Diversified Cover Crops and No-Till Enhanced Soil Total Nitrogen and Arbuscular Mycorrhizal Fungi Diversity: A Case Study from the Karst Area of Southwest China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Sites
2.2. Experimental Design
2.3. Soil Sampling
2.4. Soil Analyses
2.5. Plant Yield
2.6. DNA Extraction and PCR Amplification
2.7. Illumina NovaSeq Sequencing
2.8. Bioinformatics Analysis
2.9. Statistical Analysis
3. Results
3.1. Soil Nutrient Status
3.2. Analysis of the AMF Community Composition in the Soils with the Different Management Practices
3.3. Soil Microbiological Properties
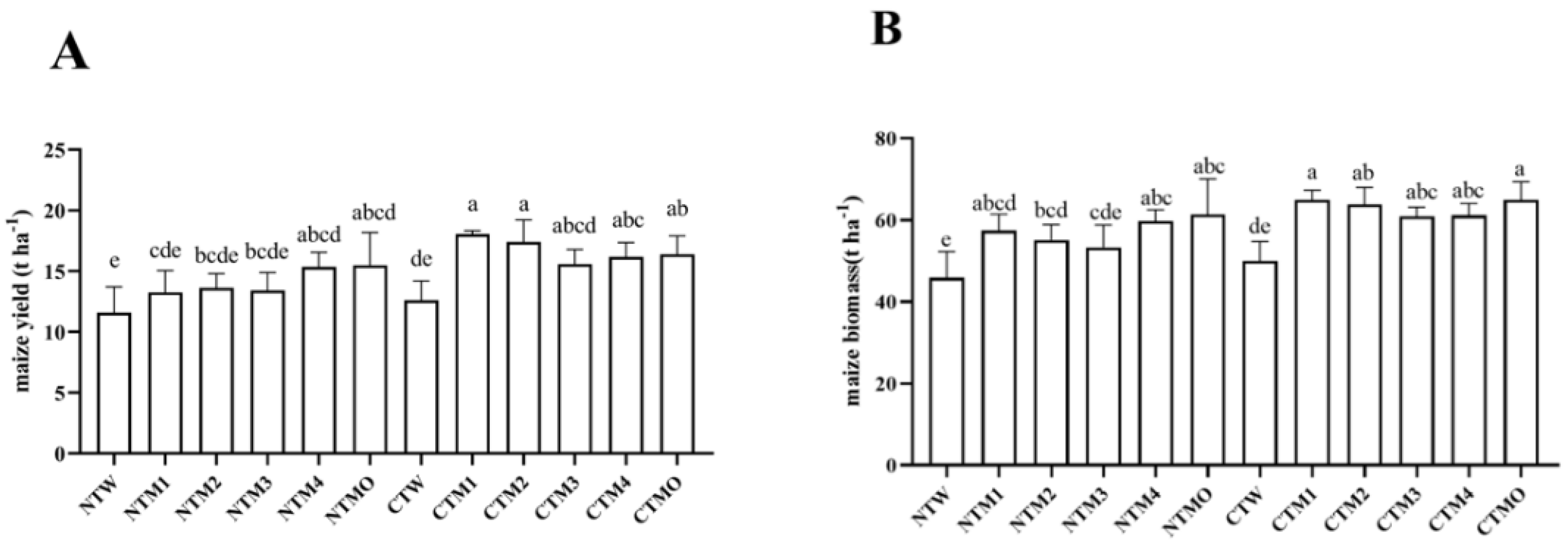
3.4. Plant Yield
3.5. The Relationship between the AMF and the Soil Properties
4. Discussion
4.1. Effects of Tillage Methods and Different Forage Mixture Combinations on Soil Nutrients
4.2. Effects of Tillage Methods and Different Forage Mixture Combinations on Soil AMF
4.3. Effects of Tillage Methods and Different Forage Mixture Combinations on Soil Enzymes and Their Microbial Properties
4.4. Effects of Tillage Methods and Different Forage Mixture Combinations on Plant Yield
4.5. Correlation between Soil Enzyme Activity and AMF
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil health in agricultural systems. Philos. Trans. R. Soc. B-Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Somenahally, A.; DuPont, J.I.; Brady, J.; McLawrence, J.; Northup, B.; Gowda, P. Microbial communities in soil profile are more responsive to legacy effects of wheat-cover crop rotations than tillage systems. Soil Biol. Biochem. 2018, 123, 126–135. [Google Scholar] [CrossRef]
- Hao, X.; Abou, M.; Steenwerth, K.L.; Nocco, M.A.; Basset, C.; Daccache, A. Are there universal soil responses to cover cropping? A systematic review. Sci. Total Environ. 2023, 861, 160600. [Google Scholar] [CrossRef]
- Williams, A.; Hunter, M.C.; Kammerer, M.; Kane, D.A.; Jordan, N.R.; Mortensen, D.A.; Smith, R.G.; Snapp, S.; Davis, A.S. Soil Water Holding Capacity Mitigates Downside Risk and Volatility in US Rainfed Maize: Time to Invest in Soil Organic Matter? PLoS ONE 2016, 11, e0160974. [Google Scholar] [CrossRef] [PubMed]
- Davis, C.J.; Presley, D.R.; Rivard, C.L.; Griffin, J.J.; Tomlinson, P.J. Conservation systems influence on soil properties in pumpkin production. Soil Sci. Soc. Am. J. 2022, 86, 435–449. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, S.; Sainju, U.M.; Ghimire, R.; Zhao, F. A meta-analysis on cover crop impact on soil water storage, succeeding crop yield, and water-use efficiency. Agric. Water Manag. 2021, 256, 107085. [Google Scholar] [CrossRef]
- Six, J.; Elliott, E.T.; Paustian, K. Aggregate and Soil Organic Matter Dynamics under Conventional and No-Tillage Systems. Soil Sci. Soc. Am. J. 1999, 63, 1350–1358. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Chen, Q.; Wen, X.; Liao, Y. Conservation tillage increases soil bacterial diversity in the dryland of northern China. Agron. Sustain. Dev. 2016, 36, 28. [Google Scholar] [CrossRef]
- Ayuke, F.O.; Kihara, J.; Ayaga, G.; Micheni, A.N. Conservation Agriculture Enhances Soil Fauna Richness and Abundance in Low Input Systems: Examples from Kenya. Front. Environ. Sci. 2019, 7, 97. [Google Scholar] [CrossRef]
- Klironomos, J.N.; Hart, M.M. Colonization of roots by arbuscular mycorrhizal fungi using different sources of inoculum. Mycorrhiza 2002, 12, 181–184. [Google Scholar] [CrossRef]
- Oehl, F.; Silva, G.A.; Goto, B.T.; Maia, L.C.; Sieverding, E. Glomeromycota: Two new classes and a new order. Mycotaxon 2011, 116, 365–379. [Google Scholar] [CrossRef]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Baltruschat, H.; Santos, V.M.; da Silva, D.K.A.; Schellenberg, I.; Deubel, A.; Sieverding, E.; Oehl, F. Unexpectedly high diversity of arbuscular mycorrhizal fungi in fertile Chernozem croplands in Central Europe. Catena 2019, 182, 104135. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; Van, D.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Wetzel, K.; Silva, G.; Matczinski, U.; Oehl, F.; Fester, T. Superior differentiation of arbuscular mycorrhizal fungal communities from till and no-till plots by morphological spore identification when compared to T-RFLP. Soil Biol. Biochem. 2014, 72, 88–96. [Google Scholar] [CrossRef]
- Araya, T.; Gebremedhin, A.; Baudron, F.; Hailemariam, M.; Birhane, E.; Nyssen, J.; Govaerts, B.; Cornelis, W. Influence of 9 years of permanent raised beds and contour furrowing on soil health in conservation agriculture based systems in Tigray region, Ethiopia. Land Degrad. Dev. 2020, 32, 1525–1539. [Google Scholar] [CrossRef]
- Maiga, A.; Alhameid, A.; Singh, S.; Polat, A.; Singh, J.; Kumar, S.; Osborne, S. Responses of soil organic carbon, aggregate stability, carbon and N fractions to 15 and 24 years of no-till diversified crop rotations. Soil Res. 2019, 57, 149–157. [Google Scholar] [CrossRef]
- Njeru, E.M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. First evidence for a major cover crop effect on arbuscular mycorrhizal fungi and organic maize growth. Agron. Sustain. Dev. 2014, 34, 841–848. [Google Scholar] [CrossRef]
- Rosner, K.; Bodner, G.; Hage-Ahmed, K.; Steinkellner, S. Long-term soil tillage and cover cropping affected arbuscular mycorrhizal fungi, nutrient concentrations, and yield in sunflower. Agron. J. 2018, 110, 2664–2672. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Kahl, K.; Carlson, B.; Huggins, D.R.; Paulitz, T. Fungal community composition and diversity vary with soil depth and landscape position in a no-till wheat-based cropping system. FEMS Microbiol. Ecol. 2018, 94, 7. [Google Scholar] [CrossRef]
- Schmidt, R.; Mitchell, J.; Scow, K. Cover cropping and no-till increase diversity and symbiotroph:saprotroph ratios of soil fungal communities. Soil Biol. Biochem. 2019, 129, 99–109. [Google Scholar] [CrossRef]
- Towns, T.G. Determination of Aqueous Phosphate by Ascorbic Acid Reduction of Phosphomolybdic Acid. Anal. Chem. 1986, 58, 223–229. [Google Scholar] [CrossRef]
- Hurwitz, S. Estimation of net P utilization by the “slope” method. J. Nutr. 1964, 84, 83–92. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, J.; Zhang, X.; Shen, Z.; Shi, L.; Chen, Y. The great potential for phytoreme diation of abandoned tailings pond using ectomycorrhizal Pinus sylvestris. Sci. Total Environ. 2020, 719, 137475.1–137475.11. [Google Scholar] [CrossRef]
- Gao, M.; Song, W.; Zhou, Q.; Ma, X.; Chen, X. Interactive effect of oxytetracycline and lead on soil enzymatic activity and microbial biomass. Environ. Toxicol. Pharmacol. 2013, 36, 667–674. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total Carbon, Organic Carbon, and Organic Matter. In Methods Of Soil Analysis. Part I. Physical And Mineralogical Properties; Klute, A., Ed.; American Society of Agronomy, Inc.: Madison, WI, USA, 1986; pp. 961–1010. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Blodgett, R.J.; Moruzzi, G. The expanded application of most probable number to the quantitative evaluation of extremely low microbial count. PDA J. Pharm. Sci. Technol. 2006, 60, 335–336. [Google Scholar]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2′ s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Treseder, K.K.; Allen, E.B.; Egerton-Warburton, L.M.; Hart, M.M.; Klironomos, J.N.; Maherali, H.; Tedersoo, L. Arbuscular mycorrhizal fungi as mediators of ecosystem responses to N deposition: A trait-based predictive framework. J. Ecol. 2018, 106, 480–489. [Google Scholar] [CrossRef]
- Lu, Y.W.; Liu, X.; Zhou, S.R. N Addition and Arbuscular Mycorrhizal Fungi Beta Diversity: Patterns and Mechanisms. Front. Environ. Sci. 2021, 9, 701653. [Google Scholar] [CrossRef]
- Tatewaki, Y.; Higo, M.; Isobe, K. Impacts of Tillage Practices on Growth, P Uptake, and Yield of Maize in Controlled and Field-Based Studies in Relation to Arbuscular Mycorrhizal Fungi. J. Appl. Microbiol. 2023, 3, 358–374. [Google Scholar] [CrossRef]
- Mhlanga, B.; Pellegrino, E.; Thierfelder, C.; Ercoli, L. Conservation agriculture practices drive maize yield by regulating soil nutrient availability, arbuscular mycorrhizas, and plant nutrient uptake. Field Crop. Res. 2022, 277, 108403. [Google Scholar] [CrossRef]
- Agnihotri, R.; Bharti, A.; Ramesh, A.; Prakash, A.; Sharma, M.P. Glomalin related protein and C16:1ω5 PLFA associated with AM fungi as potential signatures for assessing the soil C sequestration under contrasting soil management practices. Eur. J. Soil Biol. 2021, 103, 1164–5563. [Google Scholar] [CrossRef]
- Finn, D.R.; Lee, S.; Lanzen, A.; Bertrand, M.; Nicol, G.W.; Hazard, C. Cropping systems impact changes in soil fungal, but not prokaryote, alpha-diversity and community composition stability over a growing season in a long-term field trial. FEMS Microbiol. Ecol. 2021, 97, 10. [Google Scholar] [CrossRef]
- Sharma-Poudyal, D.; Schlatter, D.; Yin, C.T.; Hulbert, S.; Paulitz, T. Long-term no-till: A major driver of fungal communities in dryland wheat cropping systems. PLoS ONE 2017, 12, e0184611. [Google Scholar] [CrossRef]
- Bolo, P.; Kihara, J.; Mucheru-Muna, M.; Njeru, E.M.; Kinyua, M.; Sommer, R. Application of residue, inorganic fertilizer and lime affect P solubilizing microorganisms and microbial biomass under different tillage and cropping systems in a Ferralsol. Geoderma 2021, 390, 114962. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Sun, X.; Jia, S.; Zhang, X.; Liang, W. Conservation tillage positively influences the microflora and microfauna in the black soil of Northeast China. Soil Tillage Res. 2015, 149, 46–52. [Google Scholar] [CrossRef]
- Lu, X.; Lu, X.; Liao, Y. Effect of Tillage Treatment on the Diversity of Soil Arbuscular Mycorrhizal Fungal and Soil Aggregate-Associated Carbon Content. Front. Microbiol. 2018, 9, 2986. [Google Scholar] [CrossRef]
- Gosling, P.; Proctor, M.; Jones, J.; Bending, G.D. Distribution and diversity of Paraglomus spp. in tilled agricultural soils. Mycorrhiza 2014, 24, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Morton, J.B.; Bever, J.D.; Pfleger, F.L. Taxonomy of Acaulospora gerdemannii and Glomus leptotichum, synanamorphs of an arbuscular mycorrhizal fungus in Glomales. Mycol. Res. 1997, 101, 625–631. [Google Scholar] [CrossRef]
- Lienhard, P.; Terrat, S.; PrévostBouré, N.C.; Nowak, V.; Régnier, T.; Sayphoummie, S.; Panyasiri, K.; Tivet, F.; Mathieu, O.; Levêque, J.; et al. Pyrosequencing evidences the impact of cropping on soil bacterial and fungal diversity in Laos tropical grassland. Agron. Sustain. Dev. 2013, 34, 525–533. [Google Scholar] [CrossRef]
- Souza, R.C.; Hungria, M.; Cantão, M.E.; Vasconcelos, A.T.R.; Nogueira, M.A.; Vicente, V.A. Meta-genomic analysis reveals microbial functional redundancies and specificities in a soil under different tillage and cropmanagement regimes. Appl. Soil Ecol. 2015, 86, 106–112. [Google Scholar] [CrossRef]
- Ma, X.C.; Xu, X.; Geng, Q.H.; Luo, Y.Q.; Ju, C.H.; Li, Q.; Zhou, Y. Global arbuscular mycorrhizal fungal diversity and abundance decreases with soil available P. Glob. Ecol. Biogeogr. 2023, 32, 1423–1434. [Google Scholar] [CrossRef]
- Moitinho, M.R.; Fernandes, C.; Truber, P.V.; Marcelo, A.V.; Corá, J.E.; Bicalho, E. Arbuscular mycorrhizal fungi and soil aggregation in a no-tillage system with crop rotation. J. Plant Nutr. Soil Sci. 2020, 183, 482–491. [Google Scholar] [CrossRef]
- Bargaz, A.; Faghire, M.; Abdi, N.; Farissi, M.; Sifi, B.; Drevon, J.; Cherkaoui Ikbal, M.; Ghoulam, C. Low Soil P Availability Increases Acid Phosphatases Activities and Affects P Partitioning in Nodules, Seeds and Rhizosphere of Phaseolus vulgaris. Agriculture 2012, 2, 139–153. [Google Scholar] [CrossRef]
- Zhou, J.; Xia, B.; Treves, D.S.; Wu, L.-Y.; Marsh, T.L.; O’Neill, R.V.; Palumbo, A.V.; Tiedje, J.M. Spatial and resource factors influencing high microbial diversity in soil. Appl. Environ. Microbiol. 2002, 68, 326–334. [Google Scholar] [CrossRef] [PubMed]
- Roldán, A.; Salinas-García, J.R.; Alguacil, M.M.; Díaz, E.; Caravaca, F. Soil enzyme activities suggest advantages of conservation tillage practices in sorghum cultivation under subtropical conditions. Geoderma 2005, 129, 178–185. [Google Scholar] [CrossRef]
- Wen, L.S.; Peng, Y.; Zhou, Y.R.; Cai, G.; Lin, Y.Y.; Li, B.Y. Effects of conservation tillage on soil enzyme activities of global cultivated land: A meta-analysis. J. Environ. Manag. 2023, 345, 118904. [Google Scholar] [CrossRef]
- Li, C.H.; Ma, B.L.; Zhang, T.Q. Soil bulk density effects on soil microbial populations and enzyme activities during the growth of maize (Zea mays L.) planted in large pots under field exposure. Plant Sci. 2002, 82, 147–154. [Google Scholar] [CrossRef]
- Xomphoutheb, T.; Jiao, S.; Guo, X.; Mabagala, F.S.; Sui, B.; Wang, H.; Zhao, L.P.; Zhao, X.M. The effect of tillage systems on P distribution and forms in rhizosphere and non-rhizosphere soil under maize (Zea mays L.) in North-east China. Sci. Rep. 2020, 10, 6574. [Google Scholar] [CrossRef]
- Halstead, R.L. Phosphatase activity of soils as influenced by lime and other treatments. Can. J. Soil Sci. 1964, 44, 137–144. [Google Scholar] [CrossRef]
- Morra, M.J.; Kirkegaard, J.A. Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol. Biochem. 2002, 34, 1683–1690. [Google Scholar] [CrossRef]
- Jones, D.L.; Nguyen, C.; Finlay, R.D. Carbon flow in the rhizosphere: Carbon trading at the soilroot interface. Plant Soil 2009, 321, 5–33. [Google Scholar] [CrossRef]
- Higo, M.; Tatewaki, Y.; Gunji, K.; Kaseda, A.; Isobe, K. Cover cropping can be a stronger determinant than host crop identity for arbuscular mycorrhizal fungal communities colonizing maize and soybean. PeerJ. 2019, 7, e6403. [Google Scholar] [CrossRef]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total N in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]
- Cissé, G.; van Oort, F.; Chenu, C.; Essi, M.; Staunton, S. Is the operationally defined fraction of soil organic matter, “GRSP” (glomalin-related soil protein), stable in soils? Evidence from trends in long-term bare fallow soil. Eur. J. Soil Biol. 2021, 72, 1101–1112. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, T.; Fu, Y.; Chen, B.; Crotty, F.; Murray, P.J.; Yu, S.Q.; Xu, C.; Liu, W. Spatial change in glomalin-related soil protein and its relationships with soil enzyme activities and microbial community structures during urbanization in Nanchang, China. Geoderma 2023, 434, 116476. [Google Scholar] [CrossRef]
- Schneider, F.; Don, A.; Hennings, I.; Schmittmann, O.; Seidel, S.J. The effect of deep tillage on crop yield—What do we really know? Soil Tillage Res. 2017, 174, 193–204. [Google Scholar] [CrossRef]
- Cui, S.; Zilverberg, C.J.; Allen, V.G.; Brown, C.P.; Phillips, N. Carbon and N responses of three old world blue stems to N fertilization or inclusion of a legume. Field Crop. Res. 2014, 164, 45–53. [Google Scholar] [CrossRef]
- Inwood, S.E.E.; Bates, G.E.; Butler, D.M. Forage Performance and Soil Quality in Forage Systems under Organic Management in the Southeastern United States. Agron. J. 2015, 107, 1641–1652. [Google Scholar] [CrossRef]
- Wu, S.W.; Shi, Z.Y.; Chen, X.N.; Gao, J.K.; Wang, X.G. Arbuscular mycorrhizal fungi increase crop yields by improving biomass under rainfed condition: A meta-analysis. PeerJ 2022, 10, e1286. [Google Scholar] [CrossRef]
- Li, Z.; Cui, S.; Zhang, Q.; Xu, G.; Feng, Q.; Chen, C.; Li, Y. Optimizing Wheat Yield, Water, and Nitrogen Use Efficiency with Water and Nitrogen Inputs in China: A Synthesis and Life Cycle Assessment. Front. Plant Sci. 2022, 13, 930484. [Google Scholar] [CrossRef]
- Nopphakat, K.; Runsaeng, P.; Klinnawee, L. Acaulospora as the Dominant Arbuscular Mycorrhizal Fungi in Organic Lowland Rice Paddies Improves P Availability in Soils. Sustain. Sci. 2022, 14, 31. [Google Scholar] [CrossRef]


| Treatment | SOM (g kg−1) | TN (g kg−1) | TP (g kg−1) | AN (mg kg−1) | AP (mg kg−1) | pH |
|---|---|---|---|---|---|---|
| NTW | 20.32a | 1.31a | 0.63ab | 115.20b | 20.38e | 5.63cde |
| NTM1 | 19.80abc | 1.22bcd | 0.64abc | 115.71b | 26.44bc | 5.43e |
| NTM2 | 16.24f | 1.23bc | 0.63bc | 112.18bc | 28.23abc | 6.25a |
| NTM3 | 19.59abcd | 1.28ab | 0.62cd | 112.69bc | 26.62bc | 5.86bcd |
| NTM4 | 18.36bcde | 1.23bc | 0.66ab | 125.26a | 30.91ab | 6.17ab |
| NTMO | 19.36abcd | 1.22bcd | 0.59de | 112.69bc | 24.79cd | 5.58de |
| CTW | 17.85def | 1.18cd | 0.61bc | 91.06f | 24.35cde | 5.99cde |
| CTM1 | 19.95ab | 1.26ab | 0.62abc | 103.63d | 28.63abc | 6.01ab |
| CTM2 | 17.37ef | 1.28ab | 0.63bc | 97.60def | 31.35a | 6.05ab |
| CTM3 | 17.91cdef | 1.09e | 0.57e | 100.11de | 21.22de | 5.90bc |
| CTM4 | 18.36bcde | 1.16d | 0.67a | 104.64cd | 27.87abc | 6.10ab |
| CTMO | 16.75ef | 1.16d | 0.63bc | 95.08ef | 27.60abc | 5.54de |
| Phylum | Class | Order | Family | Genus | Species |
|---|---|---|---|---|---|
| Glomeromycota | Glomeromycetes | Archaeosporales | Archaeosporaceae | Archaeospora | Archaeospora VTX00005 |
| Archaeospora VTX00245 | |||||
| Acaulosporaceae | Acaulospora | Acaulospora VTX00030 | |||
| Acaulospora VTX00037 | |||||
| Diversisporales | Diversisporaceae | Diversispora | Diversispora VTX00062 | ||
| Diversispora VTX00054 | |||||
| Glomerales | Glomeraceae | Glomus | Glomus VTX00150 | ||
| Glomus VTX00280 | |||||
| Glomus VTX00114 | |||||
| Glomus VTX00143 | |||||
| Glomus VTX00125 | |||||
| Glomus VTX00222 | |||||
| Glomus VTX00113 | |||||
| Glomus VTX00310 | |||||
| Glomus VTX00309 | |||||
| Glomus VTX00195 | |||||
| Glomus VTX00092 | |||||
| Glomus VTX00248 | |||||
| Glomus VTX00056 | |||||
| Glomus VTX00278 | |||||
| Glomus VTX00057 | |||||
| Glomus VTX00193 | |||||
| Glomus VTX00279 | |||||
| Glomus VTX00307 | |||||
| Glomus VTX00333 | |||||
| Glomus VTX00063 | |||||
| Paraglomerales | Paraglomeraceae | Paraglomus | Paraglomus VTX00337 |
| Treatment | S-ACP (U g−1) | S-DHA (U g−1) | S-SC (U g−1) | Spore Density (g soil−1) | GRSP (mg g−1) | EGRSP (mg g−1) |
|---|---|---|---|---|---|---|
| NTW | 82.25d | 300.10f | 2.73g | 8.52f | 101.80d | 1.94a |
| NTM1 | 60.51e | 295.87f | 3.13f | 25.58b | 106.20bc | 1.90a |
| NTM2 | 78.23d | 324.89e | 3.32e | 15.47c | 108.90ab | 1.89a |
| NTM3 | 34.56f | 245.83h | 2.37h | 15.77c | 105.26bcd | 1.99a |
| NTM4 | 76.33d | 274.23g | 2.61g | 24.03b | 105.92bcd | 1.94a |
| NTMO | 40.92f | 253.46h | 2.65g | 29.92a | 104.27cd | 1.95a |
| CTW | 106.58c | 383.68c | 3.86cd | 4.83f | 105.45bcd | 1.94a |
| CTM1 | 125.73b | 396.94b | 4.33b | 11.40cde | 106.18bc | 1.96a |
| CTM2 | 113.85c | 378.93e | 4.02c | 12.68cde | 107.72abc | 1.94a |
| CTM3 | 133.14ab | 343.71d | 4.27b | 14.33cd | 110.37a | 1.97a |
| CTM4 | 137.69a | 387.35c | 3.72d | 11.22cde | 107.20abc | 1.96a |
| CTMO | 126.57b | 411.51a | 4.73a | 10.23de | 105.74bcd | 1.97a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, L.; Wang, T.; Cui, S.; Li, Y.; Gui, W.; Yang, F.; Chen, J.; Dong, R.; Gu, X.; Zhao, X.; et al. Diversified Cover Crops and No-Till Enhanced Soil Total Nitrogen and Arbuscular Mycorrhizal Fungi Diversity: A Case Study from the Karst Area of Southwest China. Agriculture 2024, 14, 1103. https://doi.org/10.3390/agriculture14071103
Tian L, Wang T, Cui S, Li Y, Gui W, Yang F, Chen J, Dong R, Gu X, Zhao X, et al. Diversified Cover Crops and No-Till Enhanced Soil Total Nitrogen and Arbuscular Mycorrhizal Fungi Diversity: A Case Study from the Karst Area of Southwest China. Agriculture. 2024; 14(7):1103. https://doi.org/10.3390/agriculture14071103
Chicago/Turabian StyleTian, Lihua, Tao Wang, Song Cui, Yuan Li, Weiyang Gui, Feng Yang, Jihui Chen, Rui Dong, Xinyao Gu, Xuechun Zhao, and et al. 2024. "Diversified Cover Crops and No-Till Enhanced Soil Total Nitrogen and Arbuscular Mycorrhizal Fungi Diversity: A Case Study from the Karst Area of Southwest China" Agriculture 14, no. 7: 1103. https://doi.org/10.3390/agriculture14071103
APA StyleTian, L., Wang, T., Cui, S., Li, Y., Gui, W., Yang, F., Chen, J., Dong, R., Gu, X., Zhao, X., Zhang, M., Chen, C., & Li, Z. (2024). Diversified Cover Crops and No-Till Enhanced Soil Total Nitrogen and Arbuscular Mycorrhizal Fungi Diversity: A Case Study from the Karst Area of Southwest China. Agriculture, 14(7), 1103. https://doi.org/10.3390/agriculture14071103







