gEO).
2.2.4. Enzymes’ (Tyrosinase and Cholinesterases) Inhibitory Capacity
The evaluation of Tyr, AChE, and BChE’s inhibitory activities was performed according to Gonçalves et al. [12] with slight modifications. For Tyr, EOs were dissolved in 86% ethanol and mixed with 20 mM of sodium phosphate buffer (pH 6.8) and Tyr solution (prepared in buffer). L-DOPA (2.5 mM) was added after 10 min and the absorbance was read at 475 nm 10 min later. Kojic acid was used as a positive control. For cholinesterases, EOs were dissolved in 20% ethanol and mixed with DTNB solution (3 mM), acetylthiocholine iodide (15 mM, for AChE) or S-butyrylthiocholine (15 mM, for BChE), and phosphate buffer. AChE or BChE (0.28 U/mL) was added, and the absorbance read at 405 nm. Galantamine was used as a positive control. All results were expressed as IC50 (μg/mL).
2.3. Statistical Analysis
Data are expressed as the mean ± standard error (SE) and analyzed by one-way analysis of variance (ANOVA) and Duncan’s New Multiple Range Test (p < 0.05). Pearson correlation (p < 0.01) was performed using IBM SPSS Statistics for Windows (version 26.0, IBM Corporation, Armonk, NY, USA). Principal component analysis (PCA) was performed using OriginPro software, version 2022 (OriginLab Corporation, Northampton, MA, USA).
3. Results and Discussion
3.1. EO Extraction Yield and Chemical Profile
Figure 1 presents the yields of L. viridis EO, expressed as the weight of EO in relation to the weight of the starting plant material. The extraction yield of the control plants was higher compared to that obtained in other studies with L. viridis [11,20,28], in vitro shoot cultures, or micropropagated plants [28]. When comparing the results of the various environmental treatments, it was found that the highest yield was obtained in the UV-B treatment (2.75% w/w), followed by moderate and severe heat treatments (10% less) and salinity treatment (13% less).
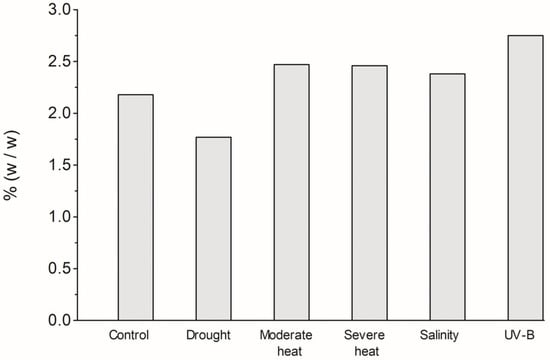
Figure 1.
Extraction yield (%, w/w) of Lavandula viridis L’Hér essential oils isolated from plants subjected to different environmental conditions.
In Manukyan’s [33] investigation, intensive UV-B radiation had a positive effect on the total EO yield from Nepeta cataria L., Melissa officinalis L., and Salvia officinalis L. of the Lamiaceae family. Additionally, the oil yield of Ocimum sanctum L. increased significantly by 42% after UV-B treatment [34]. In 2019, the same author exposed Thymus transcaucasicus Ronn. to different temperatures (15, 20, and 25 °C) and found that the highest EO yield was achieved at the highest temperature [35]. Other authors have reported an increase in extraction yield after exposing plants of different Lamiaceae genera to salinity, including Ocimum basilicum L. [36,37], Origanum majorana L. [38], and Rosmarinus officinalis L. [39], which is consistent with our results. In the present study, the lowest extraction yield was obtained when plants were subjected to drought. This resulted in a 19% reduction compared to the control and a 36% reduction compared to the UV-B experiment. Similarly, in Thymus vulgaris L. (Lamiaceae), the EO yield also decreased with drought stress [40]. However, in Sideritis perfoliata L. (Lamiaceae) and Melissa officinalis L., drought had a favorable impact on EO yield.
Lavandula spp. are aromatic shrubs commonly used in alternative medicine and in the cosmetic industry for the production of fragrances. This study analyzed the chemical profile of L. viridis EO obtained from both control plants and plants exposed to different abiotic factors (Table 1). To the best of our knowledge, this is the first study to investigate the effects of abiotic factors on the EO of this species. The EO appeared yellow in all treatments, with no discernible differences in color.

Table 1.
Chemical composition (relative %) of the Lavandula viridis L’Hér essential oils isolated from plants subjected to different environmental conditions.
Numerous factors such as the genotype, plant phenological stage of the plant, botanical organ, geographical location, environmental conditions, propagation method, harvesting time, processing of the plant material, nature (fresh or dried), and extraction techniques influence the quality and chemical composition of EOs [41,42]. Table 1 shows that 82 compounds were identified in the L. viridis EO. Some of these compounds were identified for the first time in this species [11,16,20,28,30,31]. Significant differences in the concentration of volatile compounds were observed after subjecting plants to different environmental stresses. The monoterpene fraction was predominant in all six EOs studied, ranging from 78.88 to 84.54%. The main components were oxygen-containing monoterpenes, which accounted for 63.90 to 72.05%. The oxygenated monoterpenes 1,8-cineole (eucalyptol) (21.56–28.98%) and camphor (10.74–13.21%) were the primary components of all L. viridis EOs, consistent with previous studies on this species [11,16,20,28,30,31]. The valuable scent of lavender is attributed to the sensory characteristics of oxygenated terpenes [41]. A study conducted by Xiao et al. [43] used gas chromatography–olfactometry (GC-O) and descriptive sensory analysis to identify the characteristic aroma components of five different lavender EOs. The study found that woody and camphor odors were related to two specific compounds. Camphor was identified as one of the characteristic aroma components of the lavender EOs, conferring “musty, penetrating, slightly minty notes” [41]. According to Aprotosoaie et al. [41], EOs from stems and leaves have a high content of 1,8-cineole and camphor, which results in a harsher note and consequently reduces the quality of true lavender flower EO. In fact, the most valued lavender EOs in the perfumery and cosmetics are those with a high concentration of linalool (and its esters) and a low concentration of camphor. On the other hand, EOs with high camphor content are mainly valued for their therapeutic properties and are used in aromatherapy and phytotherapy [42]. The levels of 1,8-cineole and camphor increased after exposure to severe heat and salinity conditions. Aćimović et al. [44] demonstrated a positive correlation between high temperature and the accumulation of 1,8-cineole, the main component of Nepeta nuda L. However, salinity, the most studied abiotic stress in the Lamiaceae family, has produced highly variable results. For instance, in Mentha x piperita L. [45], O. basilicum [46,47], R. officinalis [48,49], and S. officinalis [50], the concentration of 1,8-cineole decreased after the plants were exposed to salt stress. In contrast, this stress had a positive effect on the production of this compound in O. basilicum [51], S. officinalis [52], and Salvia mirzayanii Rech. [53,54]. Drought had a negative impact on 1,8-cineole production in L. viridis, which is consistent with different Lamiaceae species, such as R. officinalis [55], Salvia reuterana [56], and Lavandula angustifolia [57]. However, several authors have found that drought is an effective strategy for increasing the production of 1,8-cineole in other species [55,56,57,58,59,60,61]. In accordance with our results, salinity improved the concentration of camphor in other Lamiaceae species, such as R. officinalis [48] and S. officinalis [62].
As previously observed [11,16,20,28,30,31], α-pinene (monoterpene hydrocarbon) was the third most abundant compound in the control plants. The concentration of this compound, which has a major influence on the aroma of Lavandula officinalis L. [43], decreased in response to the environmental factors tested. Other authors found distinct results showing that UV-B radiation [33,63], drought [55], and salinity [62] positively influenced α-pinene production. Cis-verbenol was the fourth most abundant compound in control plants, with a concentration of 4.27 ± 0.10%. This concentration was maintained after stress exposure, with the exception of moderate heat, where a decrease was observed (3.45 ± 0.03%). At comparable levels, pinocarvone is also produced and its accumulation is significantly stimulated by all abiotic factors, especially by salinity (Table 1). In fact, the abiotic factors tested led to an improvement in the accumulation of 44% of the identified compounds compared to the control.
The sesquiterpene fraction, which ranged from 8.67 to 13.12%, was predominantly composed of oxygenated compounds (5.59–8.12%) (Table 1). Viridiflorol (identified for the first time in L. viridis) was the predominant oxygenated sesquiterpene (1.01–2.42%) present in the six EOs. However, its concentration decreased significantly after exposure to different abiotic treatments, especially salinity and severe heat. Viridiflorol concentration increased in response to drought conditions [58] but decreased in response to salt stress [52] in S. officinalis. The sesquiterpene hydrocarbons were found in small amounts (2.96–4.99%), with β-selinene being the main compound of this fraction. A significant decrease in the concentration of this compound was observed in plants exposed to severe heat and salinity.
In order to assess the impact of the five distinct environmental factors on the individual volatile components of L. viridis EOs, a biplot PCA was constructed utilizing the sample scores and the variable loadings derived from GC-MS results (Figure 2).
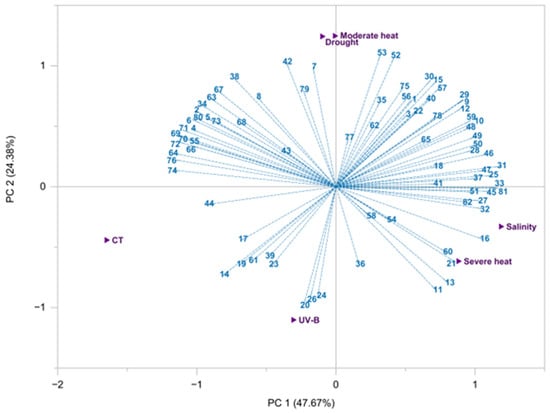
Figure 2.
Principal component analysis (PCA) biplot of the eighty-two compounds identified and quantified in the Lavandula viridis L’Hér essential oils (Table 1) isolated from plants subjected to different environmental conditions.
The first two PCs (PC1 and PC2) were found to account for 72.05% of the total variation within the dataset. The results indicate that moderate heat and drought exert a comparable influence on EOs’ composition, with the content of borneol, dehydro-1,8-cineole, myrtenyl acetate, eucarvone, 2-phenyl ethyl propanoate, and nerol acetate being particularly noteworthy. Furthermore, drought and moderate heat also exerted a significant influence on the monoterpene hydrocarbons (α-pinene, camphene, sabinene, β-pinene, δ-3-carene, p-cymene, γ-terpinene, tricyclene, myrcene, and p-cymenene), all of which were positioned at positive PC2 values (third and fourth quadrants). Similarly, salinity and severe heat (located at the first quadrant, positive PC1 and negative PC2) demonstrated a comparable impact on the EOs’ profiles, with the most representative compounds belonging to the oxygenated monoterpene family (pinocarvone, verbenone, terpinene-4-ol, cis-chrysanthenol, α-campholenal, 6-camphenol, trans-linalool oxide, (-)-carvone, cis-p-mentha-1(7),8-diene-2-ol, p-mentha-1,4-dien-7-ol, α-pinene oxide, p-cymen-7-ol, trans-carvone oxide, and p-mentha-1,8-dien-7-ol), including the two most abundant compounds (1,8-cineole and camphor). Conversely, control and UV-B, despite being in the same quadrant, exhibited a notable disparity in their chemical response. Abiotic factors exhibited a minimal impact on the majority of the sesquiterpenes (both hydrocarbons and oxygenated compounds), which were primarily situated in the third quadrant (negative PC1; positive PC2). UV-B was the treatment that exerted the least influence on the chemical profile of EO L. viridis.
3.2. Antioxidant Potential of EOs
Due to the abundance of highly bioactive chemicals in EOs, including monoterpenes and sesquiterpenes, they have been increasingly used as natural antioxidants and antibacterial agents [64]. In this study, the antioxidant activity of L. viridis EOs derived from plants subjected to five distinct environmental factors was evaluated using two distinct analytical methodologies (DPPH and ABTS) (Figure 3).
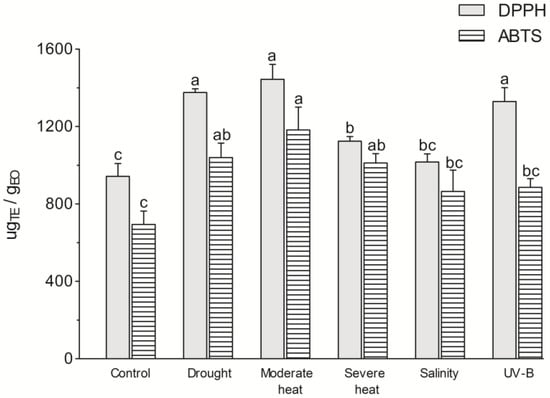
Figure 3.
Antioxidant activity (DPPH, 2,2-diphenyl-1-picrylhydrazyl; ABTS, 2,2-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) of Lavandula viridis L’Hér essential oils isolated from plants subjected to different environmental conditions. Values are presented as mean ± SE. Different letters in each method indicate significant differences (p < 0.05), (Duncan’s new multiple range test).
The results indicate that EOs from plants grown under control conditions exhibited a higher potential to scavenge the DPPH radical (943.23 ± 65.82 μgTE/gEO) than the radical ABTS (693.92 ± 69.22 μgTE/gEO). The results were different for EOs from other Lavandula species, such as Lavandula coronopifolia Poir. [65], L. angustifolia, Lavandula latifolia Medik., and Lavandula hybrida L. [66], that displayed a higher anti-ABTS activity. The L. viridis EO demonstrated superior performance in scavenging DPPH radicals in comparison to L. coronopifolia [65], L. angustifolia, L. latifolia, and L. hybrida EOs [66] and ABTS radicals in comparison to L. latifolia and L. hybrida EOs [66].
Overall, EOs from L. viridis plants exposed to abiotic factors exhibited higher antioxidant potential than the EO from control plants (Figure 3). This is not surprising since EOs are rich in compounds with antioxidant potential, which play an important role in combating abiotic stress in plants. The highest results in both DPPH (1444.37 ± 77.34 μgTE/gEO) and ABTS (1181.96 ± 118.86 μgTE/gEO) assays were observed in the EO from plants subjected to moderate heat, with a 41 and 34% increase in the capacity to scavenge ABTS and DPPH free radicals, respectively, compared to the control. Furthermore, drought, severe heat, and UV-B radiation also enhanced the antioxidant capacity of the EO.
The antioxidant capacity of plant extracts is typically higher than that of EOs, mainly due to the chemical structure and redox properties of the phenolic compounds usually present in the extracts, which are essential for neutralizing reactive oxygen species (ROS), such as free radicals [60]. This is consistent with our findings, in which L. viridis extracts rich in phenolic compounds exhibited a greater antioxidant activity (approximately 70–180 mgTE/gextract) [7] than the EOs evaluated in this study (approximately 0.7–1.4 mgTE/gEO). Only one study was identified in the literature that examined the influence of abiotic factors on the antioxidant activity of Lamiaceae EOs. In contrast to our findings, the aforementioned study demonstrated that the antioxidant capacity of Thymus daenensis Celak EO was higher under normal irrigation conditions than under drought [60]. Interestingly, salinity, despite being a favorable stimulus for enhancing the production of the main compounds of L. viridis EO (1,8-cineole and camphor), did not demonstrate a pronounced effect on the antioxidant capacity. These findings may indicate the potential involvement of minor components of the EO in the antioxidant activity. Pearson’s correlation (Figure 4) indicates that borneol (identified in a range of 2.31–2.76%) was the compound that most contributed to ABTS scavenging (0.745, p ≤ 0.01), followed by p-cymenene (0.643, p ≤ 0.05) (0.05–0.09%), 2-phenyl ethyl propanoate (0.622, p ≤ 0.05) (0.13–0.18%), p-cymen-8-ol (0.613, p ≤ 0.05) (0.17–0.31%), humulane-1,6-dien-3-ol (0.579, p ≤ 0.05) (0.15–0.18%), and germacrene D (0.578, p ≤ 0.05) (0.15–0.18%). Eugenol (0.637, p ≤ 0.05) (0.36–0.49%) and myrtenol (0.599, p ≤ 0.05) (1.08–1.25%) were the most important components responsible for the DPPH scavenging capacity. Moreover, numerous other compounds demonstrate a significant or moderate correlation with ABTS and DPPH scavenging potential. This suggests that the antioxidant efficacy of the investigated EOs may depend on the collective action of multiple compounds, rather than a single component.
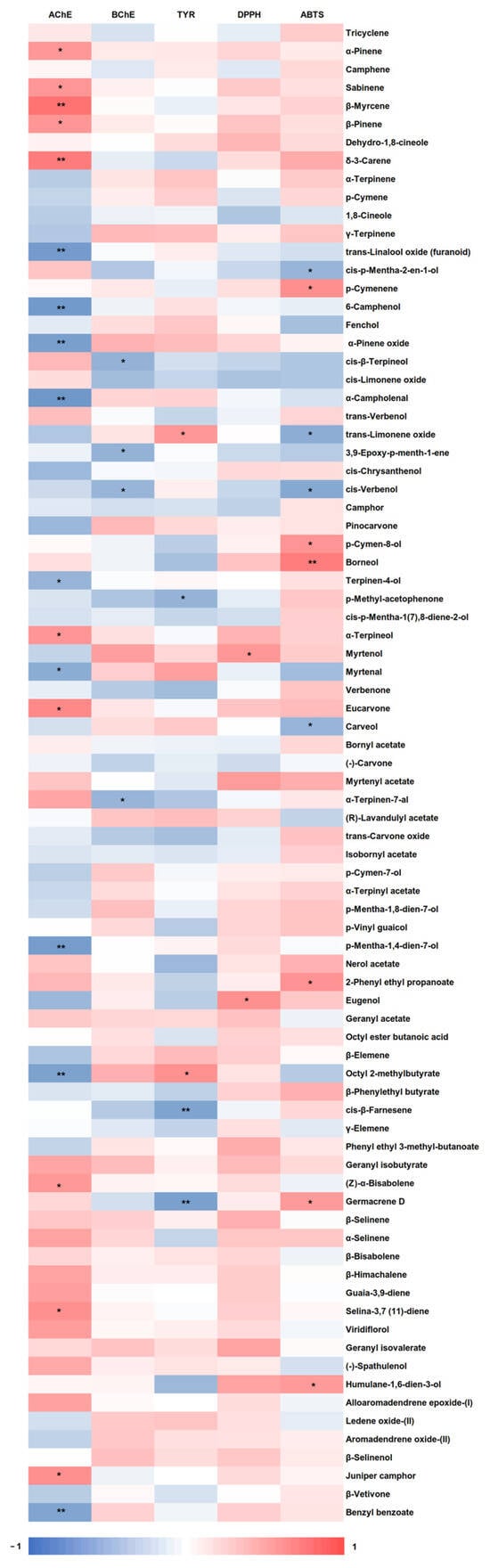
Figure 4.
Heatmap representing the Pearson’s correlation coefficients between the 82 compounds identified in the Lavandula viridis L’Hér essential oils and the inhibition of three enzymes involved in neurodegenerative diseases (AChE, BChE, and Tyr), as well as antioxidant activity (DPPH and ABTS). Correlation is significant at p ≤ 0.01 (**) and p ≤ 0.05 (*).
3.3. Enzyme Inhibitory Assays
The ability of L. viridis EOs to inhibit the activity of three enzymes (AChE, BChE, and Tyr) is shown in Table 2. This is the first report on the ability of L. viridis EOs to inhibit Tyr. Furthermore, to the best of our knowledge, this is also the first study reporting the influence of abiotic factors on the inhibitory activities of EOs against the three enzymes. In vitro inhibition assays of AChE and BChE, which are responsible for the hydrolysis of the neurotransmitter acetylcholine, represent a valuable approach to identify new inhibitors of these enzymes from natural sources with potential applications in the treatment of Alzheimer’s disease [67]. In humans, excessive melanin production can cause hyperpigmentation pathologies, such as melanoma [68], as well as neurodegenerative processes leading to Parkinson’s disease [69]. Consequently, the identification of novel Tyr inhibitors may facilitate the development of novel cosmetic products and innovative therapeutic strategies for the treatment of Parkinson’s disease and cancer. The neuroprotective effects of EOs derived from Lamiaceae species and their components have been documented [70,71]. Furthermore, EOs have been employed in aromatherapy to mitigate the symptoms of dementia and improve memory and cognition in patients [72]. The present study demonstrated that L. viridis EOs exhibited greater efficacy in inhibiting AChE than BChE and Tyr (Table 2).

Table 2.
Acetylcholinesterase (AChE), butyrylcholinesterase (BChE), and tyrosinase (Tyr) inhibitory activities of the Lavandula viridis L’Hér essential oils isolated from plants subjected to different environmental conditions.
EOs derived from plants exposed to drought, salinity, and UV-B radiation showed superior inhibitory activity against AChE compared to the control EO. The most effective result was observed for UV-B radiation (338.80 ± 9.11 μg/mL), which caused a twofold higher inhibition compared to the control (781.33 ± 31.70 μg/mL). Furthermore, the UV-B result was superior to that reported by Costa et al. [11], in which the EO of L. viridis exhibited an IC50 of 411.33 ± 72.73 μg/mL. Furthermore, the L. viridis EOs showed greater AChE inhibitory activity than that shown by other Lavandula species, including L. angustifolia [64]. The highest inhibition results of BChE and Tyr were observed when severe heat was applied (IC50 = 2250.22 ± 205.42 μg/mL for BChE and IC50 = 3551.01 ± 315.98 μg/mL for Tyr). Similarly to our results, L. angustifolia EOs also exhibited a low anti-tyrosinase activity [64,73], with Lavandula stoechas L. EO [74] being more effective in inhibiting this enzyme.
The results presented in the heatmap (Figure 4) indicate that α-campholenal was the compound with the strongest correlation with AChE inhibition (−0.813, p ≤ 0.01), followed by 6-camphenol (−0.799, p ≤ 0.01), trans-linalool oxide (−0.787, p ≤ 0.01), p-mentha-1,4-dien-7-ol (−0.779, p ≤ 0.01), α-pinene oxide (−0.759, p ≤ 0.01), octyl 2-methylbutyrate (−0.731, p ≤ 0.01), benzyl benzoate (−0.711, p ≤ 0.01), myrtenal (−0.656, p ≤ 0.05), and terpinen-4-ol (−0.589, p ≤ 0.05). The literature indicates that benzyl benzoate has the potential to reduce the activity of AChE in Haemaphysalis longicornis [75]. Myrtenal has been shown to possess neuroprotective properties [76], while terpinene-4-ol has demonstrated a high binding affinity towards the active binding site of AChE in a molecular docking study [77]. The compounds with the strongest correlations with BChE were cis-β-terpineol (−0.610, p ≤ 0.05), 3,9-epoxy-p-menth-1-ene (−0.607, p ≤ 0.05), α-terpinen-7-al (−0.583, p ≤ 0.05), and cis-verbenol (−0.582, p ≤ 0.05). Furthermore, the results demonstrated that germacrene D (−0.751, p ≤ 0.01), cis-β-farnesene (−0.722, p ≤ 0.01), and ρ-methyl-acetophenone (−0.597, p ≤ 0.05) exhibited a high degree of correlation with Tyr inhibition. Recently, Tran-Trung et al. [78] demonstrated that germacrene D interacts with the residue His381 within the active site of the Tyr enzyme, suggesting that this compound may be a potential candidate for Tyr inhibitors. Similarly, all these correlated compounds are present in L. viridis EOs in lower concentrations (0.05–4.07%), indicating that synergism may also be related to the potential of EOs to inhibit different enzymes.
4. Conclusions
This is the first report studying the effect of abiotic factors on the EOs’ composition of L. viridis. In general, the environmental conditions tested resulted in enhanced EO extraction yield and caused some quantitative changes in the chemical composition. The oxygenated monoterpenes were the most abundant components of the essential oils studied, with 1,8-cineole being the major compound. Furthermore, the application of heat and salinity resulted in an enhanced production of 1,8-cineole, which could be of significant interest to the cosmetic and biomedical industries in the context of climate change. Furthermore, the environmental conditions tested in this study demonstrated an increase in the biological properties of L. viridis EO, including its capacity to inhibit AChE, BChE, and Tyr, as well as its antioxidant activity. Nevertheless, since EOs are composed of numerous chemical compounds with diverse structures and modes of action, it is crucial to investigate the influence of the synergistic mechanisms on the composition and bioactivity of the plant products in future studies.
Author Contributions
I.M.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Software, Validation, Visualization, Roles/Writing—original draft. S.G.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Visualization, Funding acquisition, Project administration, Resources, Supervision, Writing—review and editing. R.R.-S.: Formal analysis, Investigation, Methodology, Software, Validation, Writing—review and editing. J.M.M.-R.: Validation, Funding acquisition, Project administration, Resources, Writing—review and editing. A.R.: Conceptualization, Investigation, Validation, Funding acquisition, Project administration, Resources, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Funds through FCT-Foundation for Science and Technology under the Projects UIDB/05183/2020 (https://doi.org/10.54499/UIDB/05183/2020) and LA/P/0121/2020 (https://doi.org/10.54499/LA/P/0121/2020). Inês Mansinhos (https://doi.org/10.54499/SFRH/BD/145243/2019) and Sandra Gonçalves (CEECINST/00052/2021) acknowledge the financial support from FCT. Raquel Rodríguez Solana was supported by the grant RYC2022-036888-I, funded by MCIU/AEI/10.13039/501100011033 and by the FSE+ as well as by a Juan de la Cierva—Incorporation contract from the Spanish Ministry of Science, Innovation, and Universities (IJC2018-036207-I).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Ballesteros, G.I.; Newsham, K.K.; Acuña-Rodríguez, I.S.; Atala, C.; Torres-Díaz, C.; Molina-Montenegro, M.A. Extreme Environments as Sources of Fungal Endophytes Mitigating Climate Change Impacts on Crops in Mediterranean-Type Ecosystems. Plants People Planet 2023, 6, 148–161. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Romano, A. How Climate Change-Related Abiotic Factors Affect the Production of Industrial Valuable Compounds in Lamiaceae Plant Species: A Review. Front. Plant Sci. 2024, 15, 1370810. [Google Scholar] [CrossRef]
- Verma, N.; Shukla, S. Impact of Various Factors Responsible for Fluctuation in Plant Secondary Metabolites. J. Appl. Res. Med. Aromat. Plants 2015, 2, 105–113. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C.M. Drought Stress Effects and Olive Tree Acclimation under a Changing Climate. Plants 2019, 8, 232. [Google Scholar] [CrossRef] [PubMed]
- Barut, M.; Tansı, L.S.; Karaman, S. Essential oil Composition of Lavender (Lavandula angustifolia Mill.) at Various Plantation Ages and Growth Stages in the Mediterranean Region. Turk. J. Agric.-Food Sci. Technol. 2022, 10, 746–753. [Google Scholar] [CrossRef]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A Review of the Bioactive Components and Pharmacological Properties of Lavandula Species. Naunyn-Schmiedebergs Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef] [PubMed]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Impact of Temperature on Phenolic and Osmolyte Contents in In Vitro Cultures and Micropropagated Plants of Two Mediterranean Plant Species, Lavandula viridis and Thymus lotocephalus. Plants 2022, 11, 3516. [Google Scholar] [CrossRef] [PubMed]
- Bussotti, F.; Pollastrini, M. Opportunities and Threats of Mediterranean Evergreen Sclerophyllous Woody Species Subjected to Extreme Drought Events. Appl. Sci. 2020, 10, 8458. [Google Scholar] [CrossRef]
- Costa, P.; Sarmento, B.; Gonçalves, S.; Romano, A. Protective Effects of Lavandula viridis L’Hér Extracts and Rosmarinic Acid against H2O2-Induced Oxidative Damage in A172 Human Astrocyte Cell Line. Ind. Crops Prod. 2013, 50, 361–365. [Google Scholar] [CrossRef]
- Costa, P.; Gonçalves, S.; Valentão, P.; Andrade, P.B.; Romano, A. Accumulation of Phenolic Compounds in in Vitro Cultures and Wild Plants of Lavandula viridis L’Hér and Their Antioxidant and Anti-Cholinesterase Potential. Food Chem. Toxicol. 2013, 57, 69–74. [Google Scholar] [CrossRef]
- Costa, P.; Grosso, C.; Gonçalves, S.; Andrade, P.B.; Valentão, P.; Gabriela Bernardo-Gil, M.; Romano, A. Supercritical Fluid Extraction and Hydrodistillation for the Recovery of Bioactive Compounds from Lavandula viridis L’Hér. Food Chem. 2012, 135, 112–121. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Rodríguez-Solana, R.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Romano, A. Impact of Metallic Nanoparticles on In Vitro Culture, Phenolic Profile and Biological Activity of Two Mediterranean Lamiaceae Species: Lavandula viridis L’Hér and Thymus lotocephalus G. López and R. Morales. Molecules 2021, 26, 6427. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical Composition and Antifungal Activity of the Essential oils of Lavandula viridis L’Hér. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef]
- Costa, P.; Grevenstuk, T.; Rosa da Costa, A.M.; Gonçalves, S.; Romano, A. Antioxidant and Anti-Cholinesterase Activities of Lavandula viridis L’Hér Extracts after in Vitro Gastrointestinal Digestion. Ind. Crops Prod. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Costa, P.; Medronho, B.; Gonçalves, S.; Romano, A. Cyclodextrins Enhance the Antioxidant Activity of Essential oils from Three Lamiaceae Species. Ind. Crops Prod. 2015, 70, 341–346. [Google Scholar] [CrossRef]
- Costa, S.; Cavadas, C.; Cavaleiro, C.; Salgueiro, L.; do Céu Sousa, M. In Vitro Susceptibility of Trypanosoma Brucei Brucei to Selected Essential oils and Their Major Components. Exp. Parasitol. 2018, 190, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.; Lima, A.S.; Vieira, P.; Dias, L.S.; Tinoco, M.T.; Barroso, J.G.; Pedro, L.G.; Figueiredo, A.C.; Mota, M. Nematicidal Activity of Essential oils and Volatiles Derived from Portuguese Aromatic Flora against the Pinewood Nematode, Bursaphelenchus Xylophilus. J. Nematol. 2010, 42, 8–16. [Google Scholar]
- Machado, M.; Martins, N.; Salgueiro, L.; Cavaleiro, C.; Sousa, M.C. Lavandula luisieri and Lavandula viridis Essential oils as Upcoming Anti-Protozoal Agents: A Key Focus on Leishmaniasis. Appl. Sci. 2019, 9, 3056. [Google Scholar] [CrossRef]
- Mateus, D.M.R.; Ferraz, E.; Perna, V.; Sales, P.; Hipólito-Correia, V. Essential oils and Extracts of Plants as Biocides against Microorganisms Isolated from the Ruins of the Roman City of Conímbriga (Portugal). Environ. Sci. Pollut. Res. 2024, 31, 40669–40677. [Google Scholar] [CrossRef]
- Zuzarte, M.; Francisco, V.; Neves, B.; Liberal, J.; Cavaleiro, C.; Canhoto, J.; Salgueiro, L.; Cruz, M.T. Lavandula viridis L’Hér Essential oil Inhibits the Inflammatory Response in Macrophages through Blockade of NF-KB Signaling Cascade. Front. Pharmacol. 2022, 12, 695911. [Google Scholar] [CrossRef]
- ISO 3515:2002; Oil of Lavender (Lavandula angustifolia Mill). ISO: Geneva, Switzerland, 2002.
- ISO 8902:2009; Oil of Lavandin Grosso (Lavandula angustifolia Mill. x Lavandula Latifolia Medik.), French Type. ISO: Geneva, Switzerland, 2009.
- Green Lavender Essential Oil. Available online: https://otefarm.eu/product/green-lavender-essential-oil/ (accessed on 21 June 2024).
- Green Lavender Essential Oil, 19.50 €. Available online: https://www.fontepenedo.com/en/essential-oils/lavandula-viridis-oil (accessed on 21 June 2024).
- Óleo Essencial de Rosmaninho Verde (Branco) SELVAGEM (Lavandula viridis). Available online: https://info714569.wixsite.com/btiquantum/product-page/óleo-essencial-de-lavandula-viridis-selvagem (accessed on 21 June 2024).
- Óleo Essencial Lavanda-Branca GT Portugal 5 mL. Available online: https://www.laszlo.com.br/oleo-essencial-lavanda-laszlo-lavanda-branca-gt-portugal-sku-7799-laszlo.html (accessed on 21 June 2024).
- Aceite Esencial de Lavanda Verde Silvestre y BIO. Available online: https://www.micosmeticacasera.es/aceite-esencial-de-lavanda-verde-bio/ (accessed on 21 June 2024).
- Nogueira, J.M.F.; Romano, A. Essential oils from Micropropagated Plants of Lavandula viridis. Phytochem. Anal. 2002, 13, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.-M.; Peng, J.-Q.; Chen, Y.; Tao, L.; Zhang, Y.-Y.; Fu, L.-Y.; Long, Q.-D.; Shen, X.-C. 1,8-Cineole: A Review of Source, Biological Activities, and Application. J. Asian Nat. Prod. Res. 2021, 23, 938–954. [Google Scholar] [CrossRef]
- Gonçalves, S.; Serra, H.; Nogueira, J.M.F.; Almeida, R.; Custódio, L.; Romano, A. Headspace-SPME of in Vitro Shoot-Cultures and Micropropagated Plants of Lavandula viridis. Biol. Plant. 2008, 52, 133–136. [Google Scholar] [CrossRef]
- Matos, F.; Miguel, M.G.; Duarte, J.; Venâncio, F.; Moiteiro, C.; Correia, A.I.D.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Antioxidant Capacity of the Essential oils from Lavandula luisieri, L. stoechas subsp. lusitanica, L. stoechas subsp. lusitanica x L. luisieri and L. viridis Grown in Algarve (Portugal). J. Essent. Oil Res. 2009, 21, 327–336. [Google Scholar] [CrossRef]
- Mansinhos, I.; Gonçalves, S.; Rodríguez-Solana, R.; Ordóñez-Díaz, J.L.; Moreno-Rojas, J.M.; Romano, A. Ultrasonic-Assisted Extraction and Natural Deep Eutectic Solvents Combination: A Green Strategy to Improve the Recovery of Phenolic Compounds from Lavandula pedunculata subsp. lusitanica (Chaytor) Franco. Antioxidants 2021, 10, 582. [Google Scholar] [CrossRef] [PubMed]
- Manukyan, A. Effects of PAR and UV-B Radiation on Herbal Yield, Bioactive Compounds and Their Antioxidant Capacity of Some Medicinal Plants Under Controlled Environmental Conditions. Photochem. Photobiol. 2013, 89, 406–414. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Agrawal, S.B. Comparative Analysis of Essential oil Composition and Oil Containing Glands in Ocimum sanctum L. (Holy Basil) under Ambient and Supplemental Level of UV-B through Gas Chromatography–Mass Spectrometry and Scanning Electron Microscopy. Acta Physiol. Plant. 2011, 33, 1093–1101. [Google Scholar] [CrossRef]
- Manukyan, A. Secondary Metabolites and Their Antioxidant Capacity of Caucasian Endemic Thyme (Thymus Transcaucasicus Ronn.) as Affected by Environmental Stress. J. Appl. Res. Med. Aromat. Plants 2019, 13, 100209. [Google Scholar] [CrossRef]
- Akbari, G.A.; Soltani, E.; Binesh, S.; Amini, F. Cold Tolerance, Productivity and Phytochemical Diversity in Sweet Basil (Ocimum basilicum L.) Accessions. Ind. Crops Prod. 2018, 124, 677–684. [Google Scholar] [CrossRef]
- Tarchoune, I.; Baâtour, O.; Harrathi, J.; Cioni, P.L.; Lachaâl, M.; Flamini, G.; Ouerghi, Z. Essential oil and Volatile Emissions of Basil (Ocimum basilicum) Leaves Exposed to NaCl or Na2SO4 Salinity. J. Plant Nutr. Soil Sci. 2013, 176, 748–755. [Google Scholar] [CrossRef]
- Baatour, O.; Kaddour, R.; Aidi Wannes, W.; Lachaâl, M.; Marzouk, B. Salt Effects on the Growth, Mineral Nutrition, Essential oil Yield and Composition of Marjoram (Origanum majorana). Acta Physiol. Plant. 2010, 32, 45–51. [Google Scholar] [CrossRef]
- Bidgoli, D.R.; Azarnezhad, N.; Akhbari, M.; Ghorbani, M. Salinity Stress and PGPR Effects on Essential oil Changes in Rosmarinus officinalis L. Agric. Food Secur. 2019, 8, 2. [Google Scholar] [CrossRef]
- Said-Al Ahl, H.A.H.; Sabra, A.S.; Alataway, A.; Astatkie, T.; Mahmoud, A.A.; Bloem, E. Biomass Production and Essential oil Composition of Thymus Vulgaris in Response to Water Stress and Harvest Time. J. Essent. Oil Res. 2019, 31, 63–68. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula Genus: A Systematic Review of Their Chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, Q.; Niu, Y.; Zhou, X.; Liu, J.; Xu, Y.; Xu, Z. Odor-Active Compounds of Different Lavender Essential oils and Their Correlation with Sensory Attributes. Ind. Crops Prod. 2017, 108, 748–755. [Google Scholar] [CrossRef]
- Aćimović, M.; Lončar, B.; Pezo, M.; Stanković Jeremić, J.; Cvetković, M.; Rat, M.; Pezo, L. Volatile Compounds of Nepeta nuda L. from Rtanj Mountain (Serbia). Horticulturae 2022, 8, 85. [Google Scholar] [CrossRef]
- Aziz, E.E.; Al-Amier, H.; Craker, L.E. Influence of Salt Stress on Growth and Essential oil Production in Peppermint, Pennyroyal, and Apple Mint. J. Herbs Spices Med. Plants 2008, 14, 77–87. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed Priming with Melatonin Effects on Growth, Essential oil Compounds and Antioxidant Activity of Basil (Ocimum basilicum L.) under Salinity Stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Elhindi, K.M.; Al-Suhaibani, N.A.; El-Din, A.F.S.; Yakout, S.M.; Al-Amri, S.M. Effect of Foliar-Applied Iron and Zinc on Growth Rate and Essential oil in Sweet Basil (Ocimum basilicum L.) under Saline Conditions. Progr. Nutr. 2016, 18, 288–298. [Google Scholar]
- El-Esawi, M.A.; Elansary, H.O.; El-Shanhorey, N.A.; Abdel-Hamid, A.M.E.; Ali, H.M.; Elshikh, M.S. Salicylic Acid-Regulated Antioxidant Mechanisms and Gene Expression Enhance Rosemary Performance under Saline Conditions. Front. Physiol. 2017, 8, 716. [Google Scholar] [CrossRef]
- Tounekti, T.; Vadel, A.M.; Bedoui, A.; Khemira, H. NaCl Stress Affects Growth and Essential oil Composition in Rosemary (Rosmarinus officinalis L.). J. Hortic. Sci. Biotechnol. 2008, 83, 267–273. [Google Scholar] [CrossRef]
- Es-sbihi, F.Z.; Hazzoumi, Z.; Aasfar, A.; Amrani Joutei, K. Improving Salinity Tolerance in Salvia officinalis L. by Foliar Application of Salicylic Acid. Chem. Biol. Technol. Agric. 2021, 8, 25. [Google Scholar] [CrossRef]
- Masoudniaragh, A.; Oraei, M.; Gohari, G.; Akbari, A.; Faramarzi, A. Using Halloysite Nanotubes as Carrier for Proline to Alleviate Salt Stress Effects in Sweet Basil (Ocimum basilicum L.). Sci. Hortic. 2021, 285, 110202. [Google Scholar] [CrossRef]
- Taarit, B.M.; Msaada, K.; Hosni, K.; Hammami, M.; Kchouk, M.E.; Marzouk, B. Plant Growth, Essential oil Yield and Composition of Sage (Salvia officinalis L.) Fruits Cultivated under Salt Stress Conditions. Ind. Crops Prod. 2009, 30, 333–337. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V.; Niazi, A.; Moghadam, A. Effect of Salt Stress on Terpenoid Biosynthesis in Salvia Mirzayanii: From Gene to Metabolite. J. Hortic. Sci. Biotechnol. 2019, 94, 389–399. [Google Scholar] [CrossRef]
- Valifard, M.; Mohsenzadeh, S.; Kholdebarin, B.; Rowshan, V. Effects of Salt Stress on Volatile Compounds, Total Phenolic Content and Antioxidant Activities of Salvia Mirzayanii. S. Afr. J. Bot. 2014, 93, 92–97. [Google Scholar] [CrossRef]
- Kulak, M. Recurrent Drought Stress Effects on Essential oil Profile of Lamiaceae Plants: An Approach Regarding Stress Memory. Ind. Crops Prod. 2020, 154, 112695. [Google Scholar] [CrossRef]
- Ramezani, S.; Abbasi, A.; Sobhanverdi, S.; Shojaeiyan, A.; Ahmadi, N. The Effects of Water Deficit on the Expression of Monoterpene Synthases and Essential oils Composition in Salvia Ecotypes. Physiol. Mol. Biol. Plants 2020, 26, 2199–2207. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of Water Stress on Lavender and Sage Biomass Production, Essential oil Composition and Biocidal Properties against Tetranychus Urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Bettaieb, I.; Hamrouni-Sellami, I.; Bourgou, S.; Limam, F.; Marzouk, B. Drought Effects on Polyphenol Composition and Antioxidant Activities in Aerial Parts of Salvia officinalis L. Acta Physiol. Plant. 2011, 33, 1103–1111. [Google Scholar] [CrossRef]
- Llorens-Molina, J.A.; Vacas, S. Effect of Drought Stress on Essential oil Composition of Thymus vulgaris L. (Chemotype 1, 8-Cineole) from Wild Populations of Eastern Iberian Peninsula. J. Essent. Oil Res. 2017, 29, 145–155. [Google Scholar] [CrossRef]
- Pirbalouti, A.G.; Samani, M.R.; Hashemi, M.; Zeinali, H. Salicylic Acid Affects Growth, Essential oil and Chemical Compositions of Thyme (Thymus Daenensis Celak.) under Reduced Irrigation. Plant Growth Regul. 2014, 72, 289–301. [Google Scholar] [CrossRef]
- Radwan, A.; Kleinwächter, M.; Selmar, D. Impact of Drought Stress on Specialised Metabolism: Biosynthesis and the Expression of Monoterpene Synthases in Sage (Salvia officinalis). Phytochemistry 2017, 141, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in Growth Parameter and Essential oil Composition of Sage (Salvia officinalis L.) Leaves in Response to Various Salt Stresses. Ind. Crops Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Chang, X.; Alderson, P.G.; Wright, C.J. Enhanced UV-B Radiation Alters Basil (Ocimum basilicum L.) Growth and Stimulates the Synthesis of Volatile oils. J. Hortic. For. 2009, 1, 27–31. Available online: https://academicjournals.org/journal/JHF/article-stat/58E7FAC4938 (accessed on 26 June 2024).
- El Kharraf, S.; Faleiro, M.L.; Abdellah, F.; El-Guendouz, S.; El Hadrami, E.M.; Miguel, M.G. Simultaneous Hydrodistillation-Steam Distillation of Rosmarinus Officinalis, Lavandula angustifolia and Citrus Aurantium from Morocco, Major Terpenes: Impact on Biological Activities. Molecules 2021, 26, 5452. [Google Scholar] [CrossRef]
- Eltayeb, L.M.H.; Yagi, S.; Mohamed, H.M.M.; Zengin, G.; Shariati, M.A.; Rebezov, M.; Uba, A.I.; Lorenzo, J.M. Essential oils Composition and Biological Activity of Chamaecyparis Obtusa, Chrysopogon Nigritanus and Lavandula Coronopifolia Grown Wild in Sudan. Molecules 2023, 28, 1005. [Google Scholar] [CrossRef]
- Carrasco, A.; Tomas, V.; Tudela, J.; Miguel, M.G. Comparative Study of GC-MS Characterization, Antioxidant Activity and Hyaluronidase Inhibition of Different Species of Lavandula and Thymus Essential oils. Flavour Fragr. J. 2016, 31, 57–69. [Google Scholar] [CrossRef]
- Gok, M.; Cicek, C.; Sari, S.; Bodur, E. Novel Activity of Human BChE: Lipid Hydrolysis. Biochimie 2023, 204, 127–135. [Google Scholar] [CrossRef]
- Pham, T.-N.; Cazier, E.A.; Gormally, E.; Lawrence, P. Valorization of Biomass Polyphenols as Potential Tyrosinase Inhibitors. Drug Discov. Today 2024, 29, 103843. [Google Scholar] [CrossRef] [PubMed]
- Vittorio, S.; Dank, C.; Ielo, L. Heterocyclic Compounds as Synthetic Tyrosinase Inhibitors: Recent Advances. Int. J. Mol. Sci. 2023, 24, 9097. [Google Scholar] [CrossRef]
- Ayaz, M.; Sadiq, A.; Junaid, M.; Ullah, F.; Subhan, F.; Ahmed, J. Neuroprotective and Anti-Aging Potentials of Essential oils from Aromatic and Medicinal Plants. Front. Aging Neurosci. 2017, 9, 168. [Google Scholar] [CrossRef]
- Gonçalves, S.; Mansinhos, I.; Romano, A. Neuroprotective Compounds from Plant Sources and Their Modes of Action: An Update. In Plant-Derived Bioactives: Chemistry and Mode of Action; Swamy, M.K., Ed.; Springer: Singapore, 2020; pp. 417–440. ISBN 9789811523618. [Google Scholar]
- Chen, W.N.; Chin, K.W.; Tang, K.S.; Agatonovic-Kustrin, S.; Yeong, K.Y. Neuroprotective, Neurite Enhancing, and Cholinesterase Inhibitory Effects of Lamiaceae Family Essential oils in Alzheimer’s Disease Model. J. Herb. Med. 2023, 41, 100696. [Google Scholar] [CrossRef]
- Kharraf, S.E.; El-Guendouz, S.; Farah, A.; Mateus, M.C.; Hadrami, E.M.E.; Miguel, M.G. Impact of Fifteen Combinations of the Main Components of Rosemary, Lavender and Citrus Essential oils on in Vitro Biological Activities. S. Afr. J. Bot. 2023, 156, 162–168. [Google Scholar] [CrossRef]
- El Omari, N.; Balahbib, A.; Bakrim, S.; Benali, T.; Ullah, R.; Alotaibi, A.; Naceiri El Mrabti, H.; Goh, B.H.; Ong, S.-K.; Ming, L.C.; et al. Fenchone and Camphor: Main Natural Compounds from Lavandula stoechas L., Expediting Multiple in Vitro Biological Activities. Heliyon 2023, 9, e21222. [Google Scholar] [CrossRef]
- Nwanade, C.F.; Wang, M.; Li, H.; Masoudi, A.; Yu, Z.; Liu, J. Individual and Synergistic Toxicity of Cinnamon Essential oil Constituents against Haemaphysalis Longicornis (Acari: Ixodidae) and Their Potential Effects on Non-Target Organisms. Ind. Crops Prod. 2022, 178, 114614. [Google Scholar] [CrossRef]
- Dragomanova, S.; Lazarova, M.; Munkuev, A.; Suslov, E.; Volcho, K.; Salakhutdinov, N.; Bibi, A.; Reynisson, J.; Tzvetanova, E.; Alexandrova, A.; et al. New Myrtenal-Adamantane Conjugates Alleviate Alzheimer’s-Type Dementia in Rat Model. Molecules 2022, 27, 5456. [Google Scholar] [CrossRef] [PubMed]
- Prabu, S.; Jing, D.; Chandran, V.; Mathew, P. Insecticidal Activity of Origanum majorana L. Essential oil as Anti-Cholinergic. Agent. Entomol. Res. 2020, 50, 402–413. [Google Scholar] [CrossRef]
- Tran-Trung, H.; Thang, T.D.; Nguyen, T.H.D.; Vu, D.C.; Tuan, N.H.; Ha, N.X.; Chen, T.V.; Oanh, H.T.; Giang, N.T.T.; Thuy, P.T. Essential oils From the Trunks and Leaves of Paramignya Scandens (Griff.) Craib From Vietnam: Phytochemical Composition, In Vitro α-Amylase and Tyrosinase Inhibitory Activities and In Silico Molecular Docking Studies. Nat. Prod. Commun. 2023, 18, 1934578X231222383. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).