Abstract
Increasing soybean productivity can be achieved by treating seeds with biostimulants. To this end, an investigation was conducted into the potential of a formulation prepared with clove es-sential oil (CEO) diluted in soybean oil for seed treatment. Soybean seeds were treated with CEO concentrations between 0.5 to 3.0 mL/L, and subjected to germination, vigor, and sanity analyses. The CEO at 1.6 mL/L exhibited favorable outcomes regarding germination, root length, and re-duced fungal infection. In this way, a two-crop field experiment evaluated soybean seeds treated with CEO at 1.6 mL/L. Soybean seeds treated with CEO in the field in 2021/2022 were not different from the controls. However, in 2019/2020, there was a higher percentage of emergence, nodulation, and production of 749 kg/ha more than in the industrial treatment. These results highlight the potential use of CEO as a biostimulant.
1. Introduction
Soybean (Glycine max L. Merril) is an important oilseed crop as its grains have high nutritional and industrial potential. This species is also easily adaptable to different soil types and climatic conditions [1]. Soybean productivity is linked to the implementation of appropriate management practices, the use of advanced technologies, and the adoption of high-quality seeds. These provide a high emergence of vigorous plantules in the cultivation field [2,3,4,5,6].
The physiological performance of seeds can be optimized through seed treatment, which involves the application of products containing insecticides, fungicides, and biostimulants that may be of synthetic or natural origin. Furthermore, using biological products, inoculants, and nutrients helps preserve or improve seed performance. This approach favors emergence and creates more favorable conditions for seedling development, enabling the full expression of the crop’s genetic potential [7,8]
However, despite their significant contribution to crop production, seed treatment with synthetic chemical agents can cause environmental disturbances, such as selecting resistant pathogens, releasing secondary pests, and contaminating soil and groundwater [9,10,11]. Furthermore, growing concern about consuming healthier foods produced in an environmentally conscious manner makes chemical synthetic products questionable [12,13]. Sustainable methods like essential oils have gained prominence in this scenario. Some of these oils are already used in agriculture as fungicides, bactericides, insecticides, and biostimulants [14,15,16]. Essential oils are renewable resources that exhibit lower natural selection pressure, rapid environmental degradation, low toxicity, and a broad spectrum of action [17,18,19,20].
Seed-treatment biostimulants are a recent innovation. The results obtained from their use have shown that they contribute to the absorption of nutrients and improve plant tolerance to stress [15]. This approach contributes to seeds expressing their full genetic potential, substantially increasing crop productivity [21,22,23].
Bulgari et al. (2019) and Teklic et al. (2021) emphasized that biostimulants enhance plants’ ability to withstand adverse climatic conditions, impacting both primary and secondary metabolism [24,25]. However, it is essential to note that, despite the benefits obtained from biostimulant products, there are still gaps in the understanding of the effects of these products on grain productivity and plant physiology. However, it is essential to note that, despite the benefits obtained from biostimulant products, there are still gaps in the understanding of their effects on grain productivity and plant physiology [26,27,28].
Clove essential oil (CEO) (Syzygium aromaticum, L.) is a promising alternative for developing new biostimulants for seed treatment. Indians, the Chinese, and Egyptians have used this oil for centuries because of its antioxidant, insecticidal, antimicrobial, and antiseptic properties [29,30,31,32,33,34,35]. CEO can be extracted from leaves, twigs, and stalks, constituting a mixture of metabolites, mainly eugenol (70–90%), eugenol acetate, β-caryophyllene (5–15%), and α-humulene (up to 2.1%) [36].
Eugenol, the predominant component of clove essential oils, is a metabolite involved in several plant interactions with the environment. However, the effect of this compound on plants depends on the quantities applied, and it may be harmful in high doses [35,37,38,39]. Nascimento et al. (2020) used clove essential oil to treat pepper seeds and observed that it reduced the incidence of fungi without affecting the seeds’ physiological quality [40].
Several studies have emphasized the importance of evaluating the physiological quality of treated seeds from biostimulants to ensure optimal results. Hence, it is crucial to determine the appropriate doses of biostimulants by evaluating the germination and emergence of treated seeds [41]. Therefore, this study aimed to evaluate the effectiveness of different CEO concentrations on the germination and emergence of soybean seeds under laboratory and field conditions, contributing to the generation of an ecological and low-cost product intended to treat soybean seeds.
2. Materials and Methods
The study was divided into two stages: laboratory analysis and field testing. In the first stage, tests were conducted to analyze the CEO’s chemical composition and evaluate its phytotoxicity in soybean seeds. The experiments were performed at the Crop Physiology and Metabolism Lab (LAFIMEPRO) and the Laboratory of Development Natural Agrochemicals (LDAN) at the Federal University of Viçosa (UFV), Campus Rio Paranaíba, MG.
In the second stage, planting was carried out under field conditions during 2019/2020 and 2021/2022. The experiment was conducted in the city of São Gotardo, MG, Brazil, located at cartographic co-ordinates 19°15′33″ S and 46°05′28” W. According to the Köppen classification, the climate classification is Aw, which is tropical and dry in winter. This tropical savannah has soil-type dystrophic red-yellow oxysol. The fertility was adjusted based on the cropping pattern in the Cerrado Mineiro environment.
2.1. Analysis of the Chemical Composition of Clove Essential Oil
The CEO used for this study was purchased from Ferquima Indústria e Comércio LTDA (Vargem Grande Paulista, São Paulo, Brazil). It consisted of eugenol (76.18%), (E)-caryophyllene (19.9%), α-humulene (2.26%), aromadendrene (0.19%), and eugenol acetate (1.02%), similar to that used by Silva (2022) [35].
2.2. Evaluation of the Biostimulant Effect of Clove Essential Oil
A germination test using soybean seeds (M5917-IPRO) was conducted according to the Standards for Seed Analysis (RAS) [42]. The CEO was evaluated in concentrations of 0.5, 0.7, 1.0, 1.6, 2.0, and 3.0 mL/L dissolved in soybean oil. Distilled water and pure soybean oil were tested as controls. Previously, the seeds were sanitized with sodium hypochlorite, dried, and treated sequentially with CEO solution (0.5 to 3.0 mL/L), which was applied to the dose at 40 mL/kg. The treatment used plastic bags with agitation to obtain a film on the seeds [35].
The germination tests were performed using three sheets of Germitest® paper, sterilized and moistened with distilled water at a proportion of 2.5× its dry weight. Fifty seeds were placed in this, with the leaves rolled in sequence and added to a germination chamber at a temperature of 25 °C and a photoperiod of 12 h. Every 24 h, the germination rate was checked for seven days, and the number of seedlings that showed radicle protrusion was recorded. On the seventh day, the total number of germinated seeds was counted to determine the germination percentage (G), the quantity of dead seeds (DS), and the quantity of abnormal seedlings (AS).
With data on seeds germinated per day, the Germination Speed Index (GSI) was calculated using the formula proposed by Maguire (1962) [43]. At the end of the test, ten seedlings were randomly selected from each replicate per treatment to evaluate root length (RL), number of secondary roots (NSR), Root Wet Mass (RWM), and Dry mass of the roots (DMR). Root length was measured, using a millimeter ruler, from the top to the bottom of the roots. The number of secondary roots was counted, and the fresh mass was obtained by weighing the root system of the plants on a precision scale. The roots were then dried in an oven (Nova Instruments, Piracicaba, São Paulo, Brazil) at 65 °C for 72 h, and the dry mass was determined by weighing the roots again. This meticulous process ensured accurate and reliable data for analysis.
2.3. Sanity Test
The sanity test, called the “Blotter test”, was conducted following the Rules for Seed Analysis (RAS) [44]. The experiment was designed in a 2 × 3 factorial arrangement, considering two types of seeds (sanitized and unsanitized) and three treatment modes (CEO 1.6 mL/L at a dose of 40 mL/kg soybean seed, and the controls: distilled water and pure soybean oil), each replicated 16 times. Each replicate was represented by a Gerbox containing 25 seeds, totaling 400 seeds per treatment. The test was structured in Gerbox using two sheets of Germitest® paper. A total of 2.5 mL of sodium 2,4-dichlorophenoxyacetate (2,4-D) was added at a concentration of 5 mg/L to inhibit germination. In the distilled water control groups, a water–agar solution was applied to Germitest® paper to fix the seeds, thus preventing any movement during the incubation period. Afterward, they were placed in an incubator at 25°, with a 12-h photoperiod over 10 days. After incubation, the number of contaminated seeds was counted and identified based on fungal growth.
2.4. Evaluation of the Biostimulant Effect of CEO Applied to Soybean Seeds in Field Experiments, 2019/2020 and 2021/2022
The seeds of soybean cultivar Monsoy M5917-IPRO (Agro Bayer, Piracicaba, São Paulo, Brazil) were treated with CEO formulated with soybean oil in a concentration of 1.6 mL/L applied to 40 mL/kg of seeds. They were then inoculated with Bradyrhizobium elkanii and B. japonicum. The control groups included seeds treated with soybean oil plus Bradyrhizobium elkanii and B. japonicum, as well as seeds with an industrial treatment (fungicide: pyraclostrobin; insecticide: thiamethoxam; long-life inoculant: Nitragin Power 200; protection polymer: Incotec®; drying: drying powder). In 2021/2022, in addition to industrial treatment and soybean oil, another control treatment was added: non-treated seeds.
The parameter emergence, plant height at 20 days after emergency (DAE), and the end of cultivation, stem diameter, insertion of the first pod, number of pods and seeds per plant, seed weight per plant, and production in bags per hectare were measured.
The experimental design used in 2019/2020 was Completely Randomized Blocks (CRB), with two blocks and three replicates each. Each replication consisted of a 5-m-long planting line with a seeding density of 18 seeds per linear meter. The percentage of plant emergence was measured 20 days after planting (DAP), and the number of nodules was quantified by collecting from 5 plants randomly collected per replication 50 DAP. After the cycle (146 DAP), we evaluated the productivity by harvesting all plants. According to the productivity parameters, such as the number of pods and grains per plant, 20 plants were randomly selected for each replication. The 2021/2022 experiment was conducted in five blocks with one replication. Each repetition consisted of three lines of a 5-m-long planting line with 18 seeds per linear meter seeding density. The adopted cultural management followed the farmer’s practices. In 2019/2020, the parameters of emergence, nodulation, number of pods and seeds per plant, and production in bags per hectare were evaluated.
2.5. Biometric Analysis, Leaf-Gas Exchange
Gas exchange analyses were performed 38 d after DAP planting at the V2 phenological stage with the aid of a gas-exchange analyzer, model LI-6400 XT (Li-Cor Biosciences, Lincoln, NE, USA), coupled to a fluorometer (6400-40), with a photon-flux density of 1200 µmol m−2 s−1. TGF analyses were composed of the following parameters: stomatal conductance (Gs), transpiration (E), evapotranspiration (ETR), quantum Efficiency of photosystem 2 (PSII), net photosynthesis rate (A), intrinsic water use efficiency (WUE i), instantaneous water use efficiency (WUE), and internal carbon concentration (Ci). These parameters were verified under photosynthetically active radiation (PAR) of 1200 μmol m−2 s−1 at the leaf level and under environmental conditions of temperature, carbon dioxide (CO2) concentration, and vapor pressure deficit (VPD).
2.6. Extraction of Metabolites and GC-MS Analysis
The experiment was carried out in just one block; each replicate consisted of three leaves collected from different plants. The leaves were stored in a thermal box and transported to the laboratory, where they were immediately macerated in liquid nitrogen. To extract metabolites, 10 mg of macerated leaves were weighed in Eppendorf tubes. Then, 1 mL of methanol containing 25 μg/mL of ribitol was added as an internal standard, and the mixture was vortexed for 5 s and sonicated for 15 min. Subsequently, the samples were centrifuged at 13.000 rpm for 15 min, and the supernatant was transferred to a tube. The residue extraction procedure was repeated using 1 mL of water, and the supernatants were combined in a single vial. The internal standard physically normalized the mass of soybean leaves extracted from each replicate.
Then, 400 μL of the sample was transferred to a glass bottle, and the solvent was dried in a forced air recirculation oven at 40 °C for 12 h. The dried aliquots were subjected to methoxylation in pyridine, and 50 μL of 15 mg/mL methoxyamine hydrochloride was added, followed by trimethylation with 50 μL of N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA) + 1% TMCS reagent (trichloromethyl silane). For quality control (QC), aliquots of all soybean leaf samples were collected and processed using the same procedure as the experimental samples. QC samples were analyzed by GC-MS before, during, and after sample injection to ensure that instrumental drift was maintained at a minimum level.
2.7. GC-MS-Based Untargeted Metabolomics Analysis
Derivatized soybean leaf extract samples were analyzed by GC-MS using a chromatography system coupled to a Shimadzu GCMS-QP2010 mass spectrometer (Shimadzu, Kyoto, Japan). An RTX-5MS capillary column (30 m × 250 μm internal diameter, 0.25 μm film thickness; Restek Co., Bellefonte, PA, USA) was used. Chromatographic separation was performed using a column heating temperature ramp at 80 °C for 2 min, with an increase of 5 °C/min until 315 °C was reached, and it was maintained at this temperature for 12 min. Helium was the carrier gas, with a constant 1.0 mL/min flow. One microliter of the derivatized sample was injected at a split ratio 15:1. Mass spectra were obtained using a scan between masses from 50 to 650 m/z, in full scan mode, operated with sampling rates of up to 5 scans/s. The interface temperature was 280 °C, and the ion source temperatures were 280 and 240 °C. The detector voltage was 1.2 kV, and the electron impact (EI) model had an ionization energy of 70 eV. Retention indices (AI) were obtained by injecting a standard mixture of linear alkanes from C9 to C30, which were used to identify leaf metabolites.
2.8. GC-MS Data Processing
The raw data obtained from the GC-MS analysis were converted to the “abf” format using the ABF converter software (AbfConverter, Yokohama City, Kanagawa, Japan). The converted data were processed using the MS-DIAL software (Yokohama City, Kanagawa, Japan), where alignment deconvolution and peak identification were performed [45]. The parameters of the average peak width equal to 20 scans were used to process the data, with an amplitude (minimum peak height) equal to 1000. The deconvolution parameters were a sigma window of 0.5. To identify the peaks, the RI retention index was inserted into the software via the MSP File and compared with the FiehnLib database, [46] adopting RI tolerances of 30, a retention time (RT) of 0.5, a mass/charge (m/z) of 0.5, and minimum similarity of 70%.
2.9. Statistical Analysis
The data obtained via the Ms-DIAL Software were exported to a txt spreadsheet, normalized, and subjected to statistical and metabolic pathway analyses using the opensource software Metaboanalyst 4.9 (http://www.metaboanalyst.ca, accessed on 28 November 2022), a program based on R and designed for metabolomic analysis [47]. Values absent in 50% of the data were excluded from the study. Missing values were replaced with a small value (half of the minimum positive value in the original data), and data were relative to the filtered standard deviation, sum normalized, log2 transformed, and autoscaled. Statistical analyses were conducted to compare metabolites in the three seed treatments (soybean oil, industrial, and CEO) using the Student’s t-test with a p-value of less than 0.05. A multivariate statistical approach was used, including Principal component analysis (PCA) and PERMANOVA tests, to identify differences in metabolic profiles among treatments using MetaboAnalyst 4.9. Additionally, a graphical heat map was generated based on hierarchical clustering to visualize the similarity of the compounds, which was evaluated using the Euclidean distance measure.
The significance level was set at p < 0.05. Fifty-eight potential biomarkers identified in Arabidopsis thaliana were used to determine the metabolic pathways altered by industrial seed treatment. Therefore, the Student’s t-test was performed using MetaboAnalyst 5.0, based on the KEGG metabolic pathway library. All results were subjected to statistical analysis using the false discovery rate (FDR).
3. Results
3.1. Preparation of the Formulated CEO and Application to the Seeds
Seeds treated with CEO, ranging from 0.5 mL/L to 3.0 mL/L, showed germination rates between 97.5% and 100%, as evidenced in Table 1. These rates were significantly different from the two controls. The treated soybean seeds with CEO presented higher results than those observed in controls, with the highest GVI obtained with T6 (1.6 mL/L).

Table 1.
Assessment of the phytotoxicity of CEO applied in different concentrations to soybean seeds’ germination and vigor.
Seeds subjected to CEO treatment revealed a significant increase in root length compared to the control groups, with the best performance being observed at a concentration of 1.6 mL/L. In the NSR analysis, the CEO at 0.5 mL/L performed better than the control group, although it did not differ significantly from the CEO at 1.6 mL/L. In the RWM and DMR trials, treatments with CEO stood out compared to the control groups. However, there was variation according to the concentration of CEO used.
3.2. Sanity Test
Soybean seeds treated with CEO 1.6 mL/L (as shown in Table 2) and sanity-test investigations significantly reduced the contamination percentage compared to the two controls—distilled water and soybean oil. However, soybean oil also showed a significant reduction in contamination rate compared to the water control.

Table 2.
Filamentous fungal incidence on seeds.
3.3. The Influence of Seed Treatment with CEO on Soybean Crops in Field Experiments
The CEO at 1.6 mL/L demonstrated the most promising results as the appropriate dose for biostimulant activity in germination and sanity tests, and was selected for field-level tests. To this end, two field experiments were conducted in 2019/2020 and 2021/2022.
3.3.1. Field Experiment 2019/2020
Seeds treated with CEO (1.6 mL/L, at a dose of 40 mL/kg of seeds) showed a higher percentage of emergence, nodulation, and production than those treated with industrial treatment and soybean oil controls. However, the number of pods and seeds per plant was lower than in the industrial treatment, highlighting the importance of establishing an adequate plant stand in the field to ensure high productivity at the end of the crop (Table 3).

Table 3.
Evaluation of the biostimulant effect of CEO applied to soybean seeds in a field experiment in 2019/2020.
3.3.2. Field Experiment 2021/2022
The percentage of emergence and height at 20 DAE and the end of the crop in 2021/2022 (Table 4) were higher in the industrial treatment. The CEO did not promote a significant increase in emergence. Still, the height parameters at seven days and at the end of cultivation during the harvest did not differ from the industrial treatment or controls. The parameters of stem diameter, insertion of the first pod, number of pods and seeds per plant, seed weight per plant, and production in bags per hectare showed no significant differences, although the seeds treated industrially and with CEO produced 300 and 252 kg/ha more than the control.

Table 4.
Evaluation of the biostimulant effect of CEO applied to soybean seeds in a field experiment in 2021/22.
3.3.3. Comparison between Field Experiments in 2019/2020 and 2021/2022
Statistical analysis was performed to compare the productivity of the experiments for both harvests, excluding the control treatment from 2021/2022 and a repetition of the 2019/2020 experiment. In 2019/2020, the highest productivity was observed with a CEO of 1.6 mL/L, demonstrating superiority compared to the controls (Table 5).

Table 5.
Comparison of the productivity (Kg/ha) of soybean plants treated with CEO in the field in the two harvests.
3.3.4. Evaluation of the Effects of CEO Treatment on Gas Exchange and Metabolomics in Soybean Plants in the 2019/2020 Experiment
Table 6 shows that the gas exchange parameters in the V2 phenological stage did not show significant differences.

Table 6.
Assimilation rate of CO2 (A) (μmol CO2 m2 s−1), transpiration (E) (mmol H2O m−2 s−1), internal CO2 concentration (Ci) (μmol CO2 mol−1), stomatal conductance (Gs) (mmol m2 s−1), efficiency instantaneous carboxylation (EiC) (μmol CO2m2s−1/μmolmol−1), photosynthesis/concentration internal (A/Ci) (μmol CO2 m−2 s−1/μmol CO2 mol−1).
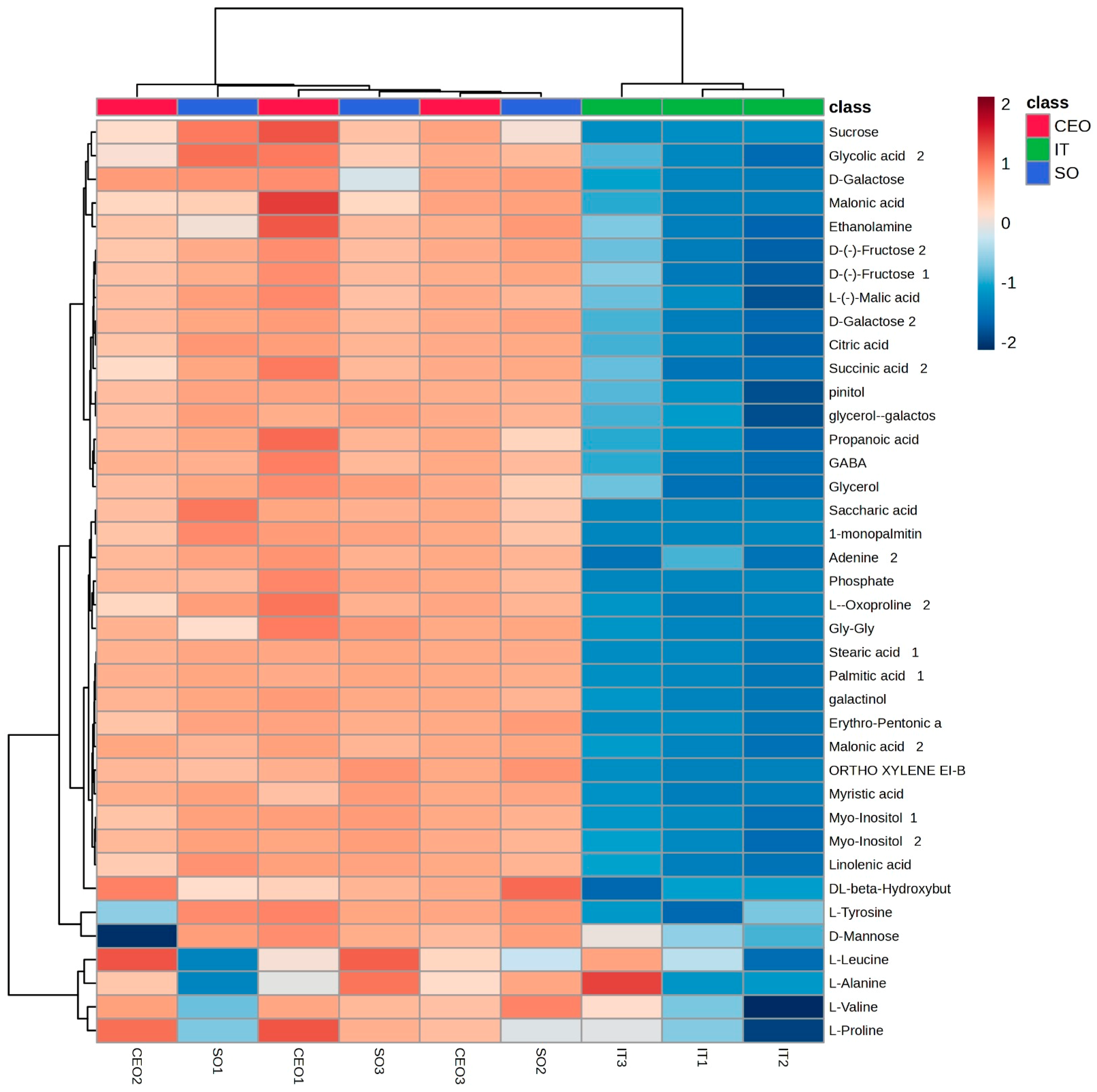
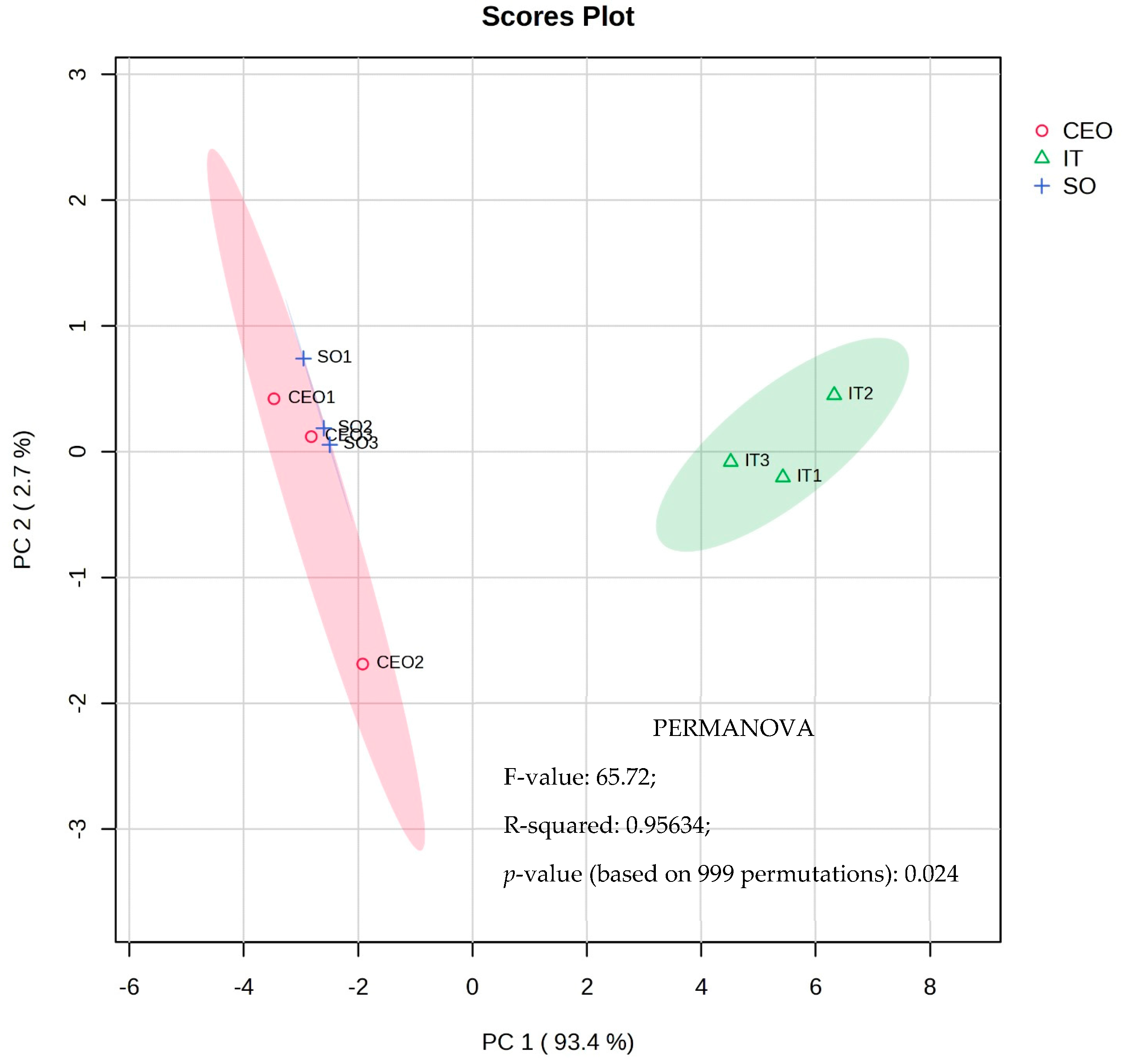
In the metabolomic analysis of plants in the V2 phenological stage in soybean leaf samples, 38 metabolites showed a significant difference (p < 0.05) in relative concentrations between the treatments. After this verification, a heat map was generated based on the groups of the main components of the 58 identified metabolites, classified by their p-values using the Student’s t-test (Figure 1). These data revealed a clear and distinct grouping, one formed by industrial seed treatment and the other by treatments with soybean oil and CEO (Figure 1 and Figure 2).
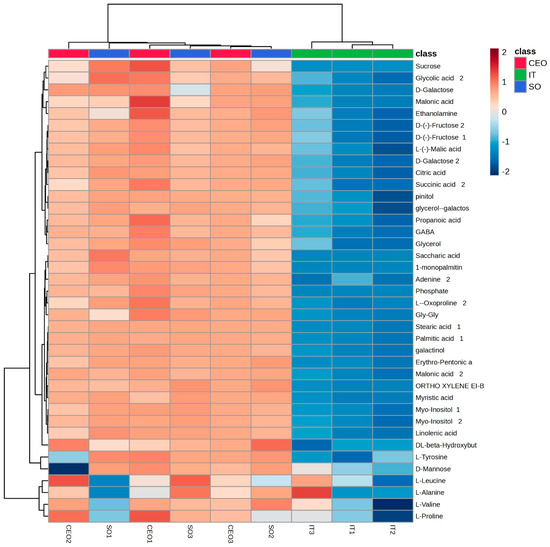
Figure 1.
Heatmap generated using Euclidean distance for metabolite clustering with significant differences between groups (p < 0.05). The columns represent the samples from each treatment (green = industrial treatment; blue = soybean oil; red = CEO). Each line represents a metabolite. The colored squares (heat map) represent the abundance of metabolites, where dark red is the highest abundance and dark blue is the lowest.
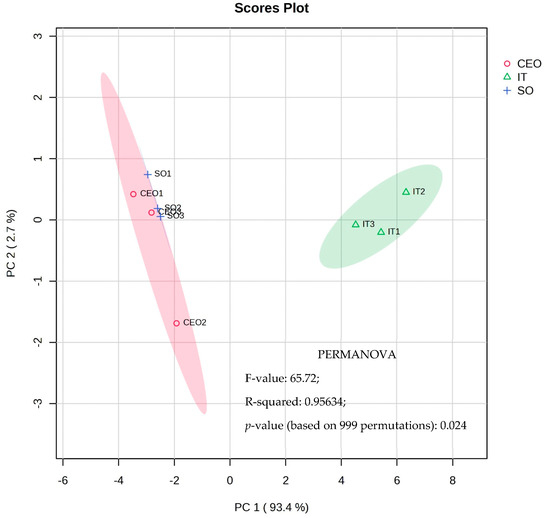
Figure 2.
Multivariate principal component analysis (PCA) and PERMANOVA test of metabolic profiles found in soybean leaf extracts whose seeds were subjected to CEO, soybean oil, and industrial treatment.
Graph 1 of the PCA analysis reveals a clear separation between the industrial treatment and treatments with soybean oil and CEO. Main component 1, responsible for explaining 93.4% of the total variance, stands out in this differentiation. Industrial treatment plants exhibit lower relative concentrations of amino acids, such as L-proline, L-valine, L-tyrosine, and glycine-glycine dipeptide, as well as malonic, myristic, linolenic, and propanoic organic acids. Furthermore, they had lower relative sucrose, D-mannose, and D-galactose levels.
4. Discussion
The formulated CEO and application to the seeds showed germination rates between 97.5% and 100%. It is crucial to note that, as Gonzalez et al. (2002) emphasize, the concentration of allelochemicals influences the response and can manifest itself as a biostimulant or allelopathic potential [48]. High doses of CEO can be toxic and damage seed germination [35,49,50,51]. Therefore, the formulation and concentration-optimization stages were essential to promote germination, GVI, and root development gains in seeds treated with CEO.
The GVI results for CEO (1.6 mL/L) were higher than those observed in controls. Santos (2014) observed no difference in the GVI of soybean seeds treated with CEO emulsion by immersion for 5 min at a concentration of 2.5 mL/L or (0.25%). Differences can be justified by the use of different vehicles when applying the CEO. A higher rate of GVI is correlated with faster emergence in the field, reducing the exposure of seeds to adverse environmental conditions, which can harm germination and facilitate the action of micro-organisms, causing deterioration of seeds or seedlings [48,52,53].
Seeds that received 1.6 mL/L of CEO treatment increased in root length and number of secondary roots compared to the control groups. A study by Gomes et al. (2016) evaluated the size of shoots and roots of lima bean plants subjected to different CEO dosages. Comparing these dosages, the researchers found that both parameters presented superior results compared to the control group [13]. The increase in these parameters due to the CEO highlights the compound’s biostimulant potential, favoring the initial development of the root system. This makes the CEO highly promising, as plants with well-developed root systems can better explore deeper soil layers. This capacity facilitates adaptation to soils with low fertility, and increases the absorption of nutrients and water [54,55].
Soybean seeds treated with 1.6 mL/L of CEO reduced their contamination percentage compared to the two controls—distilled water and soybean oil. CEO applied to pea seeds also reduced the rate of seed contamination by fungi that cause bitter roots [56]. The CEO’s fungicidal action has yet to be fully established. Still, according to Wang et al. (2010), eugenol present in CEO can bind to the fungal membrane and alter its permeability, leading to rupture [57]. This statement is based on the hydrophobic properties of CEO, which can adhere to the walls of the sclerotium, causing morphological changes [58,59].
The CEO and soybean oil treatments showed a higher relative concentration of amino acids, such as L-proline and L-valine, organic acids like malonic, myristic, linolenic, and propanoic, and sugars such as sucrose, D-mannose, and D-galactose. These sugars and amino acids are the first-response metabolites produced when plants face stressful conditions [60,61,62]. Considering that the CEO presented more nodules than the other treatments, we suggest that such trends may be related to changes caused by plant–microorganism interactions, which require further elucidation studies.
Soybean seeds reated with CEO at 1.6 mL/L in the field experiments in 2021/2022 were not different from the controls. However, in 2019/2020, there was a higher percentage of emergence, nodulation, and production than in the industrial treatment and soybean-oil controls. However, the number of pods and seeds per plant was lower than in the industrial treatment, showing that establishing an adequate plant stand in the field is essential for high productivity at the end of the crop. Silva et al. (2019) evaluated the emergence of rice seeds protected with CEO, and observed increases from 60% to 87% in protected seeds compared to the control [63]. Treatment with CEO in the field in 2019/2020 promoted a significant increase in the number of nodules in soybeans; according to Hungria et al. (2001), biological nitrogen fixation by soybean crops is directly related to the number of nodules present in the plant [64]. In other words, abundant nodulation tends to fix more nitrogen and, consequently, greater nutrient availability for the plant, reflecting productivity. These results highlight the potential use of the CEO as a biostimulant.
5. Conclusions
These results highlight the impact of clove essential oil on soybean-seed germination. The data revealed that incorporating this formulation, made with clove essential oil solubilization in soybean oil in agricultural practices can significantly increase germination and early seedling development. Given these discoveries, clove essential oil is a promising biostimulant in sustainable agriculture. Considering the growing challenges related to food production and the need for more efficient agricultural practices, the strategic use of this oil is a viable and ecologically sensible alternative. Therefore, investing in additional research and integrating clove essential oil into agricultural practices could significantly advance the search for sustainable and effective methods to promote healthy plant growth, thus contributing to innovative solutions in contemporary agriculture.
Author Contributions
J.P.C., V.G.N. and W.R.M. were responsible for formal analysis, investigation, methodology, writing—review, and editing. G.H.S. was responsible for conceptualization, funding acquisition, resources, and supervision. M.F.C.S. was responsible for funding acquisition and writing the original draft. All authors have read and agreed to the published version of the manuscript.
Funding
The authors gratefully acknowledge financial support from the National Council for Scientific and Technological Development (CNPq, Conselho Nacional de Desenvolvimento Científico e Tecnológico), National Institute of Science and Technology—INCT BioNat, grant # 465637/2014-0, Brazil and Fundação de Apoio à Pesquisa do Espírito Santo (FAPES).
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hasanuzzaman, M.; Fujita, M.; Oku, H.; Islam, M.T.; Ali, Q.; Athar, H.-R.; Haider, M.Z.; Shahid, S.; Aslam, N.; Shehzad, F.; et al. Role of Amino Acids in Improving Abiotic Stress Tolerance to Plants. In Plant Tolerance to Environmental Stress; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Bakhshandeh, E.; Gholamhossieni, M. Quantification of Soybean Seed Germination Response to Seed Deterioration under PEG-Induced Water Stress Using Hydrotime Concept. Acta Physiol. Plant 2018, 40, 126. [Google Scholar] [CrossRef]
- Marcos Filho, J. Fisiologia de Sementes de Plantas Cultivadas; Abrates: Londrina, Brazil, 2015. [Google Scholar]
- Pinthus, M.J.; Kimel, U. Speed of Germination as a Criterion of Seed Vigor in Soybeans 1. Crop Sci. 1979, 19, 291–292. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, W.Q.; Liu, S.J.; Møller, I.M.; Song, S.Q. Proteome Analysis of Poplar Seed Vigor. PLoS ONE 2015, 10, e0132509. [Google Scholar] [CrossRef] [PubMed]
- Ebone, L.A.; Caverzan, A.; Chavarria, G. Physiologic Alterations in Orthodox Seeds Due to Deterioration Processes. Plant Physiol. Biochem. 2019, 145, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Ramesh, R.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Bhutia, T.L.; Devi, S.H.; Thounaojam, A.S.; et al. Role of Biostimulants in Mitigating the Effects of Climate Change on Crop Performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.T.; Liu, W.; Olhoft, P.; Crafts-Brandner, S.J.; Pennycooke, J.C.; Christiansen, N. Soybean Yield Formation Physiology—A Foundation for Precision Breeding Based Improvement. Front. Plant Sci. 2021, 12, 719706. [Google Scholar] [CrossRef] [PubMed]
- Soylu, E.M.; Kurt, Ş.; Soylu, S. In Vitro and in Vivo Antifungal Activities of the Essential Oils of Various Plants against Tomato Grey Mould Disease Agent Botrytis Cinerea. Int. J. Food Microbiol. 2010, 143, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting Sustainability of Fungiside Seed Treatments for Field Crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef]
- Moumni, M.; Brodal, G.; Romanazzi, G. Recent Innovative Seed Treatment Methods in the Management of Seedborne Pathogens. Food Secur. 2023, 15, 1365–1382. [Google Scholar]
- Tandon, A.; Jabeen, F.; Talwar, S.; Sakashita, M.; Dhir, A. Facilitators and Inhibitors of Organic Food Buying Behavior. Food Qual. Prefer. 2021, 88, 104077. [Google Scholar] [CrossRef]
- Gomes, R.S.S.; Nunes, M.C.; Nascimento, L.C.; Souza, J.O.; Porcino, M.M. Eficiência de Óleos Essenciais Na Qualidade Sanitária e Fisiológica Em Sementesde Feijão-Fava (Phaseolus lunatus L.). Rev. Bras. Plantas Med. 2016, 18, 279–287. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Fierascu, I.C.; Dinu-Pirvu, C.E.; Fierascu, I.; Paunescu, A. The Application of Essential Oils as a Next-Generation of Pesticides: Recent Developments and Future Perspectives. Z. Fur Naturforschung Sect. C J. Biosci. 2020, 75, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Wazeer, H.; Shridhar Gaonkar, S.; Doria, E.; Pagano, A.; Balestrazzi, A.; Macovei, A. Plant-Based Biostimulants for Seeds in the Context of Circular Economy and Sustainability. Plants 2024, 13, 1004. [Google Scholar] [CrossRef] [PubMed]
- Knaak, N.; Fiuza, L. Potencial Dos Óleos Essenciais de Plantas No Controle de Insetos e Microrganismos. Neotrop. Biol. Conserv. 2010, 5, 120–132. [Google Scholar] [CrossRef]
- Šernaitė, L.; Rasiukevičiūtė, N.; Dambrauskienė, E.; Viškelis, P.; Valiuškaitė, A. Biocontrol of Strawberry Pathogen Botrytis Cinerea Using Plant Extracts and Essential Oils. Zemdirbyste 2020, 107, 147–152. [Google Scholar] [CrossRef]
- Lyubenova, A.; Nikolova, M.; Slavov, S.B. Inhibitory Effect of Greek Oregano Extracts, Fractions and Essential Oil on Economically Important Plant Pathogens on Soybean. Agric. Sci. Technol. 2023, 15, 61–66. [Google Scholar] [CrossRef]
- Proto, M.R.; Biondi, E.; Baldo, D.; Levoni, M.; Filippini, G.; Modesto, M.; Di Vito, M.; Bugli, F.; Ratti, C.; Minardi, P.; et al. Essential Oils and Hydrolates: Potential Tools for Defense against Bacterial Plant Pathogens. Microorganisms 2022, 10, 702. [Google Scholar] [CrossRef] [PubMed]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Lagzian, A.; Saberi Riseh, R.; Khodaygan, P.; Sedaghati, E.; Dashti, H. Introduced Pseudomonas fluorescens VUPf5 as an Important Biocontrol Agent for Controlling Gaeumannomyces graminis var. tritici the Causal Agent of Take-All Disease in Wheat. Arch. Phytopathol. Plant Prot. 2013, 46, 785123. [Google Scholar] [CrossRef]
- Taylor, A.G.; Harman, G.E. Concepts and Technologies of Selected Seed Treatments. Annu. Rev. Phytopathol. 1990, 28, 321–339. [Google Scholar] [CrossRef]
- Guleria, G.; Thakur, S.; Shandilya, M.; Sharma, S.; Thakur, S.; Kalia, S. Nanotechnology for Sustainable Agro-Food Systems: The Need and Role of Nanoparticles in Protecting Plants and Improving Crop Productivity. Plant Physiol. Biochem. 2023, 194, 533–549. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants Application in Horticultural Crops under Abiotic Stress Conditions. Agronomy 2019, 9, 306. [Google Scholar] [CrossRef]
- Parađiković, N.; Teklić, T.; Zeljković, S.; Lisjak, M.; Špoljarević, M. Biostimulants Research in Some Horticultural Plant Species—A Review. Food Energy Secur. 2019, 8, e00162. [Google Scholar] [CrossRef]
- Johnson, R.; Joel, J.M.; Puthur, J.T. Biostimulants: The Futuristic Sustainable Approach for Alleviating Crop Productivity and Abiotic Stress Tolerance. J. Plant Growth Regul. 2024, 43, 659–674. [Google Scholar] [CrossRef]
- Ma, Y.; Freitas, H.; Dias, M.C. Strategies and Prospects for Biostimulants to Alleviate Abiotic Stress in Plants. Front. Plant Sci. 2022, 13, 1024243. [Google Scholar] [CrossRef] [PubMed]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, J.D.F.; Paroul, N.; Czyewski, E.; Lerin, L.; Rotava, I.; Cansian, R.L.; Mossi, A.; Toniazzo, G.; de Oliveira, D.; Treichel, H. Perfil Da Composição Química e Atividades Antibacteriana e Antioxidante Do Óleo Essencial Do Cravo-Da-Índia (Eugenia caryophyllata Thunb.). Rev. Ceres 2010, 57, 589–594. [Google Scholar] [CrossRef]
- Affonso, R.S.; Rennó, M.N.; Slana, G.B.C.A.; França, T.C.C. Chemical and Biological Aspects of the Essential Oil of Indian Cloves. Rev. Virtual Quim. 2012, 4, 146–161. [Google Scholar] [CrossRef]
- Lima, T.S.; da Silva França, K.R.; de Azevedo, P.T.M.; Paiva, Y.F.; Silva, J.C.S.; Silva, K.O.; Santos, A.B.; de Sousa Galdino, J.A.A.; de Mendonça Júnior, A.F.; Cardoso, T.A.L. Control of Some Phytopathogenic Fungi Using Clove Essential Oil (Syzygium aromaticum L.). J. Exp. Agric. Int. 2019, 39, 1–11. [Google Scholar] [CrossRef]
- Hashem, A.H.; Abdelaziz, A.M.; Hassanin, M.M.H.; Al-Askar, A.A.; AbdElgawad, H.; Attia, M.S. Potential Impacts of Clove Essential Oil Nanoemulsion as Bio Fungicides against Neoscytalidium Blight Disease of Carum carvi L. Agronomy 2023, 13, 1114. [Google Scholar] [CrossRef]
- Yang, C.-J.; Gao, Y.; Du, K.-Y.; Luo, X.-Y. Screening of 17 Chinese Medicine Plants against Phytopathogenic Fungi and Active Component in Syzygium aromaticum. J. Plant Dis. Prot. 2019, 127, 237–244. [Google Scholar] [CrossRef]
- Santamarina, M.P.; Roselló, J.; Giménez, S.; Blázquez, M.A. Commercial Laurus nobilis L. and Syzygium aromaticum L. Merr. & Perry Essential Oils against Post-Harvest Phytopathogenic Fungi on Rice. LWT Food Sci. Technol. 2016, 65, 325–332. [Google Scholar] [CrossRef]
- Silva, A.A.; Pereira, F.A.C.; de Souza, E.A.; de Oliveira, D.F.; Nobre, D.A.C.; Macedo, W.R.; Silva, G.H. Inhibition of Anthracnose Symptoms in Common Bean by Treatment of Seeds with Essential Oils of Ocimum gratissimum and Syzygium aromaticum and Eugenol. Eur. J. Plant Pathol. 2022, 163, 865–874. [Google Scholar] [CrossRef]
- Alma, M.H.; Ertaş, M.; Nitz, S.; Kollmannsberger, H. Chemical Composition and Content of Essential Oil from the Bud of Cultivated Turkish Clove (Syzygium aromaticum L.). Bioresources 2007, 2, 265–269. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A Precious Spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Gupta, I.; Singh, R.; Muthusamy, S.; Sharma, M.; Grewal, K.; Singh, H.P.; Batish, D.R. Plant Essential Oils as Biopesticides: Applications, Mechanisms, Innovations, and Constraints. Plants 2023, 12, 2916. [Google Scholar] [CrossRef]
- de Oliveira, M.S.; da Costa, W.A.; Pereira, D.S.; Botelho, J.R.S.; de Alencar Menezes, T.O.; de Aguiar Andrade, E.H.; da Silva, S.H.M.; da Silva Sousa Filho, A.P.; de Carvalho, R.N. Chemical Composition and Phytotoxic Activity of Clove (Syzygium aromaticum) Essential Oil Obtained with Supercritical CO2. J. Supercrit. Fluids 2016, 118, 185–193. [Google Scholar] [CrossRef]
- do Nascimento, D.M.; dos Santos, P.L.; Ribeiro-Junior, M.R.; Sartori, M.M.P.; Kronka, A.Z. Essential Oils Control Anthracnose in Pepper Seeds. Res. Soc. Dev. 2020, 9, e7619109028. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of Non-Microbial Biostimulants in Regulation of Seed Germination and Seedling Establishment. Plant Growth Regul. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- BRASIL. Regras Para Análise de Sementes; MAPA/ACS: Brasília, Brazil, 2009; ISBN 9788599851708. [Google Scholar]
- Maguire, J.D. Speed of Germination—Aid in Selection and Evaluation for Seedling Emergence and Vigor 1. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- de Análise, M.; de Sementes, S. Manual de Análise Sanitária de Sementes, 1st ed.; Mapa/ACS: Brasília, Brazil, 2009; Volume 1, ISBN 8599851640. [Google Scholar]
- Lai, Z.; Tsugawa, H.; Wohlgemuth, G.; Mehta, S.; Mueller, M.; Zheng, Y.; Ogiwara, A.; Meissen, J.; Showalter, M.; Takeuchi, K.; et al. Identifying Metabolites by Integrating Metabolome Databases with Mass Spectrometry Cheminformatics. Nat. Methods 2018, 15, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Kind, T.; Wohlgemuth, G.; Lee, D.Y.; Lu, Y.; Palazoglu, M.; Shahbaz, S.; Fiehn, O. FiehnLib: Mass Spectral and Retention Index Libraries for Metabolomics Based on Quadrupole and Time-of-Flight Gas Chromatography/Mass Spectrometry. Anal. Chem. 2009, 81, 10038–10048. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Soufan, O.; Li, C.; Caraus, I.; Li, S.; Bourque, G.; Wishart, D.S.; Xia, J. MetaboAnalyst 4.0: Towards More Transparent and Integrative Metabolomics Analysis. Nucleic Acids Res. 2018, 46, W486–W494. [Google Scholar] [CrossRef] [PubMed]
- González, H.R.; Mederos, D.M.; Sosa, I.H. Efectos Alelopáticos de Restos de Diferentes Espécies de Plantas Medicinales Sobre La Albahaca (Ocimum basilicum L.) Em Condiciones de Laboratório. Rev. Cuba. Plantas Med. 2002, 7, 67–72. [Google Scholar]
- Mazzafera, P. Efeito Alelopático Do Extrato Alcoólico Do Cravo-Da-Índia e Eugenol. Rev. Bras. Botânica 2003, 26, 231–238. [Google Scholar] [CrossRef]
- Grzanka, M.; Sobiech, Ł.; Danielewicz, J.; Horoszkiewicz-Janka, J.; Skrzypczak, G.; Sawinska, Z.; Radzikowska, D.; Świtek, S. Impact of Essential Oils on the Development of Pathogens of the Fusarium Genus and Germination Parameters of Selected Crops. Open Chem. 2021, 19, 884–893. [Google Scholar] [CrossRef]
- Da Silva Santos De Moura, S.; Soares, A.M.; Ursulino, M.M.; De Oliveira, R.; Nascimento, L.C.; Alves, E.U. Physiological and Sanitary Quality of Seeds of Dimorphandra Gardneriana Tul. Treated with Essential Oils. Comun. Sci. 2018, 9, 457–464. [Google Scholar] [CrossRef]
- Tao, Q.; Xing, J.; Meng, F.; Zhang, Y.; Liu, X.; Guo, S.; Shan, Y.; Zhong, S.; Sun, J.; Zhao, Y. Siberian Wildrye (Elymus sibiricus) Seed Vigor Estimation for the Prediction of Emergence Performance under Diverse Environmental Conditions. Agronomy 2024, 14, 173. [Google Scholar] [CrossRef]
- Reed, R.C.; Bradford, K.J.; Khanday, I. Seed Germination and Vigor: Ensuring Crop Sustainability in a Changing Climate. Heredity 2022, 128, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.P.; Surigaoge, S.; Yang, H.; Yu, R.P.; Wu, J.P.; Xing, Y.; Chen, Y.; Li, L. Diversified Cropping Systems with Complementary Root Growth Strategies Improve Crop Adaptation to and Remediation of Hostile Soils. Plant Soil 2024, 1–24. [Google Scholar] [CrossRef]
- Li, P.F.; Ma, B.L.; Wei, X.F.; Guo, S.; Ma, Y.Q. Deeper Root Distribution and Optimized Root Anatomy Help Improve Dryland Wheat Yield and Water Use Efficiency under Low Water Conditions. Plant Soil 2024. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzali, L.; Romani, M.; Annicchiarico, P.; Pecetti, L. Organic Seed Treatments with Essential Oils to Control Ascochyta Blight in Pea. Eur. J. Plant Pathol. 2019, 155, 831–840. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, J.; Chen, H.; Fan, Y.; Shi, Z. Antifungal Activity of Eugenol against Botrytis Cinerea. Trop. Plant Pathol. 2010, 35, 137–143. [Google Scholar] [CrossRef]
- Venturoso, L.R.; Bacchi, L.M.A.; Gavassoni, W.L.; Conus, L.A.; Pontim, B.C.A.; Bergamin, A.C. Antifungal Activity of Plant Extracts on the Development of Plant Pathogens. Summa Phytopathol. 2011, 37, 18–23. [Google Scholar] [CrossRef]
- Costa, A.R.T.; Amaral, M.F.Z.J.; Martins, P.M.; Paula, J.A.M.; Fiuza, T.S.; Tresvenzol, L.M.F.; Paula, J.R.; Bara, M.T.F. Ação Do Óleo Essencial de Syzygium aromaticum (L.) Merr. & L.M.Perry Sobre as Hifas de Alguns Fungos Fitopatogênicos. Rev. Bras. Plantas Med. 2011, 13, 240–245. [Google Scholar]
- Fàbregas, N.; Fernie, A.R. The Metabolic Response to Drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.; Foolad, M.R. Roles of Glycine Betaine and Proline in Improving Plant Abiotic Stress Resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Kavi Kishor, P.B.; Sangam, S.; Amrutha, R.N.; Sri Laxmi, P.; Naidu, K.R.; Rao, K.R.S.S.; Rao, S.; Reddy, K.J.; Theriappan, P.; Sreenivasulu, N. Regulation of Proline Biosynthesis, Degradation, Uptake and Transport in Higher Plants: Its Implications in Plant Growth and Abiotic Stress Tolerance. Curr. Sci. 2005, 88, 424–438. [Google Scholar]
- da Silva, I.N.; Christ, A.J.; Sousa, S.; Carvalho, J.W.P.; Pascuali, L.C. Qualidade Fisiológica de Sementes de Arroz Tratadas Com Óleos Essenciais e Extratos Vegetais. Rev. Destaques Acadêmicos 2019, 11, 259–271. [Google Scholar]
- Hungria, M.; Campo, R.J.; Mendes, I.C. Fixação Biológica Do Nitrogênio Na Cultura Da Soja. Embrapa Soja. Circular Técnica 2001, 35, 1–48. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).