Abstract
One of the goals of modern orcharding is to produce a high volume of fruits with uniform size, organoleptic parameters, and health characteristics. The aim of this work was to study various shoot types and their prevailing positions along the cane and to identify how shoot type can influence the quality of fruit from the Actinidia tree. The experiment was conducted over a two-year period in a commercial orchard of Actinidia chinensis, cv. Gold 3. The shoots along the cane were classified as follows: spur shoots (SPs), terminated shoots (TEs), non-terminated shoots (NTs), and cut non-terminated shoots (CNTs). The data were statistically processed using ANOVA and Principal Component Analysis (ACP). Four different categories of fruit were obtained from the four shoot types, and their various attributes were compared. The prevailing category (comprising 55% of the studied fruits) was TEs, which are characterised by a higher soluble solid content, sweetness, and excellent health characteristics, as well as the reduced hardness of their pulp, which would support the hypothesis that harvesting could be brought forward. The second most common category (comprising 19% of total fruit) with the lowest soluble solid content, but a high antioxidant capacity, was that which was detached from the CNTs, while 13% of the fruit was produced from NTs, which had the lowest health value but good sweetness perception. Finally, the category with the lowest fruit percentage over the total fruit harvested (10%) was SPs, which are characterised by their smaller size. It has yet to be determined what the performance of each category will be post-harvest; whether it is possible to assign the quality categories while harvesting the fruit or to differentiate the harvest time accordingly remains subject to debate.
1. Introduction
One of the goals of modern orcharding is to ensure that orchards produce a high volume of good-quality fruit that is more or less uniform in size and possesses valuable organoleptic and health characteristics [1]. To achieve this, the plants must be self-rooted or propagated on certified clonal rootstocks [2,3], and both soil characteristics and soil management must be consistent, along with the irrigation, fertilisation, and canopy management conditions [4,5,6]. However, although carefully controlling the growing conditions can reduce variability among plants, other factors, such as the type of branch or shoot bearing the fruit, may still lead to discrepancies.
In kiwifruit, the shoots of the current season at the axil of the leaves originate from the axillary meristems [7]. A certain number of phytomers originate in these meristems between the summer of the year of their formation and prior to the onset of winter dormancy. In the spring of the following year, the active bud begins to swell and develops into a shoot that contains a small number of leaves [8]. In many shoots, growth arrest occurs shortly afterwards, followed by the abortion of the bud tip. Other shoots continue to grow until later in the season, with the final number of phytomers exceeding the number of preformed phytomers present during sprouting [7,9]. This indicates that the initiation of new phytomers must occur in some shoots during the current growing season or ‘neoformation’ [10]. Therefore, all kiwi buds have the same potential to develop into long, non-terminated shoots [11].
Furthermore, the vine must support the rapid formation of a new canopy together with the development of flowers [8], relying on previously stored reserves [12] until the shoot becomes autotrophic 40 days after the bud breaks [13]. Some authors have reported that shoot growth is dependent on the genotype, environmental conditions (temperature) [14], and shoot competition. Indeed, studies have shown that the removal of neighbouring shoots results in a higher proportion of long shoots [15,16,17]. Therefore, the competition between shoots and reserves defines the shoot growth rate once the bud begins to break. The early shoots are more vigorous and competitive than those that follow. Insufficient satisfaction with the cold requirement accentuates the scalarity of sprouting; furthermore, in these conditions, the competition between early shoots and later ones is much more pronounced. Furthermore, in Actinidia, tip abortion of shoots can occur at any time during the extension of the preformed phytomers, giving rise to short or medium shoots. In contrast, shoot growth becomes indeterminate, giving rise to long shoots when tip abortion does not occur.
Together, these various types of shoots make up the Actinidia canopy, with different leaf numbers and leaf areas [17] that can be used to inform the classification of the shoot into one of three types: short sprouts (these have up to nine nodes and are terminated), medium shoots (which have between 10 and 18 nodes and are terminated), and long shoots (which have from 18 to as many as 90 nodes and are unterminated). Another, fourth category covers long shoots that are affected by summer pruning. A previous study on Actinidia deliciosa cv. Hayward showed that the assimilate can be easily translocated within the plant to support fruit growth on shoots with an inadequate leaf-to-fruit ratio [18,19,20], reducing the variability between fruit on vines. However, it is reported that reducing the number of sources increased the variability in fresh and dry weight [21].
The aim of our work was to study the various shoot types and their prevailing position along the cane and to determine how shoot type can affect the fruit quality of Actinidia chinensis plants, cultivar Gold 3, cultivated in one of the most important Actinidia growing areas in Italy.
2. Materials and Methods
2.1. Orchard
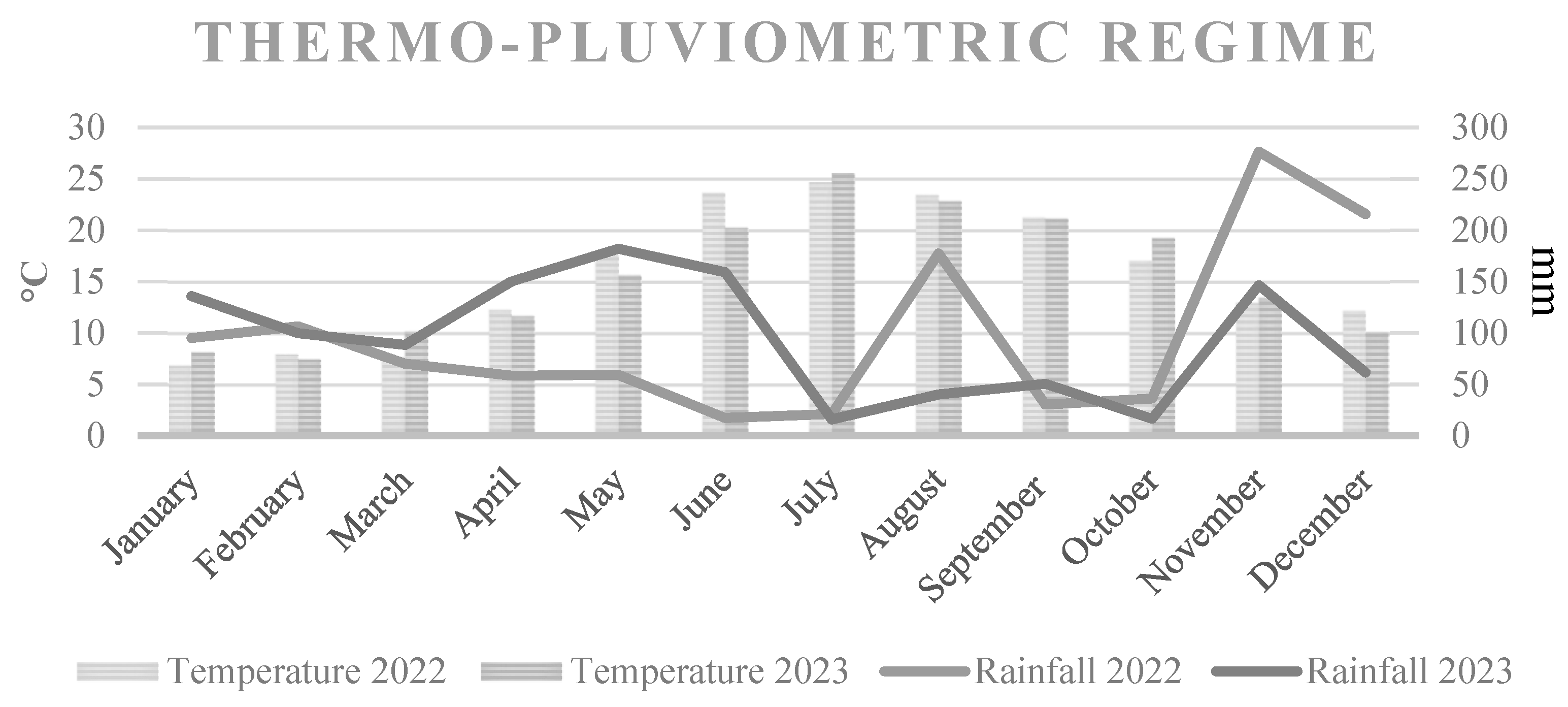
The experiment was performed over a two-year period from 2022 to 2023 in a commercial orchard (Actinidia chinensis, cv. Gold 3) located in Polistena (RC) (38°25′14.9″ N–16°01′56.9″ E), Calabria, Italy, EU. The studied vines from A. deliciosa, Hayward cv., were planted in sandy soil with 7.0 pH, 2.2% organic matter and 1.7 g kg–1 N content in 2015. The vines were spaced 5.0 m × 4.0 m apart (500 vines ha–1), and interspecific grafting was implemented in 2016 using a scion of A. chinensis Planch, cv. Gold 3, grafted onto Actinidia deliciosa (A. Chev.), cv. Hayward. The plant was trained using a pergola system. The male/female plant ratio was 1:7, and the pollinator used was cv. Bélen. The orchard was irrigated with two drippers per tree, delivering 4 litres.h−1. Each cane was pruned to 1.4 m, and twelve canes were left per plant. The yearly pruning took place in the dormant period (December) and again during the summer (July). The orchard was managed using standard integrated pest control systems and stable drip irrigation and fertilisation systems. The average maximum temperature was reached in July (Scheme 1). The precipitation was mainly concentrated in the autumn–winter period (Scheme 1).
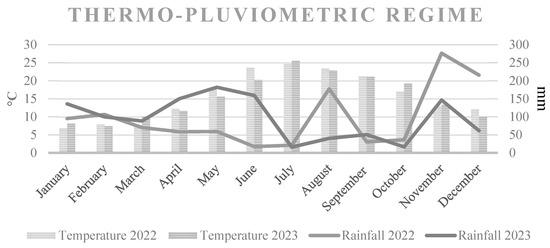
Scheme 1.
Thermo-pluviometric regime of the 2022–2023 biennium of the area of interest.
2.2. Experimental Design
We selected three vines with similar vegetative development and fruit load. The experimental design involved randomised blocks, with each vine acting as a single block. We selected three parent shoots per plant, with similar proximal diameters; along the cane, we detected the unbroken buds and the shoots for each node. The latter were classified as spur shoots (SPs), which were under 15 cm in length; terminated shoots (TEs), with lengths ranging from 15 to 60 cm; no terminated shoots (NTs), which were over 60 cm long, and cut non-terminated shoots (CNTs). The fruits were harvested and discriminated according to the shoot type, and the leading morphobiometric and qualitative indices were identified. Hydraulic resistance measurements were performed on three parent shoots of the cv. GOLD 3, distinguished by vigour.
2.3. Maturation Indices and Nutraceutical Parameters
At harvest time [164 Days After Flower Bloom (DAFB) in 2022 and 162 DAFB in 2023], the fruits were harvested and discriminated according to the shoot types.
2.4. Fruit Biometric Measures
The obtained measurements were immediately used to acquire maximum (Dmax) and minimum (Dmin) transversal diameters and longitudinal diameter (H) using a precision calibre. Fresh weight (FW) was determined using an electronic balance (Mettler-Toledo MgbH, Grelfensee, Switzerland).
2.5. Flesh Firmness
The flesh firmness (FF) was measured using a digital penetrometer, PCE-FM200 (PCE Instruments, Southampton, UK), with an 8 mm probe. The peel was removed prior to measurement, and these last measurements were taken on two opposite sides of the equatorial zone of the fruit (depth of penetration: 2.5 mm). The two measures were averaged to obtain the final result.
2.6. Soluble Solid Content
We measured the total soluble solid content (TSS) of juice drops obtained by squeezing each fruit’s apex and base using a digital refractometer (PAL-1, Atago, Tokio, Japan). The value is expressed as °brix.
2.7. Titratable Acidity
Titratable acidity (TA) was determined by titrating 10 mL of the juice diluted with distilled water (1:1) and titrated to pH 8.2 with 0.1 N NaOH (mEq. NaOH/100 g fresh fruit). The results were expressed as citric acid equivalents per 100 mL of sample juice.
2.8. Dry Matter Content
The dry matter content (DMC) was determined using a standardised sampling method. A horizontal slice of the equatorial zone fruit tissue was extracted from each fruit. The thickness of the slice was approximately 1 cm, and the fresh weight was recorded. The slice was placed in a dehydrator (Binder EED240, Tuttlingen, Germany) at 105 °C until the constant dry weight was reached. The dry matter content was expressed as a percentage of fresh weight using the following formula:
2.9. Total Polyphenol Content (TPC) and Total Antioxidant Capacity (TAC)
Five fruits per plant were collected and subjected to analysis to identify the mesocarp TPC and TAC. Fruit samples were homogenised using an Ultraturrax blender (20,000 rpm; T25 Basic, IKA-Werke GmbH & Co. KG™, Staufen, Germany, UE). TPC and TAC were analysed separately using a Kontron Uvikon 941 Plus spectrophotometer. Before TPC and TAC were measured, standard curves were prepared for each test. TPC (mg gallic acid equivalents/g fresh weight) was determined using the Folin–Ciocalteau method [22]. TAC was determined using the modified TEAC assay and expressed as mmoles Trolox equivalents/100 g fresh weight [23,24]. The TEAC assay included the hydrophilic and lipophilic contributions of the kiwifruit samples [25].
2.10. Flesh Colour
The colour of the flesh was evaluated using a Minolta spectrophotometer, CM-700d (Minolta, Inc., Tokyo, Japan), which provided measurements of L*, a* and b* at three points for each fruit. L* measures the lightness, varying from 100 for perfect white to 0 for black; a* measures the redness when its value is positive, grey when zero, and green when negative; b* measures yellowness when positive, grey when zero, and blue when negative. The colorimeter was calibrated with standard black-and-white calibration tiles.
2.11. Leaf Drop
Leaves were counted on 20 marked shoots from each vine, from 160 DAFB to 190 DAFB. For both years, leaf drop began no earlier than November, 175 DAFB.
2.12. Vegetative Measurements
During each growing season, on three parent shoots per vine, the length, the number of leaves, and the number of fruits on each shoot type were acquired from the proximal to the distal zone of the cane. The cane diameter was also measured at the base of the shoot. The evolution of the phenological stages along the cane was monitored on a weekly basis, from the bud-breaking stage to harvest time, according to the BBCH scale [26]. Only the data pertaining to the bud-breaking stage and full flower bloom are included in this study. At the end of the season, the leaves of each shoot type were detached and the leaf area was measured with an Li-3100 area meter (Li-COR, Lincoln, NE, USA). The number of leaves per fruit and average leaf area per fruit were also recorded.
2.13. Gas Exchange
Net photosynthesis (Pn) and transpiration (Tr) were measured on nine leaves × shoot (9 leaves × 3 shoots × 3 plants). The measurements were obtained using a portable photosynthesis system (Li-Cor 6400XT; LI-COR Biosciences, Lincoln, NE, USA). The gas exchange measurements were performed on clear sunny summer days (from 11:00 to 13:00) during the last week of each of the summer months (June, July, and August) in both years.
2.14. Shoot Hydraulic Conductance
A Hydraulic Conductance Flow Meter XP Gen3 (Dynamax Inc., Houston, TX, USA) was used to obtain the next set of measurements. The shoots were removed from the cordon with a clean cut and connected to the HCFM; the hydraulic conductance (HC) of the shoot was measured in quasi-steady mode at a pressure of 0.3 MPa. The sample was perfused with high-pressure water until the leaves were visibly waterlogged. Once this state of hydration was achieved, the HC was monitored until the hydration level stabilised, and a quasi-steady state (QSS) reading was taken [27]. These measurements were only performed in the second year.
2.15. Statistical Analysis
The data were statistically processed using SPSS v. 22.0 software (IBM Corp., Armonk, NY, USA) via two-way Analysis of Variance. Tukey’s test was used to discriminate averages that showed a significant effect of ANOVA. The dataset was subjected to data mining techniques such as Principal Component Analysis (ACP), using XLSTAT (Addinsoft, New York, NY, USA). The average value over the two-year study period is indicated by av.
3. Results
The bud-breaking stage began in the latter third of March in both years. The budding percentage was 82.5% in the first year and 84% in the second year, without significant differences (Table 1). The full flower bloom occurred during the second half of May for both years (12 May in 2022; 14 May in 2023). The fruit fresh weight (FW) was highest on the CNTs. The FW difference was statistically significant between the CNTs and SPs, while the FW was similar among the NTs, TEs, and other shoot types (Table 2).

Table 1.
Bud sprouting (%) over a two-year period (20022–2023) in A. chinensis trees, cv Gold 3.

Table 2.
Fruit morphometric indices of Actinidia chinensis Planch, cv. Gold 3, discriminated according to shoot type [fresh weight (FW), maximum (DMax) and minimum (DMin) transversal diameter ratio, height and transversal diameter average (H/D) ratio] measured during 2022–2023, on fruits of Actinidia chinensis Planch, cv. G3, discriminated according to shoot type [terminated shoots (TEs); non-terminated shoots (NTs), spur shoots (SPs), cut non-terminated shoots (CNTs)].
The shoot type has no influence on the ratio between the two transversal diameters of the fruit and the H/D ratio (Table 2). The differences from two observation years were recorded, but the interaction between year × shoot types was not significant (Table 2). The average value of total soluble solids over the two-year study period was significantly (approximately 27%) lower in the fruit from the SPs than in fruit from the TEs (14.28 °Brix, average value over two years). Meanwhile, the TSS average value found in the fruit of the NTs was intermediate and statistically different compared to TEs and SPs. The TSS in fruit on the CNTs was similar to the latter in the second year of the study. The titratable acidity value (such as the average between two years) was higher in the fruit of the SP sprouts and lowest in the TE sprouts, with statistically different differences; for the other types, the TA value was intermediate compared to the findings in the fruit from the preceding sprouts (SPs and TEs), which exhibited significant differences (Table 3).

Table 3.
Principal maturation indices measured on the fruit of Actinidia chinensis Planch, cv. Gold 3, discriminated according to shoot type: flesh firmness (FF), total soluble solids (TSSs), titratable acid (TA), total soluble solids and titratable acid ratio (TSS/TA), dry matter content (DMC), and dry weight fruit (DWF).
The TSS/TA ratio expressing fruit sweetness was significantly higher in the fruit from the TEs, followed by that from the NTs, CNTs, and finally the SP, and a significant difference was observed for each of the studied years. The values of the CNTs and NT fruits exhibited significant similarities, but displayed significant differences compared to the alternative shoots (Table 3). The highest DWF was recorded for the CNTs. In contrast, a statistically lower value was recorded for the SP, with a drop of around 1.40 g in the average value over the two-year study period (Table 3). The NTs and TEs showed intermediate and significantly different values compared to CNTs and SPs, without statistical differences (Table 3). The DMC (percentage of fresh weight) was significantly lower in the fruit detached from the CNTs than that harvested from NTs and SPs. The average value detected in fruit harvested from TEs did not differ from that of other types of shoots (Table 3). The effect of the year was recorded for all the above parameters; however, no significant interaction between the year and the type of shoot was observed. The firmness of the fruit pulp harvested from the TEs was significantly lower than that of the other shoot types; in the latter, the value was statistically similar, ranging from 8.00 to 8.9 kg.cm2 (Table 3). During the early stages of development, the pulp of kiwifruit had a green colour. The fruit of cv. Gold 3 should be harvested when the tint, expressed as °Hue, falls below 103 °Hue (US patent 22,355 P3). The fruit of the different shoot types reached an excellent value and was well below the prescribed limit average value on the harvest date. However, the lowest value was measured in fruit on the SP (88.95 °Hue ± 0.32), while the highest value was found in fruit pulp detached from the TEs (Table 4).

Table 4.
Principal colorimetric indices measured on the fruit of Actinidia chinensis Planch, cv. Gold 3, during 2022–2023, categorised by shoot type.
The close correlation between TPC and TAC was confirmed for the bioactive compounds. However, green pruning led to a significant increase in TAC without an equivalent increase in TPC. It is conceivable that this intervention also increased the presence of other bioactive compounds (Table 5).

Table 5.
Nutraceutical parameters in kiwifruit, Gold 3 cv., in function to shoot type: total antioxidant capacity (TAC), total polyphenol content (TPC).
The average length of the shoot over the two-year study period was highest for the NT shoots (404.33 cm ± 164.20). The shortest length was recorded for the SPs (16.273 cm ± 5.14), while intermediate values were detected for the TE (66.16 cm ± 30.36) and CNT (153.529 cm ± 31.72) shoot types (Table 6).

Table 6.
Principal vegetative indices in function to shoot type: shoot length (SL), number of leaves per Shoot (NL), total leaf area (TLA), number of fruits (NF), number of leaves per fruit (NLF), leaf area per fruit (LAF), leaf drop (LD), internode length (IL).
Differences were recorded throughout the two-year study period, and interactions of year × treatments were significant. A similar trend was observed for the number of leaves; this parameter significantly differed among the shoot types. The highest number of leaves was found for the NT shoots (60 leaves) and the lowest value for the SPs (6 leaves), while for the TEs and CNTs, the number of leaves was intermediate: 24 and 14, respectively (Table 6). The distribution of the different types of shoots on the cane showed that 33% of the shoots of the NT-type cut were present in the proximal section of the cane; they were earmarked for use as renewal canes in the following year. The SPs were found in the middle of the cane (comprising around 22% of the total shoots), while TEs (around 35% of the total shoots) and NT-type shoots (about 10% of the total shoots) were found in the more distal portion of the cane. Similar trends were recorded throughout the study period, but no significant differences were observed. The NT type was found when the cane diameter at the shoot base was over 15 mm, with TEs when the cane section was lower, about 11 mm, and SPs when the cane diameter was under 9 mm. The average leaf area of the shoot was highest in the shoots with indeterminate growth (250.00 cm2 ± 6.58), while significantly lower values were observed for the TE (177.15 cm2 ± 7.31 cm2) and CNT (175.00 cm2 ± 4.73) shoots. However, the lowest values were found for the shoots of the SP type (136.36 cm2 ± 8. 31). For this parameter, the pattern also showed similarities between the first and second year, and no significant relationship between the year and the chosen treatment was observed (Table 6). The length of the internodes was highest in the NTs (6.73 cm ± 0.99), and the value was statistically similar to those found in the TEs, while the internode length was 4 cm shorter for the SPs (2.35 cm ± 0.93). No significant differences in internode length among the shoot types were observed over the two years and no interaction between the year and the type of shoot was recorded. There were no significant differences in the leaf drop at the end of the vegetative annual cycle, although the shorter shoot had the lowest value, at about 50% (Table 6). The leaf area per fruit was lower on SPs and TEs, with 300 and 376 cm2.fruit−1 (average over two years), respectively, with 1.69 and 2.76 leaves per fruit−1 (average over two years), whereas it was higher in alternative shoots; in particular, it was over 500 cm2.fruit−1 in CNTs, with 3 leaves.fruit-1, and was significantly higher in NTs, with 2200 cm−2.fruit−1 and 8 leaves.fruit−1. The value was similar for each shoot type over the two-year study period, and no interaction of Y x type of shoot was observed. The gas exchange was also analysed, and greater photosynthetic activity (An), stomatal conductance (gs), and transpiration (E) values were observed in TEs with a lower Ci value. An, gs, and E were significantly lower in the leaves of NTs and CNTs, with a higher Ci compared to leaves of TEs; however, the lowest values of An, gs, and E with the highest significance values of Ci were found in the leaves of SPs. VPD exhibited no significant differences between treatments. The leaf temperature was similar between treatments, with the exception of SPs, for which a higher temperature was recorded (Table 7). The values of all gas exchange parameters and leaf temperatures were different for both years, but no interaction of year × treatment was observed for any of the recorded parameters.

Table 7.
Principal ecophysiological indices discriminated according to shoot type: (An: assimilation net; E: transpiration rate; gs: stomatal conductance; Ci: internal CO2 concentration; Leaf T: leaf temperature; VPD: vapour pressure deficit; HC: hydraulic conductance).
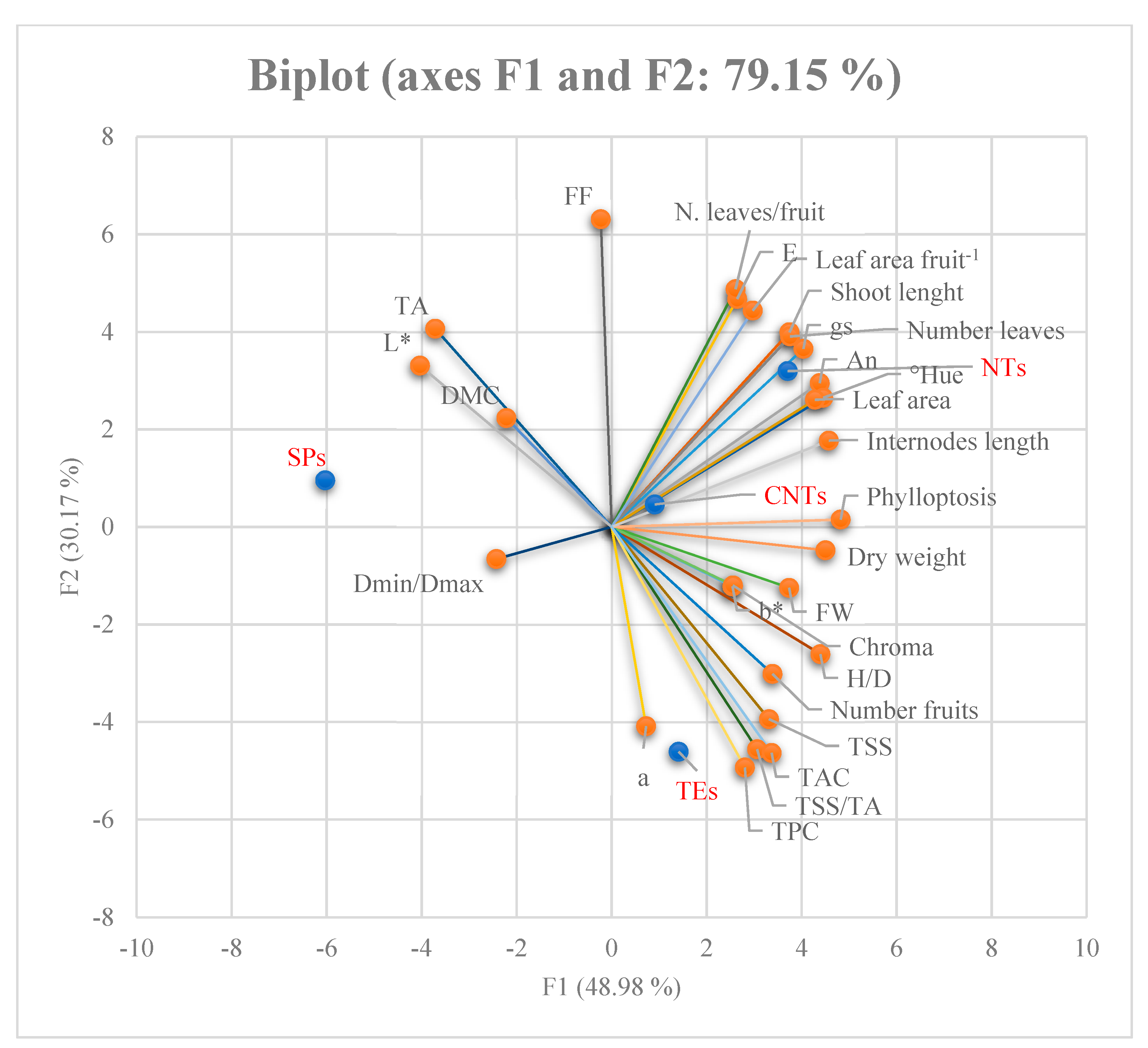
The hydraulic conductance values were similar for the TEs and NTs, but significantly lower for the SPs. No measurement was recorded for CNTs (Table 7). Principal Component Analysis (PCA) allowed us to study the relationships between the original variables to find a new, smaller set that expressed commonalities between the original items; this facilitated the identification of factors that are not directly observable (latent variables or common factors) while maintaining a high explained variability. The variables strongly correlated with and belonging to factor F1 related to characteristics of the fruit, such as its fresh weight, H/D ratio, TSS, DWF, colour parameters L* and Hue, and antioxidant capacity. Leaf photosynthesis (An) and other variables of the shoot such as conductance, length, leaf area, internode length, and leaf drop also exhibited a strong correlation with factor F1. Fruit variables such as pulp firmness, dry weight content (%), polyphenol total content, the number of leaves per fruit, and the leaf area per fruit were strongly correlated and were categorised as factor 2. Finally, the colorimetric variables of the pulp a, b, and chroma, the minimum and maximum diameter ratio of the fruit, and the number of fruits per shoot were strongly correlated and were assigned to F3 (Figure 1).
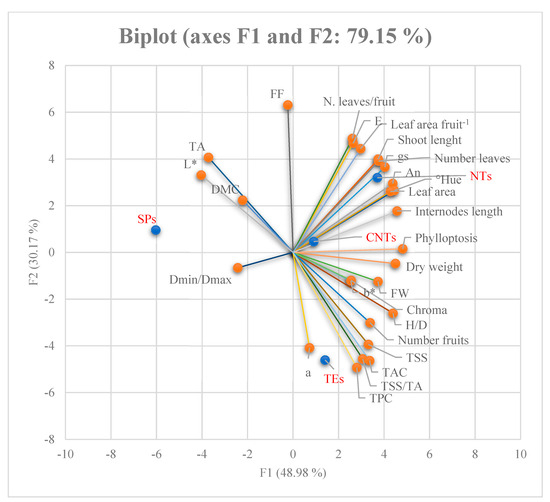
Figure 1.
Biplot with centroid and variable vectors on F1 and F2 planes.
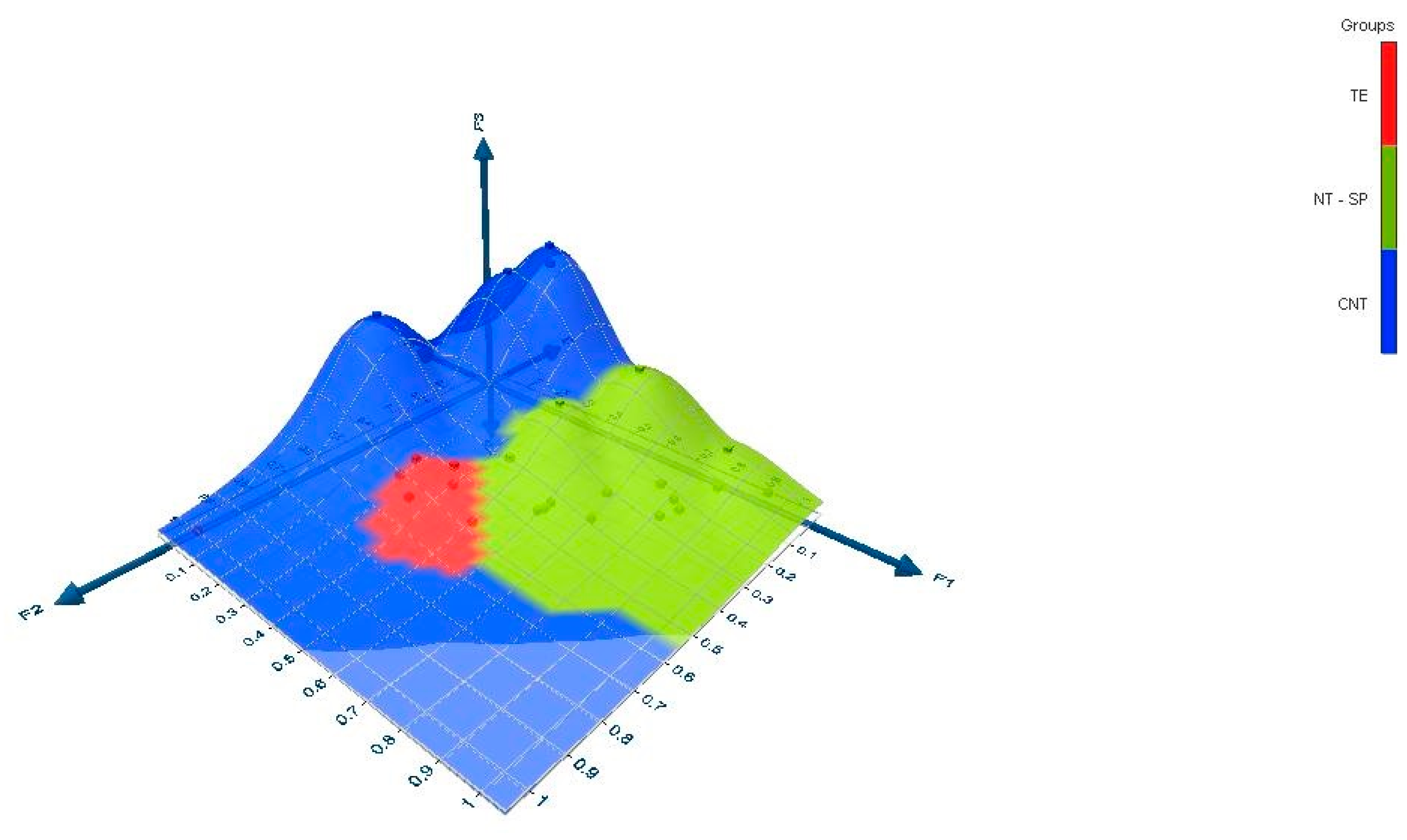
The dataset was used to make a three-dimensional graph with Excel 2021 using the XLSTAT (2021.5) add-on statistical software (Figure 2). The data correspond to the outputs (rows points and columns points) of the correspondence analysis.
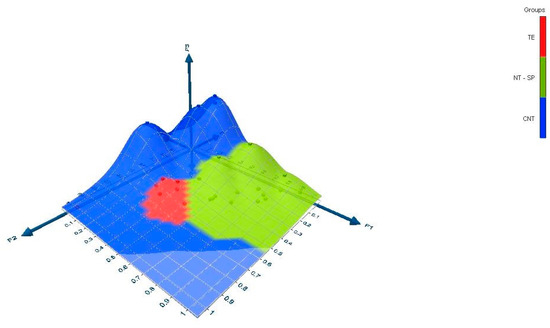
Figure 2.
Three-dimensional representation of latent variables discriminated according to shoot type.
4. Discussion
The bud-break percentage of Actinidia chinensis was similarly high for both of the studied years. This result was achieved using an Actinidia cultivar (Gold 3, cv.) with low chilling unit requirements, which is used in southern Italy, where the winters are mild.
This aspect and the bend of the cane in the pergola system adopted in the orchard reduced the acrotony habitus along the cane and favoured a greater proportion of bud breaking in the basal and central zone. Overall, the sprouting behaviour along the shoot was reduced in the proximal zone of the parent shoot (cane), whereas it increased in the medium and distal portions (Figure 1).
Carbohydrate sinks are described by their sink strength, that is, their ability to attract carbohydrates when there is a steady supply [28,29], and by their priority, which describes their ability to compete when the carbohydrate supply is limited. Root and shoot apices are the principal sinks during vegetative growth, while fruits become the dominant sinks during the reproductive phase. Thus, the distribution of different types of shoots along the parent shoot observed could be the complex result of many factors interacting to design the fate of the tip of shoots, such as carbohydrate supply from vine reserves and competition for reserves [10,12,16,30].
Furthermore, the increased access to reserves [13,15,31] and the photosynthetic contribution in the development by the young leaves during the transition of the shoot from heterotrophy to autotrophy favoured increased shoot development. Therefore, the reduced development of the SPs could be due to the lower reserve content, whereas the greater development of the TEs and NTs could be a result of both the higher reserve and photosynthetic contribution of the new leaves. The relationship between shoot type and cane diameter at the base of the shoot has been identified in previous works by other authors [9,17].
The spur shoot has a lower internode length and diameter than TEs and NTs, in addition to lower conductance (HC), and this can explain the lower fresh weight of the fruit observed in this shoot type. It has been reported that in fruit, water is imported by the xylem in the early stages of development in order to dominate the phloem flows during the later stages [21,32,33,34]. Some authors showed that dry matter predominantly enters the fruit through the phloem, while water enters via the xylem [21]. In that case, the lower DWF of SPs can be attributed to the reduced photosynthetic activity on the spur.
The lower activity recorded for the spur shoot, according to the authors of [11], could also be attributed to the amount of water available or reduced palisade tissue development. Finally, the lower the An, the fewer leaves grew, and the leaf surface of spur shoots reduced the amount of photosynthates produced by the shoots. However, the leaf surface of the fruit was the same for both the SPs and TEs. Therefore, the lower dry weight in the first case is attributable to the lower An of the leaf. The authors of [31] reported that nine weeks after the budburst, long shoots had a carbon surplus three times that of the short shoots; our results showed a greater leaf area per fruit and high An activity. All previous reports suggest the advantages of medium and long shoots in fruit development and explain the increased water content and DWF of the alternative shoots to spur shoots.
However, the DMC did not change compared to the other uncut shoots because the water and dry weight were lower in the fruit on the spur. For the final shoot type, the hydraulic conductance was the lowest (Table 5). In contrast, the fruits of indeterminate shoots with higher conductance had higher water content, while the conductance in TEs was intermediate between NTs and SPs. In the fruit of CNTs, the DWF and water were highest, probably due to minor competition between the fruit, tip, and new leaves in development compared to NT, but the final result led to the lowest DWF. However, the ripening stage on the shoot was longer in fruit on TEs, as shown by TSS, FF, and Hue° angle values. This indicates that the ratio TSS/TA, which corresponds to the sweetness of the fruit, was also influenced by the shoot type. Finally, shoots also influenced the values of bioactive substances such as TPC and TAC (Table 6). Indeed, the highest values were found in NTs and CNTs. Therefore, the green pruning on CNTs increased the TAC but not the TPC. Finally, the PCA showed that the fruits were strongly differentiated according to the type of shoots; in fact, the SPs and NTs were assigned to the F1 and F3 factors, respectively, while the fruits of the TEs and CNTs belong to the F2 factor.
5. Conclusions
In this study, we highlighted the qualitative variability within the same plant affected by the type of bud that supports fruiting. The cane determines the shoot type, which appears to be a function of the diameter of the cane itself at the budding bud and higher reserve content, as demonstrated by other authors. The fruit size was optimal for each bud type compared to the Gold 3 patent (US patent 22,355 P3). However, the PCA revealed that NTs and SPs buds are correlated with variables belonging to different factors. In contrast, TE and NT buds have relationships with variables belonging to the same plan. Four different fruit categories were obtained from the four shoot types, and the following factors were considered: the prevailing category was TE (55% of total fruits), which is characterised by its higher soluble solid content, sweetness, and excellent health characteristics, but also has softer pulp, leading us to conclude that it could be harvested at an earlier date. A second category of fruit (19%) originated from the CNTs, exhibiting the lowest soluble solid content but a high antioxidant capacity detached from CNTs, while 13% of the fruit was obtained from NTs. These fruits had the lowest health value but good sweetness perception. Finally, the category with the lowest fruit percentage (10% of the total harvest) was SPs, which were the smallest fruits obtained. It is necessary to evaluate the performance of each category post-harvest to determine whether it is possible to differentiate the product into quality categories during the harvesting process, or possibly to differentiate the harvest time; for example, one could anticipate production from TEs, which accounts for more than 50% of the fruit harvest.
Author Contributions
Conceptualization, A.D. and G.A.M.G.; methodology, A.D. and G.A.M.G.; software, A.D. and G.A.M.G.; validation, A.D., R.Z. and G.A.M.G.; formal analysis, A.A., A.D. and G.A.M.G.; investigation, A.A., A.D. and G.A.M.G.; resources, A.A., A.D. and G.A.M.G.; data curation, A.A., A.D. and G.A.M.G.; writing—original draft preparation, A.D. and G.A.M.G.; writing—review and editing, A.D. and G.A.M.G.; visualization, A.D. and G.A.M.G.; supervision, A.D., R.Z. and G.A.M.G.; project administration, A.D., R.Z. and G.A.M.G.; funding acquisition, A.D. and G.A.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We would like to thank: Diego Adornato for hosting the rehearsals and for his availability and collaboration; Francesco Zangari for his contribution and Fenis Girardi for his collaboration and advice.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Musacci, S.; Serra, S. Apple fruit quality: Overview on pre-harvest factors. Sci. Hortic. 2018, 234, 409–430. [Google Scholar] [CrossRef]
- Jimenes, I.M.; Mayer, N.A.; Dias, C.T.d.S.; Filho, J.A.S.; Rodrigues da Silva, S.R. Influence of clonal rootstocks on leaf nutrient content, vigor and productivity of young ‘Sunraycer’ nectarine trees. Sci. Hortic. 2018, 235, 279–285. [Google Scholar] [CrossRef]
- Milošević, T.; Milošević, N.; Mladenović, J. Combining fruit quality and main antioxidant attributes in the sour cherry: The role of new clonal rootstock. Sci. Hortic. 2020, 264, 109236. [Google Scholar] [CrossRef]
- Minas, I.S.; Tanou, G.; Molassiotis, A. Environmental and orchard bases of peach fruit quality. Sci. Hortic. 2018, 235, 307–322. [Google Scholar] [CrossRef]
- Gullo, G.; Dattola, A.; Vonella, V.; Zappia, R. Effects of photoselective colour nets on the vegetative, productive, and qualitative behaviour of kiwifruit, jintao cultivar. J. Berry Res. 2021, 11, 1–19. [Google Scholar] [CrossRef]
- Gullo, G.; Branca, V.; Dattola, A.; Zappia, R.; Inglese, P. Effect of summer pruning on some fruit quality traits in Hayward kiwifruit. Fruits 2013, 68, 315–322. [Google Scholar] [CrossRef]
- Snowball, A.M. Seasonal cycle of shoot development in selected Actinidia species. N. Z. J. Crop Hortic. Sci. 1997, 25, 221–231. [Google Scholar] [CrossRef]
- Brundell, D.J. Flower development in the Chinese gooseberry (Actinidia chinensis Planch). I. Development of the flowering shoot. N. Z. J. Bot. 1975, 13, 473–483. [Google Scholar] [CrossRef]
- Snowball, A.M. Axillary shoot bud development in selected Actinidia species. N. Z. J. Crop Hortic. Sci. 1997, 25, 233–242. [Google Scholar] [CrossRef]
- Foster, T.M.; Seleznyova, A.N.; Barnett, A.M. Independent control of organogenesis and shoot tip abortion are key factors to developmental plasticity in Kiwifruit (Actinidia). Ann. Bot. 2007, 100, 471–481. [Google Scholar] [CrossRef]
- Richardson, A.; Eyre, V.; Kashuba, P.; Ellingham, D.; Jenkins, H.; Nardozza, S. Early shoot development affects carbohydrate supply and fruit quality of red-fleshed Actinidia chinensis var. chinensis ‘Zes008’. Agronomy 2020, 11, 66. [Google Scholar] [CrossRef]
- Richardson, A.; Boldingh, H.; Kashuba, P.; Knight, G.; Ellingham, D. Flowering time determines the weight and composition of Actinidia chinensis var. chinensis ‘Zesy002’ kiwifruit. Sci. Hortic. 2019, 246, 741–748. [Google Scholar] [CrossRef]
- Greer, D.H.; Jeffares, D. Temperature-dependence of carbon acquisition and demand in relation to shoot growth of kiwifruit (Actinidia deliciosa) vines grown in controlled environments. Aust. J. Plant Physiol. 1998, 25, 843–850. [Google Scholar] [CrossRef]
- Seleznyova, A.; Halligan, L. Modelling effect of temperature on area expansion at the leaf the shoot and the whole plant level. Acta Hortic. 2006, 707, 167–174. [Google Scholar] [CrossRef]
- Piller, G.J.; Meekings, J.S. The acquisition and utilization of carbon in early spring by kiwifruit shoots. Ann. Bot. 1997, 79, 573–581. [Google Scholar] [CrossRef]
- Clearwater, M.J.; Seleznyova, A.N.; Thorp, T.G.; Blattmann, P.; Barnett, A.M.; Lowe, R.G.; Austin, P.T. Vigor-controlling rootstocks affect early shoot growth and leaf area development of kiwifruit. Tree Physiol. 2006, 26, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Seleznyova, A.N.; Thorp, T.G.; Barnett, A.M.; Costes, E. Quantitative analysis of shoot development and branching patterns in Actinidia. Ann. Bot. 2002, 89, 471–482. [Google Scholar] [CrossRef]
- Famiani, F.; Antognozzi, E.; Boco, M.; Tombesi, A.; Battistelli, A.; Moscatello, S.; Spaccino, L. Effects of altered source-sink relationships on fruit development and quality in Actinidia deliciosa. Acta Hortic. 1997, 444, 355–360. [Google Scholar] [CrossRef]
- Snelgar, W.P.; Thorp, T.G. Leaf area, final fruit weight and productivity in kiwifruit. Sci. Hortic. 1988, 36, 241–249. [Google Scholar] [CrossRef]
- Lai, R.; Wooley, D.J.; Lawes, G.S. Effect of leaf to fruit ratio on fruit growth of kiwifruit (Actinidia deliciosa). Sci. Hortic. 1989, 39, 247255. [Google Scholar] [CrossRef]
- Minchin, P.E.H.; Snelgar, W.P.; Blattmann, P.; Hall, A.J. Competition between fruit and vegetative growth in Hayward kiwifruit. N. Z. J. Crop Hortic. Sci. 2010, 38, 101–112. [Google Scholar] [CrossRef]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Viticult. 1997, 28, 49–55. [Google Scholar] [CrossRef]
- Pellegrini, N.; Re, R.; Yang, M.; Rice-Evans, C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for anti-oxidant activities applying 2,2_-azino-bis-(3-ethylenebenzothiazoline-6-sulphonic acid) radical cation decolourisation assay. Method Enzymol. 1999, 299, 379–389. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; RiceEvans, C. Antioxidant activity applying an improved ABTS radical cation decolourisation assay. Free Radic. Bio. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- Iliescu, L.M.; Stănică, F. Kiwifruit (Actinidia spp.) phenological growth stages in southern Romanian climate according to the BBCH scale. Sci. Pap. Ser. B Hortic. 2020, 64, 1. [Google Scholar]
- Bogeat-Triboulot, M.B.; Martin, R.; Chatelet, D.; Cochard, H. Hydraulic conductance of root and shoot measured with the transient and dynamic modes of the high-pressure flowmeter. Ann. For. Sci. 2010, 59, 389–396. [Google Scholar] [CrossRef]
- Grossman, Y.L.; DeJong, T.M. PEACH: A simulation model of reproductive and vegetative growth in peach trees. Tree Physiol. 1994, 14, 329–345. [Google Scholar] [CrossRef]
- Lacointe, A.; Minchin, P.E.H. Modelling phloem and xylem transport within a complex architecture. Funct. Plant Biol. 2008, 35, 772780. [Google Scholar] [CrossRef]
- Piller, G.J.; Greaves, A.J.; Meekings, J.S. Sensitivity of floral shoot growth, fruit set and early fruit size in Actinidia deliciosa to local carbon supply. Ann. Bot. 1998, 81, 723–728. [Google Scholar] [CrossRef]
- Greer, D.H.; Cirillo, C.; Norling, C.L. Temperature-dependence of carbon acquisition and demand in relation to shoot and fruit growth of fruiting kiwifruit (Actinidia deliciosa) vines grown in controlled environments. Func. Plant Biol. 2003, 30, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.C.; Grange, R.I.; Picken, A.J. An analysis of the accumulation of water and dry matter in tomato fruit. Plant Cell Environ. 1987, 10, 157–162. [Google Scholar] [CrossRef]
- Lang, A. Xylem, phloem and transpiration flows in developing apple fruits. J. Exp. Bot. 1990, 41, 645–651. [Google Scholar] [CrossRef]
- Greenspan, M.D.; Shackel, K.A.; Matthews, M.A. Developmental-changes in the diurnal water-budget of the grape berry exposed to water deficits. Plant Cell Environ. 1994, 17, 811–820. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).