Integrated Nutrient Management of Fruits, Vegetables, and Crops through the Use of Biostimulants, Soilless Cultivation, and Traditional and Modern Approaches—A Mini Review
Abstract
1. Introduction
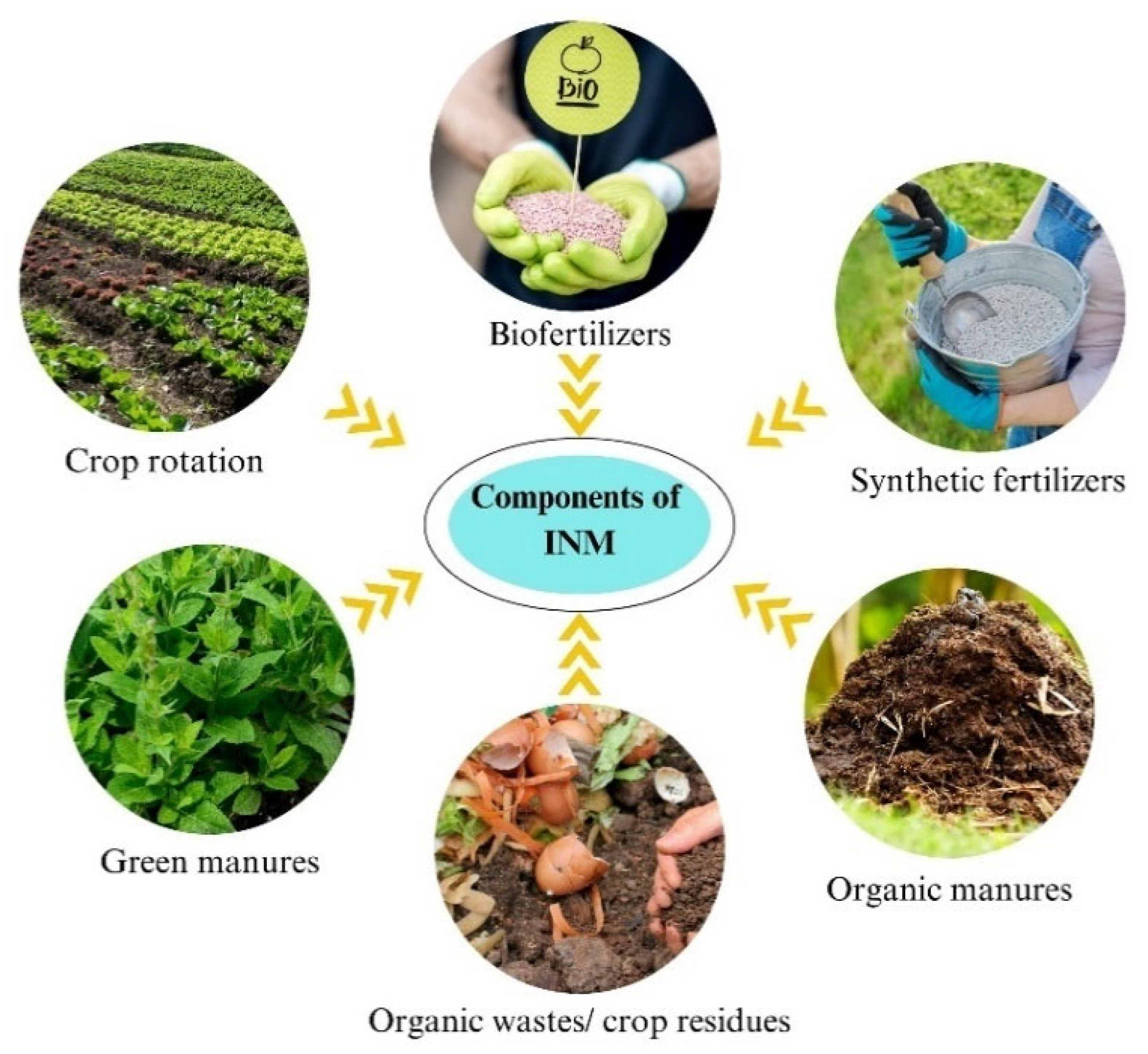
2. Conceptual Basis and Principles of INM
3. Progress of INM Technology in Fruits, Vegetables, and Crops
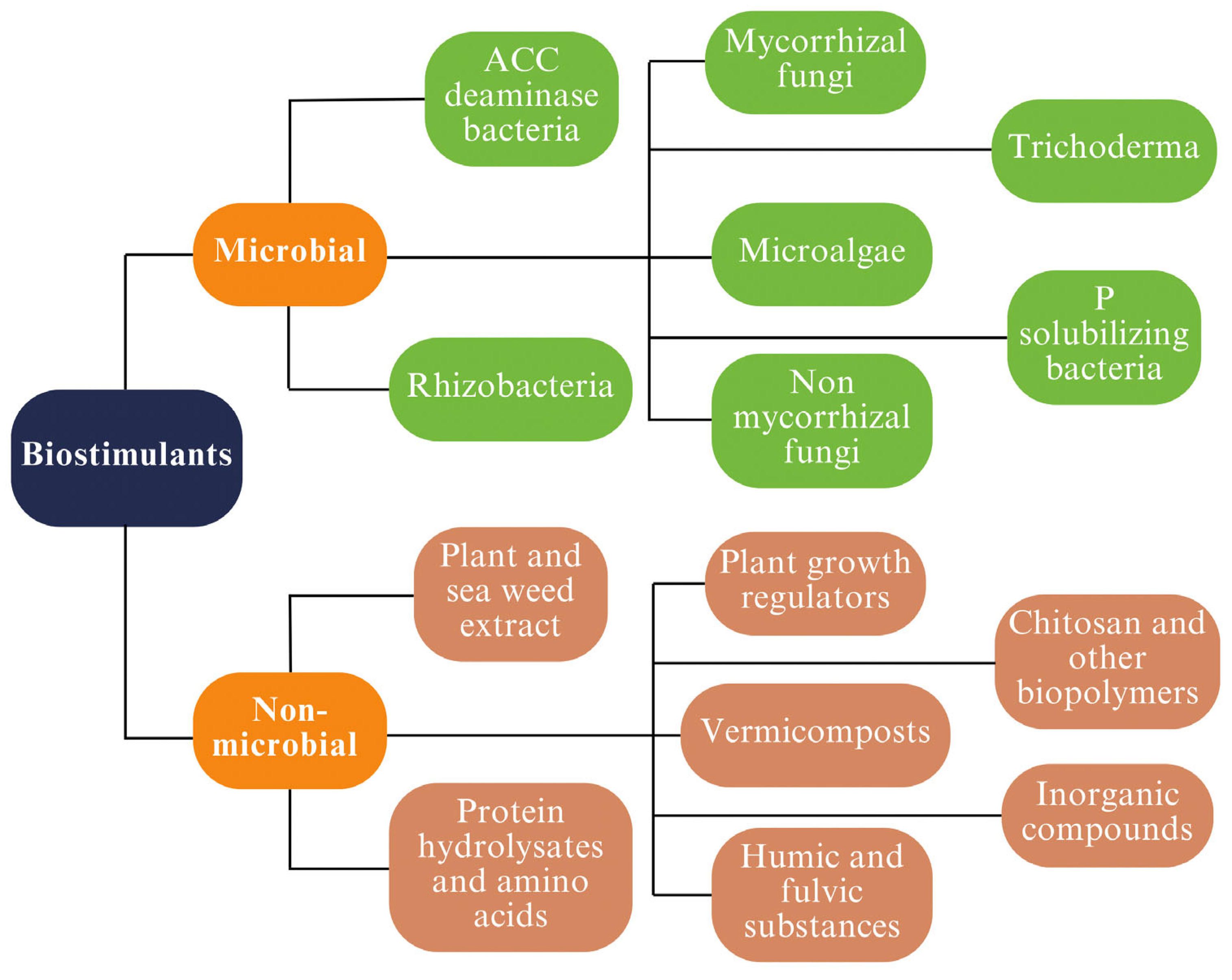
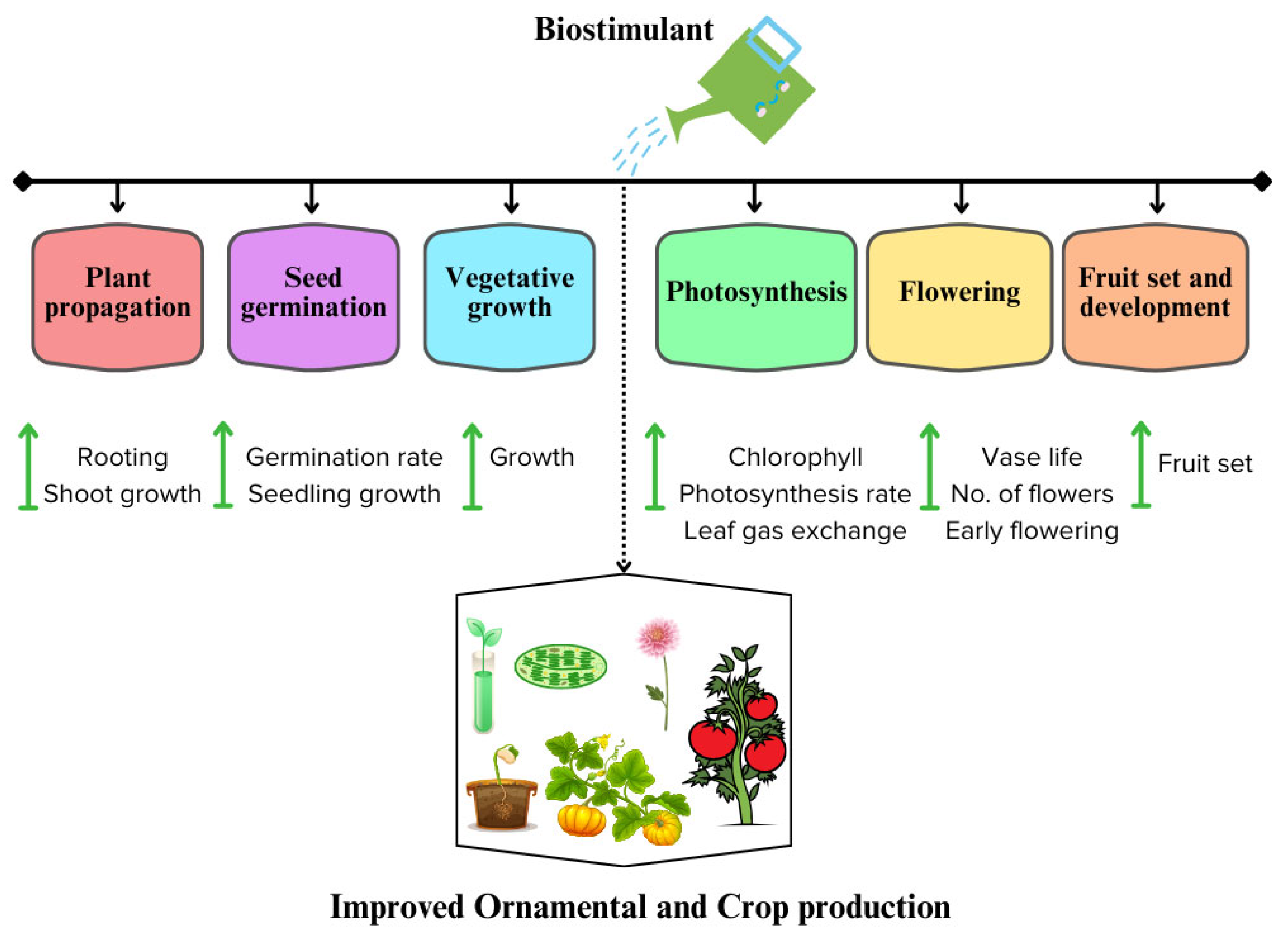
3.1. Biostimulants
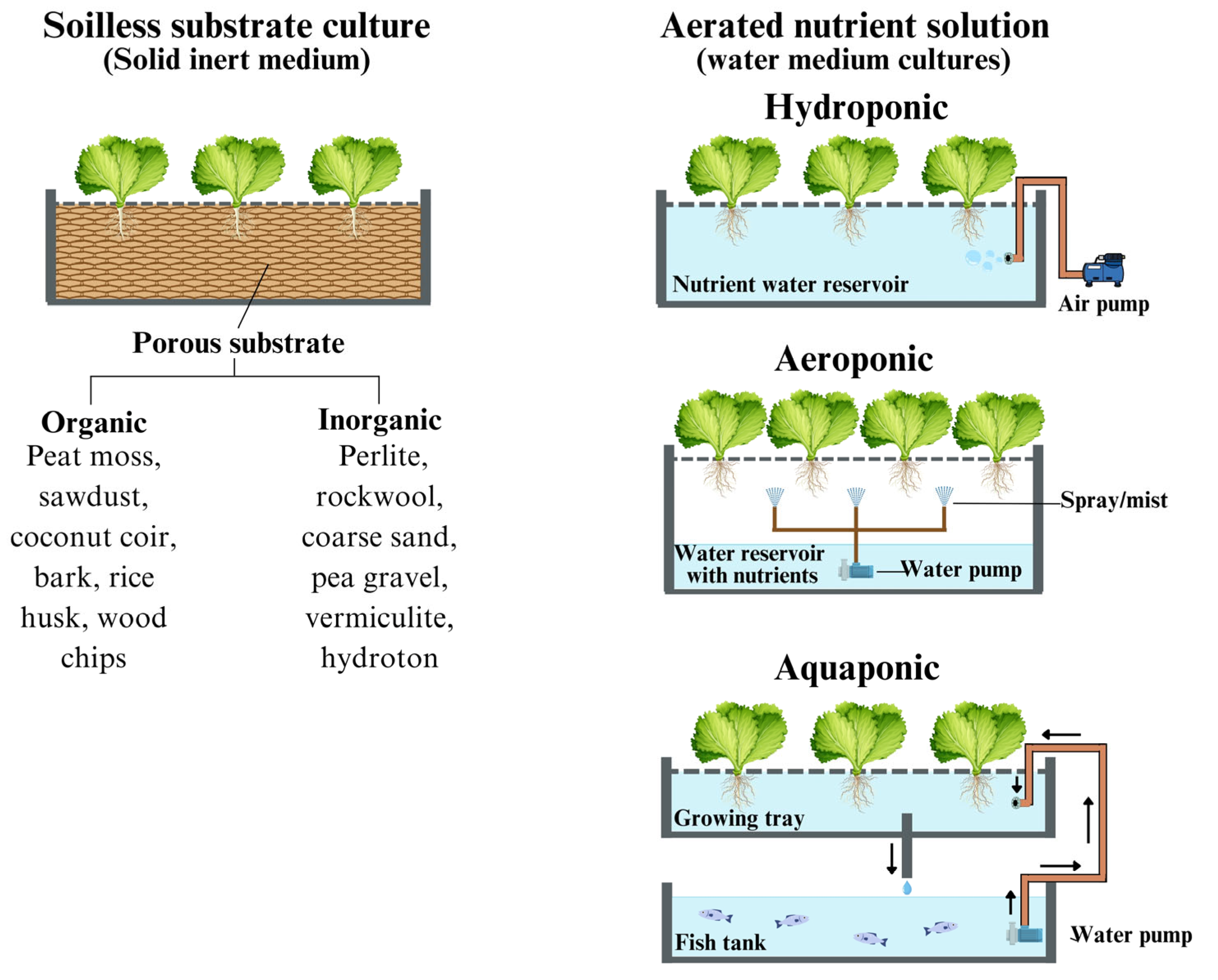
3.2. Nutrient Management and Soilless Cultivation Systems
3.2.1. Hydroponics
- Macronutrients: Carbon (C), hydrogen (H), and oxygen (O) are available in nature/the atmosphere. Nitrogen (N), phosphorus (P), potassium (K), calcium (Ca), magnesium (Mg), and sulfur (S) are required in large quantities.
- Micronutrients: Manganese (Mg), boron (B), iron (Fe), copper (Cu), zinc (Zn), molybdenum (Mo), chlorine (Cl), nickel (Ni), cobalt (Co), sodium (Na), and silicon (Si) are required in very small quantities.
3.2.2. Aeroponics
3.2.3. Aquaponics
3.3. INM Effects on the Performance of Fruits and Vegetables
INM Effects on the Performance of Tomato
3.4. INM Effects on the Performance of Field Crops
3.4.1. INM Effects on the Performance of Rice
3.4.2. INM Effects on the Performance of Wheat
3.4.3. INM Effects on the Performance of Different Crops
4. Potential Constraints in INM
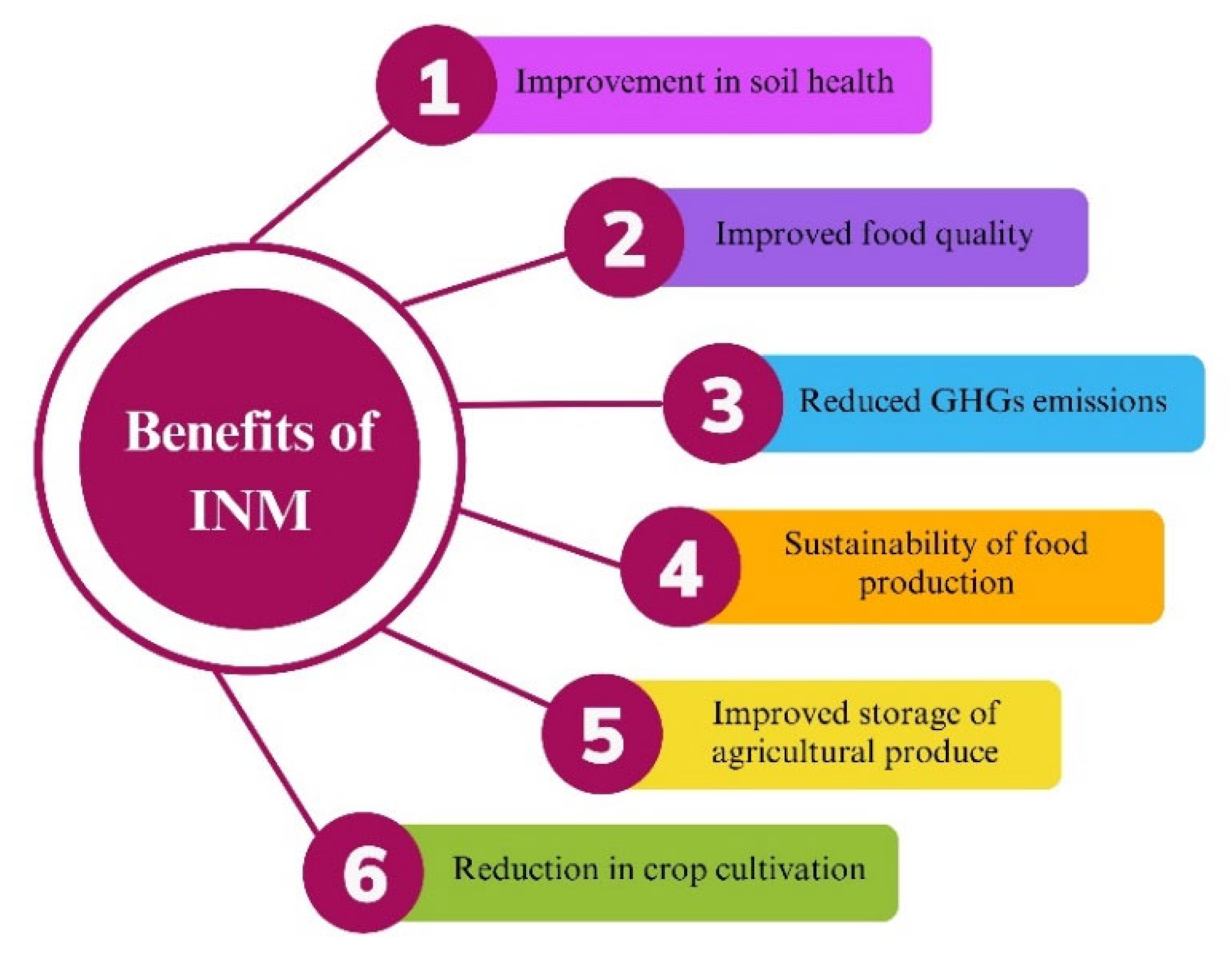
5. Conclusions and Future Perspectives on INM
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, A.; Kumar, A.; Biswas, G. Exponential population growth and global food security: Challenges and alternatives. In Bioremediation of Emerging Contaminants from Soils; Elsevier: Amsterdam, The Netherlands, 2024; pp. 1–20. [Google Scholar] [CrossRef]
- Aryal, J.P.; Manchanda, N.; Sonobe, T. Expectations for household food security in the coming decades: A global scenario. In Future Foods; Academic Press: Cambridge, MA, USA, 2022; pp. 107–131. [Google Scholar]
- Wheller, T.; Braun, J. Climate change impacts global food security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Lal, R. Soils and sustainable agriculture: A review. Agron. Sustain. Dev. 2008, 28, 57–64. [Google Scholar] [CrossRef]
- Erisman, J.W.; Sutton, M.A.; Galloway, J.; Klimont, Z.; Winiwarter, W. How a century of ammonia synthesis changed the world. Nat. Geosci. 2008, 1, 636–639. [Google Scholar] [CrossRef]
- Chen, X.; Ma, L.; Ma, W.; Wu, Z.; Cui, Z.; Hou, Y.; Zhang, F. What has caused the use of fertilizers to skyrocket in China. Nutr. Cycl. Agroecosyst. 2018, 110, 241–255. [Google Scholar] [CrossRef]
- Zhang, C.; Ju, X.; Powlson, D.; Oenema, O.; Smith, P. Nitrogen surplus benchmarks for controlling N pollution in the main cropping systems of China. Environ. Sci. Technol. 2019, 53, 6678–6687. [Google Scholar] [CrossRef] [PubMed]
- NBS. National Bureau of Statistics (China). 2020. Available online: http://www.stats.gov.cn (accessed on 7 June 2024).
- Wu, W.; Ma, B. Integrated nutrient management (INM) for sustaining crop productivity and reducing environmental impact: A review. Sci. Total Environ. 2015, 512, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.; Galloway, J.; Millar, N.; Leach, A. N-related greenhouse gases in North America: Innovations for a sustainable future. Curr. Opin. Environ. Sustain. 2014, 9–10, 1–8. [Google Scholar] [CrossRef]
- Sutton, M.A.; Howard, C.M.; Erisman, J.W.; Billen, G.; Bleeker, A.; Grennfelt, P.; Van Grinsven, H.; Grizzetti, B. The European Nitrogen Assessment: Sources, Effects and Policy Perspectives; Cambridge University Press: Cambridge, MA, USA, 2011. [Google Scholar]
- European Commission. Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Off. J. 1991, L 375, 0001–0008. Available online: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=CELEX:31991L0676:EN:HTML (accessed on 10 June 2024).
- European Commission. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy (OJ L 327 22.12.2000 p. 1). In Documents in European Community Environmental Law; European Commission: Brussels, Belgium, 2006; pp. 879–969. [Google Scholar] [CrossRef][Green Version]
- Velthof, G.L.; Lesschen, J.P.; Webb, J.; Pietrzak, S.; Miatkowski, Z.; Pinto, M.; Kros, J.; Oenema, O. The impact of the nitrates directive on nitrogen emissions from agriculture in the EU-27 during 2000–2008. Sci. Total Environ. 2014, 468–469, 1225–1233. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). FAO Database: Agriculture Production and Agri-Environmental Indicators; FAO: Rome, Italy, 2019. [Google Scholar]
- Yang, L.; Wang, L.; Chu, J.; Zhao, H.; Zhao, J.; Zang, H.; Zeng, Z. Improving soil quality and wheat yield through diversified crop rotations in the North China Plain. Soil Tillage Res. 2024, 244, 106231. [Google Scholar] [CrossRef]
- Yang, X.; Steenhuis, T.S.; Davis, K.F.; Van der Werf, W.; Ritsema, C.J.; Pacenka, S.; Du, T. Diversified crop rotations enhance groundwater and economic sustainability of food production. Food Energy Sec. 2021, 10, e311. [Google Scholar] [CrossRef]
- Liang, Z.; Xu, Z.; Cheng, J.; Ma, B.; Cong, W.F.; Zhang, C.; Groot, J.C. Designing diversified crop rotations to advance sustainability: A method and an application. Sustain. Prod. Cons. 2023, 40, 532–544. [Google Scholar] [CrossRef]
- Gou, X.; Reich, P.B.; Qiu, L.; Shao, M.; Wei, G.; Wang, J.; Wei, X. Leguminous plants significantly increase soil nitrogen cycling across global climates and ecosystem types. Glob. Chang. Biol. 2023, 29, 4028–4043. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Detto, M.; Fang, S.; Chazdon, R.L.; Li, Y.; Hau, B.C.; Yao, T.L. Soil nitrogen concentration mediates the relationship between leguminous trees and neighbor diversity in tropical forests. Commun. Biol. 2020, 3, 317. [Google Scholar] [CrossRef] [PubMed]
- Bhupenchandra, I.; Chongtham, S.K.; Devi, E.L.; Choudhary, A.K.; Salam, M.D.; Sahoo, M.R.; Khaba, C.I. Role of biostimulants in mitigating the effects of climate change on crop performance. Front. Plant Sci. 2022, 13, 967665. [Google Scholar] [CrossRef] [PubMed]
- European Biostimulants Industry Council. What Are Biostimulants? 2012. Available online: https://biostimulants.eu/ (accessed on 10 June 2024).
- Biostimulant Coalition. What Are Biostimulants? 2013. Available online: http://www.biostimulantcoalition.org/about/ (accessed on 10 June 2024).
- Zulfiqar, F.; Moosa, A.; Ali, H.M.; Bermejo, N.F.; Munné-Bosch, S. Biostimulants: A sufficiently effective tool for sustainable agriculture in the era of climate change? Plant Phys. Biochem. 2024, 211, 108699. [Google Scholar] [CrossRef]
- Palm, C.A.; Gachengo, C.N.; Delve, R.J.; Cadisch, G.; Giller, K.E. Organic inputs for soil fertility management in tropical agroecosystems: Application of an organic resource database. Agric. Ecosys. Environ. 2001, 83, 27–42. [Google Scholar] [CrossRef]
- Hindoriya, P.S.; Kumar, R.; Meena, R.K.; Ram, H.; Kumar, A.; Kashyap, S.; Bhattacharjee, S. The Impact of Integrated Nutrient Management on Trifolium alexandrinum Varietal Performance in the Indo-Gangetic Plains: A Comparative Yield and Economic Analysis. Agronomy 2024, 14, 339. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, J.C.; Shukla, Y.R.; Verma, M.L.; Singh, U.; Spehia, R.S.; Kumar, A. Nutrient Management Influences Root Characteristics and Nitrogen Use Efficiency in the Vegetable-Based Agroecosystem in the Northwestern Himalayas. Sustainability 2023, 15, 10593. [Google Scholar] [CrossRef]
- Ali, A.; Hussain, T.; Tantashutikun, N.; Hussain, N.; Cocetta, G. Application of smart techniques, internet of things and data mining for resource use efficient and sustainable crop production. Agriculture 2023, 13, 397. [Google Scholar] [CrossRef]
- Hahn, L.; Kurtz, C.; De Paula, B.V.; Feltrim, A.L.; Higashikawa, F.S.; Moreira, C.; Parent, L.É. Feature-specific nutrient management of onion (Allium cepa) using machine learning and compositional methods. Sci. Rep. 2024, 14, 6034. [Google Scholar] [CrossRef]
- Suleiman, M.K.; Shahid, S.A. Prospective of Agricultural Farming in Kuwait and Energy-Food-Water-Climate Nexus. In Terrestrial Environment and Ecosystems of Kuwait: Assessment and Restoration; Springer Nature: Cham, Switzerland, 2024; pp. 363–391. [Google Scholar] [CrossRef]
- Chamuah, S.; Amin, M.A.; Sultana, N.; Hansda, N.N.; BM, H.; Noopur, K. Protected Vegetable Crop Production for Long-term Sustainable Food Security. J. Sci. Res. Rep. 2024, 30, 660–669. [Google Scholar] [CrossRef]
- Jan, S.; Rashid, Z.; Ahngar, T.A.; Iqbal, S.; Naikoo, M.A.; Majeed, S.; Nazir, I. Hydroponics—A review. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1779–1787. [Google Scholar] [CrossRef]
- Arndt, J.A.; Aulinger, A.; Matthias, V. Quantification of lightning-induced nitrogen oxide emissions over Europe. Atmos. Environ. 2019, 202, 128–141. [Google Scholar] [CrossRef]
- Murray, L.T. Lightning NO x and impacts on air quality. Curr. Poll. Rep. 2016, 2, 115–133. [Google Scholar] [CrossRef]
- Ju, X.; Xing, G.; Chen, X.; Zhang, S.; Zhang, L.; Liu, X.; Cui, Z.; Yin, B.; Christie, P.; Zhu, Z.; et al. Reducing environmental risk by improving N management in intensive Chinese agricultural systems. Proc. Natl. Acad. Sci. USA 2009, 106, 3041–3046. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.; Dobermann, A. Toward a decision support system for site-specific nutrient management. In Increasing the Productivity of Intensive Rice Systems Through Site-Specific Nutrient Management; Dobermann, A., Witt, C., Dawe, D., Eds.; Science Publishers, Inc.: Enfield, NH, USA; International Rice Research Institute (IRRI): Los Baños, Philippines, 2004; pp. 359–395. [Google Scholar]
- Chen, X.; Cui, Z.; Vitousek, P.; Cassman, K.; Matson, P.; Bai, J.; Meng, Q.; Hou, P.; Yue, S.; Römheld, V.; et al. Integrated soil—Crop system management for food security. Proc. Natl. Acad. Sci. USA 2011, 108, 6399–6404. [Google Scholar] [CrossRef] [PubMed]
- Jambert, C.; Serca, D.; Delmas, R. Quantification of N-losses as NH3, NO, and N2O and N2 from fertilized maize fields in southwestern France. Nutr. Cycl. Agroecosyst. 1997, 48, 91–104. [Google Scholar] [CrossRef]
- Ma, B.L.; Wu, T.Y.; Tremblay, N.; Deen, W.; McLaughlin, N.B.; Morrison, M.J.; Gregorich, E.G.; Stewart, G. Nitrous oxide fluxes from corn fields: On-farm assessment of the amount and timing of nitrogen fertilizer. Glob. Chang. Biol. 2010, 16, 156–170. [Google Scholar] [CrossRef]
- Ju, X.; Lu, X.; Gao, Z.; Chen, X.; Su, F.; Kogge, M.; Römheld, V.; Christie, P.; Zhang, F. Processes and factors controlling N2O production in an intensively managed low carbon calcareous soil under sub-humid monsoon conditions. Environ. Pollut. 2011, 159, 1007–1016. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, W. Changes in topsoil organic carbon of croplands in mainland China over the last two decades. Chin. Sci. Bull. 2006, 51, 1785–1803. [Google Scholar] [CrossRef]
- Gupta, S.; Bhattacharyya, P.; Kulkarni, M.G.; Doležal, K. Growth regulators and biostimulants: Upcoming opportunities. Fro. Plant Sci. 2023, 14, 1209499. [Google Scholar] [CrossRef]
- Bulgari, R.; Franzoni, G.; Ferrante, A. Biostimulants application in horticultural crops under abiotic stress conditions. Agronomy. 2019, 9, 306. [Google Scholar] [CrossRef]
- Rakkammal, K.; Maharajan, T.; Ceasar, S.A.; Ramesh, M. Biostimulants and their role in improving plant growth under drought and salinity. Cereal Res. Comm. 2023, 51, 61–74. [Google Scholar] [CrossRef]
- Lin, K.H.; Lin, F.W.; Wu, C.W.; Chang, Y.S. Biostimulation of maize (Zea mays) and irrigation management improved crop growth and water use under controlled environment. Agronomy 2019, 9, 559. [Google Scholar] [CrossRef]
- Adoko, M.Y.; Noumavo, A.D.P.; Agbodjato, N.A.; Amogou, O.; Salami, H.A.; Aguégué, R.M.; Baba-Moussa, L. Effect of the application or coating of PGPR-based biostimulant on the growth, yield and nutritional status of maize in Benin. Front. Plant Sci. 2022, 13, 1064710. [Google Scholar] [CrossRef]
- Pacholczak, A.; Szydło, W.; Jacygrad, E.; Federowicz, M. Effect of auxins and the biostimulator AlgaminoPlant on rhizogenesis in stem cuttings of two dogwood cultivars (Cornus alba ‘Aurea’and ‘Elegantissima’). Acta Sci. Polo. Hort. Cul. 2012, 11, 93–103. [Google Scholar]
- Ritter, G.; Villa, F.; Da Silva, D.F.; Alberton, O.; Menegusso, F.J.; Eberling, T.; Dória, J. Microbiological biostimulant promotes rooting of olive cuttings. Int. J. Agric. Biol. Eng. 2021, 14, 207–212. [Google Scholar] [CrossRef]
- Corbellini, J.R.; Ribas, L.L.F.; De Maia, F.R.; Corrêa, D.D.O.; Noseda, M.D.; Suzuki, R.M.; Amano, É. Effect of microalgae Messastrum gracile and Chlorella vulgaris on the in vitro propagation of orchid Cattleya labiata. J. Appl. Phycol. 2020, 32, 4013–4027. [Google Scholar] [CrossRef]
- Wise, K.; Gill, H.; Selby-Pham, J. Willow bark extract and the biostimulant complex Root Nectar® increase propagation efficiency in chrysanthemum and lavender cuttings. Sci. Hortic. 2020, 263, 109108. [Google Scholar] [CrossRef]
- Luziatelli, F.; Gatti, L.; Ficca, A.G.; Medori, G.; Silvestri, C.; Melini, F.; Ruzzi, M. Metabolites secreted by a plant-growth-promoting Pantoea agglomerans strain improved rooting of Pyrus communis L. cv Dar Gazi cuttings. Front. Microbiol. 2020, 11, 539359. [Google Scholar] [CrossRef]
- Toscano, S.; Ferrante, A.; Branca, F.; Romano, D. Enhancing the quality of two species of baby leaves sprayed with Moringa leaf extract as biostimulant. Agronomy 2021, 11, 1399. [Google Scholar] [CrossRef]
- Rashid, N.; Wahid, A.; Ibrar, D.; Irshad, S.; Hasnain, Z.; Al-Hashimi, A.; Khan, S. Application of natural and synthetic growth promoters improves the productivity and quality of quinoa crop through enhanced photosynthetic and antioxidant activities. Plant Phys. Biochem. 2022, 182, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hassan, F.A.S.; Mazrou, R.; Gaber, A.; Hassan, M.M. Moringa extract preserved the vase life of cut roses through maintaining water relations and enhancing antioxidant machinery. Postharvest Biol. Technol. 2020, 164, 111156. [Google Scholar] [CrossRef]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.; Gaber, A.; Alsanie, W.F.; El-Shenody, R. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Lola-Luz, T.; Hennequart, F.; Gaffney, M. Effect on yield, total phenolic, total flavonoid and total isothiocyanate content of two broccoli cultivars (Brassica oleraceae var italica) following the application of a commercial brown seaweed extract (Ascophyllum nodosum). Agric. Food Sci. 2014, 23, 28–37. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; El-Nakhel, C.; Bonini, P.; D’Amelia, L.; Dell’Aversana, E.; Rouphael, Y. Biostimulant application with a tropical plant extract enhances Corchorus olitorius adaptation to sub-optimal nutrient regimens by improving physiological parameters. Agronomy 2019, 9, 249. [Google Scholar] [CrossRef]
- Fusco, G.M.; Nicastro, R.; Rouphael, Y.; Carillo, P. The effects of the microbial biostimulants approved by EU regulation 2019/1009 on yield and quality of vegetable crops. Foods 2021, 11, 2656. [Google Scholar] [CrossRef]
- Li, H.; Yue, H.; Li, L.; Liu, Y.; Zhang, H.; Wang, J.; Jiang, X. Seed biostimulant Bacillus sp. MGW9 improves the salt tolerance of maize during seed germination. AMB Exp. 2021, 11, 74. [Google Scholar] [CrossRef]
- Gupta, S.; Doležal, K.; Kulkarni, M.G.; Balázs, E.; Van Staden, J. Role of non-microbial biostimulants in regulation of seed germination and seedling establishment. Plant Growth Reg. 2022, 97, 271–313. [Google Scholar] [CrossRef]
- Amirkhani, M.; Mayton, H.S.; Netravali, A.N.; Taylor, A.G. A seed coating delivery system for bio-based biostimulants to enhance plant growth. Sustainability 2019, 11, 5304. [Google Scholar] [CrossRef]
- Amirkhani, M.; Netravali, A.N.; Huang, W.; Taylor, A.G. Investigation of soy protein–based biostimulant seed coating for broccoli seedling and plant growth enhancement. HortScience 2016, 51, 1121–1126. [Google Scholar] [CrossRef]
- Qiu, Y.; Amirkhani, M.; Mayton, H.; Chen, Z.; Taylor, A.G. Biostimulant seed coating treatments to improve cover crop germination and seedling growth. Agronomy 2020, 10, 154. [Google Scholar] [CrossRef]
- Cocetta, G.; Ertani, A.; Bulgari, R.; Franzoni, G.; Nicola, S.; Ferrante, A. Biostimulants and Plant Response under Adverse Environmental Conditions: A Functional Interplay. In Plant Performance under Environmental Stress; Husen, A., Ed.; Springer: Cham, Switzerland, 2021; pp. 417–436. [Google Scholar] [CrossRef]
- Paradiso, R.; Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Pelosi, M.E.; Rippa, M.; Mori, M. Integrating Smart Greenhouse Cover, Reduced Nitrogen Dose and Biostimulant Application as a Strategy for Sustainable Cultivation of Cherry Tomato. Plants 2024, 13, 440. [Google Scholar] [CrossRef]
- Lephatsi, M.; Nephali, L.; Meyer, V.; Piater, L.A.; Buthelezi, N.; Dubery, I.A.; Tugizimana, F. Molecular mechanisms associated with microbial biostimulant-mediated growth enhancement, priming and drought stress tolerance in maize plants. Sci. Rep. 2022, 12, 10450. [Google Scholar] [CrossRef]
- Mzibra, A.; Aasfar, A.; Benhima, R.; Khouloud, M.; Boulif, R.; Douira, A.; Meftah Kadmiri, I. Biostimulants derived from Moroccan seaweeds: Seed germination metabolomics and growth promotion of tomato plant. J. Plant Growth Regul. 2021, 40, 353–370. [Google Scholar] [CrossRef]
- Hussein, M.H.; Eltanahy, E.; Al Bakry, A.F.; Elsafty, N.; Elshamy, M.M. Seaweed extracts as prospective plant growth bio-stimulant and salinity stress alleviator for Vigna sinensis and Zea mays. J. Appl. Phycol. 2021, 33, 1273–1291. [Google Scholar] [CrossRef]
- Campobenedetto, C.; Mannino, G.; Agliassa, C.; Acquadro, A.; Contartese, V.; Garabello, C.; Bertea, C.M. Transcriptome analyses and antioxidant activity profiling reveal the role of a lignin-derived biostimulant seed treatment in enhancing heat stress tolerance in soybean. Plants 2020, 9, 1308. [Google Scholar] [CrossRef]
- Rupawalla, Z.; Shaw, L.; Ross, I.L.; Schmidt, S.; Hankamer, B.; Wolf, J. Germination screen for microalgae-generated plant growth biostimulants. Algal Res. 2022, 66, 102784. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Moosa, A.; Ferrante, A.; Darras, A.; Sheteiwy, M.S.; Ali, B.; El Sabagh, A. Borage leaf extract improves the vase life of cut gladiolus flowers by delaying the senescence process and reducing water stress. Posth. Biol. Technol. 2024, 210, 112766. [Google Scholar] [CrossRef]
- Younis, A.; Akhtar, M.S.; Riaz, A.; Zulfiqar, F.; Qasim, M.; Farooq, A.; Bhatti, Z.M. Improved cut flower and corm production by exogenous moringa leaf extract application on gladiolus cultivars. Acta Sci. Pol. Hortorum Cultus 2018, 17, 25–38. [Google Scholar] [CrossRef]
- Carillo, P.; Pannico, A.; Cirillo, C.; Ciriello, M.; Colla, G.; Cardarelli, M.; Rouphael, Y. Protein hydrolysates from animal or vegetal sources affect morpho-physiological traits, ornamental quality, mineral composition, and shelf-life of Chrysanthemum in a distinctive manner. Plants 2022, 11, 2321. [Google Scholar] [CrossRef]
- Hashemabadi, D.; Torkashvand, A.M.; Kaviani, B.; Bagherzadeh, M.; Rezaalipour, M.; Zarchini, M. Effect of Mentha pulegium extract and 8-hydroxy quinoline sulphate to extend the quality and vase life of rose (Rosa hybrid) cut flower. J. Environ. Biol. 2015, 36, 215. [Google Scholar]
- Pohl, A.; Kalisz, A.; Sekara, A. Seaweed extracts’ multifactorial action: Influence on physiological and biochemical status of Solanaceae plants. Acta Agrobot. 2019, 72, 1758. [Google Scholar] [CrossRef]
- Graziani, G.; Ritieni, A.; Cirillo, A.; Cice, D.; Di Vaio, C. Effects of biostimulants on annurca fruit quality and potential nutraceutical compounds at harvest and during storage. Plants 2020, 9, 775. [Google Scholar] [CrossRef]
- Soltaniband, V.; Brégard, A.; Gaudreau, L.; Dorais, M. Biostimulants promote plant development, crop productivity, and fruit quality of protected strawberries. Agronomy 2022, 12, 1684. [Google Scholar] [CrossRef]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef]
- Basile, B.; Brown, N.; Valdes, J.M.; Cardarelli, M.; Scognamiglio, P.; Mataffo, A.; Colla, G. Plant-based biostimulant as sustainable alternative to synthetic growth regulators in two sweet cherry cultivars. Plants 2021, 10, 619. [Google Scholar] [CrossRef]
- Putra, P.A.; Yuliando, H. Soilless culture system to support water use efficiency and product quality: A review. Agric. Agric. Sci. Procedia 2015, 3, 283–288. [Google Scholar] [CrossRef]
- Savvas, D.; Gianquinto, G.; Tuzel, Y.; Gruda, N. Soilless Culture. FAO Plant Production and Protection Paper No. 217: Good Agricultural Practices for Greenhouse Vegetable Crops. 2013. Available online: https://cris.unibo.it/handle/11585/173272 (accessed on 13 June 2024).
- Caputo, S. History, techniques and technologies of soil-less cultivation. In Small Scale Soil-Less Urban Agriculture in Europe; Springer: Cham, Switzerland, 2022; pp. 45–86. [Google Scholar] [CrossRef]
- Dalal, D.; Mainani, R.; Thakker, R.; Solanki, H. A Study of Selected Microgreens in Soil-Less Media. Int. Assoc. Biol. Comput. Dig. 2022, 1, 228–230. [Google Scholar] [CrossRef]
- Rosli, N.S.M.; Abdullah, R.; Yaacob, J.S.; Razali, R.B.R. Effect of biochar as a hydroponic substrate on growth, colour and nutritional content of red lettuce (Lactuca sativa L.). Bragantia 2023, 82, e20220177. [Google Scholar] [CrossRef]
- Cedeño, J.M.; Magán, J.J.; Thompson, R.B.; Fernández, M.D.; Gallardo, M. Comparison of Methods to Determine Nutrient Uptake of Tomato Grown in Free-Draining Perlite Substrate—Key Information for Optimal Fertigation Management. Horticulturae 2024, 10, 232. [Google Scholar] [CrossRef]
- Radtke Wieth, A.; Dutra Pinheiro, W.; Da Silva Duarte, T. Commercial substrates and nutrient concentrations in the production of arugula microgreens. Agron. Colomb. 2021, 39, 5–11. [Google Scholar] [CrossRef]
- Saleh, R.; Gunupuru, L.R.; Lada, R.; Nams, V.; Thomas, R.H.; Abbey, L. Growth and biochemical composition of microgreens grown in different formulated soilless media. Plants 2022, 11, 3546. [Google Scholar] [CrossRef] [PubMed]
- Ritonga, S.; Vinolina, N.S. Response of wheatgrass and ricegrass growth on various types of planting media. IOP Conf. Ser. Earth Environ. Sci. 2022, 977, 012034. [Google Scholar] [CrossRef]
- Pamungkas, P.B.; Nadia, L.S.; Amrih, D. Study of microgreens growth on various planting medium. AIP Conf. Proc. 2023, 2491, 020018. [Google Scholar] [CrossRef]
- Sharma, A.; Hazarika, M.; Heisnam, P.; Pandey, H.; Devadas, V.S.; Wangsu, M.; Kartha, B.D. Factors affecting production, nutrient translocation mechanisms, and LED emitted light in growth of microgreen plants in soilless culture. ACS Agric. Sci. Technol. 2023, 3, 701–719. [Google Scholar] [CrossRef]
- Paradiso, R.; Buonomo, R.; Dixon, M.A.; Barbieri, G.; De Pascale, S. Soybean cultivation for Bioregenerative Life Support Systems (BLSSs): The effect of hydroponic system and nitrogen source. Adv. Space Res. 2014, 53, 574–584. [Google Scholar] [CrossRef]
- Maru, R.N.; Wesonga, J.; Okazawa, H.; Kavoo, A.; Neondo, J.O.; Mazibuko, D.M.; Orsini, F. Evaluation of Growth, Yield and Bioactive Compounds of Ethiopian Kale (Brassica carinata A. Braun) Microgreens under Different LED Light Spectra and Substrates. Horticulturae 2024, 10, 436. [Google Scholar] [CrossRef]
- Maboko, M.M. Effect of plant density and harvesting frequency on yield components of hydroponically grown mustard spinach (Brassica juncea). In II All Africa Horticulture Congress. Acta Hortic. 2012, 1007, 515–521. [Google Scholar] [CrossRef]
- Sinaga, A.N.; Zahra, A.M.; Nugroho, E.; Simatupang, H.K.; Pitaloka, N.D.; Annisa, H.N.; Pahlawan, M.F. Hydroponic NFT-Based Indoor Farming of Red and Green Lettuce Microgreens in Response to Artificial Lighting. In Proceedings of the 3rd International Conference on Smart and Innovative Agriculture (ICoSIA 2022), Yogyakarta, Indonesia, 22–23 November 2022; Atlantis Press: Amsterdam, The Netherlands, 2023; pp. 625–634. [Google Scholar] [CrossRef]
- Singh, B. New Systems of Vegetable Production: Protected Cultivation, Hydroponics, Aeroponics, Vertical, Organic, Microgreens. In Vegetables for Nutrition and Entrepreneurship; Springer Nature: Singapore, 2023; pp. 31–56. [Google Scholar] [CrossRef]
- Soufi, H.R.; Roosta, H.R.; Hamidpour, M. The plant growth, water and electricity consumption, and nutrients uptake are influenced by different light spectra and nutrition of lettuce. Sci. Rep. 2023, 13, 20766. [Google Scholar] [CrossRef]
- Da Silva, M.G.; Soares, T.M.; Gheyi, H.R. Production of Coriander Using the Hydroponic Technique. In Handbook of Coriander (Coriandrum sativum); CRC Press: Boca Raton, FL, USA, 2023; pp. 39–62. [Google Scholar]
- Srivastava, U.; Mathur, A. Recent Advancements in Prevalent Practices for Plant Cultivation by Hydroponics. Def. Life Sci. J. 2023, 8, 255–268. [Google Scholar] [CrossRef]
- Puspitahati, P.; Andica, F. Floating Raft Hydroponic System Using Spray Bars Pumps On Pakcoy Cultivation Growth (Brassica rapa L.). In Proceedings of the 3rd Sriwijaya International Conference on Environmental Issues, SRICOENV 2022, Palembang, South Sumatera, Indonesia, 5 October 2022. [Google Scholar] [CrossRef]
- Atherton, H.R.; Li, P. Hydroponic cultivation of medicinal plants—Plant organs and hydroponic systems: Techniques and trends. Horticulturae 2023, 9, 349. [Google Scholar] [CrossRef]
- Wortman, S.E. Crop physiological response to nutrient solution electrical conductivity and pH in an ebb-and-flow hydroponic system. Sci. Hortic. 2015, 194, 34–42. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.J.; Gang, M.S.; Kim, D.W.; Cho, W.J.; Jang, J.K. Closed hydroponic nutrient solution management using multiple water sources. J. Biosyst. Eng. 2023, 48, 215–224. [Google Scholar] [CrossRef]
- Nalwade, R.; Mote, T. Hydroponics farming. In Proceedings of the 2017 IEEE International Conference on Trends in Electronics and Informatics (ICEI), Tirunelveli, India, 11–12 May 2017; pp. 645–650. [Google Scholar] [CrossRef]
- Nxawe, S.; Ndakidemi, P.A.; Laubscher, C.P. Possible effects of regulating hydroponic water temperature on plant growth, accumulation of nutrients and other metabolites. Afr. J. Biotechnol. 2010, 9, 9128–9134. [Google Scholar]
- Ali, A.; Cavallaro, V.; Santoro, P.; Mori, J.; Ferrante, A.; Cocetta, G. Quality and physiological evaluation of tomato subjected to different supplemental lighting systems. Sci. Hortic. 2023, 323, 112469. [Google Scholar] [CrossRef]
- Rajendiran, G.; Rethnaraj, J. Smart Aeroponic Farming System: Using IoT with LCGM-Boost Regression Model for Monitoring and Predicting Lettuce Crop Yield. Int. J. Intell. Eng. Syst. 2023, 16, 251–262. [Google Scholar]
- Ouyang, Z.; Tian, J.; Yan, X.; Yang, Z. Micro-nano oxygenated irrigation improves the yield and quality of greenhouse cucumbers under-film drip irrigation. Sci. Rep. 2023, 13, 19453. [Google Scholar] [CrossRef]
- Farawn, K.K.; Leunov, V.I.; Tereshonkova, T.A.; Salman, A.H.; Al-Rukabi, M.N.M.; Shaaban, F. Evaluate tomato growing in the conditions of subirrigation aeroponics at Fitopiramida greenhouse. IOP Conf. Ser. Earth Environ. Sci. 2022, 1010, 012034. [Google Scholar] [CrossRef]
- Regmi, A.; Rueda-Kunz, D.; Liu, H.; Trevino, J.; Kathi, S.; Simpson, C. Comparing resource use efficiencies in hydroponic and aeroponic production systems. Technol. Hortic. 2024, 4, e005. [Google Scholar] [CrossRef]
- Sobczak, A.; Kućko, A.; Pióro-Jabrucka, E.; Gajc-Wolska, J.; Kowalczyk, K. Effect of Salicylic Acid on the Growth and Development of Sweet Pepper (Capsicum annum L.) under Standard and High EC Nutrient Solution in Aeroponic Cultivation. Agronomy 2023, 13, 779. [Google Scholar] [CrossRef]
- Cayambe, J.; Heredia-R, M.; Torres, E.; Puhl, L.; Torres, B.; Barreto, D.; Diaz-Ambrona, C.G. Evaluation of sustainability in strawberry crops production under greenhouse and open-field systems in the Andes. Int. J. Agric. Sustain. 2023, 21, 2255449. [Google Scholar] [CrossRef]
- Buckseth, T.; Sharma, S.; Tiwari, J.K.; Kumar, V.; Sharma, A.K.; Challam, C.; Singh, R.K. Plant Sources Identify Variations in Potato Production Potential Under Aeroponics. Potato Res. 2023. [Google Scholar] [CrossRef]
- He, J. Enhancing Productivity and Improving Nutritional Quality of Subtropical and Temperate Leafy Vegetables in Tropical Greenhouses and Indoor Farming Systems. Horticulturae 2024, 10, 306. [Google Scholar] [CrossRef]
- Tunio, M.H.; Gao, J.; Mohamed, T.M.; Ahmad, F.; Abbas, I.; Shaikh, S.A. Comparison of nutrient use efficiency, antioxidant assay, and nutritional quality of butter-head lettuce (Lactuca sativa L.) in five cultivation systems. Int. J. Agric. Biol. Eng. 2023, 16, 95–103. [Google Scholar] [CrossRef]
- Verma, A.K.; Chandrakant, M.H.; John, V.C.; Peter, R.M.; John, I.E. Aquaponics as an integrated agri-aquaculture system (IAAS): Emerging trends and future prospects. Technol. Forecast. Soc. Chang. 2023, 194, 122709. [Google Scholar] [CrossRef]
- Masabni, J.; Niu, G. Aquaponics. In Plant Factory Basics, Applications and Advances; Academic Press: Cambridge, MA, USA, 2022; pp. 167–180. [Google Scholar] [CrossRef]
- Angkha, B.; Verma, A.K.; Kumar, S.H.; Prakash, C.; Thomas, R.M. Mobilization of mica by Bacillus sp. and its effect on Nile tilapia (Oreochromis niloticus) cum holy basil (Ocimum tenuiflorum)–based aquaponic system. Aquac. Int. 2020, 28, 2045–2058. [Google Scholar] [CrossRef]
- Stathopoulou, P.; Tsoumalakou, E.; Levizou, E.; Vanikiotis, T.; Zaoutsos, S.; Berillis, P. Iron and potassium fertilization improve rocket growth without affecting tilapia growth and histomorphology characteristics in aquaponics. Appl. Sci. 2021, 11, 5681. [Google Scholar] [CrossRef]
- Yıldız, H.Y.; Bekcan, S. Role of stocking density of tilapia (Oreochromis aureus) on fish growth, water quality and tomato (Solanum lycopersicum) plant biomass in the aquaponic system. Int. J. Environ. Agriic. Biotechnol. 2017, 2, 238971. [Google Scholar] [CrossRef]
- Roosta, H.R. Effects of foliar spray of K on mint, radish, parsley and coriander plants in aquaponic system. J. Plant Nut. 2014, 37, 2236–2254. [Google Scholar] [CrossRef]
- Rakocy, J.E. Aquaponics—Integrating fish and plant culture. In Aquaculture Production Systems; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 344–386. [Google Scholar] [CrossRef]
- Siqwepu, O.; Salie, K.; Goosen, N. Evaluation of potassium diformate and potassium chloride in the diet of the African catfish, Clarias gariepinus in a recirculating aquaculture system. Aquaculture 2020, 526, 735414. [Google Scholar] [CrossRef]
- John, V.C.; Verma, A.K.; Krishnani, K.K.; Chandrakant, M.H.; Varghese, T.; Pathak, M.S. Effect of potassium supplementation on osmoregulatory and stress response of Pangasianodon hypophthalmus (Sauvage, 1878) with Spinacia oleracea L. in aquaponics. J. Fish Biol. 2022, 101, 249–261. [Google Scholar] [CrossRef]
- Pasch, J.; Ratajczak, B.; Appelbaum, S.; Palm, H.W.; Knaus, U. Growth of basil (Ocimum basilicum) in DRF, raft, and grow pipes with effluents of African catfish (Clarias gariepinus) in decoupled aquaponics. AgriEngineering 2021, 3, 92–109. [Google Scholar] [CrossRef]
- Ani, J.S.; Manyala, J.O.; Masese, F.O.; Fitzsimmons, K. Effect of stocking density on growth performance of monosex Nile Tilapia (Oreochromis niloticus) in the aquaponic system integrated with lettuce (Lactuca sativa). Aquacult. Fish. 2022, 7, 328–335. [Google Scholar] [CrossRef]
- Mamatha, D.; Verma, A.K.; Tiwari, V.K.; Chandrakant, M.H.; Nayak, S.K.; Javed, H. Biointegration of rohu (Labeo rohita) fry and lemon grass (Cymbopogon citratus) in a recirculating aquaponic System. J. Ind. Soc. Coast. Agric. Res. 2020, 38. [Google Scholar]
- Kaburagi, E.; Yamada, M.; Baba, T.; Fujiyama, H.; Murillo-Amador, B.; Yamada, S. Aquaponics using saline groundwater: Effect of adding microelements to fish wastewater on the growth of Swiss chard (Beta vulgaris L. spp. cicla). Agri. Water Manag. 2020, 227, 105851. [Google Scholar] [CrossRef]
- Bailey, D.S.; Ferrarezi, R.S. Valuation of vegetable crops produced in the UVI Commercial Aquaponic System. Aquacult. Rep. 2017, 7, 77–82. [Google Scholar] [CrossRef]
- Peter, R.M.; Verma, A.K.; Saharan, N.; Tiwari, V.K.; Chandrakant, M.H.; Thomas, R.M. Effect of hydraulic loading rate on production of tomato (Solanum lycopersicum) with pearlspot (Etroplus suratensis) in recirculating aquaponic system. J. Agric. Eng. 2021, 58, 300–308. [Google Scholar] [CrossRef]
- Walling, I.; Kanaujia, S.P.; Changini, M. Response of broccoli (Brassica oleracea var, italica) to integrated nutrient management. Ann. Plant Soil Res. 2022, 24, 106–109. [Google Scholar] [CrossRef]
- Yuan, X.; Tajima, R.; Matsumoto, M.; Fujiwara, A.; Aoyama, T.; Okada, C.; Takimoto, H. Analysing food groups and nutrient intake in adults who met and did not meet the daily recommended vegetable intake of 350 g: The 2016 National Health and Nutrition Survey in Japan. J. Nutr. Sci. 2024, 13, e12. [Google Scholar] [CrossRef]
- Kumar, M.; Chaudhary, V.; Naresh, R.K.; Maurya, O.P.; Pal, S.L. Does integrated sources of nutrients enhance growth, yield, quality and soil fertility of vegetable crops. Int. J. Curr. Microb. App. Sci. 2018, 7, 125–155. [Google Scholar] [CrossRef]
- Chopra, A.K.; Payum, T.; Sachin, S.; Vinod, K. Effect of integrated nutrient management on agronomical attributes of tomato under field conditions. Arch. Agric. Environ. Sci. 2017, 2, 86–91. [Google Scholar]
- Gokul, D.; Poonkodi, P.; Angayarkanni, A. Effect of integrated nutrient management on the growth and nutrient content of chilli. Int. J. Chem. Stud. 2020, 8, 2647–2651. [Google Scholar] [CrossRef]
- Ankit, P.; Choudhary, A.; Raj, S.; Pooja, S.; Forum, B. Effect of integrated nutrient management on yield of brinjal. Int. J. Agric. Food Sci. 2022, 4, 12–16. [Google Scholar]
- Kumar, P.; Kumar, A.; Kumar, N.; Atik, A.; Verma, M. Effect of integrated nutrient management on productivity and nutrient availability of potato. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1429–1436. [Google Scholar] [CrossRef][Green Version]
- Tomar, A.; Sangeeta, M.; Asati, K.; Swati, B. Integrated nutrient management in bottle gourd variety Kashi Ganga on the plant disease, TSS and economics under Malwa condition of MP. Int. J Curr. Sci. Res. Rev. 2022, 5, 2581–8341. [Google Scholar] [CrossRef]
- Singh, J.; Singh, M.; Kumar, M.; Gupta, A.; Singh, K. Growth, yield and quality parameters of cucumber as influence by INM applications. Int. J Curr. Microbilol. Appl. Sci. 2020, 9, 2319–7706. [Google Scholar] [CrossRef]
- Dudhat, M.A.; Patel, K.D. Evaluation of INM on performance of quality and yield attributes of hybrid bitter gourd. J. Pharmacogn. Phytochem. 2020, 9, 1643–1645. [Google Scholar]
- Patel, H.S.; Patel, N.B.; Sarvaiya, J.P.; Chawla, S.L. INM on growth and yield of ridge gourd cv. GARG-1. Pharma Innov. 2021, 10, 1064–1069. [Google Scholar]
- Chaudhari, V.M.; Patel, N.K.; Barot, D.C.; Solanki, K.S. Impact of biofertilizers based nutrient management on growth and yield of cauliflower cv. Pusa Snowball KT-25. Pharma Innov. 2023, 12, 3037–3040. [Google Scholar]
- Kumari, K.; Singh, S.K.; Mehmi, V.; Kumar, U.; Kaur, K. Influence of plant growth regulators and bio-fertilizers on growth and yield of broccoli under central region of Punjab. J. Agriways 2019, 7, 44–49. [Google Scholar]
- Kumar, D.; Kumar, S.; Kumar, R.; Verma, S. Effect of organic and inorganic fertilizer on growth, yield and quality of cabbage. Int. J. Pure Appl. Biosci. 2017, 5, 1590–1593. [Google Scholar] [CrossRef]
- Abou, M.M.; Zaki, M.F.; Sedera, S.A. Bio-fertilizers and foliar application of milagro biostimulants in relation to growth, head yield and quality as mineral K requirement of Chinese cabbage. Middle East J. Agric. 2018, 7, 1310–1322. [Google Scholar]
- Kaur, B.; Boparai, A.K.; Singh, K. Effect of integrated nutrient sources on agronomic performance of onion (Onion cepa L.) and soil properties. Ind. J. Ecol. 2023, 50, 367–371. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, H.R. Effect of Integrated Nutrient Management on Growth, Yield and Quality in Garlic (Allium sativum L.). Int. J. Plant Soil Sci. 2023, 35, 648–654. [Google Scholar] [CrossRef]
- Amartey, J.N.A.; Sarkordie-Addo, J.; Essilfie, M.E.; Dapaah, H.K. Growth and yield of carrots affected by integrated nutrient management of organic and inorganic fertilizers. Afr. J. Agric. Res. 2022, 18, 576–585. [Google Scholar] [CrossRef]
- Shilpa, D.; Sharma, M.; Kaur, M.; Kumar Sharma, A.; Sharma, P.; Chauhan, M. Soil fertility, growth, yield and root quality of radish (Raphanus sativus L.) as influenced by integrated nutrient management practices. Comm. Soil Sci. Plant Anal. 2023, 54, 1316–1333. [Google Scholar] [CrossRef]
- Dong, M.; Xin, R.; Li, Z.Y.; Li, Y.L.; Huang, X.H.; Dong, X.P.; Qin, L. Simultaneously quantification of organic acids metabolites by HPLC mass spectrometry to reveal the postharvest quality change in cherry tomato. J. Food Comp. Anal. 2023, 117, 105105. [Google Scholar] [CrossRef]
- Miah, M.G.R.; Karim, A.S.; Islam, M.M.; Islam, M.D.; Kamal, M.Z.U. Effects of water stress and nutrient management on the performance of tomato. Int. J. Environ. Agric. Biotechnol. 2023, 8. Available online: https://i.agriculturejournals.org/index.php/ijeab/article/view/73 (accessed on 21 May 2024). [CrossRef]
- Saroha, A.; Kotiyal, A.; Thakur, A. Climate-Resilient Fertilizer Management for Crop Production. In Climate-Resilient Agriculture, Vol 2: Agro-Biotechnological Advancement for Crop Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 61–79. [Google Scholar] [CrossRef]
- Elbasiouny, H.; El-Ramady, H.; Elbehiry, F.; Rajput, V.D.; Minkina, T.; Mandzhieva, S. Plant nutrition under climate change and soil carbon sequestration. Sustainability 2022, 14, 914. [Google Scholar] [CrossRef]
- Charishma, K.V.; Chatterjee, S.; Supriya, T.; Bera, M.; Barman, S.; Datta, N. Integrated nutrient management on the growth, yield, nutrient uptake and soil nutrient status of tomato (Solanum lycopersicum) cv.“Arka Rakshak” under north-eastern ghats region of India. Crop Res. 2022, 57, 266–274. [Google Scholar] [CrossRef]
- Singh, D.P.; Maurya, B.K.; Kumari, M.; Singh, R.P.; Singh, D.B.; Kumar, P. Response of integrated nutrient management (INM) on growth and yield attributes in tomato (Solanum lycopersicum L.). Ann. Agric. Res. 2023, 44, 120–126. Available online: https://epubs.icar.org.in/index.php/AAR/article/view/134818 (accessed on 21 May 2024).
- Singh, D.; Kushum, S. Effect of integrated nutrient management on the growth, yield and quality in tomato (Solanum lycopersicum L.). Int. J. Environ. Clim. Chang. 2022, 12, 2802–2811. [Google Scholar] [CrossRef]
- Jethava, B.A.; Patel, K.M.; Rathva, V.D.; Macwan, S.J. Effect of integrated nutrient management and micronutrients on growth, yield and economics of tomato cv. GAT-5. Pharma Innov. J. 2023, 12, 4462–4466. [Google Scholar]
- Sharma, H.L.; Tailor, S.P.; Rajawat, K.S.; Kurmi, K.P. Effect of integrated nutrient management on the growth, yield parameters and economics in tomato (Lycopersicon esculentum L.) under Southern Rajasthan conditions. Pharma Innov. J. 2023, 12, 321–326. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Cui, D.; Li, Y.; Zou, G.; Thompson, R.; Yang, J. Integrated crop-nitrogen management improves tomato yield and root architecture and minimizes soil residual N. Agronomy 2022, 12, 1617. [Google Scholar] [CrossRef]
- Chaurasiya, P.C.; Kumar, S. Effect of integrated nutrients management on growth, yield and quality of tomato (Solanum lycopersicum L.) under Chhattisgarh plains. Pharma Innov. 2023, 12, 857–860. [Google Scholar]
- Pavani, Y.; Janaki, P.; Jagadeeswaran, R.; Sankari, A.; Ramalakshmi, A.; Arthanari, P.M. Response of tomato to fertilizer nutrients integration and herbicides spray: Evaluating growth, yield, fruit quality and herbicides residue. Plant Sci. Today 2024, 11, 93–101. [Google Scholar] [CrossRef]
- Tao, Y.; Liu, T.; Wu, J.; Wu, Z.; Liao, D.; Shah, F.; Wu, W. Effect of combined application of chicken manure and inorganic nitrogen fertilizer on yield and quality of cherry tomato. Agronomy 2022, 12, 1574. [Google Scholar] [CrossRef]
- Seth, A.; Sarkar, D.; Datta, A.; Mandal, B.; Chowdhury, A.; Masto, R.E.; Saha, S. Suitability of complex extractants for assessment of available soil zinc for nutrition of rice (Oryza sativa L.) in subtropical India. Soil Sci. 2017, 182, 28–35. [Google Scholar] [CrossRef]
- Sarkar, D.; Baishya, L.K.; Meitei, C.B.; Naorem, G.C.; Thokchom, R.C.; Singh, J.; Rahman, F.H. Can sustainability of maize-mustard cropping system be achieved through balanced nutrient management? Field Crops Res. 2018, 225, 9–21. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Malik, K.; Chejara, S.; Rajwar, D.; Narjary, B.; Chandra, P. Integration of organics in nutrient management for rice-wheat system improves nitrogen use efficiency via favorable soil biological and electrochemical responses. Front. Plant Sci. 2023, 13, 1075011. [Google Scholar] [CrossRef]
- Biswas, P.; Bohra, J.S.; Kumar, N. Effect of nutrient management on quality, NPK content and uptake in rice (Oryza sativa L.) under rice-wheat cropping system. J. Pharmacogn. Phytochem. 2020, 9, 1254–1258. [Google Scholar]
- Tomar, R.; Singh, N.B.; Singh, V.; Kumar, D. Effect of planting methods and integrated nutrient management on growth parameters, yield and economics of rice. J. Pharmacaogn. Phytochem. 2018, 7, 520–527. [Google Scholar]
- Ram, M.S.; Shankar, T.; Maitra, S.; Duvvada, S.K. Effect of Integrated Nutrient Management on Growth, Yield, Nutrient Content and Economics of Summer Rice (Oryza sativa L.). Int. J. Pure Appl. Biosci. 2020, 8, 421–427. [Google Scholar] [CrossRef]
- Rao, A.; Singh, N.B.; Pandey, D.; Singh, M.P. Effect of integrated nutrient management on productivity and profitability of rice under SRI. J. Pharmacogn. Phytochem. 2020, 9, 665–667. [Google Scholar]
- Shankar, T.; Maitra, S.; Ram, M.S.; Mahapatra, R. Influence of integrated nutrient management on growth and yield attributes of summer rice (Oryza sativa L.). Crop Res. 2020, 55, 1–5. [Google Scholar] [CrossRef]
- Behera, H.S.; Pany, B.K. Impact of inorganic nitrogenous fertilizers and farmyard manure combination on grain, straw, biological yield and harvest index of rice (Oryza sativa L.). J. Pharmacogn. Phytochem. 2021, 10, 257–260. [Google Scholar] [CrossRef]
- Aasif, M.; Chinnamani, I.; Kumar, N.S.; Hemalatha, M.; Suresh, S. Influence of integrated nutrient management practices on yield and nutrient uptake of rice under system of rice intensification. Int. J. Adv. Agric. Sci. Technol. 2008, 5, 10–16. [Google Scholar]
- Apon, M.; Gohain, T.; Apon, R.; Banik, M.; Mandal, A.K. Effect of integrated nutrient management on growth and yield of local rice (Oryza sativa L.) under rainfed upland condition of Nagaland. Pharma Innov. J. 2018, 7, 426–429. [Google Scholar]
- Sharma, A.K.; Singh, T.; Patel, A.; Yadav, R.A. Influence of integrated nutrient management practices on scented rice (Oryza sativa L.) Pertaining to eastern Uttar Pradesh. J. Pharmacogn. Phytochem. 2018, 7, 1448–1453. [Google Scholar]
- Baishya, L.K.; Rathore, S.S.; Singh, D.; Sarkar, D.; Deka, B.C. Effect of integrated nutrient management on rice productivity, profitability and soil fertility. Ann. Plant Soil Res. 2015, 17, 86–90. [Google Scholar]
- Tiwari, H.; Singh, A.K.; Pandey, S.R.; Tiwari, A. Effect of Integrated nutrient management practices on nutrient content and uptake by rice (Oryza sativa L.). J. Pharmacogn. Phytochem. 2020, 9, 2131–2134. [Google Scholar]
- Sarwar, G.; Hussain, N.; Schmeisky, H.; Suhammad, S.; Ibrahim, M.; Ahmad, S. Efficiency of Various Organic Residues for Enhancing Rice-Wheat Production under Normal Soil Conditions. Pak. J. Bot. 2008, 40, 2107–2113. [Google Scholar]
- Ding, W.; Xu, X.; He, P.; Ullah, S.; Zhang, J.; Cui, Z.; Zhou, W. Improving yield and nitrogen use efficiency through alternative fertilization options for rice in China: A meta-analysis. Field Crops Res. 2018, 227, 11–18. [Google Scholar] [CrossRef]
- Bhardwaj, A.K.; Rajwar, D.; Mandal, U.K.; Ahamad, S.; Kaphaliya, B.; Minhas, P.S.; Prabhakar, M.; Banyal, R.; Singh, R.; Chaudhari, S.K.; et al. Impact of carbon inputs on soil carbon fractionation, sequestration and biological responses under major nutrient management practices for rice-wheat cropping systems. Sci. Rep. 2019, 9, 9114. [Google Scholar] [CrossRef] [PubMed]
- Chaubey, A.K.; Mishra, S.; Singh, S.K.; Chaubey, C.; Pandey, K.P. Integrated Nutrients Management for Future Production: A Review. Int. J. Environ. Clim. Chang. 2023, 13, 1770–1779. [Google Scholar] [CrossRef]
- Akhtar, N.; Ramani, V.B.; Yunus, M.; Femi, V. Effect of different nutrient management treatments on growth, yield attributes, yield and quality of wheat (Triticum aestivum L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3473–3479. [Google Scholar]
- Saini, L.H.; Saini, A.K.; Malve, S.H.; Patel, J.P.; Nand, B.; Chaudhary, H.S. Growth and yield attainment of wheat under different levels of vermicompost, biofertilizers and nitrogen. Pharma Innov. J. 2023, 12, 1245–1249. [Google Scholar]
- Patyal, A.; Shekhar, C.; Sachan, R.; Kumar, D.; Yadav, A.; Kumar, G. Effect of integrated nutrient management (INM) on growth parameters and yield of wheat (Triticum aestivum L.). Int. J. Plant Soil Sci. 2022, 34, 962–967. [Google Scholar] [CrossRef]
- Kumar, G.; Niwas, R. Effect of organic and inorganic fertilizers on growth and yield of wheat (Triticum aestivum L.). Pharma Innov. J. 2022, 11, 1005–1009. [Google Scholar]
- Kumawat, H.; Singh, D.P.; Jat, G.; Choudhary, R.; Singh, P.B.; Dhayal, S.; Khardia, N. Effect of fertility levels and liquid biofertilizers on growth and yield of wheat (Triticum aestivum L.). Pharma Innov. J. 2021, 10, 1365–1369. [Google Scholar]
- Pandey, I.B.; Dwivedi, D.K.; Pandey, R.K. Integrated nutrient management for sustaining wheat (Triticum aestivum) production under late sown condition. Ind. J. Agric. 2009, 54, 306–309. [Google Scholar]
- Desai, H.A.; Dodia, I.N.; Desai, C.K.; Patel, M.D.; Patel, H.K. Integrated nutrient management in wheat (Triticum aestivum L.). Trends Biosci. 2015, 8, 472–475. [Google Scholar]
- Devi, K.N.; Singh, M.S.; Singh, N.G.; Athokpam, H.S. Effect of integrated nutrient management on growth and yield of wheat (Triticum aestivum L.). J. Crop Weed. 2011, 7, 23–27. [Google Scholar]
- Fazily, T.; Thakral, S.K.; Dhaka, A.K. Effect of integrated nutrient management on growth, yield attributes and yield of wheat. Int. J. Adv. Agric. Sci. Technol. 2021, 8, 106–118. [Google Scholar] [CrossRef]
- Singh, G.; Kumar, S.; Singh, G.; Singh, N. Effect of integrated nutrient management on nutrient uptake and grain yield of wheat (Triticum aestivum L.) under irrigated conditions. J. Pharmacogn. Phytochem. 2019, 8, 1077–1080. [Google Scholar]
- Kumar, A.; Singh, A.K.; Kumar, S.; Kumar, D.; Gopal, T.; Pandey, D.; Pandey, V.K. Effect of nutrient management and moisture regime on growth and yield of wheat (Triticum aestivum L.). J. Pharmacogn. Phytochem. 2018, 7, 610–613. [Google Scholar]
- Dhaliwal, S.S.; Sharma, V.; Shukla, A.K.; Gupta, R.K.; Verma, V.; Kaur, M.; Behera, S.K.; Singh, P. Residual effect of organic and inorganic fertilizers on growth, yield and nutrient uptake in wheat under a basmati rice–wheat cropping system in North-Western India. Agriculture 2023, 13, 556. [Google Scholar] [CrossRef]
- Messaoudi, A.; Labdelli, F.; Rebouh, N.Y.; Djerbaoui, M.; Kucher, D.E.; Hadjout, S.; Ouaret, W.; Zakharova, O.A.; Latati, M. Investigating the Potassium Fertilization Effect on Morphological and Agrophysiological Indicators of Durum Wheat under Mediterranean Rain-Fed Conditions. Agriculture 2023, 13, 1142. [Google Scholar] [CrossRef]
- Begam, A.; Pramanick, M.; Dutta, S.; Paramanik, B.; Dutta, G.; Patra, P.S.; Biswas, A. Inter-cropping patterns and nutrient management effects on maize growth, yield and qality. Field Crops Res. 2024, 310, 109363. [Google Scholar] [CrossRef]
- Paramesh, V.; Kumar, R.M.; Rajanna, G.A.; Gowda, S.; Nath, A.J.; Madival, Y.; Toraskar, S. Integrated nutrient management for improving crop yields, soil properties, and reducing greenhouse gas emissions. Front. Sustain. Food Syst. 2023, 7, 1173258. [Google Scholar] [CrossRef]
- Li, C.; Stomph, T.J.; Makowski, D.; Li, H.; Zhang, C.; Zhang, F.; Van der Werf, W. The productive performance of intercropping. Proc. Nat. Acad. Sci. USA 2023, 120, e2201886120. [Google Scholar] [CrossRef] [PubMed]
- Velmourougane, K.; Manikandan, A.; Blaise, D.; Vellaichamy, M. Cotton stalk compost as a substitution to farmyard manure along with mineral fertilizers and microbials enhanced Bt cotton productivity and fibre quality in rainfed vertisols. Waste Biom. Valor. 2022, 13, 2847–2860. [Google Scholar] [CrossRef]
- Iraiyanban, A.A.A.; Manuel, R.I.; Joseph, P.A.; Kumar, P.D. Influence of Cropping System and Nutrient Management Practices on the Yield, and Economics of Pearl Millet [Pennisetum glaucum]. Int. J. Environ. Clim. Chang. 2023, 13, 701–709. [Google Scholar] [CrossRef]
- Prakash, S.; Reddy, S.V.V.; Prasad, R.; Ballabh, J. Effect of Integrated Nutrient Management in Chickpea (Cicer arietinum L.). J. Sur. Fish. Sci. 2022, 8, 278–282. [Google Scholar]
- Palai, J.B. Performance of Finger Millet on Residual Soil Nutrient Status of Preceding Leguminous Crops under Integrated Nutrient Management—A Review. Indian J. Nat. Sci. 2022, 13, 42631. [Google Scholar]
- Gafoor, R.A.; Pillai, S.; Nishan, M.A. Effect on integrated nutrient management on finger millet (Eleusine coracana (L.) Gaertn.): A review. J. Pharmacogn. Phytochem. 2021, 10, 292–298. [Google Scholar] [CrossRef]
- Kharsan, G.D.B.; Barman, G.D.; Shrivastava, S.; Devi, S. Integrated nutrient management studies on French bean (Phaseolus vulgaris L.) quality attributes in Vertisols of Chhattisgarh plain. Pharma Innov. 2023, 12, 4663–4665. [Google Scholar]
- Arbabi, A.; Sirousmehr, A.; Ganbari, A.; Fanaei, H.R. Effect of organic, chemical and combined fertilizers on some quantitative and qualitative characteristics of three sunflower (Heliantus annuus L.) cultivars. J. Agric. Sci. Sustain. Prod. 2023, 33, 25–37. [Google Scholar]
- Kugedera, A.T.; Kokerai, L.K. A review on the effects of mineral fertilizer, manure and water management in improving sorghum grain yields in semi-arid areas. J. Plant Nut. 2024, 47, 1175–1188. [Google Scholar] [CrossRef]
- Thakur, O.; Verma, A.; Thakur, P. Response of integrated nutrient management on Sweet Potato: A review. Int. J. Stat. Appl. Math. 2023, 8, 875–879. [Google Scholar]
- Kugedera, A.T.; Nyamadzawo, G.; Mandumbu, R.; Nyamangara, J. Potential of field edge rainwater harvesting, biomass transfer and integrated nutrient management in improving sorghum productivity in semi-arid regions: A review. Agrofor. Syst. 2022, 96, 909–924. [Google Scholar] [CrossRef]
- Ali, A.; Santoro, P.; Ferrante, A.; Cocetta, G. Investigating pulsed LED effectiveness as an alternative to continuous LED through morpho-physiological evaluation of baby leaf lettuce (Lactuca sativa L. var. Acephala). S. Afr. J. Bot. 2023, 160, 560–570. [Google Scholar] [CrossRef]
- Ali, A.; Santoro, P.; Mori, J.; Ferrante, A.; Cocetta, G. Effect of UV-B elicitation on spearmint’s (Mentha spicata L.) morpho-physiological traits and secondary metabolites production. Plant Growth Regul. 2023; 1–14. [Google Scholar] [CrossRef]
- Gonnella, M.; Renna, M. The Evolution of soilless systems towards ecological sustainability in the perspective of a circular economy. Is it really the opposite of organic agriculture? Agronomy 2021, 11, 950. [Google Scholar] [CrossRef]
- Gebreegziher, W.G. Soilless culture technology to transform vegetable farming, reduce land pressure and degradation in drylands. Cogent Food Agric. 2023, 9, 2265106. [Google Scholar] [CrossRef]
- Chejara, S.; Malik, K.; Rani, M.; Bhardwaj, A.K. Integrated nutrient management: Concept, constraints, and advantages for a sustainable agriculture. J. Nat. Res. Cons. Manag. 2021, 2, 85–94. [Google Scholar] [CrossRef]
- Srinivasarao, C.; Naik, M.R.; Lakshmi, C.S.; Kumar, G.R.; Manasa, R.; Rakesh, S.; Prasad, J.V.N.S. Economic and Environmental Benefits of Integrated Nutrient Management in Indian Agriculture. 2020. Available online: http://krishi.icar.gov.in/jspui/handle/123456789/81307 (accessed on 21 May 2024).
- Garzón, J.; Montes, L.; Garzón, J.; Lampropoulos, G. Systematic review of technology in aeroponics: Introducing the technology adoption and integration in sustainable agriculture model. Agronomy 2023, 13, 2517. [Google Scholar] [CrossRef]
- Kumar, A.; Chandel, N.; Barkha, B. Organic Farming vs. Integrated Nutrient Management: A Comparative Review of Agricultural Productivity and Sustainability. Int. J. Plant Soil Sci. 2024, 36, 460–473. [Google Scholar] [CrossRef]
- Francini, A.; Giro, A.; Ferrante, A. Biochemical and molecular regulation of phenylpropanoids pathway under abiotic stresses. In Plant Signaling Molecules; Woodhead Publishing: Sawston, UK, 2019; pp. 183–192. [Google Scholar] [CrossRef]
| No. | Focus Points of INM Strategy | Methods | Detailed Strategies |
|---|---|---|---|
| 1 | Assessing plant nutritional deficiency and soil nutrient availability | Sampling and laboratory analysis | Soil sampling and post-harvest plant tissue sampling are conducted apart from the visual observation of nutrient deficiencies in plants. Usually, the results are compared with a reference healthy plant considered as the standard |
| 2 | Evaluating the potential and limitations of soil fertility management | Monitoring the relationship between the INM strategy and nutrient diagnosis | Inspection related to overuse or underuse of nitrogen fertilizers |
| 3 | Investigating the techniques and technologies to balance nutrients | Nutrient intake and output differential inspection and computing the soil nutrient budget | Choosing an appropriate INM after analyzing the variables |
| 4 | Evaluating the productivity and sustainability of INM activities | The use of locally relevant technology | Active participation of farmers in testing and analysis |
| Biostimulants | Crops | Effect of Biostimulants | References | |
|---|---|---|---|---|
| Plant propagation | Algamino plant | White dogwood (Cornus alba L.) | Improved rooting speed in cuttings | [47] |
| Arbuscular mycorrhizal fungi | Olive (Olea europaea L.) | Enhanced rooting and seeding quality | [48] | |
| Microalgae Chlorella vulgaris and Messastrum gracile | Crimson cattleya (Cattleya labiate) | An alternative to plant growth regulators for in vitro propagation | [49] | |
| Root Nectar (willow bark extract and Nutrifield’s biostimulant complex) | Chrysanthemum, lavender (Lavandula angustifolia) | Improved development of root branching and adventitious roots | [50] | |
| Microbial metabolites | Pear (Pyrus communis L.) | Enhanced auxin production that enabled efficient rooting | [51] | |
| Vegetative growth | Moringa leaf extracts | Kale, broccoli (Brassica oleracea) | 60% increased nitrate levels in broccoli, while 70% reduced in kale | [52] |
| Moringa leaf extract | Quinoa (Chenopodium quinoa) | Improved grain yield and overall growth | [53] | |
| Seaweed-based extracts | Cucumber (Cucumis sativus) | Improved growth and fruit yield | [54] | |
| True-Algae-Max (seaweed liquid extract) | Hot peppers (Capsicum annuum) | Improved fruit composition and plant growth | [55] | |
| Photosynthesis and leaf gas exchange | Ascophyllum nodosum (seaweed extract) | Broccoli (Brassica oleracea), spinach (Spinacia oleracea) | Reduction in stomatal closure, improved water stress tolerance and gas exchange | [56] |
| FOLIAR (amino acid based) | Perennial ryegrass (Lolium perenne) | 95% increased photochemical efficiency (Fv/Fm) | [57] | |
| PE Auxym (tropical plant extract) | Nalta jute (Corchorus olitorius) | SPAD index and photosynthesis improved | [58] | |
| Moringa leaf extract | Quinoa (Chenopodium quinoa) | Improved photosynthesis and leaf gaseous exchange | [53] |
| Plant Developmental Stages | Biostimulants | Crops | Effects of Biostimulants | References |
|---|---|---|---|---|
| Seed germination | Polysaccharide-enriched extracts (PEEs) obtained from Moroccan seaweed | Cherry tomato (Solanum lycopersicum) | 0.002 mg/mL of PEEs resulted in an increased seed germination percentage and speed | [68] |
| Seaweed leaf extracts (Laurencia obtusa, Ulva fasciata, and Cystoseira compressa) | Maize (Zea mays) and cowpea (Vigna unguiculata) | Improved seed germination and enhanced seedling growth | [69] | |
| KIEM (lignin derivatives, plant-derived amino acids, molybdenum) | Cucumber (Cucumis sativus) | Improved heat stress tolerance of cucumber seeds | [70] | |
| Bacillus sp. MGW9 | Maize (Zea mays) | Stimulated salt tolerance mechanism during seed germination | [60] | |
| Micro-algae strains | Spinach (Spinacia oleracea) | Better seed germination results | [71] | |
| Flowering | Borage leaf-extract-based biostimulant | Gladiolus cut flower | Better osmotic balance and reduced oxidative stress resulted in an improved vase life | [72] |
| Moringa leaf extract | Gladiolus (white prosperity cultivar) | Improved performance of cut spikes | [73] | |
| Protein hydrolysates (both animal and plant origins) | Chrysanthemum | Improvement in the vase life | [74] | |
| Hydroxyquinoline sulfate (8-HQS) | Cut rose (Rosa hybrida L.) | Improvement in visual quality and better vase life | [75] | |
| Moringa leaf and seed extract | Cut flower (Rosa hybrida cv. “Upper class”) | Extended vase life, proline accumulation, and reduction in stomatal aperture | [54] | |
| Fruit set and quality | Seaweed extract | Eggplant (Solanum melongena) | Improved antioxidant activity, TSSs, anthocyanins, and total polyphenols | [76] |
| Protein hydrolysates | Annurca apples (Malus domestica) | Improved total polyphenol profile | [77] | |
| Seaweed extract, mycorrhiza, and Trichoderma | Strawberries (Fragaria × ananassa) | Enhanced anthocyanins, TSSs, and total polyphenols | [78] | |
| CycloFlow (mixture of yeast and sugarcane molasses) | Tomatoes (Solanum lycopersicum) | Increased vitamin C content | [79] | |
| Seaweed extract and fulvic acid based | Guava (Psidium guavaja L.) | Increased TSS, fruit size, and fruit weight | [80] |
| Media | Types | Crops | References | |
|---|---|---|---|---|
| Solid Inert Medium | Coco coir | Arugula (Eruca sativa), basil (Ocimum basilicum), sunflower (Helianthus annuus) | [84] | |
| Hydroton | Red lettuce (Lactuca sativa) | [85] | ||
| Perlite | Tomato (Solanum lycopersicum) | [86] | ||
| Vermiculite | Arugula (Eruca sativa) | [87] | ||
| Peat moss | Kale (Brassica oleracea), Swiss chard (Beta vulgaris), arugula (Eruca sativa) | [88] | ||
| Sawdust | Rice (Oryza sativa) dust, wheat (Triticum aestivum) dust, Pak choi (Brasica rapa), arugula (Eruca sativa), kale (Brassica oleracea) | [88,89] | ||
| Rockwool | Microgreens, soybean | [90,91,92] | ||
| Coarse sand | Ethiopian kale (Brassica carinata) | [93] | ||
| Pea gravel | Spinach (Spinacia oleracea L.) | [94] | ||
| Water Medium Culture | Circulating methods (closed systems) | Nutrient film technique (NFT) | Red and green lettuce (Lactuca sativa), microgreens | [95,96,97] |
| Deep flow technique (DFT) | Coriander (Coriandrum sativum), wheat microgreen (Triticum aestivum) | [98] | ||
| Non-circulating methods (open systems) | Root-dipping technique | Lettuce (Lactuca sativa), microgreens | [99] | |
| Floating technique | Pak choi (Brasica rapa) | [100] | ||
| Capillary action technique | Pak choi (Brasica rapa) | [101] | ||
| Ebb and flow system | Basil (Ocimum basilicum), kale (Brassica oleracea), cherry tomato (Solanum lycopersicum), pepper (Capsicum annuum) | [102] | ||
| Crops | pH | EC (mS/cm) |
|---|---|---|
| Pak choi (Brassica rapa L.) | 7.0 | 1.5 to 2.0 |
| Asparagus (Asparagus officinalis L.) | 6.0–7.0 | 6.0–6.8 |
| Basil (Ocimum basilicum L.) | 5.5–6.0 | 1.0–1.6 |
| Broccoli (Brassica oleracea L. var. italica) | 6.0 to 6.8 | 2.8 to 3.5 |
| Cucumber (Cucumis sativus L.) | 5.0 to 5.5 | 1.7 to 2.0 |
| Eggplant (Solanum melongena L.) | 6.0 | 2.5 to 3.5 |
| Cabbage (Brassica oleracea L.) | 6.5 to 7.0 | 2.5 to 3.0 |
| Lettuce (Lactuca sativa L.) | 6.0 to 7.0 | 1.2 to 1.8 |
| Tomato (Solanum lycopersicum L.) | 6.0 to 6.5 | 2.0 to 4.0 |
| Strawberry (Fragaria ananassa L.) | 6.0 | 1.8 to 2.2 |
| Zucchini (Cucurbita pepo L.) | 6.0 | 1.8 to 2.4 |
| Plant Species | Fish Species | References |
|---|---|---|
| Spinach (Spinacia oleracea) | Shark catfish (Pangasianodon hypophthalmus) | [124] |
| Basil (Ocimum basilicum) | African Catfish (Clarias gariepinus) | [125] |
| Lettuce (Lactuca sativa) | Tilapia (Oreochromis niloticus) | [126] |
| Lemon grass (Cymbopogon citratus) | Rohu (Labeo rohita) | [127] |
| Swiss chard (Beta vulgaris) | Tilapia (Oreochromis niloticus) | [128] |
| Lettuce (Lactuca sativa), Pak choi (Brassica campestris), Chinese cabbage (Brassica rapa), kale (Brassica oleracea), collards, Swiss chard (Beta vulgaris) | Tilapia (Oreochromis niloticus) | [129] |
| Tomato (Solanum lycopersicum) | Pearlspot (Etroplus suratensis) | [130] |
| Fruits and Vegetables | Impact of Integrated Nutrient Management | References |
|---|---|---|
| Tomato (Solanum lycopersicum L.) | 50% recommended dose of fertilizer (RDF) in combination with 5 t/ha ARV (Agro Residue Vermicompost) resulted in an increased plant height, root length, dry weight, chlorophyll content, leaf area index, number of flowers per plant, and fruits per plant, which ultimately increased crop yield. | [134] |
| Pepper (Capsicum annuum L.) | Higher maximum plant height, increased leaf area index, improved chlorophyll content, and an improvement in number of branches per plant were observed after the treatment with 75% fertilizers and poultry manure at the rate of 5 t/ha, in addition to biofertilizers and 2% magnesium sulfate (MgSO4). | [135] |
| Eggplant (Solanum melongena L.) | 100% NPK in combination with 25% N through Vermicompost yielded an enhanced number of fruits per plant. The length as well as diameter, weight, and yield of fruit per hectare improved. | [136] |
| Potato (Solanum tuberosum) | Integrated use of Tata Geo Green at 3.75 t/ha soil treatment along with 75% NPK fertilizer (150:60:100) are optimal to produce greater plant growth, net returns, and B:C ratios. | [137] |
| Bottle gourd (Lagenaria siceraria) | Inhibition of red pumpkin beetle and powdery mildew with an increment in B:C and total soluble solids (TSSs) by using 50% NPK, 25% vermicompost, and 25% compost. | [138] |
| Cucumber (Cucumis sativus L.) | Increased yield by using RDF + vermicompost at the rate of 5 t/ha in addition to Azotobacter at the rate of 5 Kg/ha and adding phosphate-solubilizing bacteria (PSBs) at the rate of 5 kg/ha. | [139] |
| Bitter gourd (Momordica charantia) | Increased total soluble solids, protein content, ascorbic acid, shelf life, and total fruit yield were achieved using 100% RDF of NPK in addition to FYM 5 t/ha and biofertilizers at 4 kg/ha (Azotobacter and PSBs). | [140] |
| Ridge gourd (Luffa acutangular) | Use of 25% recommended dose of nutrients (RDN) in combination with 50% RDF from Azotobacter + Bio-compost (2.5 L/ha + PSB 2.5 L/ha) was found to be optimal for ridge gourd growth and yield metrics. | [141] |
| Cauliflower (Brassica oleracea var. botrytis) | 100% RDF in combination with Azospirillium (5 L/ha), PSBs (5 L/ha), and potash-mobilizing bacteria (KMBs) (5 L/ha) enhanced morphological and quality attributes. | [142] |
| Broccoli (Brassica oleracea L. var. italica) | Gibberellic acid (GA3) application at 50 ppm in combination with Azotobacter at 5 kg/ha enhanced the maximum head yield per plant, head yield per plot, and total head yield. | [143] |
| Cabbage (Brassica oleracea L. var. capitata) | Application of farmyard manure (FYM) 50% with Azotobacter 50% to the soil improved plant spread, leaves per plant, stalk length, leaf area, and leaf length and width, along with minimizing the days to maturity. | [144] |
| Chinese cabbage (Brassica rapa) | Mineral potassium 100% with the addition of potassium biofertilizer yielded the maximum head diameter, height, and yield. | [145] |
| Onion (Allium cepa) | Combination of FYM at 20 tons, vermicompost at 5 tons, poultry manure at 2 tons, and 100% recommended NPK enhanced the bulb weight, neck thickness, plant height, and number of leaves. | [146] |
| Garlic (Allium sativum) | 100% NPK in combination with 50 kg sulfur (S)/ha and 5% Jeevamrit (Jv) at the rate of 1 L/m2 yielded an increased plant height, number of leaves per plant, and bulb weight and diameter, ultimately positively affecting the overall bulb yield. | [147] |
| Carrot (Daucus carota) | Carrot growth and yield increased after the combined treatment of organic manures and inorganic fertilizers (5 t/ha cow dung (CD) + 5 t/ha poultry manure (PM)). | [148] |
| Radish (Raphanus sativus) | 90% recommended fertilizer dose in combination with 10% Spent Mushroom Compost (SMC), apart from FYM, Azotobacter, and PSB, resulted in a higher leaf number and size, root size, weight, and yield. | [149] |
| Tomato Parameter | Modes of INM | Impact of Integrated Nutrient Management | References |
|---|---|---|---|
| Morphological parameters | 50% RDN in combination with 25% N through VC and 25% N through FYM treatment | All growth parameters for tomato improved | [154] |
| NPK (120:60:80 kg/ha) application in combination with FYM 10 t/ha, S at 25 kg/ha, Azotobacter, and mixed micronutrients | Increased tomato plant height and leaf length | [155] | |
| Combination of 75% N through urea, muriate of potash (MOP), single superphosphate (SSP), 25% through vermicompost, B, Zn, Azotobacter + PSB | Plant spread and height improved | [156] | |
| 50% RDF in combination with 50% N from FYM and Bio NPK | Improved crop growth rate, relative crop growth rate, and increased number of primary branches and plant height | [157] | |
| Yield and yield attributes | 75% N through urea, MOP, SSP, 25% through vermicompost, B, Zn, Azotobacter + PSB | Enhanced yield and maximum B:C ratio | [156] |
| 50% RDF + 50% N from vermicompost + Bio NPK | Maximum number of fruits per plant, fruit yield per plot, and maximum fruit yield per hectare and better B:C ratio | [157] | |
| 75% RDF + 25% organic (FYM + VC + PM) | Maximum fruit yield | [158] | |
| Integrated crop nitrogen management compared to traditional management | Improved tomato yield by 32.1% | [159] | |
| Nutrient contents and nutrient uptake | 50% RDN in combination with 25% N through VC and 25% N through FYM | Increased uptake of N, P, and K | [154] |
| 50% RDN, 25% N through VC and FYM | 39.7% increase in the N uptake | [159] | |
| Physio-chemical properties | 75% RDF and 50% vermicompost | Maximum TSS, titratable acidity (TA), pH, and ascorbic acid content | [160] |
| Combined treatment of NPK and FYM | Elevated ascorbic acid contents | [161] | |
| Chicken manure and inorganic N fertilizer | Increases in soluble protein and TA by 124% and 118% | [162] |
| Rice Parameters | Modes of INM | Impact of Integrated Nutrient Management | References |
|---|---|---|---|
| Growth parameters | 100% RDF + S40Zn5B1.5 kg ha−1 | Accumulation of dry matter and plant height | [166] |
| 75% NPK + 25% FYM | Plant maximum height | [167] | |
| 75% RDN + 25% N | Maximum plant height recorded at 90 days after treatment (DAT) | [168] | |
| 125% RDF + 25% vermicompost | Dry matter accumulation | [169] | |
| 75% RDN + 25% poultry manure | Dry matter accumulation | [170] | |
| Yield and yield attributes | Integrated effect of fertilizer and FYM | Increase in grain yield | [171] |
| Application of poultry manure as soil and panchakavya as foliar application | Increase in grain yield | [172] | |
| Application of 100% RDF in combination with 5 t ha−1 FYM | Highest number of panicles, increased panicle length and test weight, and higher grain and straw yields | [173] | |
| 50% recommended NPK + 50% N as FYM in addition to 5 kg zinc ha−1 | All yield attributes influenced through INM such as number of effective tillers, length of panicle, grains per panicle, filled and unfilled grains per panicle, and test weight | [174] | |
| 2.5 t poultry manure ha−1 along with 75 kg N + 16.5 kg P and 31.3 kg K ha−1 | Higher crop growth and improved grain yield | [175] | |
| Nutrient contents and nutrient uptakes | 75% RDN and 25% N through vermicompost | Increased N contents of both grain and straw | [168] |
| 100% RDF through inorganic fertilizer + 25% RDN through Neem Cake | Increased uptake of nutrient contents (%) of grain and straw | [176] | |
| Synchronized treatment of organic manure and chemical fertilizer | Significant uptake of N, P, and K | [175] | |
| Physio-chemical properties | Combination of organic manure and fertilizer | Improved various physio-chemical properties, improved uptake, and raised nutrient absorption | [176] |
| Increased compost concentration along with fertilizer | Reduced pH and sodium absorption ratio | [177] | |
| Addition of inorganic fertilizers with organic manures | Improved mineralization | [175] |
| Wheat Parameters | Modes of INM | Impact of Integrated Nutrient Management | References |
|---|---|---|---|
| Growth parameters | - | Increased plant height and accumulation of dry matter | [181] |
| Combined application of 4 t/ha vermicompost and Azotobacter chroococcum inoculation at the rate of 5 mL/kg seed and 100% RDN | Accumulation of dry matter and increased plant height | [182] | |
| 100% RDF and 25% N through vermicompost + ZnSO4 at 25 kg/ha | Improved plant height (92.25 cm) and dry matter accumulation (274.65 g m−2) were achieved | [183] | |
| Application of 100% NPK + 5 t/ha FYM + 5 t/ha vermicompost | Higher leaf area, dry matter, and plant height | [184] | |
| RDF 100% in combination with Azotobacter + PSB | Significantly improved wheat plant height | [185] | |
| Yield and yield attributes | Application of 150% RDF together with 10 tons of FYM + 25 kg ZnSO4/ha | Maximum grain yield of 3.8–3.9 t/ha was achieved | [186] |
| - | Increased length of spike, number of grains per spike, grain weight per spike, and 1000-seed weight | [181] | |
| Application of inorganic fertilizer in combination with higher/lower dose of FYM, biofertilizer, and sulfur | Improved spike length and number of grains per spike | [187] | |
| 75% RDF + vermicompost at the rate of 1 t/ha·1 + PSB | Higher yield attributes and ultimately yield of wheat, which led to higher uptake of NPK by the crop | [188] | |
| 100% RDN + 25% N through vermicompost | Higher number of effective tillers (94%), longer spike length (34%), higher grain yield (165%), and greater straw yield (157%) of wheat over control | [189] | |
| Nutrient contents and nutrient uptakes | RDF 100% + vermicompost (2 t/ha) in addition to PSB | Significant nutrient uptake was registered | [190] |
| 75% RDF, in addition to vermicompost at 1 t/ha and PSB | Enhanced NPK availability in soil for the wheat crop compared to control | [188] | |
| 75% RDF and 25% N through FYM | Efficient nutrient supply system for wheat variety Malviya 234 was achieved | [191] | |
| Physio-chemical properties | Combine application of FYM and 75% RDN | Sustained soil quality and ultimately wheat productivity can be achieved | [192] |
| - | Increased protein content | [181] | |
| Applied potassium at the rate of 100 kg K2O/ha | Under Mediterranean rain-fed conditions (Algeria), durum wheats’ physiological indices improved | [193] |
| Crops | Impacts of Integrated Nutrient Management | References |
|---|---|---|
| Cotton (Gossypium arboreum) | Soil fertility status improved using cotton stalks, less dependency of FYM, and reduced costs of inorganic nutrients by 20–25 USD/h | [197] |
| Pearl millet (Pennisetum glaucum) | Economically viable and environmentally friendly recommended dose of inorganic fertilizer (25%) with the combination of Azospirillum biofertilizers, PSB, and 2% foliar application of urea is suitable for increasing pearl millet yield | [198] |
| Chickpea (Cicer arietinum L.) | 75% RDF with vermicompost and Rhizobium resulted in an increased growth and yield of the crop | [199] |
| Fenugreek (Trigonella foenum-graecum) | Growing finger millet with the residual soil fertility of the previous leguminous crop can result in adequate development and output of this less nutrient-demanding crop | [200] |
| Finger millet (Eleusine coracana) | Increased nutritional quality of the grains, nutrient uptake, and nitrogen use efficiency indices were achieved through INM | [201] |
| French bean (Phaseolus vulgaris) | 75% RDF in addition to 1 t vermicompost was responsible for an increased ascorbic acid content and dry matter content of green pods | [202] |
| Sunflower (Helianthus annuus) | In addition to increasing the head diameter, the biological yields of the 25 cultivars of Shams, Ghasem, and Haysan sunflowers were enhanced by 39.2%, 31.5%, and 34.5%, respectively, when treated with humic acid plus chemical fertilizer | [203] |
| Sorghum (Sorghum bicolor) | If manure and mineral fertilizer are combined, sorghum yields can be increase by 500–5000 kg/ha depending on the type of soil and amount of rainfall in the area | [204] |
| Sweet potato (Ipomoea batatas) | Incorporation of organic manures plus chemical fertilizers enriched the crop yield and enhanced the water use efficiency and economic return to farmers | [205] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Niu, G.; Masabni, J.; Ferrante, A.; Cocetta, G. Integrated Nutrient Management of Fruits, Vegetables, and Crops through the Use of Biostimulants, Soilless Cultivation, and Traditional and Modern Approaches—A Mini Review. Agriculture 2024, 14, 1330. https://doi.org/10.3390/agriculture14081330
Ali A, Niu G, Masabni J, Ferrante A, Cocetta G. Integrated Nutrient Management of Fruits, Vegetables, and Crops through the Use of Biostimulants, Soilless Cultivation, and Traditional and Modern Approaches—A Mini Review. Agriculture. 2024; 14(8):1330. https://doi.org/10.3390/agriculture14081330
Chicago/Turabian StyleAli, Awais, Genhua Niu, Joseph Masabni, Antonio Ferrante, and Giacomo Cocetta. 2024. "Integrated Nutrient Management of Fruits, Vegetables, and Crops through the Use of Biostimulants, Soilless Cultivation, and Traditional and Modern Approaches—A Mini Review" Agriculture 14, no. 8: 1330. https://doi.org/10.3390/agriculture14081330
APA StyleAli, A., Niu, G., Masabni, J., Ferrante, A., & Cocetta, G. (2024). Integrated Nutrient Management of Fruits, Vegetables, and Crops through the Use of Biostimulants, Soilless Cultivation, and Traditional and Modern Approaches—A Mini Review. Agriculture, 14(8), 1330. https://doi.org/10.3390/agriculture14081330









