Abstract
Sea buckthorn berries (SBB) are well known for being rich in natural bioactive compounds with high pharmacological activity. In this study, the optimization of extraction parameters was performed to recover phenolic compounds with high antioxidant activity from SBB. This study involved a systematic optimization approach, including screening for a variety of parameters, including temperature, time, ethanol concentration, agitation, and solid loading. On the basis of Plackett–Burman design (PBD) model, the two most significant parameters (agitation and solid loading) were selected, and the correlation model between those parameters and multiple responses was derived via response surface methodology (RSM). As a result, the optimal extraction condition for maximizing phenolic content and antioxidant activity was determined to be agitation at 109.54 rpm and a solid loading of 172.67 g/L. Under optimal conditions, SBB extract showed a total phenolic content of 0.21 mg/mL and ABTS and DPPH activities of 27.27% and 58.16%, respectively. The SBB extract prepared under optimal conditions was found to contain caffeic acid, vanillic acid, rutin, and vitamin B1 (thiamine). This work is the first challenge to design an optimization model for the efficient recovery of antioxidants from SBB and is significant in that the model can be applied simply and economically to conventional extraction processes.
1. Introduction
Sea buckthorn (Hippophae rhamnoides L.), a member of the Elaeagnaceae family, is widely planted in China, Mongolia, Russia, and Northern Europe [1]. It has been reported to have health-promoting benefits, such as outstanding antioxidant, anti-inflammatory, anti-aging, and intestinal protection [2]. The parts of the sea buckthorn (especially berries) have been used in traditional medicine for centuries to treat a variety of ailments [3]. In Tibetan and Mongolian, sea buckthorn berries (SBB) have been traditionally used to treat phlegm and cough [4]. Additionally, in Central Asia (Tajikistan and Afghanistan), SBB has been known to be effective in treating high blood pressure, digestive system, and skin diseases [5].
Sea buckthorn is well known to be rich in polyphenols such as flavonoids, phenolic acids, and anthocyanins and has been used in various industries [6,7]. Sea buckthorn oil is produced from the seeds or fruit pulp and is a valuable source of fatty acids, especially unsaturated fatty acids [8]. The oil has been reported to exert cosmeceutical activities, such as photoprotection of the skin, and pharmacological activities, such as cardiovascular disease prevention [9,10]. However, sea buckthorn oil is unstable and can be oxidized by oxygen, light, high temperature, and humidity, which can lead to the loss of useful substances or give it an unpleasant taste [11]. SBB contains high amounts of polyphenols as well as fatty acids, which have been reported to protect against high blood pressure, coronary heart disease, and decreased heart function [12]. However, fresh SBBs have a very short shelf life after harvesting due to their thin skin and high water content (80–90%) and are susceptible to microbial infections, resulting in spoilage and deterioration [13]. To avoid these problems, berries such as SBB are usually processed into liquid products such as juices and extracts [14]. Here, maceration can be considered mainly for the industrial production of berry extracts [15]. Maceration, one of the traditional techniques, has the advantage of low investment costs and simple operation but has the disadvantage of having a longer reaction time compared to non-traditional techniques such as ultrasonic or microwave extraction [16]. However, as previous studies have shown, optimizing the maceration process parameters can lead to high yields and energy-efficient recovery of antioxidants such as polyphenols and carotenoids from plant resources [17,18].
In the process of the phenolic compound extraction, the extraction yield can be influenced by different parameters (i.e., independent variables), such as the reaction temperature, time, extraction solvent (e.g., type and concentration), agitation, and solid loading [19,20,21]. Additionally, parameters can affect the extraction reaction individually or interactively, making it difficult to select a major factor to optimize. The basic single-factor method cannot identify interactions between parameters and lacks comprehensive information about the effects of all determinants [22]. To overcome these drawbacks, many studies have been conducted to obtain appropriate results in process design using various statistical methods [23,24,25,26].
Plackett–Burman design (PBD) is a useful parameter screening method that is efficient and economical [27]. PBD ensures that only the most meaningful parameters are selected, significantly reduces the number of experiments, and provides knowledge to evaluate target parameters for further optimization [28]. Response surface methodology (RSM) is a mathematical and statistical method that is used in designing experiments, establishing models, evaluating the effect of parameters, and obtaining optimum conditions for responses [29]. RSM is useful for understanding the relationship between one or more responses and a parameter [30]. Consequently, the combination of PBD and RSM (PBD-RSM) is an attractive approach used to evaluate numerous parameters and estimate their interactions based on numerical data [31].
The objectives of this study were (i) to design an extraction process of SBB, which is rich in phenolic compounds with high antioxidant activity, and (ii) to validate the predicted model with experimental results. For systematic optimization, parameters were selected based on PBD analysis, and additional optimization of significant parameters was performed using Central Composite Design (CCD), a type of RSM. To the best of our knowledge, no study has adopted PBD-RSM to design an extraction process using a maceration technique to recover phenolic compounds with antioxidant activity from SBB.
2. Materials and Methods
2.1. Materials
The SBB was purchased from Xi’an Yuensun Biological Technology Co., Ltd. (Xi’an, China). The SBB was made by freeze-drying whole fresh fruit to remove moisture and then powdering it. For the total phenolic content (TPC) measurement, Folin–Ciocalteu’s phenol reagent (CAS No. 12111-13-6), sodium carbonate (CAS No. 497-19-8), and gallic acid (CAS No. 149-91-7) were acquired from Sigma-Aldrich (St. Louis, MO, USA). For the evaluation of antioxidant activities, 2,2′-azino-bis(3-ethylbenzothiozoline)-6-sulfonic acid (ABTS; CAS No. 30931-67-0), 2,2-diphenyl-1-picrylhydrazyl radical (DPPH; CAS No. 1898-66-4), potassium persulfate (CAS No. 7727-21-1), and sodium nitrate (CAS No. 7632-00-0) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Ethanol and methanol were purchased from Samchun Chemical (Seoul, Republic of Korea). To determine the presence of phytochemical compounds in SBB extracts, catechin (CAS No. 7295-85-4), caffeic acid (CAS No. 331-39-5), syringic acid (CAS No. 530-57-4), vanillic acid (CAS No. 121-34-6), rutin (CAS No. 153-18-4), hesperidin (CAS No. 520-26-3), luteolin (CAS No. 491-70-3), vitamin B-12 (CAS No. 68-19-9), vitamin B7 (CAS No. 68-19-9), vitamin B1 (CAS No. 59-43-8), and vitamin C (CAS No. 50-81-7) were acquired Sigma-Aldrich (St. Louis, MO, USA). Also, myricetin (CAS No. 529-44-2) and kaempferol (CAS No. 520-18-3) were purchased from Tokyo Chemical Industry (Tokyo, Japan).
2.2. Antioxidant Extraction from SBB
The antioxidant extraction experiments from SBB were performed according to the experimental conditions suggested in PBD or RSM. According to the proposed experimental conditions, a certain amount of SBB was loaded into an equal volume of ethanol (20 mL) and reacted for the specified time. Afterward, 1 mL was sampled and centrifuged at 13,000 rpm for 5 min. The supernatant was appropriately diluted and used as a sample for evaluating antioxidant activities. Here, the extract was diluted so that the absorbance of the reaction solution mixed with the extract and ABTS or DPPH radicals was detected within 0.2 to 1.5. Antioxidant activity was then calculated by multiplying the dilution factor of each extract.
2.3. Experimental Design
2.3.1. Plackett–Burman Design
PBD was used to screen the significant parameters affecting antioxidant extraction from SBB. The effects of five parameters such as temperature, time, ethanol concentration, agitation, and solid loading were investigated based on PBD (Table 1). The two levels of each parameter are listed in Table 1. After the extraction according to the PBD, evaluation of antioxidant activities was performed. PBD was established using the first-order polynomial model below:
where Y is the response (ABTS activity and DPPH activity), β0 is model intercept, βi is the linear factor coefficient, and Xi is the coded independent variables. The parameters with a low p-value in the regression analysis were considered to have a significant effect on the response and were further optimized by RSM.

Table 1.
Parameters and the levels in Plackett–Burman design.
2.3.2. Response Surface Methodology
CCD was applied to optimize the significant parameter (from PBD experiment) to obtain maximum TPC and antioxidant activity. The significant parameters were modified independently, while the others were maintained at constant conditions (temperature, 50 °C; time, 1 h; ethanol concentration, 75%). The significant parameters were assessed at five levels (−2, −1, 0, 1, 2), as shown in Table 2. The second-order polynomial equation below was used to fit the experimental data and investigate the associations between variables:
where Y is the predicted response (TPC, ABTS activity, and DPPH activity), β0 is model intercept, Xi and Xj are significant parameters, βi and βj are linear regression coefficients, βij is interactive regression coefficient, and βii and βjj are quadratic regression coefficients.

Table 2.
Parameters and the levels in response surface design.
2.4. Quantification of Total Phenolic Content
The total phenolic contents in SBB extracts were determined spectrophotometrically according to the Folin–Ciocalteau method [32]. A total of 10 μL of aliquot SBB extracts was diluted with 790 μL of deionized water (DW), and 50 μL of Folin–Ciocalteu’s phenol reagent was added. The blank contains the same constituents, with the exception that DW replaces the extracts. All samples were mixed and reacted for 8 min at 30 °C. Additionally, 150 μL of sodium carbonate (20%, w/v) was added to the samples and left to react at 25 °C. After 60 min, the absorbance (A) of the samples was measured at 765 nm. The calibration curve for total phenolic content was obtained using gallic acid with different concentrations as standard solutions. The total phenolic contents in the SBB extracts were calculated by the calibration curve and expressed as the average mg gallic acid equivalent (GAE) per g of extract.
2.5. Evaluation of ABTS Cation Radical-Scavenging Activity
ABTS cation radical-scavenging activity of SBB extracts was measured by following the protocols [33]. All experiments were performed in triplicate. A total of 7 mM of ABTS stock solution was prepared by dissolving ABTS in DW, and 2.45 mM of potassium persulfate was added to the stock solution at a ratio of 1:1. Subsequently, the mixture was mixed with methanol at a ratio of 1:40 to prepare the ABTS reaction solution. A total of 950 μL of ABTS reaction solution was added to 50 μL of aliquot of SBB extracts, and 50 μL of DW was used instead of the extracts as a control. Then, they were mixed and left at 25 °C for 30 min for radical scavenging reaction. After the reaction was completed, the absorbance (A) of the samples was measured at 734 nm. ABTS cation radical scavenging activity of the extracts was calculated using Equation (3).
Radical scavenging activity (%) = (1 − Acontrol/Asample) × 100
2.6. Evaluation of DPPH Free Radical-Scavenging Activity
DPPH radical-scavenging activity of SBB extracts was measured by following the protocols [17]. All experiments were performed in triplicate. A total of 500 μL aliquot of SBB extracts was mixed with 500 μL of 0.25 mM of DPPH solution (dissolved in methanol). As a control, 500 μL of DW was used instead of the sample. After reaction for 30 min at 25 °C, the absorbance (A) of all samples was measured at 517 nm. DPPH free radical-scavenging activity of the extracts was calculated using Equation (3).
2.7. Detection of Phytochemical Compounds by HPLC
The phytochemical compounds of SBB were detected by high-performance liquid chromatography (HPLC) with a diode array detector (G7117C, Agilent, Santa Clara, CA, USA). The following chemicals were used as standards for phytochemicals: catechin, caffeic acid, syringic acid, vanillic acid, rutin, hesperidin, myricetin, luteolin, kaempferol, vitamin B-12 (cobalamin), vitamin B7 (biotin), vitamin B1 (thiamin), and vitamin C (ascorbic acid). The chemicals were selected because they were reported to be present or chemically identified in SBB in various literature [34,35,36,37]. The flow rate of gradient mobile phases (A: 100% acetonitrile; B: 0.03% phosphoric acid (v/v)) was 0.6 mL/min. The gradient elution method was adopted as follows: 0 min (10% A), 0–5 min (10–20% A), 5–20 min (20–40% A), 20–45 min (40–75% A), 45–47 min (75–10% A), and 47–50 min (10% A). INNO column C18 (5 µm, 4.6 mm × 250 mm, Young Jin Biochrom, Seongnam, Republic of Korea) was used at 25 °C. The injection volume and monitoring wavelength were set to 5 µL sample and 250 nm, respectively.
3. Results and Discussion
3.1. Selection of Significant Parameters in Antioxidant Extraction from Sea Buckthorn Using Plackett–Burman Design
To recover antioxidants from SBB, PBD-based screening was first performed for parameters mainly considered in maceration. The selection of variables via PBD was based on their significant effect on the antioxidant activity of SBB extracts. Temperature (A), time (B), ethanol concentration (C), agitation (D), and solid loading (E) were selected as variables. Table 3 enlists the designed experimental conditions (12 runs) and the actual experimental values (ABTS and DPPH activity). The ABTS and DPPH activity of SBB extracts varied from 48.66 to 94.03% (run 3 and run 2, respectively) and 9.73 to 32.38% (run 3 and run 2, respectively), respectively.

Table 3.
Total phenol content and antioxidant activity of sea buckthorn berries extracts prepared under the conditions of the Plackett–Burman design.
An analysis of variance (ANOVA) was performed to confirm the adequacy of PBD, as shown in Table 4. The parameters with low p-values were considered significant and included in further optimization based on RSM [38,39]. For both ABTS and DPPH activity, the order of relative importance of the parameters was found to be as follows: C > E > D > A > B. It could be stated that the ethanol concentration (C), agitation (D), and solid loading (E) were found to have a significant effect on the antioxidant activity of SBB extracts. Based on the ANOVA results, the ethanol concentration (C) has a significant effect on all reactions; however, in practice, samples extracted with 25% ethanol produced an excess of precipitate and significantly interfered with the measurement of antioxidant activities (ABTS and DPPH radicals). Therefore, the data were labeled as not available (n.a.), and the ethanol concentration (C) was not considered as a parameter for RSM-based optimization. Conversely, Danielski et al. successfully extracted phenolic compounds from sea buckthorn pomace extract produced using low ethanol concentrations (e.g., 30%) and confirmed its antioxidant activity [40]. However, in many studies, 75% ethanol was found to be a more useful extraction solvent for the various phenolic compounds from plant resources than pure ethanol and water [41]. Lohvina et al. reported that 70% ethanol extract of fenugreek seed had higher antioxidant activity, such as iron-chelating ability and transition metal-reducing ability, compared to 30%, 50%, and 96% ethanol extracts [42]. Therefore, in the subsequent extraction optimization, 75% ethanol was set as a constant parameter. Agitation can contribute to increasing the extraction yield of bioactive compounds by increasing the surface area of the liquid (i.e., extraction solvent) and solids. Das et al. reported that agitation treatment showed the same extraction efficiency as ultrasonic treatment for the recovery of phenolics and flavonoid compounds from green tea [43]. Solid loading (i.e., the solid-to-liquid ratio) is one of the variables that have a major impact on bioconversion processes, including extraction [44]. A sufficient amount of solvent is required for proper hydration and swelling of the biomass, and the addition of solvent leads to increased extraction efficiency [45]. High solid loading is an operational strategy that can increase product yields while lowering operating costs [46]. In order to efficiently and economically recover antioxidants from SBB, optimal conditions with high solid loading and appropriate agitation should be explored. Therefore, agitation and solid loading, which are critical parameters for extraction, were further optimized based on RSM.

Table 4.
Analysis of variance for the Plackett–Burman design model.
3.2. Relationship between Extraction Parameters and Multiple Responses (Antioxidant Activity and Total Phenolic Content) by RSM
Response surface modeling was performed to optimize two important parameters (D: agitation; E: solid loading). The other parameters were set to constant conditions (temperature, 50 °C; time, 1 h; ethanol concentration, 75%). Since the antioxidant activity is mainly due to phenolic compounds in the extract, RSM-based optimization was performed by including the total phenolic content as a response along with the antioxidant activity. Table 5 illustrates the set of 13 experimental runs suggested by the CCD model of RSM. The TPC, ABTS activity, and DPPH activity of SBB extracts varied from 0.09 to 0.2 mg/mL, 14.34 to 29.09%, and 35.20 to 59.38%, respectively. All experimental values for TPC, ABTS activity, and DPPH activity were lowest in run 7 and highest in run 8.

Table 5.
Actual response values under the conditions of the response surface design.
Multiple regression analysis was applied, and the coefficients of the polynomial model were derived using experimental values to estimate responses. The following second-order polynomial equations were obtained to describe the TPC, ABTS activity, and DPPH activity, respectively:
TPC (mg/mL) = 0.1559 − 0.0019D + 0.0264E +0.0165DE + 0.0009 D2 − 0.0019E2
ABTS activity (%) = 23.37 − 0.383D + 3.69E + 2.99DE − 0.239D2 − 0.4663E2
DPPH activity (%) = 50.89 − 1.41D + 5.88E + 4.69DE − 0.0254D2 − 1.1E2
Based on the regression coefficient value (β), the term with a positive sign indicates that the response value increases as the variable level increases, and a negative sign indicates the opposite [47]. For all responses, the linear effects of agitation (D) and solid loading (E) were negative and positive, respectively. Table 6 shows the ANOVA results for regression validation of Equations (4)–(6). In general, if a model term has a low p-value (<0.05), it means that the term has a statistically significant effect [48]. For TPC, significant effects were confirmed in all terms, including linear (D and E), interaction (DE), and quadratic (D2 and E2). For ABTS activity and DPPH activity, all terms excluding D2 had a significant effect. The coefficient of variation (C.V.) of TPC, ABTS activity, and DPPH activity were observed to be 1.17%, 2.22%, and 3.32%, respectively. A lower C.V. than 10% indicates a moderate precision and reliability of the experiments [49]. R2 of TPC, ABTS activity, and DPPH activity were found to be 0.9976, 0.9914, and 0.9967, respectively. R2 close to 1 indicates an agreement between the experimental and the predicted values for the response [50]. Because the value of R2 increases as the number of parameters increases, the adjusted R2 is generally used to determine the accuracy of the model more correctly [51]. Specifically, if the difference between the predicted and adjusted R2 value is less than 0.2, the model is highly statistically significant and has good fitness [52,53]. The predicted R2 is in reasonable agreement with the adjusted R2 for all models, including TPC (predicted R2: 0.9788; adjusted R2: 0.9959), ABTS activity (predicted R2: 0.9302; adjusted R2: 0.9853), and DPPH activity (predicted R2: 0.7509; adjusted R2: 0.9429). The adequacy precision of TPC, ABTS activity, and DPPH activity was observed to be 85.840, 43.275, and 21.515, respectively. The adequacy precision measures the signal-to-noise ratio, and high adequate precision (i.e., >4.0) means that the model can be used to navigate the design space [54]. Overall, the ANOVA results are suitable for predicting TPC and antioxidant activity.

Table 6.
Analysis of variance for the response surface model.
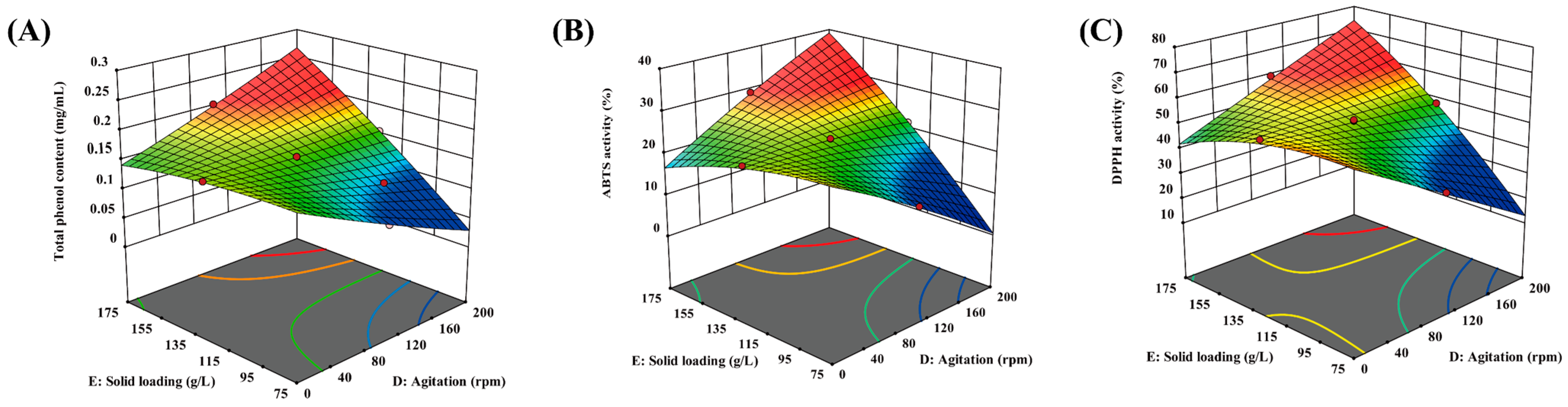
As shown in Figure 1A–C, 3D surface plots are useful for visualizing the relationship between parameters for a response. The interaction terms (i.e., agitation–solid loading, DE) were statistically significant for all responses (Table 6). Accordingly, it was observed that there were synergistic effects of agitation–solid loading for TPC (Figure 1A), ABTS activity (Figure 1B), and DPPH activity (Figure 1C). The highest value of TPC, ABTS activity, and DPPH activity was found at agitation (D) = 200 rpm and solid loading (E) = 175 g/L, while the lowest value was at agitation (D) = 200 rpm and solid loading (E) = 75 g/L.
Figure 1.
A 3D surface plot showing the effect of agitation–solid loading on the TPC (A), ABTS activity (B), and DPPH activity (C).
3.3. Experimental Validation of the Predicted Model: TPC and Antioxidant Activity
Numerical optimization was performed using Equations (1)–(3) to determine the optimal extraction conditions for maximizing TPC, ABTS, and DPPH activity from SBB. Accordingly, the goals of parameters and response were set as follows: agitation and solid loading, “is in range”; TPC, ABTS activity, and DPPH activity “maximized”. As a result, the optimal extraction conditions were derived with an agitation of 109.54 rpm and a solid loading of 172.67 g/L (Table 7). The predicted TPC, ABTS, and DPPH activity was estimated to be 0.20 mg/mL, 29.72%, and 59.53%, respectively. In order to verify the model, an extraction experiment was performed under optimal conditions, and then the TPC, ABTS, and DPPH activity were evaluated. As a result, the actual TPC, ABTS, and DPPH activity were 0.21 mg/mL, 27.27%, and 58.16%, respectively. Also, the errors between the predicted and experimental values were 5.0% (for TPC), 6.26% (for ABTS activity), and 0.38% (for DPPH activity), which indicates the successful prediction of the responses in the SBB-extraction process.

Table 7.
Model-predicted and experimental response values under optimal conditions.
3.4. Detection of Phytochemical Compounds in SBB Extracts
Phytochemicals in the SBB extract produced under optimal extraction conditions were detected using HPLC (Figure S1). Here, the following chemicals were used as authentic standards for phytochemicals: catechin, caffeic acid, syringic acid, vanillic acid, rutin, hesperidin, myricetin, luteolin, kaempferol, vitamin B-12, vitamin B7, vitamin B1, and vitamin C. As shown in Table 8, it was confirmed that the SBB extract contained caffeic acid, vanillic acid, rutin, and vitamin B1. In a report by Criste et al., rutin, luteolin, kaempferol, quercetin, quercitrin, and gallic acid were observed in SBB extract homogenized with water [34]. Guo et al. also investigated the phytochemical composition of SBB acetone extract and reported that it contained gallic acid, ferulic acid, catechins, epicatechins, and kaempferol [35]. Similar to our study, Danielski et al. reported that phenolic compounds such as p-hydroxybenzoic acid, protocatechuic acid, p-coumaric acid, caffeic acid, O-methyl gallic acid, ferulic acid, and apigenin were detected in ethanolic extracts of sea buckthorn pomace [40]. In the same literature, sea buckthorn by-product extract was reported to have in vitro anti-diabetic and anti-obesity activities by confirming that it inhibits β-glucosidase and pancreatic lipase enzymes. Differences in the phytochemical types of SBB extracts reported in the literature can be attributed to the origin of the fruit, extraction solvent (e.g., type, concentration), and extraction method [13]. Phytochemicals within SBB are responsible for a variety of physiological effects. Caffeic acid is a hydroxycinnamic acid reported to have antithrombotic effects by downregulating platelet aggregation molecules [55]. Vanillic acid is one of the polyphenolic substances extracted from various medicinal plants and helps manage/reduce oxidative stress due to its powerful antioxidant activity [56]. Rutin has been reported to have pharmacological activities, including anticancer, antioxidant, anti-inflammatory, antibacterial and antifungal activities, neuroprotection, and cardioprotection [57]. In addition, Khan et al. reported that sea buckthorn protein emulsifiers treated with sonication and various proteases could be an eco-friendly alternative to synthetic emulsifiers used in the food industry [58]. Our study highlights that SBB produced from an optimized extraction process contains caffeic acid, vanillic acid, rutin, and vitamin B1 and has the potential to be utilized as an antioxidant for nutraceutical and pharmaceutical materials.

Table 8.
Phytochemical compounds in SBB extract.
4. Conclusions
This is the first study applying PBD-RSM to efficiently recover phenolic compounds with high antioxidant activity from SBB. Based on PBD analysis, agitation and solid loading were confirmed as significant parameters affecting antioxidant activity. To maximize the recovery of phenolic compounds with high antioxidant activity from SBB, the two parameters were further optimized through RSM, and the optimal extraction conditions were an agitation of 109.54 rpm and a solid loading of 172.67 g/L. This study highlights the improvement in the potential of SBB as a resource for antioxidant production through systematic process optimization and evaluation. Subsequently, the application, harvesting, and transportation strategies of SBB extracts should be explored. In the near future, antioxidant-rich SBB extracts are expected to be useful for the manufacture of functional products such as bioactive films and nutraceuticals.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/agriculture14071095/s1, Figure S1: Chromatogram of phytochemical compounds in SBB extracts.
Author Contributions
Conceptualization, S.K. and H.Y.Y.; methodology, S.K., J.L., H.S. and H.Y.Y.; validation, K.H.L., Y.C., J.H.L., T.L. and H.Y.Y.; formal analysis, J.L., H.S. and K.H.L.; writing—original draft preparation, S.K. and J.L. writing—review and editing, T.L. and H.Y.Y.; project administration, H.Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science and ICT (MSIT) (RS-2023-00213287).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ciesarová, Z.; Murkovic, M.; Cejpek, K.; Kreps, F.; Tobolková, B.; Koplík, R.; Belajová, E.; Kukurová, K.; Daško, L.; Panovská, Z.; et al. Why is sea buckthorn (Hippophae rhamnoides L.) so exceptional? A review. Food Res. Int. 2020, 133, 109170. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, W.; Kallio, H.; Yang, B. Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn (Hippophaë rhamnoides). Crit. Rev. Food Sci. Nutr. 2022, 62, 3798–3816. [Google Scholar] [CrossRef] [PubMed]
- Guliyev, V.B.; Gul, M.; Yildirim, A. Hippophae rhamnoides L.: Chromatographic methods to determine chemical composition, use in traditional medicine and pharmacological effects. J. Chromatogr. B 2004, 812, 291–307. [Google Scholar] [CrossRef]
- Dong, K.; Binosha Fernando, W.M.; Durham, R.; Stockmann, R.; Jayasena, V. Nutritional value, health-promoting benefits and food application of sea buckthorn. Food Rev. Int. 2023, 39, 2122–2137. [Google Scholar] [CrossRef]
- Suryakumar, G.; Gupta, A. Medicinal and therapeutic potential of Sea buckthorn (Hippophae rhamnoides L.). J. Ethnopharmacol. 2011, 138, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Akhter, P.; Bhatti, T.Y.; Shafiq, I.; Jamil, F.; Nazar, R.; Nazir, M.S.; Hassan, S.U.; Hassan, M.; Park, Y. Antioxidant activity of sea buckthorn (Hippophae rhamnoides) seed oil extracted using various organic solvents. Korean J. Chem. Eng. 2023, 40, 2914–2920. [Google Scholar] [CrossRef]
- Sytařová, I.; Orsavová, J.; Snopek, L.; Mlček, J.; Byczyński, Ł.; Mišurcová, L. Impact of phenolic compounds and vitamins C and E on antioxidant activity of sea buckthorn (Hippophaë rhamnoides L.) berries and leaves of diverse ripening times. Food Chem. 2020, 310, 125784. [Google Scholar] [CrossRef] [PubMed]
- Olas, B. Sea buckthorn as a source of important bioactive compounds in cardiovascular diseases. Food Chem. Toxicol. 2016, 97, 199–204. [Google Scholar] [CrossRef]
- Gęgotek, A.; Jastrząb, A.; Jarocka-Karpowicz, I.; Muszyńska, M.; Skrzydlewska, E. The effect of sea buckthorn (Hippophae rhamnoides L.) seed oil on UV-induced changes in lipid metabolism of human skin cells. Antioxidants 2018, 7, 110. [Google Scholar] [CrossRef] [PubMed]
- Vashishtha, V.; Barhwal, K.; Kumar, A.; Hota, S.K.; Chaurasia, O.P.; Kumar, B. Effect of seabuckthorn seed oil in reducing cardiovascular risk factors: A longitudinal controlled trial on hypertensive subjects. Clin. Nutr. 2017, 36, 1231–1238. [Google Scholar] [CrossRef]
- Lyu, X.; Wang, Y.; Gao, S.; Wang, X.; Cao, W.; Cespedes-Acuña, C.L. Sea buckthorn leaf extract on the stability and antioxidant activity of microencapsulated sea buckthorn oil. Food Biosci. 2022, 48, 101818. [Google Scholar] [CrossRef]
- Sayegh, M.; Miglio, C.; Ray, S. Potential cardiovascular implications of Sea Buckthorn berry consumption in humans. Int. J. Food Sci. Nutr. 2014, 65, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Wang, J.; Zhu, L.; Yu, X.; Zhang, Q.; Li, M.; Hu, B.; Yang, X. Metabolomics provide a novel interpretation of the changes in flavonoids during sea buckthorn (Hippophae rhamnoides L.) drying. Food Chem. 2023, 413, 135598. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.; Cruz, R.M.; Díez-Méndez, A.; Albertos, I. Valorization of berries’ agro-industrial waste in the development of biodegradable pectin-based films for fresh salmon (Salmo salar) shelf-life monitoring. Int. J. Mol. Sci. 2022, 23, 8970. [Google Scholar] [CrossRef]
- Albuquerque, B.R.; Prieto, M.A.; Barreiro, M.F.; Rodrigues, A.; Curran, T.P.; Barros, L.; Ferreira, I.C. Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crops Prod. 2017, 95, 404–415. [Google Scholar] [CrossRef]
- Jha, A.K.; Sit, N. Extraction of bioactive compounds from plant materials using combination of various novel methods: A review. Trends Food Sci. Technol. 2022, 119, 579–591. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Jung, J.; Heo, J.W.; Lee, K.H.; Kim, S.; Son, H.; Chun, Y.; Yoo, H.Y. Valorization of persimmon calyx, an industrial biowaste, as a potential resource for antioxidant production. Environ. Technol. Innov. 2023, 30, 103038. [Google Scholar] [CrossRef]
- Kim, S.; Son, H.; Pang, S.Y.; Yang, J.J.; Lee, J.; Lee, K.H.; Lee, J.H.; Park, C.; Yoo, H.Y. Optimization of Major Extraction Variables to Improve Recovery of Anthocyanins from Elderberry by Response Surface Methodology. Processes 2022, 11, 72. [Google Scholar] [CrossRef]
- Wang, R.; Wang, L.; Zhang, L.; Wan, S.; Li, C.; Liu, S. Solvents effect on phenolics, iridoids, antioxidant activity, antibacterial activity, and pancreatic lipase inhibition activity of noni (Morinda citrifolia L.) fruit extract. Food Chem. 2022, 377, 131989. [Google Scholar] [CrossRef]
- Kim, S.; Lee, K.H.; Lee, J.; Lee, S.K.; Chun, Y.; Lee, J.H.; Yoo, H.Y. Efficient Recovery Strategy of Luteolin from Agricultural Waste Peanut Shells and Activity Evaluation of Its Functional Biomolecules. Int. J. Mol. Sci. 2023, 24, 12366. [Google Scholar] [CrossRef]
- Elhag, H.E.E.A.; Naila, A.; Nour, A.H.; Ajit, A.; Sulaiman, A.Z.; Abd Aziz, B. Optimization of protein yields by ultrasound assisted extraction from Eurycoma longifolia roots and effect of agitation speed. J. King Saud Univ. Sci. 2019, 31, 913–930. [Google Scholar] [CrossRef]
- Jawale, J.P.; Nandre, V.S.; Latpate, R.V.; Kulkarni, M.V.; Doshi, P.J. Isolation, characterization and optimizations of laccase producer from soil: A comprehensive study of application of statistical approach to enhance laccase productivity in Myrothecium verrucaria NFCCI 4363. Bioresour. Technol. Rep. 2021, 15, 100751. [Google Scholar] [CrossRef]
- Kim, D.S.; Lee, J.H.; Shin, H.J. Optimization of Vacuum Frying Process for Sweet Potato Chip Manufacturing Using Response Surface Methodology and Artificial Neural Network Model. Biotechnol. Bioprocess Eng. 2023, 28, 554–567. [Google Scholar] [CrossRef]
- Bellebcir, A.; Merouane, F.; Chekroud, K.; Bounabi, H.; Vasseghian, Y.; Kamyab, H.; Chelliapan, S.; Klemeš, J.J.; Berkani, M. Bioprospecting of biosurfactant-producing bacteria for hydrocarbon bioremediation: Optimization and characterization. Korean J. Chem. Eng. 2023, 40, 2497–2512. [Google Scholar] [CrossRef]
- Bhamare, H.M.; Jadhav, H.P.; Sayyed, R.Z. Statistical optimization for enhanced production of extracellular laccase from Aspergillus sp. HB_RZ4 isolated from bark scrapping. Environ. Sustain. 2018, 1, 159–166. [Google Scholar] [CrossRef]
- Boateng, I.D.; Yang, X.M. Process optimization of intermediate-wave infrared drying: Screening by Plackett–Burman; comparison of Box-Behnken and central composite design and evaluation: A case study. Ind. Crops Prod. 2021, 162, 113287. [Google Scholar] [CrossRef]
- Guo, N.; Song, X.R.; Kou, P.; Zang, Y.P.; Jiao, J.; Efferth, T.; Liu, Z.M.; Fu, Y.J. Optimization of fermentation parameters with magnetically immobilized Bacillus natto on Ginkgo seeds and evaluation of bioactivity and safety. LWT 2018, 97, 172–179. [Google Scholar] [CrossRef]
- Luiz, M.T.; Viegas, J.S.R.; Abriata, J.P.; Viegas, F.; de Carvalho Vicentini, F.T.M.; Bentley, M.V.L.B.; Chorilli, M.; Marchetti, J.M.; Tapia-Blacido, D.R. Design of experiments (DoE) to develop and to optimize nanoparticles as drug delivery systems. Eur. J. Pharm. Biopharm. 2021, 165, 127–148. [Google Scholar] [CrossRef]
- Li, Z.; Lu, D.; Gao, X. Optimization of mixture proportions by statistical experimental design using response surface method-A review. J. Build. Eng. 2021, 36, 102101. [Google Scholar] [CrossRef]
- Shirazi, M.; Khademalrasoul, A.; Safieddin Ardebili, S.M. Multi-objective optimization of soil erosion parameters using response surface method (RSM) in the Emamzadeh watershed. Acta Geophys. 2020, 68, 505–517. [Google Scholar] [CrossRef]
- Boateng, I.D.; Mustapha, A.; Kuehnel, L.; Daubert, C.R.; Kumar, R.; Agliata, J.; Flint-Garcia, S.; Wan, C.; Somavat, P. From purple corn waste (pericarp) to polyphenol-rich extract with higher bioactive contents and superior product qualities using two-step optimization techniques. Ind. Crops Prod. 2023, 200, 116871. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total phenolic content, flavonoid content and antioxidant potential of wild vegetables from Western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, K.; Charles, A.L. In vitro antioxidant activity of Kyoho grape extracts in DPPH and ABTS assays: Estimation methods for EC50 using advanced statistical programs. Food Chem. 2019, 275, 41–49. [Google Scholar] [CrossRef]
- Criste, A.; Urcan, A.C.; Bunea, A.; Pripon Furtuna, F.R.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical composition and biological activity of berries and leaves from four Romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef]
- Guo, R.; Guo, X.; Li, T.; Fu, X.; Liu, R.H. Comparative assessment of phytochemical profiles, antioxidant and antiproliferative activities of Sea buckthorn (Hippophaë rhamnoides L.) berries. Food Chem. 2017, 221, 997–1003. [Google Scholar]
- Li, W.; Hydamaka, A.W.; Lowry, L.; Beta, T. Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and seabuckthorn. Cent. Eur. J. Biol. 2009, 4, 499–506. [Google Scholar] [CrossRef]
- Zompra, A.A.; Chasapi, S.A.; Karagkouni, E.C.; Karamouzi, E.; Panopoulos, P.; Spyroulias, G.A. Metabolite and bioactive compounds profiling of meteora Sea buckthorn berries through high-resolution NMR analysis. Metabolites 2021, 11, 822. [Google Scholar] [CrossRef]
- Moslemi, F.; Ehrampoush, M.H.; Mehralian, M.; Dalvand, A. Modeling of heterogeneous fenton process using catalyst produced from date palm waste for dye removal: Catalyst characterization and process optimization. Korean J. Chem. Eng. 2023, 40, 2671–2682. [Google Scholar] [CrossRef]
- Marathe, K.; Chaudhari, A.; Kamalaja, K.; Maheshwari, V. Magnesium dependent proteinaceous protease inhibitor with pesticidal potential from alkali-halotolerant Streptomyces spp.: Optimization of production using statistical tools. Biocatal. Agric. Biotechnol. 2016, 5, 58–68. [Google Scholar] [CrossRef]
- Danielski, R.; Shahidi, F. Phenolic composition and bioactivities of sea buckthorn (Hippophae rhamnoides L.) fruit and seeds: An unconventional source of natural antioxidants in North America. J. Sci. Food Agric. 2024, 104, 5553–5564. [Google Scholar] [CrossRef]
- Sun, C.; Wu, Z.; Wang, Z.; Zhang, H. Effect of ethanol/water solvents on phenolic profiles and antioxidant properties of Beijing propolis extracts. Evi. Based Comp. Altern Med. 2015, 2015, 595393. [Google Scholar] [CrossRef] [PubMed]
- Lohvina, H.; Sándor, M.; Wink, M. Effect of Ethanol Solvents on Total Phenolic Content and Antioxidant Properties of Seed Extracts of Fenugreek (Trigonella foenum-graecum L.) varieties and determination of phenolic Composition by HPLC-ESI-MS. Diversity 2021, 14, 7. [Google Scholar] [CrossRef]
- Das, P.R.; Eun, J.B. A comparative study of ultra-sonication and agitation extraction techniques on bioactive metabolites of green tea extract. Food Chem. 2018, 253, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Rashid, T.; Taqvi, S.A.A.; Sher, F.; Rubab, S.; Thanabalan, M.; Bilal, M.; ul Islam, B. Enhanced lignin extraction and optimisation from oil palm biomass using neural network modelling. Fuel 2021, 293, 120485. [Google Scholar] [CrossRef]
- Mahindrakar, K.V.; Rathod, V.K. Ultrasonic assisted aqueous extraction of catechin and gallic acid from Syzygium cumini seed kernel and evaluation of total phenolic, flavonoid contents and antioxidant activity. Chem. Eng. Process. Process Intensif. 2020, 149, 107841. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Martínez-Zarzoso, I.; Bengochea-Morancho, A.; Morales-Lage, R. Does environmental policy stringency foster innovation and productivity in OECD countries? Energy Policy 2019, 134, 110982. [Google Scholar] [CrossRef]
- Shin, H.; Lee, J.; Bae, J.; Lee, K.H.; Yoo, H.Y.; Park, C. Enhancement of dieckol extraction yield from Ecklonia cava through optimization of major variables in generally recognized as safe solvent-based process. Front. Mar. Sci. 2023, 10, 1287047. [Google Scholar] [CrossRef]
- Abdulgader, M.; Yu, Q.J.; Zinatizadeh, A.A.; Williams, P.; Rahimi, Z. Application of response surface methodology (RSM) for process analysis and optimization of milk processing wastewater treatment using multistage flexible fiber biofilm reactor. J. Environ. Chem. Eng. 2020, 8, 103797. [Google Scholar] [CrossRef]
- Hassan, W.N.F.W.; Ismail, M.A.; Lee, H.S.; Meddah, M.S.; Singh, J.K.; Hussin, M.W.; Ismail, M. Mixture optimization of high-strength blended concrete using central composite design. Constr. Build. Mater. 2020, 243, 118251. [Google Scholar] [CrossRef]
- He, C.; Ma, M.; Wang, P. Extract interpretability-accuracy balanced rules from artificial neural networks: A review. Neurocomputing 2020, 387, 346–358. [Google Scholar] [CrossRef]
- Ranga, M.; Sinha, S.; Biswas, P. Rhodamine B dye degradation by fabricated Ti/RuO2 anode: Optimization by RSM, reaction mechanism, study of sludge. Korean J. Chem. Eng. 2023, 40, 2219–2238. [Google Scholar] [CrossRef]
- Khan, K.; Johari, M.A.M.; Amin, M.N.; Khan, M.I.; Iqbal, M. Optimization of colloidal nano-silica based cementitious mortar composites using RSM and ANN approaches. Results Eng. 2023, 20, 101390. [Google Scholar] [CrossRef]
- Kunnan Singh, J.S.; Ching, Y.C.; Abdullah, L.C.; Ching, K.Y.; Razali, S.; Gan, S.N. Optimization of mechanical properties for polyoxymethylene/glass fiber/polytetrafluoroethylene composites using response surface methodology. Polymers 2018, 10, 338. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.S.; Nam, K.S.; Park, H.J. Caffeic Acid Diminishes the Production and Release of Thrombogenic Molecules in Human Platelets. Biotechnol. Bioprocess Eng. 2018, 23, 641–648. [Google Scholar] [CrossRef]
- Kaur, J.; Gulati, M.; Singh, S.K.; Kuppusamy, G.; Kapoor, B.; Mishra, V.; Gupta, S.; Arshad, M.F.; Porwal, O.; Jha, N.K. Discovering multifaceted role of vanillic acid beyond flavours: Nutraceutical and therapeutic potential. Trends Food Sci. Technol. 2022, 122, 187–200. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The pharmacological potential of rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.S.; Sodhi, N.S.; Fayaz, S.; Wani, S.A.; Bhat, M.S.; Mishra, H.N.; Bakshi, R.A.; Dar, B.N.; Dhillon, B. Seabuckthorn seed protein concentrate: A novel seed protein; emulsifying properties as affected by ultrasonication and enzymatic hydrolysis. Int. J. Food Sci. 2023, 58, 1621–1630. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).