Advancements in Real-Time Monitoring of Enteric Methane Emissions from Ruminants
Abstract
1. Introduction
Enteric CH4 Emissions

2. Monitoring of Biomarkers and Proxies for Ruminant Health and CH4 Emissions
2.1. Feed Intake and Feeding Behaviour
2.2. Rumination Time
2.3. Rumen Status
2.3.1. Reticulo-Ruminal pH
2.3.2. Reticulo-Ruminal Temperature
2.3.3. Reticulo-Rumen Motility
3. Animal-Based Techniques for Measuring Enteric CH4
3.1. Respiration Chambers
3.2. Sulphur Hexafluoride (SF6) Tracer Method
3.3. Spot Sampling
3.4. Laser CH4 Detectors
3.5. Open-Path Laser
4. Emerging Technologies and Techniques
4.1. Portable Accumulation Chamber
4.2. CO2 Tracer Method
4.3. Optical Gas Imaging (OGI)
5. Discussion and Future Outlook
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- European Environment Agency. Progress and Prospects for Decarbonisation in the Agriculture Sector and Beyond; European Environment Agency: Brussels, Brussels, 2022.
- Statista Agriculture Emissions Worldwide—Statistics & Facts. Available online: https://www.statista.com/topics/10348/agriculture-emissions-worldwide/#topicOverview (accessed on 24 June 2024).
- Environmental Protection Agency. Ireland’s National Inventory Report 2021; Environmental Protection Agency: Wexford, Ireland, 2021.
- O’Connor, S. Meeting Ireland’s Sustainability Challenges and Obligations: The Potential and Viability of Small-Scale Anaerobic Digestion; Atlantic Technological University Sligo: Sligo, Ireland, 2022. [Google Scholar]
- O’Connor, S.; Ehimen, E.; Pillai, S.C.; Black, A.; Bartlett, J. Biogas Production from Small-Scale Anaerobic Digestion Plants on European Farms. Renew. Sustain. Energy Rev. 2021, 139, 110580. [Google Scholar] [CrossRef]
- Helwatkar, A.; Riordan, D.; Walsh, J. Sensor Technology For Animal Health Monitoring. In Proceedings of the International Journal On Smart Sensing and Intelligent Systems, Liverpool, UK, 2–4 September 2014. [Google Scholar]
- Goopy, J.P.; Chang, C.; Tomkins, N. A Comparison of Methodologies for Measuring Methane Emissions from Ruminants. In Methods for Measuring Greenhouse Gas Balances and Evaluating Mitigation Options in Smallholder Agriculture; Springer: Berlin/Heidelberg, Germany, 2016; pp. 97–117. [Google Scholar] [CrossRef]
- Raynor, E.J.; Schilling-Hazlett, A.; Place, S.E.; Martinez, J.V.; Thompson, L.R.; Johnston, M.K.; Jorns, T.R.; Beck, M.R.; Kuehn, L.A.; Derner, J.D.; et al. Snapshot of Enteric Methane Emissions from Stocker Cattle Grazing Extensive Semiarid Rangelands. Rangel. Ecol. Manag. 2024, 93, 77–80. [Google Scholar] [CrossRef]
- Beauchemin, K.A.; Kreuzer, M.; O’Mara, F.; McAllister, T.A. Nutritional Management for Enteric Methane Abatement: A Review. Aust. J. Exp. Agric. 2008, 48, 21–27. [Google Scholar] [CrossRef]
- Bačėninaitė, D.; Džermeikaitė, K.; Antanaitis, R. Global Warming and Dairy Cattle: How to Control and Reduce Methane Emission. Animals 2022, 12, 2687. [Google Scholar] [CrossRef] [PubMed]
- Chagunda, M.G. Opportunities and Challenges in the Use of the Laser Methane Detector to Monitor Enteric Methane Emissions from Ruminants. Animal 2013, 7, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Muñoz, C.; Yan, T.; Wills, D.A.; Murray, S.; Gordon, A.W. Comparison of the Sulfur Hexafluoride Tracer and Respiration Chamber Techniques for Estimating Methane Emissions and Correction for Rectum Methane Output from Dairy Cows. J. Dairy Sci. 2012, 95, 3139–3148. [Google Scholar] [CrossRef] [PubMed]
- Tedeschi, L.O.; Abdalla, A.L.; Álvarez, C.; Anuga, S.W.; Arango, J.; Beauchemin, K.A.; Becquet, P.; Berndt, A.; Burns, R.; De Camillis, C.; et al. Quantification of Methane Emitted by Ruminants: A Review of Methods. J. Anim. Sci. 2022, 100, skac197. [Google Scholar] [CrossRef] [PubMed]
- Moraes, L.E.; Strathe, A.B.; Fadel, J.G.; Casper, D.P.; Kebreab, E. Prediction of Enteric Methane Emissions from Cattle. Glob. Chang. Biol. 2007, 20, 2140–2148. [Google Scholar] [CrossRef] [PubMed]
- Džermeikaitė, K.; Krištolaitytė, J.; Antanaitis, R. Relationship between Dairy Cow Health and Intensity of Greenhouse Gas Emissions. Animals 2024, 14, 829. [Google Scholar] [CrossRef]
- Bobade, M.; Khune, V.; Mishra, S.; Dubey, A.; Yadav, A.; Soni, A.; Bhagat, S.; Das, S.; Krishnan, K. New Age Dairy Farming: Precision Dairy Farming (PDF): A Review. Int. J. Chem. Stud. 2020, 8, 1041–1046. [Google Scholar] [CrossRef]
- Negussie, E.; de Haas, Y.; Dehareng, F.; Dewhurst, R.J.; Dijkstra, J.; Gengler, N.; Morgavi, D.P.; Soyeurt, H.; van Gastelen, S.; Yan, T.; et al. Large-Scale Indirect Measurements for Enteric Methane Emissions in Dairy Cattle: A Review of Proxies and Their Potential for Use in Management and Breeding Decisions. J. Dairy Sci. 2017, 100, 2433–2453. [Google Scholar] [CrossRef] [PubMed]
- Tomkins, N.W.; McGinn, S.M.; Turner, D.A.; Charmley, E. Comparison of Open-Circuit Respiration Chambers with a Micrometeorological Method for Determining Methane Emissions from Beef Cattle Grazing a Tropical Pasture. Anim. Feed Sci. Technol. 2011, 166–167, 240–247. [Google Scholar] [CrossRef]
- Ellis, J.L.; Kebreab, E.; Odongo, N.E.; McBride, B.W.; Okine, E.K.; France, J. Prediction of Methane Production from Dairy and Beef Cattle. J. Dairy Sci. 2007, 90, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Lovendahll, P.; Difford, G.F.; Li, B.; Chagunda, M.G.G.; Huhtanen, P.; Lidauer, M.H.; Lassen, J.; Lund, P. Review: Selecting for Improved Feed Efficiency and Reduced Methane Emissions in Dairy Cattle. Animal 2018, 12, s336–s349. [Google Scholar] [CrossRef] [PubMed]
- Negussie, E.; Lehtinen, J.; Mäntysaari, P.; Bayat, A.R.; Liinamo, A.E.; Mäntysaari, E.A.; Lidauer, M.H. Non-Invasive Individual Methane Measurement in Dairy Cows. Animal 2017, 11, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Zehner, N.; Umstätter, C.; Niederhauser, J.J.; Schick, M. System Specification and Validation of a Noseband Pressure Sensor for Measurement of Ruminating and Eating Behavior in Stable-Fed Cows. Comput. Electron. Agric. 2017, 136, 31–41. [Google Scholar] [CrossRef]
- Gonzalez, L.A.; Kyriazakis, I.; Tedeschi, L.O. Review: Precision Nutrition of Ruminants: Approaches, Challenges and Potential Gains. Animal 2018, 12, S246–S261. [Google Scholar] [CrossRef] [PubMed]
- Braun, U.; Trösch, L.; Nydegger, F.; Hässig, M. Evaluation of Eating and Rumination Behaviour in Cows Using a Noseband Pressure Sensor. BMC Vet. Res. 2013, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Leiber, F.; Holinger, M.; Zehner, N.; Dorn, K.; Probst, J.K.; Spengler Neff, A. Intake Estimation in Dairy Cows Fed Roughage-Based Diets: An Approach Based on Chewing Behaviour Measurements. Appl. Anim. Behav. Sci. 2016, 185, 9–14. [Google Scholar] [CrossRef]
- Rombach, M.; Münger, A.; Niederhauser, J.; Südekum, K.H.; Schori, F. Evaluation and Validation of an Automatic Jaw Movement Recorder (RumiWatch) for Ingestive and Rumination Behaviors of Dairy Cows during Grazing and Supplementation. J. Dairy Sci. 2018, 101, 2463–2475. [Google Scholar] [CrossRef]
- Zehner, N.; Niederhauser, J.J.; Nydegger, F.; Grothmann, A.; Keller, M.; Hoch, M.; Haeussermann, A.; Schick, M. Validation of a New Health Monitoring System (RumiWatch) for Combined Automatic Measurement of Rumination, Feed Intake, Water Intake and Locomotion in Dairy Cows. In Proceedings of the International Conference of Agricultural Engineering CIGR-Ageng 2012, Valencia, Spain, 8–12 July 2012. [Google Scholar]
- Galli, J.R.; Cangiano, C.A.; Milone, D.H.; Laca, E.A. Acoustic Monitoring of Short-Term Ingestive Behavior and Intake in Grazing Sheep. Livest. Sci. 2011, 140, 32–41. [Google Scholar] [CrossRef]
- Hajnal, É.; Kovács, L.; Vakulya, G. Dairy Cattle Rumen Bolus Developments with Special Regard to the Applicable Artificial Intelligence (AI) Methods. Sensors 2022, 22, 6812. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Okada, H.; Sawada, H.; Takahashi, Y.; Kimura, K.; Itoh, T. Evaluation of Ruminal Motility in Cattle by a Bolus-Type Wireless Sensor. J. Vet. Med. Sci. 2019, 81, 1835–1841. [Google Scholar] [CrossRef]
- Gesler, P. Chapter 10: Rumen Bolus Technology at Commercial Farms. In Practical Precision Livestock Farming: Hands-on Experiences with PLF Technologies in Commercial and R&D Settings; Waheningen Academic Publishers: Wageningen, The Netherlands, 2022; pp. 165–173. [Google Scholar] [CrossRef]
- Moonsyst International Ltd. Available online: https://moonsyst.com/home (accessed on 17 May 2024).
- Mottram, T.; Lowe, J.; McGowan, M.; Phillips, N. Technical Note: A Wireless Telemetric Method of Monitoring Clinical Acidosis in Dairy Cows. Comput. Electron. Agric. 2008, 64, 45–48. [Google Scholar] [CrossRef]
- Mikuła, R.; Pszczola, M.; Rzewuska, K.; Mucha, S.; Nowak, W.; Strabel, T. The Effect of Rumination Time on Milk Performance and Methane Emission of Dairy Cows Fed Partial Mixed Ration Based on Maize Silage. Animals 2022, 12, 50. [Google Scholar] [CrossRef]
- Paudyal, S. Using Rumination Time to Manage Health and Reproduction in Dairy Cattle: A Review. Vet. Q. 2021, 41, 292–300. [Google Scholar] [CrossRef]
- Lindgren, E. Validation of Rumination Measurement Equipment and the Role of Rumination in Dairy Cow Time Budgets; Swedish University of Agricultural: Uppsala, Sweden, 2009. [Google Scholar]
- Huang, H.; Szumacher-Strabel, M.; Patra, A.K.; Ślusarczyk, S.; Lechniak, D.; Vazirigohar, M.; Varadyova, Z.; Kozłowska, M.; Cieślak, A. Chemical and Phytochemical Composition, in Vitro Ruminal Fermentation, Methane Production, and Nutrient Degradability of Fresh and Ensiled Paulownia Hybrid Leaves. Anim. Feed Sci. Technol. 2021, 279, 115038. [Google Scholar] [CrossRef]
- Han, C.S.; Kaur, U.; Bai, H.; Roqueto dos Reis, B.; White, R.; Nawrocki, R.A.; Voyles, R.M.; Kang, M.G.; Priya, S. Sensor Technologies for Real-Time Monitoring of the Rumen Environment. J. Dairy Sci. 2022, 105, 6379–6404. [Google Scholar] [CrossRef]
- Penner, G.B.; Aschenbach, J.R.; Gäbel, G.; Oba, M. Technical Note: Evaluation of a Continuous Ruminal PH Measurement System for Use in Noncannulated Small Ruminants. J. Anim. Sci. 2009, 87, 2363–2366. [Google Scholar] [CrossRef]
- González, L.A.; Manteca, X.; Calsamiglia, S.; Schwartzkopf-Genswein, K.S.; Ferret, A. Ruminal Acidosis in Feedlot Cattle: Interplay between Feed Ingredients, Rumen Function and Feeding Behavior (a Review). Anim. Feed Sci. Technol. 2012, 172, 66–79. [Google Scholar] [CrossRef]
- Dijkstra, J.; Van Gastelen, S.; Dieho, K.; Nichols, K.; Bannink, A. Review: Rumen Sensors: Data and Interpretation for Key Rumen Metabolic Processes. Animal 2020, 14, s176–s186. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, A.W.; Davison, C.; Tachtatzis, C.; Andonovic, I.; Michie, C.; Ferguson, H.J.; Somerville, L.; Jonsson, N.N. Identification of the Rumination in Cattle Using Support Vector Machines with Motion-Sensitive Bolus Sensors. Sensors 2019, 19, 1165. [Google Scholar] [CrossRef] [PubMed]
- DePeters, E.J.; George, L.W. Rumen Transfaunation. Immunol. Lett. 2014, 162, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P. Precision Dairy Farming: The Next Dairy Marvel. J. Vet. Sci. Technol. 2014, 5. [Google Scholar] [CrossRef]
- Lee, M.; Seo, S. Wearable Wireless Biosensor Technology for Monitoring Cattle: A Review. Animals 2021, 11, 2779. [Google Scholar] [CrossRef] [PubMed]
- Burnett, T.A.; Kaur, M.; Polsky, L.; Cerri, R.L.A. Rumen-Reticular Temperature During Estrus and Ovulation Using Automated Activity Monitors in Dairy Cows. Front. Vet. Sci. 2020, 7, 597512. [Google Scholar] [CrossRef] [PubMed]
- Alzahal, O.; Alzahal, H.; Steele, M.A.; Van Schaik, M.; Kyriazakis, I.; Duffield, T.F.; McBride, B.W. The Use of a Radiotelemetric Ruminal Bolus to Detect Body Temperature Changes in Lactating Dairy Cattle. J. Dairy Sci. 2011, 94, 3568–3574. [Google Scholar] [CrossRef]
- Kim, H.; Min, Y.; Choi, B. Real-Time Temperature Monitoring for the Early Detection of Mastitis in Dairy Cattle: Methods and Case Researches. Comput. Electron. Agric. 2019, 162, 119–125. [Google Scholar] [CrossRef]
- Costa, J.B.G.; Ahola, J.K.; Weller, Z.D.; Peel, R.K.; Whittier, J.C.; Barcellos, J.O.J. Reticulo-Rumen Temperature as a Predictor of Calving Time in Primiparous and Parous Holstein Females. J. Dairy Sci. 2016, 99, 4839–4850. [Google Scholar] [CrossRef]
- Ahn, G.; Ricconi, K.; Avila, S.; Klotz, J.L.; Harmon, D.L. Ruminal Motility, Reticuloruminal Fill, and Eating Patterns in Steers Exposed to Ergovaline. J. Anim. Sci. 2020, 98, skz374. [Google Scholar] [CrossRef]
- Ammer, S.; Lambertz, C.; Gauly, M. Is Reticular Temperature a Useful Indicator of Heat Stress in Dairy Cattle? J. Dairy Sci. 2016, 99, 10067–10076. [Google Scholar] [CrossRef] [PubMed]
- Lees, A.M.; Lees, J.C.; Lisle, A.T.; Sullivan, M.L.; Gaughan, J.B. Effect of Heat Stress on Rumen Temperature of Three Breeds of Cattle. Int. J. Biometeorol. 2018, 62, 207–215. [Google Scholar] [CrossRef]
- Aoki, M.; Kimura, K.; Suzuki, O. Predicting Time of Parturition from Changing Vaginal Temperature Measured by Data-Logging Apparatus in Beef Cows with Twin Fetuses. Anim. Reprod. Sci. 2005, 86, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cooper-Prado, M.J.; Long, N.M.; Wright, E.C.; Goad, C.L.; Wettemann, R.P. Relationship of Ruminal Temperature with Parturition and Estrus of Beef Cows. J. Anim. Sci. 2011, 89, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Humer, E.; Ghareeb, K.; Harder, H.; Mickdam, E.; Khol-Parisini, A.; Zebeli, Q. Peripartal Changes in Reticuloruminal PH and Temperature in Dairy Cows Differing in the Susceptibility to Subacute Rumen Acidosis. J. Dairy Sci. 2015, 98, 8788–8799. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Ha, J.; Kwon, W.S.; Moon, J.; Gim, G.M.; Yi, J. Change of Ruminoreticular Temperature and Body Activity before and after Parturition in Hanwoo (Bos Taurus Coreanae) Cows. Sensors 2021, 21, 7892. [Google Scholar] [CrossRef] [PubMed]
- Kovács, L.; Kézér, F.L.; Ruff, F.; Szenci, O. Rumination time and reticuloruminal temperature as possible predictors of dystocia in dairy cows. J. Dairy Sci. 2017, 100, 1568–1579. [Google Scholar] [CrossRef] [PubMed]
- Firk, R.; Stamer, E.; Junge, W.; Krieter, J. Automation of Oestrus Detection in Dairy Cows: A Review. Livest. Prod. Sci. 2002, 75, 219–232. [Google Scholar] [CrossRef]
- Vicentini, R.R.; Bernardes, P.A.; Ujita, A.; Oliveira, A.P.; Lima, M.L.P.; El Faro, L.; Sant’Anna, A.C. Predictive Potential of Activity and Reticulo-Rumen Temperature Variation for Calving in Gyr Heifers (Bos Taurus Indicus). J. Therm. Biol. 2021, 95, 102793. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, R.R.; Oliveira, A.P.; Veroneze, R.; Montanholi, Y.R.; Lima, M.L.P.; Ujita, A.; Alves, S.F.; de Lima, A.C.N.; El Faro, L. Reticulo-Rumen Temperature as a Predictor of Estrus in Dairy Gir Heifers. In Proceedings of the 11th World Congress on Genetics Applied to Livestock Production, Auckland, New Zealand, 11–16 February 2018. [Google Scholar]
- Boehmer, B.H.; Pye, T.A.; Wettemann, R.P. Ruminal Temperature as a Measure of Body Temperature of Beef Cows and Relationship with Ambient Temperature. Prof. Anim. Sci. 2015, 31, 387–393. [Google Scholar] [CrossRef]
- Bewley, J.M.; Grott, M.W.; Einstein, M.E.; Schutz, M.M. Impact of Intake Water Temperatures on Reticular Temperatures of Lactating Dairy Cows. J. Dairy Sci. 2008, 91, 3880–3887. [Google Scholar] [CrossRef]
- Haubro Andersen, P. Bovine Endotoxicosis--Some Aspects of Relevance to Production Diseases. A Review. Acta Vet. Scand. Suppl. 2003, 98, 141–155. [Google Scholar] [CrossRef]
- Rose, M.K. Metabolic Alterations in Buffaloes Suffering from Digestive Disorders. Haryana Vet. 2013, 52, 71–72. [Google Scholar]
- Song, X.; van der Tol, P.P.J.; Groot Koerkamp, P.W.G.; Bokkers, E.A.M. Hot Topic: Automated Assessment of Reticulo-Ruminal Motility in Dairy Cows Using 3-Dimensional Vision. J. Dairy Sci. 2019, 102, 9076–9081. [Google Scholar] [CrossRef]
- McSweeney, C.S.; Kennedy, P.M.; John, A. Reticulo-Ruminal Motility in Cattle (Bos Indicus) and Water Buffaloes (Bubalus Bubalis) Fed a Low Quality Roughage Diet. Comp. Biochem. Physiol. A Physiol. 1989, 94, 635–638. [Google Scholar] [CrossRef]
- Scheurwater, J.; Hostens, M.; Nielen, M.; Heesterbeek, H.; Schot, A.; van Hoeij, R.; Aardema, H. Pressure Measurement in the Reticulum to Detect Different Behaviors of Healthy Cows. PLoS ONE 2021, 16, e0254410. [Google Scholar] [CrossRef]
- Braun, U.; Rauch, S. Ultrasonographic Evaluation of Reticular Motility during Rest, Eating, Rumination and Stress in 30 Healthy Cows. Vet. Rec. 2008, 163, 571–574. [Google Scholar] [CrossRef]
- Foster, D. Disorders of Rumen Distension and Dysmotility. Vet. Clin. N. Am. Food Anim. Pract. 2017, 33, 499–512. [Google Scholar] [CrossRef]
- Okine, E.K.; Mathison, G.W.; Kaske, M.; Kennelly, J.J.; Christopherson, R.J. Current Understanding of the Role of the Reticulum and Reticulo-Omasal Orifice in the Control of Digesta Passage from the Ruminoreticulum of Sheep and Cattle. Can. J. Anim. Sci. 2011, 78, 15–21. [Google Scholar] [CrossRef]
- Braun, U.; Schweizer, A. Ultrasonographic Assessment of Reticuloruminal Motility in 45 Cows. Schweiz. Arch. Tierheilkd 2015, 157, 87–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arai, S.; Haritani, M.; Sawada, H.; Kimura, K. Effect of Mosapride on Ruminal Motility in Cattle. J. Vet. Med. Sci. 2019, 81, 1017. [Google Scholar] [CrossRef]
- Nogami, H.; Arai, S.; Okada, H.; Zhan, L.; Itoh, T. Minimized Bolus-Type Wireless Sensor Node with a Built-In Three-Axis Acceleration Meter for Monitoring a Cow’s Rumen Conditions. Sensors 2017, 17, 687. [Google Scholar] [CrossRef]
- Humer, E.; Aschenbach, J.R.; Neubauer, V.; Kröger, I.; Khiaosa-ard, R.; Baumgartner, W.; Zebeli, Q. Signals for Identifying Cows at Risk of Subacute Ruminal Acidosis in Dairy Veterinary Practice. J. Anim. Physiol. Anim. Nutr. 2018, 102, 380–392. [Google Scholar] [CrossRef]
- Andersson, L.M.; Arai, S.; Okada, H. Orally Administrable Wireless Activity and PH Probe for Cattle Reticulum. Sens. Mater. 2018, 30, 3029–3038. [Google Scholar] [CrossRef]
- Rose-Dye, T.K.; Burciaga-Robles, L.O.; Krehbiel, C.R.; Step, D.L.; Fulton, R.W.; Confer, A.W.; Richards, C.J. Rumen Temperature Change Monitored with Remote Rumen Temperature Boluses after Challenges with Bovine Viral Diarrhea Virus and Mannheimia Haemolytica. J. Anim. Sci. 2011, 89, 1193–1200. [Google Scholar] [CrossRef]
- Hill, J.; McSweeney, C.; Wright, A.D.G.; Bishop-Hurley, G.; Kalantar-zadeh, K. Measuring Methane Production from Ruminants. Trends Biotechnol. 2016, 34, 26–35. [Google Scholar] [CrossRef]
- Storm, I.M.L.D.; Hellwing, A.L.F.; Nielsen, N.I.; Madsen, J. Methods for Measuring and Estimating Methane Emission from Ruminants. Animals 2012, 2, 160–183. [Google Scholar] [CrossRef]
- Hammond, K.J.; Waghorn, G.C.; Hegarty, R.S. The GreenFeed System for Measurement of Enteric Methane Emission from Cattle. Anim. Prod. Sci. 2016, 56, 181–189. [Google Scholar] [CrossRef]
- Rosenstock, T.S.; Rufino, M.C.; Butterbach-Bahl, K.; Wollenberg, E.; Richards, M. Methods for Measuring Greenhouse Gas Balances and Evaluating Mitigation Options in Smallholder Agriculture; Springer Nature: Berlin/Heidelberg, Germany, 2016; pp. 1–203. [Google Scholar] [CrossRef]
- Klein, L.; Wright, A.D.G. Construction and Operation of Open-Circuit Methane Chambers for Small Ruminants. Aust. J. Exp. Agric. 2006, 46, 1257–1262. [Google Scholar] [CrossRef]
- Grainger, C.; Clarke, T.; McGinn, S.M.; Auldist, M.J.; Beauchemin, K.A.; Hannah, M.C.; Waghorn, G.C.; Clark, H.; Eckard, R.J. Methane Emissions from Dairy Cows Measured Using the Sulfur Hexafluoride (SF6) Tracer and Chamber Techniques. J. Dairy Sci. 2007, 90, 2755–2766. [Google Scholar] [CrossRef] [PubMed]
- Goopy, J.P.; Donaldson, A.; Hegarty, R.; Vercoe, P.E.; Haynes, F.; Barnett, M.; Oddy, V.H. Low-Methane Yield Sheep Have Smaller Rumens and Shorter Rumen Retention Time. Br. J. Nutr. 2014, 111, 578–585. [Google Scholar] [CrossRef]
- Huhtanen, P.; Ramin, M.; Hristov, A.N. Enteric Methane Emission Can Be Reliably Measured by the GreenFeed Monitoring Unit. Livest. Sci. 2019, 222, 31–40. [Google Scholar] [CrossRef]
- Hellwing, A.L.F.; Lund, P.; Weisbjerg, M.R.; Brask, M.; Hvelplund, T. Technical Note: Test of a Low-Cost and Animal-Friendly System for Measuring Methane Emissions from Dairy Cows. J. Dairy Sci. 2012, 95, 6077–6085. [Google Scholar] [CrossRef] [PubMed]
- McGinn, S.M.; Beauchemin, K.A.; Flesch, T.K.; Coates, T. Performance of a Dispersion Model to Estimate Methane Loss from Cattle in Pens. J. Environ. Qual. 2009, 38, 1796–1802. [Google Scholar] [CrossRef]
- Kebreab, E.; Clark, K.; Wagner-Riddle, C.; France, J. Methane and Nitrous Oxide Emissions from Canadian Animal Agriculture: A Review. Can. J. Anim. Sci. 2011, 86, 135–158. [Google Scholar] [CrossRef]
- Negussie, E.; González-Recio, O.; Battagin, M.; Bayat, A.R.; Boland, T.; de Haas, Y.; Garcia-Rodriguez, A.; Garnsworthy, P.C.; Gengler, N.; Kreuzer, M.; et al. Integrating Heterogeneous Across-Country Data for Proxy-Based Random Forest Prediction of Enteric Methane in Dairy Cattle. J. Dairy Sci. 2022, 105, 5124–5140. [Google Scholar] [CrossRef]
- Teagasc. TResearch Autumn 2022: Cleaning the Air; Teagasc: Carlow, Ireland, 2022. [Google Scholar]
- Boadi, D.A.; Wittenberg, K.M. Methane Production from Dairy and Beef Heifers Fed Forages Differing in Nutrient Density Using the Sulphur Hexafluoride (SF6) Tracer Gas Technique. Can. J. Anim. Sci. 2011, 82, 201–206. [Google Scholar] [CrossRef]
- Machmüller, A.; Hegarty, R.S. Alternative Tracer Gases for the ERUCT Technique to Estimate Methane Emission from Grazing Animals. Int. Congr. Ser. 2006, 1293, 50–53. [Google Scholar] [CrossRef]
- Bekele, W.; Guinguina, A.; Zegeye, A.; Simachew, A.; Ramin, M. Contemporary Methods of Measuring and Estimating Methane Emission from Ruminants. Methane 2022, 1, 82–95. [Google Scholar] [CrossRef]
- Garnsworthy, P.C.; Difford, G.F.; Bell, M.J.; Bayat, A.R.; Huhtanen, P.; Kuhla, B.; Lassen, J.; Peiren, N.; Pszczola, M.; Sorg, D.; et al. Comparison of Methods to Measure Methane for Use in Genetic Evaluation of Dairy Cattle. Animals 2019, 9, 837. [Google Scholar] [CrossRef] [PubMed]
- Broucek, J. Methods of Methane Measurement in Ruminants. Anim. Sci. 2014, 47, 51–60. [Google Scholar]
- Pinares-Patiño, C.; Gere, J.; Williams, K.; Gratton, R.; Juliarena, P.; Molano, G.; MacLean, S.; Sandoval, E.; Taylor, G.; Koolaard, J. Extending the Collection Duration of Breath Samples for Enteric Methane Emission Estimation Using the SF6 Tracer Technique. Animals 2012, 2, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Lassey, K.R. Livestock Methane Emission: From the Individual Grazing Animal through National Inventories to the Global Methane Cycle. Agric. For. Meteorol. 2007, 142, 120–132. [Google Scholar] [CrossRef]
- Ghassemi Nejad, J.; Ju, M.S.; Jo, J.H.; Oh, K.H.; Lee, Y.S.; Lee, S.D.; Kim, E.J.; Roh, S.; Lee, H.G. Advances in Methane Emission Estimation in Livestock: A Review of Data Collection Methods, Model Development and the Role of AI Technologies. Animals 2024, 14, 435. [Google Scholar] [CrossRef] [PubMed]
- Hegarty, R.S. Applicability of Short-Term Emission Measurements for on-Farm Quantification of Enteric Methane. Animal 2013, 7 (Suppl. S2), 401–408. [Google Scholar] [CrossRef] [PubMed]
- Huhtanen, P.; Cabezas-Garcia, E.H.; Utsumi, S.; Zimmerman, S. Comparison of Methods to Determine Methane Emissions from Dairy Cows in Farm Conditions. J. Dairy Sci. 2015, 98, 3394–3409. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.J.; Saunders, N.; Wilcox, R.H.; Homer, E.M.; Goodman, J.R.; Craigon, J.; Garnsworthy, P.C. Methane Emissions among Individual Dairy Cows during Milking Quantified by Eructation Peaks or Ratio with Carbon Dioxide. J. Dairy Sci. 2014, 97, 6536–6546. [Google Scholar] [CrossRef]
- Agri Data Analytics. Available online: https://agridataanalytics.com/ (accessed on 25 June 2024).
- Zelp. 2024. Zelp Website. Available online: https://www.zelp.co/ (accessed on 23 May 2024).
- Van Well, B.; Murray, S.; Hodgkinson, J.; Pride, R.; Strzoda, R.; Gibson, G.; Padgett, M. An Open-Path, Hand-Held Laser System for the Detection of Methane Gas. J. Opt. A Pure Appl. Opt. 2005, 7, S420. [Google Scholar] [CrossRef]
- Hristov, A.N.; Kebreab, E.; Niu, M.; Oh, J.; Bannink, A.; Bayat, A.R.; Boland, T.M.; Brito, A.F.; Casper, D.P.; Crompton, L.A.; et al. Symposium Review: Uncertainties in Enteric Methane Inventories, Measurement Techniques, and Prediction Models. J. Dairy Sci. 2018, 101, 6655–6674. [Google Scholar] [CrossRef]
- Ricci, P.; Chagunda, M.G.G.; Rooke, J.; Houdijk, J.G.M.; Duthie, C.A.; Hyslop, J.; Roehe, R.; Waterhouse, A. Evaluation of the Laser Methane Detector to Estimate Methane Emissions from Ewes and Steers. J. Anim. Sci. 2014, 92, 5239–5250. [Google Scholar] [CrossRef]
- Sorg, D.; Muhlbach, S.; Rosner, F.; Kuhla, B.; Derno, M.; Meese, S.; Schwarm, A.; Kreuz, M.; Swalve, H. The Agreement between Two Next-Generation Laser Methane Detectors and Respiration Chamber Facilities in Recording Methane Concentrations in the Spent Air Produced by Dairy Cows. Comput. Electron. Agric. 2017, 143, 262–272. [Google Scholar] [CrossRef]
- Sorg, D. Measuring Livestock CH4 Emissions with the Laser Methane Detector: A Review. Methane 2021, 1, 38–57. [Google Scholar] [CrossRef]
- Patra, A.K. Recent Advances in Measurement and Dietary Mitigation of Enteric Methane Emissions in Ruminants. Front. Vet. Sci. 2016, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.A.; Johnson, D.E. Methane Emissions from Cattle. J. Anim. Sci. 1995, 73, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Weerasekara, C.; Morris, L.C.; Malarich, N.A.; Giorgetta, F.R.; Herman, D.I.; Cossel, K.C.; Newbury, N.R.; Owensby, C.E.; Welch, S.M.; DePaola, B.D.; et al. Using Open-Path Dual-Comb Spectroscopy to Monitor Methane Emissions from Simulated Grazing Cattle. EGUsphere 2024, 1181. [Google Scholar] [CrossRef]
- Sun, K.; Tao, L.; Miller, D.J.; Zondlo, M.A.; Shonkwiler, K.B.; Nash, C.; Ham, J.M. Open-Path Eddy Covariance Measurements of Ammonia Fluxes from a Beef Cattle Feedlot. Agric. For. Meteorol. 2015, 213, 193–202. [Google Scholar] [CrossRef]
- Herman, D.I.; Weerasekara, C.; Hutcherson, L.C.; Giorgetta, F.R.; Cossel, K.C.; Waxman, E.M.; Colacion, G.M.; Newbury, N.R.; Welch, S.M.; DePaola, B.D.; et al. Precise Multispecies Agricultural Gas Flux Determined Using Broadband Open-Path Dual-Comb Spectroscopy. Sci. Adv. 2021, 7, 9765–9796. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Loh, Z.; Griffith, D.W.T.; Turner, D.; Eckard, R.; Edis, R.; Denmead, O.T.; Bryant, G.W.; Paton-Walsh, C.; Tonini, M.; et al. Performance of Open-Path Lasers and Fourier Transform Infrared Spectroscopic Systems in Agriculture Emissions Research. Atmos. Meas. Tech. 2022, 15, 3593–3610. [Google Scholar] [CrossRef]
- Phillips, F.A.; Naylor, T.; Forehead, H.; Griffith, D.W.T.; Kirkwood, J.; Paton-Walsh, C. Vehicle Ammonia Emissions Measured in An Urban Environment in Sydney, Australia, Using Open Path Fourier Transform Infra-Red Spectroscopy. Atmosphere 2019, 10, 208. [Google Scholar] [CrossRef]
- Laubach, J.; Kelliher, F.M. Methane Emissions from Dairy Cows: Comparing Open-Path Laser Measurements to Profile-Based Techniques. Agric. For. Meteorol. 2005, 135, 340–345. [Google Scholar] [CrossRef]
- Gao, Z.; Desjardins, R.L.; Flesch, T.K. Assessment of the Uncertainty of Using an Inverse-Dispersion Technique to Measure Methane Emissions from Animals in a Barn and in a Small Pen. Atmos. Environ. 2010, 44, 3128–3134. [Google Scholar] [CrossRef]
- DeBruyn, Z.J.; Wagner-Riddle, C.; VanderZaag, A. Assessment of Open-Path Spectrometer Accuracy at Low Path-Integrated Methane Concentrations. Atmosphere 2020, 11, 184. [Google Scholar] [CrossRef]
- Baldé, H.; Vander Zaag, A.; Smith, W.; Desjardins, R.L. Ammonia Emissions Measured Using Two Different GasFinder Open-Path Lasers. Atmosphere 2019, 10, 261. [Google Scholar] [CrossRef]
- Hacker, J.M.; Chen, D.; Bai, M.; Ewenz, C.; Junkermann, W.; Lieff, W.; McManus, B.; Neininger, B.; Sun, J.; Coates, T.; et al. Using Airborne Technology to Quantify and Apportion Emissions of CH4 and NH3 from Feedlots. Anim. Prod. Sci. 2016, 56, 190–203. [Google Scholar] [CrossRef]
- Loh, Z.; Chen, D.; Bai, M.; Naylor, T.; Griffith, D.; Hill, J.; Denmead, T.; McGinn, S.; Edis, R. Measurement of Greenhouse Gas Emissions from Australian Feedlot Beef Production Using Open-Path Spectroscopy and Atmospheric Dispersion Modelling. Aust. J. Exp. Agric. 2008, 48, 244–247. [Google Scholar] [CrossRef]
- O’Connor, E.; Mchugh, N.; Boland, T.M.; Dunne, E.; Mcgovern, F.M. Investigation of Intra-Day Variability of Gaseous Measurements in Sheep Using Portable Accumulation Chambers. J. Anim. Sci. 2021, 99, skab132. [Google Scholar] [CrossRef] [PubMed]
- Levrault, C.M.; Difford, G.F.; Steinheim, G.; Groot Koerkamp, P.W.G.; Ogink, N.W.M. Validation of the Methane Production Measurement Accuracy and Ranking Capacity of Portable Accumulation Chambers for Use with Small Ruminants. Biosyst. Eng. 2023, 236, 201–211. [Google Scholar] [CrossRef]
- Robinson, D.L.; Goopy, J.P.; Hegarty, R.S.; Oddy, V.H. Comparison of Repeated Measurements of Methane Production in Sheep over 5 Years and a Range of Measurement Protocols. J. Anim. Sci. 2015, 93, 4637–4650. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Tamayo, R.; Ramírez Agudelo, J.F.; Dewhurst, R.J.; Miller, G.; Vernon, T.; Kettle, H. A Parsimonious Software Sensor for Estimating the Individual Dynamic Pattern of Methane Emissions from Cattle. Animal 2019, 13, 1180–1187. [Google Scholar] [CrossRef]
- Lassen, J.; Løvendahl, P.; Madsen, J. Accuracy of Noninvasive Breath Methane Measurements Using Fourier Transform Infrared Methods on Individual Cows. J. Dairy Sci. 2012, 95, 890–898. [Google Scholar] [CrossRef]
- Madsen, J.; Bjerg, B.S.; Hvelplund, T.; Weisbjerg, M.R.; Lund, P. Methane and Carbon Dioxide Ratio in Excreted Air for Quantification of the Methane Production from Ruminants. Livest. Sci. 2010, 129, 223–227. [Google Scholar] [CrossRef]
- Hellwing, A.; Lund, P.; Madsen, J.; Weisberg, M.R. Comparison of Enteric Methane Production from the CH4/CO2 Ratio and Measured in Respiration Chambers. Adv. Anim. Biosci. 2013, 4. [Google Scholar] [CrossRef]
- Huhtanen, P.; Bayat, A.R.; Lund, P.; Hellwing, A.L.F.; Weisbjerg, M.R. Short Communication: Variation in Feed Efficiency Hampers Use of Carbon Dioxide as a Tracer Gas in Measuring Methane Emissions in on-Farm Conditions. J. Dairy Sci. 2020, 103, 9090–9095. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Q. Assessing Bovine Methane Emissions: Respiratory Simulation and Optical Gas Imaging Methods; Massachusetts Institute of Technology: Cambridge, UK, 2023. [Google Scholar]
- Kang, R.; Liatsis, P.; Kyritsis, D.C. Emission Quantification via Passive Infrared Optical Gas Imaging: A Review. Energies 2022, 15, 3304. [Google Scholar] [CrossRef]
- Ravikumar, A.P.; Wang, J.; Brandt, A.R. Are Optical Gas Imaging Technologies Effective for Methane Leak Detection? Environ. Sci. Technol. 2017, 51, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Asadzadeh, S.; de Oliveira, W.J.; de Souza Filho, C.R. UAV-Based Remote Sensing for the Petroleum Industry and Environmental Monitoring: State-of-the-Art and Perspectives. J. Pet. Sci. Eng. 2022, 208, 109633. [Google Scholar] [CrossRef]
- Moonen, A.J.; Sufian, B.A. Introducing New Drone-Based Inspection Technologies to Safely and Consistently Deliver High Value Results. In Proceedings of the Offshore Technology Conference Asia 2020, OTCA 2020, Kuala Lumpur, Malaysia, 27 October 2020. [Google Scholar]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock—A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italiy, 2013. [Google Scholar]
- Appuhamy, J.A.D.R.N.; France, J.; Kebreab, E. Models for Predicting Enteric Methane Emissions from Dairy Cows in North America, Europe, and Australia and New Zealand. Glob. Chang. Biol. 2016, 22, 3039–3056. [Google Scholar] [CrossRef] [PubMed]
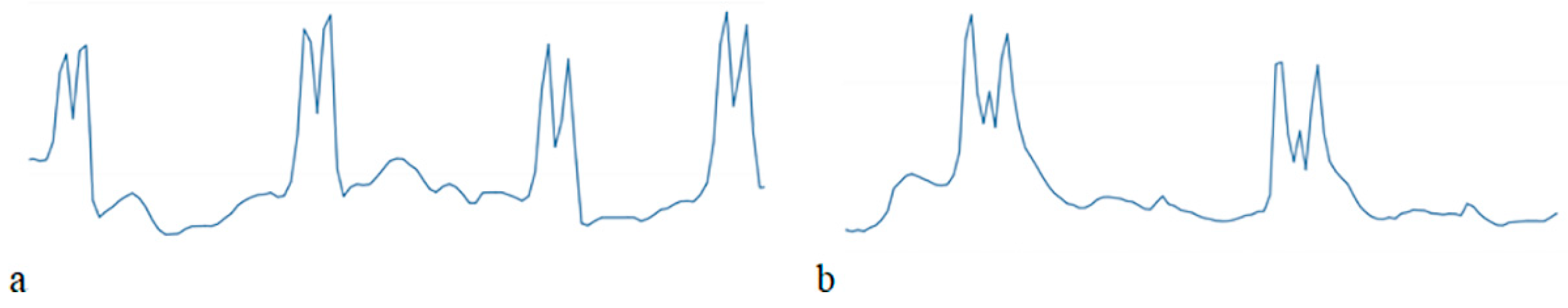

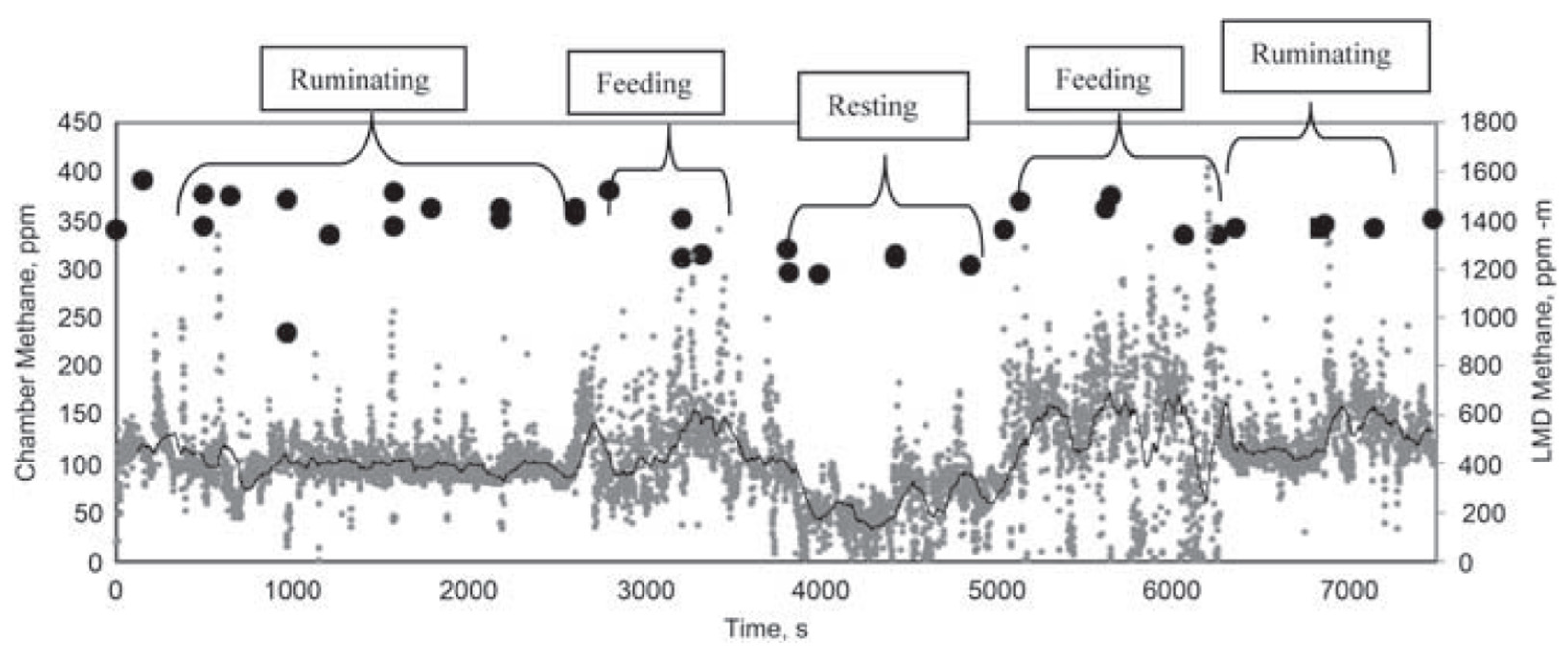
| Technique | Cost | Application | Suitability | Advantages | Disadvantages |
|---|---|---|---|---|---|
| Respiration chamber | Generally high | Research | Requires controlled conditions; measurements are limited to a single animal at a time. | Highly accurate data collection using a controlled environment; individual animal monitoring; precise measurement of dry matter intake. | Expensive to construct and maintain; requires an animal acclimation period; may disrupt animal normal behaviour. |
| Sulphur hexafluoride (SF6) tracer method | Moderate | Research | Applicable for grazing animals; suitable for a large number of individual animals. | Provides accurate data; suitable for enclosed and free-range animals; few interferences by other gases; far less intrusive than respiration chambers. | Relies on a highly potent GHG; high risk of equipment failure; depends on spot concentration measurements; may disrupt animal normal behaviour. |
| Spot sampling | Moderate | Research and Commercial | Applicable for grazing animals; suitable for multiple animals at once. | More affordable and simpler than SF6 tracer and respiration chamber methods; non-invasive technique. | Restricted measurement periods; the GreenFeed method requires an animal bait. |
| Laser CH4 detector | Moderate | Research and Commercial | Requires semi-controlled conditions; suitable for a large number of individual animals. | Immediate results; reduced labour requirement; minimal stress on the animal. | Sensitive to environmental factors; animal needs to stay relatively still. |
| Open-path laser | High | Research | Suitable for grazing animals; able of collecting measurements from a large herd of animals; emissions cannot be attributed to a single animal. | Non-intrusive; large-scale monitoring; suitable for enclosed and free-range animals. | Requires expensive equipment; utilises sensitive instrumentation; sensitive to environmental factors; requires animal cooperation. |
| Portable accumulation chamber | Moderate | Research | Requires controlled conditions; measurements are limited to a single animal at a time. | Relatively simple and portable; short-term measurement. | Restricted measurement periods; may disrupt animal normal behaviour. |
| CO2 tracer method | Moderate | Research | Applicable for a large number of individual animals. | Suitable for enclosed and free-range animals; far less intrusive than respiration chambers. | Less accurate for short-term variations, sensitive to background CO2, requires careful calibration. |
| Optical gas imaging (OGI) | High | Research | Requires controlled conditions; applicable for a large number of individual animals. | Non-intrusive; minimal animal discomfort, suitable for enclosed and free-range animals. | Requires expensive equipment; technology is still under development; sensitive to environmental factors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Connor, S.; Noonan, F.; Savage, D.; Walsh, J. Advancements in Real-Time Monitoring of Enteric Methane Emissions from Ruminants. Agriculture 2024, 14, 1096. https://doi.org/10.3390/agriculture14071096
O’Connor S, Noonan F, Savage D, Walsh J. Advancements in Real-Time Monitoring of Enteric Methane Emissions from Ruminants. Agriculture. 2024; 14(7):1096. https://doi.org/10.3390/agriculture14071096
Chicago/Turabian StyleO’Connor, Seán, Flannagán Noonan, Desmond Savage, and Joseph Walsh. 2024. "Advancements in Real-Time Monitoring of Enteric Methane Emissions from Ruminants" Agriculture 14, no. 7: 1096. https://doi.org/10.3390/agriculture14071096
APA StyleO’Connor, S., Noonan, F., Savage, D., & Walsh, J. (2024). Advancements in Real-Time Monitoring of Enteric Methane Emissions from Ruminants. Agriculture, 14(7), 1096. https://doi.org/10.3390/agriculture14071096






