Abstract
The aim of this study was to develop ready-to-eat vegetable–herb mixes with high nutritional and sensory values as well as good storability. In this regard, the suitability of fresh herbs (peppermint, oregano, green basil, red basil, and parsley) was tested for their use in mixes with fresh-cut iceberg lettuce. Lettuce–herb mixtures were stored for 6 days at 5 °C. The reason for the decrease in the appearance of the salads was the browning of the cut surface of the lettuce, as well as discoloration on the cut herbs. Comparing the storage abilities of the cut herbs, red basil and parsley retained the best appearance for 6 d at 5 °C. A small addition of herbs to fresh-cut iceberg lettuce caused a significant increase (p < 0.05) in the contents of pro-health ingredients such as chlorophyll, carotenoids, L-ascorbic acid, and polyphenols in the mixes. There were large discrepancies in the sensory quality of the mixes, but the highest quality and consumer acceptance were found for salads with parsley (5% and 10%) and red basil (5%). After harvest, the fresh herbs were more contaminated by molds than the iceberg lettuce. Bacterial, yeast, and mold contamination increased during storage, but the rate of mold growth was much lower in the mixes with parsley compared to lettuce alone. In conclusion, the addition of parsley and mint contributed the most to the health-promoting and microbiological properties of iceberg lettuce salads. However, according to sensory evaluation, parsley and red basil contributed the most to improving the acceptability of the product in terms of best taste and shelf life.
1. Introduction
The demand for fresh-cut vegetables is increasing, especially in metropolitan cities. They are perishable products with a short shelf life. Physiological decomposition, microbial growth, and sensory deterioration are the main reasons for quality reduction and shelf-life shortening. The industry is constantly launching new species and varieties to meet consumer demands for convenience, freshness, safety, and health-promoting values. Increasing the consumption of fresh-cut fruits and vegetables is associated with numerous reports of foodborne disease outbreaks [1,2,3,4,5]. Therefore, the need to optimize anti-contamination conditions for produce has become a global problem [6,7,8].
Currently, chlorine is the most widely used disinfectant in the light processing of horticultural products [7,9,10,11]; however, the use of synthetic additives to reduce the spoilage of fresh-cut vegetables has raised consumer concerns due to chemical residues and their effect on human health. In contrast, the technologies based on natural additives to reduce losses and ensure microbiological safety are widely accepted. Aromatic herbs have been used for centuries for seasoning dishes because of their distinctive flavor and properties that improve well-being and reduce the risk of developing diseases [12,13]. Santos et al. [14] stated that from a global standpoint, the addition of fresh-cut aromatic herbs to vegetable salads seems to be a good direction because of the incremental nutritional value and antimicrobial activity. Most vegetables compose very well with fresh herbs, so they can be considered as adding value both in terms of culinary seasoning and natural preservative properties. Freshly chopped broccoli, cabbage, carrots, onions, cucumber, and tomatoes can be mixed with aromatic species such as the following: thyme, oregano, rosemary, cilantro, ginger, bay, and garlic [15].
According to Scollard et al. [16], some cut herbs exhibit antiradical activity, while according to Ninfali et al. [17], a small addition of herbs to lettuce results in an increase in oxidative radical scavenging capacity. Santos et al. [14] demonstrate that fresh-cut herbs show only minor changes in phytonutrient content during storage. The aim of this study was to determine the health-promoting properties, microbiological quality, and storage ability of mixes of fresh-cut iceberg lettuce with fresh herbs. An important challenge was to develop a ready-to-eat product with improved nutritional and health properties, as well as high sensory qualities acceptable to consumers. Estimating the storage life of lettuce–herb mixes was also an important part of this study.
2. Material and Methods
2.1. Plant Material
The study was conducted on iceberg lettuce (Lactuca sativa L. var. capitata) obtained from a commercial farm near Skierniewice (Poland). The lettuce cv. Ice Wave F1 was grown for summer harvest. The herbs that were used as admixtures to fresh-cut lettuce were cultivated in the experimental field of the National Institute of Horticultural Research in Skierniewice. There were two perennial species, peppermint (Mentia piperita) and oregano (Origanum vulgare), and three annuals, red basil (Ocinum basilicum purpurascens), green basil (Ocimum basilicum L.), and flat-leaf parsley (Petroselinum crispum). Immediately after harvest, the iceberg lettuce and herbs were transported to the storage laboratory and stored at 0–1 °C.
2.2. Setting up Storage Experiments
Storage experiments were set up one day after the harvesting of lettuce and herbs. The lettuce was transferred from cold storage to room temperature (16–20 °C). The inner part of the head was taken for testing after removing the outer leaves, which protected them from the direct influence of the environment during growth. This is usually how lettuce heads are prepared for cutting. Next, the lettuce was cut into strips 0.5–1.0 cm wide. The herbs were also acclimated to room temperature and washed in tap water that was 5 °C warmer than the temperature of the herbs. After washing, the herbs were placed on special sieves to dry and then cut into 2–3 mm strips. Lettuce–herb mixes were prepared separately for each sample, weighing the appropriate amount of cut lettuce and cut herbs. The number of herbs added to the iceberg lettuce was previously determined by the sensory panel based on a comparison of the taste and aroma of mixes containing 3%, 5%, and 10% of herbal additives. Flavor evaluation was carried out on samples immediately after cutting. The sensory panel recommended that thyme, rosemary, and marjoram be completely eliminated from the study due to unacceptable sensory quality (unpleasant taste in the mixture with lettuce). As for other herbs, mixes with an acceptable flavor and aroma were selected and subjected to a storage experiment. The following lettuce–herb mixes were prepared:
- -
- Mix with red basil: 95% fresh-cut lettuce and 5% fresh-cut red basil;
- -
- Mix with green basil: 97% fresh-cut lettuce and 3% fresh-cut green basil;
- -
- Mix with peppermint: 95% fresh-cut lettuce and 5% fresh-cut peppermint;
- -
- Mix with oregano: 97% fresh-cut lettuce and 3% fresh-cut oregano;
- -
- Mix with leaf parsley (90%): 90% fresh-cut lettuce and 10% fresh-cut leaf parsley;
- -
- Mix with leaf parsley (95%): 95% fresh-cut lettuce and 5% fresh-cut leaf parsley;
- -
- Control: 100% fresh-cut lettuce.
The overall weight of each sample was 100 g. The mixes were packed into transparent polypropylene boxes recommended for food products, including fruit, vegetables, and salads. The boxes were 154 mm × 98 mm × 70 mm (L × W × H) with a capacity of 1.0 L (GUILLIN W1/059C). The boxes were covered with lids (GUILLIN W2/001) with 77 punctures, each 120 µm in diameter, for air exchange (Figure 1).

Figure 1.
Mix of fresh-cut iceberg lettuce with parsley packed in GUILLIN W1/059C box and covered with GUILLIN W2/001 lid.
Each individual storage experiment was conducted in four replicates. The experiments were repeated three times, resulting in a total of 12 replicates for each experimental object. In the second experiment, an additional two samples each were prepared for chemical and sensory analyses and four for microbiological analyses. In the third experiment, an additional two samples each were prepared for chemical and sensory analyses and six for microbiological analyses. All samples were stored at 5 °C. The storage times were as follows: 6 days for storage samples, 3 days for chemical samples, 4 days for sensory samples, 0 days for half of the microbiological samples, and 3 days for the remaining microbiological samples.
2.3. Quality Assessment
A quality assessment was performed every two days during storage, for up to 6 d. The following vegetable characteristics were visually assessed: wilting/softening, browning/discoloration of cut surface, rotting, and marketable value. The evaluation was conducted on the basis of the 9-grade scoring scale:
- -
- Wilting/softening: 1—no signs of wilting and softening, 2—very light, 3—light, 4—light to medium, 5—medium, 6—medium to strong, 7—strong, 8—strong to very strong, 9—very strong;
- -
- Browning/discoloration of the cut surface: 1—no signs of discoloration, 2—very beginning of discoloration, 3—very light discoloration, 4—creamy-beige, 5—light brown, 6—quite strong, 7—medium brown, 8—strong to very strong, 9—brown;
- -
- Rotting: 1—no signs of rotting, 2—1 small spot, 3—up to 3 small spots, 4—up to 5 small spots, 5—light (1–5% of spoiled material), 6—quite strong (5–10% of spoiled material), 7—strong (10–20% of spoiled material), 8—strong to very strong (20–50% of spoiled material), 9—very strong (>50% of spoiled material);
- -
- Marketable value: 1—no marketable and useable value, 2—not marketable but useable for feed, 3—limited, 4—limited to fair, 5—fair (appearance of slight to moderate defects—marketability threshold), 6—fair to good, 7—good, 8—very good, 9—excellent.
The visual quality of the lettuce and herbs was evaluated separately. The weight loss of the whole sample was calculated based on the difference between the initial weight and the weight measured every two days during storage. The difference was divided by the initial weight and expressed as a percentage.
2.4. Chemical Analysis
Chemical analyses were performed on whole samples after 3 d of storage at 5 °C. The chlorophyll and carotenoids were measured via a spectrophotometric method according to Lichtenthaler and Wellburn [18], with modifications by Sumanta et al. [19]. The plant samples (2 g) were homogenized with 10 mL cold 80% acetone and centrifuged at 8500 relative centrifugal force (RCF) at 4 °C for 15 min. The supernatant (0.5 mL) was mixed with 4.5 mL of 80% acetone. The absorbance was measured using a Cary 300 Bio UV-Vis spectrophotometer (Varian, Mulgrave, Australia) at the following wavelengths: 646.8 nm for chlorophyll a, 663.2 nm for chlorophyll b, and 470 nm for carotenoids. The total chlorophyll is the sum content of chlorophyll a and chlorophyll b. The results were recalculated and expressed in mg kg−1 fresh weight of the analyzed sample.
The polyphenol content was measured using the modified spectrophotometric method of Tsao and Yang [20]. The plant samples (10 g) were homogenized with 50 mL of 70% ethanol and centrifuged at 20,000 RCF for 10 min. The collected supernatant (0.4 mL) was mixed with 2 mL of Folin–Ciocalteu’s phenol reagent. Then, 7.5% sodium carbonate solution (1.6 mL) was added and the mixture was shaken. After 30 min, the absorbance was read against the prepared blank at 765 nm at an ambient temperature in dark conditions. The polyphenol content was expressed as mg of gallic acid equivalents per mg kg−1 fresh weight of the analyzed plant material.
The ascorbic acid content was determined using high-performance liquid chromatography. The samples (5 g) were homogenized with 50 mL of 6% HPO3, then the collected supernatant was filtered through a 0.45 µm Teflon filter. The Agilent 1200 HPLC system, equipped with a diode array detector (DAD), was used. The separation was conducted on two Supelcosil LC-18 columns (250 mm × 4.6 mm; 5 µm) with a precolumn according to IFU (Instructions for Use) [21] procedures. A phosphate-buffered solution (1% KH2PO4; pH 2.5) was used as the mobile phase. The column temperature was kept at 30 °C with a flow rate of 0.8 mL/min. The detection of ascorbic acid was performed using absorbance at a 244 nm wavelength. The samples (5 g) for acid determinations were homogenized with 50 mL of 6% HPO3, then filtered through a 0.45 µm Teflon filter. The results were expressed as mg kg−1 fresh weight.
2.5. Sensory Analysis
Sensory analyses were performed on whole samples after 4 d of storage at 5 °C. The evaluation was carried out according to the accredited quantitative descriptive analysis (QDA) method in accordance with the implementation procedure described in ISO 13299:2016 [22]. The following quality attributes were selected and defined: color, crispness, lettuce flavor, herbal aroma, sweet flavor, overall quality, and consumer acceptance. An unstructured linear scale was used with marked threshold values from 0 to 10 contract units (CU), where 0 meant no intensity and 10 meant a high intensity of the attribute. Analsens computer software was used during the evaluations. The evaluations were carried out at the sensory analysis laboratory, which meets all the requirements specified in PN-EN ISO 8589:2010/A1:2014-07 [23] for sensory analysis laboratories. A sensory characterization of samples was carried out in two independent repetitions by a 10-member evaluation team. The judges had received methodological training in sensory methods and are qualified as expert evaluators in accordance with PN-EN ISO 8586:2014-3 [24]. The unit samples of the test product were placed in coded plastic containers (250 mL) covered with lids. Non-carbonated water was used between the evaluated samples as a taste neutralizer.
2.6. Microbiological Analysis
Microbiological analyses were conducted on samples of the lettuce–herb mixes after 0 and 3 d of storage. The analyses were performed according to standard methodologies: PN-EN ISO 4833-2:2013-12 [25] and PN-ISO 21527-1:2009 [26]. A total of 25 g from each 100 g sample of analyzed material was transferred into 225 mL of peptone water in sterile stomacher filter bags (400 mL). The samples were homogenized in a stomacher BagMixer® 400 P with a fixed speed of 8 stroke/s for 10 min. Furthermore, decimal dilutions were made with the same diluent and analyzed for aerobic mesophilic bacteria, yeasts, and molds. Mesophilic aerobic bacteria were enumerated on plate count agar (Darmstadt, Germany) after incubation at 30 °C for four days. Yeasts and molds were counted on yeast extract glucose chloramphenicol agar (YGC agar, Merck, Darmstadt, Germany) after incubation at 25 °C for seven days. Each object was analyzed in 2 (second experiment) and 3 (third experiment) replications. The results were expressed as colony-forming units per gram of plant material (CFU/g) and transformed for statistical calculations to decimal logarithm (log10).
2.7. Data Analysis
The experiments were set up in a one factorial design in the study, considering the herbal additive as differentiating factor of the experimental objects. The results were statistically analyzed using one-factor analysis of variance (ANOVA). The obtained mean values were compared using the Tukey HSD (Honestly Significant Difference) procedure at p = 0.05. The sensory characteristics of the evaluated plant material were described using principal component analysis (PCA) based on a correlation matrix. Calculations were carried out in the statistical package STATISTICA 13 (Dell Inc., Round Rock, TX, USA).
3. Results
3.1. Weight Loss and Storage Ability of Mixes of Fresh-Cut Iceberg Lettuce with Fresh-Cut Herbs
The weight losses of the lettuce–herb mixes were very low. After 2 d, they ranged from 0.01% to 0.04%, after 4 d from 0.04% to 0.12%, and after 6 d from 0.06% to 0.16%. The differences after 2 and 4 d were statistically insignificant. After 6 d, the statistically lowest weight loss was found for the mix of lettuce with red basil (0.06%), while the highest was found for the mix with oregano (0.16%) (Table 1).

Table 1.
The weight loss of fresh-cut iceberg lettuce in mixes with fresh-cut herbs during storage at 5 °C.
During 6 d storage, no signs of wilting or rotting were observed on either the lettuce or herbs. The decrease in the marketable value of lettuce was due to the browning of the cut surface. After the first two days, the lettuce presented as high quality (Table 2). However, significantly greater browning occurred and the lowest marketable value was estimated for the control lettuce, while the lowest browning and highest marketable value was found for the lettuce mixed with oregano. The differences between the other combinations were not significant, both in terms of browning and marketable value. Extending the storage to 4 d, the greatest browning was found for lettuce mixed with green basil, which also translated into the lowest quality of this object, although this was not statistically significant. Over the next two days, the browning of the cut surface in all objects increased, reaching an almost light brown color, and the marketable value dropped close to the score of 5 (marketability threshold). In the last days of storage, the quality of the lettuce from all mixes reached statistically equal marks for both browning and marketable value.

Table 2.
The quality of fresh-cut iceberg lettuce in mixes with fresh-cut herbs during storage at 5 °C.
Discoloration was also the main reason for the decrease in quality of the fresh-cut herbs (Table 3). There were significant differences among the tested species in susceptibility to discoloration during storage. Green basil and oregano already showed clear discoloration after two days, lowering the quality from excellent to good. These two herbs stood out from the others with the greatest discoloration and the lowest marketable value throughout the entire storage period. The specifics of these discolorations varied because the green basil became discolored all over, while the oregano only discolored on the cut edges. The darkening of the cut surface of the peppermint contributed to lowering its quality to above good after 4 d and to below good after 6 d. Fresh-cut red basil and parsley showed the best appearance during storage. These herbs showed no discoloration and maintained an excellent quality for up to 4 d. After another two days, the quality of the red basil continued to be excellent, while the freshly cut parsley showed very slight signs of yellowing. After 6 d of storage at 5 °C, the freshly cut herbs in mixes with iceberg lettuce presented with very different qualities: the red basil was excellent, the parsley was very good, and the peppermint was between good and fair, while the green basil and oregano were fair.

Table 3.
The quality of fresh-cut herbs in mixes with fresh-cut iceberg lettuce during storage at 5 °C.
It turned out that red basil, peppermint, and parsley retained better quality than lettuce during short-term storage, while the green basil and oregano had a worse quality than lettuce (Table 2 and Table 3). Despite the small addition of herbs, they were clearly visible in the mixes; the red basil and parsley throughout the storage period especially added to the visual attractiveness of the obtained product.
3.2. Effect of Herbal Additives on the Chemical Composition of Mixes with Iceberg Lettuce
The small addition of herbs to the fresh-cut lettuce had a very significant effect on improving the health-promoting properties of the created salads (Figure 2). The increase in chlorophyll content depended on the admixed herb and its proportion in the mix (Figure 2A). The smallest increase of almost 86% (not statistically significant compared to the control) was found in the mix with green basil (3% share). In the other mixes, the increase was statistically significant. Mixes with 5% added herbs (red basil, peppermint, and leaf parsley) had 3–4-fold more chlorophyll than the control samples. The mixture with 10% leaf parsley contained the most chlorophyll—7-fold higher than the control lettuce. A very similar trend regarding the effect of herbs on chlorophyll content was also found for carotenoids (Figure 2B). Comparing the experimental objects, the 3% portion of green basil contributed the least to the increase in carotenoid content in mixes with iceberg lettuce, although the increase was 65% and was statistically significant. Oregano, also applied at 3%, contributed to an increase of 126%. The higher growth of carotenoids was found in mixes with 5% herbs, and the highest was in the mix with 10% of leaf parsley (3.5-fold).
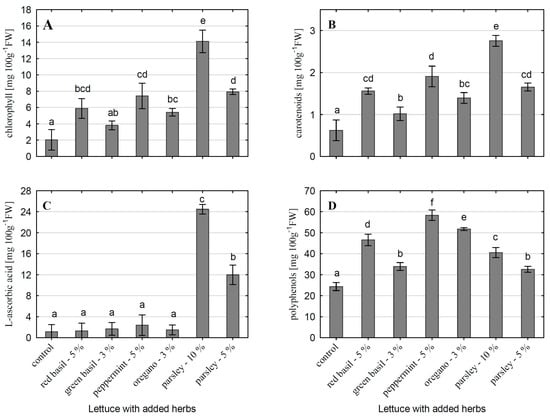
Figure 2.
The content of chemical compounds in mixes of iceberg lettuce with fresh herbs: (A)—chlorophyll, (B)—carotenoids, (C)—L-ascorbic acid, (D)—polyphenols. The analyses were carried out after 3 d of storage at 5 °C. The vertical bars represent means of 4 samples. Vertical lines represent standard deviation (SD). Each sample has a total weight of 100 g. The results are expressed in mg kg−1 fresh weight. Means followed by the different letter are significantly different (p < 0.05, Tukey test). The letter “a” means the lowest content and the further letters of the alphabet indicate a higher content.
Green and red basil, like peppermint and oregano, appeared to be poor in ascorbic acid, and their addition to fresh-cut lettuce did not affect the content of this bioactive compound (Figure 2C). Leaf parsley greatly enriched the created mixes in vitamin C. The addition of 5% resulted in about a 10.5-fold increase, and the addition of 10% resulted in an approximately 21-fold increase.
The polyphenol content of lettuce–herb mixes was significantly higher than in lettuce alone (Figure 2D). The mix with peppermint added at 5% was the richest in polyphenols (an increase of about 140%), while the mix with oregano (3%) was in second place (an increase of about 110%). In the case of the addition of green basil (3%) and parsley (5%), the increase in polyphenols in salads was the smallest in comparison with other mixes (about 34%).
3.3. Sensory Evaluation of Mixes of Fresh-Cut Lettuce with Fresh Herbs
The sensory quality of the evaluated lettuce with fresh herbs is shown in Figure 3. The map space was defined by the first two principal components, explaining 60% and 34.5% of the overall variation, respectively. The overall quality was positively related to the product acceptance score, while the herbal aroma was correlated with the flavor of fresh herbs (vectors aligned in the same direction and in close proximity). The mixes of lettuce with parsley and red basil were in the closest position in the vectors of overall quality and product acceptability ratings, which indicates their high sensory scores. Comparing salads with parsley, a higher acceptability was obtained when the admixture was 5% rather than 10%. The lettuce mixed with oregano was at the furthest distance and the opposite side of the vectors of overall quality and consumer acceptability, indicating its low sensory score.
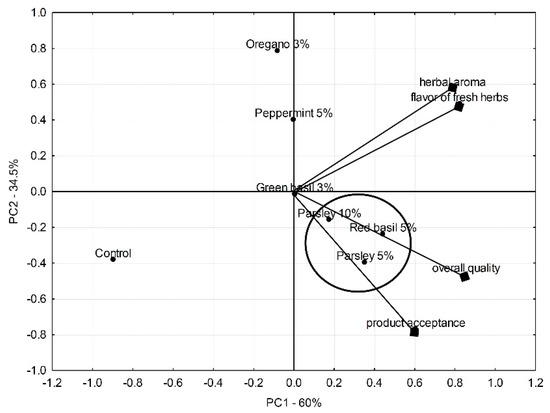
Figure 3.
Principal component analysis of sensory profiling results of fresh-cut iceberg lettuce mixed with fresh herbs: control, red basil (5%), green basil (3%), peppermint (5%), parsley (5%), and parsley (10%).
3.4. Microbiological Assessment of Fresh-Cut Iceberg Lettuce with Additives of Fresh Herbs
The addition of herbs to fresh-cut lettuce enhanced the microbial load in the mixes compared to control lettuce samples; however, the differences were significant only in the case of molds (Figure 4). The highest contamination with molds was noted for the parsley additive, especially when it was used in a dose of 10% (4.3 log10 cfu g−1) (Figure 4C). The lowest contamination of the mix was noted with the addition of oregano (1.6 log10 cfu g−1).
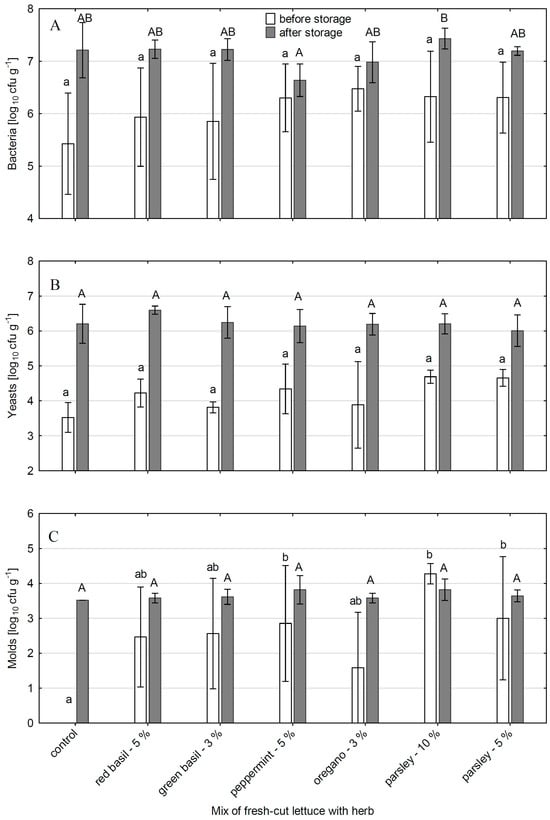
Figure 4.
The microbiological contamination of (A) bacteria, (B) yeasts, and (C) molds on iceberg lettuce with fresh herbs. The vertical bars represent means of 5 samples (white bars—after 0 days of storage, gray bars—after 3 d of storage at 5 °C). The vertical lines represent standard deviation (SD) (n = 5). From each 100 g sample, 25 g of material was taken for microbiological analysis. Means followed by the different small letter are significantly different for the value on day 0. Means followed by the different capital letter are significantly different for the value on day 3 (p ˂ 0.05 Tukey test). The letter “a” or “A” means the lowest contamination and further letters of the alphabet indicate higher contamination.
After three days of storage at 5 °C, the densities of microorganisms, estimated in lettuce without additives and in lettuce mixed with herbs, were comparable and reached an average of 7.1 log10 cfu g−1 for bacteria, 6.2 log10 cfu g−1 for yeasts, and 3.7 log10 cfu g−1 for molds (Figure 4). The differences between salads in microbial load were generally not significant. However, the addition of peppermint to lettuce lowered the microbial growth rate during storage, especially bacteria and molds. In the control, the number of bacteria increased for three days by 1.79 log10 cfu g−1, while in the mix with peppermint it rose only by 0.34 log10 cfu g−1. In the case of molds, the increase was by 3.52 log10 cfu g−1 and 0.96 log10 cfu g−1 for the control and the peppermint mix respectively (Figure 4A,C). The proliferation of molds was also inhibited in the mix of lettuce with parsley. In combination with 10% parsley, there was a decrease of 0.46 log10 cfu g−1 compared to the initial molds number estimated in this mix. In the mix with 5% parsley, the increase in the molds number was only by 0.64 log10 cfu g−1. Trends toward slower bacterial and yeast growth were also noted (although statistically unproven) (Figure 4A,B). The bacterial growth over 3 d of storage in the control object was 1.79 log10 cfu g−1, while in the mixes it was 0.34–1.37 log10 cfu g−1. The yeast growth in the control was 2.68 log10 cfu g−1, while in the mixes it was 1.35–2.43 log10 cfu g−1.
4. Discussion
The launch of mixes of fresh-cut vegetables with fresh herbs is in line with the market trend in expanding ready-to-eat products. Among minimally processed vegetables and fruits (MPVF), lettuces and other leafy vegetables occupy a prominent place as important ingredients in salad. The lettuce–herb mixes meet the requirements for convenience food, which is characterized by increased health-promoting properties compared to lettuce alone. This coincides with dietary recommendations and is becoming increasingly important in the era of fast-food bars and the growing problem of an overweight population. Mixes are still a perishable product due to rapid spoilage, but in the current study, the main ingredient, which was fresh-cut iceberg lettuce, showed slightly less discoloration of the cut surface at the very beginning of storage compared to lettuce without the herb additive. This proved the positive effect of herbs on inhibiting physiological changes in lettuce. This effect was attributed to the action of volatile substances secreted by the herbs. Similar to the results obtained by Lopresti and Tomkins [27] and Cantwell and Reid [28], the herbs differed in their shelf life during storage. The riskiest is the use of green basil and oregano due to their tendency to discolor quickly. The green basil darkening mostly occurred due to the susceptibility to chilling injury (CI). A temperature of 5 °C was too low for this species, as it is a plant of tropical origin and exposure to temperatures below 12 °C creates brown spots and black necrosis on the leaves [29]. In the case of oregano, one of the reasons for discoloration can be chilling sensibility, although Cantwell and Reid [30] suggest that leaf discoloration only occurs in plant material cultivated under a cool climate. Another reason may be relatively high ethylene production, which can eventually lead to aging, senescence, and tissue browning. A distinctly different behavior was exhibited by red basil, which maintained its proper color, gloss, and fresh appearance during short-term storage. This was indicative of resistance to low-temperature stress, which was most likely due to high anthocyanin content. In the study of Baczek-Kwinta et al. [31], red basil “Rubin” contained 10-fold more anthocyanins than green-leaf plants, and this may be a stress protection factor. Parsley showed a relatively good shelf life, with a slight decline in quality at the end of storage due to yellowing. Cantwell and Reid [30] also reported a decrease in the chlorophyll content in parsley leaves during storage. The above authors emphasize the poorer shelf life of mint than parsley, which also appeared in the current study.
A very-low weight loss showed that both lettuce and herbs maintained good firmness, and there was no shrinkage of leaf tissue. The plastic boxes used to package lettuce with herbs protected the plant material from moisture loss.
Including culinary herbs in meals is a big nutritional advantage, considering the metabolites that have health-promoting properties. Santos et al. [14] also showed high levels of health-promoting components in fresh-cut chives, cilantro, mint, and parsley, and stated their minor changes during 10 d storage at 3 °C. In the study by Ninfali et al. [17], the addition of 1.5% marjoram to the salad with lettuce and tomato resulted in a 4-fold increase in oxygen radical absorbance capacity. As in the current study, cut parsley was found to be particularly rich in vitamin C and peppermint was found to be rich in polyphenols, while the carotene content of both herbs was similar. The assertion by Martinez-Gracia et al. [32] that small amounts of herbs and spices do not contribute to the food has not been confirmed. The results of our study showed that the addition of herbs at a dose of 3–10% considerably enriched the salads in vitamins and other important nutrients. Vitamin C is a stimulator of immune system activity in the human body [33]. Carotenoids contribute to the increased protection of the skin from UV radiation. They also protect against diabetes, cancer, and inflammatory diseases [34]. Polyphenols have the ability to prevent lipid overproduction, liver dysfunction, inflammation, infections, and cancer [35].
The addition of fresh-cut culinary herbs to fresh vegetables increases the variety of flavors and expands the market offering in the ready-to-eat salad department [32]. Toivonen and Brummell [36] have paid attention to an important feature, which is the sensory quality of the products on the market. In the current study, the mixes of iceberg lettuce with the addition of leaf parsley and red basil in the amounts of 5–10% and 5%, respectively, gained high sensory evaluation, so they should be accepted by consumers. According to Pollack [37], fresh-cut vegetables are generally used as an accompaniment to other dishes or as ready-to-eat salads with dressing, so the addition of fresh herbs can further enhance the flavor of the meal.
In our study, the addition of herbs increased the microbial load in the mixes. However, in the case of bacteria and yeasts, the increase was not significant compared to the control. The strongest increase after herbs application was observed for molds. Kowalska and Szczech [38] reported that among the leafy green vegetables (rocket, spinach, lamb’s lettuce, iceberg lettuce, celery, chive, dill, and parsley), the most contaminated vegetables with molds were parsley and rocket, but the least contaminated was iceberg lettuce. This was related to the structure of the plants, as the inner leaves in lettuce heads are less likely to be colonized by microorganisms [39] than the leaves of plants with a spreading, open habit (like parsley and other herbs). Straight after mixing, the bacteria counts in the salads did not exceed the value of 107 cfu g−1, which is in line with the results previously obtained for fresh leafy greens [40,41,42]. During storage, the number of microorganisms rose in all objects, which is what is usually observed in minimally processed leafy products. Jackson et al. [43] have found that the total cultured bacteria in lettuce and spinach stored in a modified chilled atmosphere increased to 5.5 × 108 cfu g−1. Kowalska and Szczech [38] reported that in leafy vegetables sold in supermarkets, the mesophilic bacteria were the dominant group in all products and reached values as high as 8.4 log10 cfu g−1, and for iceberg lettuce the bacterial load ranged from 2.08 to 7.16 log10 cfu g−1. Similarly, Sun et al. [44] found that after storage for 3 days the number of bacteria in lettuce increased by more than 5 log10 cfu g−1. The other dominant group of microorganisms were yeasts. According to Kowalska and Szczech [38], their number in iceberg lettuce ranged from 3.4 to 4.0 log10 cfu g−1, while in other leafy greens the value was higher—about 6.0 log10 cfu g−1—and these findings are in accordance with the presented data. In the case of molds, their number increased during storage, especially in the control lettuce. In the mixes with herbs, the increase in mold numbers was markedly less extensive than in lettuce alone. It is also worth noting that during storage there was a general tendency for a greater increase in the number of microorganisms in lettuce alone than in the mixes with herbs. This indicates a slowdown in the growth of microorganisms during storage with fresh herbs. Thus, the reports of Holley and Patel [45] about the antimicrobial effect of herbs were confirmed.
5. Conclusions
Iceberg lettuce is a very popular vegetable around the world, and there is a high demand for both whole heads and fresh-cut versions. As a minimally processed food, it should meet the requirements of convenience, sanitary safety, nutrition, and good taste and smell. Improving the storage life of fresh-cut horticultural products is still a big challenge for food technologists and nutritional researchers.
The addition of fresh herbs significantly improves the health-promoting properties of the new mixes. Depending on the herb, the contents of chlorophyll, carotenes, vitamin C, and polyphenols were even several times higher in the mixes than in the lettuce itself. Of the tested herbs (peppermint, oregano, green basil, red basil, and leaf parsley), red basil and parsley proved to be the best additions in terms of sensory and storage qualities. It is also important that the addition of herbs does not significantly raise the contamination of lettuce–herb mixes by bacteria and yeasts, and that the microbial load does not exceed the level typical for marketable products. Moreover, the growth rate of the molds during the storage of the mixes, especially with parsley, was lower than in the control lettuce samples. This shows the great potential for launching a new, valuable, ready-to-eat product—lettuce mixes with herbs.
Author Contributions
Conceptualization, M.G.; investigation, M.G., M.S., B.K., A.W. and M.M.-F.; methodology, M.G., M.S., B.K., A.W. and M.M.-F.; writing—original draft, M.G.; resources, T.S.; writing—review and editing, M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of Horticultural Research, statutory project number ZPiPOiW/3/2018.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chatziprodromidou, I.P.; Bellou, M.; Vantarakis, G.; Vantarakis, A. Vital outbreaks linked to fresh produce consumption: A systematic review. J. Appl. Microbiol. 2018, 124, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Carstens, C.K.; Salazar, J.K.; Darkoh, C. Multistate Outbreaks of Foodborne Illness in United States Associated with Fresh Produce from 2010 to 2017. Front. Microbiol. 2019, 10, 2667. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Foodborne Illnesses and Outbreaks from Fresh Produce. Congressional Research Service. Informing the Legislative Debate Since 1914. Available online: https://sgp.fas.org/crs/misc/IF11092.pdf (accessed on 24 July 2024).
- McDaniel, C.; Jadeja, R. A review of fresh produce outbreaks, current interventions, food safety concerns and potential benefits of novel antimicrobial sodium acid sulfate. MOJ Food Process. Technol. 2019, 7, 59–67. [Google Scholar] [CrossRef]
- Zhang, H.; Yamamoto, E.; Murphy, J.; Locas, A. Microbiological safety of ready-to-eat fresh-cut fruits and vegetables sold on the Canadian retail market. Int. J. Food Microbiol. 2020, 335, 1108855. [Google Scholar] [CrossRef] [PubMed]
- Dulce, M.; Antunes, C.; Cavaco, A.M. The use of essential oils for postharvest decay control. A review. Flavour Fragr. J. 2010, 25, 351–366. [Google Scholar] [CrossRef]
- Castro-Ibanez, I.; Gil, M.I.; Allende, A. Ready to eat vegetables: Current problems and potential solutions to reduce microbial risk in the production chain. LWT Food Sci. Technol. 2016, 85, 284–292. [Google Scholar] [CrossRef]
- Balali, G.I.; Yar, D.D.; Dela, V.G.A.; Adjei-Kusi, P. Microbial contamination an increasing treat to the consumption of fresh fruits and vegetables in today’s world. Int. J. Microbiol. 2020, 2020, 3029295. [Google Scholar] [CrossRef] [PubMed]
- Rico, D.; Martin-Diana, A.B.; Barat, J.M.; Barry-Ryan, C. Extending and measuring the quality of fresh-cut and vegetables: A review. Trends Food Sci. Technol. 2007, 18, 373–386. [Google Scholar] [CrossRef]
- Xylia, P.; Chrysargyris, A.; Botsaris, G.; Tzortzakis, N. Potential application of spermint and lavender essential oils for assuring endive quality and safety. Crop Prot. 2017, 102, 94–103. [Google Scholar] [CrossRef]
- Xylia, P.; Clark, A.; Chrysargyris, A.; Romanazzi, G.; Tzortzakis, N. Quality and safety attributes on shredded carrots by using Origanum majorana and ascorbic acid. Postharvest Biol. Technol. 2019, 155, 120–129. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Hosseini, H.; Nasrollahzadeh, J.; Khaneghah, A.M.; Rismanchi, M.; Chaves, R.D.; Shahraz, F.; Azizkhani, M.; Mahmoudzadeh, L.; Haslberger, A.G. Antibacterial activity of Carum copticum essential oil against Escherichia coli O157: H7 in meat: Stx genes expression. Curr. Microbiol. 2016, 73, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Romanazzi9, G.; Sanzani, S.M.; Bi, Y.; Tian, S.; Martinez, P.G.; Alkan, N. Induced resistance to control Postharvest Decay of fruit and vegetables. Postharvest Biol. Technol. 2016, 122, 82–94. [Google Scholar] [CrossRef]
- Santos, J.; Herrero, M.; Mendiola, J.A.; Oliva-Teles, M.T.; Ibanez, E.; Delerue-Matos, C.; Oliveira, M.B.P.P. Fresh-cut aromatic herbs: Nutritional quality stability during shelf-life. LWT Food Sci. Technol. 2014, 59, 101–107. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Gonzales-Aguilar, G.A.; Del-Toro-Sanchez, L. Enhancing safety and aroma appealing of fresh-cut fruits and vegetables using the antimicrobial and aromatic power of essential oils. J. Food Sci. 2009, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Scollard, J.; Francis, G.A.; O’Beirne, D. Some conventional and latent anti-listerial effects of essential oils, herbs, carrot and cabbage in fresh-cut vegetable systems. Postharvest Biol. Technol. 2013, 77, 87–93. [Google Scholar] [CrossRef]
- Ninfali, P.; Mea, G.; Giorgini, S.; Roschi, M.; Bacchiocca, M. Antioxidant capacity of vegetables, spices and dressings relevant to nutrition. Br. J. Nutr. 2005, 93, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b leaf extracts in different solvents. Biochem. Soc. Trans 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Sumanta, N.; Haque, C.I.; Nishika, J.; Suprakash, R. Spectrophotometric analysis of chlorophylls and carotenoids from commonly grown fern species by using various extracting solvents. Res. J. Chem. Sci. 2014, 4, 63–69. [Google Scholar] [CrossRef]
- Tsao, R.; Yang, R. Optimization of a new mobile phase to know the complex and real polyphenolic composition: Towards a total phenolic index using high-performance liquid chromatography. J. Chromatogr. A 2003, 1018, 29–40. [Google Scholar] [CrossRef] [PubMed]
- IFU. Determination of L-Ascorbic Acid in Fruit Juices by HPLC. Method 17a. International Fruit and Vegetable Juice Association 2005. Available online: https://ifu-fruitjuice.com/page/ListofIFUMethods (accessed on 24 July 2024).
- ISO 13299: 2016; Sensory Analysis—Methodology—General Guidance for Establishing a Sensory Profile. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/standard/58042.html (accessed on 24 July 2024).
- PN-EN ISO 8589:2010/A1: 2014-07; Sensory Analysis-General Guidelines for Design of Sensory Analysis. ISO: Geneva, Switzerland, 2014. Available online: https://sklep.pkn.pl/pn-en-iso-8589-2010e.html (accessed on 24 July 2024). (In Polish)
- PN-EN ISO 8586:2014-03; Sensory Analysis-General Guidelines for the Selection, Training and Monitoring of Selected Assessors and Expert Sensory Assessors. ISO: Geneva, Switzerland, 2014. Available online: https://sklep.pkn.pl/pn-en-iso-8586-2014-03e.html (accessed on 24 July 2024).
- PN-EN ISO 4833-2:2013-12; Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms—Part 2: Enumeration by Surface Seeding at 30 Degrees. ISO: Geneva, Switzerland, 2013. Available online: https://sklep.pkn.pl/pn-en-iso-4833-2-2013-12e.html (accessed on 24 July 2024). (In Polish)
- PN-ISO 21527-1:2009; Microbiology of Food and Feed—Horizontal Method for the Determination of Yeast and Mold Counts—Part 1: Method for Counting Colonies in Products with Water Activity Higher than 0.95. ISO: Geneva, Switzerland, 2009. Available online: https://sklep.pkn.pl/pn-iso-21527-1-2009p.html (accessed on 24 July 2024). (In Polish)
- Lopresti, J.; Tomkins, B. Postharvest handling and packaging of fresh herbs. Rural Ind. Res. Dev. Corp. Res. Pap. 1997, 97, 5. [Google Scholar]
- Cantwell, M.; Reid, M. Herbs (Fresh Culinary). Recomm. Maint. Postharvest Qual. 2001. Available online: https://postharvest.ucdavis.edu/files/259450.pdf (accessed on 24 July 2024). (In Polish).
- Aharoni, N.; Kenigsbuch, D.; Chalupowicz, D.; Faura-Mlinski, M.; Aharon, Z.; Maurer, D.; Ovadia, A.; Lers, A. Reducing chilling injury and decay in stored sweet basil. Isr. J. Plant Sci. 2010, 58, 167–181. [Google Scholar] [CrossRef]
- Cantwell, M.I.; Reid, M.S. Postharvest physiology and handling of fresh culinary herbs. J. Herbs Spices Med. Plants 1993, 3, 93–125. [Google Scholar] [CrossRef]
- Baczek-Kwinta, R.; Serek, B.; Wator, A.; Hura, K. The comparison of antioxidant activity of basil cultivars assayed by different methods. Zesz. Probl. Postępów Nauk Rolniczych. 2009, 539, 55–56. (In Polish) [Google Scholar]
- Martinez-Gracia, C.; Gonzalez-Bermudez, C.A.; Cabellero-Valcarcel Santaella-Pascual, M.; Frontela-Saseta, C. Use of herbs and spices for food preservation: Advantages and limitations. Curr. Opin. Food Sci. 2015, 6, 38–43. [Google Scholar] [CrossRef]
- Carr, A.C.; Maggini, S. Vitamin C and Immune Function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Swapnil, P.; Meena, M.; Singh, S.K.; Dhuldhaj, U.P.; Harish Marwal, A. Vital roles of carotenoids in plants and humans to deteriorate stress with its structure, biosynthesis, metabolic engineering and functional aspects. Curr. Plant Biol. 2021, 26, 100203. [Google Scholar] [CrossRef]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and their benefits: A review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Toivonen, P.M.A.; Brummell, D.A. Biochemical bases of appearance and texture changes in fresh-cut fruit and vegetables. Postharvest Biol. Technol. 2008, 48, 1–14. [Google Scholar] [CrossRef]
- Pollack, S. Consumer demand for fruit and vegetables: The US example. In: The changing structure of global food consumption and trade. U.S. Dept. of Agriculture. Econ. Resour. Serv. 2001, 6, 49–54. [Google Scholar]
- Kowalska, B.; Szczech, M. Differences in microbiological quality of leafy green vegetables. Ann. Agric. Environ. Med. 2022, 29, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Sequino, G.; Valentino, V.; Torreri, E.; De Filippis, F. Specific microbial communities are selected in minimally-processed fruit and vegetables according to the type of product. Foods 2022, 11, 2164. [Google Scholar] [CrossRef] [PubMed]
- Johnston, L.M.; Jaykus, L.A.; Moll, D.; Martinez, M.C.; Anciso, J.; Mora, B.; Moe, C.L. A field study of the microbiological quality of fresh produce. J. Food Prot. 2005, 68, 1840–1847. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Song, J. Microbial quality assessment methods for fresh-cut fruits and vegetables. Stewart Postharvest Rev. 2008, 4, 1–9. [Google Scholar] [CrossRef]
- Kłapeć, T.; Wójcik-Fatla, A.; Cholewa, A.; Cholewa, G.; Dutkieewicz, J. Microbiological characterization of vegetables and their rhizosphere soil in Eastern Poland. Ann. Agric. Environ. Med. 2016, 23, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Jackson, C.R.; Randolph, K.C.; Osborn, S.L.; Tyler, H.L. Culture dependent and independent analysis of bacterial communities associated with commercial salad leaf vegetables. BMC Microbiol. 2013, 13, 27. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhao, X.; Ma, Y.; Ma, Z.; He, Z.; Zhao, W.; Wang, P.; Zhao, S.; Wang, D. Investigation on the Microbial Diversity of Fresh-Cut Lettuce during Processing and Storage Using High Throughput Sequencing and Their Relationship with Quality. Foods 2022, 11, 1683. [Google Scholar] [CrossRef] [PubMed]
- Holley, R.A.; Patel, D. Improvement in shelf-life and safety of perishable foods by plant essential oils and smoke antimicrobials. Food Microbiol. 2005, 22, 273–292. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).