The Influence of Nitrogen and Sulfur Fertilization on Oil Quality and Seed Meal in Different Genotypes of Winter Oilseed Rape (Brassica napus L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Experiment
2.3. Biochemical Analysis
2.3.1. Determination of Oil and Protein
2.3.2. Determination of Fatty Acids
2.3.3. Determination of Glucosinolates
2.4. Statistical Analysis
3. Results
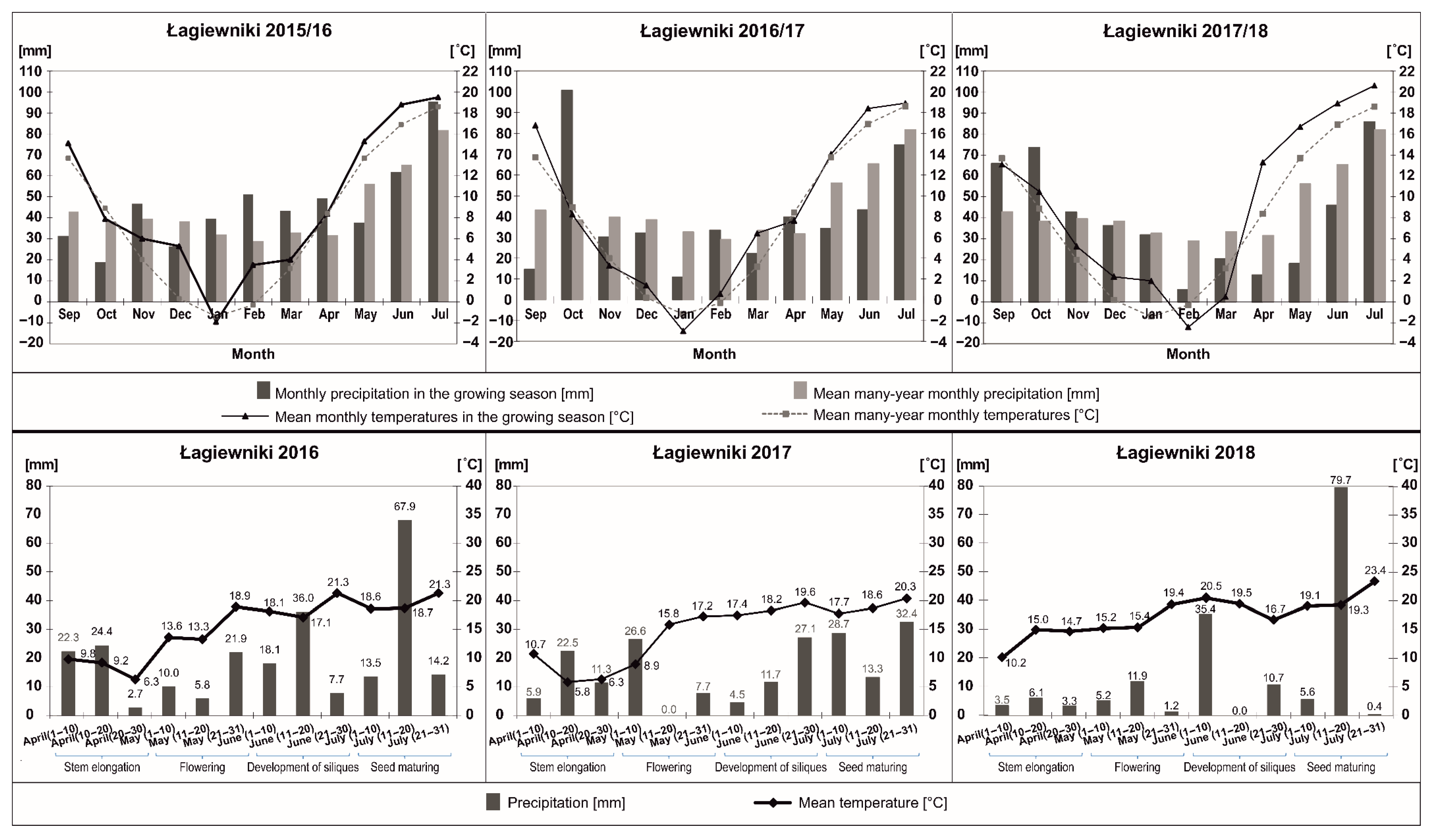
3.1. Weather Conditions
3.2. Total-Protein and Crude-Fat Content
3.3. Composition of Fatty Acids in Oil
3.4. Glucosinolate (GLS) Content in Seeds
4. Discussion
4.1. Total-Protein and Crude-Fat Content
4.2. Composition of Fatty Acids in Oil
4.3. Glucosinolate (GLS) Content in Seeds
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO. Food and Agriculture Organization of the United Nations. Faostat Agriculture data. Available online: https://www.fao.org/faostat/en/#data (accessed on 23 September 2023).
- McVetty, P.B.E.; Duncan, R.W. Canola, Rapeseed and Mustard: For Biofuels and Bioproducts. In Industrial Crops, Handbook of Plant Breeding; Crus, V.M.V., Dierig, D.A., Eds.; Springer: New York, NY, USA, 2015; p. 9. [Google Scholar]
- Weymann, W.; Bottcher, U.; Sieling, K.; Kage, H. Effects of weather conditions during different growth phases on yield formation of winter oilseed rape. Field Crops Res. 2015, 173, 41–48. [Google Scholar] [CrossRef]
- Spasibionek, S. New mutants of winter rapeseed (Brassica napus L.) with changed fatty acid composition. Plant Breed. 2006, 125, 259–267. [Google Scholar] [CrossRef]
- Matthäus, B.; Haase, N.; Unbehend, G. Impact of HOLL rapeseed oil during frying on product quality during storage. In Proceedings of the 13th International Rapeseed Congress, Prague, Czech Republic, 5–9 July 2011; pp. 528–531. Available online: https://www.irc2011.org (accessed on 5 June 2011).
- Spasibionek, S. Genetic and breeding study of winter oilseed rape mutants (Brassica napus L.) with changed fatty acid composition. PBAI-NRI Monogr. Disseratations 2013, 47, 1–106. (In Polish) [Google Scholar]
- Rakow, G.; Relf-Eckstein, J.-A.; Raney, J.P. Rapeseed genetic research to improve its agronomic performance and seed quality. Helia 2007, 30, 199–206. [Google Scholar]
- Szulc, P.M.; Drozdowska, L.; Kachlicki, P. Effect of sulphur on the yield and content of glucosinolates in spring oilseed rape seeds. Electron. J. Pol. Agric. Univ. 2003, 6, 1–8. Available online: http://www.ejpau.media.pl/volume6/issue2/agronomy/art-01 (accessed on 5 June 2011).
- Faraji, A. Oil concentration in canola (Brassica napus L.) as a function of environmental conditions during seed filling period. Int. J. Plant Prod. 2012, 6, 267–277. [Google Scholar]
- Liersch, A.; Bocianowski, J.; Nowosad, K.; Mikołajczyk, K.; Spasibionek, S.; Wielebski, F.; Matuszczak, M.; Szała, L.; Cegielska-Taras, T.; Sosnowska, K.; et al. Effect of Genotype × Environment Interaction for Seed Traits in Winter Oilseed Rape (Brassica napus L.). Agriculture 2020, 10, 607. [Google Scholar] [CrossRef]
- Yahbi, M.; Keli, A.; El Alami, N.; Nabloussi, A.; Maataoui, A.; Daoui, K. Chemical composition and quality of rapeseed meal as affected by genotype and nitrogen fertilization. OCL 2024, 31, 5. [Google Scholar] [CrossRef]
- White, C.A.; Roques, S.E.; Berry, P.M. Effects of foliar-applied nitrogen fertilizer on oilseed rape (Brassica napus L.). J. Agric. Sci. 2015, 153, 42–55. [Google Scholar] [CrossRef]
- Narits, L. Effect of nitrogen rate and application time to yield and quality of winter oilseed rape (Brassica napus L. var. oleifera subvar. biennis). Agron. Res. 2010, 8, 671–686. [Google Scholar]
- Groth, D.A. Agronomic and Economic Effectiveness of Nitrogen and Sulphur Fertilization of Different Winter Oilseed Rape Morphotypes. Ph.D. Thesis, Uniwersytet Warmińsko Mazurski w Olsztynie, Olsztyn, Poland, 2019. (In Polish). [Google Scholar]
- Jankowski, K.J.; Sokólski, M.; Kordan, B. Camelina: Yield and quality response to nitrogen and sulfur fertilization in Poland. Ind. Crops Prod. 2019, 141, 111776. [Google Scholar] [CrossRef]
- Chen, X.J.; Zhu, Z.J.; Ni, X.L.; QIAN, Q.Q. Effect of nitrogen and sulfur supply on glucosinolates in Brassica campestris ssp. Chinensis. Agric. Sci. China. 2006, 5, 603–608. [Google Scholar] [CrossRef]
- Šiaudinis, G.; Butkutė, B. Responses of spring oilseed rape seed yield and quality to nitrogen and sulfur fertilization. Commun. Soil Sci. Plan. 2013, 44, 145–157. [Google Scholar] [CrossRef]
- Ahmad, G.; Jan, A.; Arif, M.; Jan, M.T.; Khattak, R.A. Influence of nitrogen and sulfur fertilization on quality of canola (Brassica napus L.) under rainfed conditions. J. Zhejiang Univ. Sci. 2007, 8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Malhi, S.S.; Gill, K.S. Interactive effects of N and S fertilizers on canola yield and seed quality on S-deficient Gray Luvisol soils in northeastern Saskatchewan. Can. J. Plant Sci. 2007, 87, 211–222. [Google Scholar] [CrossRef]
- Chahal, H.S.; Sing, A.; Malhi, G.S. Role of Sulphur nutrition in oilseed crop production—A review. J. Oilseeds Res. 2020, 11, 95–102. [Google Scholar]
- Manaf, A.; Hassan, F.U. Effects of sulphur on fatty acid accumulation in Brassica Cultivars. Int. J. Agric. Biol. 2006, 8, 588–592. [Google Scholar]
- Zhao, F.; Evans, E.; Bilsborrow, P.E.; Syers, K. Influence of sulphur and nitrogen on seed yield and quality of low glucosinolate oilseed rape (Brassica napus L.). J. Sci. Food Agric. 1993, 63, 29–37. [Google Scholar] [CrossRef]
- Jankowski, K.; Budzyński, W.; Kijewski, Ł.; Zając, T. Biomass quality of Brassica oilseed crops in response to sulfur fertilization. Agron. J. 2015, 107, 1377–1391. [Google Scholar] [CrossRef]
- Skwierawska, M.; Krzebietke, S.; Jankowski, K.; Benedycka, Z.; Mackiewicz-Walec, E. Sulphur in the Polish fertilization diagnostics. J. Elem. 2014, 19, 299–312. [Google Scholar] [CrossRef]
- GUS. Statistics Poland. 2019. Available online: https://www.stat.gov.pl (accessed on 5 June 2011).
- Klikocka, H. The sulphur status in biosphere and plant fertilization. Przemysł Chem. 2010, 89, 903–908. (In Polish) [Google Scholar]
- Sattar, A.; Cheema, M.A.; Wahid, M.A.; Saleem, M.F.; Hassan, M. Interactive effect of sulphur and nitrogen on growth, yield and quality of canola. Crop Environ. 2011, 2, 32–37. [Google Scholar]
- Szatkowski, A.; Antoszkiewicz, Z.; Purwin, C.; Jankowski, K.J. Oilseed Radish: Nitrogen and Sulfur Management Strategies for Seed Yield and Quality—A Case Study in Poland. Agriculture 2024, 14, 755. [Google Scholar] [CrossRef]
- Malhi, S.S.; Gan, Y.; Raney, J.P. Yield, seed quality, and sulfur uptake of Brassica oilseed crops in response to sulfur fertilization. Agron. J. 2007, 99, 570–577. [Google Scholar] [CrossRef]
- Wielebski, F. The effect of sulphur fertilization on chemical composition of seeds of different breeding forms of winter oilseed rape in the conditions of diverse nitrogen rates. Oilseed Crops 2011, 32, 79–95. (In Polish) [Google Scholar]
- Poisson, E.; Brunel-Muguet, S.; Kauffmann, F.; Trouverie, J.; Avice, J.-C.; Mollier, A. Sensitivity analyses for improving sulfur management strategies in winter oilseed rape. PLoS ONE 2018, 13, e0204376. [Google Scholar] [CrossRef] [PubMed]
- Ijaz, M.; Honermeier, B. Effect of triazole and strobilurin fungicides on seed yield formation and grain quality of winter rapeseed (Brassica napus L.). Field Crops Res. 2012, 130, 80–86. [Google Scholar] [CrossRef]
- Grant, C.A.; Mahli, S.S.; Karamanos, R.E. Sulfur management for rapeseed. Field Crops Res. 2012, 128, 119–128. [Google Scholar] [CrossRef]
- Filipek-Mazur, B.; Tabak, M.; Gorczyca, O.; Lisowska, A.A. Effect of sulfur-containing fertilizers on the quantity and quality of spring oilseed rape and winter wheat yield. J. Elem. 2019, 24, 1383–1394. [Google Scholar]
- Ahmad, A.; Abdin, M.Z. Effect of sulphur application on lipid, RNA and fatty acid content in developing seeds of rapeseed (Brassica campestris L.). Plant Sci. 2000, 150, 71–76. [Google Scholar] [CrossRef]
- Barczak, B. Sulphur as a nutrient determining the yield size and quality of selected crop species. UTP Bydg. Monogr. Disseratation 2010, 144, 1–131. (In Polish) [Google Scholar]
- Ahmad, A.; Abdin, M.Z. Interactive effect of sulphur and nitrogen on the oil and protein contents and fatty profiles of oil in the seeds of rapeseed (Brassica campestris L.) and mustard (Brassica juncea L. Czern. And Coss). J. Agron. Crop Sci. 2000, 185, 49–54. [Google Scholar] [CrossRef]
- Zhao, F.J.; Bilsborrow, P.E.; Evans, E.J.; McGrath, S.P. Nitrogen to sulphur ratio in rapeseed and rapeseeds protein and its use in diagnosing sulphur deficiency. J. Plant Nutr. 1997, 20, 549–558. [Google Scholar] [CrossRef]
- Joshi, N.I.; Mali, P.C.; Saxena, A. Effect of nitrogen and sulphur application on yield and fatty acid composition of mustard (Brassica juncea L.) oil. J. Agron. Crop Sci. 1998, 180, 59–63. [Google Scholar] [CrossRef]
- Ma, B.L.; Zheng, Z.; Whalen, J.K.; Caldwell, C.; Vanasse, A.; Pageau, D.; Scott, P.; Earl, H.; Smith, D.L. Uptake and nutrient balance of nitrogen, sulfur, and boron for optimal canola production in eastern Canada. J. Plant. Nutr. Soil Sci. 2019, 182, 252–264. [Google Scholar] [CrossRef]
- Poisson, E.; Trouverie, J.; Brunel-Muguet, S.; Akmouche, Y.; Pontet, C.; Pinochet, X.; Avice, J.-C. Seed Yield Components and Seed Quality of Oilseed Rape Are Impacted by Sulfur Fertilization and Its Interactions With Nitrogen Fertilization. Front. Plant Sci. 2019, 10, 458. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, S.; Paulsen, H.M.; Gupta, A.K.; Bloem, E.; Schnug, E. Influence of sulphur fertlilization on yield and quality of oilseed rape and mustard. In Proceedings of the 10th International Rapeseed Congres, Camberra, Australia, 26–29 September 1999. CD rom. [Google Scholar]
- Zhao, F.J.; Evans, E.J.; Bilsborrow, P.E. Varietal differences in sulphur uptake and utilization in relation to glucosinolate accumulation in oilseed rape. In Proceedings of the 9th International Rapeseed Congress, Cambridge, UK, 4–7 July 1995; Volume 1, pp. 271–273. [Google Scholar]
- Jankowski, K.; Budzyński, W.; Szymanowski, A. Influence of the rate and timing of sulphur fertilisation on winter oilseed rape yield. Oilseed Crops 2008, 29, 75–89. (In Polish) [Google Scholar]
- Gugała, M.; Sikorska, A.; Zarzecka, K. The effect of fertilization with sulphur, boron, and amino acids on the content of glucosinolate in winter rape seeds. Agronomy 2020, 10, 519. [Google Scholar] [CrossRef]
- Wielebski, F.; Wójtowicz, M. Effect of spring sulphur fertilization on yield and glucosinolate content in seeds of winter oilseed rape composite hybrids. Oilseed Crops 2003, 24, 109–119. (In Polish) [Google Scholar]
- Zhao, F.J.; McGrath, S.P.; Blake-Kalff, M.M.A.; Link, A.; Tucker, M. Crop responses to sulphur fertilization. Fertil. Fertil. 2003, 3, 26–51. [Google Scholar]
- Mailer, R.J.; Cornish, P.S. Effects of water stress on glucosinolate and oil concentration in the seeds of rape (Brassica napus L.) and turnip (Brassica rapa L. var. silvestris (Lam) Briggs). Aust. J. Exp. Agric. 1987, 27, 207–211. [Google Scholar]
- Baux, A.; Hebeisen, T.; Pellet, D. Effects of minimal temperatures on low-linolenic rapeseed oil fatty-acid composition. Eur. J. Agron. 2008, 29, 102–107. [Google Scholar] [CrossRef]
- Ghobadi, M.; Bakhshandeh, M.; Fathi, G.; Gharineh, M.H. Short and long periods of water stress during different growth stages of canola (Brassica napus L.): Effect on yield, yield components, seed oil and protein contents. J. Agron. 2006, 5, 336–341. [Google Scholar]
- Morrison, M.J.; Stewart, D.W. Heat stress during flowering in summer Brassica. Crop Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Wójtowicz, M. Effect of environmental and agronomical factors on quantity and quality of yield of winter oilseed rape (Brassica napus L.). PBAI-NRI Monogr. Disseratations 2013, 45, 1–111. (In Polish) [Google Scholar]
- Merrien, A.; Krouti, M.; Dechambre, J.; Garnon, V.; Evrard, J. Contribution to understand the fluctuation of linolenic acid profile in winter oilseed rape grown in France. In Proceedings of the 12th International Rapeseed Congress, Wuhan, China, 26–30 March 2007; Volume 5, pp. 95–97. [Google Scholar]
- Spasibionek, S.; Mikołajczyk, K.; Cwiek-Kupczyńska, H.; Piętka, T.; Krótka, K.; Matuszczak, M. Marker assisted selection of new high oleic and low linolenic winter oilseed rape (Brassica napus L.) inbred lines revealing good agricultural value. PLoS ONE 2020, 15, e0233959. [Google Scholar] [CrossRef] [PubMed]
- Sokólski, M.; Załuski, D.; Szatkowski, A.; Jankowski, K.J. Winter Oilseed Rape: Agronomic Management in Different Tillage Systems and Seed Quality. Agronomy 2023, 13, 524. [Google Scholar] [CrossRef]
- Oleszek, W. Glucosinolates—Occurrence and ecological significance. Wiadomości Bot. 1995, 39, 49–58. (In Polish) [Google Scholar]
- Jensen, C.R.; Mogensen, V.O.; Mortensen, G.; Fieldsend, J.K.; Milford, G.F.J.; Andersen, M.N.; Thage, J.H. Seed glucosinolate, oil and protein contents of field-grown rape (Brassica napus L.) affected by soil drying and evaporative demand. Field Crop Res. 1996, 47, 93–105. [Google Scholar] [CrossRef]
- Verkerk, R.; Schreiner, M.; Krumbein, A.; Ciska, E.; Holst, B.; Rowland, I.; De Schrijver, R.; Hansen, M.; Gerhäuser, C.; Mithen, R.; et al. Glucosinolates in Brassica vegetables: The influence of the food supply chain on intake, bioavailability and human health. Mol. Nutr. Food Res. 2009, 53, 219–265. [Google Scholar] [CrossRef]
- Zhao, F.J.; Evans, E.J.; Bilsborrow, P.E.; Syers, J.K. Influence of nitrogen and sulphur on the glucosinolate profile of rapeseed (Brassica napus L.). J. Sci. Food Agric. 1994, 64, 295–304. [Google Scholar] [CrossRef]
- Krzymański, J. Determination of fat and water content in oilseeds by MNR method. Tłuszcze Środki Piorące I Kosmet. 1970, 14, 202–208. (In Polish) [Google Scholar]
- Wielebski, F.; Wójtowicz, M. Influence of water deficit and differentiated nitrogen fertilization on winter double low oilseed rape seed yield and glucosinolate content. Oilseed Crops 1994, 15, 27–34. (In Polish) [Google Scholar]
- Bečka, D.; Bečková, L.; Tomášek, J.; Mikšík, V.; Vicianová, M. Effects of various nitrogen fertilisers applied in autumn on growth parameters, yield and quality of winter oilseed rape. Plant Soil Environ. 2024, 70, 317–325. [Google Scholar] [CrossRef]
- Fismes, J.; Vong, P.C.; Guckert, A.; Frossard, E. Influence of sulfur on apparent N-use efficiency, yield and quality of oilseed rape (Brassica napus L.) grown on a calcareous soil. Eur. J. Agron. 2000, 12, 127–141. [Google Scholar] [CrossRef]
- Egesel, C.Ö.; Gül, M.K.; Kahrıman, F. Changes in yield and seed quality traits in rapeseed genotypes by sulphur fertilization. Eur. Food Res. Technol. 2009, 229, 505–513. [Google Scholar] [CrossRef]
- Ijaz, M.; Mahmood, K.; Honermeier, B. Interactive Role of Fungicides and Plant Growth Regulator (Trinexapac) on Seed Yield and Oil Quality of Winter Rapeseed. Agronomy 2015, 5, 435–446. [Google Scholar] [CrossRef]
- Fazli, L.S.; Abdin, M.Z.; Jamal, A.; Ahmad, S. Interactive effect of sulphur and nitrogen on lipid accumulation, acetyl-CoA concentration and acetyl-CoA carboxylase activity in developing seeds of oilseed crops (Brassica campestris L. and Eruca sativa Mill.). Plant Sci. 2005, 168, 29–36. [Google Scholar] [CrossRef]
- Rathke, G.W.; Christen, O.; Diepenbrock, W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crops Res. 2005, 94, 103–113. [Google Scholar] [CrossRef]
- Wójtowicz, M. Effect of nitrogen fertilization and environment conditions on biological and commercial characters of oilseed rape composite hybrids Kaszub and Mazur. Oilseed Crops 2004, 25, 109–123. (In Polish) [Google Scholar]
- Zhen-hua, Z.; Hai-xing, S.; Qiang, L.; Xiang-min, R.; Jian-wei, P.; Gui-xian, X.; Yu-ping, Z.; Li-ru, C.; Chun-yun, G.; Ji-dong, G. Responses of Seed Yield and Quality to Nitrogen Application Levels in Two Oilseed Rape (Brassica napus L.) Varieties Differing in Nitrogen Efficiency. Plant Prod. Sci. 2012, 15, 265–269. [Google Scholar] [CrossRef]
- Zapletalová, A.; Ducsay, L.; Varga, L.; Sitkey, J.; Javoreková, S.; Hozlár, P. Influence of Nitrogen Nutrition on Fatty Acids in Oilseed Rape (Brassica napus L.). Plants 2022, 11, 44. [Google Scholar] [CrossRef] [PubMed]
- Krauze, A.; Bowszys, T. Effect of time of sulphur fertilization of spring oilseed rape cv. Star on seed yield, sulphur content and crude oil. Oilseed Crops 2001, 22, 285–290. (In Polish) [Google Scholar]
- Kozłowska-Strawska, J. Fat content and fatty acid composition in oilseed rape grown in the Lubelski Region under different levels of soil sulphur fertility. Ecol. Chem. Eng. 2012, A 19, 191–201. [Google Scholar]
- Omidi, H.; Tahmasebi, Z.; Badi, H.A.N.; Torabi, H.; Miransari, M. Fatty acid composition of canola as affected by agronomical, genotypic and environmental parameters. C. R. Biol. 2010, 333, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz, M.; Wójtowicz, A. The effect of climate change on linolenic fatty acid in oilseed rape. Agronomy 2020, 10, 2003. [Google Scholar] [CrossRef]
- Bilsborrow, P.E.; Evans, E.J.; Milford, G.F.J.; Fieldsend, J.K. The effects of S and N on the yield and quality of oilseed rape in the UK. In Proceedings of the 9th Intern. Rapeseed Congress, Cambridge, UK, 4–7 July 1995; Volume 1, pp. 280–283. [Google Scholar]
- Söchting, H.P.; Verreet, J.-A. Effects of different cultivation system (soil management, nitrogen fertilization) on the epidemics of fungal diseases in oilseed rape (Brassica napus L. var. napus). J. Plant Dis. Prot. 2004, 111, 1–29. [Google Scholar] [CrossRef]
- Wielebski, F.; Muśnicki, C. Influence of the increasing doses and different methods of sulphur fertilization on seed yield and glucosinolate content in two double low oilseed rape cultivars in field experiments. Rocz. Akad. Rol. W Pozn. 1998, 303, 149–167. (In Polish) [Google Scholar]
- Zukalová, H.; Matula, J.; Kuchtova, P.; Miksik, V. Influence of Sulphur on the yield and quality of winter oilseed rape. Oilseed Crops 2001, 22, 587–596. (In Polish) [Google Scholar]
- Drozdowska, L.; Szulc, P.; Łukanowski, A.; Sadowski, C. Glucosinolate content and Pathogenic fungi occurrence in seeds of spring oilseed rape fertilized with sulphur. Plant Breed. Seed Sci. 2002, 46, 3–9. [Google Scholar]

| Parameter | Y | N | S | C | Y × N | Y × S | N × S | Y × C | N × C | S × C | Y × N × S | N × S × C | Y × N × C | Y × S × C | Y × N × S × C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Content of seeds [g kg−1 DM] | |||||||||||||||
| crude fat | 497.14 ** | 15.99 ** | 2.98 * | 284.29 ** | 0.22 ns | 1.26 ns | 2.42 * | 20.95 ** | 1.20 ns | 0.48 ns | 0.89 ns | 0.93 ns | 0.55 ns | 0.52 ns | 0.87 ns |
| total protein | 159.81 ** | 27.69 ** | 5.40 ** | 240.67 ** | 1.71 ns | 1.26 ns | 3.35 ** | 4.37 ** | 1.55 ns | 0.47 ns | 0.74 ns | 1.38 ns | 1.92 ns | 0.37 ns | 0.87 ns |
| Fatty acids [%]: | |||||||||||||||
| C16 | 38.91 ** | 0.30 ns | 0.67 ns | 4626.51 ** | 0.05 ns | 2.41 * | 1.03 ns | 34.16 ** | 1.62 ns | 0.97 ns | 1.04 ns | 0.82 ns | 0.60 ns | 1.46 ns | 1.52 ns |
| C18 | 328.47 ** | 1.74 ns | 3.96 * | 67.35 ** | 1.96 ns | 1.45 ns | 0.90 ns | 41.2 ** | 0.71 ns | 1.61 ns | 0.70 ns | 0.57 ns | 1.71 ns | 0.76 ns | 0.71 ns |
| C18:1 | 121.15 ** | 0.34 ns | 1.06 ns | 8071.52 ** | 4.45 * | 1.36 ns | 0.57 ns | 71.00 ** | 0.48 ns | 1.96 ns | 1.03 ns | 1.34 ns | 0.61 ns | 1.29 ns | 0.97 ns |
| C18:2 | 122.97 ** | 0.85 ns | 1.45 ns | 8098.36 ** | 1.49 ns | 1.04 ns | 0.85 ns | 67.05 ** | 0.75 ns | 1.13 ns | 0.84 ns | 1.39 ns | 0.40 ns | 1.45 ns | 1.06 ns |
| C18:3 | 426.96 ** | 2.29 ns | 0.95 ns | 1630.55 ** | 2.74 ns | 0.95 ns | 0.35 ns | 25.36 ** | 0.30 ns | 0.92 ns | 1.39 ns | 0.98 ns | 1.08 ns | 0.44 ns | 0.71 ns |
| C20:1 | 18.82 ** | 14.86 ** | 0.59 ns | 953.84 ** | 0.40 ns | 1.07 ns | 0.12 ns | 3.88 ** | 2.30 ns | 0.57 ns | 1.15 ns | 0.55 ns | 0.51 ns | 0.64 ns | 0.92 ns |
| C22:1 | 2.20 ns | 3.39 ns | 0.16 ns | 1.89 ns | 1.57 ns | 0.99 ns | 0.69 ns | 0.57 ns | 0.84 ns | 1.26 ns | 0.48 ns | 1.43 ns | 0.60 ns | 1.04 ns | 0.86 ns |
| SFA | 71.35 ** | 0.99 ns | 2.98 * | 3037.88 ** | 0.44 ns | 0.75 ns | 1.14 ns | 47.25 ** | 0.55 ns | 0.97 ns | 1.06 ns | 0.69 ns | 1.02 ns | 1.26 ns | 1.05 ns |
| UFA | 60.66 ** | 0.59 ns | 1.67 ns | 663.55 ** | 0.29 ns | 1.06 ns | 0.84 ns | 32.25 ** | 1.28 ns | 1.04 ns | 0.80 ns | 0.80 ns | 0.61 ns | 0.85 ns | 1.02 ns |
| MUFA | 120.91 ** | 0.39 ns | 1.19 ns | 8586.24 ** | 3.87 * | 1.37 ns | 0.56 ns | 72.18 ** | 0.60 ns | 1.77 ns | 1.13 ns | 1.25 ns | 0.53 ns | 1.17 ns | 1.39 ns |
| PUFA | 188.16 ** | 0.27 ns | 1.03 ns | 7587.9 ** | 3.68 * | 1.49 ns | 0.43 ns | 80.02 ** | 0.45 ns | 1.67 ns | 1.09 ns | 1.23 ns | 0.68 ns | 1.23 ns | 1.49 ns |
| Glucosinolate content [μM g−1 seeds] | |||||||||||||||
| glnap | 68.29 ** | 7.03 ** | 20.26 ** | 157.23 ** | 0.27 ns | 1.44 ns | 1.00 ns | 51.73 ** | 0.99 ns | 2.61 * | 0.72 ns | 0.76 ns | 0.84 ns | 1.63 ns | 0.97 ns |
| glbra | 38.46 ** | 9.12 ** | 24.83 ** | 906.02 ** | 1.19 ns | 1.08 ns | 0.85 ns | 135.09 ** | 2.48 * | 1.67 ns | 0.42 ns | 0.80 ns | 0.61 ns | 1.22 ns | 1.07 ns |
| progo | 4.54 ns | 16.88 ** | 16.14 ** | 36.47 ** | 0.65 ns | 0.58 ns | 1.36 ns | 44.46 ** | 2.01 ns | 2.34 * | 0.55 ns | 0.76 ns | 1.07 ns | 1.63 ns | 0.93 ns |
| napol | 2.27 ns | 2.38 ns | 14.74 ** | 320.82 ** | 0.90 ns | 1.10 ns | 0.90 ns | 23.48 ** | 3.49 ** | 7.01 ** | 0.52 ns | 0.76 ns | 0.34 ns | 2.06 * | 1.56 ns |
| ind | 1.40 ns | 1.51 ns | 1.56 ns | 21.01 ** | 1.20 ns | 0.49 ns | 0.78 ns | 8.53 ** | 1.81 ns | 0.84 ns | 0.98 ns | 1.14 ns | 1.50 ns | 0.49 ns | 0.64 ns |
| 4-OH | 5.66 * | 3.21 * | 0.67 ns | 36.67 ** | 0.71 ns | 0.83 ns | 0.65 ns | 5.51 ** | 1.36 ns | 1.28 ns | 0.66 ns | 0.31 ns | 0.36 ns | 0.51 ns | 1.14 ns |
| alkenyl | 15.2 ** | 13.27 ** | 20.40 ** | 29.67 ** | 0.45 ns | 0.60 ns | 1.20 ns | 61.57 ** | 0.98 ns | 2.69 * | 0.63 ns | 0.64 ns | 0.94 ns | 1.72 ns | 0.96 ns |
| indole | 5.31 * | 3.61 * | 0.56 ns | 35.61 ** | 0.78 ns | 1.05 ns | 0.62 ns | 6.14 ** | 1.36 ns | 1.17 ns | 0.65 ns | 0.32 ns | 0.42 ns | 0.52 ns | 1.09 ns |
| total | 1.35 ns | 13.28 ** | 11.89 ** | 52.04 ** | 0.81 ns | 0.68 ns | 1.17 ns | 24.71 ** | 0.08 ns | 2.66 * | 0.82 ns | 0.64 ns | 0.61 ns | 1.33 ns | 1.08 ns |
| Content | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor/Level | g kg−1 DM | Fatty Acids [%] | |||||||||||
| Crude Fat | Total Protein | C16 | C18 | C18:1 | C18:2 | C18:3 | C20:1 | C22:1 | SFA | UFA | MUFA | PUFA | |
| 2015/2016 | 469.0 a† | 208.2 c | 4.18 a | 1.65 c | 71.6 c | 13.7 a | 7.65 a | 1.20 b | 0.01 | 5.83 c | 92.9 a | 72.8 c | 21.4 a |
| 2016/2017 | 461.5 b | 223.4 b | 3.91 b | 2.12 b | 74.8 a | 11.2 c | 6.66 c | 1.27 a | 0.00 | 6.03 b | 92.7 b | 76.1 a | 17.9 c |
| 2017/2018 | 448.7 c | 227.9 a | 3.93 b | 2.29 a | 72.9 b | 12.6 b | 6.96 b | 1.31 a | 0.01 | 6.22 a | 92.5 c | 74.2 b | 19.6 b |
| N100 | 465.7 a | 214.2 b | 4.02 | 2.03 | 73.1 | 12.5 | 7.15 | 1.23 b | 0.00 | 6.05 | 92.7 | 74.3 | 19.6 |
| N160 | 459.2 b | 221.1 a | 4.00 | 2.03 | 73.1 | 12.5 | 7.06 | 1.26 a | 0.01 | 6.03 | 92.7 | 74.4 | 19.6 |
| N220 | 455.2 b | 224.3 a | 4,00 | 2.01 | 73.1 | 12.6 | 7.06 | 1.28 a | 0.02 | 6.00 | 92.7 | 74.4 | 19.6 |
| S0 | 458.4 b | 221.6 a | 4.02 | 2.04 a | 73.0 | 12.6 | 7.05 | 1.26 | 0.01 | 6.06 a | 92.7 | 74.3 | 19.7 |
| S30 | 461.7 a | 218.6 b | 4.01 | 2.02 ab | 73.2 | 12.4 | 7.09 | 1.27 | 0.01 | 6.02 ab | 92.7 | 74.4 | 19.5 |
| S60 | 459.5 ab | 219.4 b | 4.00 | 2.00 b | 73.1 | 12.5 | 7.13 | 1.25 | 0.01 | 6.00 b | 92.7 | 74.3 | 19.7 |
| S90 | 460.5 ab | 219.9 ab | 4,00 | 2.02 ab | 73.2 | 12.4 | 7.08 | 1.25 | 0.01 | 6.02 ab | 92.7 | 74.4 | 19.5 |
| Monolit | 470.9 a | 212.3 b | 4.67 a | 2.07 a | 66.3 c | 17.7 a | 8.21 a | 1.02 c | 0.01 | 6.74 a | 92.2 c | 67.3 c | 25.9 a |
| Polka | 450.0 c | 222.9 a | 3.42 c | 1.97 c | 77.3 a | 8.25 c | 7.55 b | 1.51 a | 0.00 | 5.39 c | 93.1 a | 78.8 a | 15.8 c |
| PN440 | 459.2 b | 224.3 a | 3.93 b | 2.02 b | 75.7 b | 11.6 b | 5.51 c | 1.24 a | 0.02 | 5.95 b | 92.8 b | 76.9 b | 17.1 b |
| Growing Season | Factor/Level | Content | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g kg−1 DM | Fatty Acids [%] | |||||||||||||
| Crude Fat | Total Protein | C16 | C18 | C18:1 | C18:2 | C18:3 | C20:1 | C22:1 | SFA | UFA | MUFA | PUFA | ||
| 2015/2016 | Monolit | 476.8 a† | 201.7 f | 4.92 a | 1.76 e | 65.1 g | 18.4 a | 8.74 a | 0.99 f | 0.01 | 6.68 b | 92.3 de | 66.1 h | 27.2 a |
| Polka | 464.9 cd | 211.7 e | 3.60 g | 1.62 f | 75.9 c | 9.56 e | 7.85 b | 1.46 b | 0.00 | 5.22 g | 93.3 a | 77.4 d | 17.4 d | |
| PN440 | 468.0 bc | 211.4 e | 4.03 d | 1.56 g | 73.8 e | 13.1 c | 6.35 e | 1.16 d | 0.03 | 5.59 e | 93.2 ab | 74.9 f | 19.5 c | |
| 2016/2017 | Monolit | 471.4 b | 216.7 d | 4.61 b | 2.14 c | 67.1 f | 17.2 b | 8.00 b | 1.0 f | 0.00 | 6.75 ab | 92.2 ef | 68.1 g | 25.2 b |
| Polka | 451.7 e | 225.5 c | 3.30 i | 2.04 d | 78.8 a | 7.11 g | 7.22 d | 1.52 a | 0.00 | 5.34 f | 93.1 b | 80.3 a | 14.3 f | |
| PN440 | 461.5 d | 228.0 b | 3.81 f | 2.18 c | 78.6 a | 9.38 e | 4.76 g | 1.28 c | 0.00 | 6.00 d | 92.8 c | 79.9 b | 14.1 f | |
| 2017/2018 | Monolit | 464.6 cd | 218.6 d | 4.48 c | 2.31 a | 66.8 f | 17.5 b | 7.89 b | 1.07 e | 0.02 | 6.79 a | 92.1 f | 67.8 g | 25.4 b |
| Polka | 433.4 g | 231.7 a | 3.36 h | 2.25 b | 77.2 b | 8.08 f | 7.58 c | 1.55 a | 0.00 | 5.61 e | 92.8 c | 78.7 c | 15.7 e | |
| PN440 | 448.1 f | 233.5 a | 3.95 e | 2.31 a | 74.7 d | 12.3 d | 5.42 f | 1.29 c | 0.02 | 6.26 c | 92.4 d | 76.0 e | 17.7 d | |
| 2015/2016 | N100 | 474.5 | 204.7 | 4.19 | 1.64 | 71.7 d | 13.60 | 7.67 | 1.17 | 0.00 | 5.83 | 93.0 | 72.9 d | 21.3 a |
| N160 | 470.0 | 208.2 | 4.19 | 1.64 | 71.5 d | 13.74 | 7.66 | 1.21 | 0.02 | 5.83 | 92.9 | 72.8 d | 21.4 a | |
| N220 | 465.3 | 211.8 | 4.17 | 1.66 | 71.5 d | 13.79 | 7.61 | 1.23 | 0.03 | 5.83 | 92.9 | 72.8 d | 21.4 a | |
| 2016/2017 | N100 | 468.2 | 216.0 | 3.92 | 2.14 | 74.7 a | 11.20 | 6.80 | 1.24 | 0.00 | 6.06 | 92.7 | 75.9 b | 18.0 d |
| N160 | 459.6 | 227.0 | 3.90 | 2.12 | 74.8 a | 11.29 | 6.66 | 1.27 | 0.00 | 6.01 | 92.7 | 76.0 ab | 17.9 de | |
| N220 | 456.8 | 227.2 | 3.91 | 2.10 | 75.0 a | 11.18 | 6.51 | 1.29 | 0.00 | 6.01 | 92.7 | 76.3 a | 17.7 e | |
| 2017/2018 | N100 | 454.6 | 221.8 | 3.95 | 2.30 | 72.9 bc | 12.63 | 6.98 | 1.29 | 0.00 | 6.25 | 92.5 | 74.2 c | 19.6 bc |
| N160 | 448.0 | 228.1 | 3.93 | 2.32 | 73.1 b | 12.50 | 6.88 | 1.30 | 0.00 | 6.25 | 92.5 | 74.4 c | 19.4 c | |
| N220 | 443.5 | 234.0 | 3.92 | 2.26 | 72.7 c | 12.72 | 7.04 | 1.33 | 0.04 | 6.18 | 92.4 | 74.1 c | 19.8 b | |
| 2015/2016 | S0 | 469.1 | 208.8 | 4.22 a | 1.65 | 71.5 | 13.8 | 7.61 | 1.18 | 0.00 | 5.88 | 92.9 | 72.7 | 21.4 |
| S30 | 473.6 | 206.6 | 4.19 ab | 1.66 | 71.7 | 13.6 | 7.60 | 1.22 | 0.02 | 5.84 | 92.9 | 73.0 | 21.2 | |
| S60 | 468.9 | 208.8 | 4.16 b | 1.64 | 71.4 | 13.8 | 7.72 | 1.21 | 0.03 | 5.80 | 92.9 | 72.7 | 21.5 | |
| S90 | 468.1 | 208.9 | 4.16 b | 1.64 | 71.7 | 13.7 | 7.66 | 1.20 | 0.01 | 5.81 | 93.0 | 72.9 | 21.3 | |
| 2016/2017 | S0 | 459.9 | 225.2 | 3.92 cd | 2.15 | 74.6 | 11.4 | 6.69 | 1.27 | 0.00 | 6.07 | 92.7 | 75.8 | 18.1 |
| S30 | 462.1 | 222.4 | 3.92 cd | 2.10 | 74.8 | 11.3 | 6.66 | 1.27 | 0.00 | 6.02 | 92.7 | 76.1 | 17.9 | |
| S60 | 461.2 | 223.3 | 3.91 d | 2.10 | 74.8 | 11.2 | 6.71 | 1.25 | 0.00 | 6.01 | 92.7 | 76.1 | 17.9 | |
| S90 | 462.9 | 222.7 | 3.89 c | 2.13 | 75.1 | 11.0 | 6.59 | 1.26 | 0.00 | 6.02 | 92.7 | 76.4 | 17.6 | |
| 2017/2018 | S0 | 446.2 | 230.9 | 3.92 cd | 2.32 | 72.9 | 12.6 | 6.87 | 1.32 | 0.03 | 6.23 | 92.4 | 74.3 | 19.5 |
| S30 | 449.5 | 226.7 | 3.91 d | 2.30 | 72.9 | 12.5 | 7.02 | 1.31 | 0.00 | 6.21 | 92.5 | 74.2 | 19.6 | |
| S60 | 448.4 | 226.1 | 3.94 cd | 2.27 | 72.9 | 12.7 | 6.98 | 1.29 | 0.00 | 6.20 | 92.5 | 74.2 | 19.6 | |
| S90 | 450.6 | 228.0 | 3.97 c | 2.28 | 72.8 | 12.7 | 6.99 | 1.30 | 0.03 | 6.25 | 92.4 | 74.1 | 19.7 | |
| Factor/Level | Glucosinolate Content [μM g−1 Seeds] | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| glnap | glbra | progo | naplo | indol | 4-OH | Alkenyl GLS | Indole GLS | Total GLS | ||||
| rel | rel | rel | ||||||||||
| 2015/2016 | 1.84 c† | 0.63 b | 4.38 | 0.19 | 0.11 | 5.59 a | 7.04 b | 100 | 5.70 a | 100 | 12.7 | 100 |
| 2016/2017 | 2.07 b | 0.85 a | 4.76 | 0.16 | 0.10 | 4.98 b | 7.84 a | 112 | 5.08 b | 89 | 12.9 | 102 |
| 2017/2018 | 2.53 a | 0.80 a | 4.73 | 0.12 | 0.11 | 4.89 b | 8.18 a | 116 | 5.00 b | 88 | 13.2 | 104 |
| N100 | 2.06 b | 0.72 b | 4.34 b | 0.16 | 0.10 | 5.03 b | 7.28 b | 100 | 5.13 b | 100 | 12.4 b | 100 |
| N160 | 2.18 a | 0.76 ab | 4.70 a | 0.15 | 0.10 | 5.14 ab | 7.79 a | 107 | 5.24 ab | 102 | 13.0 a | 105 |
| N220 | 2.21 a | 0.79 a | 4.82 a | 0.17 | 0.11 | 5.30 a | 7.99 a | 110 | 5.41 a | 106 | 13.4 a | 108 |
| S0 | 2.04 c | 0.68 c | 4.36 c | 0.13 b | 0.10 | 5.14 | 7.21 c | 100 | 5.24 | 100 | 12.4 b | 100 |
| S30 | 2.12 bc | 0.75 b | 4.59 b | 0.16 a | 0.11 | 5.22 | 7.62 b | 106 | 5.33 | 102 | 12.9 a | 104 |
| S60 | 2.18 ab | 0.79 ab | 4.69 ab | 0.17 a | 0.11 | 5.10 | 7.83 ab | 109 | 5.21 | 100 | 13.0 a | 105 |
| S90 | 2.25 a | 0.81 a | 4.84 a | 0.17 a | 0.11 | 5.15 | 8.07 a | 112 | 5.26 | 101 | 13.3 a | 107 |
| Monolit | 1.91 c | 0.45 c | 4.89 a | 0.22 a | 0.09 b | 5.07 b | 7.47 b | 100 | 5.16 b | 100 | 12.6 b | 100 |
| Polka | 2.38 a | 1.02 a | 4.53 b | 0.14 b | 0.11 a | 5.49 a | 8.09 a | 108 | 5.60 a | 108 | 13.7 a | 109 |
| PN440 | 2.15 b | 0.80 b | 4.44 b | 0.11 c | 0.12 a | 4.90 c | 7.50 b | 100 | 5.02 b | 97 | 12.5 b | 99 |
| Factor/Level | Glucosinolate Content [μM g−1 seeds] | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| glnap | glbra | progo | naplo | indol | 4-OH | Alkenyl GLS | Indole GLS | Total GLS | |||||
| rel | rel | rel | |||||||||||
| Monolit | S0 | 1.83 h† | 0.38 | 4.61 c | 0.17 b | 0.09 | 5.02 | 6.99 e | 100 | 5.11 | 100 | 12.1 f | 100 |
| S30 | 1.88 h | 0.46 | 4.92 ab | 0.24 a | 0.10 | 5.23 | 7.50 cd | 107 | 5.33 | 104 | 12.8 cde | 106 | |
| S60 | 1.91 gh | 0.45 | 4.90 b | 0.24 a | 0.10 | 4.93 | 7.50 cd | 107 | 5.02 | 98 | 12.5 ef | 103 | |
| S90 | 2.02 fg | 0.48 | 5.15 a | 0.24 a | 0.09 | 5.11 | 7.89 bc | 113 | 5.20 | 102 | 13.1 cd | 108 | |
| Polka | S0 | 2.22 cd | 0.93 | 4.22 d | 0.12 def | 0.11 | 5.47 | 7.49 cd | 100 | 5.58 | 100 | 13.1 cde | 100 |
| S30 | 2.31 bc | 1.02 | 4.41 cd | 0.15 bc | 0.11 | 5.42 | 7.89 bc | 105 | 5.51 | 99 | 13.4 bc | 102 | |
| S60 | 2.43 b | 1.05 | 4.60 c | 0.14 cd | 0.11 | 5.55 | 8.22 b | 110 | 5.66 | 101 | 13.9 ab | 106 | |
| S90 | 2.57 a | 1.10 | 4.91 ab | 0.17 b | 0.11 | 5.54 | 8.75 a | 117 | 5.65 | 101 | 14.4 a | 110 | |
| PN440 | S0 | 2.06 ef | 0.76 | 4.24 d | 0.09 g | 0.11 | 4.92 | 7.15 de | 100 | 5.03 | 100 | 12.2 f | 100 |
| S30 | 2.15 def | 0.77 | 4.45 cd | 0.11 efg | 0.11 | 5.04 | 7.48 cd | 105 | 5.15 | 102 | 12.6 def | 103 | |
| S60 | 2.20 cd | 0.87 | 4.58 c | 0.13 cde | 0.12 | 4.83 | 7.78 c | 109 | 4.95 | 98 | 12.7 def | 101 | |
| S90 | 2.18 cde | 0.82 | 4.48 cd | 0.10 fg | 0.12 | 4.81 | 7.58 c | 106 | 4.93 | 98 | 12.5 ef | 102 | |
| Monolit | N100 | 1.78 | 0.44 e | 4.49 | 0.23 a | 0.09 | 5.03 | 6.94 | 100 | 5.12 | 100 | 12.1 | 100 |
| N160 | 1.94 | 0.45 e | 5.03 | 0.21 a | 0.09 | 5.04 | 7.63 | 110 | 5.13 | 100 | 12.8 | 106 | |
| N220 | 1.99 | 0.45 e | 5.18 | 0.22 a | 0.10 | 5.17 | 7.84 | 113 | 5.27 | 103 | 13.1 | 109 | |
| Polka | N100 | 2.33 | 0.98 b | 4.34 | 0.14 bc | 0.11 | 5.28 | 7.79 | 100 | 5.38 | 100 | 13.2 | 100 |
| N160 | 2.42 | 1.03 ab | 4.61 | 0.13 c | 0.11 | 5.44 | 8.19 | 105 | 5.55 | 103 | 13.7 | 104 | |
| N220 | 2.41 | 1.07 a | 4.65 | 0.16 b | 0.11 | 5.75 | 8.29 | 106 | 5.88 | 109 | 14.2 | 108 | |
| PN440 | N100 | 2.06 | 0.75 d | 4.19 | 0.10 d | 0.12 | 4.77 | 7.10 | 100 | 4.89 | 100 | 12.0 | 100 |
| N160 | 2.17 | 0.79 cd | 4.48 | 0.10 d | 0.11 | 4.95 | 7.54 | 106 | 5.06 | 103 | 12.6 | 105 | |
| N220 | 2.22 | 0.86 c | 4.65 | 0.12 cd | 0.12 | 4.97 | 7.85 | 111 | 5.09 | 104 | 12.9 | 108 | |
| 2015/2016 | Monolit | 1.73 g | 0.49 c | 4.73 cd | 0.28 a | 0.09 d | 5.29 bc | 7.23 d | 100 | 5.38 c | 100 | 12.6 c | 100 |
| Polka | 2.20 cd | 0.98 b | 4.73 cd | 0.19 c | 0.10 cd | 5.95 a | 8.10 b | 112 | 6.05 a | 113 | 14.2 a | 113 | |
| PN440 | 1.58 h | 0.43 cd | 3.69 f | 0.10 ef | 0.13 a | 5.55 b | 5.80 e | 80 | 5.68 b | 106 | 11.5 d | 91 | |
| 2016/2017 | Monolit | 1.85 f | 0.44 cd | 5.16 a | 0.23 b | 0.10 cd | 5.12 cd | 7.68 c | 100 | 5.22 cd | 100 | 12.9 c | 100 |
| Polka | 2.31 c | 1.12 a | 4.50 de | 0.14 d | 0.10 cd | 5.28 bc | 8.07 b | 105 | 5.38 c | 103 | 13.4 b | 104 | |
| PN440 | 2.03 e | 0.99 b | 4.62 cd | 0.12 de | 0.10 cd | 4.58 e | 7.78 bc | 101 | 4.67 e | 90 | 12.4 c | 96 | |
| 2017/2018 | Monolit | 2.15 d | 0.41 d | 4.79 bc | 0.17 c | 0.09 d | 4.83 de | 7.52 cd | 100 | 4.92 de | 100 | 12.4 c | 100 |
| Polka | 2.64 b | 0.98 b | 4.38 e | 0.10 ef | 0.13 a | 5.25 c | 8.10 b | 108 | 5.38 c | 109 | 13.5 b | 109 | |
| PN440 | 2.82 a | 0.99 b | 5.01 ab | 0.09 f | 0.12 b | 4.57 e | 8.91 a | 119 | 4.69 e | 95 | 13.6 b | 110 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spasibionek, S.; Wielebski, F.; Liersch, A.; Walkowiak, M. The Influence of Nitrogen and Sulfur Fertilization on Oil Quality and Seed Meal in Different Genotypes of Winter Oilseed Rape (Brassica napus L.). Agriculture 2024, 14, 1232. https://doi.org/10.3390/agriculture14081232
Spasibionek S, Wielebski F, Liersch A, Walkowiak M. The Influence of Nitrogen and Sulfur Fertilization on Oil Quality and Seed Meal in Different Genotypes of Winter Oilseed Rape (Brassica napus L.). Agriculture. 2024; 14(8):1232. https://doi.org/10.3390/agriculture14081232
Chicago/Turabian StyleSpasibionek, Stanisław, Franciszek Wielebski, Alina Liersch, and Magdalena Walkowiak. 2024. "The Influence of Nitrogen and Sulfur Fertilization on Oil Quality and Seed Meal in Different Genotypes of Winter Oilseed Rape (Brassica napus L.)" Agriculture 14, no. 8: 1232. https://doi.org/10.3390/agriculture14081232
APA StyleSpasibionek, S., Wielebski, F., Liersch, A., & Walkowiak, M. (2024). The Influence of Nitrogen and Sulfur Fertilization on Oil Quality and Seed Meal in Different Genotypes of Winter Oilseed Rape (Brassica napus L.). Agriculture, 14(8), 1232. https://doi.org/10.3390/agriculture14081232






