An Investigation and Invasiveness Analysis of Two Species of Giant African Snail in a Coastal City of Southern China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Survey Sample Area
2.2. Test Snail
2.3. Phylogenetic Analysis
2.4. Food Intake and Preference
2.5. Cold Adaptation Treatment
2.6. Drought Resistance Treatment
3. Results
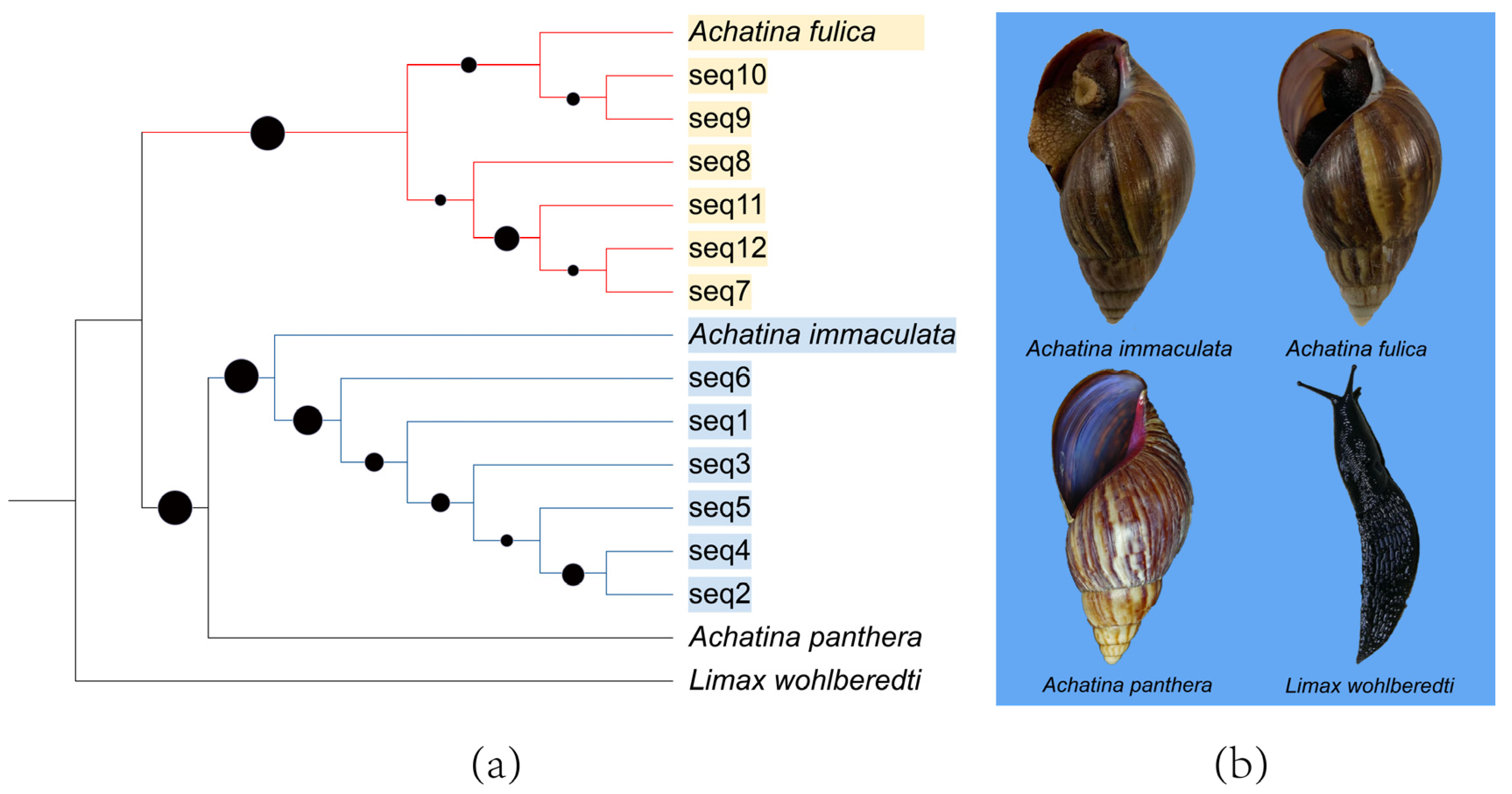
3.1. Phylogenetic Analyses
3.2. Distribution and Population Survey
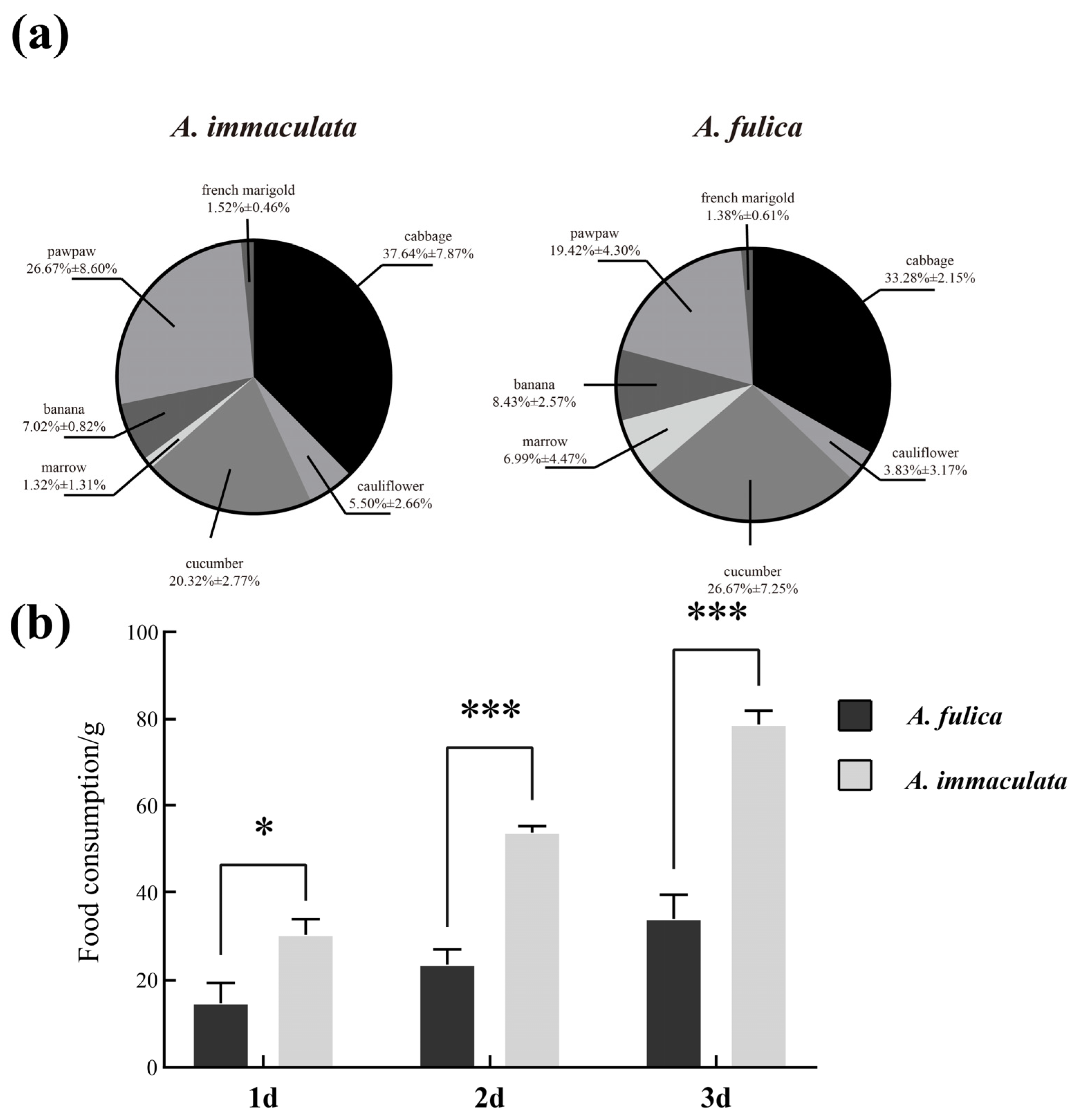
3.3. Food Intake and Dietary Preferences
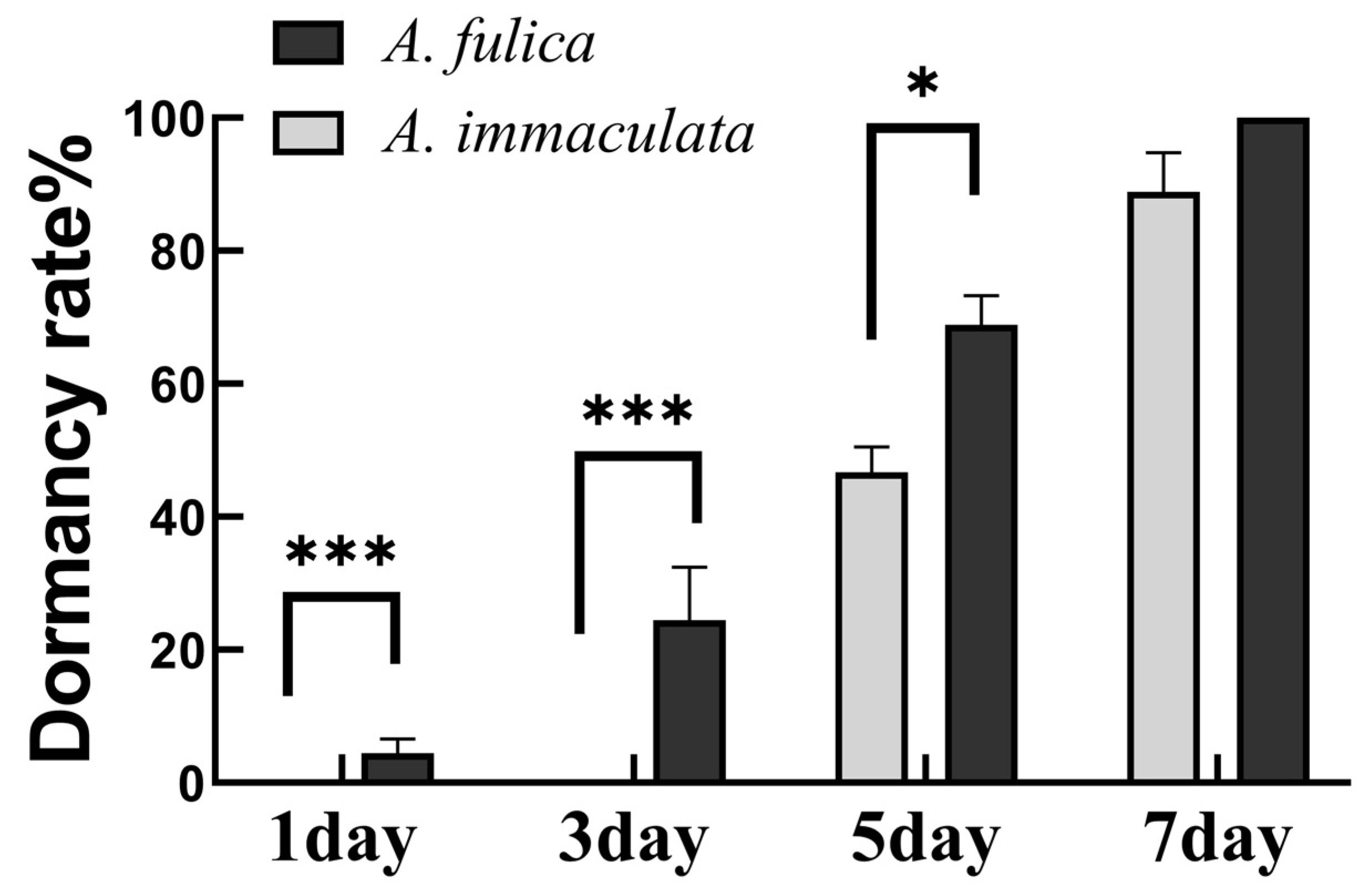
3.4. Cold Adaptation
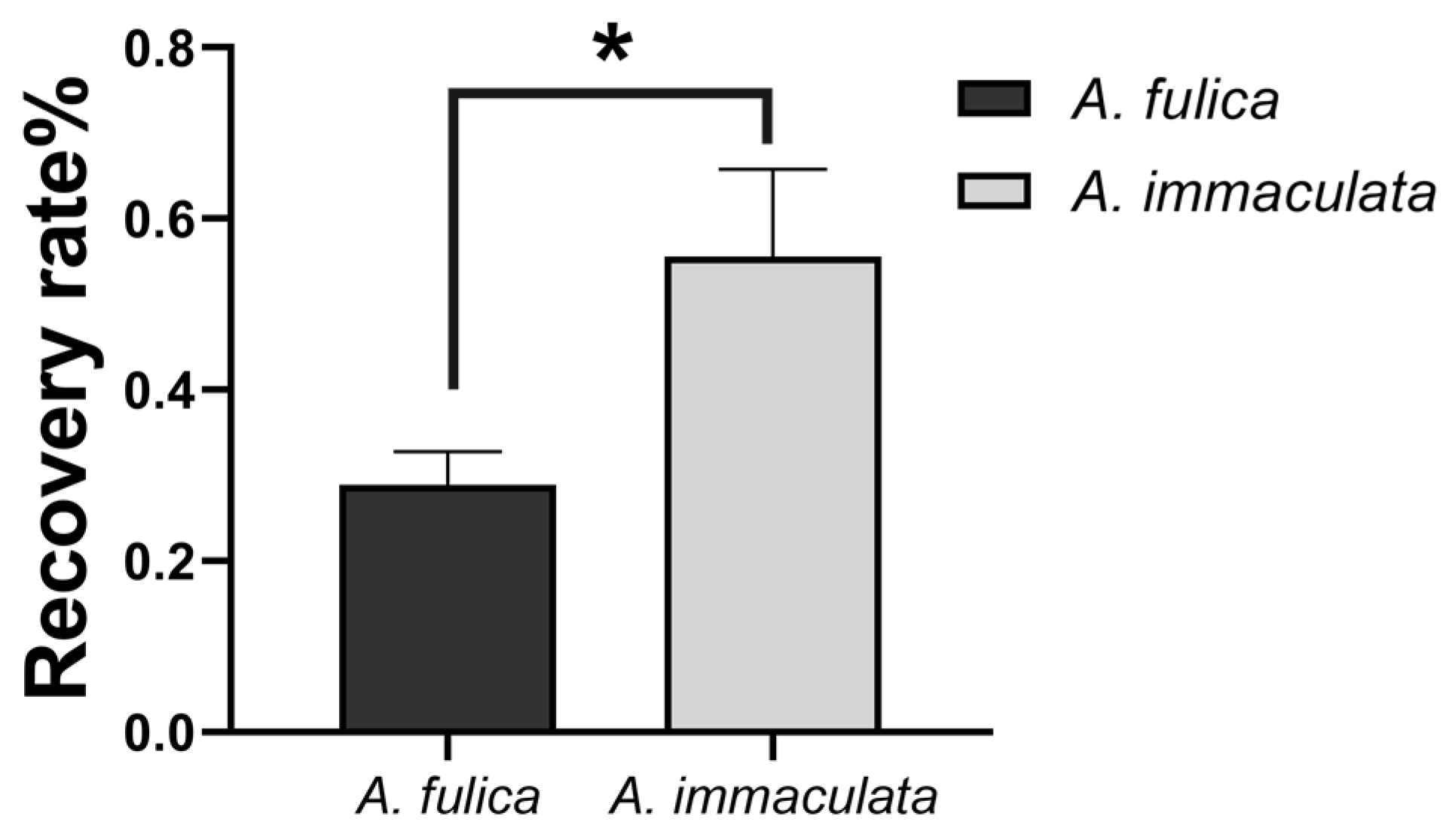
3.5. Drought Resistance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wan, F.H.; Xie, B.Y.; Yang, G.Q. Invasion Biology. J. Biosaf. Biosecur. 2011, 20, 85–86. [Google Scholar]
- Zhang, C.Q.; Dai, J.R. Progress of research on biologically invasive medical molluscs in China. Chin. J. Schistosomiasis Control 2019, 31, 441–445. [Google Scholar] [CrossRef]
- You, Y. The situation Analysis and countermeasures on situation of transmission and damage control for Giant Africa snail (Chinese). J. Guangxi Agric. 2016, 31, 46–48. [Google Scholar]
- Ma, Y.B.; Zhou, H.L.; Hu, Y.; Zhou, M.Y.; Lu, Z.Y. Potential geographical distribution of Achatina fulica in China based on Maxent model. J. Trop. Med.-US 2021, 21, 535–539. [Google Scholar]
- Wang, X.F.; Li, Z.Y.; Liu, B.; Qiao, X.; Wan, F.H.; Qian, W.Q.; Liu, C.H. Research the new pesticide for Achatina fulica based on invasive plants and snail pheromone. J. Biosaf. 2022, 31, 289–294. [Google Scholar]
- Guo, Y.H.; Zhang, Y.; Liu, Q.; Huang, Y.; Mao, G.Y.; Yue, Z.Y.; Abe, E.M.; Li, J.; Wu, Z.D.; Li, S.Z.; et al. A chromosomal-level genome assembly for the giant African snail Achatina fulica. Gigascience 2019, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- GGuo, J.; Zhang, J.E.; Wu, R.S.; Yang, Y.H. Overview and perspective on the current research status for the giant snail Achatina fulica in China. J. South. Agric. 2015, 46, 626–630. [Google Scholar]
- Solorzano Alava, L.; Hernandez Alvarez, H.; Bedoya Pilozo, C.; Rodriguez, M.; Rojas Rivero, L. Achatina fulica infected by Angiotrongylus cantonensis in Napo, Ecuador. Rev. Peru. Med. Exp. Salud Publica 2022, 39, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Ramdwar, M.N.A.; Harripersad, J.; Badal, S. Examining the predatory relationship between the indigenous firefly (Aspisoma ignitum) and the invasive giant African snail (Achatina fulica) in Trinidad, West Indies. J. Agric. Sci. 2023, 15, 47–56. [Google Scholar] [CrossRef]
- Tang, L.N.; Li, A.M.; Lu, L.D.; Xv, L.N.; Lin, G.C.; Wang, S.H.; Zhang, X.S. Survey of infections status of Angiostrongylus cantonensis in intermediate host in Guiyang city. Chin. J. Vector Biol. 2008, 19, 342–344. [Google Scholar]
- Rodrigues, P.S.; Gomes, S.R.; Montresor, L.C.; Ramos-de-Souza, J.; Barros, L.A.; Fernandez, M.A.; Thiengo, S.C. The giant African snail Achatina (Lissachatina) fulica Bowdich, 1822 as an intermediate host of Aelurostrongylus abstrusus (Railliet, 1898) in the Rio de Janeiro state, Brazil. Vet. Parasitol. Reg. Stud. Rep. 2022, 30, 8. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.G.; Augusto, R.D.; Pinheiro, J.; Thiengo, S.C. Physiology and immunity of the invasive giant African snail, Achatina (Lissachatina) fulica, intermediate host of Angiostrongylus cantonensis. Dev. Comp. Immunol. 2020, 105, 10. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service of the United States. Available online: https://www.aphis.usda.gov/aphis/ourfocus/planthealth/plant-pest-and-disease-programs/pests-and-diseases/giant-african-snail (accessed on 8 July 2023).
- Celis-Ramirez, M.; Angel, M.Q.; Varela-M, R.E. Control of invasive alien species: The Giant African snail (Lissachatina fulica) a difficult urban public management challenge. J. Environ. Manag. 2022, 322, 11. [Google Scholar] [CrossRef]
- Hu, M.; Wang, P.; Yang, H.; Li, J.; Liu, R.; Xu, M.; Liang, F.; Chen, S.; Zhou, W. Quarantine and identification of a new major invasive species Achatina panthera in Taiwan. Plant Prot. 2023, 49, 263–268. [Google Scholar]
- Yang, H.X.; Tian, M.; Deng, X.H.; Wang, Y.B. Dongguan port intercepts African snails from imported logs for the first time, Guangdong (Chinese). Plant Prot. 2012, 26, 3. [Google Scholar]
- Liu, C.H.; Ren, Y.W.; Li, Z.Y.; Hu, Q.; Yin, L.J.; Wang, H.C.; Qiao, X.; Zhang, Y.; Xing, L.S.; Xi, Y.; et al. Giant African snail genomes provide insights into molluscan whole-genome duplication and aquatic-terrestrial transition. Mol. Ecol. Resour. 2021, 21, 478–494. [Google Scholar] [CrossRef]
- Baily, J.L. The giant African snail: A problem in economic malacology. Q. Rev. Biol. 1961, 37, 270. [Google Scholar]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutiérrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Gutiérrez, J.J.C. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Neglected Trop. Dis. 2019, 13, 18. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.X. The African giant snail, akin to a “pathogen blind box”, is slowly crawling northward from South China. The Beijing News, 20 May 2024. [Google Scholar]
- Steinke, D.; Prosser, S.W.J.; Hebert, P.D.N. DNA Barcoding of Marine Metazoans. Methods Mol. Biol. 2016, 1452, 155–168. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mar. Biol. 1994, 3, 294–299. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11 Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef] [PubMed]
- Vogler, R.E.; Beltramino, A.A. Achatina fulica (giant African land snail). CABI Int. 2013, 2013, 2640. [Google Scholar]
- Maheshini, P.W.D.B.; Thilakarathne, K.G.D.D.; Hirimuthugoda, G.N.; Ranawana, K.B.; Kumburegama, S. The distribution of terrestrial pest gastropods and their damage to agricultural crops in Kandy and Nuwara Eliya districts in Sri Lanka. Ceylon J. Sci. 2019, 48, 177–184. [Google Scholar] [CrossRef]
- Reddy, K.B.; Sreedharan, K. Record of giant African snail, Achatina fulica Bowdich on coffee in Visakha agency areas, Andhra Pradesh. Indian Coffee 2006, 70, 17–19. [Google Scholar]
- Sheela Thakur, S.T. Food consumption and growth potential of giant African snail, Achatina fulica. J. Ecobiol. 2004, 16, 455–461. [Google Scholar]
- Jav Aregowda, J.A.; Aregowda, J. Incidence of the giant African snail, Achatina fulica (Bowdich), on horticultural crops. Pest Manag. Sci. 2004, 12, 221–222. [Google Scholar]
- Fontanilla, I.K.; Naggs, F.; Wade, C.M. Molecular phylogeny of the Achatinoidea (Mollusca: Gastropoda). Mol. Phylogenet. Evol. 2017, 114, 382–385. [Google Scholar] [CrossRef]
- Adler, K.A.; De Nault, D.L.; Cardoza, C.M.; Womack, M. Evolutionary rates and shape variation along the anuran vertebral column with attention to phylogeny, body size, and ecology. Evolution 2022, 76, 2724–2738. [Google Scholar] [CrossRef]
- Lindenfors, P. Sexually antagonistic selection on primate size. J. Evol. Biol. 2002, 15, 595–607. [Google Scholar] [CrossRef]
- Harwood, J.D.; Obrycki, J.J. Web-construction behavior of linyphiid spiders (Araneae, Linyphiidae): Competition and co-existence within a generalist predator guild. J. Insect Behav. 2005, 18, 593–607. [Google Scholar] [CrossRef]
- Raut, S.K.; Barker, G.M. Achatina fulica Bowdich and Other Achatinidae as Pests in Tropical Agriculture; CABI Publishing: Wallingford, UK, 2002; pp. 55–114. [Google Scholar]
- Ringeisen, B.R.; Souza, C.D.; Njuguna, E.W.; Ronald, P.C. We need to act now to ensure global food security, and reduce agricultural greenhouse gas emissions. Bull. At. Sci. 2024, 80, 162–167. [Google Scholar] [CrossRef]
- Li, C.Y.; Xu, M.Y.; Thammaratsuntorn, J.; Zhao, J.L.; Qian, Y.Z.; Wu, C.; Qian, D. Comparison of growth, food intake, pepsin activity and pepsinogen genes expression among siniperca species. J. Shanghai Ocean Univ. 2016, 25, 1–7. [Google Scholar]
- Wei, S.Y.; Zhou, L.; Luo, Z.Q.; She, Y.L.; Wang, Q.Z.; Chen, J.Y.; Qu, S.; Wei, Y.M. Economic impacts of multiple natural disasters and agricultural adaptation measures on supply chains in China. J. Clean. Prod. 2023, 418, 138095. [Google Scholar] [CrossRef]
- Guo, K.; Pysek, P.; van Kleunen, M.; Kinlock, N.L.; Lucanová, M.; Leitch, I.J.; Pierce, S.; Dawson, W.; Essl, F.; Kreft, H.; et al. Plant invasion and naturalization are influenced by genome size, ecology and economic use globally. Nat. Commun. 2024, 15, 10. [Google Scholar] [CrossRef]
- Evans, M.V.; Drake, J.M.; Jones, L.; Murdock, C.C. Assessing temperature-dependent competition between two invasive mosquito species. Ecol. Appl. 2021, 31, 12. [Google Scholar] [CrossRef] [PubMed]

| Species | Family | Reference |
|---|---|---|
| Cabbage (Brassica oleracea var. Capitata) | Brassicaceae | Maheshini et al. [25] |
| Cauliflower (Brassica oleracea var. Botrytis) | Brassicaceae | Reddy et al. [26] |
| Cucumber (Cucumis sativus) | Cucurbitaceae | Maheshini et al. [25] |
| Marrow (Cucurbita pepo) | Cucurbitaceae | Thakur et al. [27] |
| Banana (Musa nana) | Musaceae | Aregowda et al. [28] |
| Pawpaw (Carica papaya) | Caricaceae | Thakur et al. [27] |
| French marigold (Tagetes patula) | Asteraceae | Thakur et al. [27] |
| Similarity to Achatina fulica | Similarity to Achatina immaculata | Similarity to Achatina panthera | |
|---|---|---|---|
| seq1 | 74.42% | 97.33% | 83.89% |
| seq2 | 73.33% | 96.90% | 83.38% |
| seq3 | 74.49% | 97.05% | 83.94% |
| seq4 | 73.33% | 96.47% | 83.55% |
| seq5 | 74.76% | 97.05% | 84.06% |
| seq6 | 76.41% | 97.63% | 84.58% |
| seq7 | 93.18% | 75.60% | 72.34% |
| seq8 | 93.17% | 75.32% | 72.06% |
| seq9 | 93.59% | 75.46% | 72.20% |
| seq10 | 93.87% | 75.95% | 72.59% |
| seq11 | 93.18% | 76.10% | 72.83% |
| seq12 | 93.48% | 76.45% | 72.18% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Wang, X.; Tang, Y.; Wang, L.; Han, R.; Qiao, X.; Wan, F.; Qian, W.; Liu, C. An Investigation and Invasiveness Analysis of Two Species of Giant African Snail in a Coastal City of Southern China. Agriculture 2024, 14, 1217. https://doi.org/10.3390/agriculture14081217
Zhang Y, Wang X, Tang Y, Wang L, Han R, Qiao X, Wan F, Qian W, Liu C. An Investigation and Invasiveness Analysis of Two Species of Giant African Snail in a Coastal City of Southern China. Agriculture. 2024; 14(8):1217. https://doi.org/10.3390/agriculture14081217
Chicago/Turabian StyleZhang, Yongzhe, Xinfeng Wang, Yuzhe Tang, Linjing Wang, Rui Han, Xi Qiao, Fanghao Wan, Wanqiang Qian, and Conghui Liu. 2024. "An Investigation and Invasiveness Analysis of Two Species of Giant African Snail in a Coastal City of Southern China" Agriculture 14, no. 8: 1217. https://doi.org/10.3390/agriculture14081217






