Abstract
Phosphorus (P) is an essential macro-element for plants, and understanding the characteristics of the absorption and transport of P in crops is essential. The low availability of P restricts the growth, nitrogen fixation, and yield of soybean plants. In this research, the radioisotope 33P was supplied to the culture solution to trace the absorption and transport of P in nodulated soybean plants monitored using an imaging plate. The absorption rate of 33P was almost the same under the light and dark conditions. The absorption rate of 33P in the decapitated roots was near to that of the intact plants under light. These results indicate that the P absorption is not affected by evapotranspiration over a short time period. Conversely, the 33P transport from the roots to the shoot was significantly lower under dark conditions than it was under light conditions, although some 33P reached the top of the shoots under both the light and dark conditions. The transport of P to the shoots depends on the transpiration supplemented by the root pressure. The multiplication value of the 33P concentration in the xylem sap and transpiration rate was almost equivalent to the transport rate of 33P in the intact shoots. This value may be adaptable and used to estimate the transport rate of P for the diagnosis.
1. Introduction
Phosphorus is an essential macro-element, and the growth and yield of crops are sometimes restricted by the availability of P and its low mobility in soil [1,2,3]. Plant roots absorb inorganic P (Pi) mainly in the form of H2PO4− under natural pH conditions; this process is associated with phosphate transporters (PHTs) localized in the cell membranes. There are at least 14 PTH1 family gene families in soybean plants [4,5]. The production of soybeans has been increasing because of their nutritional value for humans and livestock. P nutrition is essential for soybean plants because P deficiency represses plant growth, nodule growth, and nitrogen-fixation activity [6,7,8]. On the other hand, excess P causes a decrease in the plant growth and yellowing of the lower leaves [9]. Therefore, it is important to understand the characteristics of P absorption and transport in nodulated soybean plants.
Higher plant roots absorb the mineral nutrients dissolved in the soil solution. Nutrient ions such as Pi in the soil solution move from the soil to the root surface and penetrate into the cortex via the apoplast pathway. Pi is absorbed into the epidermal or cortex cells mediated by transporters such as PHT1, localized on the plasma membrane. Then, they move radially and symplastically to the stele. Pi passes through the endodermis to the parenchyma cells of the stele, and it is released from the cytoplasm into the stele apoplast by the efflux transporter (PHO1) [2,3]. Then, Pi dissolved in water is loaded into the xylem vessels. The nutrient ions are transported upward through the xylem vessels by two motive forces; one is transpiration by the leaves, and the other is the root pressure. The evaporation of water through the stomata decreases the water potential in the leaves, and the negative pressure pulls up the solution in the xylem vessels from the roots. The root pressure is a positive driving force of xylem transport; it pushes up the solution from the roots to the shoot and is driven by the osmotic potential difference between the solution in the xylem vessels and that in the surrounding apoplast of the stele [10].
There have been several methods for collecting xylem sap [10,11,12,13]. Bollard [11] collected xylem sap evacuated from a cut stem to investigate the translocation forms of nitrogen among various dicotyledons, monocotyledons, and gymnosperms. Glutamine and asparagine were dominant in the xylem sap of most of the species, but some plants contained citrulline or allantoic acid as a principal component. Another method to obtain xylem sap depends on the decapitation of shoots and the collection of the sap that exudates from the cut end of the stump [11]. This “root pressure method” is the simplest way to collect xylem sap, and this technique can be readily applied to field experiments [14,15]. Under light conditions, the transpiration from the leaves causes a negative pressure in the xylem. Thus, the xylem sap needs a few minutes to start the exudation from the cut stump. The exudation of xylem sap from a cut stump depends on the root pressure because there is no transpiration without a shoot.
There has been a question about the use of the nutrient concentration in the xylem sap to estimate of the real transport rate in intact plants. The concentrations of nutrients in the xylem sap collected from the cut stump may be diluted or concentrated compared with the xylem sap in intact plants. However, it is difficult to answer the question because there is no non-invasive method to collect the real xylem sap from the intact plant. In addition, how to estimate the flux or transport rate of nutrients through the xylem vessels using xylem sap concentrations is an open question.
Regarding the P absorption and transport in soybean plants, the responses of these two processes were not the same in terms of the Pi concentrations in the culture solution. A previous study [16] reported that the Pi absorption rates increased with the higher concentration of Pi in the culture solution after 3 days of 0–500 μM Pi treatments. However, the concentrations of Pi in the xylem sap increased in response to the Pi concentrations from 0 to 50 μM but were relatively constant at higher Pi concentrations up to 500 μM Pi. These results indicated that the absorption of Pi in the roots and the subsequent P transport process from the roots to the shoots might be differently controlled during the response to external Pi concentrations.
Isotopes are beneficial tools for investigating the transport of nutrients in plants because isotopes can distinguish the newly absorbed nutrients in a plant from those that are already possessed. In addition, radioisotopes can visualize the 2D distribution of the labeled nutrients as images. Nakanishi et al. [17,18,19,20,21] investigated the water-transport processes in soybean stems using radioactive 15O-labeled water. When the H215O was supplied to the roots, the flow rate of 15O-labeled water in the stem was rapid at 4 mm/s, and the radioactivity was first detected at 20 s after H215O was supplied at 35 mm above the cotyledon node [21]. They also observed that 15O-labeled water in xylem vessels leaked horizontally to the surrounding stem tissues [21]. Regarding the 15NO3− absorption and transport in soybean plants, 15N was detected in the xylem sap collected from the root stump during the first 0–15 min after 15NO3− supply to the culture solution [22]. Moreover, 13N radioactivity was initially detected in the first trifoliate leaf within 6–10 min after 13NO3− feeding in the culture solution [23]. The transport rate of photoassimilates from the soybean leaves to the roots via phloem was relatively slow compared with the xylem transport of H2O and NO3−. When the first trifoliate leaf of soybean was exposed to 11CO2, the 11C was first detected in the roots 40 min after the 11CO2 supply [24]. Regarding the translocation form of P in the xylem vessels, only inorganic 32PO4 was detected in the xylem sap of soybean after 32PO4 was supplied to the culture solution [25]. Sasaki et al. [26] reported that labeled P was partly assimilated into some organic P compounds, such as ATP and G6P in the maize root cortex, but only inorganic Pi was uploaded into the xylem vessel.
In this study, we investigated the Pi absorption and transport from the roots to the shoots in nodulated soybean by monitoring the radioactivity from 33P-labeled Pi in the culture solution for a short period of up to 4 h. We particularly focused on the effect of transpiration and root pressure on the absorption and transport of 33P. Plant roots absorb and transport water and nutrients through the xylem vessels connecting the roots, stems, and leaves. This movement depends on both the evapotranspiration through leaf stomata, in which a negative water potential in leaves pulls water from the roots and root pressure, which is a positive pressure from the roots. The xylem sap obtained from the cut root stump depends only on the root pressure. Moreover, under dark conditions, plants do not transpire the water from the leaves because the stomata close. In this research, we investigated the effect of light and dark conditions on the absorption and transport of 33P. Then, the 33P transport in xylem sap collected from the cut stump was compared with that in the intact shoot. Finally, we evaluated the new estimation method of the P flow rate using the concentration of Pi in the xylem sap and the transpiration rate.
2. Materials and Methods
2.1. Plant Cultivation
The soybean (Glycine max [L.] Merr., cv. Williams) plants were inoculated with a suspension of Bradyrhizobium diazoefficience (strain USDA110, 108 cells/mL), and they were cultivated with hydroponically in a biophotochamber (LH-350S; Nippon Medical and Chemical instruments Co., Ltd., Osaka, Japan) under 28 °C day/18 °C night temperatures, 55% relative humidity, and under a photon flux density of 228 μmol m−2 s−1 with a 16 h photoperiod and an 8 h dark period, as same as in the previous study [9,16]. Each plant was cultivated in 800 mL of N-free culture solution in a 900 mL glass bottle. The Pi concentration was adjusted to 50 μM. The pH of the culture solution was adjusted to 6.0 ± 0.2. The culture solution was continuously aerated using an air pump at about 0.5L/min and changed every 2 or 3 days.
2.2. 33P Tracer Experiments
At 29 or 30 days after planting (DAP), the soybean plants were supplied with 33P-labeled orthophosphate (33Pi) in the Isotope Center at the Tokyo University of Agriculture. 33P is a radioisotope that decays by the β-decay mode with a maximum β-energy of 0.249 MeV and a half life of 25.4 days. Another radioisotope, 32P, is more popular than 33P for tracer experiments and has a maximum β-energy of 1.71 MeV and a half life of 14.3 days. We selected 33P as a tracer in this experiment due to the longer half life and the lower energy than those of 32P. H333PO4 (PerkinElmer, Inc., Boston, MA, USA) was purchased from the Japan Radioisotope Association. The 0.2 mL 33Pi isotope solution contained 1.85 × 108 Bq/mL (5.0 mCi/mL) in water. The solution was diluted with a 1 mM H3PO4 carrier solution and filled up to 2 mL. Then, 0.1 mL of diluted solution, which initially contained 9.25 × 106 Bq/mL, was used for each plant.
2.3. Absorption and Transport of 33P under Light and Dark Conditions
A tracer experiment was conducted to compare the absorption and transport of 33P by intact plants under light or dark conditions. The soybean plants were pre-cultivated under the same conditions at 1 h before feeding 33Pi. Light and dark cultivations started 1 h before feeding 33Pi into the 800 mL culture solution. The concentration of Pi in the culture solution was 50 μM. Six LED lamps (LED DESK STAND 120~140 μmol/m2s Yazawa corporation, Tokyo, Japan) were used for illumination under light conditions. The plants under dark conditions were covered with black plastic sheets under dark conditions. Under light conditions, after 1, 2, 3, or 4 h of 33Pi supply to the plants, the shoot was separated at 1 cm below the cotyledonary node. Then, the underground parts were washed twice in 800 mL of culture solution to release the 33Pi in the apoplast of the roots to the culture solution. The extra water attached to the roots was wiped off using a paper towel. Then, they were separated into the roots and nodules. Under dark conditions, the plants were supplied with 33Pi for 2 and 4 h. Then, the shoot and the roots were separated in the same way as those under light conditions. The shoot and roots were pressed and heated with an iron. Then, the distribution of radioactivity was measured with autoradiography using a rectangular imaging plate (IP) with length and width of 40 cm × 20 cm. The exposure time was 1 h for the roots and 16 h for the shoot because the radioactivity in the roots was much higher than that in the shoot. The radioactivity recorded on the IP was scanned using the BAS system (BAS-2500; Fujifilm Corporation, Tokyo, Japan). To calibrate the radioactivity in the plant parts, serial dilutions of 33P standard solution were spotted on filter paper, which amounted to 3.56 Bq, 35.6 Bq, and 356 Bq per spot. The amount of P originating from 33P was calculated in each part.
2.4. Transport of 33P in Xylem Sap
The soybean plants were pre-cultivated under light conditions for 1 h before feeding 33Pi into the solution. After the plants were transferred to the culture solution containing 33Pi for 1, 2, 3, or 4 h, the shoot was removed at 1 cm below the cotyledonary node, and the xylem sap exudated from the decapitated stump was collected in quartz wool in a plastic tube for 1 h. The shoot and washed roots and nodules were heated with an iron and were exposed on an IP. A 5 μL sample of xylem sap was spotted on a filter paper and the radioactivity was monitored using the IP. The amount of P originating from 33P in the region of interest was calculated in each part using BAS-2500 software Multi Gauge Version 3.11.
2.5. Estimation of P-Flux of Soybean Plants Grown in the Field
The new estimation method to determine the P flux rate using the concentration of Pi and the transpiration rate was applied to soybean plants cultivated in the lysimeter soil at the Tokyo University of Agriculture [27]. Basal dressings of N (16 kgN/ha), P (60 kg P2O5/ha), and K (80 kg K2O/ha) were supplied to the surface soil just before planting. The soybean seeds were inoculated with B. diazoefficience and planted on 15 June 2021. Exudating xylem sap from a cut stump was collected for 1 h (Figure 1). The samplings were carried out three times at 10–12 AM and 2–4 PM on 27 July at the beginning of the bloom stage (R1 stage) [28], at 10–12 AM on 31 August at the beginning of the seed stage (R5 stage), and on 28th September at the beginning of the maturity stage (R7 stage). The basal part of the main stem below the cotyledon node was cut using pruning shears, and an aliquot of quartz wool in a plastic tube was used to hold the xylem sap exudated from the cut stump (Figure 1B). The main stem of the shoot was re-cut in water in a bucket to prevent air-block in the xylem vessels, wiped with a paper towel, and then put into the 50 mL plastic tube containing 20 mL of water (Figure 1C). After 15 min, the plant was removed from the tube, the tube was weighed, and the loss of the water per 1 h was calculated as the transpiration rate. The Pi concentrations in the xylem sap were analyzed using a modified ascorbic acid–molybdenum blue method [16].
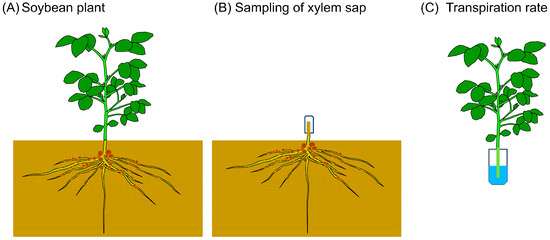
Figure 1.
Field estimation of P flux using the P concentration of xylem sap and the transpiration rate measured using the detached plant shoot. (A) Soybean plant cultivated in a field. The shoot was separated from the underground part. (B) Quartz wool in a plastic tube was put on the cut root stump, and the xylem sap was collected for 1 h. (C) The basal part of the main stem of the decapitated shoot was re-cut in water and put in 20 mL of water in a plastic tube. After 15 min, the shoot was removed from the tube. The water loss in the tube was determined by weighing the tube before and after transpiration measurement.
2.6. Statistics
The experiments were conducted with three biological replications. The plants were cultivated using a random arrangement in a growth chamber. Statistical significance using Student’s t-test and Tukey’s test was determined using the statistical analysis program of Biomedical Statistics, Graduate School of Medicine, Osaka University [29].
3. Results
3.1. Absorption and Transport of 33P in Soybean Plants under Light and Dark Conditions
3.1.1. Distribution Images of 33P Radioactivity in Soybean Plants
Figure 2A,B show the distribution images of 33P radioactivity in the shoot and underground parts of soybean plants at 2 h and 4 h after 33Pi supply, respectively. Under light and dark conditions, the 33P radioactivity was distributed uniformly in the roots after 2 h and 4 h of 33Pi supply, except for the uppermost part of the roots, where the culture solution did not have direct contact. Under light conditions, a part of the 33P reached the top fourth of the leaves and buds at 2 h of 33Pi supply (Figure 2A). The radioactivity of 33P was high in the developing fourth leaf, the third mature leaf, and the second mature leaf, compared with the primary and the first leaf. The distribution image of 33P radioactivity in the roots under dark conditions was similar to that under light conditions. However, the 33P radioactivity in the shoots was much lower under dark conditions than it was under light conditions. Under dark conditions, 33P reached the uppermost buds and the developing fourth leaf at 2 h of 33Pi supply. The 33P was distributed in the mature third, second, and first trifoliate leaves, as well as primary leaves, although the radioactivity in the mature leaves appeared to be lower than that under light conditions. In addition, the distribution of 33P in the mature leaves under dark conditions appeared to be restricted in the leaf veins and did not distribute in the mesophyll cells. At 4 h after 33Pi supply (Figure 2B), the radioactivity in the shoots was higher than that 2 h after supply, but the trends of 33P distributions among the parts were similar between the 2 h and the 4 h 33Pi supply.
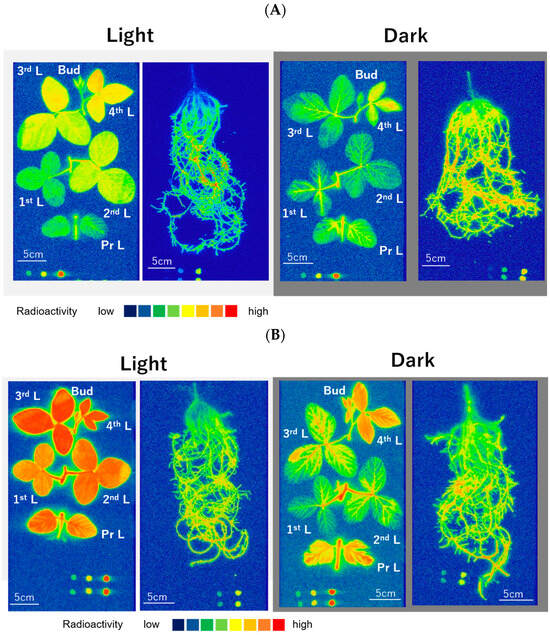
Figure 2.
Distribution images of 33P in soybean plants after 2 h (A) and 4 h (B) 33Pi supply. Color index: The radioactivity is higher in red > orange > yellow > green > blue in this sequence. Pr L, primary leaf; 1st L, first leaf; 2nd L, second leaf; 3rd L, third leaf; 4th L, fourth leaf; Bud, bud. Nodules were detached and placed near the upper right corner. Dots at the bottom show the standard 33P spots. The exposure time for the roots was 1 h and that for the shoot was 16 h because the radioactivity was much higher in the roots than the shoot.
3.1.2. Comparison of the Radioactivity in the Shoots and Roots under Light and Dark Conditions
Figure 3A,B shows the changes in the accumulation of 33P-labeled P in the upper part (sum of the leaves, stems, and buds) and in the lower part (sum of the roots and nodules). The total amount of 33P (sum of the roots and shoot) increased linearly during the 33Pi supply period in either light or dark conditions. In addition, the average amounts of 33P were 6.51 μmol under light conditions and 6.30 μmol/plant under dark conditions, and those values were not statistically significant. These results mean that the absorption rates of Pi under light and dark conditions are the same, suggesting that evapotranspiration may not contribute to the promotion of the absorption of Pi in the culture solution.
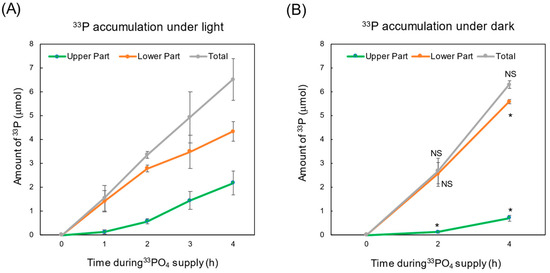
Figure 3.
Comparison of 33P accumulation in the upper and lower part of soybean under light (A) and dark (B) conditions. * and NS indicate statistically significant at p < 0.05 and not significant using Student’s t-test between light conditions (A) and dark conditions (B). n = 3.
On the other hand, the accumulation of 33P in the shoot under dark conditions was significantly (p < 0.05) lower than that under light conditions after 2 h and 4 h of 33P supply. After 4 h of 33P supply, the amount of 33P under dark conditions was 0.72 μmol/plant, and that under light conditions was 2.18 μmol/plant, which indicates that the former accounted for 33% of the latter. Under light conditions, the evapotranspiration might have accelerated the 33P transport from the roots to the shoots, while under dark conditions without evapotranspiration, an appreciable amount of 33P was transported to the shoot and was mainly driven by the root pressure. The amount of 33P in the lower part under dark conditions was 5.58 μmol/plant, and it was significantly higher than that under light conditions (4.33 μmol/plant) after 4 h of 33Pi supply. This higher accumulation of 33P in the roots under dark conditions was primarily due to the lower transport of 33P to the shoot due to the lack of evapotranspiration.
3.1.3. Comparison of the Radioactivity in Each Part of the Shoots under Light and Dark Conditions
The amount of 33P in each part of the shoot under the light and dark conditions after 2 h and 4 h of 33Pi supply is shown in Figure 4. After 2 h of 33Pi supply under light conditions (Figure 4A), the amount of 33P in the third leaf was the highest among the organs, followed by the fourth leaf and the second leaf. On the other hand, under dark conditions, the amount of 33P in each leaf was lower than 0.04 μmol/plant at 2 h, and the 33P amounts under dark conditions were significantly lower in the second, third, and fourth leaves and buds than those under light conditions. The amount of 33P after 4 h of 33Pi supply (Figure 4B) was higher than that after 2 h, but the 33P labeling trends were similar to those of the results of 2 h. These results confirmed that a part of the P absorbed from the roots was transported to the shoot during a short period of 2 h to each leaf and bud, and some parts reached the apical bud either under light or dark conditions. Under light conditions, recently developed leaves, such as the second and the third leaves, accumulated a high amount of 33P compared with the older leaves (primary leaf and the first leaf) and developing leaf (the fourth leaf). Under dark conditions, a small amount of 33P was transported to each leaf and bud. However, the amounts were not high in the second, third, and fourth leaves compared with those under light conditions. This may be due to the lack of evapotranspiration under dark conditions.
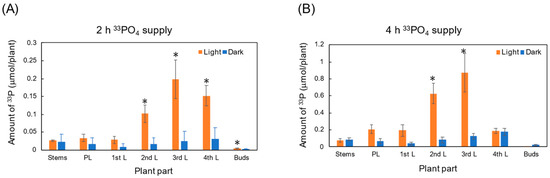
Figure 4.
Comparison of 33P accumulation in each part of soybean shoot after 2 h (A) and 4 h (B) 33Pi supply under light and dark conditions. * indicates statistical significance at p < 0.05 using Student’s t-test between light and dark conditions. n = 3.
3.1.4. Comparison of the Radioactivity in Roots and Nodules under Light and Dark Conditions
From the IP images of the nodulated roots, it was clear that the radioactivity in the nodules was not detected by the high radioactivity of the roots. So, we separated the nodules and roots, and the radioactivity was separately determined. Figure 5 shows the 33P concentration based on the dry weight in the roots and nodules after 4 h of 33Pi supply under the light and dark conditions. There were no significant differences in the roots and nodules between the light and dark conditions. The average 33P concentration in the nodules was about 1/10 of the roots. Therefore, roots are the primary absorption site of Pi, although some small portion of Pi may be absorbed from the nodule surface.
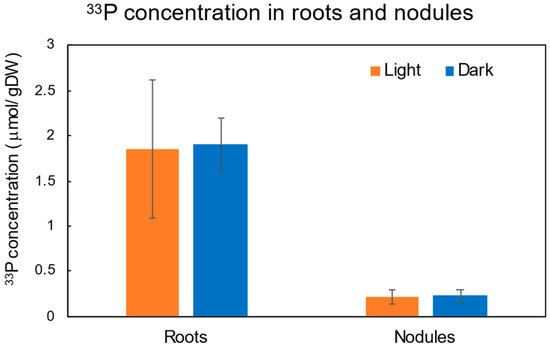
Figure 5.
Comparison of 33P concentration in roots and nodules based on the dry weight under light and dark conditions after 4 h of 33PO4 supply. n = 3.
3.2. Transport of 33P in Xylem Sap
To investigate the relationship between 33P transport in intact plants and that in the xylem sap, the 33P-labeled Pi was supplied to the soybean plants, the shoot was decapitated, and the xylem sap bleeding from the cut surface was collected for a 1 h period (Figure 6). Because the sampling time of the roots was 1 h after the shoot sampling due to the xylem sap collection, the data of the roots at 1, 2, 3, and 4 h, and those of the shoot at 0, 1, 2, and 3 h, came from the same plants. From 0 to 1 h, the decapitated soybean roots continued to absorb 33P (1.22 μmol/plant) at a comparable rate to that of the intact plants (1.42 μmol/plant). Moreover, the accumulation of the 33P amount in the underground part increased linearly to 4 h (5.26 μmol/plant), like the intact plants under the light (4.33 μmol/plant) and under dark (5.58 μmol/plant) conditions (Figure 3). These results strongly suggest that short-term Pi absorption in the roots does not depend on the presence of shoots because decapitation did not affect the accumulation of 33P in the roots.
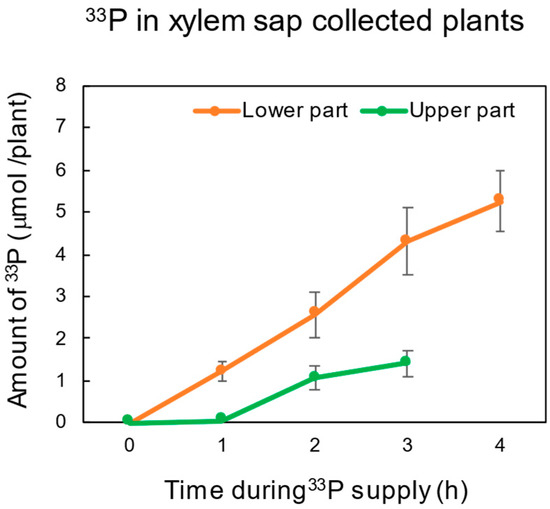
Figure 6.
Amount of 33P accumulation in the lower and upper part of soybean plants used for xylem sap collection under light conditions. The shoot was decapitated at 0, 1, 2, or 3 h after 33Pi supply, and the xylem sap was collected from the cut stump for 1 h. The lower part at 1, 2, 3, and 4 h, and the upper part at 0, 1, 2, and 3 h, were from the same plant, respectively. n = 3.
Figure 7A shows the changes in the volume of xylem sap collected for 1 h. The volume fluctuated from 100 to 300 μL. Figure 7B shows the 33P concentration in the xylem sap. No radioactivity was detected during the initial 1 h period from 0 to 1 h after 33Pi supply. 33P was detected in the xylem sap collected in the period from 1 to 2 h, but it was low at 0.028 mmol/L. The radioactivity in the xylem sap increased in the periods from 2 to 3 h and 3 to 4 h to about 0.3 mmol/L.
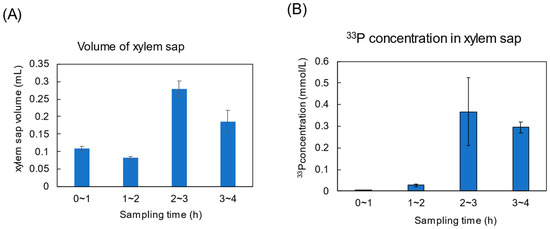
Figure 7.
Changes in xylem sap volumes and the 33P concentration in xylem sap under light conditions. (A) volume of xylem sap, and (B) 33P concentration. n = 3.
The 33P flux rate in the xylem sap vessels was estimated using the 33P concentration in the xylem sap and the xylem sap exudation rate or transpiration rate was compared with the 33P flux rate in the intact shoot (Figure 7). The exudation rate of the xylem sap collected for 1 h, from 3 h to 4 h after the 33P supply, was 0.214 mL/h, and the value was 10 times lower than the transpiration rate of the intact plants (1.84 mL/h) (Figure 8A). The 33P flux was estimated based on the xylem sap exudation rate (0.214 mL/h) multiplied by 33P concentration in xylem sap (0.334 μmol/mL) (Figure 8B), and the estimated 33P flux was 0.071 μmol/h. This was much lower than the 33P flow rate in the intact shoot (0.657 μmol/h). However, the 33P flux was estimated based on the transpiration rate (1.84 mL/h) multiplied by the 33P concentration in the xylem sap, and the value was almost the same (0.614 μmol/h) as that in the intact shoot (0.657 μmol/h). This result indicates that the concentration of 33P in the collected xylem sap was almost the same as that in the intact xylem sap when the xylem sap was collected for 1 h after the decapitation of the soybean plant.
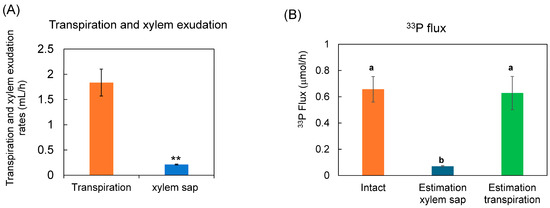
Figure 8.
Estimation of 33P flux using 33P concentration of xylem sap in comparison with the 33P flux in the intact plant. (A) The transpiration rate and xylem sap exudation rate. (B) Estimation of the 33P flux based on xylem sap exudation rate and that based on transpiration rate. n = 3. ** indicates statistically significant at p < 0.01 using Student’s t-test. Different letters indicate the significant difference in the values among treatments using Tukey’s method.
3.3. Estimation of P Flux of Soybean Plants Cultivated in Soil
Figure 9A shows the exudation rates of the xylem sap during AM and PM on 27 July, AM on 31 August, and AM on 28 September. The xylem sap exudation rate between AM and PM on 27 July were similar, but they decreased on 31 August and on 28 September. The transpiration rates from the cut shoot during AM and PM on 27 July were the same, but they increased on 31 August and on 28 September. The Pi concentration in the xylem sap collected during the afternoon of 27 July tended to be lower than that collected during the morning. The P concentration in the xylem sap significantly decreased along with the growth stages. Figure 9D shows the estimated PO4-flux in at the various stages. The estimation of the P flux is based on the P concentration in the xylem sap and the xylem exudation rate plus the transpiration rate. The P flux during AM on 27 July was 34.2 μmol/h, and it was higher than that during PM on 27 July (19.6 μmol/h), on 31 August AM (15.8 μmol/h), and AM on 28 September (9.3 μmol/h).
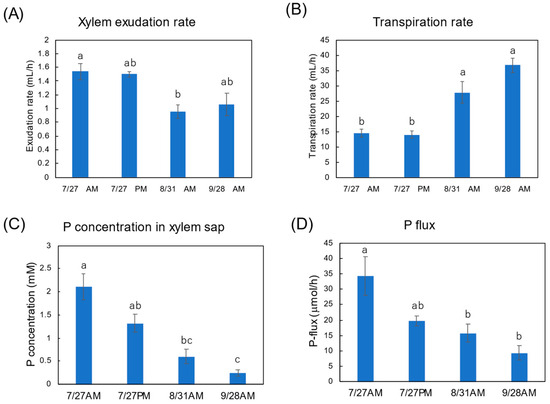
Figure 9.
Estimation of P flux using the P concentration of xylem sap and the transpiration rate. (A) Xylem sap exudation rate from cut stump, (B) transpiration rate from decapitated shoot, (C) the PO4 concentration in the xylem sap, (D) estimation of the P-flux based on multiplying the nutrient concentration value in xylem sap collected (C) by the sum of the xylem exudation rate (A), plus transpiration rate (B). Different letters indicate the significant difference in the values among treatments using Tukey’s method. n = 4.
4. Discussion
4.1. Absorption of 33P in Soybean Plants under Light and Dark Conditions and Decapitation
The transport of anions across the cell membrane requires an energy supply to transport against the electrochemical potential gradient [1]. The Pi in the soil solution is absorbed into the root cells, mediated by a high-affinity phosphate transporter PHT1 across the plasma membrane of the root cells, co-transported with 2–4 H+ [3,30].
The results showing that the total 33P absorptions in the intact plants at 2 h and 4 h of 33Pi supply were the same under the light and dark conditions (Figure 2) indicate that the Pi absorption did not require transpiration because the transpiration rate was negligible under dark conditions. Moreover, the photoassimilates in the roots did not deplete in 4 h, and they provided the energy to support Pi absorption. Under more severe conditions than darkness, the decapitated roots absorbed Pi at the same rate as the intact plants under light conditions, which supports the above discussion. Yamawaki et al. [31] reported that the absorption rate of 32P-Pi in Lotus japonicus roots was the same in the daytime and nighttime and that the transport of 32P in the shoot was higher in the daytime compared with nighttime. Their results in lotus were the same as our results in soybean provided here.
4.2. Transport of 33P from Roots to Shoot under Light and Dark Conditions
Although there were similar 33P absorption rates under light/dark conditions, the 33P transport rate from the roots to the shoot was significantly lower under dark conditions than it was under light conditions as shown in Figure 2. After 4 h of 33P supply, the 33P amount in the shoot under dark conditions accounted for 33% compared with that under light conditions. This result indicated that transpiration may play an important role in the P transport from the roots to the shoots. Under dark conditions, some 33P translocated to the top of the plants despite the lack of transpiration (Figure 1). This result suggests that sole root pressure alone can push up xylem sap from the roots to the shoot.
As shown in Figure 3, the 33P radioactivity was high in the developed leaves in the third and second leaves under light conditions due to the more active transpiration from these leaves. Under dark conditions, 33P radioactivity was much lower in the third and second leaves compared with that under light conditions due to the lack of transpiration. The distribution of 33P radioactivity in the mature leaf blades under dark conditions was restricted in the leaf veins and the 33P did not spread in the mesophyll tissues, although the 33P did distribute in the mesophyll under light conditions. This result may be due to the transpiration flow promoting the mass flow of water and 33P from the vein to the mesophyll.
4.3. Distribution of 33P in Roots and Nodules under Light and Dark Conditions
The concentrations of 33P based on dry weight in both the roots and nodules were the same between the light and dark conditions after 4 h of 33P supply (Figure 4). These results indicated that the absorption of 33P in the roots and nodules was independent of transpiration and the photoassimilates supply from the leaves, at least within 4 h. The transport process mediated by PHTs with protons could operate without transpiration. In addition, the photoassimilate storage for the energy supply for P absorption was not exhausted during this period.
The concentration of 33P was much lower in the nodules and was about 1/10 of that in the roots. This means that the roots are the primary organ used to absorb Pi, although some Pi can be absorbed directly from the nodule surface mediated by GmPT7 [32]. When 15N-labeled NO3− was supplied to the nodulated soybean, some 15N was directly absorbed from the nodule surface [33]. After 1 h of 15N-NO3− supply to the intact nodulated roots, the 15N abundance in the nodules was almost 1/10 compared with that in the roots. The 15N abundance in the roots decreased by about 1/10 due to the decapitation, which means that the dependence of NO3− absorption in soybean roots on transpiration is different from Pi absorption in this research. In addition to the direct absorption of 33P from the nodule surface, some 33P might be transported from the roots to the nodules via phloem through a recycling process from the shoots [34]. The high-affinity P transporter GmPT5 may contribute to the transport of Pi from the roots to the nodules [2].
4.4. Estimation of P Flux Rate Using Xylem PO4 Concentration and Transpiration Rate
In his review, Schurr [9] pointed out in his review that the flux of nutrients in xylem vessels was more important than the concentration of the xylem sap itself. However, various xylem sap collecting methods are destructive, and they disturb the xylem flux and may change the nutrient concentration. When the bleeding xylem sap is collected from the cut stump, the transpiration and photoassimilate supply will stop due to decapitation. In this experiment, the average transpiration rate of the intact plant was 1.84 mL/h, as measured from the decrease in the solution weight, and it was about 10 times higher than the xylem sap exudation rate of 0.267 mL/h. The total transported 33P in the shoot of the intact plant was 0.675 μmol/h, about 10 times higher than the 33P flux (0.071 μmol/h) estimated by multiplying the 33P concentration and the xylem exudation rate. On the other hand, when the flux rate was calculated by multiplying the 33P concentration and transpiration rate, the flux rate was 0.614 μmol/h. Conversely, the concentration of 33P in the xylem sap of the intact plant was estimated as 0.376 μmol/mL from the total 33P in the shoot and transpiration and was almost equivalent to the concentration in xylem sap collected from the stump (0.334 μmol/mL). This result indicates that the concentration of newly absorbed 33P in the xylem sap collected for 1 h from the young soybean plant appeared to be the same as that in the xylem sap in the intact plant. Goodger et al. [35] compared the concentrations of xylem sap constituents of maize collected using three different sampling techniques: a “root pressure” technique from the cut stump and a “pressurized” technique from the root stump or from a wedge of the leaf. The sap flow rate was about twice as high in the pressurized root stump compared as in the non-pressurized root stump. However, the concentrations of the constituents were slightly higher or similar using the root-pressure techniques compared with those in the pressurized root stump.
Therefore, we propose a novel and practical method to estimate the nutrient flux (μmol/h) by multiplying the nutrient concentration in the xylem sap collected (μmol/mL) by the sum of the xylem sap exudation rate (mL/h) and the transpiration rate (mL/h) (Figure 1). In laboratory experiments using hydroponics, the transpiration rate is measured as the weight loss of the culture solution. However, this method cannot be applied in field research. In contrast, our method can be easily implemented in the field by using a detached shoot to measure the shoot transpiration rate. The latter method is easily achievable, as the shoot is placed in a small tube with about 20 mL of water, and the water loss is measured by weighing before and after transpiration. The field trial for the estimation of P flux in soybean plants shows the feasibility of using this method to estimate the P flux in the xylem vessels (Figure 9). The estimation made using the calculation that multiplies the xylem sap concentration and the xylem sap exudation rate results in an underestimation, as shown in Figure 8B. However, the calculation that multiplies the xylem sap concentration and the transpiration rate seems to be more reliable. More research is necessary to confirm the feasibility of this method.
5. Conclusions
The total radioactivity of 33P in the plants increased linearly over time up to 4 h, and that under light and dark conditions, it showed the same absorption rate. On the other hand, the transport rate of 33P from the roots to the shoot was higher under light conditions compared with that under dark conditions, suggesting that transpiration contributes to the transport of P from the roots to the shoot. In addition, the cut stump without a shoot for collecting xylem sap absorbed 33P at the same rate as the intact plants. These results indicate that Pi absorption is independent of the transpiration rate and the photoassimilate supply during a short time period of a few hours. The estimated flux of 33P, determined by multiplying the 33P concentration in the collected xylem sap, and the transpiration rate was equal to the transport rate in the intact plants. This technique may be applied to the diagnosis of the nutrient flux of the soybean plants grown in the field.
Author Contributions
Conceptualization, Y.Y. and T.O.; methodology, Y.Y. and T.O.; software, Y.Y.; validation, Y.Y., K.H., A.S. and T.O.; formal analysis, Y.Y. and S.N.; investigation, Y.Y. and S.N.; resources, K.H. and A.S.; data curation, Y.Y.; writing—original draft preparation, Y.Y. and T.O.; writing—review and editing, K.H. and A.S.; visualization, Y.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We appreciate Kazunari Shoji for his kind help in the RI experiments.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Marschner, H. Functions of mineral nutrients: Macronutrients. In Mineral Nutrition of Higher Plants, 2nd ed.; Academic Press: London, UK, 1986; pp. 229–312. [Google Scholar]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to the roots. Annu. Plant Rev. 2015, 48, 3–22. [Google Scholar]
- Lambers, H. Phosphorus acquisition and utilization in plants. Annu. Rev. Plant Biol. 2022, 73, 17–42. [Google Scholar] [CrossRef]
- Qin, L.; Guo, Y.; Chen, L.; Liang, R.; Gu, M.; Xu, G.; Zhao, J.; Walk, T.; Liao, H. Functional characterization of 14 Pht1 family genes in yeast and their expressions in response to nutrient starvation in soybean. PLoS ONE 2012, 7, e47726. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kobae, Y.; Mizuno, T.; Hata, S. Identification and expression analysis of arbuscular mycorrhiza-inducible phosphate transporter genes of soybean. Biosci. Biotechnol. Biochem. 2012, 76, 309–313. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cassman, K.G.; Whitney, A.S.; Stockinger, K.R. Root growth and dry matter distribution of soybean as affected by phosphorus stress, nodulation, and nitrogen source. Crop Sci. 1980, 20, 239–244. [Google Scholar] [CrossRef]
- Israel, D.W. Investigation of the role of phosphorus in symbiotic dinitrogen fixation. Plant Physiol. 1987, 84, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yuan, H.; Wu, G.; Ma, C.; Gong, Z. Proteome analysis of the soybean nodule phosphorus response mechanism and characterization of stress-induced ribosome structural and protein expression changes. Front. Plant Sci. 2022, 13, 908889. [Google Scholar] [CrossRef] [PubMed]
- Ohyama, T.; Takayama, K.; Akagi, A.; Saito, A.; Higuchi, K.; Sato, T. Development of an N-free culture solution for cultivation of nodulated soybean with less pH fluctuation by the addition of potassium bicarbonate. Agriculture 2023, 13, 739. [Google Scholar] [CrossRef]
- Schurr, U. Xylem sap sampling-new approaches to an old topic. Trends Plant Sci. 1998, 3, 293–298. [Google Scholar] [CrossRef]
- Bollard, E.G. Translocation of organic nitrogen in the xylem. Aust. J. Biol. Sci. 1957, 10, 292–301. [Google Scholar] [CrossRef][Green Version]
- Alexou, M.; Peuke, A.D. Methods for xylem sap collection. Chapter 13. In Plant Mineral Nutrients: Methods and Protocols, Methods in Molecular Biology; Maathuis, J.M., Ed.; Springer Science+Business Media, LLC: Berlin/Heidelberg, Germany, 2013; Volume 953. [Google Scholar] [CrossRef]
- Takamatsu, T.; Watanabe, M.; Koshikawa, M.K. Convenient sampling of xylem sap from adult tree trunks and analysis of its components. Forests 2023, 14, 389. [Google Scholar] [CrossRef]
- Takahashi, Y.; Chinushi, T.; Nakano, T.; Ohyama, T. Evaluation of N2 fixation activity and N absorption activity by relative ureide methods in field-grown soybean plants with deep placement of coated urea. Soil Sci. Plant Nutr. 1992, 38, 699–708. [Google Scholar] [CrossRef]
- Sakazume, T.; Tanaka, K.; Aida, H.; Ishikawa, S.; Nagumo, Y.; Takahashi, Y.; Ohtake, N.; Sueyoshi, K.; Ohyama, T. Estimation of nitrogen fixation rate of soybean (Glycine max (L.) Merr.) by micro-scale relative ureide analysis using root bleeding xylem sap and apoplast fluid in stem. Bull. Facul. Agric. Niigata Univ. 2014, 67, 27–41. [Google Scholar]
- Yamamura, Y.; Higuchi, K.; Saito, A.; Ohyama, T. Absorption and transport of phosphorus in nodulated soybean plants and diagnosis of phosphorus status using xylem sap analysis. Agriculture 2024, 14, 403. [Google Scholar] [CrossRef]
- Nakanishi, T.M.; Yokota, H.; Tanoi, K.; Furukawa, J.; Ikeue, N.; Ookuni, N.; Uchida, H.; Tsuji, A. Circadian Rhythm in 15O-Labeled Water Uptake Manner of a Soybean Plant by PETIS (Positron Emitting Tracer Imaging System). Radioisotopes 2001, 50, 163–168. [Google Scholar] [CrossRef]
- Tanoi, K.; Hojo, J.; Nishioka, M.; Nakanishi, T.M.; Suzuki, K. New technique to trace [15O]water uptake in a living plant with an imaging plate and a BGO detector system. J. Radioanal. Nucl. Chem. 2005, 263, 547–552. [Google Scholar] [CrossRef]
- Ohya, T.; Tanoi, K.; Iikura, H.; Rai, H.; Nakanishi, T.M. Effect of rhizosphere pH condition on cadmium movement in a soybean plant. J. Radioanal. Nucl. Chem. 2008, 275, 247–251. [Google Scholar] [CrossRef]
- Ohya, T.; Tanoi, K.; Hamada, Y.; Okabe, H.; Rai, H.; Hojo, J.; Suzuki, K.; Nakanishi, T.M. An analysis of long-distance water transport in the soybean stem using H215O. Plant Cell Physiol. 2008, 49, 718–729. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakanishi, T.M. Novel Plant Imaging and Analysis, Water, Elements and Gas, Utilizing Radiation and Radioisotopes; Springer: Gateway East, Singapore, 2021; pp. 1–214. [Google Scholar] [CrossRef]
- Ohyama, T.; Kato, N.; Saito, K. Nitrogen transport in xylem of soybean plant supplied with 15NO3−. Soil Sci. Plant Nutr. 1989, 35, 131–137. [Google Scholar] [CrossRef]
- Sato, T.; Ohtake, N.; Ohyama, T. Analysis of nitrate absorption and transport in non-nodulated and nodulated soybean plants with 13NO3− and 15NO3−. Radioisotopes 1999, 48, 450–458. [Google Scholar] [CrossRef]
- Fujikake, H.; Yamazaki, A.; Ohtake, N.; Sueyoshi, K.; Matsuhashi, S.; Ito, T.; Mizuniwa, C.; Kume, T.; Hashimoto, S.; Ishioka, N.-S.; et al. Quick and reversible inhibition of soybean root nodule growth by nitrate involves a decrease in sucrose supply to nodules. J. Exp. Bot. 2003, 54, 1379–1388. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.M. Xylem sap composition in woody plants. Nature 1965, 205, 1027. [Google Scholar] [CrossRef]
- Sasaki, Y.; Arima, Y.; Kumazawa, K. Radial transport of phosphate in corn roots. Mechanism of transport of phosphate estimated by the turnover rates of intermediate compounds. Soil Sci. Plant Nutr. 1984, 30, 137–144. [Google Scholar] [CrossRef]
- Ohyama, T.; Ikebe, K.; Okuoka, S.; Ozawa, T.; Nishiura, T.; Ishiwata, T.; Yamazaki, A.; Tanaka, F.; Takahashi, T.; Umezawa, T.; et al. A deep placement of lime nitrogen reduces the nitrate leaching and promotes soybean growth and seed yield. Crop Environ. 2022, 1, 221–230. [Google Scholar] [CrossRef]
- Fehr, W.R.; Caviness, C.E. Stages of Soybean Development; Special Report 87. Iowa State University Digital Repository; Iowa Agricultural and Home Economics Experiment Station Publications, 1977; pp. 1–11. Available online: https://dr.lib.iastate.edu/handle/20.500.12876/90239 (accessed on 6 July 2024).
- MEdical and PHarmaceutical Statistics (MEPHAS). Available online: www.gen-info.osaka-u.ac.jp/MEPHAS/mokuji1-e.html (accessed on 24 May 2002).
- Sakano, K. Proton/phosphate stoichiometry in uptake of inorganic phosphate by cultured cells of Catharanthus roseus (L.) G. Don. Plant Physiol. 1990, 93, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Yamawaki, M.; Kanno, S.; Ishibashi, H.; Noda, A.; Hirose, A.; Tanoi, K.; Nahanishi, T.M. A study of 32P-phosphate uptake in a plant by a real-time RI imaging system. Proc. Radiochem. 2011, 1, 289–293. [Google Scholar] [CrossRef]
- Chen, L.; Qin, L.; Zhou, L.; Li, X.; Chen, Z.; Sun, L.; Wang, W.; Lin, Z.; Zhao, J.; Yamaji, N.; et al. A nodule-localized phosphate transporter GmPT7 plays an important role in enhancing symbiotic N2 fixation and yield in soybean. New Phytol. 2016, 221, 2013–2025. [Google Scholar] [CrossRef]
- Mizukoshi, K.; Nishiwaki, T.; Ohtake, N.; Minagawa, R.; Ikarashi, T.; Ohyama, T. Nitrate transport pathway into soybean nodules traced by tungstate and 15NO3−. Soil Sci. Plant Nutr. 1995, 41, 75–88. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Liang, Q.; Lyu, X.; Li, S.; Gong, Z.; Dong, S.; Yan, C.; Ma, C. Regulation of phosphorus supply on nodulation and nitrogen fixation in soybean plants with dual-root systems. Agronomy 2021, 11, 2354. [Google Scholar] [CrossRef]
- Goodger, J.Q.D.; Sharp, R.E.; Marsh, E.L.; Schachtman, D.P. Relationships between xylem sap constituents and leaf conductance of well-watered and water-stressed maize across three xylem sap sampling techniques. J. Exp. Bot. 2005, 56, 2389–2400. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).