Carbon Sequestration by Tropical Trees and Crops: A Case Study of Oil Palm
Abstract
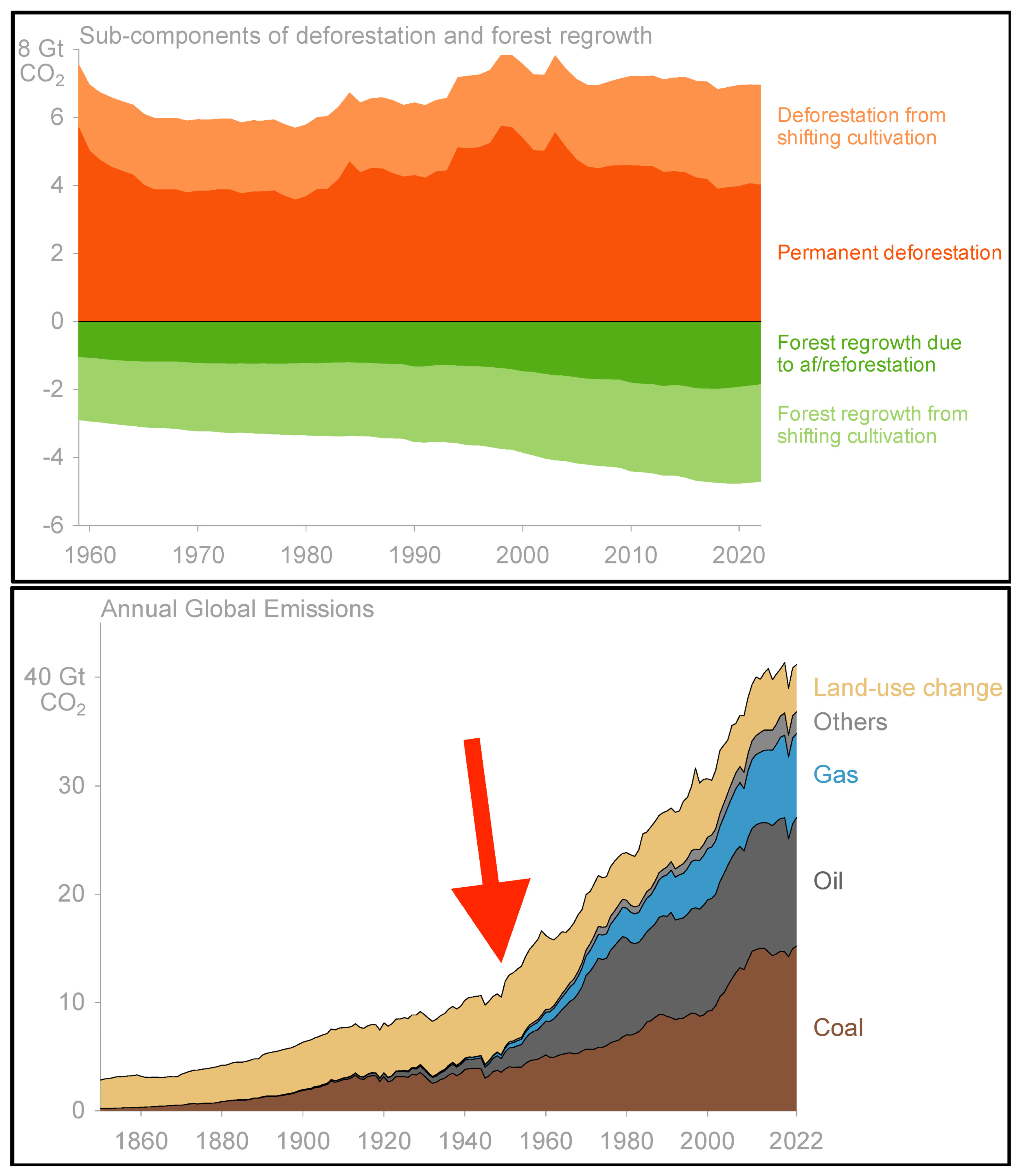
:1. Introduction
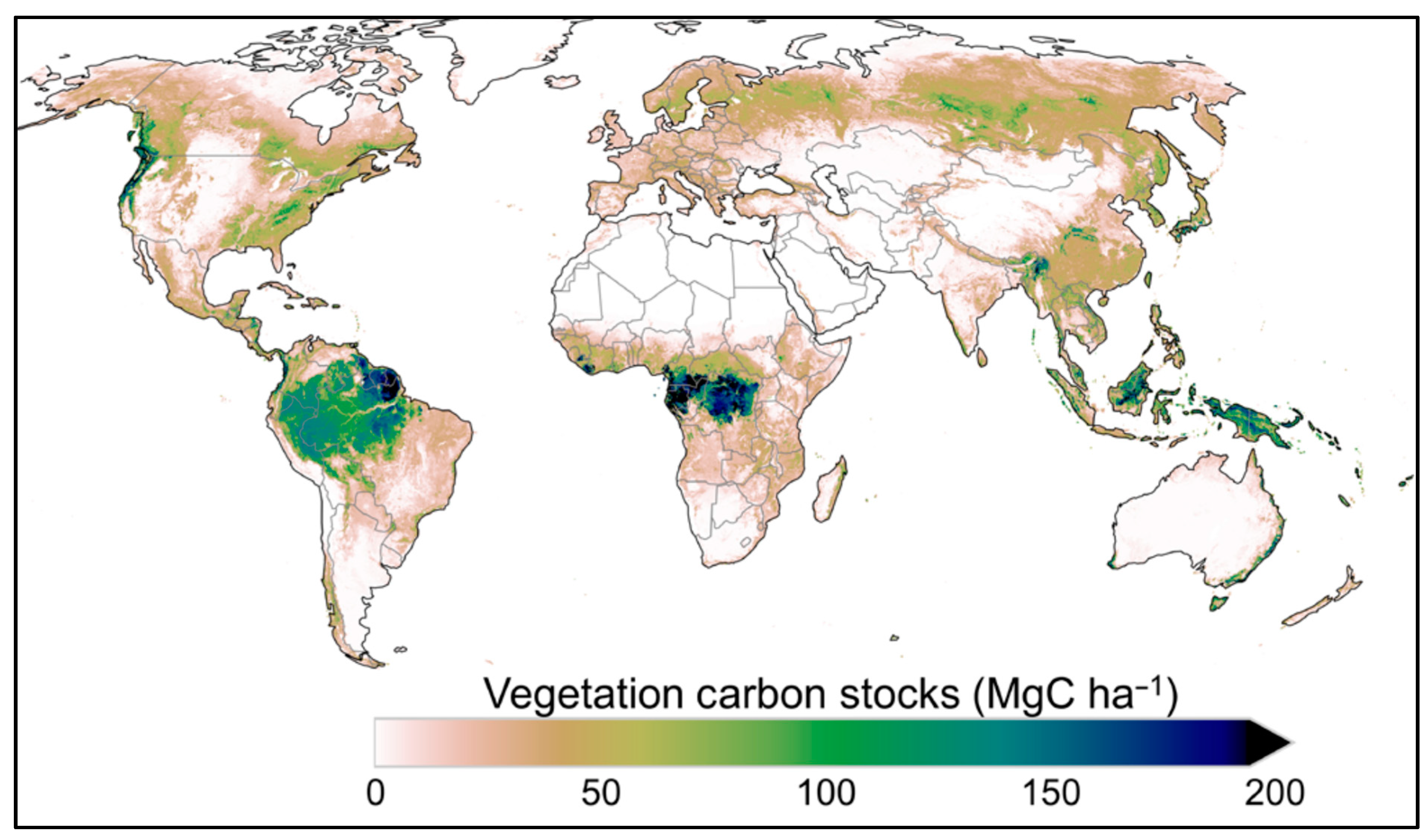
2. Carbon Sequestration by Tropical Vegetation
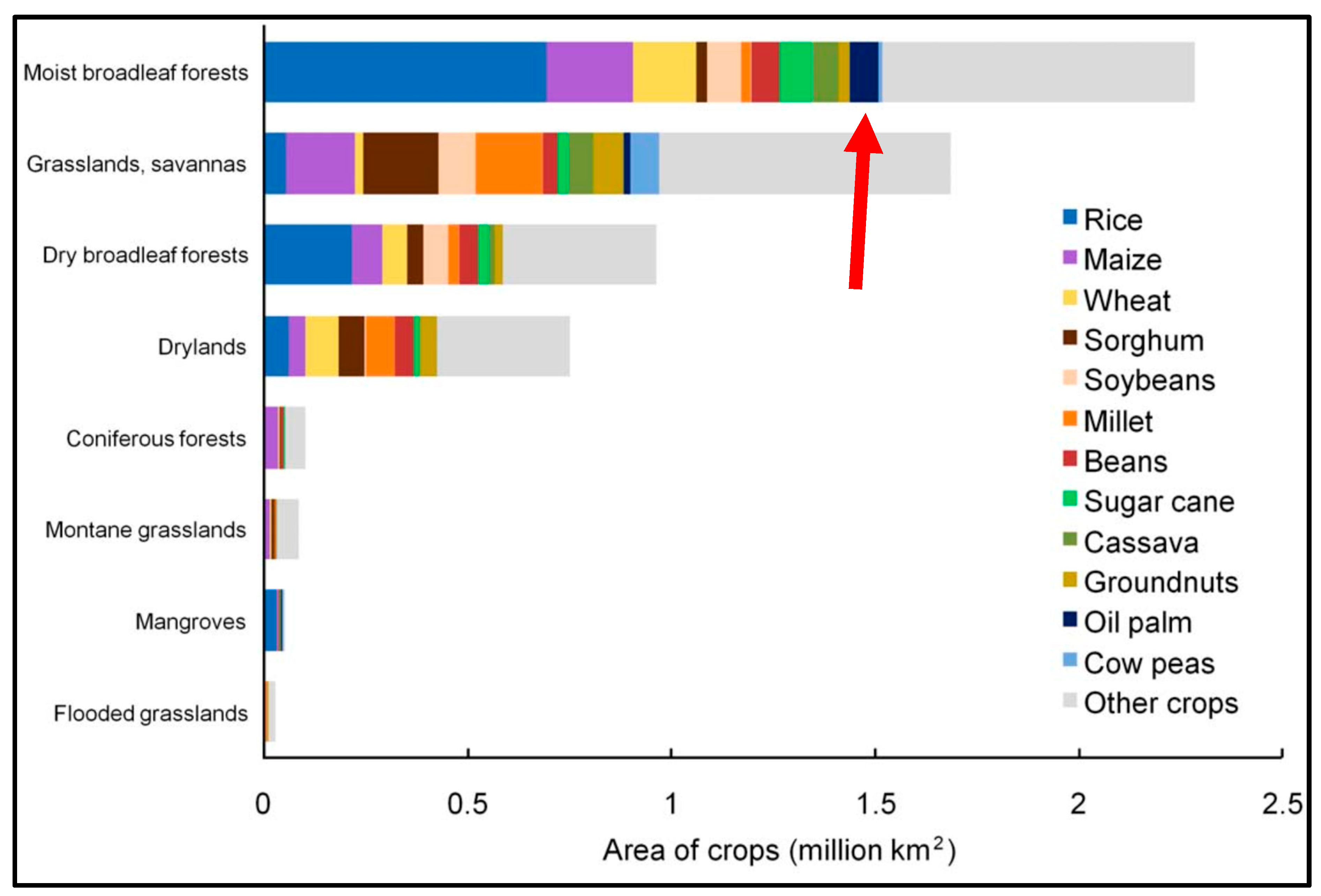
3. Carbon Sequestration by Tropical Crops
4. Oil Palm as a Tropical Tree Crop
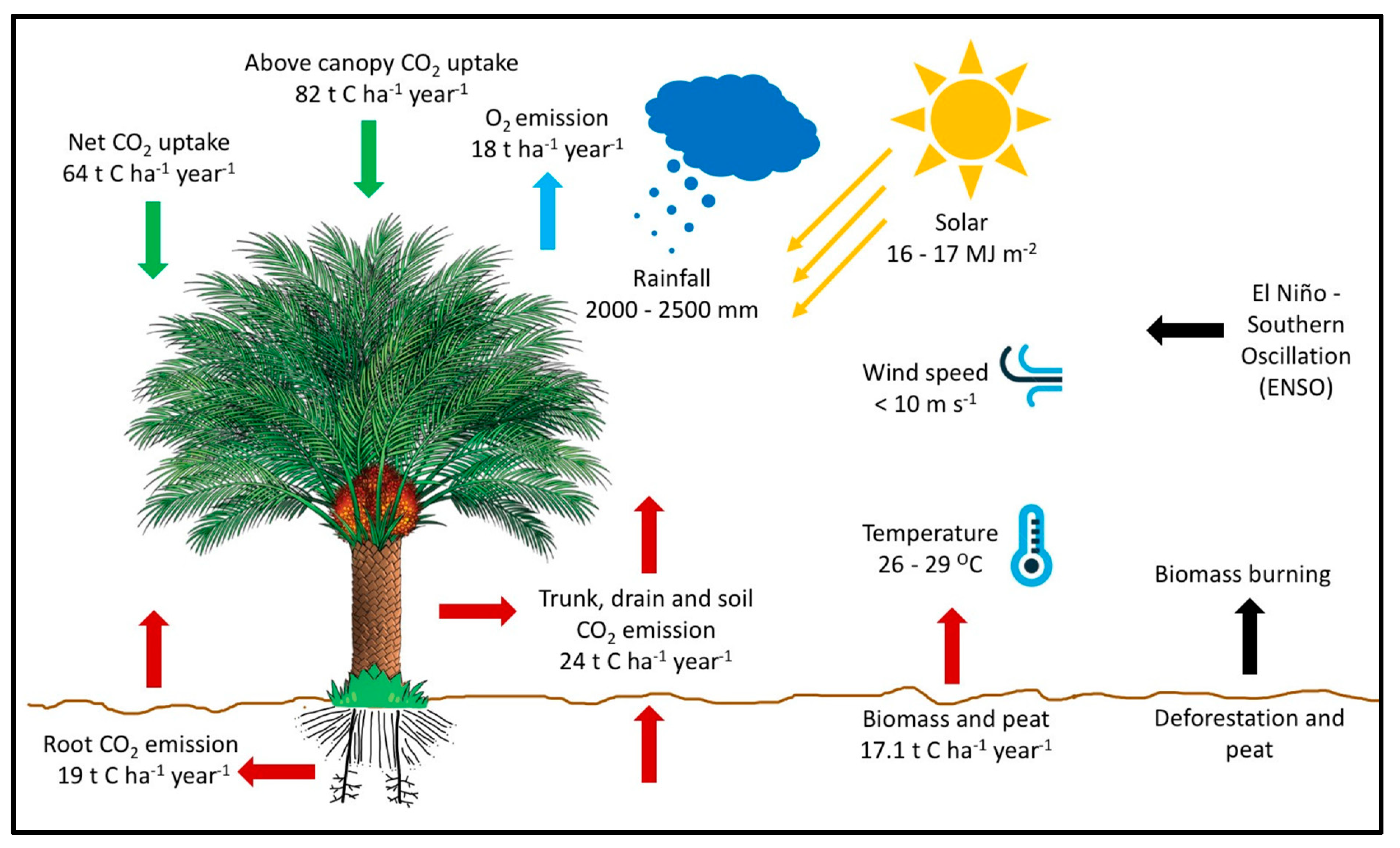
5. Carbon Sequestration Potential of Oil Palm versus Tropical Forests
6. Tropical Peatland Impacts on Carbon Sequestration
7. Climate Change Impacts on Carbon Sequestration
8. Land Use Conversion (LUC) Impacts on Carbon Sequestration
9. Biomass Sequestration Potential of Oil Palm
9.1. Biomass for Fuel
9.2. Biomass for Structural and Other Materials
10. Oil Palm Carbon Sequestration in Comparison with Other Major Oil Crops
11. Conclusions and Outlook
Funding
Acknowledgments
Conflicts of Interest
References
- Murphy, D.J.; Cardona, T. Photosynthetic Life. Origin, Evolution and Future; Oxford University Press: Oxford, UK, 2022. [Google Scholar]
- Murphy, D.J. Biological carbon sequestration: From deep history to the present day. Earth 2024, 5, 195–213. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Bernhardt, E.S. The Global Carbon Cycle. In Biogeochemistry, An Analysis of Global Change, 3rd ed.; Academic Press: Cambridge, MA, USA, 2013; pp. 419–444. [Google Scholar]
- NOAA. Doesn’t Carbon Dioxide in the Atmosphere Come from Natural Sources? 2020. Available online: https://www.climate.gov/news-features/climate-qa/doesnt-carbon-dioxide-atmosphere-come-natural-sources#:~:text=Yes%2C%20there%20are%20natural%20sources,even%20belches%20from%20ruminant%20animals (accessed on 7 July 2024).
- Yue, X.L.; Gao, Q.X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim. Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Rackley, S.A.; Sewel, A.; Clery, C.; Dowson, G.; Styring, P.; Andrews, G.; McCord, S.; Knops, P.; de Richter, R.; Ming, T. The Global Carbon Cycle. In Negative Emissions Technologies for Climate Change Mitigation; Elsevier: Amsterdam, The Netherlands, 2023; ISBN 978-0-12-819663-2. [Google Scholar]
- Ito, A. Disequilibrium of terrestrial ecosystem CO2 budget caused by disturbance-induced emissions and non-CO2 carbon export flows: A global model assessment. Earth Syst. Dynam. 2019, 10, 685–709. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2023, Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Core Writing Team, Lee, H., Romero, J., Eds.; IPCC: Geneva, Switzerland, 2023; pp. 35–115. [Google Scholar] [CrossRef]
- Jones, M.W.; Peters, G.P.; Gasser, T.; Andrew, R.M.; Schwingshackl, C.; Gütschow, J.; Houghton, R.A.; Friedlingstein, P.; Pongratz, J.; Le Quéré, C. National Contributions to Climate Change. 2023. Available online: https://ourworldindata.org/grapher/methane-emissions (accessed on 29 May 2024).
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.T.; Peters, G.P.; et al. Global Carbon Budget 2023. 2024 Data Updated on Co2.Earth May 2024. Available online: https://www.co2.earth (accessed on 1 July 2024).
- Albrecht, A.; Kandji, S.T. Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 2003, 99, 15–27. [Google Scholar] [CrossRef]
- Le Quéré, C.; Andrew, R.M.; Friedlingstein, P.; Sitch, S.; Pongratz, J.; Manning, A.C.; Korsbakken, J.I.; Peters, G.P.; Canadell, J.G.; Jackson, R.B.; et al. Global Carbon Budget. Earth Syst. Sci. Data 2018, 10, 405–448. [Google Scholar] [CrossRef]
- Mahli, Y.; Grace, J. Tropical forests and atmospheric carbon dioxide. Trends Ecol. Evol. 2000, 15, 332–337. [Google Scholar]
- Mendelsohn, R.; Sedjo, R.; Sohgen, B. Forest Carbon Sequestration. In Fiscal Policy to Mitigate Climate Change; De Mooij, R.A., Ed.; International Monetary Fund: New York, NY, USA, 2012; Chapter 5. [Google Scholar] [CrossRef]
- Harris, N.; Gibbs, D. Forests Absorb Twice as Much Carbon as They Emit Each Year; World Resources Institute: Washington, DC, USA, 2021. Available online: https://www.wri.org/insights/forests-absorb-twice-much-carbon-they-emit-each-year (accessed on 7 July 2024).
- Harris, N.L.; Gibbs, D.A.; Baccini, A.; Birdsey, R.A.; Bruin, S.; Farina, M.; Fatoyinbo, L.; Hansen, M.C.; Herold, M.; Houghton, R.A.; et al. Global maps of twenty-first century forest carbon fluxes. Nat. Climate Chang. 2021, 11, 234–240. [Google Scholar] [CrossRef]
- FAO. Land Use in Agriculture by the Numbers; FAO: Rome, Italy, 2020; Available online: https://www.fao.org/sustainability/news/detail/en/c/1274219/ (accessed on 7 July 2024).
- Lewis, S.L.; Lopez-Gonzalez, G.; Sonké, B.; Affum-Baffoe, K.; Baker, T.R.; Ojo, L.O.; Phillips, O.L.; Reitsma, J.M.; White, L.; Comiskey, J.A.; et al. Increasing carbon storage in intact African tropical forests. Nature 2009, 457, 1003–1006. [Google Scholar] [CrossRef]
- Ratnasingam, J.; Ramasamy, G.; Toong, W.; Ioras, F.; Canja, C.M.; Lupu, M.I.; Abrudan, I.V. Carbon Stocking in the Natural Forests—The Case of Malaysia. Not. Bot. Horti Agrobot. 2015, 43, 1842–4309. [Google Scholar]
- Xu, L.; Saatchi, S.S.; Yang, Y.; Yu, Y.; Pongratz, J.; Bloom, A.A.; Bowman, K.; Worden, J.; Liu, J.; Yin, Y.; et al. Changes in global terrestrial live biomass over the 21st century. Sci. Adv. 2021, 7, eabe9829. [Google Scholar] [CrossRef]
- Winkler, A.J.; Myneni, R.B.; Alexandrov, G.A.; Brovkin, V. Earth system models underestimate carbon fixation by plants in the high latitudes. Nat. Commun. 2019, 10, 885. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Ju, W.; Chen, J.M.; Ciais, P.; Cescatti, A.; Sardans, J.; Janssen, s.I.A.; Wu, M.; Berry, J.A.; et al. Recent global decline of CO2 fertilization effects on vegetation photosynthesis. Science 2020, 370, 1295–1300. [Google Scholar] [CrossRef] [PubMed]
- Frankenberg, C.; Yin, Y.; Byrne, B.; He, L.; Gentine, P. Comment on “Recent global decline of CO2 fertilization effects on vegetation photosynthesis”. Science 2021, 373, eabg5673. [Google Scholar] [CrossRef] [PubMed]
- Sang, Y.; Huang, L.; Wang, X.; Keenan, T.F.; Wang, C.; He, Y. Comment on “Recent global decline of CO2 fertilization effects on vegetation photosynthesis”. Science 2021, 373, eabg4420. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Salazar, P.; Medrano-Vizcaíno, P. The effects of climate change on decomposition processes in Andean Paramo ecosystem-synthesis, a systematic review. Appl. Ecol. Environ. Res. 2019, 17, 4957–4970. [Google Scholar] [CrossRef]
- Stuble, K.L.; Ma, S.; Liang, J.; Luo, Y.; Classen, A.T.; Souza, L. Long-term impacts of warming drive decomposition and accelerate the turnover of labile, not recalcitrant, carbon. Ecosphere 2019, 10, e02715. [Google Scholar] [CrossRef]
- Dawson-Glass, E.; Hewins, C.R.; Burke, D.J.; Souza, L.; Stuble, K.L. Warming-induced functional shifts in the decomposer community interact with plant community compositional shifts to impact litter decomposition. Funct. Ecol. 2023, 37, 2583–2597. [Google Scholar] [CrossRef]
- Zuidema, P.A.; Babst, F.; Groenendijk, P.; Trouet, V.; Abiyu, A.; Acuna-Soto, R.; Adenesky-Filho, E.; Alfaro-sánchez, R.; Arago, J.R.; Assis-Pereira, R.; et al. Tropical tree growth driven by dry-season climate variability. Nat. Geosci. 2022, 15, 269–276. [Google Scholar] [CrossRef]
- Weisse, M.; Goldman, E.; Carter, S. Tropical Primary Forest Loss Worsened in 2022, Despite International Commitments to End Deforestation; World Resources Institute: Washington, DC, USA, 2023; Available online: https://research.wri.org/gfr/latest-analysis-deforestation-trends (accessed on 7 July 2024).
- Gaveau, D.L.A.; Locatelli, B.; Salim, M.A.; Husnayaen; Manurung, T.; Descals, A.; Angelsen, A.; Meijaard, E.; Sheil, D. Slowing deforestation in Indonesia follows declining oil palm expansion and lower oil prices. PLoS ONE 2022, 17, e0266178. [Google Scholar] [CrossRef]
- Paris, B. Energy use in open-field agriculture in the EU: A critical review recommending energy efficiency measures and renewable energy sources adoption. Renew. Sustain. Energy Rev. 2022, 158, 112098. [Google Scholar] [CrossRef]
- Safitri, L.; Galdos, M.V.; Alexis Comber, A.; Challinor, A. Potential for low-emissions oil palm production in Indonesia: Insights from spatiotemporal dynamics. Environ. Res. Lett. 2024, 19, 054045. [Google Scholar] [CrossRef]
- Daud, N.N.; Chinenyenwa, A.S.; Rhys, T.H.; Ken, L.; Lee, H. Carbon Sequestration in Malaysian Oil Palm Plantations—An Overview: Towards a Sustainable Geoenvironment. In Proceedings of the 8th International Congress on Environmental Geotechnics Volume 3: Towards a Sustainable Geoenvironment 8th 2019, Hangzhou, China, 28 October–1 November 2018; Volume 3, pp. 49–56. [Google Scholar]
- Scholz, I.; von Broock, A.; Peters, J.; Kopansky, D. Peatland Atlas 2023, Facts and Figures about Wet Climate Guardians; Heinrich Böll Stiftung: Brussels, Belgium, 2023. [Google Scholar]
- Yunus, M. Unraveling the Dilemma of Tropical Peatland Restoration in Indonesia. Modern Diplomacy, 31 January 2024. Available online: https://moderndiplomacy.eu/2024/01/31/unraveling-the-dilemma-of-tropical-peatland-restoration-in-indonesia/(accessed on 7 July 2024).
- Lamade, E.; Bouillet, J.P. Carbon storage and global change: The role of oil palm. Oilseeds Fats Crops Lipids 2005, 12, 154–160. [Google Scholar] [CrossRef]
- Phalan, B.; Bertzky, M.; Butchart, S.H.; Donald, P.F.; Scharlemann, J.P.; Stattersfield, A.J.; Balmford, A. Crop expansion and conservation priorities in tropical countries. PLoS ONE 2018, 8, e51759. [Google Scholar] [CrossRef] [PubMed]
- Patthanaissaranukool, W.; Polprasert, C. Carbon Mobilization in Oil Palm Plantation and Milling Based on a Carbon-Balanced. Environ. Asia 2011, 24, 17–26. [Google Scholar]
- Field, C.B.; Behrenfeld, M.J.; Randerson, J.T.; Falkowski, P. Primary production of the biosphere: Integrating terrestrial and oceanic components. Science 1998, 281, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Raich, J.W.; Lambers, H.; Oliver, D.J. 10.16—respiration in terrestrial ecosystems. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 613–649. [Google Scholar]
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.; Bakker, D.; Hauck, J.; Landschützer, P.; Le Quéré, C.; Luijkx, I.; Peters, G.P.; et al. Global Carbon Budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. [Google Scholar] [CrossRef]
- Joshi, J.; Amthor, J.S.; McCarty, D.R.; Messina, C.D.; Wilson, M.A.; Millar, A.H.; Hanson, A.D. Why cutting respiratory CO2 loss from crops is possible, practicable, and prudential. Mod. Agric. 2023, 1, 16–26. [Google Scholar] [CrossRef]
- Ritchie, H. Is Our Appetite for Soy Driving Deforestation in the Amazon? OurWorldInData.org. 2021. Available online: https://ourworldindata.org/soy (accessed on 7 July 2024).
- Rudorff, B.F.T.; Adami, M.; Aguiar, D.A.; Moreira, M.A.; Mello, M.P.; Fabiani, L.; Amaral, D.F.; Pires, B.M. The Soy Moratorium in the Amazon Biome Monitored by Remote Sensing Images. Remote Sens. 2011, 3, 185–202. [Google Scholar] [CrossRef]
- Tyukavina, A.; Hansen, M.C.; Potapov, P.V.; Stehman, S.V.; Smith-Rodriguez, K.; Okpa, C.; Aguilar, R. Types and rates of forest disturbance in Brazilian Legal Amazon, 2000–2013. Sci. Adv. 2017, 3, e1601047. [Google Scholar] [CrossRef]
- Soterroni, A.C.; Ramos, F.M.; Mosnier, A.; Fargione, J.; Andrade, P.R.; Baumgarten, L.; Pirker, J.; Obersteiner, M.; Kraxner, F.; Câmara, G. Expanding the Soy Moratorium to Brazil’s Cerrado. Sci. Adv. 2019, 5, eaav7336. [Google Scholar] [CrossRef]
- Kuschnig, N.; Cuaresma, J.C.; Krisztin, T.; Giljum, S. Spatial spillover effects from agriculture drive deforestation in Mato Grosso, Brazil. Sci. Rep. 2021, 11, 21804. [Google Scholar] [CrossRef] [PubMed]
- De Lima, C.Z.; Estevam, C.G.; Pavao, E.P.; Pinto, T.P.; Assad, E.D. Greenhouse Gases Mitigation Potential of Soy Farming Decarbonization Actions to be Taken by 2030; Observatório de Conhecimento e Inovação em Bioeconomia: São Paulo, Brazil, 2022. [Google Scholar]
- Toloi, M.N.V.; Bonilla, S.H.; Toloi, R.C.; Alencar Nääs, I. Potential for carbon sequestration in different biomes and CO2 emissions in soybean crop. Environ. Dev. Sustain. 2024, 26, 3331–3347. [Google Scholar] [CrossRef]
- Leijten, F.; Lantz CBaldos, U.; Johnson, J.A.; Sim, S.; Verburg, P.H. Projecting global oil palm expansion under zero-deforestation commitments: Direct and indirect land use change impacts. iScience 2023, 26, 106971. [Google Scholar] [CrossRef]
- Villoria, N.; Garrett, R.; Gollnow, F.; Carlson, K. Leakage does not fully offset soy supply-chain efforts to reduce deforestation in Brazil. Nat. Commun. 2022, 13, 5476. [Google Scholar] [CrossRef] [PubMed]
- Bausano, G.; Masiero, M.; Migliavacca, M. Food, biofuels or cosmetics? Land-use, deforestation and CO2 emissions embodied in the palm oil consumption of four European countries: A biophysical accounting approach. Agric. Econ. 2023, 11, 35. [Google Scholar] [CrossRef]
- Joint Research Centre. The Amazon Region in 2022 and 2023, Deforestation, Forest Degradation and the Risk of Growing Soy Production. EU Science Hub. 2024. Available online: https://joint-research-centre.ec.europa.eu/jrc-news-and-updates/amazon-region-2022-and-2023-deforestation-forest-degradation-and-risk-growing-soy-production-2024-02-28_en (accessed on 7 July 2024).
- WTO. Certain Measures Concerning Palm Oil and Oil Palm Crop-Based Biofuels. World Trade Organization Dispute Settlement DS593; European Union, 2024; Available online: https://www.wto.org/english/tratop_e/dispu_e/600r_a_e.pdf (accessed on 7 July 2024).
- FAO. Global Forest Resources Assessment 2020—Key Findings; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Divya, M.P.; Mathuram, A.G.; Baranidharan, K.; Ravi, R.; Manivasakan, S.; Packialakshmi, M. Assessing the biomass productivity of Eucalyptus plantations at different age gradations. Pharma Innov. J. 2022, 11, 1548–1551. [Google Scholar]
- FAO. The Global Outlook for Future Wood Supply from Forest Plantations. Forest Plantation Yields in the Tropical and Subtropical Zone; FAO: Rome, Italy, 2000; Available online: https://www.fao.org/3/X8423E/X8423E08.htm (accessed on 7 July 2024).
- Kumar, B.M.; Nair, P.K.R. Carbon Sequestration Potential of Agroforestry Systems: Opportunities and Challenges. In Advances in Agroforestry 8; Springer: Singapore, 2011. [Google Scholar]
- Dhyani, S.K.; Ram, A.; Newaj, R.; Handa, A.K.; Dev, I. Agroforestry for Carbon Sequestration in Tropical India. In Carbon Management in Tropical and Sub-Tropical Terrestrial Systems; Ghosh, P., Ed.; Springer: Singapore, 2020. [Google Scholar]
- Ghale, B.; Mitra, E.; Sodhi, H.S. Carbon Sequestration Potential of Agroforestry Systems and Its Potential in Climate Change Mitigation. Water Air Soil Pollut. 2022, 233, 228. [Google Scholar] [CrossRef]
- Cheah, L.W.; Gan, H.H.; Goh, K.J. Potential carbon stock and management of carbon in oil palm plantations on mineral soils. AAR Newsl. 2015, 2015, 4–7. [Google Scholar]
- Ritchie, H. Palm Oil. OurWorldInData.org. 2021. Available online: https://ourworldindata.org/palm-oil (accessed on 7 July 2024).
- Gunsaro, P.; Hartoyo, M.E.; Agus, F.; Killeen, T.J. Oil Palm and Land Use Change in Indonesia, Malaysia and Papua New Guinea. Technical Panels of the 2nd Greenhouse Gas Working Group of RSPO. 2013. Available online: https://www.researchgate.net/publication/288658092_Oil_palm_and_land_use_change_in_Indonesia_Malaysia_and_Papua_New_Guinea (accessed on 7 July 2024).
- Rehman, S.A.U.; Sudadi, U.; Anwar, S.; Sabiham, S. Land use changes and above-ground biomass estimation in peatlands of Riau and West Kalimantan, Indonesia. J. Int. Soc. Southeast Asian Agric. Sci. 2015, 21, 123–135. [Google Scholar]
- Santosa, Y.; Sunkar, A.; Kwatrina, R.T. Is it true that oil palm plantations are the main driver of Indonesia’s tropical forest deforestation? J. Oil Palm Res. 2020, 3, 1–10. [Google Scholar] [CrossRef]
- Parker, D. Forest Loss Trajectories and Palm Oil Extent in Indonesia. Ph.D. Thesis, University of Maryland, College Park, MD, USA, 2022. [Google Scholar] [CrossRef]
- Sullivan, C.; Rausch, L.; Rojas, A.C.; Gibbs, H. Zero Deforestation Palm Oil in Colombia. In Gibbs Land Use and Environment Report Series; 2020; Available online: https://gibbs-lab.wisc.edu/publications.html (accessed on 7 July 2024).
- Henson, I.; Ruiz, R.; Romero, H.M. The greenhouse gas balance of the oil palm industry in Colombia: A preliminary analysis. II. Greenhouse gas emissions and the carbon budget. Agron. Colomb. 2012, 30, 370–378. [Google Scholar]
- Quezada, J.C.; Etter, A.; Ghazoul, J.; Buttler, A.; Guillaume, T. Carbon neutral expansion of oil palm plantations in the Neotropics. Sci. Adv. 2019, 20, eaaw4418. [Google Scholar] [CrossRef] [PubMed]
- Solidaridad. Barometer on Sustainable Palm Oil Production and Trade in Colombia 2022. 2023. Available online: https://solidaridadlatam.org/wp-content/uploads/2023/11/FINALBarometerPalmOil_2023_EN.pdf (accessed on 7 July 2024).
- Global Carbon Project. Available online: https://www.globalcarbonproject.org (accessed on 7 July 2024).
- Tan, Q.T.; Rajakal, J.P.; Lim, C.H.; Mimi, M.H.; Ng Denny, K.S. Toward Carbon Neutrality: Systematic Approach to Decarbonize Palm Oil Value Chain. Ind. Eng. Chem. Res. 2024, 63, 1903–1925. [Google Scholar] [CrossRef]
- Critchley, W.R.S.; Bruijnzeel, L.A. Environmental Impacts of Converting Moist Tropical Forest to Agriculture and Plantations. IHP Humid Tropics Programme Series. UNESCO. 1999. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000109608 (accessed on 7 July 2024).
- Odhiambo, C.R. Up to Half of Tropical Forestland Cleared for Agriculture Isn’t Put to Use, Research Shows. Mongabay. 2022. Available online: https://news.mongabay.com/2022/12/half-of-tropical-forestland-cleared-for-agriculture-isnt-put-to-use-research-shows/ (accessed on 7 July 2024).
- Pendrill, F.; Gardner, T.A.; Meyfroidt, P.; Persson, U.M.; Adams, J.; Azevedo, T.; Bastos Lima, M.G.; Baumann, M.; Curtis, P.G.; De Sy, V.; et al. Disentangling the numbers behind agriculture-driven tropical deforestation. Science 2022, 377, eabm9267. [Google Scholar] [CrossRef] [PubMed]
- Veloo, R.; van Ranst, E.; Selliah, P. Peat Characteristics and its Impact on Oil Palm Yield. Wagening. J. Life Sci. 2014, 72–73, 33–40. [Google Scholar] [CrossRef]
- Goh, N. EU, Malaysia, Indonesia Create Task Force over Deforestation Rule. Nikkei Asia, 30 June 2023. Available online: https://asia.nikkei.com/Editor-s-Picks/Interview/EU-Malaysia-Indonesia-create-task-force-over-deforestation-rule(accessed on 7 July 2024).
- Corley, R.H.V.; Tinker, P.B. The Oil Palm; Wiley Blackwell: Chichester, UK, 2015. [Google Scholar]
- Henson, I.E. A review of models for assessing carbon stocks and carbon sequestration in oil palm plantations. J. Oil Palm. Res. 2017, 29, 1–10. [Google Scholar] [CrossRef]
- Murphy, D.J.; Goggin, K.A.; Patterson, R. Oil palm crops in the 2020s and beyond: Challenges and solutions. CABI J. Agric. Biosci. 2021, 2, 39. [Google Scholar]
- Sibhatu, K.T. Oil palm boom: Its socioeconomic use and abuse. Front. Sustain. Food Syst. 2023, 7, 1083022. [Google Scholar] [CrossRef]
- Henson, I.E. Comparative Ecophysiology of Oil Palm and Tropical Rainforest. In Oil Palm and the Environment: A Malaysian Perspective; Singh, G., Huan, L.K., Leng, T., Kow, D.L., Eds.; Malaysian Palm Oil Board: Kuala Lumpur, Malaysia, 1999; pp. 9–39. [Google Scholar]
- Henson, I.E. Notes on oil palm productivity. IV. Carbon dioxide gradients and fluxes and evapotranspiration, above and below the canopy. J. Oil Palm. Res. 1999, 11, 33–40. [Google Scholar]
- Lamade, E.; Setiyo, I.E. Characterisation of carbon pools and dynamics for oil palm and forest ecosystems: Application to environmental evaluation. In Proceedings of the 2002 International Oil Palm Conference, Nusa Dua, Bali, Indonesia, 8–12 July 2002. [Google Scholar]
- Sheil, D.; Casson, A.; Meijaard, E.; van Nordwijk, M.; Gaskell, J.; Sunderland-Groves, J.; Wertz, K.; Kanninen, M. The Impacts and Opportunities of Oil Palm in Southeast Asia: What Do We Know and What Do We Need to Know? Occasional paper 51; CIFOR: Bogor, Indonesia, 2009. [Google Scholar]
- Henson, I.; Ruiz, R.; Romero, H.M. The greenhouse gas balance of the oil palm industry in Colombia: A preliminary analysis. I. Carbon sequestration and carbon. Agron. Colomb. 2012, 30, 359–369. [Google Scholar]
- Pulhin, F.B.; Lasco, R.D.; Urquiola, J.P. Carbon Sequestration Potential of Oil Palm in Bohol, Philippines. Ecosyst. Dev. J. 2014, 4, 14–19. [Google Scholar]
- Uning, R.; Latif, M.T.; Othman, M.; Juneng, L.; Hanif, N.M.; Nadzir, M.; Maulud, K.N.; Shafrina, W.; Jaafar, W.M.; Said, N.F.S. A Review of Southeast Asian Oil Palm and Its CO2 Fluxes. Sustainability 2020, 12, 5077. [Google Scholar] [CrossRef]
- PAPSI-Monitor. Palm Oil Industry Will Become a Net Carbon Sink. Palm Oil J. II, 47. 2021. Available online: https://palmoilina.asia/wp-content/uploads/2021/12/2.47.-PALM-OIL-INDUSTRY-WILL-BECOME-A-NET-CARBON-SINK-2.pdf (accessed on 7 July 2024).
- Ariesca, R.; Sau, A.A.W.T.; Adinugroho, W.C.; Setiawan, A.A.R.; Ahamed, T.; Noguchi, R. Land Swap Option for Sustainable Production of Oil Palm Plantations in Kalimantan, Indonesia. Sustainability 2023, 15, 2394. [Google Scholar] [CrossRef]
- Johnson, D. Tree crops and tropical development: The oil palm as a successful example. Agric. Adm. 1980, 7, 107–112. [Google Scholar] [CrossRef]
- USDA. Palm Oil World Production. 2024. Available online: https://ipad.fas.usda.gov/cropexplorer/cropview/commodityView.aspx?cropid=4243000 (accessed on 7 July 2024).
- FAO. World Food and Agriculture—Statistical Yearbook 2023; FAO: Rome, Italy, 2023. [Google Scholar] [CrossRef]
- Descals, A.; Gaveau, D.L.A.; Wich, S.; Szantoi, Z.; Meijaard, E. Global mapping of oil palm planting year from 1990 to 2021. Earth Syst. Sci. Data Discuss. 2024, preprint. [Google Scholar] [CrossRef]
- Botânico, M.P.; Angyalossy, V. Is the secondary thickening in palms always diffuse? An. Acad. Bras. Cienc. 2013, 85, 1461–1472. [Google Scholar] [CrossRef] [PubMed]
- Cunha Neto, I.L. Vascular variants in seed plants-a developmental perspective. Ann. Bot. Plants 2023, 15, plad036. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.; Mamat, M.N. The optimal age of oil palm replanting. Oil Palm Ind. Econ. J. 2002, 2, 11–18. [Google Scholar]
- Voora, V.; Bermúdez, J.; Farrell, J.; Larrea, C.; Luna, E. Palm Oil Prices and Sustainability. IISD Market Report, June 2023. [Google Scholar]
- Sasaki, N.; Putz, F.E. Critical need for new definitions of “forest” and “forest degradation” in global climate change agreements. Conserv. Lett. 2009, 2, 226–232. [Google Scholar] [CrossRef]
- Ashton-Butt, A.; Aryawan, A.A.K.; Hood, A.S.C.; Naim, M.; Purnomo, D.; Suhardi; Wahyuningsih, R.; Willcock, S.; Poppy, G.M.; Caliman, J.P.; et al. Understory Vegetation in Oil Palm Plantations Benefits Soil Biodiversity and Decomposition Rates. Front. For. Glob. Change 2018, 1, 10. [Google Scholar] [CrossRef]
- Hart, T.B.; Hart, J.A.; Murphy, P.G. Monodominant and species-rich forests of the humid tropics: Causes for their co-occurrence. Am. Nat. 1989, 133, 613–633. [Google Scholar] [CrossRef]
- Torti, S.D.; Coley, P.D. Tropical Monodominance: A Preliminary Test of the Ectomycorrhizal Hypothesis. Biotropica 1999, 31, 220–228. [Google Scholar] [CrossRef]
- Purwanto, E.; Santoso, H.; Jelsma, I.; Widayati, A.; Nugroho, H.Y.S.H.; van Noordwijk, M. Agroforestry as policy option for forest-zone oil palm production in Indonesia. Land 2020, 9, 531. [Google Scholar] [CrossRef]
- Khasanah, N.; van Noordwijk, M.; Slingerland, M.; Sofiyudin, M.; Stomph, D.; Migeon, A.F.; Hairiah, K. Oil Palm Agroforestry Can Achieve Economic and Environmental Gains as Indicated by Multifunctional Land Equivalent Ratios. Front. Sustain. Food Syst. 2020, 3, 122. [Google Scholar] [CrossRef]
- Pacheco, P.; Gnych, S.; Dermawan, A.; Komarudin, H.; Okarda, B. The Palm Oil Global Value Chain: Implications for Economic Growth and Social and Environmental Sustainability; CIFOR Working Paper 220; CIFOR: Bogor, Indonesia, 2017. [Google Scholar]
- Hanafiah, K.M.; Mutalib, A.H.A.; Miard, P.; Goh, C.S.; Sah, S.A.M.; Ruppert, N. Impact of Malaysian palm oil on sustainable development goals: Co-benefits and trade-offs across mitigation strategies. Sustain. Sci. 2021, 10, 1007. [Google Scholar] [CrossRef] [PubMed]
- Chiriacò, M.V.; Bellotta, M.; Jusić, J.; Perugini, L. Palm oil’s contribution to the United Nations sustainable development goals: Outcomes of a review of socio-economic aspects. Environ. Res. Lett. 2022, 17, 063007. [Google Scholar] [CrossRef]
- Shanahan, M. Palm Oil: The Pros and Cons of a Controversial Commodity. China Dialogue. 2023. Available online: https://chinadialogue.net/en/food/11627-palm-oil-the-pros-and-cons-of-a-controversial-commodity/ (accessed on 7 July 2024).
- Global Methane Initiative. Resource Assessment for Livestock and Agro-Industrial Wastes—Indonesia. 2015. Available online: https://www.globalmethane.org/documents/ag_indonesia_res_assessment.pdf (accessed on 7 July 2024).
- Loh, S.K.; Nasrin, A.B.; Mohamad Azri, S.; Nurul Adela, B.; Muzzammil, N.; Jay, T.; Stasha, R.A.; Lim, W.S.; Choo, Y.M.; Kaltschmitt, M. First Report on Malaysia’s experiences and development in biogas capture and utilization from palm oil mill effluent under the Economic Transformation Programme. Renew. Sust. Energ. Rev. 2017, 74, 1257–1274. [Google Scholar] [CrossRef]
- Loh, S.K.; Nasrin, A.B.; Mohamad Azri, S.; Nurul Adela, B.; Muzzammil, N.; Jay, T.; Stasha, R.A. Biogas Capturing Facilities in Palm Oil Mills: Current Status and Way Forward. Palm Oil Eng. Bull. 2019, 132, 13–17. [Google Scholar]
- Schmidt, J.; De Rosa, M. Certified palm oil reduces greenhouse gas emissions compared to non-certified. J. Clean. Prod. 2020, 277, 124045. [Google Scholar] [CrossRef]
- Alcock, T.D.; Salt, D.E.; Wilson, P.; Ramsden, S.J. More sustainable vegetable oil: Balancing productivity with carbon storage opportunities. Sci. Total Environ. 2022, 829, 154539. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Sun, L.; Hansen, M.C. Oil palm reconciliation in Indonesia: Balancing rising demand and environmental conservation towards 2050. J. Clean. Prod. 2022, 380, 135087. [Google Scholar] [CrossRef]
- Frianto, D.; Sutrisno, E.; Wahyudi., A.; Novriyanti, E.; Adinugroho, W.C.; Yunianto, A.S.; Kurniawan, H.; Khotimah, H.; Windyoningrum, A.; Dharmawan, I.W.S.; et al. Carbon stock dynamics of forest to oil palm plantation conversion for ecosystem rehabilitation planning. Global J. Environ. Sci. Manag. 2024, 10, 1–22. [Google Scholar]
- Henson, I.E. Estimating ground CO2 flux and its components in a stand of oil palm. PORIM Bull. 1994, 28, 1–12. [Google Scholar]
- Henson, I.E. Modelling carbon sequestration and greenhouse gas emissions associated with oil palm cultivation and land-use change in Malaysia. A re-evaluation and a computer model. MPOB Technol. 2009, 31, 116. [Google Scholar]
- Syahrinudin. The potential of oil palm and forest plantations for carbon sequestration on degraded land in Indonesia. In Ecology and Development Series 28; Vlek, P., Ed.; Cuvillier Verlag: Gottingen, Germany, 2005. [Google Scholar]
- Kho, L.K.; Jepsen, M.R. Carbon stock of oil palm plantations and tropical forests in Malaysia: A review. Singap. J. Trop. Geogr. 2015, 36, 249–266. [Google Scholar] [CrossRef]
- Septiwibowo, B.; Hadiwijiaya, B.; Caliman, J.P. CO2 balance on oil palm agrosystem in Sumatra, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2019, 336, 012022. [Google Scholar] [CrossRef]
- Fowler, D. Effects of land use on surface–atmosphere exchanges of trace gases and energy in Borneo: Comparing fluxes over oil palm plantations and a rainforest. Phil. Trans. R. Soc. B 2011, 366, 3196–3209. [Google Scholar] [CrossRef]
- Hardter, R.; Chow, W.Y.; Hock, O.S. Intensive plantation cropping, a source of sustainable food and energy production in the tropical rain forest areas in Southeast Asia. For. Ecol. Manag. 1997, 91, 93–102. [Google Scholar] [CrossRef]
- Fairhurst, T.H.; Härdter, R. (Eds.) Oil Palm: Management for Large and Sustainable Yields; International Plant Nutrition Institute: Saskatoon, Canada, 2003; ISBN 981-04-8485-2. [Google Scholar]
- Zhao, Y.; Goldberg, S.; Xu, J.C.; Harrison, R.D. Spatial and seasonal variation in soil respiration along a slope in a rubber plantation and a natural forest in Xishuangbanna, Southwest China. J. Material. Sci. 2018, 15, 695–707. [Google Scholar] [CrossRef]
- Khasanah, N. The carbon footprint of Indonesian palm oil production. In Technical Brief No 25, Palm Oil Series; World Agroforestry Centre—ICRAF, SEA Regional Office: Bogor, Indonesia, 2012. [Google Scholar]
- Rieley, J.O.; Ahmad-Shah, A.A.; Brady, M.A. The extent and nature of tropical peat swamps. In Tropical Lowland Peatlands of South East Asia, Proceedings of a Workshop on Tropical Lowland Peatlands; Maltby, E., Ed.; CABI: Cisarua, Indonesia, 1996. [Google Scholar]
- Rieley, J.O.; Wüst, R.A.J.; Jauhiainen, J.; Page, S.E.; Wösten, J.H.M.; Hooijer, A.; Siegert, E.; Limin, S.H.; Vasander, H.; Stahlhut, M.; et al. Tropical peatlands: Carbon stores, carbon gas emissions and contribution to climate change processes. In Peatlands and Climate Change; Strack, M., Ed.; International Peat Society: Jyväskylä, Finland, 2008; pp. 129–162. Available online: https://edepot.wur.nl/41970 (accessed on 7 July 2024).
- Koh, L.P.; Miettinen, J.; Liew, S.C.; Ghazoul, J. Remotely sensed evidence of tropical peatland conversion to oil palm. Proc. Natl. Acad. Sci. USA 2011, 108, 5127–5132. [Google Scholar] [CrossRef] [PubMed]
- Page, S.E.; Rieley, J.O.; Wust, R. Lowland Tropical Peatlands of Southeast Asia. Peatlands: Evolution and Records of Environmental and Climate Changes; Martini, I.P., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; Chapter 7. [Google Scholar]
- Page, S.E.; Rieley, J.O.; Banks, C.J. Global and regional importance of the tropical peatland carbon pool. Glob. Chang. Biol. 2011, 17, 798–818. [Google Scholar] [CrossRef]
- Miettinen, J.; Hooijer, A.; Tollenaar, D.; Page, S.; Malins, C.; Vernimmen, R.; Shi, C.; Liew, S.C. Historical Analysis and Projection of Oil Palm Plantation Expansion on Peatland in Southeast Asia; ICCT White Paper No 17; Feb 2012 Indirect Effects of Biofuel Production; ICCT: Singapore; Available online: https://theicct.org/sites/default/files/publications/ICCT_palm-expansion_Feb2012.pdf (accessed on 7 July 2024).
- Moore, S.; Evans, C.; Page, S.; Garnett, M.H.; Jones, T.G.; Freeman, C.; Hooijer, A.; Wiltshire, A.J.; Limin, S.H.; Gauci, V. Deep instability of deforested tropical peatlands revealed by fluvial organic carbon fluxes. Nature 2013, 493, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Bandla, A.; Mukhopadhyay, S.; Mishra, S.; Sudarshan, A.S.; Swarup, S. Genome-resolved carbon processing potential of tropical peat microbiomes from an oil palm plantation. Sci. Data 2023, 10, 373. [Google Scholar] [CrossRef]
- Dargie, G.C.; Lewis, S.L.; Lawson, I.T.; Mitchard, E.T.A.; Page, S.E.; Bocko, Y.E.; Ifo, S.A. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 2017, 542, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Paramananthan, S.; Zauyah, S.; Lim, C.P.; Chan, Y.K.; Boaklan, D. Proposals for a unified classification of organic soils in Malaysia. Proc. workshop on classification and management of peat in Malaysia. Mal. Soc. Soil Sci. March 1984. [Google Scholar]
- Paramananthan, S. Organic Soils of Malaysia; Malaysian Palm Oil Council: Selangor, Malaysia, 2016; Available online: https://seap.ipni.net/article/SEAP-3208 (accessed on 7 July 2024).
- Hooijer, A.; Page, S.; Jauhiainen, J.; Lee, W.A.; Lu, X.X.; Idris, A.; Anshari, G. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 2012, 9, 1053–1071. [Google Scholar] [CrossRef]
- Dariah, A.; Marwanto, S.; Agus, F. Root- and peat-based CO2 emissions from oil palm plantations. Mitig. Adapt. Strateg. Glob. Chang. 2014, 19, 831–843. [Google Scholar] [CrossRef]
- Novita, N.; Kauffman, J.B.; Hergoualc’h, K.; Murdiyarso, D.; Tryanto, D.H.; Jupesta, J. Carbon Stocks from Peat Swamp Forest and Oil Palm Plantation in Central Kalimantan, Indonesia. In Climate Change Research, Policy and Actions in Indonesia; Springer Climate: Berlin/Heidelberg, Germany, 2021; pp. 203–227. [Google Scholar] [CrossRef]
- Matysek, M.; Evers, S.; Samuel, M.K.; Sjogersten, S. High heterotrophic CO2 emissions from a Malaysian oil palm plantations during dry-season. Wetl. Ecol. Manag. 2017, 26, 415–424. [Google Scholar] [CrossRef]
- Kiew, F.; Hirata, R.; Hirano, T.; Xhuan, W.G.; Aries, E.B.; Kemudang, K.; Melling, L. Carbon dioxide balance of an oil palm plantation established on tropical peat. Agric. For. Meteorol. 2020, 295, 108189. [Google Scholar] [CrossRef]
- Lewis, K.; Rumpang, E.; Kho, L.K.; McCalmont, J.; Teh, Y.A.; Gallego-Sala, A.; Hill, T.C. An assessment of oil palm plantation aboveground biomass stocks on tropical peat using destructive and non-destructive methods. Sci. Rep. 2020, 10, 2230. [Google Scholar] [CrossRef] [PubMed]
- Waters, K.; Altiparmak, S.O.; Shutters, S.T.; Thies, C. The Green Mirage: The EU’s Complex Relationship with Palm Oil Biodiesel in the Context of Environmental Narratives and Global Trade Dynamics. Energies 2024, 17, 343. [Google Scholar] [CrossRef]
- EUR-Lex. Directive 2003/30/EC of the European Parliament and of the Council of 8 May 2003 on the Promotion of the Use of Biofuels or Other Renewable Fuels for Transport. EU 2003. 2003. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/ (accessed on 7 July 2024).
- Pendrill, F.; Persson, U.M.; Godar, J.; Kastner, T. Deforestation Risk Embodied in Production and Consumption of Agricultural and Forestry Commodities 2005–2018. 2022. Available online: https://zenodo.org/record/5886600#.Y1K4GXZBxPY (accessed on 7 July 2024).
- Miettinen, J.; Hooijer, A.; Vernimmen, R.; Liew, S.C.; Page, S.E. From carbon sink to carbon source: Extensive peat oxidation in insular Southeast Asia since 1990. Environ. Res. Lett. 2017, 12, 024014. [Google Scholar] [CrossRef]
- Bandla, A.; Akhtar, H.; Lupascu, M.; Sukri, R.S.; Swarup, S. Elevated methane flux in a tropical peatland post-fire is linked to depth-dependent changes in peat microbiome assembly. NPJ Biofilms Microbiomes 2024, 10, 8. [Google Scholar] [CrossRef] [PubMed]
- Matson, P.A.; Naylor, R.; Ortiz-Monasterio, I. Integration of environmental, agronomic and economic aspects of fertilizer management. Science 1998, 280, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.; Powlson, D.S. Considering manure and carbon sequestration. Science 2000, 287, 428–429. [Google Scholar] [CrossRef]
- Nunes, L.J.R. The Rising Threat of Atmospheric CO2, A Review on the Causes, Impacts, and Mitigation Strategies. Environments 2023, 10, 66. [Google Scholar] [CrossRef]
- Afriyanti, D.; Hein, L.; Kroese, C.; Zuhdi, M.; Saad, A. Scenarios for withdrawal of oil palm plantations from peatlands in Jambi Province, Sumatra, Indonesia. Reg. Environ. Chang. 2019, 19, 1201–1215. [Google Scholar] [CrossRef]
- Beyer, R.; Rademacher, T. Species richness and carbon footprints of vegetable oils: Can high yields outweigh palm oil’s environmental impact? Sustainability 2021, 13, 1813. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Ganoderma boninense disease deduced from simulation modelling with large data sets of future Malaysian oil palm climate. Phytoparasitica 2019, 47, 255–262. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Ganoderma boninense disease of oil palm is expected to significantly reduce production after in if projected climate change occurs. Microorganisms 2019, 7, 24. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Future scenarios for oil palm mortality and infection by Phytophthora palmivora in Colombia, Ecuador and Brazil, extrapolated to Malaysia and Indonesia. Phytoparasitica 2020, 48, 513–523. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Oil palm survival under climate change in Malaysia with future basal stem rot assessments. For. Pathol. 2020, 50, e12641. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Depletion of Indonesian oil palm plantations implied from modeling oil palm mortality and Ganoderma boninense rot under future climate. AIMS Environ. Sci. 2020, 7, 366–379. [Google Scholar] [CrossRef]
- Paterson, R.R.M. Longitudinal trends of future climate change and oil palm growth: Empirical evidence for tropical Africa. Environ. Sci. Pollut. Res. 2021, 28, 21193–21203. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Sanz-Saez, A. Photosynthesis in a changing global climate: A matter of scale. Front. Plant Sci. 2023, 14, 1158816. [Google Scholar] [CrossRef]
- De Kauwe, M.G.; Medlyn, B.E.; Prentice, I.C.; Smith, N.G.; Terrer, C.; Wang, H.; Zhang, Y.; Zhou, S. A constraint on historic growth in global photosynthesis due to rising CO2. Nat. Clim. Chang. 2023, 13, 1376–1381. [Google Scholar]
- Ruehr, S.; Keenan, T.F.; Williams, C.; Zhou, Y.; Lu, X.; Bastos, A.; Canadell, J.G.; Prentice, I.C.; Sitch, S.; Terrer, C.; et al. Publisher Correction: Evidence and attribution of the enhanced land carbon sink. Nat. Rev. Earth Environ. 2023, 4, 864. [Google Scholar] [CrossRef]
- Beringer, T.; Müller, C.; Chatterton, J.; Kulak, M.; Schaphoff, S.; Jans, Y. CO2 fertilization effect may balance climate change impacts on oil palm cultivation. Environ. Res. Lett. 2023, 18, 054019. [Google Scholar] [CrossRef]
- Woittiez, L.S.; van Wijk, M.T.; Slingerland, M.; van Noordwijk, M.; Giller, K.E. Yield gaps in oil palm: A quantitative review of contributing factors. Eur. J. Agron. 2017, 83, 57–77. [Google Scholar] [CrossRef]
- Fleiss, S. Potential Impacts of Climate Change on Oil Palm Cultivation. SEnSOR Programme; University of York, 2020. Available online: https://www.sensorproject.net/wp-content/uploads/2018/01/Climate-change-report-FINAL.pdf (accessed on 7 July 2024).
- Abubukar, A.; Gambo, J.; Ishak, M.Y. Navigating climate challenges: Unraveling the effects of climate change on oil palm cultivation and adaptation strategies. Adv. Food Secur. Sustain. 2023, 8, 95–116. [Google Scholar]
- Hermanto, A.; Gan, S.H.; Mustopa, I.R.; Wong, W.C.; Ng, P.H.; Tan, N.P.; Chang, C.W. Use of multiseasonal oil palm yield data to assess drought tolerance. Sci. Hortic. 2023, 308, 111603. [Google Scholar] [CrossRef]
- Anwar, R.; Rahman, A. The Impact of Rainfall on Oil Palm Production: A Case Study in Berau Regency, East Borneo, Indonesia. Asian J. Adv. Agric. Res. 2024, 24, 1–9. [Google Scholar] [CrossRef]
- Manorama, K.; Mathur, R.K.; Suresh, K. Strategies for enhancing water productivity in oil palm (Elaeis guineensis Jacq.)—Present status and way forward. Int. J. Innov. Hortic. 2021, 9, 88–94. [Google Scholar] [CrossRef]
- Demmink, L. Increasing Water Efficiency in Colombian Palm Oil Production by Implementing Efficient Irrigation Systems. Delphy, 26 July 2022. Available online: https://www.agroberichtenbuitenland.nl/landeninformatie/colombia/achtergrond/delphy---increased-water-efficiency-in-palm-oil---colombia(accessed on 7 July 2024).
- Martínez-Arteaga, D.; Arias, N.A.; Darghan, A.E.; Rivera, C.; Beltran, J.A. Typology of Irrigation Technology Adopters in Oil Palm Production: A Categorical Principal Components and Fuzzy Logic Approach. Sustainability 2023, 15, 9944. [Google Scholar] [CrossRef]
- Wicke, B.; Sikkema, R.; Dornburg, V.; Junginger, H.M.; Faaij, A. Drivers of Land Use Change and the Role of Palm Oil Production in Indonesia and Malaysia; Copernicus Institute Science: Utrecht, Netherlands, 2008. [Google Scholar]
- Flynn, H.C.; Canals, L.M. Quantifying global greenhouse gas emissions from land-use change for crop production. Glob. Chang. Biol. 2012, 18, 1622–1635. [Google Scholar] [CrossRef]
- Persson, U.M.; Henders, S.; Cederberg, C. A method for calculating a land-use change carbon footprint (LUC-CFP) for agricultural commodities—applications to Brazilian beef and soy, Indonesian palm oil. Glob. Chang. Biol. 2014, 20, 3482–3491. [Google Scholar] [CrossRef] [PubMed]
- Khasanah, N.; van Noordwijk, M.; Ningsih, H. Aboveground carbon stocks in oil palm plantations and the threshold for carbon-neutral vegetation conversion on mineral soils. Cogent Environ. Sci. 2015, 1, 1119964. [Google Scholar] [CrossRef]
- UN. Sustainable Development Goals; United Nations: New York, NY, USA, 2022. Available online: https://sdgs.un.org/goals (accessed on 7 July 2024).
- UN. Global Sustainable Development Report; United Nations: New York, NY, USA, 2023. Available online: https://sdgs.un.org/gsdr/gsdr2023 (accessed on 7 July 2024).
- Luke, S.H.; Purnomo, D.; Advento, A.D.; Aryawan, A.A.K.; Naim, M.; Pikstein, R.N.; Ps, S.; Rambe, T.D.S.; Soeprapto; Caliman, J.P.; et al. Effects of Understory Vegetation Management on Plant Communities in Oil Palm Plantations in Sumatra, Indonesia. Front. For. Glob. Chang. 2019, 2, 33. [Google Scholar] [CrossRef]
- ETA; TBI. Intercropping in Oil Palm Plantations: A Technical Guide; Ecological Trends Alliance, Kampala, Uganda, and Tropenbos International: Wageningen, The Netherlands, 2021. [Google Scholar]
- Ahirwal, J.; Sahoo, U.K.; Thangjam, U.; Thong, P. Oil Palm Agroforestry Enhances Crop Yield and Ecosystem Carbon Stock in Northeast India: Implications for UN Sustainable Development Goals. Sustain. Prod. Consum. 2022, 30, 478–487. [Google Scholar] [CrossRef]
- Gutteridge, R.C.; Shelton, H.M. Forage Tree Legumes in Tropical Agriculture. CAB International: Wallingford, UK, 1994. Available online: https://assets.publishing.service.gov.uk/media/57a08dc7ed915d622c001b83/Forage_Tree_Legumes.pdf. (accessed on 7 July 2024).
- Gardner, T.; Rylander, Y. Indonesia Makes Progress towards Zero Palm Oil Deforestation, But Gains in Forest Protection Are Fragile. Stockholm Environment Institute. Stockholm 2022. Available online: https://www.sei.org/featured/zero-palm-oil-deforestation/ (accessed on 7 July 2024).
- Rainforest Action Network. Palm Oil Fact Sheet. n.d. Available online: https://www.ran.org/palm_oil_fact_sheet/ (accessed on 7 July 2024).
- Ritchie, H.; Roser, M. Palm Oil. Our World in Data. 2020. Available online: https://ourworldindata.org/palm-oil (accessed on 7 July 2024).
- Voora, V.; Bermúdez, J.; Le, H.; Larrea, C.; Luna, E. Soybean Prices and Sustainability. IISD Market Report, February 2024. Available online: https://www.iisd.org/system/files/2024-02/2024-global-market-report-soybean.pdf(accessed on 7 July 2024).
- Lusiana, B.; Lusiana, B.; Slingerland, M.; Miccolis, A.; Khasanah, N.; Leimona, B. Oil palm production, instrumental and relational values: The public relations battle for hearts, heads, and hands along the value chain. Curr. Opin. Environ. Sustain. 2023, 64, 101321. [Google Scholar] [CrossRef]
- Ingram, V.; Behagel, J.; Mammadova, A.; Verschuur, X. The Outcomes of Deforestation-Free Commodity Value Chain Approaches; Forest and Nature Conservation Policy Group, Wageningen University & Research: Wageningen, The Netherlands, 2020. [Google Scholar]
- Hambali, E.; Rivai, M. The Potential of Palm Oil Waste Biomass in Indonesia in 2020 and 2030 The Potential of Palm Oil Waste Biomass in Indonesia in 2020 and 2030. In International Conference on Biomass: Technology, Application, and Sustainable Development; IOP Conf. Series; IOP Publishing: Bogor, Indonesia, 2017. [Google Scholar]
- Malaysia Innovation Agency (AIM). National Biomass Strategy 2020; New Wealth Creation for Malaysia’s Palm Oil Industry. 2011. Available online: https://www.poram.org.my/v1/poram/ad/12_1.pdf (accessed on 7 July 2024).
- Ng Denny, K.S.; Ng Rex, T.L. Applications of process system engineering in palm-based biomass processing industry. Curr. Opin. Chem. Eng. 2013, 2, 448–454. [Google Scholar] [CrossRef]
- Rajakal, J.P.; Hwang, J.Z.H.; Hassim, M.H.; Andiappan, V.; Tan, Q.T.; Ng, D. Integration and Optimisation of Palm Oil Sector with Multiple-Industries to Achieve Circular Economy. Sustain. Prod. Consum. 2023, 40, 31–46. [Google Scholar] [CrossRef]
- Noerrizki, A.M.; Putri, T.N. Utilization of palm oil waste as bioenergy. J. Environ. Sustain. 2019, 3, 48–66. [Google Scholar] [CrossRef]
- UkrAgroConsult. Biodiesel Production in the World Will Grow by 8% This Year—Forecast. OleoScope. 2023. Available online: https://ukragroconsult.com/en/news/biodiesel-production-in-the-world-will-grow-by-8-this-year-forecast/ (accessed on 7 July 2024).
- Carroll, S.G. EU Lawmakers Vote to Blacklist Soy Biodiesel over Sustainability Concerns. Euractive. 2024. Available online: https://www.euractiv.com/section/biofuels/news/eu-lawmakers-vote-to-blacklist-soy-biodiesel-over-sustainability-concerns/ (accessed on 7 July 2024).
- S&P Global. Malaysia’s B20 mandate could boost biodiesel output by 79%: Trade body head. S&P, 6 March 2024. [Google Scholar]
- Nasution, M.A.; Herawan, T.; Rivani, M. Analysis of Palm Biomass as Electricity from Palm Oil Mills in North Sumatera. Energy Procedia 2014, 47, 166–172. [Google Scholar] [CrossRef]
- Sari, D.A.P.; Fadiilah, D.; Azizi, A. Utilization of Palm Oil Mill Effluent (POME) for Biogas Power Plant; Its Economic Value and Emission Reduction. J. Adv. Res. Dyn. Control. Syst. 2019, 11, 465–470. [Google Scholar]
- Zamr, i.M.F.; Milano, J.; Shamsuddin, A.H.; Roslan, M.E.; Salleh, S.F.; Rahman, A.A.; Bahru, R.; Fattah, I.M.; Mahlia, T.M. An overview of palm oil biomass for power generation sector decarbonization in Malaysia: Progress, challenges, and prospects. Wiley Interdiscip. Rev. Energy Environ. 2022, 11, e437. [Google Scholar]
- Tachev, V. Malaysia’s Energy Transition: Challenges and Opportunities. Energy Tracker Asia. 2023. Available online: https://energytracker.asia/malaysia-energy-transition/ (accessed on 7 July 2024).
- IRENA. Malaysia Energy Transition Outlook; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2023. [Google Scholar]
- Sterman, J.D.; Siegel, L.; Rooney-Varga, J. Does Replacing Coal with Wood Lower CO2 Emissions? Dynamic Lifecycle Analysis of Wood Bioenergy. Environ. Res. Lett. 2018, 13, 015007. [Google Scholar] [CrossRef]
- Booth, M.S. The Great Biomass Boondoggle. New York Review of Books, 2019. Available online: https://www.nybooks.com/online/2019/10/14/the-great-biomass-boondoggle/(accessed on 7 July 2024).
- Buchholz, T.; Gunn, J.S.; Sharma, B. When Biomass Electricity Demand Prompts Thinnings in Southern US Pine Plantations: A Forest Sector Greenhouse Gas Emissions Case Study. Front. For. Glob. Chang. 2021, 4, 642569. [Google Scholar] [CrossRef]
- NRDC. A Bad Biomass Bet. Issue Brief. NRDC. 2021. Available online: https://www.nrdc.org/sites/default/files/bad-biomass-bet-beccs-ib.pdf (accessed on 7 July 2024).
- Wang, N.; Akimbo, K.; Nemet, G. What went wrong? Learning from three decades of carbon capture, utilization and sequestration (CCUS) pilot and demonstration projects. Energy Policy 2021, 158, 112546. [Google Scholar] [CrossRef]
- Sterman, J.D.; Moomaw, W.; Rooney-Varga, J.; Siegel, L. Does wood bioenergy help or harm the climate? Bull. At. Sci. 2022, 78, 128–138. [Google Scholar] [CrossRef]
- Snowdon, C. Trees for Burning: The Biomass Controversy; Institute of Economic Affairs; Working Paper; 2024; Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4716864 (accessed on 7 July 2024).
- National Audit Office. The Government’s Support for Biomass. 2024. Available online: www.nao.org.uk (accessed on 7 July 2024).
- Lawson, D. Time’s Up for Drax’s Tree-Burning Racket. Sunday Times, 4 June 2023. Available online: https://www.thetimes.co.uk/article/times-up-for-draxs-tree-burning-racket-drw2gd23x(accessed on 7 July 2024).
- Mavrokefalidis, D. NAO: Government Fails to Ensure Biomass Sustainability in £20bn Support. Energy Live News, 24 January 2024. [Google Scholar]
- Millard, R. UK Cannot Prove Sustainability of Biomass Power Plants, Warns Watchdog. Financial Times, 24 January 2024. Available online: https://www.ft.com/content/adee9de4-c36f-435c-8551-f28b77737246(accessed on 7 July 2024).
- Putro, L.H.S. Emissions of CH4 and CO2 from Wastewater of Palm Oil Mills: A Real Contribution to Increase the Greenhouse Gas and Its Potential as Renewable Energy Sources. Environ. Nat. Resour. J. 2021, 20, 61–72. [Google Scholar] [CrossRef]
- Yacob, S.; Hassan, M.A.; Shirai, Y.; Wakisaka, M.; Subash, S. Baseline study of methane emission from open digesting tanks of palm oil mill effluent treatment. Chemosphere 2005, 59, 1575–1581. [Google Scholar] [CrossRef] [PubMed]
- Poh, P.E.; Yong, W.J.; Chong, M.F. Palm Oil Mill Effluent (POME) Characteristic in High Crop Season and the Applicability of High-Rate Anaerobic Bioreactors for the Treatment of POME. Ind. Eng. Chem. Res. 2010, 49, 11732–11740. [Google Scholar] [CrossRef]
- Okoli, I.C. Oil Palm Tree Wastes 7, The Composition and Treatment of the Palm Oil Mill Effluent. Research Tropica. 2020. Available online: https://researchtropica.com/oil-palm-tree-wastes-7-the-composition-and-treatment-of-the-palm-oil-mill-effluent/ (accessed on 7 July 2024).
- Efeca. Carbon emissions and palm oil. Efeca Briefing Note. Jan 2022. Bournemouth, UK. Available online: https://www.efeca.com/wp-content/uploads/2022/01/Palm-Oil-and-Carbon-Emissions_final.pdf (accessed on 7 July 2024).
- De Rosa, M.; Schmidt, J.; Pasang, H. Industry-driven mitigation measures can reduce GHG emissions of palm oil. J. Clean. Prod. 2022, 365, 132565. [Google Scholar] [CrossRef]
- Foong, S.Z.; Ng, D.K. A Systematic Approach for Synthesis and Optimisation of Sustainable Oil Palm Value Chain (OPVC). S. Afr. J. Chem. Eng. 2022, 4, 65–78. [Google Scholar] [CrossRef]
- Rajakal, J.P.; Ng, F.Y.; Zulkifli, A.; How, B.S.; Sunarso, J.; Ng, D.K.S.; Andiappan, V. Analysis of Current State, Gaps, and Opportunities for Technologies in the Malaysian Oil Palm Estates and Palm Oil Mills towards Net-Zero Emissions. Heliyon 2024, 10, e30768. [Google Scholar] [CrossRef]
- Azwan, M.; Ludin, N.A.; Rahim, A.; Norman, K.; Salmah, J. Analysis of energy utilisation in Malaysian oil palm mechanisation operation. J. Oil Palm. Res. 2016, 28, 485–495. [Google Scholar] [CrossRef]
- Bušić, A.; Marđetko, N.; Kundas, S.; Morzak, G.; Belskaya, H.; Ivančić Šantek, M.; Komes, D.; Novak, S.; Šantek, B. Bioethanol Production from Renewable Raw Materials and Its Separation and Purification: A Review. Food Technol. Biotechnol. 2018, 56, 289–311. [Google Scholar] [CrossRef]
- Rizal, N.F.A.A.; Ibrahim, M.F.; Zakaria, M.R.; Abd-Aziz, S.; Yee, P.L.; Hassan, M.A. Pre-treatment of Oil Palm Biomass for Fermentable Sugars Production. Molecules 2018, 23, 1381. [Google Scholar] [CrossRef]
- New, E.G.; Wu, T.Y.; Tnah, S.K.; Procentese, A.; Cheng, C.K. Pretreatment and sugar recovery of oil palm fronds using choline chloride:calcium chloride hexahydrate integrated with metal chloride. Energy 2023, 277, 127486. [Google Scholar] [CrossRef]
- Hossain, N.; Jalil, R. Sugar and Bioethanol Production from Oil Palm Trunk (OPT). Asia Pac. J. Energy Environ. 2017, 4, 13–16. [Google Scholar] [CrossRef]
- Kong, S.H.; Loh, S.K.; Bachmann, R.T.; Rahim, S.A.; Salimon, J. Biochar from oil palm biomass: A review of its potential and challenges. Renew. Sustain. Energy Rev. 2014, 39, 729–739. [Google Scholar] [CrossRef]
- Sulaiman, O.; Salim, N.; Nordin, N.A.; Hashim, R.; Ibrahim, M.; Sato, M. The potential of oil palm trunk biomass as an alternative source for compressed wood. BioResources 2012, 7, 2688–2706. [Google Scholar] [CrossRef]
- Saka, S.; Munusamy, M.; Shibata, M.; Tono, Y.; Miyafuji, H. Chemical Constituents of the Different Anatomical Parts of the Oil Palm (Elaeis guineensis) for Their Sustainable Utilization. Seminar Proceedings—Natural Resources & Energy Environment JSPS-VCC Program on Environmental Science, Kyoto, 2008; pp. 19–34. Available online: https://core.ac.uk/download/pdf/162013768.pdf (accessed on 7 July 2024).
- Akmar, P.F.; Kennedy, J.F. The potential of oil and sago palm trunk wastes as carbohydrate resources. Wood Sci. Technol. 2001, 35, 467–473. [Google Scholar] [CrossRef]
- Ho, L.S.; Tan, B.A.; Noh, N.A.; Talib, S.S.; Ithnin, N.; Daim, L.D.; Keong, T.O.; Yusof, H. Preliminary analysis of lignocellulose content and monolignol composition of oil palm trunk from two different genetic backgrounds. Bio Res. 2015, 10, 8194–8207. [Google Scholar] [CrossRef]
- Powney, S. Oil Palm Wood—An Untapped Resource. WBPI Online. 2017. Available online: https://www.wbpionline.com/features/oil-palm-wood-an-untapped-resource-6012167/ (accessed on 7 July 2024).
- Mamiński, M.; Hong, P.S.; Chai, L.Y.; Chin, K.L. Oil Palm Wood (Elaeis guineensis Jacq.) as an Underutilized Resource of Raw Materials. Ann. Wars. Univ. Life Sci. SGGW For. Wood Technol. 2010, 71, 235–239. Available online: https://wulsannals.com/resources/html/article/details?id=110101&language=en (accessed on 7 July 2024).
- IOI. High-Performance Palm Wood Panels, Produced Sustainably. 2020. Available online: https://ioipalmwood.com (accessed on 7 July 2024).
- Fitch, P. How Sustainable Is Palm Wood? 2022. Available online: https://ioipalmwood.com/IOIPW/NEWS/PDF/PFA%20-%202022%20Nov_Dec.pdf (accessed on 7 July 2024).
- Ji, X.; Usman, A.; Razalli, N.H.; Sambanthamurthi, R.; Gupta, S.V. Oil palm phenolics (OPP) inhibit pancreatic cancer cell proliferation via suppression of NF-κB pathway. Anticancer Res. 2015, 35, 97–106. [Google Scholar] [PubMed]
- Leow, S.S.; Sekaran, S.D.; Sundram, K.; Tan, Y.; Sambanthamurthi, R. Gene expression changes in spleens and livers of tumour-bearing mice suggest delayed inflammation and attenuated cachexia in response to oil palm phenolics. J. Nutr. Nutr. 2016, 6, 305–326. [Google Scholar] [CrossRef] [PubMed]
- Fairus, S.; Leow, S.S.; Mohamed, I.N.; Tan, Y.A.; Sundram, K. A phase I single-blind clinical trial to evaluate the safety of oil palm phenolics (OPP) supplementation in healthy volunteers. Sci. Rep. 2018, 8, 8217. [Google Scholar] [CrossRef]
- Febriani, A.; Syafriana, V.; Afriyando, H.; Djuhariah, T.S. The Utilization of Oil Palm Leaves (Elaeis guineensis Jacq.) Waste as an Antibacterial Solid Bar Soap. IOP Conf. Ser. Earth Environ. Sci. 2020, 572, 012038. [Google Scholar] [CrossRef]
- Ayyildiz, H.F.; Shoaib, H.; Kara, H. Bioactive Phytochemicals from Palm Oil Processing By-products. In Bioactive Phytochemicals from Vegetable Oil and Oilseed Processing By-Products; Ramadan Hassanien, M.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2023. [Google Scholar]
- Meijaard, E.; Virah-Sawmy, M.; Newing, H.S.; Ingram, V.J.; Holle, M.J.M.; Pasmans, T.; Omar, S.; van de Hombergh, H.; Unus, N.; Forsch, A.; et al. Exploring the Future of Vegetable Oils. Oil Crop Implications—Fats, Forests, Forecasts, and Futures; IUCN, 2024; Available online: https://portals.iucn.org/library/node/51451 (accessed on 7 July 2024).
- Schmidt, J.H. Life cycle assessment of five vegetable oils. J. Clean. Prod. 2015, 87, 130.e138. [Google Scholar] [CrossRef]
- Sadras, V.O.; Grassini, P.; Steduto, P. Status of water use efficiency of main crops. In SOLAW Background Thematic Report; FAO: Rome, Italy, 2008; Available online: https://www.fao.org/fileadmin/templates/solaw/files/thematic_reports/TR_07_web.pdf (accessed on 7 July 2024).
- Mekonnen, M.M.; Hoekstra, A.Y. National water footprint accounts: The green, blue and grey water footprint of production and consumption. In Value of Water Research Report Series, No. 50; UNESCO-IHE: Delft, The Netherlands, 2011. [Google Scholar]
- Safitri, L.; Hermantoro, H.; Purboseno, S.; Kautsar, V.; Saptomo, S.K.; Kurniawan, A. Water Footprint and Crop Water Usage of Oil Palm (Eleasis guineensis) in Central Kalimantan: Environmental Sustainability Indicators for Different Crop Age and Soil Conditions. Water 2019, 11, 35. [Google Scholar] [CrossRef]
- Boev, P.; Rijk, G.; Stravens, M. The Environmental Impact of Food, on Climate, Forests, Land, Water, and Air, Profundo. Amsterdam, The Netherlands. 2024. Available online: https://wwfeu.awsassets.panda.org/downloads/240429_report-profundo-wwf_final.pdf (accessed on 7 July 2024).
- Muñoz, I.; Schmidt, J.H.; Dalgaard, R. Comparative Life Cycle Assessment of Five Different Vegetable Oils. In 9th International Conference LCA of Food; LCA Press: San Francisco, CA, USA, 2014; Available online: https://lca-net.com/files/Paper-no-165_Munoz_et_al.pdf (accessed on 7 July 2024).
- O’Connell, A.; Kousoulidou, M.; Lonza, L.; Weindorf, W. Considerations on GHG emissions and energy balances of promising aviation biofuel pathways. Renew. Sustain. Energy Rev. 2018, 101, 504.e515. [Google Scholar] [CrossRef]
- Parish, F.; Serena Lew, S.; Linda Archibald, L. Southeast Asia: Progress for Peatlands; Heinrich Böll Stiftung: Brussels, Belgium, 2023; Available online: https://eu.boell.org/en/2023/09/11/southeast-asia-progress-peatlands (accessed on 7 July 2024).
- Slingerland, M.; Khasanah, N.; van Noordwijk, M.; Susanti, A.; Meilantina, M. Improving smallholder inclusivity through integration of oil palm with crops. In Exploring Inclusive Palm Oil Production; Jezeer, R., Pasiecznik, N., Eds.; ETFRN and Tropenbos International: Wageningen, The Netherlands, 2019; pp. 147–154. [Google Scholar]
- Harmen Smit, H.; Meijaard, E.; van der Laan, C.; Mantel, S.; Budiman, A.; Verweij, P. Breaking the link between environmental degradation and oil palm expansion: A method for enabling sustainable oil palm expansion. PLoS ONE 2013, 8, e68610. [Google Scholar]
- FAO. How Much Cropland Has the World Spared Due to Increases in Crop Yields? Our World in Data; FAO: Rome, Italy, 2020; Available online: https://ourworldindata.org/grapher/land-sparing-by-crop (accessed on 7 July 2024).


| Indicator | Tropical Forest | Oil Palm Plantation |
|---|---|---|
| Gross assimilation, ton CO2/ha/yr | 163.5 | 161.0 |
| Total respiration, ton CO2/ha/yr | 121.1 | 96.5 |
| Net assimilation, ton CO2/ha/yr | 42.4 | 64.5 |
| Oxygen production ton O2/ha/yr | 7.09 | 18.7 |
| Leaf area index | 7.3 | 5.6 |
| Photosynthetic efficiency, % | 1.73 | 3.18 |
| Radiation conversion efficiency, g/mj | 0.86 | 1.68 |
| Total area biomass, ton/ha | 431 | 100.0 |
| Incremental biomass, ton/ha/yr | 5.8 | 8.3 |
| Dry matter productivity, ton/ha/yr | 25.7 | 36.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murphy, D.J. Carbon Sequestration by Tropical Trees and Crops: A Case Study of Oil Palm. Agriculture 2024, 14, 1133. https://doi.org/10.3390/agriculture14071133
Murphy DJ. Carbon Sequestration by Tropical Trees and Crops: A Case Study of Oil Palm. Agriculture. 2024; 14(7):1133. https://doi.org/10.3390/agriculture14071133
Chicago/Turabian StyleMurphy, Denis J. 2024. "Carbon Sequestration by Tropical Trees and Crops: A Case Study of Oil Palm" Agriculture 14, no. 7: 1133. https://doi.org/10.3390/agriculture14071133





