Evidence of Cooperative Interactions between Rhizobacteria and Wood-Decaying Fungi and Their Effects on Maize Germination and Growth
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacteria Cultures
2.2. Fungi Cultures
2.3. Emergence and Growth Assays of Zea mays
2.4. Chlorophyll Determination
2.5. Enzymatic Determination
2.6. Data Analysis
3. Results
3.1. Soil Physico-Chemical Parameters
3.2. Germination Rates
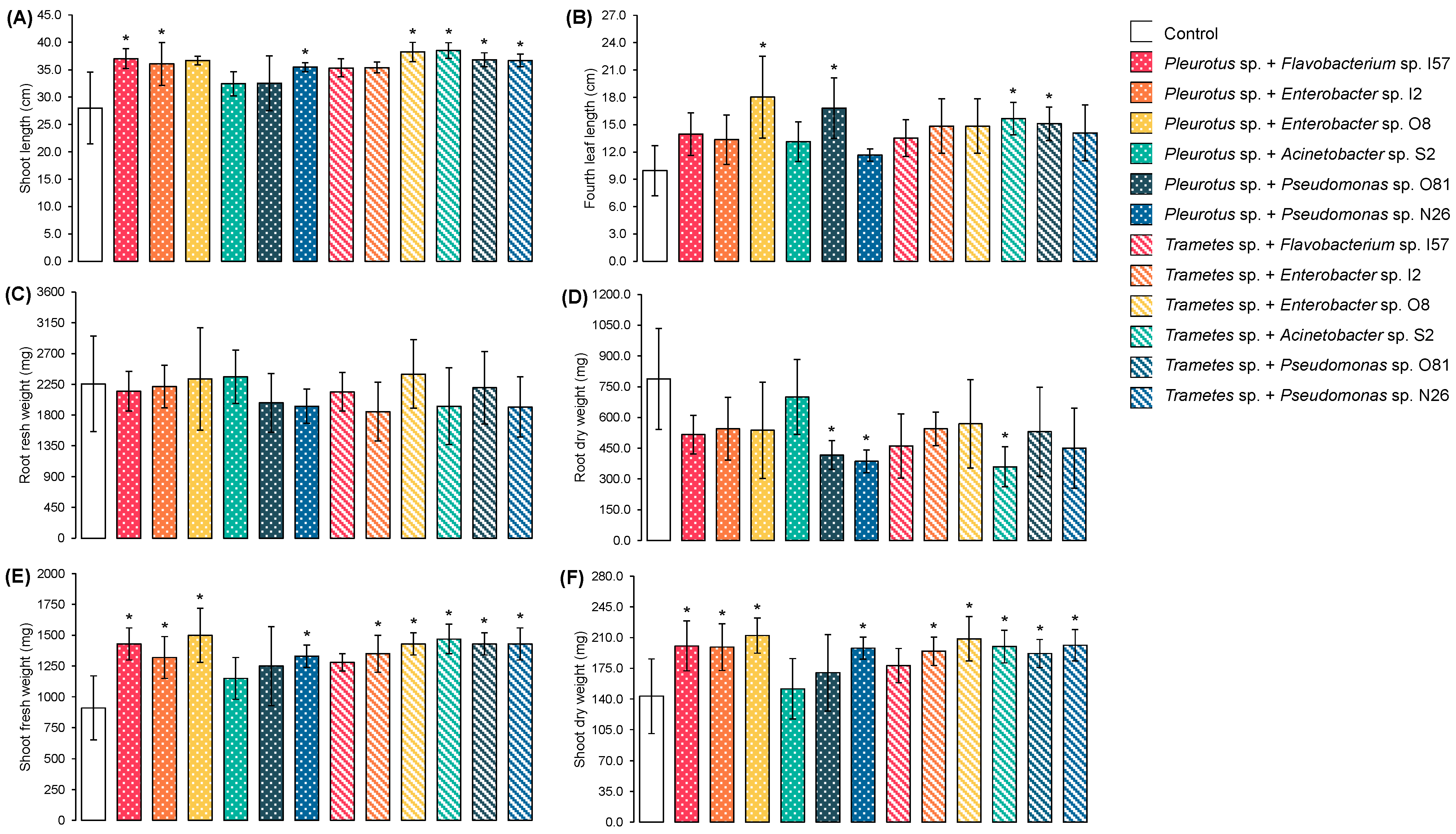
3.3. Zea mays Morphometric Parameters
3.4. Zea mays Photosynthetic Pigments
3.5. Soil Enzymatic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission. Questions and Answers on a Directive on Soil Monitoring and Resilience. 2023, p. 4. Available online: https://ec.europa.eu/commission/presscorner/detail/en/qanda_23_3637 (accessed on 13 May 2024).
- Timmis, K.; Ramos, J.L. The soil crisis: The need to treat as a global health problem and the pivotal role of microbes in prophylaxis and therapy. Microb. Biotechnol. 2021, 14, 769–797. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, A.; Basile, A.; Bouma, J. Targeting the soil quality and soil health concepts when aiming for the United Nations Sustainable Development Goals and the EU Green Deal. Soil Discuss. 2020, 6, 453–466. [Google Scholar] [CrossRef]
- Carneiro, B.; Cardoso, P.; Figueira, E.; Lopes, I.; Venâncio, C. Forward-looking on new microbial consortia: Combination of rot fungi and rhizobacteria on plant growth-promoting abilities. Appl. Soil Ecol. 2023, 182, 104689. [Google Scholar] [CrossRef]
- Khan, A.; Singh, A.V.; Gautam, S.S.; Agarwal, A.; Punetha, A.; Upadhayay, V.K.; Kukreti, B.; Bundela, V.; Jugran, A.K.; Goel, R. Microbial bioformulation: A microbial assisted biostimulating fertilization technique for sustainable agriculture. Front. Plant Sci. 2023, 14, 1270039. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Bell, T. Experimental tests of the bacterial distance–decay relationship. ISME J. 2010, 4, 1357–1365. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Jiao, S.; Li, Q.; Du, N. Dispersal limitation relative to environmental filtering governs the vertical small-scale assembly of soil microbiomes during restoration. J. Appl. Ecol. 2020, 57, 402–412. [Google Scholar] [CrossRef]
- Tisdall, J.M. Possible role of soil microorganisms in aggregation in soils. Plant Soil 1994, 159, 115–121. [Google Scholar] [CrossRef]
- Ambriz, E.; Báez-Pérez, A.; Sánchez-Yáñez, J.M.; Moutoglis, P.; Villegas, J. Fraxinus–Glomus–Pisolithus symbiosis: Plant growth and soil aggregation effects. Pedobiologia 2010, 53, 369–373. [Google Scholar] [CrossRef]
- Kohlmeier, S.; Smits, T.H.; Ford, R.M.; Keel, C.; Harms, H.; Wick, L.Y. Taking the fungal highway: Mobilization of pollutant-degrading bacteria by fungi. Environ. Sci. Technol. 2005, 39, 4640–4646. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J.P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef]
- Rojas-Higuera, N.S.; Pava-Sánchez, A.M.; Pinzón Rangel, D.L.; Díaz-Ariza, L.A.; Quevedo-Hidalgo, B.; Pedroza-Rodríguez, A.M. Bio-transformed sawdust by white rot fungi used as a carrier for plant growth-promoting bacteria. Eur. J. Wood Wood Prod. 2017, 75, 263–273. [Google Scholar] [CrossRef]
- Rocha, R.; Lopes, T.; Fidalgo, C.; Alves, A.; Cardoso, P.; Figueira, E. Bacteria associated with the roots of common bean (Phaseolus vulgaris L.) at different development stages: Diversity and plant growth promotion. Microorganisms 2022, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Venâncio, C.; Pereira, R.; Freitas, A.C.; Rocha-Santos, T.A.P.; da Costa, J.P.; Duarte, A.C.; Lopes, I. Salinity induced effects on the growth rates and mycelia composition of basidiomycete and zygomycete fungi. Environ. Pollut. 2017, 231, 1633–1641. [Google Scholar] [CrossRef] [PubMed]
- Borges, J.; Cardoso, P.; Lopes, I.; Figueira, E.; Venâncio, C. Exploring the Potential of White-Rot Fungi Exudates on the Amelioration of Salinized Soils. Agriculture 2023, 13, 382. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Agricultural Production Statistics: 2000–2021. FAO Analytical Brief. 2022, p. 17. Available online: https://www.fao.org/3/cc3751en/cc3751en.pdf (accessed on 12 May 2024).
- Brígido, C.; Menéndez, E.; Paço, A.; Glick, B.R.; Belo, A.; Félix, M.R.; Oliveira, S.; Carvalho, M. Mediterranean native leguminous plants: A reservoir of endophytic bacteria with potential to enhance chickpea growth under stress conditions. Microorganisms 2019, 7, 392. [Google Scholar] [CrossRef] [PubMed]
- ISO 11269-2: 7; Soil Quality—Determination of the Effects of Pollutants on Soil Flora—Part 2: Effects of Chemicals on the Emergence of Higher Plants. ISO—The International Organization for Standardization: Geneve, Switzerland, 1995.
- OECD 208; Terrestrial Plant Test: Seedling Emergence and Seedling Growth Test. OECD—Organization for Economic Cooperation and Development: Paris, France, 2006; p. 6.
- ISO 10390; Soil quality—Determination of pH. Institut za Standardizaciju Srbije: Belgrade, Serbia, 2007.
- FAO UN. Food and agriculture organization of the United Nations—physical and chemical methods of soil and water analysis. In Proceedings of the Rotterdam Convention on the Prior Informed Consent Procedure for Certain Hazardous Chemicals and Pesticides in International Trade Chemical Review Committee, Geneva, Switzerland, 27 June–6 July 1984; Volume 10, pp. 1–275.
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Htwe, A.Z.; Moh, S.M.; Soe, K.M.; Moe, K.; Yamakawa, T. Effects of biofertilizer produced from Bradyrhizobium and Streptomyces griseoflavus on plant growth, nodulation, nitrogen fixation, nutrient uptake, and seed yield of mung bean, cowpea, and soybean. Agronomy 2019, 9, 77. [Google Scholar] [CrossRef]
- Mondani, F.; Khani, K.; Honarmand, S.J.; Saeidi, M. Evaluating effects of plant growth-promoting rhizobacteria on the radiation use efficiency and yield of soybean (Glycine max) under water deficit stress condition. Agric. Water Manag. 2019, 213, 707–713. [Google Scholar] [CrossRef]
- Masood, S.; Zhao, X.Q.; Shen, R.F. Bacillus pumilus promotes the growth and nitrogen uptake of tomato plants under nitrogen fertilization. Sci. Hortic. 2020, 272, 109581. [Google Scholar] [CrossRef]
- Katsenios, N.; Andreou, V.; Sparangis, P.; Djordjevic, N.; Giannoglou, M.; Chanioti, S.; Stergiou, P.; Xanthou, M.Z.; Kakabouki, I.; Vlachakis, D.; et al. Evaluation of plant growth promoting bacteria strains on growth, yield and quality of industrial tomato. Microorganisms 2021, 9, 2099. [Google Scholar] [CrossRef] [PubMed]
- Daraz, U.; Ahmad, I.; Li, Q.S.; Zhu, B.; Saeed, M.F.; Li, Y.; Ma, J.; Wang, X.B. Plant growth promoting rhizobacteria induced metal and salt stress tolerance in Brassica juncea through ion homeostasis. Ecotoxicol. Environ. Saf. 2023, 267, 115657. [Google Scholar] [CrossRef]
- Ringman, R.; Beck, G.; Pilgård, A. The importance of moisture for brown rot degradation of modified wood: A critical discussion. Forests 2019, 10, 522. [Google Scholar] [CrossRef]
- López Nava, J.A.; Méndez González, J.; Ruelas Chacón, X.; Nájera Luna, J.A. Assessment of edible fungi and films bio-based material simulating expanded polystyrene. Mater. Manuf. Process. 2016, 31, 1085–1090. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Lankinen, P.; Hietala, S.; Mikkonen, K.S. Dense and continuous networks of aerial hyphae improve flexibility and shape retention of mycelium composite in the wet state. Compos. Part A Appl. Sci. Manuf. 2022, 152, 106688. [Google Scholar] [CrossRef]
- Charpentier-Alfaro, C.; Benavides-Hernández, J.; Poggerini, M.; Crisci, A.; Mele, G.; Della Rocca, G.; Emiliani, G.; Frascella, A.; Torrigiani, T.; Palanti, S. Wood-decaying fungi: From timber degradation to sustainable insulating biomaterials production. Materials 2023, 16, 3547. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikov, M.; Gerasko, T.; Paschenko, Y.; Pokoptseva, L.; Onyschenko, O.; Kolesnikova, A. Effect of water deficit on maize seeds (Zea mays L.) during germination. Agron. Res. 2023, 21, 156–174. [Google Scholar]
- Vogel, C.; Sekine, R.; Huang, J.; Steckenmesser, D.; Steffens, D.; Huthwelker, T.; Borca, C.N.; Del Real, A.E.P.; Castillo-Michel, H.; Adam, C. Effects of a nitrification inhibitor on nitrogen species in the soil and the yield and phosphorus uptake of maize. Sci. Total Environ. 2020, 715, 136895. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Chen, X.X.; Zhang, W.; Wang, Q.; Liu, Y.M.; Liu, D.Y.; Zou, C.Q. Zinc nutrition of wheat in response to application of phosphorus to a calcareous soil and an acid soil. Plant Soil 2019, 434, 139–150. [Google Scholar] [CrossRef]
- Sinsabaugh, R.; Carreiro, M.; Repert, D. Allocation of extracellular enzymatic activity in relation to litter composition, N deposition, and mass loss. Biogeochemistry 2002, 60, 1–24. [Google Scholar] [CrossRef]
- Haq, I.U.; Hillmann, B.; Moran, M.; Willard, S.; Knights, D.; Fixen, K.R.; Schilling, J.S. Bacterial communities associated with wood rot fungi that use distinct decomposition mechanisms. ISME Commun. 2022, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yan, M.; Qian, X.; Yang, Z.; Xu, Y.; Wang, T.; Cao, J.; Sun, S. Bacterial community composition in the growth process of Pleurotus eryngii and growth-promoting abilities of isolated bacteria. Front. Microbiol. 2022, 13, 787628. [Google Scholar] [CrossRef] [PubMed]
- Borràs, E.; Caminal, G.; Sarrà, M.; Novotný, Č. Effect of soil bacteria on the ability of polycyclic aromatic hydrocarbons (PAHs) removal by Trametes versicolor and Irpex lacteus from contaminated soil. Soil Biol. Biochem. 2010, 42, 2087–2093. [Google Scholar] [CrossRef]
- Heilmann-Clausen, J.; Boddy, L. Inhibition and Stimulation Effects in Communities of Wood Decay Fungi: Exudates from Colonized Wood Influence Growth by Other Species. Microb. Ecol. 2005, 49, 399–406. [Google Scholar] [CrossRef]
- Prylutskyi, O.; Yatsiuk, I.; Savchenko, A.; Kit, M.; Solodiankin, O.; Schigel, D. Strict Substrate Requirements Alongside Rapid Substrate Turnover May Indicate an Early Colonization: A Case Study of Pleurotus calyptratus (Agaricales, Basidiomycota). Fungal Ecol. 2021, 59, 101098. [Google Scholar] [CrossRef]
- Xiang, L.; Harindintwali, J.D.; Wang, F.; Redmile-Gordon, M.; Chang, S.X.; Fu, Y.; He, C.; Muhoza, B.; Brahushi, F.; Bolan, N.; et al. Integrating biochar, bacteria, and plants for sustainable remediation of soils contaminated with organic pollutants. Environ. Sci. Technol. 2022, 56, 16546–16566. [Google Scholar] [CrossRef]
- Mori, T.; Terashima, T.; Matsumura, M.; Tsuruta, K.; Dohra, H.; Kawagishi, H.; Hirai, H. Construction of white-rot fungal-bacterial consortia with improved ligninolytic properties and stable bacterial community structure. ISME Commun. 2023, 3, 61. [Google Scholar] [CrossRef]
- Délano-Frier, J.P.; Flores-Olivas, A.; Valenzuela-Soto, J.H. Bio-Inoculation of Tomato (Solanum lycopersicum L.) and Jalapeño Pepper (Capsicum annuum L.) with Enterobacter sp. DBA51 Increases Growth and Yields under Open-Field Conditions. Agronomy 2024, 14, 702. [Google Scholar] [CrossRef]
- Etesami, H.; Maheshwari, D.K. Use of plant growth promoting rhizobacteria (PGPRs) with multiple plant growth promoting traits in stress agriculture: Action mechanisms and future prospects. Ecotoxicol. Environ. Saf. 2018, 156, 225–246. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Malla, M.A.; Khan, F.; Chowdhary, K.; Yadav, S.; Kumar, A.; Sharma, S.; Khare, P.K.; Khan, M.L. Soil microbiome: A key player for conservation of soil health under changing climate. Biodivers. Conserv. 2019, 28, 2405–2429. [Google Scholar] [CrossRef]



| Photosynthetic Pigments (Combinations) | |||||
|---|---|---|---|---|---|
| Conditions | Chlorophyll a (mg g−1 dw) | Chlorophyll b (mg g−1 dw) | Chlorophyll a+b (mg g−1 dw) | Chlorophyll x+c (mg g−1 dw) | Ratio a/b |
| Control | 9.54 ± 1.89 a | 2.48 ± 0.55 a | 12.0 ± 2.42 a | 2.55 ± 0.57 a | 3.87 ± 0.29 a |
| Pleurotus sp. | N/A | N/A | N/A | N/A | N/A |
| Trametes sp. | 6.87 ± 2.64 a | 1.96 ± 0.57 a | 8.83 ± 3.17 a | 2.16 ± 0.54 a | 3.48 ± 0.48 a |
| Flavobacterium sp. I57 | 10.6 ± 1.47 a | 2.57 ± 0.36 a | 13.1 ± 1.83 a | 2.88 ± 0.17 a | 4.10 ± 0.09 a |
| Pleurotus sp. + Flavobacterium sp. I57 | 6.46 ± 2.50 b | 1.94 ± 0.42 a | 8.41 ± 2.82 a | 1.68 ± 0.65 b | 3.29 ± 0.82 a,b |
| Trametes sp. + Flavobacterium sp. I57 | 8.26 ± 1.43 a,b | 2.20 ± 0.40 a | 10.5 ± 1.81 a | 2.24 ± 0.37 b | 3.76 ± 0.23 b |
| Enterobacter sp. I2 | 7.76 ± 1.97 a | 1.87 ± 0.56 a | 9.63 ± 2.52 a | 2.16 ± 0.40 a | 4.21 ± 0.24 a |
| Pleurotus sp. + Enterobacter sp. I2 | 8.29 ± 3.27 a | 2.21 ± 0.80 a | 10.5 ± 4.07 a | 2.20 ± 0.84 a | 3.71 ± 0.12 b |
| Trametes sp. + Enterobacter sp. I2 | 7.70 ± 0.18 a | 2.13 ± 0.05 a | 9.83 ± 0.13 a | 1.96 ± 0.21 a | 3.61 ± 0.17 a,b |
| Enterobacter sp. O8 | 10.4 ± 1.24 a | 2.71 ± 0.30 a | 13.2 ± 1.54 a | 2.80 ± 0.50 a | 3.96 ± 0.03 a |
| Trametes sp. + Enterobacter sp. O8 | 5.70 ± 0.09 b | 1.45 ± 0.08 b | 7.15 ± 0.08 b | 1.79 ± 0.10 a | 3.94 ± 0.27 a |
| Pleurotus sp. + Enterobacter sp. O8 | 9.70 ± 1.15 a | 2.62 ± 0.09 a | 12.3 ± 1.21 a | 2.56 ± 0.37 a | 3.69 ± 0.37 a |
| Acinetobacter sp. S2 | 9.48 ± 1.62 a | 2.34 ± 0.44 a | 11.8 ± 2.05 a | 2.35 ± 0.31 a | 4.06 ± 0.18 a |
| Pleurotus sp. + Acinetobacter sp. S2 | 8.12 ± 2.04 a | 2.20 ± 0.53 a | 10.3 ± 2.52 a | 2.00 ± 0.34 a | 3.68 ± 0.47 a |
| Trametes sp. + Acinetobacter sp. S2 | 8.68 ± 2.81 a | 2.12 ± 0.61 a | 10.8 ± 3.41 a | 2.41 ± 0.46 a | 4.03 ± 0.19 a |
| Pseudomonas sp. O81 | 9.32 ± 1.86 a | 2.35 ± 0.48 a | 11.7 ± 2.34 a | 2.46 ± 0.44 a | 3.96 ± 0.08 a |
| Pleurotus sp. + Pseudomonas sp. O81 | 8.35 ± 2.23 a | 2.09 ± 0.49 a | 10.4 ± 2.72 a | 2.16 ± 0.55 a | 3.98 ± 0.18 a |
| Trametes sp. + Pseudomonas sp. O81 | 9.52 ± 2.78 a | 2.53 ± 0.40 a | 12.0 ± 3.16 a | 2.34 ± 0.53 a | 3.69 ± 0.67 a |
| Pseudomonas sp. N26 | 10.2 ± 2.23 a | 2.67 ± 0.32 a | 12.9 ± 2.54 a | 2.65 ± 0.54 a | 3.77 ± 0.46 a |
| Pleurotus sp. + Pseudomonas sp. N26 | 8.82 ± 2.12 a | 2.39 ± 0.58 a | 11.2 ± 2.68 a | 2.10 ± 0.47 a | 3.70 ± 0.24 a |
| Trametes sp. + Pseudomonas sp. N26 | 9.34 ± 2.60 a | 2.47 ± 0.59 a | 11.8 ± 3.19 a | 2.46 ± 0.62 a | 3.75 ± 0.28 a |
| Phosphatase (µg p NP/Soil g/hour) | ||||||
|---|---|---|---|---|---|---|
| Conditions | Day 0 | Day 7 | Day 14 | |||
| Phosphatase | Acid | Alkaline | Acid | Alkaline | Acid | Alkaline |
| Control | 98.88 ± 11.1 A | 31.91 ± 9.04 a | 67.85 ± 4.85 A | 20.78 ± 5.35 a | 120.7 ± 57.8 A | 25.06 ± 4.48 a |
| Pleurotus sp. | 78.75 ± 5.57 B | 25.45 ± 4.00 a | 82.04 ± 18.6 A,B | 29.15 ± 3.24 b | 148.4 ± 48.1 A | 27.86 ± 6.84 a |
| Trametes sp. | 98.84 ± 12.3 A | 25.98 ± 3.03 a | 103.7 ± 20.9 A | 22.88 ± 0.62 a | 137.7 ± 18.7 A | 21.93 ± 6.32 a |
| Flavobacterium sp. I57 | 94.08 ± 11.4 A | 25.65 ± 1.58 a | 90.23 ± 12.3 A | 20.97 ± 4.19 a | 127.1 ± 12.3 A | 28.15 ± 1.44 a |
| Pleurotus sp. + Flavobacterium sp. I57 | 113.2 ± 38.7 A | 26.43 ± 2.90 a | 131.1 ± 47.6 A | 24.55 ± 3.58 a | 111.9 ± 54.7 A | 31.32 ± 8.83 a |
| Trametes sp. + Flavobacterium sp. I57 | 107.3 ± 9.00 A | 29.55 ± 4.51 a | 118.7 ± 34.9 A | 36.89 ± 20.3 a | 94.46 ± 41.4 A | 28.58 ± 1.83 b |
| Enterobacter sp. I2 | 94.40 ± 4.35 A | 24.79 ± 2.66 a | 66.28 ± 11.0 A | 20.37 ± 2.13 a | 122.6 ± 23.5 A | 26.22 ± 5.34 a |
| Pleurotus sp. + Enterobacter sp. I2 | 151.9 ± 37.3 B | 30.81 ± 7.94 a | 142.1 ± 31.9 B | 24.51 ± 1.57 b | 168.9 ± 34.9 A | 48.44 ± 12.9 b |
| Trametes sp. + Enterobacter sp. I2 | 133.8 ± 20.6 B | 21.36 ± 4.59 a | 121.4 ± 11.7 B | 26.84 ± 1.24 b | 52.78 ± 25.7 B | 21.83 ± 2.74 a |
| Enterobacter sp. O8 | 90.22 ± 6.31 A | 26.19 ± 1.90 a | 84.13 ± 3.70 A | 23.79 ± 3.82 a | 148.1 ± 44.2 A | 22.29 ± 4.36 a |
| Pleurotus sp. + Enterobacter sp. O8 | 94.68 ± 6.46 A | 33.93 ± 6.17 a | 132.8 ± 34.9 A | 30.15 ± 2.40 a | 165.0 ± 84.3 A | 38.66 ± 14.1 a |
| Trametes sp. + Enterobacter sp. O8 | 139.8 ± 19.9 B | 53.63 ± 41.6 a | 80.79 ± 13.6 A | 26.60 ± 3.61 a | 100.3 ± 21.4 A | 32.66 ± 8.74 a |
| Acinetobacter sp. S2 | 97.79 ± 11.1 A | 27.08 ± 3.89 a | 86.78 ± 17.3 A | 21.70 ± 2.33 a,b | 188.1 ± 90.1 A | 29.03 ± 3.65 a |
| Pleurotus sp. + Acinetobacter sp. S2 | 110.0 ± 22.9 A | 23.41 ± 2.03 a | 87.71 ± 15.6 A,B | 35.99 ± 8.61 b | 130.4 ± 28.5 A | 34.28 ± 4.68 b |
| Trametes sp. + Acinetobacter sp. S2 | 120.1 ± 34.8 A | 23.86 ± 0.82 a | 132.9 ± 23.7 B | 34.63 ± 1.82 b | 93.27 ± 19.3 A | 36.35 ± 8.84 b |
| Pseudomonas sp. O81 | 99.36 ± 9.08 A | 38.22 ± 19.9 a | 91.54 ± 6.84 A | 23.93 ± 3.73 a | 199.1 ± 53.1 A,B | 28.21 ± 5.99 a |
| Pleurotus sp. + Pseudomonas sp. O81 | 96.32 ± 10.5 A | 24.89 ± 3.64 a | 203.8 ± 139 A | 30.66 ± 7.91 a,b | 60.04 ± 13.4 A | 23.84 ± 3.04 b |
| Trametes sp. + Pseudomonas sp. O81 | 137.5 ± 41.2 A | 20.42 ± 4.58 a | 169.9 ± 107 A | 33.17 ± 1.65 a | 124.2 ± 26.2 B | 41.70 ± 22.0 a |
| Pseudomonas sp. N26 | 106.7 ± 14.9 A | 23.12 ± 2.09 a | 87.54 ± 7.93 A | 21.45 ± 2.83 a | 212.4 ± 55.0 A | 33.98 ± 8.05 a |
| Pleurotus sp. + Pseudomonas sp. N26 | 137.6 ± 19.1 A | 25.41 ± 2.00 a,b | 92.13 ± 15.4 A,B | 29.22 ± 2.85 b | 110.3 ± 44.9 A | 23.68 ± 1.01 b |
| Trametes sp. + Pseudomonas sp. N26 | 135.8 ± 16.9 A | 30.11 ± 3.70 a | 120.8 ± 8.20 A | 24.94 ± 2.07 a,b | 109.5 ± 51.5 A | 19.22 ± 0.74 a |
| Dehydrogenase Activity (ng TPF/g Soil/hour) | |||
|---|---|---|---|
| Conditions | Day 0 | Day 7 | Day 14 |
| Control | 4.32 ± 1.11 a | 3.89 ± 0.55 a | 9.11 ± 4.53 b |
| Pleurotus sp. | 6.13 ± 1.45 a | 3.75 ± 0.35 b | 11.8 ± 1.26 c |
| Trametes sp. | 5.24 ± 1.67 a | 4.10 ± 0.59 a | 13.4 ± 6.48 b |
| Flavobacterium sp. I57 | 3.63 ± 0.14 a | 3.68 ± 0.35 a | 3.94 ± 0.43 a |
| Pleurotus sp. + Flavobacterium sp. I57 | 4.17 ± 0.28 a | 4.41 ± 0.58 a | 8.81 ± 1.19 b |
| Trametes sp. + Flavobacterium sp. I57 | 8.51 ± 0.87 a | 4.66 ± 0.35 b | 9.79 ± 4.91 a,b |
| Enterobacter sp. I2 | 3.95 ± 0.30 a | 4.41 ± 0.82 a | 6.03 ± 0.99 b |
| Pleurotus sp. + Enterobacter sp. I2 | 7.00 ± 2.69 a | 9.57 ± 4.53 a | 11.7 ± 4.13 a |
| Trametes sp. + Enterobacter sp. I2 | 4.66 ± 0.60 a | 4.74 ± 0.46 a | 7.00 ± 2.31 a |
| Enterobacter sp. O8 | 3.82 ± 0.31 a | 3.82 ± 0.05 a | 6.10 ± 1.04 b |
| Pleurotus sp. + Enterobacter sp. O8 | 4.58 ± 0.60 a | 6.46 ± 1.54 a | 16.1 ± 7.82 a |
| Trametes sp. + Enterobacter sp. O8 | 9.28 ± 5.99 a | 7.49 ± 2.31 a | 10.0 ± 2.82 a |
| Acinetobacter sp. S2 | 3.75 ± 0.30 a | 3.90 ± 0.23 a | 10.2 ± 0.94 b |
| Pleurotus sp. + Acinetobacter sp. S2 | 9.94 ± 3.54 a,b | 4.59 ± 0.21 a | 7.52 ± 0.59 b |
| Trametes sp. + Acinetobacter sp. S2 | 6.19 ± 1.31 a | 4.62 ± 0.63 a | 11.4 ± 3.69 a |
| Pseudomonas sp. O81 | 4.05 ± 0.67 a | 3.46 ± 0.51 a | 11.6 ± 4.00 b |
| Pleurotus sp. + Pseudomonas sp. O81 | 6.87 ± 0.93 a | 5.80 ± 1.15 a | 14.5 ± 9.56 a |
| Trametes sp. + Pseudomonas sp. O81 | 4.01 ± 0.48 a | 4.80 ± 1.35 a | 13.6 ± 7.13 a |
| Pseudomonas sp. N26 | 4.17 ± 0.75 a | 3.52 ± 0.22 a | 12.3 ± 5.33 b |
| Pleurotus sp. + Pseudomonas sp. N26 | 5.59 ± 0.18 a | 5.80 ± 1.15 b | 8.23 ± 1.23 c |
| Trametes sp. + Pseudomonas sp. N26 | 3.77 ± 0.47 a | 4.44 ± 0.41 a | 10.8 ± 1.89 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, R.; Venâncio, C.; Cardoso, P.; Lourenço, J.; Figueira, E. Evidence of Cooperative Interactions between Rhizobacteria and Wood-Decaying Fungi and Their Effects on Maize Germination and Growth. Agriculture 2024, 14, 1170. https://doi.org/10.3390/agriculture14071170
Rocha R, Venâncio C, Cardoso P, Lourenço J, Figueira E. Evidence of Cooperative Interactions between Rhizobacteria and Wood-Decaying Fungi and Their Effects on Maize Germination and Growth. Agriculture. 2024; 14(7):1170. https://doi.org/10.3390/agriculture14071170
Chicago/Turabian StyleRocha, Ricardo, Cátia Venâncio, Paulo Cardoso, João Lourenço, and Etelvina Figueira. 2024. "Evidence of Cooperative Interactions between Rhizobacteria and Wood-Decaying Fungi and Their Effects on Maize Germination and Growth" Agriculture 14, no. 7: 1170. https://doi.org/10.3390/agriculture14071170
APA StyleRocha, R., Venâncio, C., Cardoso, P., Lourenço, J., & Figueira, E. (2024). Evidence of Cooperative Interactions between Rhizobacteria and Wood-Decaying Fungi and Their Effects on Maize Germination and Growth. Agriculture, 14(7), 1170. https://doi.org/10.3390/agriculture14071170








