Abstract
In chicken diet with dietary fat, adding plant polyphenols as a natural antioxidant is recommended to enhance the n-3 polyunsaturated fatty acid (n-3 PUFA) content and improve oxidative stability in meat and eggs. However, high plant polyphenol doses could act as a pro-oxidant and interfere with the absorption of n-3 PUFAs. The study aimed to determine the effects of Dodoneae angustifolia (D. angustifolia) polyphenol levels in flaxseed-enriched diets on fatty acid content and oxidative stability in the meat and eggs of Sasso chickens. Chickens received 0, 200, 500, or 800 mg of D. angustifolia extract/kg diet designated as DA0, DA2, DA5, and DA8 treatments, respectively. Results showed that the breast muscle content of docosahexaenoic acid (DHA, C22:6n-3) in 200 and 500 mg extract/kg diet and eicosapentaenoic acid (EPA, C20:5n-3) in 800 mg extract/kg diet increased (p < 0.05) compared to those who did not receive. Feeding D. angustifolia polyphenol levels had no significant effect on egg yolk n-3 PUFA content. However, a decrease (p < 0.05) in egg yolk n-6 PUFAs was observed in hens with an increase in the dose of D. angustifolia polyphenol extract. In breast muscle, feeding on a 500 mg extract/kg diet decreased lipid peroxidation (p < 0.05) compared to the control diet. However, feeding different doses of D. angustifolia extracts had no effect on egg yolk lipid peroxidation.
1. Introduction
The health benefits of polyunsaturated fatty acids (PUFAs) over saturated fatty acids (SFAs) are well recognized [1]. Eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and alpha-linolenic acid (ALA) are the main essential omega-3 fatty acids for humans. Fish and seafood are preferred food groups with a significant source of omega-3 fatty acids, such as EPA and DHA, to meet human needs [2]. Fish, a source of omega-3 fatty acids, is inaccessible and unaffordable for most communities, and consumption is raising the issue of long-term sustainability [3,4]. In Ethiopia, the contribution of fish is less than 1% of PUFA intake [5], and omega-3 intake is below the recommended range for infants and young children and below the minimum recommended range for pregnant and lactating women [6]. Alternative land-based sources of omega-3 fatty acids might represent a solution to meet human needs.
As conventional eggs and meat are poor sources of omega-3 fatty acids [7,8], contain low ALA, and have limited content of EPA and DHA [9], dietary strategies to enrich meat and eggs have been tested by the inclusion of fish oil, flaxseed, canola, and rapeseed as source of n-3 fatty acids in the chicken diet [10,11,12]. Chicken products have a tremendous potential to become sustainable omega-3 functional foods [7,8,9,13]. Chickens can utilize dietary ALA as a precursor for EPA and DHA synthesis in the liver [14]. Flaxseed (Linum usitatissimum L.) is the richest source of ALA [15]. However, previous attempts to enrich eggs and meat using flaxseed as an n-3 fatty acid source reported challenges such as (1) decreased oxidative stability [16] and (2) poor conversion efficiency of ALA into LC n-3 PUFAs [17]. The inclusion of flaxseed in chicken diet exerted oxidative stress in chickens and negatively affected the oxidative stability of meat and eggs [18]. Lipid oxidation leads to the development of toxic compounds with detrimental biological effects associated with the occurrence of cancer, cardiovascular and neurodegenerative diseases [19,20].
Subsequent bioconversion of alpha-linolenic acid (ALA) in the liver into longer n-3 fatty acids was not found to be linear with the amount of flaxseed added to chicken diet [21]. Moreover, the conversion of ALA into EPA and DHA requires the involvement of various desaturase and elongase enzymes [22]. These enzymes are directly or indirectly responsive to dietary intervention [23], such as the inclusion of polyphenol antioxidants in chicken diet [24,25]. In chickens, supplementation of dietary polyphenol antioxidants showed a modulatory effect on fatty acid metabolism [26]. Following the ban of synthetic antioxidants in animal feed in several countries [27], attention is given to the potential effects of supplementing natural antioxidants on the fatty acid composition and oxidative stability in meat [22,28] and eggs [25,29]. Recently, an increase in the total omega-3 fatty acids and oxidative stability of breast muscle was reported in turkeys fed 5% dried apple, blackcurrant, strawberry, and seedless strawberry pomaces as polyphenol antioxidants in diets containing 2.5% linseed oil [30].
In Ethiopian traditional medicine, Dodonaea angustifolia has been used to treat lymphatic swelling and burns [31] and is widely used to treat oxidative stress [32]. Its high phenolic content and efficient in vitro antioxidant activity [33] suggested the use of D. angustifolia as a novel antioxidant agent to counteract oxidative stress. However, high doses of dietary antioxidants were reported to act as pro-oxidants that could affect lipid metabolism, although the condition and mechanisms are not well understood [34]. Although the potential role of polyphenol extract of D. angustifolia in lipid metabolism in poultry is not yet elucidated, studies previously showed that hydrolyzable polyphenols can limit lipid solubility and absorption of fat [35]. Moreover, gut-level uptake of fatty acids according to their molecular structure and a decrease in a desaturase index were reported following supplementation of tannin extract in chicken diet [36]. Furthermore, in flaxseed-enriched diet, including higher alpha-tocopherol contents [37,38] and polyphenolic antioxidants from dried tomato waste [39] was reported to suppress the absorption of n-3 fatty acids in egg yolk. The explanation provided for this was hypothetical, where higher-dose polyphenols could interfere with the deposition of certain LC n-3 PUFAs and might play a role as pro-oxidants.
Thus, the inclusion of natural antioxidants from plants rich in polyphenols at different doses in n-3 PUFA-enriched chicken diets needs to be carefully investigated to determine the optimal dose/inclusion rate that enhances n-3 PUFA content while decreasing lipid oxidation in breast meat and eggs. Therefore, this study aimed to evaluate the effect of the D. angustifolia polyphenol extract at doses of 0 mg, 200 mg, 500 mg, and 800 mg as a polyphenol antioxidant in a flaxseed-enriched diet on fatty acid content and oxidative stability in breast muscle and eggs of slow-growing Sasso chickens.
2. Materials and Methods
2.1. Chemicals and Materials
Chemicals: The following chemicals were purchased from different suppliers. Wash solution (CaCl2, 0.02%, Sigma-Aldrich® 82041, GmbH, Deisenhofen, Germany), dry reagent (20 g K2CO3, + 200 g Na2SO4, AppliChem D-6429, Darmstadt, GmbH, Germany), toluene (≥99.8%, Carl Roth + Co. KG, GmbH, Karlsruhe, Germany), sodium methylate (NaOCH3 (Cats No. 124-414, Merck KGaA Darmstadt, Germany), boron trifluoride methanol solution (Carl Roth + Co. KG, GmbH, Germany), n-hexane (≥98%, Carl Roth GmbH Co. KG. Germany), n-heptane (Carl Roth + Co. KG, GmbH, Germany), fatty acid methyl esters (FAMEs), Adrenic Acid (C22:4n-6), and QuantiChromTM TBARS Assay Kit (DTBA-100, BioAssay Systems, Hayward, USA).
Materials: The following materials were obtained from different suppliers. Atomic Absorption Spectrometer (Perkin Elmer, Aanalyst 300, Burladingen, Germany), Multi Reax Mixer (Heldolph, GmbH and Co. KG, Schwabach, Germany), filter paper (110 mm, Macherey-Angel GmbH and Co. KG, Düren, Germany), centrifuge (Sigma 3K30 Laboratory Centrifuge, GmbH, Nussloch, Germany), vacuum centrifuge (SCANVAC Vacuum Speed Concentrator, LaboGene™ Aps industrivej 6-8, DK3540, Lynege, Denmark), lab mill (Model A 11 Basic, IKA GmbH; Staufen, Germany), homogenizer (Precellys Evolution, Bertin Instruments Technologies, Montigny-le-Bretonneux, France), Pyrex tubes (Pyrex, Hayes, UK), CP-Sil 88 CB column (100 m × 0.25 mm, Agilent, Santa Clara, CA, USA), PerkinElmer gas chromatograph CLARUS 680 (PerkinElmer Instruments, Waltham, MA, USA), and 96-well plate reader (SynergyTM MX, BioTek, Bad Friedrichshall, Germany).
2.2. Experimental Design, Diets, and Chickens
Before conducting the experiment, all protocols for the handling, sampling, and management of chicken were approved by the Institutional Animal Care and Use Committee (IACUC) of the International Livestock Research Institute (ILRI), Addis Ababa, Ethiopia, with approval number IACUC2019-17-2. Using a complete randomized block design, a total of 48 Sasso T451A chickens aged 35 weeks were divided among four nutritional regimens with 3 chickens per pen (12 chickens per treatment, 4 replicates of 3 chickens). The number of replicates was computed using the variance and standard deviation from previous study findings for an increase in egg yolk n-3 polyunsaturated in hens fed flaxseed with no added plant extracts [40] while considering the potential increase in parameters due to the inclusion of D. angustifolia polyphenol extract under this study. Leaves of D. angustifolia were collected from the southeast of Debre Birhan town, Woinye area at 09°39′42.24″ N, 39°31′10.5″ E, from naturally growing populations in Ethiopia. The flaxseed was purchased from 02 Kebelle local market in Addis Ababa.
The Hendrix Genetics Sasso dual-purpose chicken management guide was used to formulate a feed based on soybeans and maize to satisfy the nutritional needs of Sasso chickens (FGS, 2018, pp. 7–11) [41]. During formulation, five kg of wheat bran as a carrier was mixed with D. angustifolia plant polyphenol extract, general premix, lysine, and methionine before mixing with the rest of the ingredients in a rotating drum mixer for precise dispersion. No nutrient contribution was considered from D. angustifolia levels in each dietary group.
Chickens were fed on control, DA0: 0 mg D. angustifolia polyphenol extract + 75 g flaxseed, DA2: 200 mg D. angustifolia polyphenol extract + 75 g flaxseed, DA5: 500 mg D. angustifolia polyphenol extract + 75 g flaxseed and DA8: 800 mg D. angustifolia polyphenol extract + 75 g flaxseed per kg diet for 8 weeks. The hens were fed 165 g of feed per day and provided water for ad libitum consumption. Chickens received the recommended vaccines as follows: at 7 d infectious bursal diseases, at 7, 28, and 50 d Newcastle disease, and at 40 d fowl pox. In addition, booster doses of the Newcastle disease vaccine were given every other three months and provided with 15 h of light during laying. The average temperature and relative humidity were 25 °C and 69% during the feeding trial period, respectively. The chemical composition, nutrient levels, and fatty acid composition of the Sasso chicken diet are presented in Table 1 and Table 2 below.

Table 1.
Ingredients, chemical composition, and nutrient levels of Sasso chicken diets.

Table 2.
Fatty acid composition of Sasso chicken diets.
2.3. Composition and Analysis of Experimental Diets
The ash content in the diets was determined according to the procedures outlined [42]. A total of 2 g of diet samples were added into pre-weighed crucibles and kept overnight in an oven at 105 °C for 16 h. Then, the weights of crucibles with samples and weight differences due to temperature deflection were recorded. Samples were kept in a furnace at 500 °C for 16 h. Then, the furnace was cooled to 200 °C, and the samples were taken out and further kept in the oven at 105 °C for 2 h. The ash content was determined by weight differences.
Dry matter and total nitrogen content were determined according to [43] Method 962.09. In short, diet samples (0.3 g each) were digested in 5 mL of concentrated sulfuric acid at 350 °C for 1 h. Then, the samples were cooled down and diluted with 30 mL of deionized water. Then, samples were distilled using 25 mL of 4% boric acid in a Kjeltec distiller, and for titration, 0.1 N HCL was used. The crude fiber was analyzed by subsequent acidic and alkaline hydrolysis according to the procedure described by Balthrop et al. [44]. The wet ashing method was used to determine the mineral content of the diet using atomic absorption spectrometry according to the procedure of [45] with slight modifications in sample digestion temperature. The fatty acid composition of the diets was determined using gas chromatography and flame ionization detection (GC-MS-FID), as outlined in Section 2.6.
2.4. Extract Preparation and Determination of Phenolic Content and Antioxidant Capacity
In brief, the methods used in the preparation of extracts and determination of phenolic content as well as the antioxidant activity using DPPH (2, 2-diphenyl-1-picrylhydrazyl) radical scavenging assay and ABTS (2, 2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid) assay of the D. angustifolia polyphenol extract are described, and the results are presented and discussed in detail in our recent publication [24].
2.5. Breast Muscle and Egg Sampling
Eggs of uniform size laid in the 8th week of the trial were randomly selected (2 eggs/pen, 8 per treatment) and stored at +4 °C until analysis. For breast sampling, two out of three hens per replicate (eight per treatment) at the end of the 8th week of the feeding trial were killed by cervical dislocation and bleeding [46]. The skin, feathers, and internal organs were removed manually. The breast muscle was separated by making an incision by following the white fat marks that separate from the ribs and individually kept in a food saver Ziplock bag for 12 h at 4 °C. Then, a 20 g sample was taken from different breast locations and homogenized under liquid nitrogen using a Multi Reax Mixer and kept in labeled Sarstedt tubes at −20 °C until analysis.
2.6. Fatty Acid Analysis
2.6.1. Lipid Extraction and Transesterification of Diets, Breast Muscle, and Egg Yolk
Chicken Diets
Fatty acid analysis of the diets was performed according to the procedure described by [47]. The feed samples were dried in an oven at 55 °C to a dry matter content of >90%. Then, the feed samples were finely ground to 1 mm, 2 g weighed in 10 mL screw-capped Pyrex tubes, and 2.0 mL of nonadecanoic acid (4.0 mg) was added as an internal standard. For fatty acid extraction, 3 mL of 5% methanolic HCl was added and vortexed in tightly closed Pyrex tubes for 2 h at 60 °C in a water bath. After cooling, the sample solution was treated with 10 mL 6% K2CO3 solution and vortexed. Next, the solutions were centrifuged at 4 °C, 1200× g for 5 min (ScanSpeed 40, LaboGene, Allerød, Denmark), and finally, the fatty acid methyl esters (FAMEs) were extracted two times with 2 mL of n-hexane each. After being dried with 1 g Na2SO4 and cleaned with activated charcoal of the organic phase as required, the extracts were filtrated and evaporated using a vacuum centrifuge at 438× g, 30 °C, 30 min (ScanSpeed 40, LaboGene, Allerød, Denmark). Finally, the extracts were stored at −18 °C until GC analysis [48].
Chicken Breast Muscle and Egg Yolk
The sample preparation of chicken breast muscle was described in detail [36]. Briefly, after adding 3 mL methanol and nonadeconoic acid (19:0) as an internal standard to muscle samples, the mixtures (in duplicate) were homogenized 3 times at 25 sec intervals at 4 °C and 6500 rpm using the Precellys Evolution homogenizer. The homogenates were vortexed and transferred to Pyrex tubes containing 8 mL of chloroform. The organic phase (lipid extracts) was separated, and the solvent was subsequently removed. To transesterify the lipid extracts, 2 mL of 0.5 M sodium methoxide in methanol was added and agitated in a 60 °C water bath for 10 min. Subsequently, 1 mL of 14% boron trifluoride in methanol was added to the mixture, which was then shaken for an additional 10 min at 60 °C. The fatty acid methyl esters (FAMEs) were extracted twice with 2 mL of n-hexane and kept at −18 °C until high-resolution gas chromatography (HR-GC) analysis.
In eggs, the yolk was carefully rolled on filter paper to remove any albumen residues, ensuring complete separation of both egg components. The egg yolks were stored at −20 °C until analyses. After homogenization of frozen egg samples and the addition of C19:0 as an internal standard, total egg lipids were extracted in duplicate using chloroform/methanol (2:1, v/v) and an Ultra Turrax T25 (IKA, Staufen, Germany) 3 × 15 s at 15,777× g and room temperature. The detailed sample preparation procedure has been described by Stehr et al. [49]. Briefly, the final extraction mixtures were stored at 5 °C for 18 h in the dark and subsequently washed with 0.02% (w/v) CaCl2 solution. After centrifugation (530× g, 5 min), the organic phase was dried with Na2SO4 and K2CO3 (10:1, w/w), and the solvent was subsequently removed. The lipid extracts were dissolved in 150 μL of toluene for methyl ester preparation. Next, 1 mL of 0.5 M sodium methoxide in methanol was added to the samples, which were shaken in a 60 °C water bath for 10 min. Subsequently, 0.5 mL of 14% boron trifluoride (BF3) in methanol was added to the mixture, which was then shaken for an additional 10 min at 60 °C. The fatty acid methyl esters (FAMEs) were extracted three times in 2 mL of n-hexane. The FAMEs were stored at −18 °C until used for gas chromatography (GC) analysis.
2.6.2. Gas Chromatography Analysis
Separation and quantification of the fatty acid methyl esters in all extracts were conducted using a fused silica capillary column on a PerkinElmer Clarus 680 gas chromatograph equipped with an autosampler and flame ionization detector [48]. The GC oven temperature program was 150 °C for 5 min; heating rate of 2°/min until 200 °C and kept for 10 min; heating rate of 1°/min until 225 °C and kept for 20 min. As carrier gas, hydrogen was used at a flow rate of 1 mL min−1, the split ratio was 1:20, and the injector and detector were set at 260 and 280 °C, respectively. For the calibration of the reference standard mixture “Sigma FAME”, the methyl ester of C18:1cis-11, C22:5n-3, C18:2cis-9, trans-11, C22:4n-6, and C18:4n-3 were used. After GC analysis of five samples, the five-point calibration of single fatty acids ranged from 16 to 415 g/mL. The fatty acid concentration was expressed as mg/100 g of tissue.
2.7. Determination of Lipid Peroxidation in Egg Yolk and Breast Meat
The analysis of oxidative stability in egg yolk samples was performed as recently described for chicken muscle [24]. Briefly, egg yolk samples were homogenized, and 400 mg of yolk sample was weighed in a tube. Each 7 mL Precellys tube contained 5 pieces of 2.8 mm bulk beads and 1 piece of 5 mm bulk beads. The QuantiChrom TBARS Assay kit’s instructions were followed for sample preparation and measuring the oxidative stability of egg yolk samples. The extracts (in triplicate) were homogenized 2 times at 10 s intervals at 4 °C and 6500 rpm using the Precellys Evolution homogenizer. After the addition of 10% trichloroacetic acid (TCA) solution and incubation for 5 min on ice, the sample extracts were centrifuged, and 200 μL of thiobarbituric acid (TBA) was added to 200 μL sample extract and vortexed. Then, the sample extracts were incubated for 60 min at 100 °C.
For the analysis of lipid peroxidation of muscle samples, approximately 400 mg of muscle sample was weighed in a Precellys tube. The details of sample preparation for oxidative stability measurements in chicken muscle were described by Tadesse et al. [24]. After egg and/or muscle sample preparation, 100 µL of the extracts was transferred into the 96-well plate of the plate reader. Subsequently, the color intensity (OD) was measured at 535 nm. After that, malondialdehyde (MDA) standards were prepared in a concentration range from 0.0 to 1.5 μM MDA, and the color intensity was measured using the same procedure as for sample solutions. The MDA calibration procedure for egg yolk and breast muscle was performed according to the QuantiChrom TBARS Assay kit’s instructions using Gen5 software (version 3.10, 2020, SynergyTM MX, BioTek, Bad Friedrichshall, Germany). The calibration was newly conducted on each analyzing day; one example is in Supplementary Figure S1. Concentrations of reactive thiobarbituric acid substances (TBARS) were calculated using the MDA standard calibration. Finally, the TBARS concentrations were expressed in μg MDA/g of egg yolk or breast muscle.
2.8. Statistical Data Analysis
All data analysis tasks were completed in RStudio version 2021.9.0.351 [50] using the R project for statistical computing version 4.1.1 [51]. The response variables were characterized by visual inspection using the qqplot and boxplot functions. Data were further tested for satisfying the assumptions of normal distribution and equality of variances using the Shapiro test and Levene test functions, respectively. Then, the aov() function model aov_model<-aov (fatty acid concentration/oxidative stability~polyphenol extract levels, data=mydata) was used to summarize the analysis of variance for parameters such as fatty acid content and oxidative stability in egg yolk and breast muscle. Multiple comparisons of means were tested using the TukeyHSD function to determine the specific dose of D. angustifolia supplemented, showing significance.
3. Results
3.1. Fatty Acid Concentrations in Breast Muscle
The effects of D. angustifolia extract levels on feed intake and body and egg weight performances are provided in Table S1. Table 3 reports the fat content (%) and fatty acid concentrations (mg/100 g) in breast muscle. The fat content and saturated fatty acids (SFAs), such as myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0), concentrations in breast muscle were not affected (p > 0.05) by levels of D. angustifolia polyphenol extract in flaxseed-enriched diets. Hens fed doses of D. angustifolia polyphenol extract along with grounded flaxseed had no significant effect (p > 0.05) on alpha-linolenic acid (ALA, C18:3n-3) and linoleic acid (LA, C18:2n-6) concentrations in the breast muscle. Moreover, feeding hens with D. angustifolia polyphenol extract doses in DA2 and DA5 increased (p < 0.05) the breast muscle n-3 PUFA content compared to the DA0 diet. Moreover, an increase in the dose of D. angustifolia polyphenol extract in hens’ diet resulted in a slight dose-dependent decreasing trend on breast muscle n-6 PUFA content, but without significant (p > 0.05) differences.

Table 3.
Effects of feeding D. angustifolia polyphenol extract levels in diets containing grounded flaxseed on fat content and fatty acid concentrations in breast muscle of Sasso chickens.
Interestingly, the docosahexaenoic acid (DHA) content in the breast muscle increased (p < 0.05) by 40% in DA2 and 37% in DA5 compared to hens fed the DA0 diet. However, the observed increase in breast muscle DHA content was not consistent when D. angustifolia extract was increased in the DA8 diet. Among the n-3 PUFAs, the breast muscle content of eicosapentaenoic acid increased by 52% in DA8 (p < 0.05), followed by 19% in DA5 and 25% in DA2 (p > 0.05) compared to hens fed the DA0 diet.
3.2. Fatty Acid Concentration in Egg Yolks
The fat content (%) and fatty acid concentrations (mg/g) in the egg yolk are provided in Table 4. In the current study, feeding doses of D. angustifolia polyphenol extract in flaxseed-enriched diets had no significant effect on the egg yolk fat percentage. The egg yolk saturated fatty acids (SFAs) and the palmitic acid (C16:0) contents decreased (p < 0.05) in DA5 compared to hens fed the DA0 diet.

Table 4.
Effects of feeding different doses of D. angustifolia polyphenol extract in diets containing grounded flaxseed on yolk fat content and fatty acid concentrations in egg yolks.
In the present study, dietary D. angustifolia polyphenol extract doses had no significant effect (p > 0.05) on egg yolk alpha-linolenic acid (ALA), eicosapentaenoic acid (EPA), and DHA contents. However, feeding doses of D. angustifolia polyphenol extract (DA2, DA5, and DA8) caused a slight decrease (p > 0.05) in egg yolk docosapentaenoic acid (ALA) content compared to hens fed the DA0 diet. Moreover, increasing the level of D. angustifolia polyphenol extract to 800 mg/kg in DA8 slightly decreased EPA, DPA, and DHA contents in egg yolk compared to hens fed DA0, but without statistical differences.
3.3. Oxidative Stability in Breast Muscle and Egg Yolks
The results of malondialdehyde (MDA) content in breast muscle and egg yolks as a measure of lipid peroxidation are presented in Figure 1. In this study, feeding hens with DA5 reduced the breast MDA value by 54% (p < 0.05) and 11% in DA2 (p > 0.05) compared to the DA0 diet. However, increasing the dose of D. angustifolia polyphenol extract in DA8 did not influence the content of MDA breast muscle. No clear dose-dependent antioxidant/pro-oxidant effect of D. angustifolia polyphenol extract levels was observed, with 500 mg in DA5 seeming to be the most efficient in decreasing lipid peroxidation in breast muscle. The current findings showed no effect (p > 0.05) of feeding different D. angustifolia polyphenol extract doses on egg yolk content of malondialdehyde (MDA) between treatments.
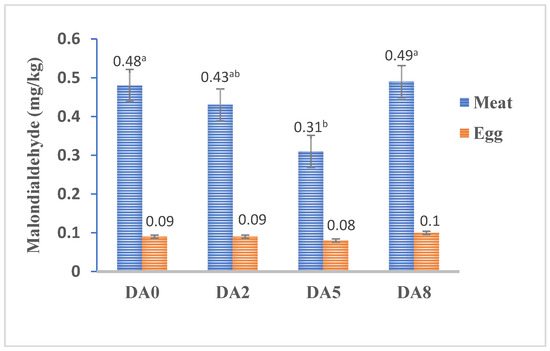
Figure 1.
Effects of feeding D. angustifolia polyphenol extract levels on malondialdehyde (MDA) content in breast muscle and egg yolks. Values on the bar with different ab superscript letters differ significantly at p < 0.05.
4. Discussion
Most studies reported the association between saturated fatty acid intake and increased risks of cardiovascular diseases [52]. In the current study, the concentrations of myristic acid (C14:0), palmitic acid (C16:0), and stearic acid (C18:0) in breast muscle from hens fed D. angustifolia extract levels in flaxseed-enriched diets were lower compared to the report by [22] in chickens fed 100 g of flaxseed with 10 g of turmeric rhizome powder. We observed no significant effect of D. angustifolia levels on alpha-linolenic acid (ALA) and linoleic acid (LA) content; however, Kumar et al. [22] found a significant increase in ALA and a decrease in LA in breast muscle of broiler chickens fed doses of turmeric powder and flaxseed.
In line with the present results, other studies reported a significant increase in breast muscle content of n-3 PUFAs (eicosapentaenoic acid and docosahexaenoic acid) in chickens fed with 10 g turmeric powder in diets containing flaxseed [22] and 6% extruded flaxseed along with 30, 40, or 50 g hemp seed meal/kg diet [53] as antioxidants in a flaxseed-enriched diet. Moreover, n-3 PUFA content in breast muscle agreed with the report by Tadesse et al. [24] in a similar dietary pattern with the inclusion of 7.5% flaxseed with 800 mg Curcuma domestica and Thymus schimperi extracts. Similar to the current findings, previous studies reported a decrease in meat n-6 PUFA content in chickens fed 10 g turmeric rhizome powder, as in Kumar et al. [22] and 2–4% grape pomace in Vlaicu et al. [25] in flaxseed-enriched diets.
The lower saturated fatty acids (SFAs) in eggs make them healthier, as the high content of myristic acid (C14:0) and 16:0 are recognized as health risk factors [54]. Consistent with the present finding, feeding hens with 6% flaxseed and 3% grape seed meal reduced the egg yolk SFA and C16:0 contents [28]. In the current study, the observed no effect among levels of D. angustifolia extract on egg yolk content of omega-3 fatty acids is in contrast to the expected. Related to this, in our previous study, feeding plant polyphenol extracts with similar phenolic content with D. angustifolia, such as T. schimperi and C. domestica, did not also affect the omega-3 fatty acid in egg yolks [24]. This may suggest the need for further study on different levels of polyphenols used in poultry diets enriched with n-3 PUFAs. Moreover, few studies previously reported possible suppressive effects on n-3 PUFA synthesis and deposition in hens fed with higher doses of alpha-tocopherol [37,38] and dried tomato waste as polyphenolic compounds [39]. However, the specific dose of polyphenols and the degree of their suppressive effects have not been well discussed in the literature.
Eggs from hens fed on conventional diets have a high content of n-6 PUFA [55]. According to the current findings, increasing the dose of D. angustifolia polyphenol extract in the hen diet caused a dose-dependent reduction in the yolk LA (C18:2n-6) and n-6 PUFA contents, with a significant reduction (p < 0.05) in hens fed DA8 compared to the DA0 diet. In agreement with the present study, the simultaneous inclusion of natural antioxidants such as different carotenoids and grape seed meal and sea buckthorn [25] in flaxseed-enriched diets was found to decrease the contents of LA and total n-6 PUFA in egg yolks.
Relatively higher malondialdehyde (MDA) levels were found in breast meat compared to egg yolks. This might be associated with the hen’s capacity to deposit dietary antioxidants preferentially in eggs, not in the body [56]. In addition, the chicken tissue, particularly breast meat, is deficient in vitamin E compared to egg yolk [53] and hence is prone to lipid peroxidative damage. Thus, oxidative stability problems could limit the reported benefits of n-3 PUFA-enriched diets if an adequate antioxidant is not included [34].
Higher doses of dietary polyphenols could act as pro-oxidants [34]. In line with the present results, there was a decrease in breast muscle MDA value in chickens fed doses of turmeric rhizome powder [22] and dried fruit pomaces [30]. However, the observed egg yolk MDA values in the current study (0.09–1.00 mg/kg yolk) are lower compared to the previous report (1.08–1.89 mg MDA/kg yolk) in hens fed 5% flaxseed and 5% dried tomato waste diets [57]. Studies found that feeding flaxseed increases the oxidative susceptibility of yolk lipids [58], while the inclusion of natural antioxidants in chicken diet showed protective effects against egg yolk lipid peroxidation [59]. In line with the present study, our recent study found no significant effect on egg yolk MDA content in hens fed on 800 mg of D. angustifolia polyphenol extract [24].
5. Conclusions
Feeding hens with different doses of Dodonaea angustifolia (D. angustifolia) polyphenol extract in flaxseed-enriched diets significantly increased the content of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), decreased lipid oxidation in breast muscle, and reduced the SFA and n-6 polyunsaturated fatty acids (n-6 PUFAs) in egg yolk. The inclusion of 500 mg of D. angustifolia polyphenol extract into flaxseed-enriched diets seems to be the optimal level to enhance the long-chain PUFA content and improve the oxidative stability in breast muscle. However, there are insufficient supporting data to suggest the most efficient dose of D. angustifolia polyphenol extract in regard to enhancing the n-3 PUFA content and decreasing lipid oxidation in eggs. Thus, inclusion of D. angustifolia polyphenol extract beyond the 800 mg/kg diet is needed to further explore its potential to improve the omega-3 fatty acid content and oxidative stability in meat and eggs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/agriculture14070993/s1, Table S1. Effect of feeding D. angustifolia polyphenol and flaxseed on body weight and egg weight performance of Sasso chickens. Figure S1. Calibration curve example for analysis of lipid peroxidation in egg yolk and breast muscle.
Author Contributions
Conceptualization: D.T., P.G. and N.R., Methodology: D.T., P.G. and D.D., Formal analysis, Data curation, Software, and Visualization: D.T. and N.N., Validation: D.D., Investigation: D.T., H.W.W. and W.E., Laboratory analysis: D.T., D.D. and S.M., Supervision: P.G., O.H. and N.R., Funding acquisition, Resources, and Project administration: O.H. and S.M. The first draft of the manuscript was written by D.T. and all authors commented on previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in part by the Bill & Melinda Gates Foundation (BMGF) and with U.K. aid from the U.K. Foreign, Commonwealth and Development Office (Grant Agreement OPP1127286) and was carried out under the auspices of the Centre for Tropical Livestock Genetics and Health (CTLGH), established jointly by the University of Edinburgh, SRUC (Scotland’s Rural College) and the International Livestock Research Institute. The findings and conclusions contained within are those of the authors and do not necessarily reflect the positions or policies of the BMGF or the U.K. Government. This research was conducted as part of the Consultative Group on International Agricultural Research (CGIAR) Research Program on Livestock and is supported by contributors to the CGIAR Trust Fund.
Institutional Review Board Statement
This animal study protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the International Livestock Research Institute (ILRI) Review Board with approval number IACUC2019-17-2.
Data Availability Statement
All data included in this study are available by contacting the corresponding author.
Acknowledgments
The authors thank Maria Dahm and Birgit Jentz for their fundamental support during lipid extraction and fatty acid analysis and lipid peroxidation measurements. The authors thank Jane Poole for her expert statistical analyses. A special acknowledgment goes to the ILRI Poultry Research Facility Team for supporting the feed trials and sample collections.
Conflicts of Interest
The authors declare that there is no conflicts of interest.
References
- Din, J.N.; Newby, D.E.; Flapan, A.D. Omega 3 Fatty Acids and Cardiovascular Disease—Fishing for a Natural Treatment. BMJ 2004, 328, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Bhalerao, S.; Hegde, M.; Katyare, S.; Kadam, S. Promotion of Omega-3 Chicken Meat Production: An Indian Perspective. Worlds Poult. Sci. J. 2014, 70, 365–374. [Google Scholar] [CrossRef]
- Irvine, K.; Etiegni, C.A.; Weyl, O.L.F. Prognosis for Long-term Sustainable Fisheries in the African Great Lakes. Fish Manag. Ecol. 2019, 26, 413–425. [Google Scholar] [CrossRef]
- Belton, B.; Little, D.C.; Zhang, W.; Edwards, P.; Skladany, M.; Thilsted, S.H. Farming Fish in the Sea Will Not Nourish the World. Nat. Commun. 2020, 11, 5804. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, K.F.; Dewey, K.G.; Perez-Exposito, A.B.; Nurhasan, M.; Lauritzen, L.; Roos, N. Food Sources and Intake of N-6 and N-3 Fatty Acids in Low-income Countries with Emphasis on Infants, Young Children (6–24 Months), and Pregnant and Lactating Women. Matern. Child Nutr. 2011, 7, 124–140. [Google Scholar] [CrossRef]
- Bhutta, Z.A.; Das, J.K.; Rizvi, A.; Gaffey, M.F.; Walker, N.; Horton, S.; Webb, P.; Lartey, A.; Black, R.E. Evidence-Based Interventions for Improvement of Maternal and Child Nutrition: What Can Be Done and at What Cost? Lancet 2013, 382, 452–477. [Google Scholar] [CrossRef]
- Rondelli, S.G.; Martinez, O.; Garcia, P.T. Effects of Different Dietary Lipids on the Fatty Acid Composition of Broiler Abdominal Fat. Braz. J. Poult. Sci. 2004, 6, 171–175. [Google Scholar] [CrossRef][Green Version]
- Imran, M.; Anjum, F.M.; Nadeem, M.; Ahmad, N.; Khan, M.K.; Mushtaq, Z.; Hussain, S. Production of Bio-Omega-3 Eggs through the Supplementation of Extruded Flaxseed Meal in Hen Diet. Lipids Health Dis. 2015, 14, 126. [Google Scholar] [CrossRef]
- Kralik, G.; Grčević, M.; Hanžek, D.; Margeta, P.; Galović, O.; Kralik, Z. Feeding to Produce N-3 Fatty Acid-Enriched Table Eggs. J. Poult. Sci. 2020, 57, 138–147. [Google Scholar] [CrossRef]
- Jia, W.; Rogiewicz, A.; Bruce, H.L.; Slominski, B.A. Feeding Flaxseed Enhances Deposition of Omega-3 Fatty Acids in Broiler Meat Portions in Different Manner. Can. J. Anim. Sci. 2010, 90, 203–206. [Google Scholar] [CrossRef]
- Rahimi, S.; Azad, S.K.; Torshizi, M.A.K. Omega-3 Enrichment of Broiler Meat by Using Two Oil Seeds. J. Agric. Sci. Technol. 2011, 13, 353–365. [Google Scholar]
- Beheshti Moghadam, M.H.; Cherian, G. Use of Flaxseed in Poultry Feeds to Meet the Human Need for N-3 Fatty Acids. Worlds Poult. Sci. J. 2017, 73, 803–812. [Google Scholar] [CrossRef]
- Hasler, C.M. Functional Foods: Their Role in Disease Prevention and Health Promotion. Food Technol. Chic. 1998, 52, 63–147. [Google Scholar]
- Bhalerao, S.; Hegde, M.; Ranade, A.S.; Avari, P. Studies in the Production of Omega Enriched Chicken Meat: II. Indian J. Poult. Sci. 2010, 45, 273–279. [Google Scholar]
- Goyal, A.; Sharma, V.; Upadhyay, N.; Gill, S.; Sihag, M. Flax and Flaxseed Oil: An Ancient Medicine & Modern Functional Food. J. Food Sci. Technol. 2014, 51, 1633–1653. [Google Scholar]
- Mir, N.A.; Tyagi; Biswas, A.K.; Tyagi, P.K.; Mandal, A.B.; Sheikh, S.A.; Deo, C.; Sharma, D.; Verma, A.K. Impact of Feeding Chromium Supplemented Flaxseed Based Diet on Fatty Acid Profile, Oxidative Stability and Other Functional Properties of Broiler Chicken Meat. J. Food Sci. Technol. 2017, 54, 3899–3907. [Google Scholar] [CrossRef]
- Omri, B.; Chalghoumi, R.; Izzo, L.; Ritieni, A.; Lucarini, M.; Durazzo, A.; Abdouli, H.; Santini, A. Effect of Dietary Incorporation of Linseed Alone or Together with Tomato-Red Pepper Mix on Laying Hens’ Egg Yolk Fatty Acids Profile and Health Lipid Indexes. Nutrients 2019, 11, 813. [Google Scholar] [CrossRef]
- Cortinas, L.; Barroeta, A.; Villaverde, C.; Galobart, J.; Guardiola, F.; Baucells, M.D. Influence of the Dietary Polyunsaturation Level on Chicken Meat Quality: Lipid Oxidation. Poult. Sci. 2005, 84, 48–55. [Google Scholar] [CrossRef]
- Cohn, J.S. Oxidized Fat in the Diet, Postprandial Lipaemia and Cardiovascular Disease. Curr. Opin. Lipidol. 2002, 13, 19–24. [Google Scholar] [CrossRef]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Caston, L.; Leeson, S. Research Note: Dietary Flax and Egg Composition. Poult. Sci. 1990, 69, 1617–1620. [Google Scholar] [CrossRef]
- Kumar, F.; Tyagi, P.K.P.K.; Mir, N.A.; Dev, K.; Begum, J.; Biswas, A.A.K.; Sheikh, S.A.; Tyagi, P.K.P.K.; Sharma, D.; Sahu, B.; et al. Dietary Flaxseed and Turmeric Is a Novel Strategy to Enrich Chicken Meat with Long Chain ω-3 Polyunsaturated Fatty Acids with Better Oxidative Stability and Functional Properties. Food. Chem. 2020, 305, 125458. [Google Scholar] [CrossRef]
- Dev, K.; Begum, J.; Biswas, A.; Mir, N.A.; Singh, J.; Prakash, R.; Sonowal, J.; Bharali, K.; Tomar, S.; Kant, R.; et al. Hepatic Transcriptome Analysis Reveals Altered Lipid Metabolism and Consequent Health Indices in Chicken Supplemented with Dietary Bifidobacterium Bifidum and Mannan-Oligosaccharides. Sci. Rep. 2021, 11, 17895. [Google Scholar] [CrossRef]
- Tadesse, D.; Retta, N.; Girma, M.; Ndiwa, N.; Dessie, T.; Hanotte, O.; Getachew, P.; Dannenberger, D.; Maak, S. In Vitro Antioxidant Activities of Plant Polyphenol Extracts and Their Combined Effect with Flaxseed on Raw and Cooked Breast Muscle Fatty Acid Content, Lipid Health Indices and Oxidative Stability in Slow-Growing Sasso Chickens. Foods 2023, 12, 115. [Google Scholar] [CrossRef]
- Vlaicu, P.A.; Panaite, T.D.; Turcu, R.P. Enriching Laying Hens Eggs by Feeding Diets with Different Fatty Acid Composition and Antioxidants. Sci. Rep. 2021, 11, 20707. [Google Scholar] [CrossRef]
- Tan, Z.; Halter, B.; Liu, D.; Gilbert, E.R.; Cline, M.A. Dietary Flavonoids as Modulators of Lipid Metabolism in Poultry. Front. Physiol. 2022, 13, 543. [Google Scholar] [CrossRef]
- Othón-Díaz, E.D.; Fimbres-García, J.O.; Flores-Sauceda, M.; Silva-Espinoza, B.A.; López-Martínez, L.X.; Bernal-Mercado, A.T.; Ayala-Zavala, J.F. Antioxidants in Oak (Quercus sp.): Potential Application to Reduce Oxidative Rancidity in Foods. Antioxidants 2023, 12, 861. [Google Scholar] [CrossRef]
- Turcu, R.P.; Panaite, T.D.; Untea, A.E.; Vlaicu, P.A.; Badea, I.A.; Mironeasa, S. Effects of Grape Seed Oil Supplementation to Broilers Diets on Growth Performance, Meat Fatty Acids, Health Lipid Indices and Lipid Oxidation Parameters. Agriculture 2021, 11, 404. [Google Scholar] [CrossRef]
- Varzaru, I.; Untea, A.; Panaite, T.D.; Panaite, T.; Olteanu, M. Effect of Dietary Phytochemicals from Tomato Peels and Rosehip Meal on the Lipid Peroxidation of Eggs from Laying Hens. Arch. Anim. Nutr. 2020, 75, 18–30. [Google Scholar] [CrossRef]
- Juskiewicz, J.; Jankowski, J.; Zielinski, H.; Zdunczyk, Z.; Mikulski, D.; Antoszkiewicz, Z.; Kosmala, M.; Zdunczyk, P. The Fatty Acid Profile and Oxidative Stability of Meat from Turkeys Fed Diets Enriched with N-3 Polyunsaturated Fatty Acids and Dried Fruit Pomaces as a Source of Polyphenols. PLoS ONE 2017, 12, e0170074. [Google Scholar] [CrossRef]
- Mesfin, F.; Demissew, S.; Teklehaymanot, T. An Ethnobotanical Study of Medicinal Plants in Wonago Woreda, SNNPR, Ethiopia. J. Ethnobiol. Ethnomed. 2009, 5, 28. [Google Scholar] [CrossRef]
- Samavati, V.; Manoochehrizade, A. Dodonaea Viscosa Var. Angustifolia Leaf: New Source of Polysaccharide and Its Anti-Oxidant Activity. Carbohydr. Polym. 2013, 98, 199–207. [Google Scholar] [CrossRef]
- Tauchen, J.; Doskocil, I.; Caffi, C.; Lulekal, E.; Marsik, P.; Havlik, J.; Van Damme, P.; Kokoska, L. In Vitro Antioxidant and Anti-Proliferative Activity of Ethiopian Medicinal Plant Extracts. Ind. Crop. Prod. 2015, 74, 671–679. [Google Scholar] [CrossRef]
- Surai, P.F. Polyphenol Compounds in the Chicken/Animal Diet: From the Past to the Future. J. Anim. Physiol. Anim. Nutr. 2014, 98, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Sun, Q.; del Rincon, S.V.; Lovato, A.; Marques, M.; Witcher, M. Gallotannin Imposes S Phase Arrest in Breast Cancer Cells and Suppresses the Growth of Triple-Negative Tumors In Vivo. PLoS ONE 2014, 9, e92853. [Google Scholar] [CrossRef]
- Minieri, S.; Buccioni, A.; Serra, A.; Galigani, I.; Pezzati, A.; Rapaccini, S.; Antongiovanni, M. Nutritional Characteristics and Quality of Eggs from Laying Hens Fed on a Diet Supplemented with Chestnut Tannin Extract (Castanea sativa Miller). Br. Poult. Sci. 2016, 57, 824–832. [Google Scholar] [CrossRef]
- Galobart, J.; Barroeta, A.C.; Baucells, M.D.; Cortinas, L.; Guardiola, F. α-Tocopherol Transfer Efficiency and Lipid Oxidation in Fresh and Spray-Dried Eggs Enriched with Ω3-Polyunsaturated Fatty Acids. Poult. Sci. 2001, 80, 1496–1505. [Google Scholar] [CrossRef]
- Meluzzi, A.; Sirri, F.; Manfreda, G.; Tallarico, N.; Franchini, A. Effects of Dietary Vitamin E on the Quality of Table Eggs Enriched with N-3 Long-Chain Fatty Acids. Poult. Sci. 2000, 79, 539–545. [Google Scholar] [CrossRef]
- Panaite, T.D.; Nour, V.; Vlaicu, P.A.; Corbu, A.R.; Saracila, M. Flaxseed and Dried Tomato Waste Used Together in Laying Hens Diet. Arch. Anim. Nutr. 2019, 73, 222–238. [Google Scholar] [CrossRef]
- Al-Nasser, A.Y.; Al-Nasser, A.; Al-Saffar, A.E.; Abdullah, F.K.; Al-Bahouh, M.E.; Ragheb, G.; Ragheb, G.; Mashaly, M.M. Effect of Adding Flaxseed in the Diet of Laying Hens on Both Production of Omega-3 Enriched Eggs and on Production Performance. Int. J. Poult. Sci. 2011, 10, 825–831. [Google Scholar] [CrossRef]
- FGS: Farmer’s Guide to Sasso Dual Purpose Chicken. Hendrix Poult. Genet. 2018, 1–8.
- Thiex, N.; Novotny, L.; Crawford, A. Determination of Ash in Animal Feed: AOAC Official Method 942.05 Revisited. J. AOAC Int. 2012, 95, 1392–1397. [Google Scholar] [CrossRef]
- AOAC Official Methods of Analysis, 15th ed.; Methods 962.09, 932.06, 925.09, 985.29, 923.03; AOAC: Arlington, VA, USA, 1990.
- Balthrop, J.; Brand, B.; Cowie, R.A.; Danier, J.; de Boever, J.L.; de Jonge, L.H.; Jackson, F.; Makkar, H.P.S.; Piotrowski, C. Quality Assurance for Animal Feed Analysis Laboratories; FAO: Rome, Italy, 2011; ISBN 9251070504. [Google Scholar]
- Plank, C.O. Plant Analysis Reference Procedures for the Southern Region of the United States. South. Coop. Ser. Bull. 1992, 368, 4–20. [Google Scholar]
- Sparrey, J.; Sandercock, D.A.A.; Sparks, N.H.C.H.C.; Sandilnds, V. Current and Novel Methods for Killing Poultry Individually On-Farm. Worlds Poult. Sci. J. 2014, 70, 737–758. [Google Scholar] [CrossRef]
- Palmquist, D.L.; Jenkins, T.C. Challenges with Fats and Fatty Acid Methods. J. Anim. Sci. 2003, 81, 3250–3254. [Google Scholar] [CrossRef]
- Kalbe, C.; Priepke, A.; Nürnberg, G.; Dannenberger, D. Effects of Long-Term Microalgae Supplementation on Muscle Microstructure, Meat Quality and Fatty Acid Composition in Growing Pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 574–582. [Google Scholar] [CrossRef]
- Stehr, M.; Grashorn, M.; Dannenberger, D.; Tuchscherer, A.; Gauly, M.; Metges, C.C.; Daş, G. Resistance and Tolerance to Mixed Nematode Infections in Relation to Performance Level in Laying Hens. Vet. Parasitol. 2019, 275, 108925. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development Environment for R; RStudio, PBC: Boston, MA, USA, 2021. [Google Scholar]
- R Core Team, R. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 19 March 2021).
- Perna, M.; Hewlings, S. Saturated Fatty Acid Chain Length and Risk of Cardiovascular Disease: A Systematic Review. Nutrients 2022, 15, 30. [Google Scholar] [CrossRef]
- Skřivan, M.; Englmaierová, M.; Taubner, T.; Skřivanová, E. Effects of Dietary Hemp Seed and Flaxseed on Growth Performance, Meat Fatty Acid Compositions, Liver Tocopherol Concentration and Bone Strength of Cockerels. Animals 2020, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Arnould, V.M.-R.; Soyeurt, H. Genetic Variability of Milk Fatty Acids. J. Appl. Genet. 2009, 50, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Sparks, N.H.C. The Hen’s Egg—Is Its Role in Human Nutrition Changing? Worlds Poult. Sci. J. 2006, 62, 308–315. [Google Scholar] [CrossRef]
- Loetscher, Y.; Kreuzer, M.; Messikommer, R.E. Late Laying Hens Deposit Dietary Antioxidants Preferentially in the Egg and Not in the Body. J. Appl. Poult. Res. 2014, 23, 647–660. [Google Scholar] [CrossRef]
- Panaite, T.D.; Nour, V.; Saracila, M.; Turcu, R.P.; Untea, A.E.; Vlaicu, P.A. Effects of Linseed Meal and Carotenoids from Different Sources on Egg Characteristics, Yolk Fatty Acid and Carotenoid Profile and Lipid Peroxidation. Foods 2021, 10, 1246. [Google Scholar] [CrossRef]
- Bou, R.; Codony, R.; Tres, A.; Decker, E.A.; Guardiola, F. Dietary Strategies to Improve Nutritional Value, Oxidative Stability, and Sensory Properties of Poultry Products. Crit. Rev. Food Sci. Nutr. 2009, 49, 800–822. [Google Scholar] [CrossRef]
- Miceli, N.; Cavò, E.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Cincotta, F.; Condurso, C.; Taviano, M.F. Phytochemical Characterization and Biological Activities of a Hydroalcoholic Extract Obtained from the Aerial Parts of Matthiola incana (L.) R. Br. Subsp. Incana (Brassicaceae) Growing Wild in Sicily (Italy). Chem. Biodivers 2019, 16, e1800677. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).