Modeling Risk in Fusarium Head Blight and Yield Analysis in Five Winter Wheat Production Regions of Hungary
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measured Variables: Weather and Crop Data
- Dry month (D): PR > P5s × 0.9;
- Wet month (W): PR > P5s × 1.1;
- Normal (N): P5s × 0.9 < PR < P5s × > 1.1;
- Warm month (H): Ta ≥ Ta5s + 1 °C;
- Cool month (C): Ta ≤ Ta5s − 1 °C;
- Normal (N): Ta5s − 1 °C < Ta < Ta5s + 1 °C.
2.2. Modeling Probability of Wheat FHB Infection by De Wolf et al. (2003)
2.3. Statistics
3. Results and Discussion
3.1. Seasonal Weather Conditions between 2017 and 2021
3.2. Wheat Yield in the Studied Seasons
3.3. Measured Infection Rates (P%) in the Counties between 2017 and 2021
3.4. Assessment of FHB
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Wolf, E.D.; Madden, L.V.; Lipps, P.E. Risk assessment models for wheat fusarium head blight epidemics based on within-season weather data. Phytopathology 2003, 93, 428–435. [Google Scholar] [CrossRef]
- Zaji, A.; Liu, Z.; Xiao, G.; Bhowmik, P.; Sangha, J.S.; Ruan, Y. Wheat spike localization and counting via hybrid UNet architectures. Comput. Electron. Agric. 2022, 203, 107439. [Google Scholar] [CrossRef]
- Bao, W.; Yang, X.; Liang, D.; Hu, G.; Yang, X. Lightweight convolutional neural network model for field wheat ear disease identification. Comput. Electron. Agric. 2021, 189, 106367. [Google Scholar] [CrossRef]
- FAOSTAT (Food and Agriculture Organization Statistics). Agricultural Data: Production and Indices Data Crop Primary. 2018. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 1 January 2023). (In Hungarian).
- KSH. 2023. Available online: https://www.ksh.hu/stadat_files/mez/hu/mez0071.html (accessed on 1 January 2023). (In Hungarian).
- Huang, L.; Wu, K.; Huang, W.; Dong, Y.; Ma, H.; Liu, Y.; Liu, L. Detection of Fusarium head blight in wheat ears using continuous wavelet analysis and PSO-SVM. Agriculture 2021, 11, 998. [Google Scholar] [CrossRef]
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A unified effort to fight an enemy of wheat and barley: Fusarium head blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Mesterházy, A.; Bartók, T.; Kászonyi, G.; Varga, M.; Tóth, B.; Varga, J. Common resistance to different Fusarium spp. causing Fusarium head blight in wheat. Eur. J. Plant Pathol. 2005, 112, 267–281. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Luo, H.S.; Wang, D.Y.; Zhou, X.G.; Li, W.F.; Gu, C.-Y.; Zhang, G.; He, F.M. Assessment of the levels of damage caused by Fusarium head blight in wheat using an improved YoloV5 method. Comput. Electron. Agric. 2022, 198, 107086. [Google Scholar] [CrossRef]
- Mengesha, G.G.; Abebe, S.M.; Mekonnen, A.A.; Esho, A.G./M.; Lera, Z.T.; Shertore, M.M.; Fedilu, K.B.; Tadesse, Y.B.; Tsakamo, Y.T.; Issa, B.T.; et al. Effects of cultivar resistances and chemical seed treatments on fusarium head blight and bread wheat yield-related parameters under field condition in southern Ethiopia. Heliyon 2023, 24, e08659. [Google Scholar] [CrossRef]
- Spolti, P.; Del Ponte, E.M.; Dong, Y.; Cummings, J.A.; Bergstrom, G.C. Triazole Sensitivity in a Contemporary Population of Fusarium graminearum from New York Wheat and Competitiveness of a Tebuconazole-Resistant Isolate. Plant Dis. 2014, 98, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Dill-Macky, R. Fusarium Head Blight (Scab). In Compendium of Wheat Diseases and Pests; APS Press: St. Paul, MN, USA, 2010; pp. 34–36. [Google Scholar]
- Xu, X.-M.; Monger, W.; Ritieni, A.; Nicholson, P. Effect of temperature and duration of wetness during initial infection periods on disease development. fungal biomass and mycotoxin concentrations on wheat inoculated with single. or combinations of. Fusarium species. Plant Pathol. 2007, 56, 943–956. [Google Scholar] [CrossRef]
- Madgwick, J.W.; West, J.S.; White, R.P.; Semenov, M.A.; Townsend, J.A.; Turner, J.A.; Fitt, B.D.L. Impacts of climate change on wheat anthesis and fusarium ear blight in the UK. Eur. J. Plant Pathol. 2011, 130, 117–131. [Google Scholar] [CrossRef]
- Vaughan, M.; Backhouse, D.; Ponte, E.D. Climate change impacts on the ecology of Fusarium graminearum species complex and susceptibility of wheat to Fusarium head blight: A review. World Mycotoxin J. 2016, 9, 685–700. [Google Scholar] [CrossRef]
- Ayers, J.E.; Pennypacker, S.P.; Nelson, P.E.; Penny-packer, P.W. Environmental factors associated with airborne ascospores of Gibberelia zeae in corn and wheat fields. Phytopathology 1975, 65, 835. [Google Scholar]
- Sutton, J.C. Epidemiolograin yield of wheat head blight and maize ear rot caused by Fusarium graminearum. Can. J. Plant. Pathol. 1982, 4, 195–209. [Google Scholar] [CrossRef]
- Shah, D.; De Wolf, E.; Paul, P.; Madden, L. Functional data analysis of weather variables linked to Fusarium head blight epidemics in the United States. Phytopathology 2019, 109, 96–110. [Google Scholar] [CrossRef]
- Bondalapati, K.D.; Stein, J.M.; Neate, S.M.; Halley, S.H.; Osborne, L.E.; Hollingsworth, C.R. Development of weather-based predictive models for Fusarium head blight and deoxynivalenol accumulation for spring malting barley. Plant Dis. 2012, 96, 673–680. [Google Scholar] [CrossRef]
- Schaafsma, A.W.; Hooker, D.C. Climatic models to predict occurrence of Fusarium toxins in wheat and maize. Int. J. Food Microbiol. 2007, 119, 116–125. [Google Scholar] [CrossRef]
- Birr, T.; Verreet, J.A.; Klink, H. Prediction of deoxynivalenol and zearalenone in winter wheat grain in a maize-free crop rotation based on cultivar susceptibility and meteorological factors. J. Plant. Dis. Protect. 2019, 126, 13–27. [Google Scholar] [CrossRef]
- Rossi, V.; Giosue, S.; Pattori, E.; Spanna, F.; Del Vecchio, A. A model estimating the risk of Fusarium head blight on wheat. EPPO Bulletin. 2003, 33, 421–425. [Google Scholar] [CrossRef]
- Anda, A.; Simon, S.; Simon-Gáspár, B. Impacts of wintertime meteorological variables on decomposition of Phragmites australis and Solidago canadensis in the Balaton System. Theor. Appl. Climatol. 2023, 151, 1963–1979. [Google Scholar] [CrossRef]
- Kocsis, T.; Kovács-Székely, I.; Anda, A. Homogeneity tests and non-parametric analyses of tendencies in precipitation time series in Keszthely; Western Hungary. Theor. Appl. Climatol. 2020, 139, 849–859. [Google Scholar] [CrossRef]
- Hay, W.T.; McCormick, S.P.; Vaughan, M.M. Effects of atmospheric CO2 and temperature on wheat and corn susceptibility to Fusarium graminearum and deoxynivalenol contamination. Plants 2021, 12, 2582. [Google Scholar] [CrossRef] [PubMed]
- Garrett, K.A.; Dendy, S.P.; Frank, E.E.; Rouse, M.N.; Travers, S.E. Climate change effects on plant disease: Genomes to ecosystems. Annu. Rev. Phytopathol. 2006, 44, 489–509. [Google Scholar] [CrossRef] [PubMed]
- Matengu, T.T.; Bullock, P.R.; Mkhabela, M.S.; Zvomuya, F.; Henriquez, M.A.; Ojo, R.E.; Fernando, D.W.G. Weather-based models for forecasting Fusarium head blight risks in wheat and barley: A review. Plant Pathol. 2023, 73, 492–505. [Google Scholar] [CrossRef]
- Nuttall, J.G.; O’Leary, G.J.; Panozzo, J.F.; Walker, C.K.; Barlow, K.M.; Fitzgerald, G.J. Models of grain quality in wheat—A review. Field Crops Res. 2017, 202, 136–145. [Google Scholar] [CrossRef]
- Regulation in Evaluation of Certain Food Additives with Sampling and Analytical Prescriptions. (In Hungarian: Rendelet az élelmiszerek mikotoxin-tartalmának hatósági ellenőrzéséhez használandó mintavételi és elemzési módszerek megállapításáról). 2006; NÉBIH, Budapest (Hungary). Available online: https://portal.nebih.gov.hu/documents/10182/21384/Fuzarium+t%C3%A1j%C3%A9koztat%C3%B3.pdf/f1a6858e-77f3-4bf0-a3a3-f42a5797bad4 (accessed on 1 January 2023).
- WHO Technical Report Series 966. Evaluation of Certain Food Additives, Seventy-fourth report of the Joint FAO/WHO Expert Committe on Food Additives, Fumonisins. 2011. Available online: http://www.inchem.org/documents/jecfa/jecmono/v966je01.pdf (accessed on 1 January 2023).
- Madden, L.V.; Hughes, G.; van den Bosch, F. Temporal Analysis I Quantifying and Comparing Epidemics. In The Study of Plant Disease Epidemics; The American Phytopathological Society: St. Paul, MN, USA, 2017; pp. 63–116. [Google Scholar]
- Landschoot, S.; Waegeman, W.; Audenaert, K.; Van Damme, P.; Vandepitte, J.; De Baets, B.; Haesaert, G. A field-specific web tool for the prediction of fusarium head blight and deoxynivalenol content in Belgium. Comput. Electron. Agr. 2013, 93, 140–148. [Google Scholar] [CrossRef]
- Giroux, M.-E.; Bourgeois, G.; Dion, Y.; Rioux, S.; Pageau, D.; Zoghlami, S.; Parent, C.; Vachon, E.; Vanasse, A. Evaluation of forecasting models for Fusarium head blight of wheat under growing conditions of Quebec, Canada. Plant Dis. 2016, 100, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 1 January 2023).
- Maaz, T.M.; Sapkota, T.B.; Eagle, A.J.; Kantar, M.B.; Bruulsema, T.W.; Majumdar, K. Meta-analysis of yield and nitrous oxide outcomes for nitrogen management in agriculture. Glob. Chang. Biol. 2021, 27, 2343–2360. [Google Scholar] [CrossRef] [PubMed]
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Babar, M.A.; et al. Climate change impact and adaptation for wheat protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef]
- Li, Y.B.; Hou, R.X.; Tao, F.L. Interactive effects of different warming levels and tillage managements on winter wheat growth. physiological processes. grain yield and quality in the North China Plain. Agric. Ecosyst. Environ. 2020, 295, 106923. [Google Scholar]
- Bai, H.Z.; Xiao, D.P.; Wang, B.; Liu, D.L.; Tang, J.Z. Simulation of wheat response to future climate change based on coupled model inter-comparison project phase 6 multi-model Ensemble projections in the North China plain. Front. Plant Sci. 2022, 13, 829580. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Peng, Y.; Lin, J.; Wang, L.; Jia, Z.; Zhang, R. Optimal nitrogen management to achieve high wheat grain yield, grain protein content, and water productivity: A meta-analysis. Agric. Water Manag. 2023; 290, 108587. [Google Scholar]
- Burton, A.; Häner, L.L.; Schaad, N.; Strebel, S.; Vuille-dit-Bille, N.; Bongiovani, P.F.; Holzkämper, A.; Pellet, D.; Herrera, J.M. Evaluating nitrogen fertilization strategies to optimize yield and grain nitrogen content in top winter wheat varieties across Switzerland. Field Crops Res. 2024, 307, 109251. [Google Scholar] [CrossRef]
- Rogger, J.; Hund, A.; Fossati, D.; Holzkämper, A. Can Swiss wheat varieties escape future heat stress? Eur. J. Agron. 2021, 131, 126394. [Google Scholar] [CrossRef]
- Giordano, N.; Sadras, V.O.; Lollato, R.P. Late-season nitrogen application increases grain protein concentration and is neutral for yield in wheat. A global meta-analysis. Field Crops Res. 2023, 290, 108740. [Google Scholar] [CrossRef]
- Prerostova, S.; Vankova, R. Phytohormone-Mediated Regulation of Heat Stress Response in Plants. In Plant Hormones and Climate Change; Ahammed, G.J., Yu, J., Eds.; Springer: Singapore, 2023. [Google Scholar]
- Zhang, D.; Liu, J.; Li, D.; Batchelor, W.D.; Wu, D.; Zhen, X.; Ju, H. Future climate change impacts on wheat grain yield and protein in the North China Region. Sci. Total Environ. 2023, 902, 166147. [Google Scholar] [CrossRef] [PubMed]
- Hasheminasab, K.S.; Shahbazi, K.; Marzi, M.; Zare, A.; Yeganeh, M.; Bazargan, K.; Kharazmi, R. A study on wheat grain zinc. iron. copper. and manganese concentrations and their relationship with grain yield in major wheat production areas of Iran. J. Agric. Food Res. 2023, 14, 100913. [Google Scholar] [CrossRef]
- Belizán, M.M.; Gomez, A.D.L.A.; Baptista, Z.P.T.; Jimenez, C.M.; Matías, M.D.H.S.; Catalán, C.A.; Sampietro, D.A. Sampietro Influence of water activity and temperature on growth and production of trichothecenes by Fusarium graminearum sensu stricto and related species in maize grains. Int. J. Food Microbiol. 2019, 305, 108242. [Google Scholar] [CrossRef]
- David, R.F.; Bozorg Magham, A.E.; Schmale, D.G.; Ross, S.D.; Marr, L.C. Identification of meteorological predictors of Fusarium graminearum ascospore release using correlation and causality analyses. Eur. J. Plant Pathol. 2016, 145, 483–492. [Google Scholar] [CrossRef]
- Gilbert, J.; Tekauz, A. Review: Recent developments in research of fusarium head blight in Canada. Can. J. Plant Pathol. 2000, 22, 1–8. [Google Scholar] [CrossRef]
- Song, Y.; Hans, W.L.; Wang, C.; Tian, J.; Huo, Z.; Gao, P.; Song, Y.; Guo, A. The influence of excess precipitation on winter wheat under climate change in China from 1961 to 2017. Sci. Total Environ. 2017, 690, 189–196. [Google Scholar] [CrossRef]
- Zhu, Y.; Xi, J.; Yao, Y.; Xu, H.; Tang, C.; Wu, L. Characterizing the dynamic linkages between environmental changes and wheat Fusarium head blight epidemics. Ecol. Inform. 2024, 80, 102524. [Google Scholar] [CrossRef]
- Shah, D.A.; Molineros, J.E.; Paul, P.A.; Willyerd, K.T.; Madden, L.V.; De Wolf, E.D. Predicting Fusarium head blight epidemics with weather-driven pre- and post-anthesis logistic regression models. Phytopathology 2013, 103, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Burke, M.B. On the use of statistical models to predict crop yield responses to climate change. Agric. Forest Meteorol. 2010, 150, 1443–1452. [Google Scholar] [CrossRef]
- Turkington, T.K.; Beres, B.L.; Kutcher, H.R.; Irvine, B.; Johnson, E.N.; O’Donovan, J.T.; Harker, K.N.; Holzapfel, C.B.; Mohr, R.; Peng, G.; et al. Winter Wheat Yields Are Increased by Seed Treatment and Fall-Applied Fungicide. Agron. J. 2016, 108, 1379–1389. [Google Scholar] [CrossRef]
- Paul, P.A.; Lipps, P.E.; De Wolf, E.; Shaner, G.; Buechley, G.; Adhikari, T.; Ali, S.; Stein, J.; Osborne, L.; Madden, L.V. A distributed lag analysis of the relationship between Gibberella zeae inoculum density on wheat spikes and weather variables. Phytopathology 2007, 97, 1608–1624. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.X.; Dong, Y.Y.; Huang, W.J.; Liu, L.Y. Regional prediction of Fusarium head blight occurrence in wheat with remote sensing based Susceptible Exposed-Infectious-Removed model. Int. J. Appl. Earth Obs. Geoinf. 2022, 114, 103043. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Huang, L.; Huang, W.; Dong, Y.; Ma, H.; Ruan, C. Development of new indices and use of CARS-Ridge algorithm for wheat fusarium head blight detection using in-situ hyperspectral data. Biosyst. Eng. 2024, 237, 13–25. [Google Scholar] [CrossRef]
- Tschanz, A.T.; Horst, R.K.; Nelson, P.E. The effect of environment on sexual reproduction of Gibberella zeae. Mycologia 1976, 68, 327–340. [Google Scholar] [CrossRef]
- Kocsis, T.; Pongrácz, R.; Hatvani, I.G.; Magyar, N.; Anda, A.; Kovács-Székely, I. Seasonal trends in the Early Twentieth Century Warming (ETCW) in a centennial instrumental temperature record from Central Europe. Hung. Geogr. Bull. 2024, 73, 3–16. [Google Scholar] [CrossRef]
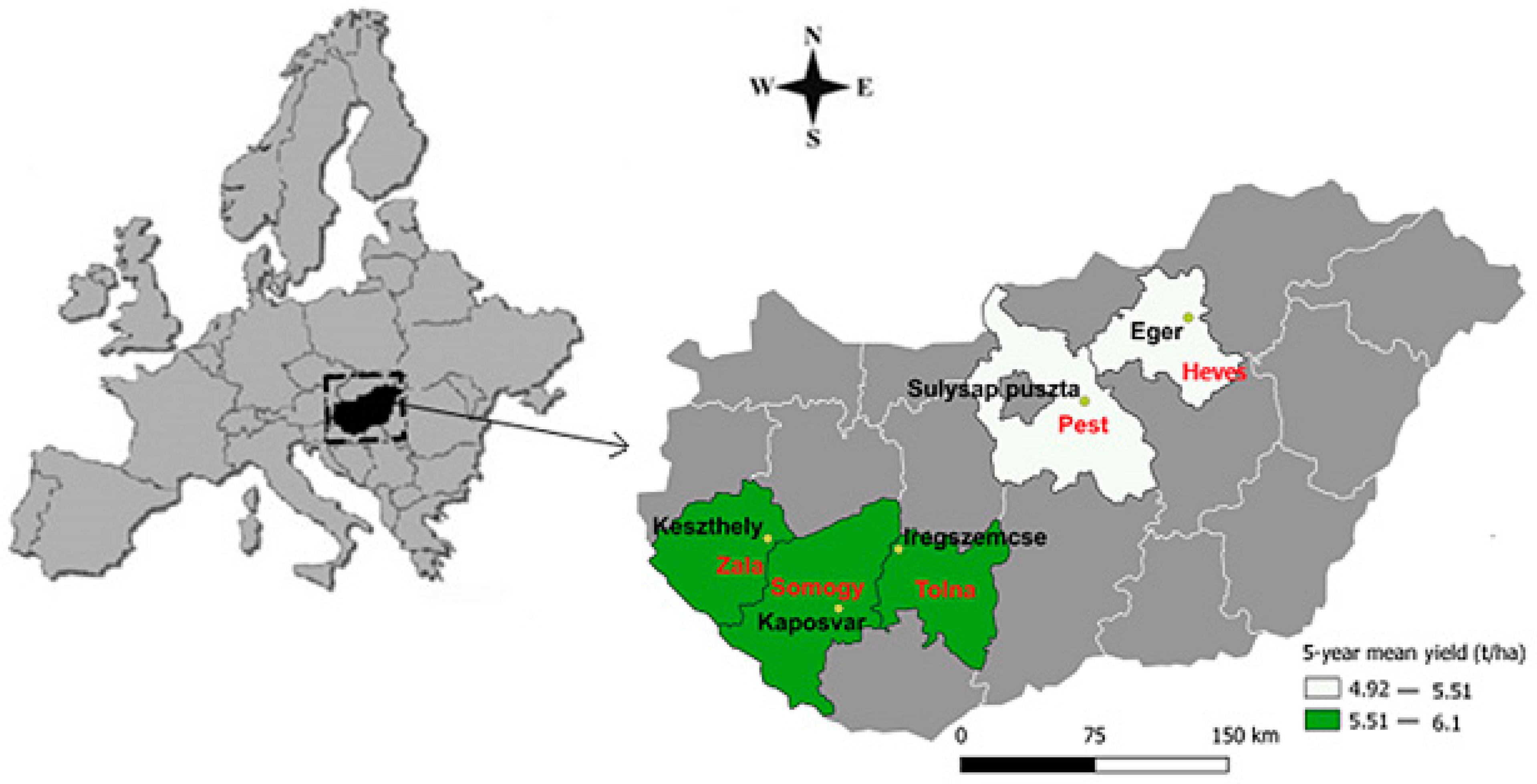
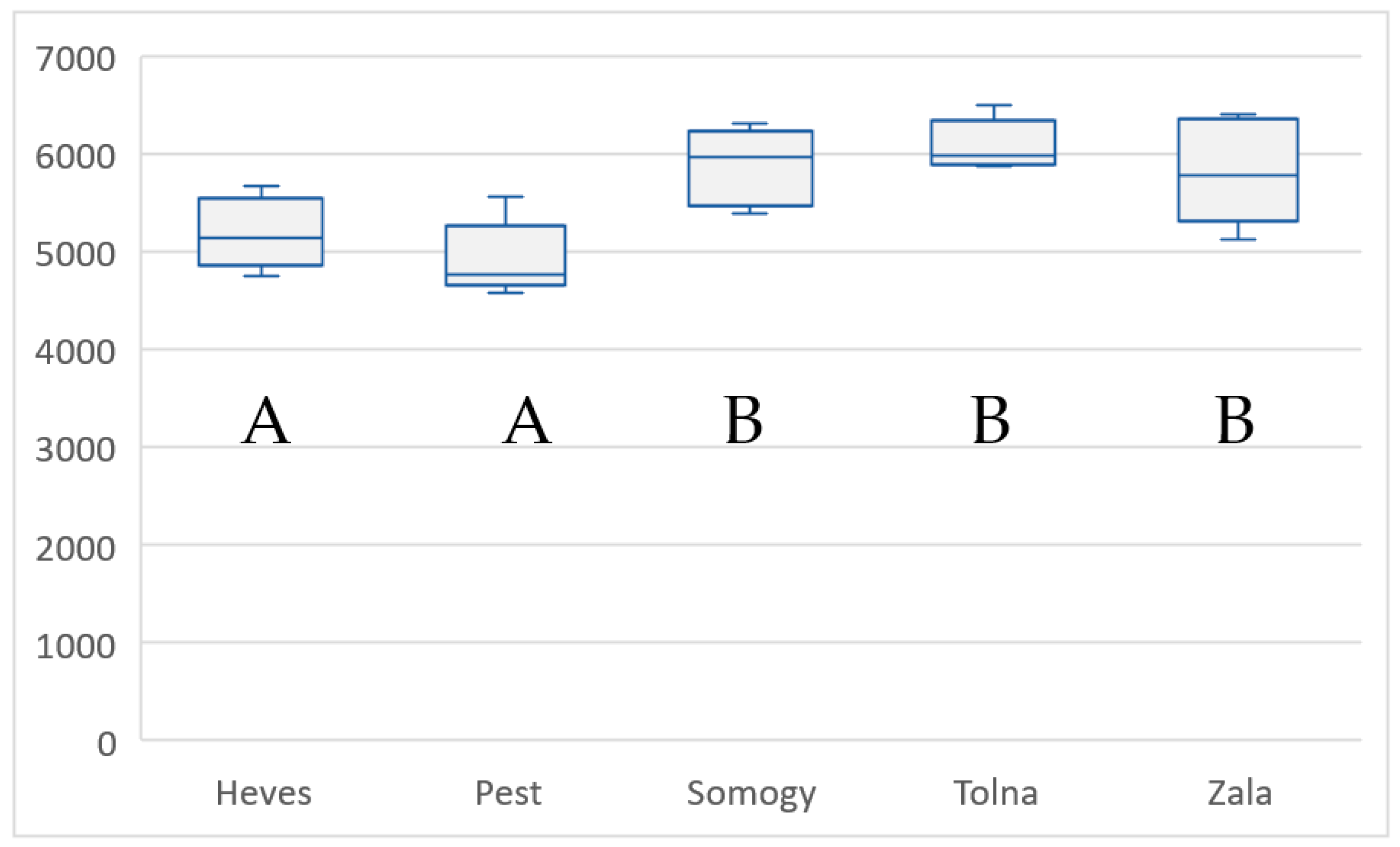
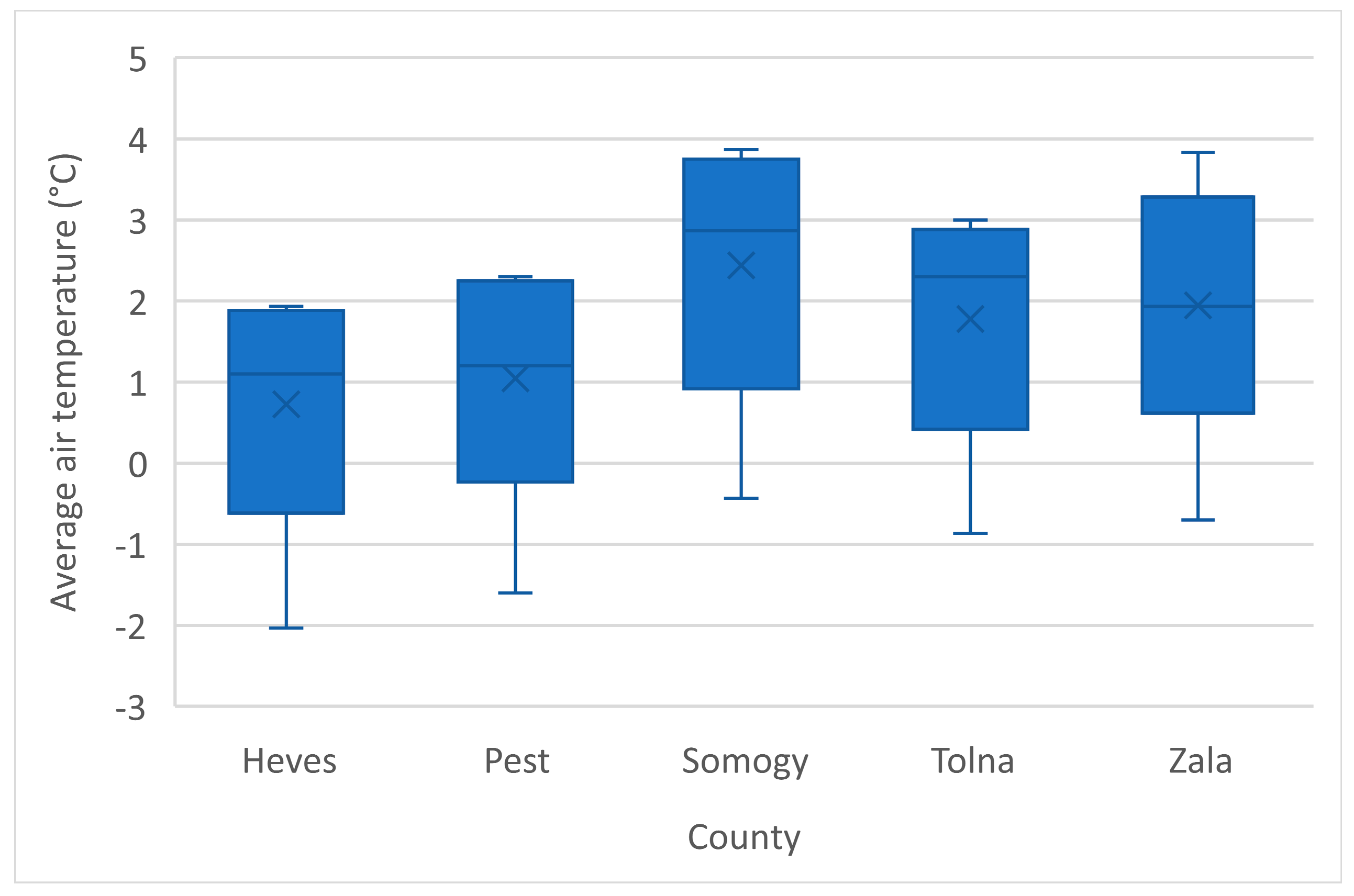

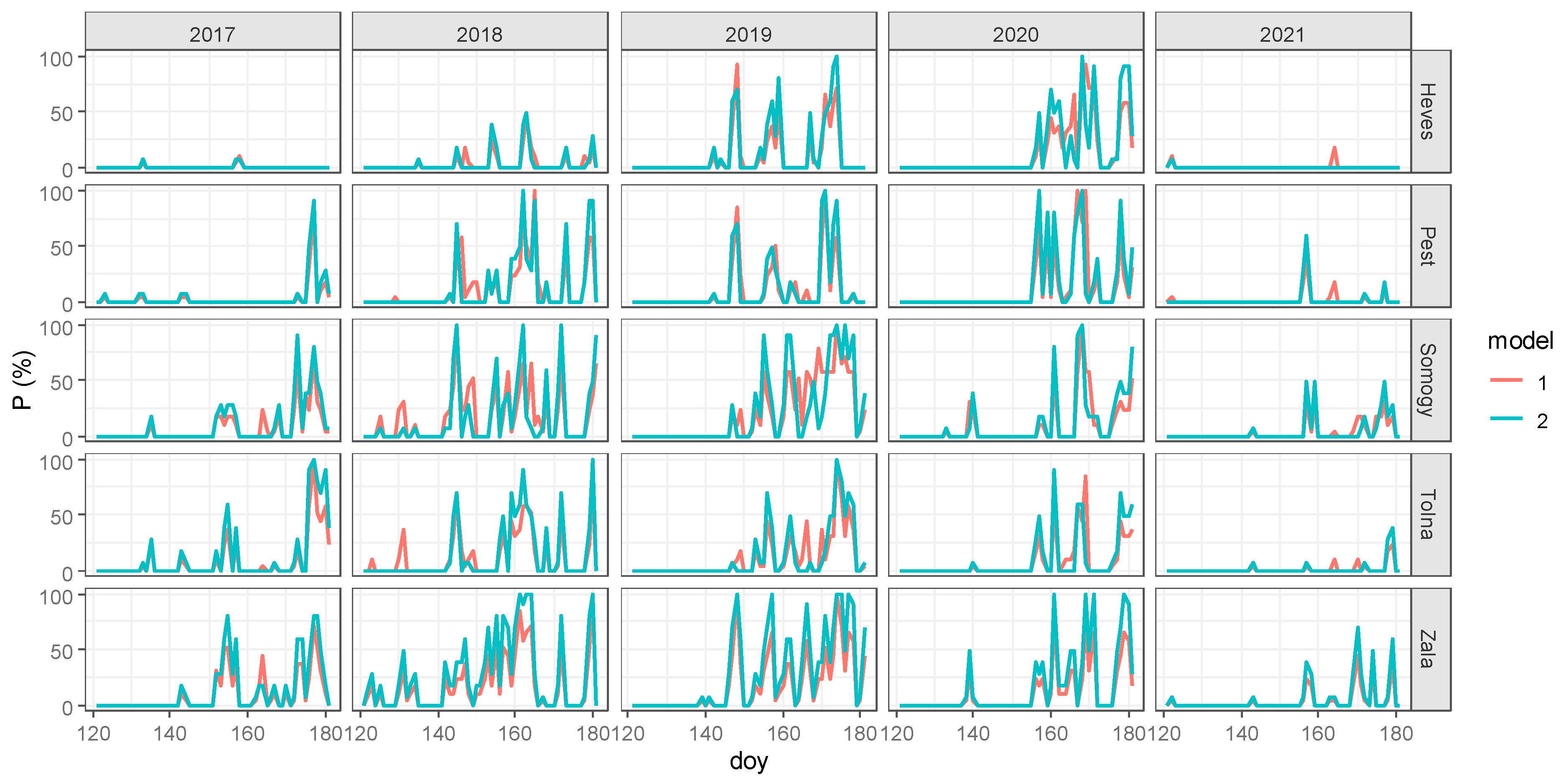


| County/Year | Zala | Heves | Tolna | Somogy | Pest |
|---|---|---|---|---|---|
| Grain yield (kg ha−1) | |||||
| 2017 | 5500 | 5140 | 6510 | 5540 | 4960 |
| 2018 | 5130 | 4760 | 5880 | 5390 | 4770 |
| 2019 | 5790 | 5440 | 5920 | 5970 | 4740 |
| 2020 | 6410 | 4980 | 6190 | 6320 | 4580 |
| 2021 | 6320 | 5670 | 5980 | 6160 | 5570 |
| Mean yield (kg/ha) | 5830 | 5198 | 6096 | 5876 | 4924 |
| SD | ±542.44 | ±361.97 | ±260.44 | ±398.66 | ±385.53 |
| CV (%) | 9.30 | 6.96 | 4.24 | 6.78 | 7.83 |
| Wheat-growing area (ha) | |||||
| 2017 | 28,030 | 43,380 | 50,693 | 58,672 | 53,993 |
| 2018 | 30,682 | 42,987 | 51,506 | 57,864 | 57,723 |
| 2019 | 29,556 | 44,172 | 49,163 | 55,078 | 57,286 |
| 2020 | 26,403 | 44,341 | 47,266 | 49,003 | 44,610 |
| 2021 | 24,862 | 35,580 | 47,072 | 53,267 | 41,013 |
| Mean Air Temperatures, Ta (°C) | |||||||||
| Zala County | October | November | December | January | February | March | April | May | June |
| 2016/2017 | 9.8 | 5.1 | −0.4 | −4.6 | 2.9 | 9.3 | 10.8 | 16.6 | 21.2 |
| 2017/2018 | 10.8 | 5.6 | 2.7 | 3.4 | −0.3 | 3.7 | 15.3 | 18.9 | 20.5 |
| 2018/2019 | 12.8 | 7.3 | 1.8 | 0.3 | 3.7 | 8.4 | 12 | 13 | 22.8 |
| 2019/2020 | 12.6 | 9 | 4.3 | 0.6 | 6.6 | 7.2 | 11.8 | 14.4 | 19.2 |
| 2020/2021 | 11.5 | 5.8 | 3.3 | 2.1 | 2.8 | 5.9 | 9.1 | 14 | 22.1 |
| Five-season mean Ta ± SD | 8.8 ± 0.6 | ||||||||
| Tolna County | |||||||||
| 2016/2017 | 9.4 | 5 | −0.5 | −5.2 | 3.1 | 9.5 | 10.8 | 16.6 | 21.5 |
| 2017/2018 | 11.5 | 5.9 | 3.3 | 3.7 | −0.1 | 3.5 | 15.8 | 19.3 | 20.6 |
| 2018/2019 | 13 | 6.7 | 1.4 | −0.2 | 3.9 | 8.7 | 12 | 13 | 22.5 |
| 2019/2020 | 12.3 | 8.2 | 3.6 | −0.5 | 5.9 | 6.7 | 12 | 14.5 | 19.5 |
| 2020/2021 | 11.7 | 5.4 | 3.1 | 2 | 3.2 | 5.7 | 8.8 | 13.9 | 22.1 |
| Five-season mean Ta ± SD | 8.7 ± 0.6 | ||||||||
| Heves County | |||||||||
| 2016/2017 | 9 | 4.7 | −1.9 | −6.1 | 1.9 | 9 | 10.4 | 16.3 | 21 |
| 2017/2018 | 10.9 | 5.3 | 1.5 | 2.2 | −0.4 | 3.1 | 16 | 19.3 | 20.4 |
| 2018/2019 | 13.3 | 7.3 | 0.2 | −1.4 | 3.6 | 8.6 | 13 | 13.9 | 22.9 |
| 2019/2020 | 13 | 9.2 | 2.4 | −1.4 | 4.8 | 7 | 12.1 | 14 | 19.6 |
| 2020/2021 | 11.4 | 4.2 | 3.4 | 0.4 | 1.7 | 5.2 | 8.3 | 14 | 22.1 |
| Five-season mean Ta ± SD | 8.3 ± 0.8 | ||||||||
| Pest County | |||||||||
| 2016/2017 | 9.3 | 4.6 | −0.7 | −6 | 1.9 | 9.1 | 10.5 | 16.6 | 21.7 |
| 2017/2018 | 11.6 | 5.5 | 2.1 | 2 | −0.5 | 2.9 | 16 | 19.4 | 20.6 |
| 2018/2019 | 13.7 | 6.7 | 0.5 | −1 | 3.9 | 8.9 | 12.7 | 13.9 | 23.0 |
| 2019/2020 | 13.2 | 8.3 | 2.7 | −1 | 5.2 | 7 | 12.3 | 14.5 | 19.8 |
| 2020/2021 | 11.6 | 4.9 | 3.1 | 1.2 | 2.3 | 5.9 | 8.7 | 13.9 | 22.6 |
| Five-season mean Ta ± SD | 8.6 ± 0.7 | ||||||||
| Somogy County | |||||||||
| 2016/2017 | 9.9 | 6.1 | −0.6 | −5 | 4.3 | 9.4 | 10.9 | 16.7 | 22.1 |
| 2017/2018 | 11.2 | 6.5 | 3.8 | 4.6 | 0.2 | 4.4 | 15.7 | 19 | 20.7 |
| 2018/2019 | 12.8 | 6.9 | 2 | 0.6 | 4.2 | 9 | 12 | 13.4 | 22.8 |
| 2019/2020 | 12.9 | 8.8 | 4.5 | 0.1 | 7 | 7.3 | 11.9 | 14.8 | 20.0 |
| 2020/2021 | 12.7 | 6 | 4 | 2.8 | 4.1 | 6.1 | 9.3 | 14.4 | 22.1 |
| Five-season mean Ta ± SD | 9.2 ± 0.6 | ||||||||
| Precipitation, PR (mm) | |||||||||
| Zala County | October | November | December | January | February | March | April | May | June |
| 2016/2017 | 97.8 | 50.9 | 4 | 25.8 | 44.6 | 15.3 | 20.9 | 38.8 | 61.1 |
| 2017/2018 | 66 | 61.8 | 72.1 | 12.9 | 53.4 | 95.2 | 13.4 | 68.1 | 101.2 |
| 2018/2019 | 23.4 | 42.8 | 11.3 | 28.2 | 17.2 | 12.8 | 28.7 | 128.8 | 50.4 |
| 2019/2020 | 25.2 | 118.6 | 90.5 | 13.2 | 30.8 | 18.6 | 27.2 | 32.7 | 93 |
| 2020/2021 | 102.3 | 11.5 | 63.7 | 22.6 | 19 | 8.5 | 27.5 | 92.5 | 3 |
| Five-season PR mean ± SD | 409.5 ± 86.7 | ||||||||
| Tolna County | |||||||||
| 2016/2017 | 63 | 40.1 | 0.7 | 17.6 | 45.2 | 16.7 | 39.7 | 48 | 86.8 |
| 2017/2018 | 77.5 | 47.1 | 58.3 | 13.6 | 58.3 | 101.2 | 10.4 | 21.3 | 118.6 |
| 2018/2019 | 11.1 | 34.9 | 15.2 | 22.9 | 18.6 | 12.9 | 45.6 | 127.9 | 49.1 |
| 2019/2020 | 28 | 91 | 73.7 | 20.6 | 36.3 | 38.3 | 15 | 34.1 | 108.6 |
| 2020/2021 | 84.7 | 7.1 | 41.5 | 16.7 | 29.7 | 12.6 | 32 | 91.1 | 14 |
| Five-season PR mean ± SD | 395.5 ± 77.2 | ||||||||
| Heves County | |||||||||
| 2016/2017 | 66.3 | 48.7 | 0.4 | 32.8 | 31.8 | 9.6 | 76.2 | 79.9 | 117.5 |
| 2017/2018 | 46.2 | 43.8 | 44.8 | 18.2 | 53.9 | 51.8 | 32.8 | 43.8 | 86.6 |
| 2018/2019 | 30 | 45.5 | 36.8 | 18.4 | 7.1 | 5.3 | 40.8 | 112.3 | 131.5 |
| 2019/2020 | 15.8 | 103.2 | 48.5 | 15.7 | 28.1 | 34.3 | 7.6 | 19.1 | 151.0 |
| 2020/2021 | 146.2 | 29.2 | 42.5 | 40.1 | 52.5 | 6.8 | 56.3 | 78 | 21.4 |
| Five-season PR mean ± SD | 441.8 ± 24.3 | ||||||||
| Pest County | |||||||||
| 2016/2017 | 58.7 | 45.3 | 2.2 | 30.1 | 33.9 | 33.3 | 66.9 | 70.5 | 39.5 |
| 2017/2018 | 72.6 | 47.6 | 37.3 | 21.6 | 66.9 | 68.9 | 16.1 | 27.9 | 121.9 |
| 2018/2019 | 14 | 53.4 | 26.7 | 21 | 7.2 | 7.4 | 30.9 | 192.3 | 33.4 |
| 2019/2020 | 8.1 | 78.6 | 54.6 | 14.7 | 28.2 | 36.5 | 5.7 | 16.4 | 144.2 |
| 2020/2021 | 102.2 | 21.1 | 37.1 | 15.1 | 35.3 | 6.7 | 32.3 | 74.8 | 22.2 |
| Five-season PR mean ± SD | 396.3 ± 50.1 | ||||||||
| Somogy County | |||||||||
| 2016/2017 | 63.2 | 63.6 | 0.6 | 18.7 | 54.6 | 18.4 | 34.6 | 78.5 | 68.6 |
| 2017/2018 | 81.7 | 56.5 | 81.2 | 29.8 | 68.9 | 121.6 | 15.5 | 69.1 | 125.6 |
| 2018/2019 | 15.6 | 36.3 | 12.4 | 27.1 | 14.3 | 18.2 | 62.7 | 130.5 | 74.1 |
| 2019/2020 | 24.2 | 106.7 | 62.7 | 17.1 | 31.5 | 22.2 | 19.9 | 39.2 | 52.8 |
| 2020/2021 | 106.2 | 6.4 | 49.3 | 29.9 | 31.5 | 12.4 | 32.8 | 74.4 | 14.2 |
| Five-season PR mean ± SD | 435.1 ± 121.2 | ||||||||
| Zala county | |||||||
| Season | Infection rate% | Ta °C | P mm | Weather classes | |||
| May | June | May | June | May | June | ||
| 2017 | 12.8 | W | W * | A * | A * | Warm–dry | Warm–dry |
| 2018 | 30.2 | W * | W | H | H * | Warm–wet | Warm–wet |
| 2019 | 25.7 | C * | W * | H * | A * | Cool–wet | Warm–dry |
| 2020 | 27.2 | C * | C | A * | H * | Cool–dry | Cool–wet |
| 2021 | 12.6 | C * | W * | H * | A * | Cool–wet | Warm–dry |
| Heves county | |||||||
| Season | Infection rate% | Ta °C | P mm | Weather classes | |||
| May | June | May | June | May | June | ||
| 2017 | 2.5 | W | N | H * | H * | Warm–wet | Normal–wet |
| 2018 | 5.3 | W * | C | A * | A | Warm–dry | Cool–dry |
| 2019 | 4.4 | C * | W * | H * | H * | Cool–wet | Warm–wet |
| 2020 | 4.0 | C * | C | A * | H * | Cool–dry | Cool–wet |
| 2021 | 3.5 | C * | W | H * | A * | Cool–wet | Warm–dry |
| Tolna county | |||||||
| Season | Infection rate% | Ta °C | P mm | Weather classes | |||
| May | June | May | June | May | June | ||
| 2017 | 9.0 | W | W | A * | H * | Warm–dry | Warm–wet |
| 2018 | 13.7 | W * | C | A * | H * | Warm–dry | Cool–wet |
| 2019 | 20.6 | C * | W * | H * | A * | Cool–wet | Warm–dry |
| 2020 | 15.5 | C * | C * | A * | H * | Cool–dry | Cool–wet |
| 2021 | 11.7 | C | W | H * | A * | Cool–wet | Warm–dry |
| Somogy county | |||||||
| Season | Infection rate% | Ta °C | P mm | Weather classes | |||
| May | June | May | June | May | June | ||
| 2017 | 5.1 | W | W | N | H | Warm–norm | Warm–wet |
| 2018 | 13.1 | W * | W * | A | H * | Warm–dry | Warm–wet |
| 2019 | 16.4 | C * | W * | H * | H | Cool–wet | Warm–wet |
| 2020 | 9.4 | C * | C * | A * | A * | Cool–dry | Cool–dry |
| 2021 | 4.8 | C * | C | A | A * | Cool–dry | Cool–dry |
| Pest county | |||||||
| Season | Infection rate% | Ta °C | P mm | Weather classes | |||
| May | June | May | June | May | June | ||
| 2017 | 2.6 | W | N | A * | A * | Warm–dry | Norm–dry |
| 2018 | 10.4 | W * | C | A * | H * | Warm–dry | Cool–wet |
| 2019 | 5.5 | C * | W * | H * | A * | Cool–wet | Warm–dry |
| 2020 | 8.1 | C * | C * | A * | H * | Cool–dry | Cool–wet |
| 2021 | 3.6 | C * | W * | A | A * | Cool–dry | Warm–dry |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anda, A.; Simon-Gáspár, B.; Simon, S.; Soós, G.; Menyhárt, L. Modeling Risk in Fusarium Head Blight and Yield Analysis in Five Winter Wheat Production Regions of Hungary. Agriculture 2024, 14, 1093. https://doi.org/10.3390/agriculture14071093
Anda A, Simon-Gáspár B, Simon S, Soós G, Menyhárt L. Modeling Risk in Fusarium Head Blight and Yield Analysis in Five Winter Wheat Production Regions of Hungary. Agriculture. 2024; 14(7):1093. https://doi.org/10.3390/agriculture14071093
Chicago/Turabian StyleAnda, Angela, Brigitta Simon-Gáspár, Szabina Simon, Gábor Soós, and László Menyhárt. 2024. "Modeling Risk in Fusarium Head Blight and Yield Analysis in Five Winter Wheat Production Regions of Hungary" Agriculture 14, no. 7: 1093. https://doi.org/10.3390/agriculture14071093
APA StyleAnda, A., Simon-Gáspár, B., Simon, S., Soós, G., & Menyhárt, L. (2024). Modeling Risk in Fusarium Head Blight and Yield Analysis in Five Winter Wheat Production Regions of Hungary. Agriculture, 14(7), 1093. https://doi.org/10.3390/agriculture14071093






