Abstract
In the context of global climate change, atmospheric nitrogen deposition is increasing, and precipitation patterns are becoming more variable. This study examines the impact of these changes on nitrogen (N) allocation mechanisms in semi-arid region tree species using one-year-old Mongolian pine (Pinus sylvestris var. mongolica) seedlings. The seedlings were planted in soil collected from the Daqinggou Sandy Ecological Experiment Station (42°54′ N, 122°25′ E). Three moisture treatments were applied (WC (normal moisture, approximately 65% ± 2.5% of the field capacity), WI (30% increased moisture), and WD (30% decreased moisture)), as well as three nitrogen treatments (NC (no nitrogen), NL (5 g·m−2·y−1 nitrogen), and NH (10 g·m−2·y−1 nitrogen)). The seedlings were sprayed with a 15N-labeled CH4N2O solution (46% N, 15N abundance 10.14%) in a pot trial, with samples taken in August and October to measure N content and 15N abundance in the seedling organs and the soil. Parameters such as Ndff (%) (the percentage of nitrogen derived from fertilizer), nitrogen content of organs, 15N absorption in organs, and 15N distribution ratio were calculated. The results showed that 15N allocation in seedlings followed the trend leaves > stems > roots. Under moisture treatments, 15N allocation ratios in leaves, stems, and roots were 63.63–71.42%, 14.89–24.14%, and 12.23–14.88% under low nitrogen, and 62.63–77.83%, 13.35–22.90%, and 7.31–19.18% under high nitrogen. Significant correlations were found in 15N abundance among the seedling organs, with coefficients ranging from 0.97 to 1.00. The main effects of moisture and nitrogen, as well as their interaction, significantly impacted 15N abundance in the seedling organs. Changes in moisture levels affected the nitrogen absorption capacity of Mongolian pine. Increased moisture significantly enhanced 15N absorption in all organs, leading to 62.63–71.42% of 15N being allocated to the leaves, maintaining an appropriate proportion with the roots and stems. Nitrogen deposition altered the nitrogen allocation strategy among different organs of Mongolian pine. Under conditions of reduced moisture and low nitrogen, a greater proportion of nitrogen was captured by the roots and stems, with an allocation increase of approximately 4.98–5.77% compared to the control group, thereby mitigating the adverse effects of water deficiency. In conditions of reduced moisture and high nitrogen, the leaves, being active organs, accumulated more limiting elements, with an increase in nitrogen allocation of 2.03–8.07% compared to the control group. To achieve an optimal allocation strategy, moderate nitrogen deposition combined with increased moisture enhanced nitrogen uptake in Mongolian pine seedlings. This study provides scientific evidence for ecological restoration, wind erosion control, and agricultural and forestry management in semi-arid regions under the context of global climate change.
1. Introduction
In recent years, economic and social development, along with human activities, have exacerbated global climate change. Among these changes, nitrogen (N) deposition and variable precipitation patterns have garnered widespread attention. Existing studies indicate that the frequency of short-term extreme increases in global summer rainfall events has risen [1,2], often followed by consecutive droughts [3]. These complex precipitation patterns alter the allocation strategies of elements within plants [4], threatening ecosystem functions and the stability of plant communities [5,6]. Anthropogenic nitrogen emissions, such as those from the industrialization of agriculture and livestock, are contributing to increasing atmospheric nitrogen deposition [7]. Current global estimates of total nitrogen deposition range between 125 and 132 TgN yr−1 [8], posing a severe air pollution problem [9]. Research has shown that atmospheric nitrogen deposition affects the growth and development of plants and animals, impairing ecosystem functions [10,11]. Continuous increases in nitrogen deposition impact biochemical processes, such as carbon and nitrogen cycling in forest ecosystems [12,13], influence nitrogen cycling in marine ecosystems, and inhibit marine productivity [14]. It is the third largest cause of global biodiversity loss [15] and a significant factor threatening the health of natural ecosystems and the biosphere [16].
Climate change has intensified the process of desertification, particularly in the border area between western Liaoning Province and the Horqin Sandy Land in eastern Inner Mongolia. This region is experiencing increasing desertification and has sensitive and fragile ecosystems [17]. The frequency of mild and moderate droughts in this area is 48.16% and 38.41%, respectively [18]. Research indicates that N, a crucial limiting element for plant growth [19], exhibits storage and distribution strategies within plants that are more sensitive to external nutrients and climate change in semi-arid environments [20,21], employing unique mechanisms [22]. Plants growing in semi-arid regions can increase nitrogen uptake to mitigate some of the water deficit [23], thereby enhancing their adaptability to water scarcity [24]. This has led us to focus our research on the response mechanisms of plants in semi-arid regions under the influences of nitrogen deposition and changes in precipitation patterns.
Mongolian pine (Pinus sylvestris var. mongolica) is an evergreen tree species known for its resistance to cold, drought, and poor soil conditions. It is currently being planted in the border area between western Liaoning Province and the Horqin Sandy Land in eastern Inner Mongolia as an ecological species to improve soil quality [25] and mitigate soil desertification [26,27]. Compared to other tree species, Mongolian pine is more sensitive to changes in precipitation and other climate variables [28,29]. This region is experiencing significant increases in nitrogen deposition [2] and overall trends of rising annual average temperatures and decreasing precipitation [30]. In individual years, there may also be brief periods of heavy rainfall or increased precipitation due to specific climatic conditions [31]. This complex precipitation pattern, combined with atmospheric nitrogen deposition, creates composite effects that warrant our attention. However, most current research predominantly focuses on drought stress in arid and semi-arid regions [32], with few studies examining the effects of increased water input. Existing studies have shown that nutrient changes due to nitrogen deposition are increasing plant nitrogen uptake while altering the distribution strategies of nitrogen and other elements [33]. Additionally, moisture availability affects the usability of nitrogen within plants [34]. Under the compounded long-term effects of atmospheric nitrogen deposition and changes in precipitation patterns, it is crucial to monitor the responses of Mongolian pine to external climatic changes such as nitrogen and moisture when using it for ecological purposes [35]. For this purpose, we employed stable-isotope tracing technology, which is widely used to reveal the dynamics of nitrogen distribution within plants [36,37], to assess the intrinsic effects of increased exogenous nitrogen concentrations from nitrogen deposition and fertilizers on plant nutrient utilization [38].
Regarding the border area between western Liaoning Province and southern Horqin Sandy Land, there are few studies on the combined effects of intensified nitrogen deposition and variable precipitation patterns on the nitrogen distribution of ecological forest trees like Mongolian pine; most focus on investigating changes in ecological stoichiometric characteristics of ecological forest trees resulting from moisture or nitrogen deposition [39]. This study employs stable-isotope tracing technology, using foliar application of 15N-labeled urea to simulate atmospheric nitrogen deposition and varying soil moisture to represent changes in precipitation patterns. The focus is on exploring the nitrogen distribution strategies of Mongolian pine under the combined influence of changing precipitation patterns and atmospheric nitrogen deposition, with some degree of novelty. The aim is to provide theoretical references for ecological restoration and desertification control in the context of global climate change.
2. Materials and Methods
2.1. Experimental Materials
This study used one-year-old Mongolian pine (Pinus sylvestris var. mongolica) seedlings as experimental materials. The soil was collected from the Daqinggou Sandy Land Ecological Experiment Station (42°54′ N, 122°25′ E) of the Institute of Applied Ecology, Chinese Academy of Sciences. This station is located at the border between western Liaoning Province and the southern part of the Horqin Sandy Land in Inner Mongolia. The area features a continental temperate semi-humid-to-semi-arid transitional climate. The mean annual temperature (MAT) is 6.4 °C, and the mean annual precipitation (MAP) is 441 mm [40]. The soil at the study site is a nutrient-poor sandy type, consisting of 90.9% sand, 5.0% silt, and 4.1% clay [41]. The soil pH is about 6.6, bulk density (BD) is 1.47 g·cm3, and total nitrogen content is 0.36 g·kg−1 [31].
2.2. Experimental Design
The experiment utilized 30 cm diameter and 30 cm high pots with a sealed bottom and no solution leakage, each filled with 15 kg of soil (approximately up to 28 cm of the pot’s height). Mongolian pine seedlings with uniform growth were transplanted into the pots. The seedlings were grown under natural conditions. On rainy days, they were placed under a rain shelter connected to the natural environment on all sides, ensuring proper ventilation. The shelter was only shaded on the upper part, which excluded external factors such as rainfall and temperature.
Based on historical soil moisture data from the Daqinggou Sandy Land Ecological Experiment Station [31,42], we established three water treatment levels based on the soil’s field capacity: WC (normal moisture, approximately 65% ± 2.5% of the field capacity), WI (30% increased moisture, approximately 85% ± 2.5% of the field capacity), and WD (30% decreased moisture, approximately 45% ± 2.5% of the field capacity). The moisture control trial commenced on May 1 and concluded on October 30 of the same year. Soil moisture content was measured using a soil moisture meter (TDR300, Spectrum Technologies, Inc., Plainfield, IL, USA) and controlled by the weighing method. Before we started the water control test, we watered it thoroughly. When the soil moisture content reached the level specified by the experimental design, the total weight of the potting bucket, soil, and seedlings (referred to as Control Weight, CW) was recorded. Due to transpiration, evaporation from the seedlings, and soil evaporation, the soil moisture content decreased over time. Therefore, every three days, we measured the total weight of the potting bucket, soil, and seedlings (referred to as Total Weight, TW) and replenished the water in the potting buckets; the amount of water added was recorded until the end of October. The amount of water replenished (referred to as Water Weight, WW) was determined using the following formula:
Referring to ambient N deposition fluxes in northern China [43] and monitoring data from the Nationwide Nitrogen Deposition Monitoring Network (NNDMN) from 2010 to 2014 in eastern Inner Mongolia and northeastern China [44], combined with data from the study site [45] and considering the ecological effects of increased nitrogen deposition in the future, three nitrogen levels were established: NC (control nitrogen treatment, no nitrogen added), NL (5 g·m−2·y−1 nitrogen added, equivalent to 50 kg·ha−1·y−1), and NH (10 g·m−2·y−1 nitrogen added, equivalent to 100 kg·ha−1·y−1), which are comparable to the treatment levels in existing research settings [40,46,47]. To mimic the process of atmospheric nitrogen deposition, a solution of 15N-labeled CH4N2O (containing 46% N with a 15N abundance of 10.14%, from Shanghai Research Institute of Chemical Industry Co., Ltd., Shanghai, China) was sprayed evenly on 15 May and 15 June, respectively, during the growing season. The total amount of nitrogen applied was equivalent to the level of the experimental design.
2.3. Measurement and Calculation of Indicators
2.3.1. Sample Collection and Dry Weight Measurement
In the summer (31 August) and autumn (31 October) of the same year, three seedlings from each treatment level were collected. The seedlings were washed with deionized water and divided into roots, stems, and leaves. Surface soil samples were also taken.
The root, stem, and leaf samples were placed in an oven, inactivated at 105 °C for 30 min, and then dried at 70 °C to a constant weight. The dry weights of the root, stem, and leaf samples were then measured and recorded.
2.3.2. N Content and 15N Abundance Measurement
The dried root, stem, and leaf samples were ground and sieved using a grinder, then stored in zip-lock bags in a dry environment. The surface soil samples were air-dried and stored in zip-lock bags in a dry environment.
The N content and 15N abundance were measured using a stable-isotope ratio mass spectrometer (Isoprime100, Isoprime Ltd., Cheadle, Greater Manchester, UK).
2.3.3. Calculation
Ndff (%) represents the proportion of nitrogen in each plant organ derived from the applied fertilizer nitrogen, reflecting the absorption capacity of 15N by each organ [48]. It is calculated according to the formula by Zhao et al. [49], with the natural abundance of 15N being approximately 0.366%:
The nitrogen content of organs (mg/plant) represents the amount of nitrogen in each plant organ and is calculated using the following formula [50]:
15N absorption in organs (mg/plant) represents the proportion of 15N in the nitrogen content of each organ absorbed from the fertilizer. The calculation formula, by Zhao et al. [38], is as follows:
15N distribution ratio (%) represents the proportion of 15N absorbed by each organ in the total 15N absorbed by the plant. The calculation formula, by Ding et al. [51] and Shi et al. [52], is as follows:
2.4. Data Processing
Data organization and processing were performed using Microsoft Excel 2016. Graph plotting and image processing were carried out using Origin 2021. Multifactor ANOVA (analysis of variance) was conducted using SPSS 26.0 to analyze the effects of moisture, nitrogen, months, and their interactions on 15N abundance in the organs and soil of Mongolian pine. Significance analysis was performed using the Waller–Duncan method and One-Way ANOVA, while correlation analysis was conducted using the Pearson index. The experimental data are presented as mean ± standard deviation.
3. Results
3.1. Impact of Nitrogen Deposition and Precipitation Patterns on 15N Abundance in Mongolian Pine
3.1.1. Changes in 15N Abundance in Seedlings
As shown in Table 1, in August, under the NC treatment, the three moisture gradient treatments did not cause significant differences in the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings. The 15N abundance was negligible. Under the NL treatment, compared to the control group, the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings increased or decreased with changes in moisture levels, exhibiting significant differences under different moisture treatments. In the NH treatment, regardless of whether moisture levels increased or decreased, the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings was higher than in the control group, with significant differences observed across the three moisture treatments. Under the WC treatment, the 15N abundance in the roots and stems was slightly lower under the high nitrogen concentration (NH) treatment compared to the low nitrogen concentration (NL) treatment, whereas it was the opposite in the leaves. Under the increased moisture treatment (WI), the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings increased by 31.98%, 47.42%, and 85.59%, respectively, in the NH treatment compared to the NL treatment, with significant differences. Under the decreased moisture treatment (WD), the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings increased by 67.51%, 73.38%, and 104.88%, respectively, under high nitrogen concentration compared to low nitrogen concentration.

Table 1.
Impact of nitrogen deposition and precipitation patterns on 15N abundance (%) in different organs of Mongolian pine seedlings on sandy land.
In October, under the NC treatment, changes in moisture levels did not cause significant differences in the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings, and the 15N abundance was negligible (see Table 1). Under the NL treatment, compared to the control, the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings significantly increased or decreased with changes in moisture levels. The same trend was observed under the NH treatment. Under the moisture control treatment, the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings increased significantly by 9.55%, 19.26%, and 20.93%, respectively, under high nitrogen concentration compared to low nitrogen concentration. Under the increased moisture treatment (WI), the 15N abundance in the roots, stems, and leaves of Mongolian pine seedlings increased significantly by 44.44%, 36.02%, and 47.02%, respectively, under the NH treatment compared to the NL treatment. Under the decreased moisture treatment (WD), the opposite trend was observed, with the 15N abundance in the roots, stems, and leaves under the high nitrogen concentration treatment being slightly lower than that under the low nitrogen concentration, with significant differences in the roots and stems.
3.1.2. Changes in Soil 15N Abundance
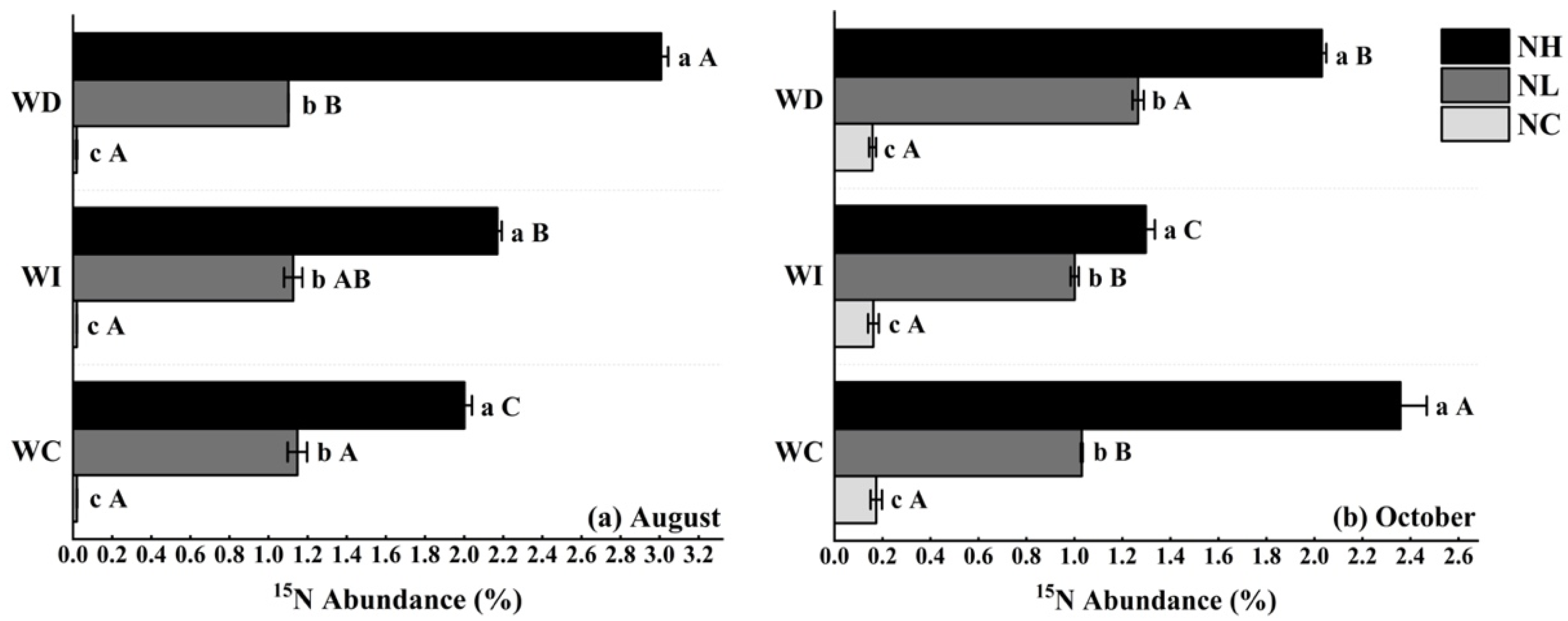
As shown in Figure 1a, in August, under the NL treatment, there were no significant differences in the soil 15N abundance of Mongolian pine seedlings on sandy land across different moisture treatments. However, under the NH treatment, there were significant differences in soil 15N abundance among the three moisture levels. For the WC, WI, and WD moisture treatments, soil 15N abundance in Mongolian pine seedlings increased with higher nitrogen concentrations, showing significant differences.
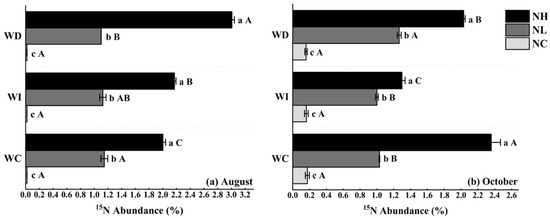
Figure 1.
(a) Impact of nitrogen deposition and precipitation patterns on soil 15N abundance of Mongolian pine seedlings on sandy land (in August); (b) impact of nitrogen deposition and precipitation patterns on soil 15N abundance of Mongolian pine seedlings on sandy land (in October). Different uppercase letters indicate significant differences between moisture treatments under the same nitrogen treatment (p < 0.05). Different lowercase letters indicate significant differences between nitrogen treatments under the same moisture treatment (p < 0.05).
In October (as shown in Figure 1b), under the NC treatment, there were no significant differences in soil 15N abundance across the three moisture levels. Under the low nitrogen treatment (NL), increased moisture did not significantly affect soil 15N abundance, but the reduced moisture treatment significantly increased soil 15N abundance by 22.33%. Under the NH treatment, soil 15N abundance in Mongolian pine seedlings significantly decreased by 45.11% and 13.62% under the increased and reduced moisture treatments, respectively, compared to the control. For the WC, WI, and WD moisture treatments, soil 15N abundance in Mongolian pine seedlings increased with higher nitrogen concentrations, showing significant differences.
3.2. Correlation and Variance Analysis of 15N Abundance in Different Organs and Soil of Mongolian Pine Seedlings Under Various Treatments of Nitrogen Deposition and Precipitation Patterns
3.2.1. Correlation Analysis
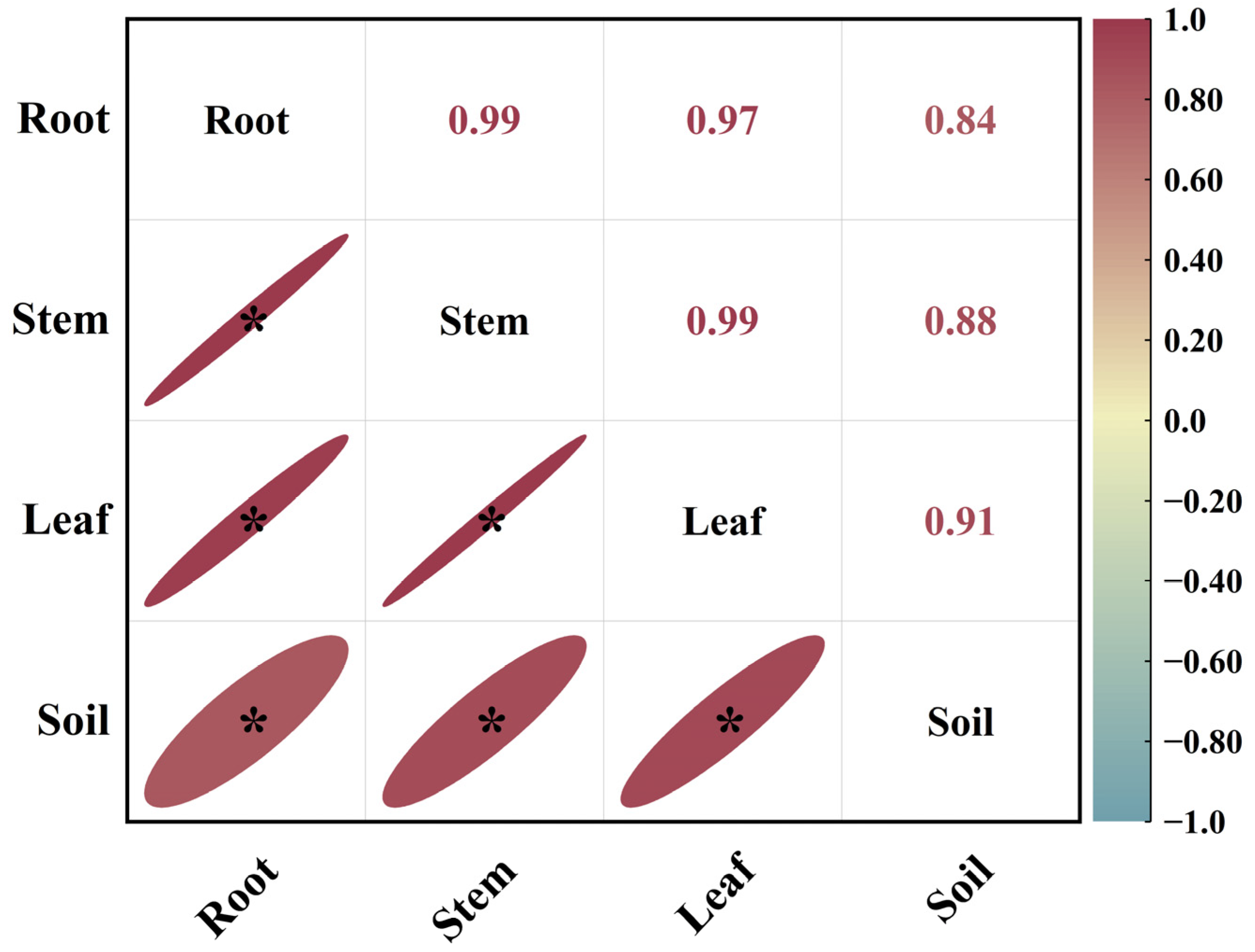
As shown in Figure 2, in August, the correlation coefficients between root 15N abundance and stem 15N abundance, leaf 15N abundance and stem 15N abundance, and leaf 15N abundance and root 15N abundance in Mongolian pine seedlings on sandy land were 0.99, 0.99, and 0.97, respectively. This indicated a significant positive correlation among the 15N abundances in different organs of the seedlings. The correlation coefficient between soil 15N abundance and leaf 15N abundance was the highest, followed by those between soil 15N abundance and stem 15N abundance and between soil 15N abundance and root 15N abundance, all showing significant positive correlations.
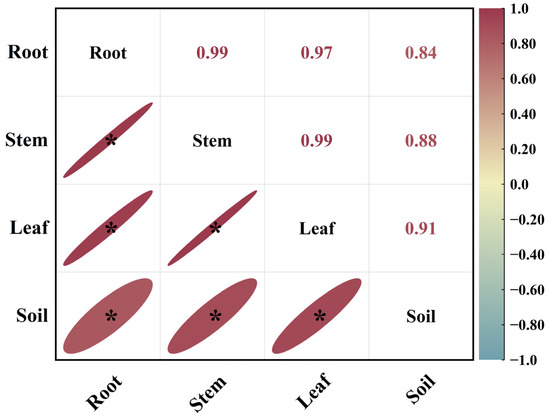
Figure 2.
Correlation analysis of 15N abundance in different organs and soil of Mongolian pine seedlings on sandy land in August. *: significant correlation at the p < 0.05 level.
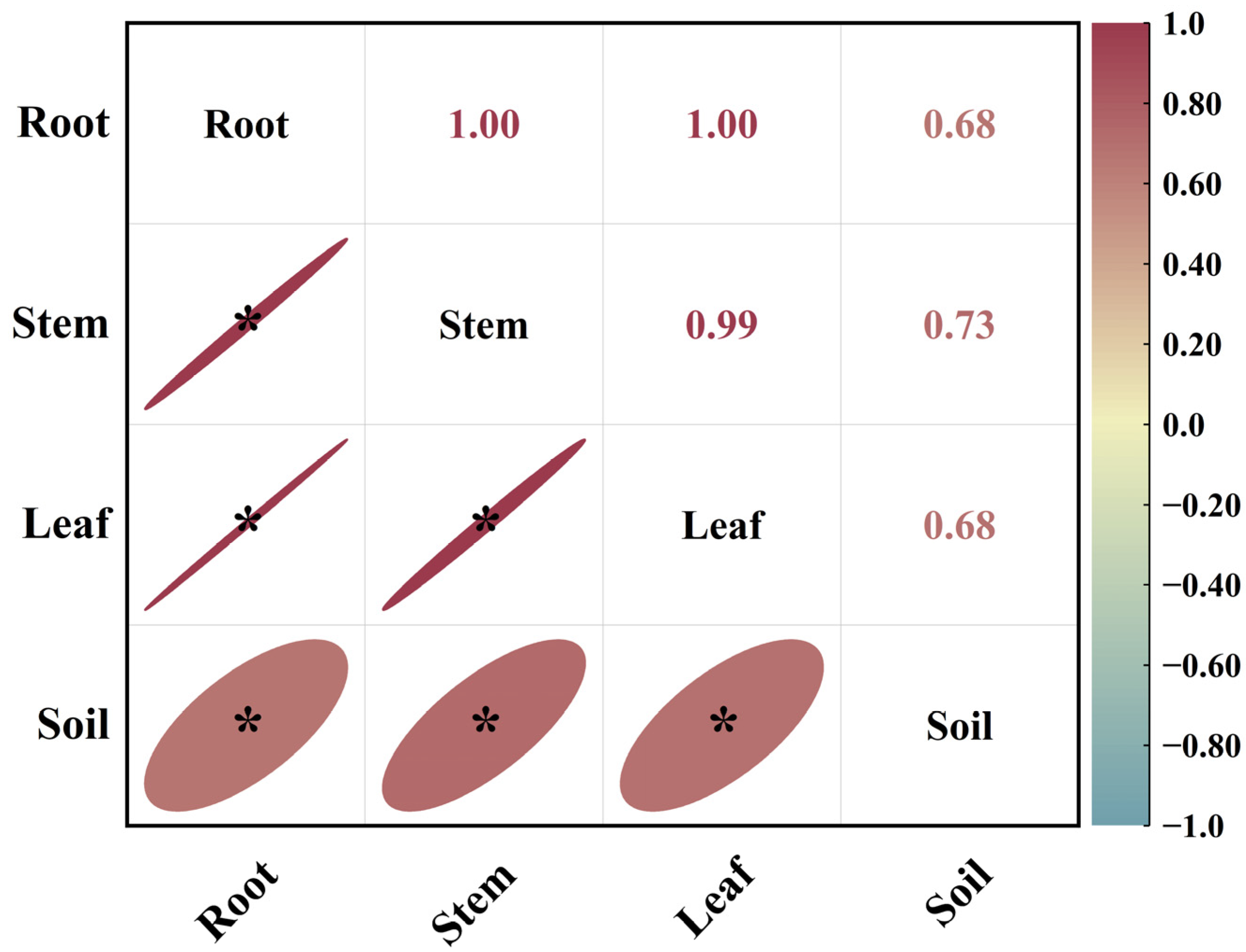
As shown in Figure 3, in October, there was a highly significant positive correlation among the 15N abundances in the roots, stems, and leaves of the Mongolian pine seedlings. Although there were significant positive correlations between the 15N abundances in the roots, stems, leaves, and soil, the correlation coefficients were lower. The correlation between stem and leaf 15N abundance and soil 15N abundance was stronger than that between root 15N abundance and soil 15N abundance.
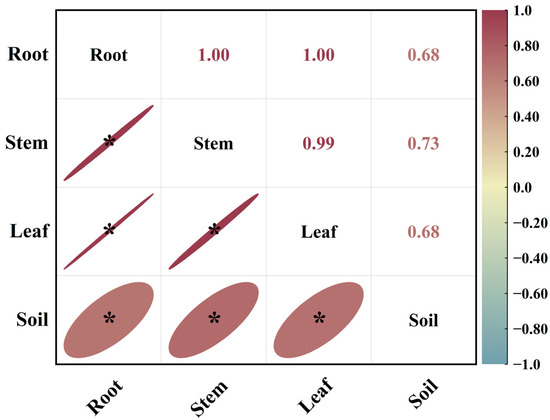
Figure 3.
Correlation analysis of 15N abundance in different organs and soil of Mongolian pine seedlings on sandy land in October. *: significant correlation at the p < 0.05 level.
3.2.2. Analysis of Variance
As shown in Table 2, based on the analysis of variance of month, moisture, and nitrogen application on the 15N abundance of Mongolian pine seedlings and soil, it was observed that month, moisture, and nitrogen application were the main effects, and their interactions had highly significant (p < 0.01) effects on the 15N abundance of each organ of Mongolian pine seedlings and soil.

Table 2.
Analysis of variance (P) for the main effects of month, moisture, nitrogen application, and their interactions on the 15N abundance of Mongolian pine seedlings and soil.
3.3. Effects of Nitrogen Deposition and Changes in Precipitation Patterns on 15N Uptake by Mongolian Pine
3.3.1. Ndff (%) in Different Organs of Mongolian Pine Seedlings and Soil
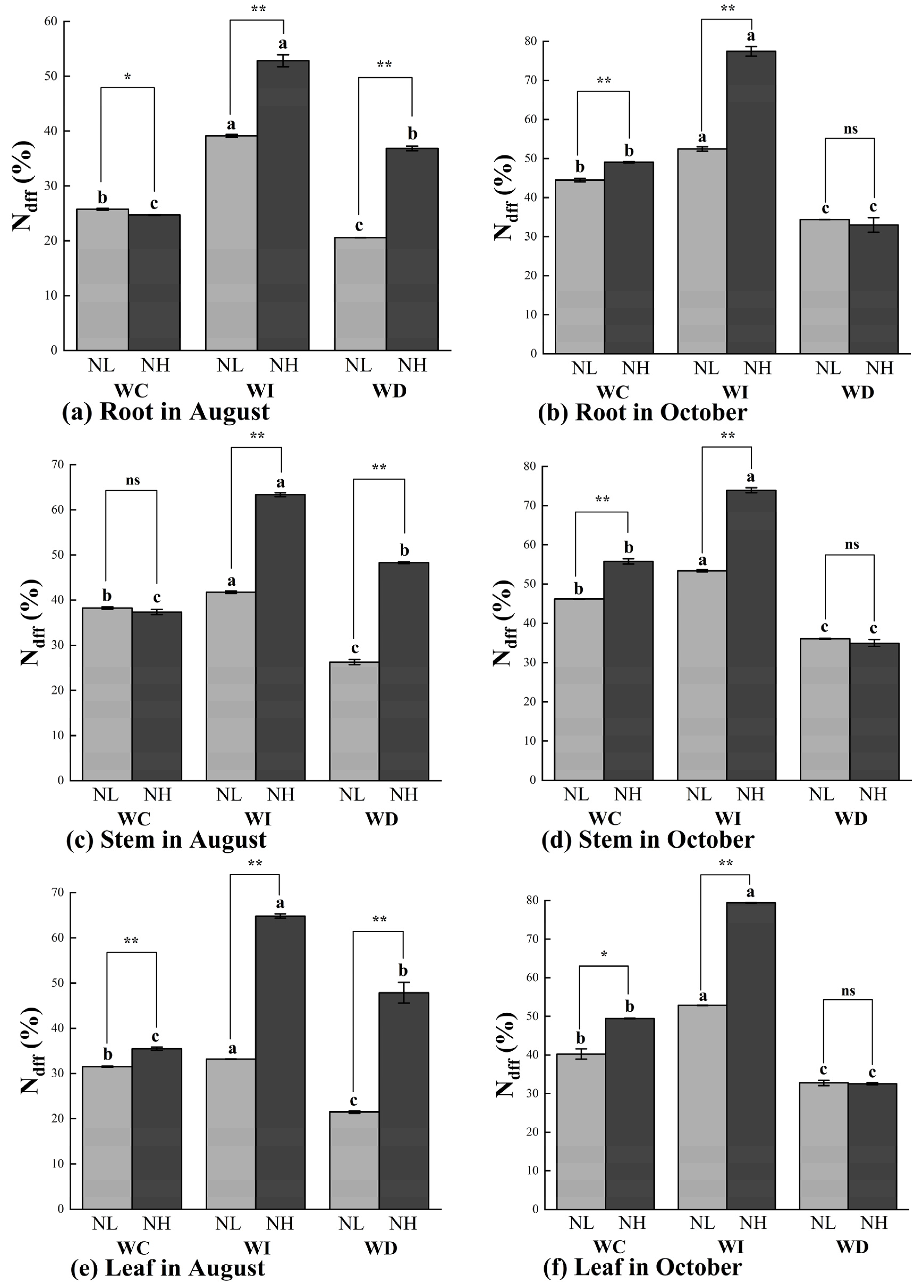
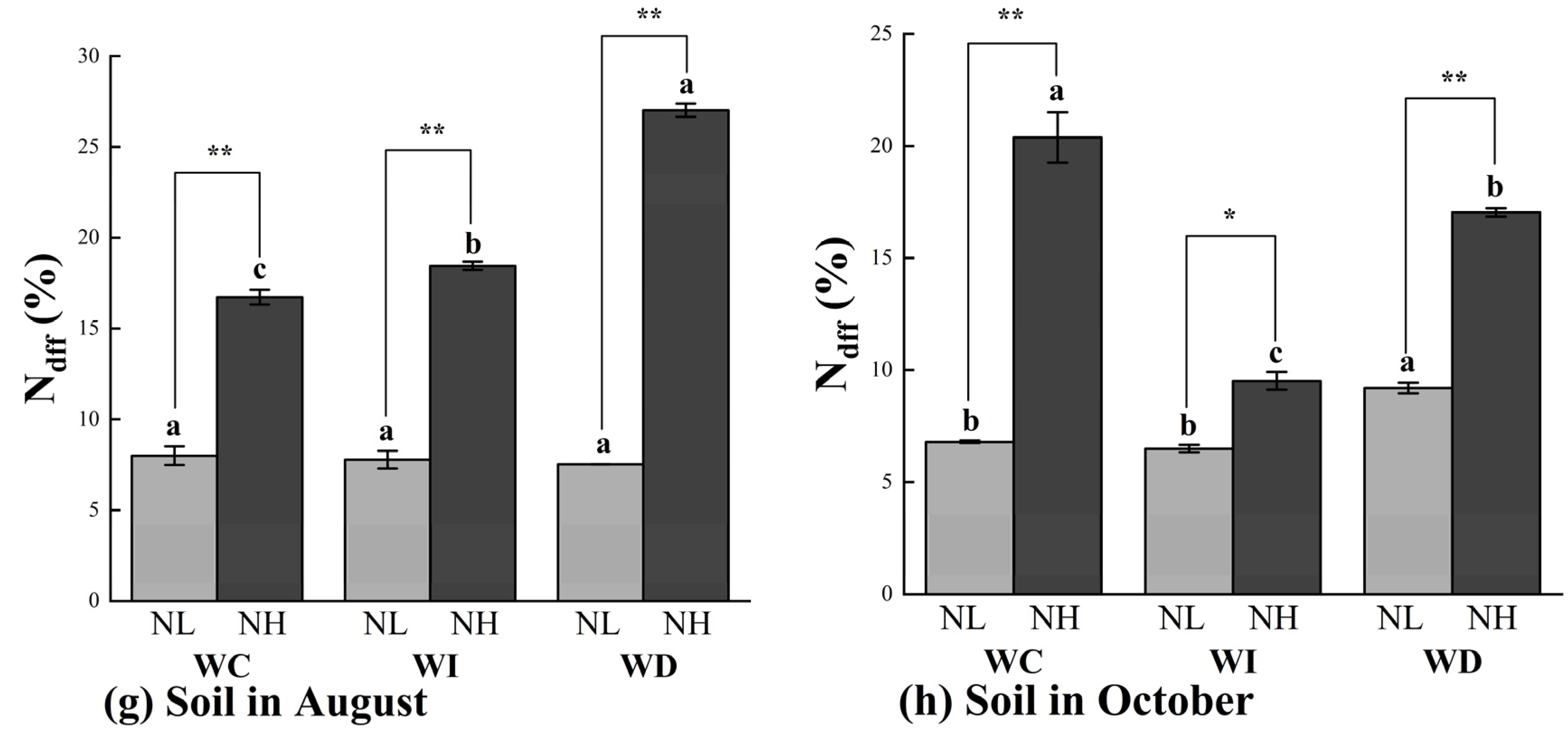
As shown in Figure 4a, in August, the Ndff (%) in the roots of Mongolian pine seedlings was generally higher under the increased moisture treatment (WI) compared to other treatments. In the NL treatment, the WI treatment significantly increased the Ndff (%) in the roots by 51.86% and 90.51% compared to the WC and WD treatments, respectively. Under the NH treatment, the increased moisture significantly raised the Ndff (%) in the roots by 114.01% and 43.43% under the WC and WD treatments, respectively. With the same moisture treatment, the NH nitrogen application significantly increased the Ndff (%) in the roots compared to the NL nitrogen application under both the WI and WD treatments. In October, the Ndff (%) in the roots generally showed an increase or decrease corresponding to the changes in moisture levels compared to the control treatment (WC) (Figure 4b). Significant differences in Ndff (%) were observed under different nitrogen application rates in the WC and WI treatments, but not under the WD treatment. In all nitrogen treatments, the Ndff (%) in the roots was significantly higher under the increased moisture treatment (WI) compared to the other two treatments (WC and WD).
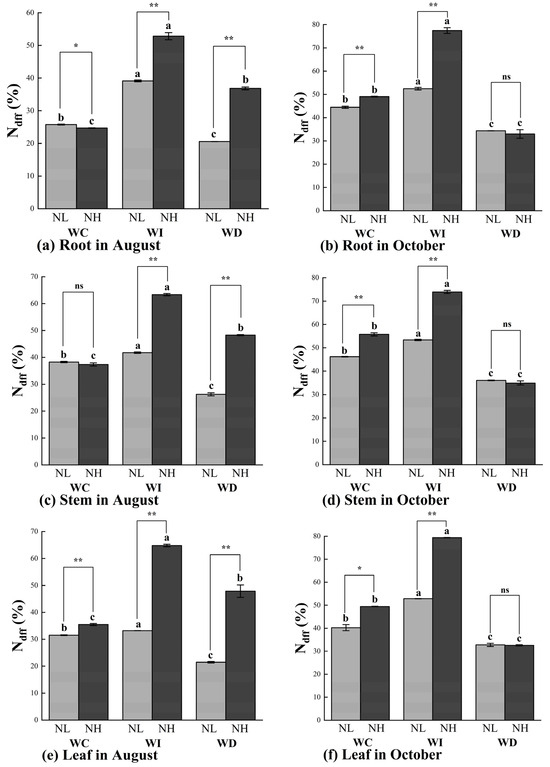
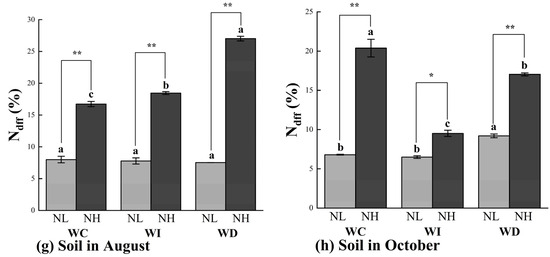
Figure 4.
Ndff (%) values of Mongolian pine seedlings under nitrogen deposition and changes in precipitation patterns. Different lowercase letters indicate that differences between moisture treatments reached significance under the same nitrogen treatment (p < 0.05). ns: not significant; *: significant correlation at the p < 0.05 level; **: significant correlation at the p < 0.01 level.
In the stems of Mongolian pine seedlings, the Ndff (%) in August was higher in all nitrogen treatments compared to the control treatment (WC). In October, the Ndff (%) in the stems showed a trend of increase or decrease corresponding to the changes in moisture levels, with significant differences observed under different nitrogen treatments (Figure 4c,d). Under the WC moisture treatment, there were no significant differences in Ndff (%) between the NL and NH nitrogen treatments. In October, the effect of the changes in moisture levels on Ndff (%) in the stems was less pronounced under the NL treatment compared to the NH treatment. Under the WD treatment, different nitrogen levels did not result in significant differences in Ndff (%) in the stems of the seedlings.
As shown in Figure 4e,f, the overall trends in Ndff (%) in the leaves of Mongolian pine seedlings in August and October were like those observed in the stems. In August, the Ndff (%) in the leaves was higher under all nitrogen treatments compared to the control treatment (WC). In October, the Ndff (%) in the leaves showed significant differences corresponding to changes in moisture levels under different nitrogen treatments. In August, the variations in Ndff (%) in the leaves were smaller under the NL treatment compared to the NH treatment. Under the WC, WI, and WD treatments, the Ndff (%) in the leaves was significantly higher by 12.56%, 95.35%, and 122.96%, respectively, under the NH treatment compared to the NL treatment. In October, under the WC moisture treatment, the Ndff (%) in the leaves was significantly higher by 22.91% under the NH treatment compared to the NL treatment. Under the WI moisture treatment, the Ndff (%) in the leaves was significantly higher by 50.21% under the high nitrogen treatment compared to the low nitrogen treatment. Under the WD treatment, there were no significant differences in Ndff (%) in the leaves between different nitrogen levels.
The patterns of Ndff (%) changes in the soil were slightly different from those in the organs of Mongolian pine seedlings (Figure 4g,h). Under the low nitrogen treatment (NL), changes in moisture levels did not significantly affect the Ndff (%) in the soil in August. In October, the reduced moisture treatment (ND) slightly increased the Ndff (%) in the soil. In August, the NH treatment significantly increased the Ndff (%) in the soil by 10.34% and 61.57% under the WI and WD treatments, respectively. Significant differences were observed between the two nitrogen treatments under the same moisture treatment. In October, the Ndff (%) in the soil was higher under the NH treatment compared to the NL treatment across all moisture treatments, with significant differences between the WC and WD treatments. Under the NL treatment, the reduced moisture treatment (WD) significantly increased the Ndff (%) in the soil compared to the control treatment (WC), while no significant differences were observed under the increased moisture treatment. Under the NH treatment, changes in moisture levels significantly reduced the Ndff (%) in the soil compared to the control group (NC).
3.3.2. Nitrogen Content and 15N Absorption
Since the nitrogen content of organs in Mongolian pine seedlings is one of the factors for calculating 15N absorption, only the results are presented here, focusing on the analysis of 15N absorption in different organs of the seedlings.
As shown in Table 3, in August, under the NL treatment, compared to the control group, changes in moisture levels increased or decreased the 15N absorption in the roots of Mongolian pine seedlings. Similar trends were observed in the stems and leaves under the NL treatment. Under the NH treatment, compared to the control group, changes in moisture levels consistently resulted in higher 15N absorption in the roots, stems, and leaves of the seedlings. Under the moisture control treatment (WC), significant differences in 15N absorption were observed in the roots, stems, and leaves of the seedlings with different nitrogen application levels. In terms of 15N absorption in the stems and leaves, under the increased moisture treatment (WI), the NH nitrogen treatment significantly increased the 15N absorption by 108.72% and 119.69%, respectively, compared to the NL nitrogen treatment. Under the reduced moisture treatment (WD), the NH nitrogen treatment significantly increased the 15N absorption in the roots, stems, and leaves by 106.12%, 109.10%, and 330.94%, respectively, compared to the NL nitrogen treatment.

Table 3.
Nitrogen content and 15N absorption in different organs of Mongolian pine seedlings under various treatments of nitrogen deposition and precipitation patterns.
In October, under the low nitrogen treatment (NL), compared to the moisture control treatment (WC), changes in moisture levels resulted in increased or decreased 15N absorption in the roots, stems, and leaves of Mongolian pine seedlings. Under the NH treatment, the 15N absorption in the roots, stems, and leaves increased by 551.43%, 83.28%, and 207.99%, respectively, under the WI treatment compared to the WC treatment, with significant differences observed in the roots and leaves. Under the WI and WD treatments, the high nitrogen treatment consistently increased the 15N absorption in the roots, stems, and leaves, except for the stems under the WI treatment, where no significant difference was observed. Under the moisture control treatment (WC), the 15N absorption in the roots and leaves was significantly lower under the high nitrogen treatment compared to the low nitrogen treatment, decreasing by 55.88% and 36.34%, respectively.
3.4. Effects of Nitrogen Deposition and Precipitation Patterns on the 15N Distribution Ratio in Organs of Mongolian Pine
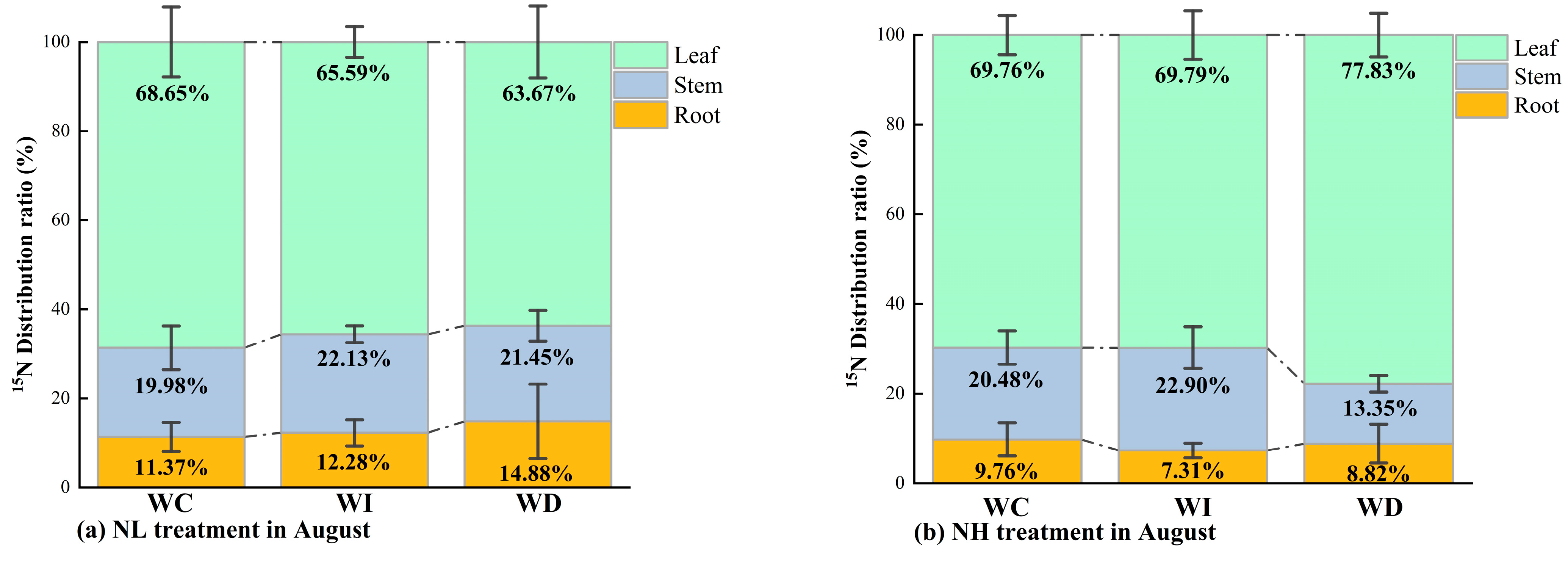
As shown in Figure 5, the distribution ratios of 15N in different organs of Mongolian pine seedlings under various nitrogen and moisture treatments in August and October were consistently in the order of leaves > stems > roots. In August, under the NL treatment (Figure 5a), changes in moisture availability (whether an increase or a decrease) affected 15N distribution. With increased moisture availability, 15N tended to transfer downward from the leaves to the stems and roots, with a higher retention in the stems compared to the control group and the WD treatment. Conversely, with decreased moisture availability, 15N in the leaves tended to transfer more towards the roots compared to the control group and the WI treatment. Under the NH treatment in August, compared to the control group (WC), the WD treatment resulted in more 15N being allocated to the leaves and less to the stems compared to both the control group (WC) and the WI treatment. When moisture was reduced, more 15N was allocated to the roots of the seedlings compared to increased moisture conditions, although it was still less than in the control group. Overall, under high nitrogen concentration, increased moisture led to relatively more 15N being allocated to the stems and leaves of the seedlings, while reduced moisture resulted in slightly more 15N being allocated to the roots.
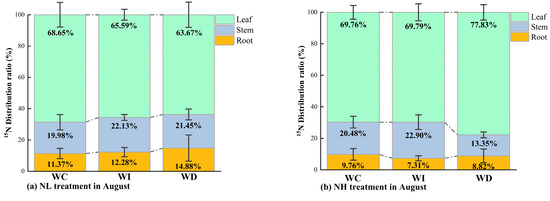
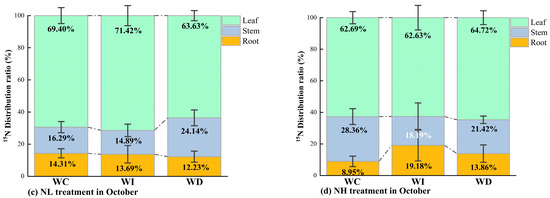
Figure 5.
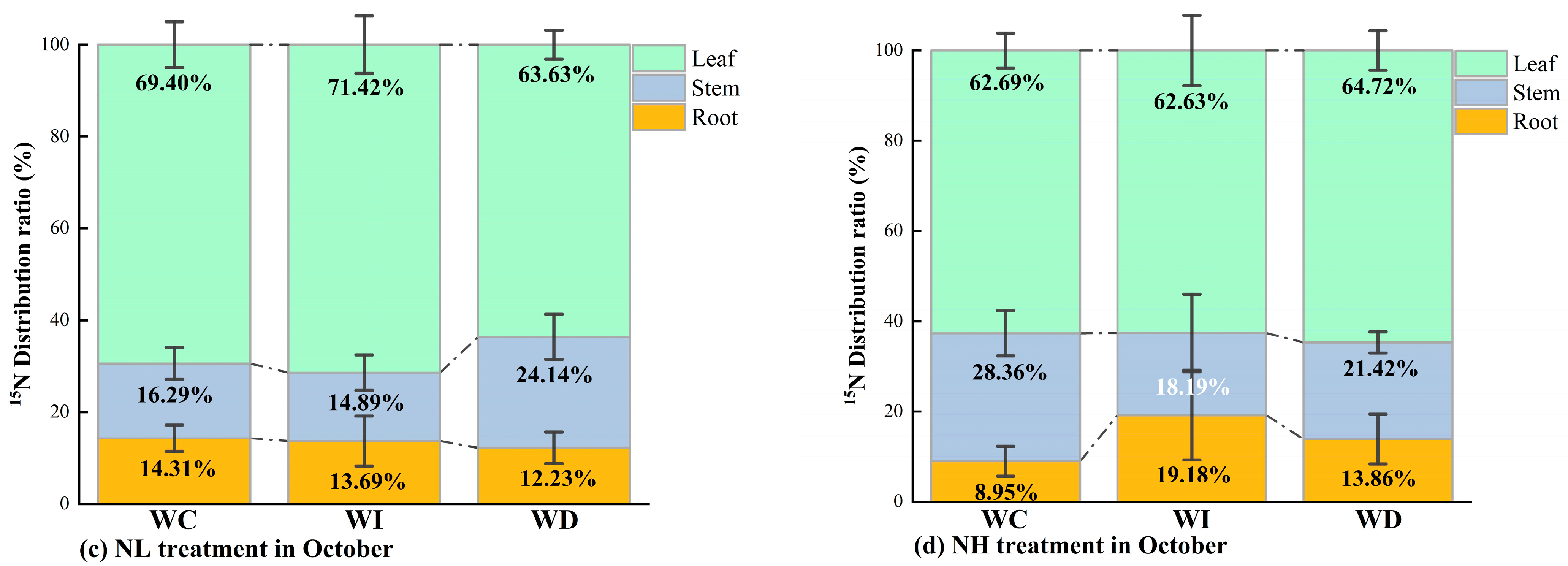
(a) 15N distribution ratio in different organs of Mongolian pine seedlings under NL treatment with changing precipitation patterns in August; (b) 15N distribution ratio in different organs of Mongolian pine seedlings under NH treatment with changing precipitation patterns in August; (c) 15N distribution ratio in different organs of Mongolian pine seedlings under NL treatment with changing precipitation patterns in October; (d) 15N distribution ratio in different organs of Mongolian pine seedlings under NH treatment with changing precipitation patterns in October.
In October, under the NL treatment (Figure 5c), the amount of 15N allocated to the roots of the seedlings increased across all moisture treatments compared to August. When moisture was increased, more 15N was allocated to the leaves compared to the control. With decreased moisture availability, the seedlings tended to allocate more 15N to the stems and roots compared to the WI treatment and the control group. As shown in Figure 5d, under the NH treatment, more 15N was allocated to the roots under both the increased and decreased moisture treatments compared to the control group. Compared to the increased and decreased moisture treatments, the control treatment had significantly more 15N allocated to the stems.
4. Discussion
This study found that atmospheric nitrogen deposition and changes in precipitation patterns influence nitrogen absorption and distribution in Mongolian pine seedlings. Correlation and variance analyses revealed significant correlations in 15N abundance among different organs of Mongolian pine seedlings, consistent with findings by Yang et al. [53] and Temme et al. [54] in other plants. Moisture and nitrogen addition, along with their interactions, significantly affected the 15N abundance in different organs of the seedlings. Similar findings were reported by Li et al. [55] in two tropical tree species, which further supported our hypothesis and provided a basis for subsequent discussions.
Increased moisture availability enhanced the 15N abundance and absorption in the roots, stems, and leaves of the seedlings. This could be due to improved nitrogen absorption efficiency in the roots and other organs with increased moisture availability [56], leading to more nitrogen being transferred to underground organs. In addition, the increased water treatment can also be viewed as years of increased mean annual precipitation (MAP), leading to a decrease in soil bulk density (BD), resulting in increased nitrogen uptake by the root system [57]. Under the low nitrogen treatment, reduced moisture availability can represent years with fewer MAP, resulting in opposite outcomes in 15N abundance and absorption. Elevated soil BD is also one of the causes we should take into consideration [58], since moisture limitation might restrict root growth and slow nutrient transport to other organs [59]. Similar findings were reported by Bethea et al. [60] in Creeping bentgrass (Agrostis stolonifera L.). Reduced moisture availability decreased nitrogen absorption efficiency, limiting nitrogen utilization, as shown in studies on wheat by Ichir et al. [61], rice by Li et al. [62], and no-till rice by Yang et al. [63] under different soil moisture levels. Climate change may lead to an increase in mean annual temperature (MAT), and the effect of MAT on MAP results in complex precipitation patterns [64]. Our findings further confirmed that moisture is a key factor influencing 15N dynamics in plants, consistent with Liu et al. [65]. Different nitrogen treatments led to varied responses in 15N absorption among different organs of the seedlings. Overall, higher nitrogen concentration resulted in greater 15N absorption in all organs compared to lower nitrogen concentration, likely due to stimulated growth and increased nitrogen demands [66]. Another reason could be that nitrogen deposition as an exogenous addition altered the nutrient supply strategy of the seedlings [67]. However, under moisture-controlled conditions, the increase in 15N absorption with the high nitrogen treatment compared to the low nitrogen treatment was not significant in the stems in August, further indicating that the interaction between moisture and nitrogen is crucial for 15N absorption in Mongolian pine seedlings. This understanding helps elucidate the response mechanism of nitrogen absorption in different organs of the seedlings under atmospheric nitrogen deposition and changing precipitation patterns.
Using a 15N-labeled urea solution to simulate atmospheric nitrogen deposition resulted in changes in 15N distribution within the plants, generally showing a pattern of leaves > stems > roots, consistent with existing research [68,69]. Under low nitrogen conditions, reduced moisture availability led to more nitrogen being allocated to the roots, possibly due to enhanced nutrient capture by the roots during moisture stress [70], limiting the transfer of nitrogen to above-ground parts [71] and reducing the adverse effects of moisture deficiency [72]. Under high nitrogen conditions, combined with reduced moisture availability, nitrogen allocation to the roots and stems decreased, possibly because nitrogen was not the preferred element under adverse conditions [73]. It may also be due to high nitrogen additions lowering soil pH and creating acidic soils [74], which affects root uptake of nutrients [75]. The allocation of nutrients to the leaves is part of the plant’s survival strategy [76]. Under low nitrogen conditions, changes in moisture availability led to reduced nitrogen allocation to the leaves, making overall nitrogen distribution more dependent on moisture availability. However, under high nitrogen conditions combined with reduced moisture availability, more 15N accumulated in the leaves, consistent with findings in poplars by Luo et al. [77]. The distribution of nitrogen among different organs in response to environmental changes might relate to the physiological characteristics, tissue structure, and nitrogen demands of the plant [5]. The consistently higher nitrogen allocation to the leaves compared to the roots and stems could be because leaves, being more active organs [78], tend to accumulate more limiting elements [6].
This study also had some limitations due to the pot experiment’s inability to account for seasonal changes in precipitation. To address this, we plan to conduct in situ experiments in the future to deepen our understanding in this field. Additionally, this experiment used one-year-old Mongolian pine seedlings. In future studies, we will include Mongolian pines of various ages, allowing us to comprehensively explore the specific differences in nitrogen allocation mechanisms among pines of different ages under varying atmospheric nitrogen deposition and precipitation patterns. In addition, we plan to incorporate other ecological factors in future experiments, such as CO2 or O3 stress, as well as the combined effects of thermal stress, both hot and cold. This approach aims to elucidate more accurately the responses and feedback mechanisms of forest ecosystems to global climate change.
5. Conclusions
This study elucidates the relationship between nitrogen deposition, moisture levels, and nitrogen allocation in Mongolian pine seedlings. The findings reveal a hierarchical nitrogen allocation pattern, with leaves receiving the highest proportion of 15N, followed by stems and roots, consistent across various treatments. Increased moisture significantly enhances 15N absorption in all organs, particularly the leaves. This allocation strategy ensures a balanced distribution among the organs, supporting the overall growth and development of the seedlings. Under reduced moisture and low nitrogen conditions, seedlings allocate more nitrogen to the roots and stems, which is crucial for mitigating water deficiency. Conversely, high nitrogen and reduced moisture conditions lead to more nitrogen being allocated to the leaves, highlighting their role in nutrient capture. This adaptive response underscores the resilience of Mongolian pine in the face of environmental challenges. The implications of these findings are profound for ecological restoration, wind erosion control, and agricultural and forestry management, especially in semi-arid regions where climate change poses significant threats. This study advocates for moderate nitrogen deposition combined with increased moisture as a strategy to bolster nitrogen uptake in Mongolian pine seedlings, thereby enhancing their survival and growth in semi-arid environments.
Author Contributions
Data curation, S.C. and Y.Z.; investigation, T.Z., S.C., Q.G. and Y.Z.; methodology, T.Z., S.C., Q.G. and X.Z.; writing—original draft, S.C.; writing—review and editing, T.Z. and X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Scientific Research Project of Liaoning Education Department (grant JYTMS20231274).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We thank Xin Guan, at Shenyang Agricultural University, for providing theoretical guidance on the mathematical and statistical analysis for this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Li, X.; Xiao, P.; Hao, S.; Wang, Z. Rainfall Erosivity Characteristics during 1961–2100 in the Loess Plateau, China. Remote Sens. 2024, 16, 661. [Google Scholar] [CrossRef]
- Dai, Z.-C.; Liu, Y.-X.; Dang, N.; Wang, Z.-R.; Cai, J.-P.; Zhang, Y.-G.; Song, Y.-B.; Li, H.; Jiang, Y. Short-term legacy effects of long-term nitrogen and water addition on soil chemical properties and micro-bial characteristics in a temperate grassland. J. Appl. Ecol. 2023, 34, 1834–1844. (In Chinese) [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Change 2013, 3, 52–58. [Google Scholar] [CrossRef]
- Verburg, P.S.J.; Kapitzke, S.E.; Stevenson, B.A.; Bisiaux, M. Carbon allocation in Larrea tridentata plant-soil systems as affected by elevated soil moisture and N availability. Plant Soil 2014, 378, 227–238. [Google Scholar] [CrossRef]
- Zhang, Z.; Chai, X.; Tariq, A.; Zeng, F.; Graciano, C.; Li, X.; Gao, Y.; Ullah, A. Coordinated Patterns in the Allocation, Composition, and Variability of Multiple Elements among Organs of Two Desert Shrubs Under Nitrogen Addition and Drought. J. Soil Sci. Plant Nutr. 2022, 22, 47–58. [Google Scholar] [CrossRef]
- Zhang, J.; He, N.; Liu, C.; Xu, L.; Yu, Q.; Yu, G. Allocation strategies for nitrogen and phosphorus in forest plants. Oikos 2018, 127, 1506–1514. [Google Scholar] [CrossRef]
- Skidmore, A.K.; Abdullah, H.; Siegenthaler, A.; Adiningrat, D.P.; Rousseau, M.; Duan, Y.; Torres-Rodriguez, A.; Neinavaz, E. Forest soils further acidify in core Natura 2000 areas amongst unaware government policy. Ecol. Indic. 2024, 159, 111621. [Google Scholar] [CrossRef]
- Kanakidou, M.; Myriokefalitakis, S.; Daskalakis, N.; Fanourgakis, G.; Nenes, A.; Baker, A.R.; Tsigaridis, K.; Mihalopoulos, N. Past, Present, and Future Atmospheric Nitrogen Deposition. J. Atmos. Sci. 2016, 73, 2039–2047. [Google Scholar] [CrossRef]
- Lovett, G.M.; Tear, T.H.; Evers, D.C.; Findlay, S.E.G.; Cosby, B.J.; Dunscomb, J.K.; Driscoll, C.T.; Weathers, K.C. Effects of Air Pollution on Ecosystems and Biological Diversity in the Eastern United States. Ann. N. Y. Acad. Sci. 2009, 1162, 99–135. [Google Scholar] [CrossRef]
- Yang, A.; Zhu, D.; Zhang, W.; Shao, Y.; Shi, Y.; Liu, X.; Lu, Z.; Zhu, Y.G.; Wang, H.; Fu, S. Canopy nitrogen deposition enhances soil ecosystem multifunctionality in a temperate forest. Glob. Chang. Biol. 2024, 30, e17250. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; Chen, W.; Wang, X.; Mao, R. Moss removal facilitates decomposition and net nitrogen loss of monospecific and mixed-species litter in a boreal peatland. Biogeochemistry 2024, 167, 121–133. [Google Scholar] [CrossRef]
- Song, Y.; Xing, J.; Hu, C.; Song, C.; Wang, Q.; Wang, S. Decomposition and Carbon and Nitrogen Releases of Twig and Leaf Litter Were Inhibited by Increased Level of Nitrogen Deposition in a Subtropical Evergreen Broad-Leaved Forest in Southwest China. Forests 2024, 15, 492. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, P.; Zhang, W.; Wang, Q.; Guo, P.; Wang, Q. Nitrogen deposition caused higher increases in plant-derived organic carbon than microbial-derived organic carbon in forest soils. Sci. Total Environ. 2024, 925, 171752. [Google Scholar] [CrossRef]
- Somes, C.J.; Landolfi, A.; Koeve, W.; Oschlies, A. Limited impact of atmospheric nitrogen deposition on marine productivity due to biogeochemical feedbacks in a global ocean model. Geophys. Res. Lett. 2016, 43, 4500–4509. [Google Scholar] [CrossRef]
- Díaz-Álvarez, E.A.; Lindig-Cisneros, R.; de la Barrera, E. Biomonitors of atmospheric nitrogen deposition: Potential uses and limitations. Conserv. Physiol. 2018, 6, coy011. [Google Scholar] [CrossRef]
- Guerrieri, R.; Cáliz, J.; Mattana, S.; Barceló, A.; Candela, M.; Elustondo, D.; Fortmann, H.; Hellsten, S.; Koenig, N.; Lindroos, A.-J.; et al. Substantial contribution of tree canopy nitrifiers to nitrogen fluxes in European forests. Nat. Geosci. 2024, 17, 130–136. [Google Scholar] [CrossRef]
- Feng, S.; Liu, X.; Zhao, W.; Yao, Y.; Zhou, A.; Liu, X.; Pereira, P. Key Areas of Ecological Restoration in Inner Mongolia Based on Ecosystem Vulnerability and Ecosystem Service. Remote Sens. 2022, 14, 2729. [Google Scholar] [CrossRef]
- Ge, D.; Gao, X.; Wei, X. Changes in spatiotemporal drought characteristics from 1961 to 2017 in northeastern maize-growing regions, China. Irrig. Sci. 2023, 42, 163–177. [Google Scholar] [CrossRef]
- Guan, L.-L.; Wen, D.-Z. More nitrogen partition in structural proteins and decreased photosynthetic nitrogen-use efficiency of Pinus massoniana under in situ polluted stress. J. Plant Res. 2011, 124, 663–673. [Google Scholar] [CrossRef]
- Aoyagi, R.; Kitayama, K. Nutrient allocation among plant organs across 13 tree species in three Bornean rain forests with contrasting nutrient availabilities. J. Plant Res. 2016, 129, 675–684. [Google Scholar] [CrossRef]
- Wu, J.; Jiao, L.; Che, X.; Zhu, X.; Yuan, X. Nutrient allocation patterns of Picea crassifolia on the eastern margin of the Qinghai-Tibet Plateau. Int. J. Biometeorol. 2024, 68, 1155–1167. [Google Scholar] [CrossRef]
- Witkowski, E.T.F.; Lamont, B.B. Disproportionate allocation of mineral nutrients and carbon between vegetative and reproductive structures in Banksia hookeriana. Oecologia 1996, 105, 38–42. [Google Scholar] [CrossRef]
- He, W.; Liu, H.; Shi, L.; Zhou, M.; Qi, Y.; Liu, F.; Zhu, X.; Zhao, P.; Xiang, C.; Shu, Y. Climate and soil change nutrient element allocation of Siberian larch in the Mongolian semiarid forest. Agric. For. Meteorol. 2022, 315, 108825. [Google Scholar] [CrossRef]
- Cleland, E.E.; Goodale, U.M. Co-limitation by nitrogen and water constrains allocation response to drought in deciduous and evergreen shrubs in a semi-arid ecosystem. Plant Ecol. 2019, 220, 213–225. [Google Scholar] [CrossRef]
- Liu, N.; Bao, G.; Bao, M. Response Characteristics of Chinese Pine (Pinus tabulaeformis Carr.) Radial Growth to Climate and Drought Variability Reconstruction in Western Liaoning, Northeast China. Forests 2019, 10, 752. [Google Scholar] [CrossRef]
- Li, Y.; Awada, T.; Zhou, X.; Shang, W.; Chen, Y.; Zuo, X.; Wang, S.; Liu, X.; Feng, J. Mongolian pine plantations enhance soil physico-chemical properties and carbon and nitrogen capacities in semi-arid degraded sandy land in China. Appl. Soil Ecol. 2012, 56, 1–9. [Google Scholar] [CrossRef]
- An, Y.; Zhang, L.; Wang, Q.; Han, Y. Soil Quality Assessment of Different Land Use Types Based on TOPSIS Method in Hilly Sandy Area of Loess Plateau, Northern China. Int. J. Environ. Res. Public Health 2022, 19, 17059. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, J.; Zhang, Y.; Qin, S.; Shao, Y.; Gao, Y. Responses of vegetation to climatic variations in the desert region of northern China. CATENA 2019, 175, 27–36. [Google Scholar] [CrossRef]
- Fang, A.; Dong, J.; Cao, Z.; Zhang, F.; Li, Y. Tempo-Spatial Variation of Vegetation Coverage and Influencing Factors of Large-Scale Mining Areas in Eastern Inner Mongolia, China. Int. J. Environ. Res. Public Health 2019, 17, 47. [Google Scholar] [CrossRef]
- You, G.; Liu, B.; Zou, C.; Li, H.; McKenzie, S.; He, Y.; Gao, J.; Jia, X.; Altaf Arain, M.; Wang, S.; et al. Sensitivity of vegetation dynamics to climate variability in a forest-steppe transition ecozone, north-eastern Inner Mongolia, China. Ecol. Indic. 2021, 120, 106833. [Google Scholar] [CrossRef]
- Song, L.; Zhu, J.; Li, M.; Yan, T.; Zhang, J. Needles stable carbon isotope composition and traits of Pinus sylvestris var. mongolica in sparse wood grassland in south edge of Keerqin Sandy Land under the conditions of different precipitation. Chin. J. Appl. Ecol. 2012, 23, 1435–1440. (In Chinese) [Google Scholar] [CrossRef]
- Meng, F.; Zhang, T.; Yin, D. The effects of soil drought stress on growth characteristics, root system, and tissue anatomy of Pinus sylvestris var. mongolica. PeerJ 2023, 11, e14578. [Google Scholar] [CrossRef]
- Zhou, X.; Ouyang, S.; Saurer, M.; Feng, M.; Bose, A.K.; Duan, H.; Tie, L.; Shen, W.; Gessler, A. Species-specific responses of C and N allocation to N addition: Evidence from dual 13C and 15N labeling in three tree species. Sci. Total Environ. 2024, 927, 172164. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.; Sugimoto, A.; Tei, S.; Bragin, I.V.; Takano, S.; Morozumi, T.; Shingubara, R.; Maximov, T.C.; Kiyashko, S.I.; Velivetskaya, T.A.; et al. Importance of soil moisture and N availability to larch growth and distribution in the Arctic taiga-tundra boundary ecosystem, northeastern Siberia. Polar Sci. 2014, 8, 327–341. [Google Scholar] [CrossRef]
- Bao, G. Mongolian pines (Pinus sylvestris var. mongolica) in the Hulun Buir steppe, China, respond to climate in adjustment to the local water supply. Int. J. Biometeorol. 2015, 59, 1–10. [Google Scholar] [CrossRef]
- Li, Q.; Zhu, L.; Zeng, Y.; Huang, Y.; Ling, L.; Peng, L.; Chun, C. Differences in fruit quality between Jinqiu Shatangju tangerine (Citrus reticulata ‘Jinqiu Shatangju’) grafted on two types of rootstocks and the relationship with absorption, distribution, and utilization of nitrogen. Sci. Hortic. 2024, 328, 112926. [Google Scholar] [CrossRef]
- Wu, H.; Wang, L.; Liu, X.; Li, Q.; Lu, C.; Dong, W. Layered-Strip Fertilization Improves Nitrogen Use Efficiency by Enhancing Absorption and Suppressing Loss of Urea Nitrogen. Agronomy 2023, 13, 2428. [Google Scholar] [CrossRef]
- Zhao, L.; Jia, Z.; Li, G.; Zhang, T.; Wei, M. N Utilization, Residual and Loss Characteristics of Spring-Topdressing (15N-urea) Pear Orchards in the Old Course of the Yellow River Area. Agronomy 2022, 12, 2682. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, G.-l.; Ding, G.-d.; Zhang, Y.; Zhao, P.-s. Patterns and environmental drivers of C, N, and P stoichiometry in the leaf-litter-soil system associated with Mongolian pine forests. Ecol. Evol. 2024, 14, e11172. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, Q.; Zheng, L.-L.; Zeng, D.-H. Responses of nutrient resorption to interannual precipitation variability and nitrogen addition in a pine plantation. Ecosphere 2023, 14, e4395. [Google Scholar] [CrossRef]
- Zeng, D.H.; Hu, Y.L.; Chang, S.X.; Fan, Z.P. Land cover change effects on soil chemical and biological properties after planting Mongolian pine (Pinus sylvestris var. mongolica) in sandy lands in Keerqin, northeastern China. Plant Soil 2009, 317, 121–133. [Google Scholar] [CrossRef]
- Fan, Z.; Tu, Z.; Li, F.; Qin, Y.; Deng, D.; Zeng, D.; Sun, X.; Zhao, Q.; Hu, Y. Experimental Manipulation of Precipitation Affects Soil Nitrogen Availability in Semiarid Mongolian Pine (Pinus sylvestris var, mongolica) Plantation. Water 2017, 9, 208. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, Y.; Han, W.; Tang, A.; Shen, J.; Cui, Z.; Vitousek, P.; Erisman, J.W.; Goulding, K.; Christie, P.; et al. Enhanced nitrogen deposition over China. Nature 2013, 494, 459–462. [Google Scholar] [CrossRef]
- Xu, W.; Luo, X.S.; Pan, Y.P.; Zhang, L.; Tang, A.H.; Shen, J.L.; Zhang, Y.; Li, K.H.; Wu, Q.H.; Yang, D.W.; et al. Quantifying atmospheric nitrogen deposition through a nationwide monitoring network across China. Atmos. Chem. Phys. 2015, 15, 12345–12360. [Google Scholar] [CrossRef]
- Song, H.-H.; Yan, T.; Zeng, D.-H. Establishment of mixed plantations of Pinus sylvestris var. mongolica and Populus xiaozhuanica may not be appropriate: Evidence from litter decomposition. J. Plant Ecol. 2019, 12, 857–870. [Google Scholar] [CrossRef]
- Yu, J.; Fu, R.; Yu, Y.; Li, C.; Tao, X. Effects of nitrogen deposition on soil respiration and its sensitivity to temperature and humidity in a Quercus acutissima forest in northern subtropics. Chin. J. Ecol. 2021, 40, 1029–1037. (In Chinese) [Google Scholar]
- Zheng, L.; Zhao, Q.; Lin, G.; Hong, X.; Zeng, D. Nitrogen addition impacts on soil phosphorus transformations depending upon its influences on soil organic carbon and microbial biomass in temperate larch forests across northern China. CATENA 2023, 230, 107252. [Google Scholar] [CrossRef]
- Wu, Y.; Sun, M.; Zhao, Z.; Liang, Z.; Liu, J.; Liu, S. Uptake and Allocation of Newly Absorbed Nitrogen in Young Pear Trees Grafted onto Vigorous Rootstocks (Pyrus betulifolia). Agronomy 2022, 12, 2303. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, J.; Jiang, Y.; Hu, D.; Yang, X.; Dong, M.; Yu, K.; Yu, S. Effect of rhizosphere aeration by subsurface drip irrigation with tanks on the growth of ‘Red Globe’ grape seedling and its absorption, distribution and utilization of urea-15N. Sci. Hortic. 2018, 236, 207–213. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, H.; Zhao, H.; Tu, Y.; Song, H.; Sheng, J. Analysis of nutrient use efficiency in cotton fields with 15N isotope labeled nitrogen fertilizer. Xinjiang Agric. Sci. 2024, 61, 34–41. (In Chinese) [Google Scholar] [CrossRef]
- Ding, N.; Chen, Q.; Zhu, Z.; Peng, L.; Ge, S.; Jiang, Y. Effects of crop load on distribution and utilization of 13C and 15N and fruit quality for dwarf apple trees. Sci. Rep. 2017, 7, 14172. [Google Scholar] [CrossRef]
- Shi, J.; Xun, M.; Song, J.; Zhang, W.; Fan, W.; Yang, H. Regulation effects of carbonized apple branches on absorption, distribution, and utilization of 15N single-labeled ammonium nitrate 15NH4NO3 or NH415NO3 in Malus hupehensis. Plant Physiol. Biochem. 2022, 186, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Shi, Z.; Sun, Y.; Wang, X.; Yang, W.; Gao, J.; Wang, X. Stoichiometric Ratios of Carbon, Nitrogen and Phosphorus of Shrub Organs Vary with Mycorrhizal Type. Agriculture 2022, 12, 1061. [Google Scholar] [CrossRef]
- Temme, A.A.; Burns, V.A.; Donovan, L.A. Element content and distribution has limited, tolerance metric dependent, impact on salinity tolerance in cultivated sunflower (Helianthus annuus). Plant Direct 2020, 4, e00238. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Cisse, E.-H.M.; Miao, L.; Li, D.; Yang, F. Ecological stoichiometry and adaptations to drought and nitrogen in Hevea brasiliensis and Dalbergia odorifera in different planting patterns. Environ. Exp. Bot. 2024, 220, 105694. [Google Scholar] [CrossRef]
- Liu, R.; Yang, Y.; Wang, Y.-s.; Wang, X.-C.; Rengel, Z.; Zhang, W.-J.; Shu, L.-Z. Alternate partial root-zone drip irrigation with nitrogen fertigation promoted tomato growth, water and fertilizer-nitrogen use efficiency. Agric. Water Manag. 2020, 233, 106049. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, B.; Sun, X.; Yue, Y.; Ma, W.; Zhao, M. Soil Tillage Management Affects Maize Grain Yield by Regulating Spatial Distribution Coordination of Roots, Soil Moisture and Nitrogen Status. PLoS ONE 2015, 10, e0129231. [Google Scholar] [CrossRef]
- Shah, A.N.; Tanveer, M.; Shahzad, B.; Yang, G.; Fahad, S.; Ali, S.; Bukhari, M.A.; Tung, S.A.; Hafeez, A.; Souliyanonh, B. Soil compaction effects on soil health and crop productivity: An overview. Environ. Sci. Pollut. Res. 2017, 24, 10056–10067. [Google Scholar] [CrossRef]
- Schonbeck, L.; Li, M.-H.; Lehmann, M.M.; Rigling, A.; Schaub, M.; Hoch, G.; Kahmen, A.; Gessler, A. Soil nutrient availability alters tree carbon allocation dynamics during drought. Tree Physiol. 2021, 41, 697–707. [Google Scholar] [CrossRef]
- Bethea, F.G.; Park, D.; Mount, A.; Menchyk, N.; Liu, H. Effects of Acute Moisture Stress on Creeping Bentgrass Cuticle Morphology and Associated Effects on Foliar Nitrogen Uptake. HortScience 2014, 49, 1582–1587. [Google Scholar] [CrossRef]
- Ichir, L.L.; Ismaili, M.; Van Cleemput, O. Effect of organic and mineral fertilizers on N-use by wheat under different irrigation frequencies. Comptes Rendus Biol. 2003, 326, 391–399. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.; Li, Z.; Ye, R.; Chen, W.; Huang, Y.; Yuan, Y.; Zhang, Y.; Hu, H.; Zheng, P.; et al. Mitigating growth-stress tradeoffs via elevated TOR signaling in rice. Mol. Plant 2024, 17, 240–257. [Google Scholar] [CrossRef]
- Yang, C.; Xu, S.; Liu, L.; Huang, M.; Zheng, T.; Wei, S.; Zhang, Y.; Deng, G.; Jiang, L. Nitrogen Uptake and Utilization by No-Tillage Rice under Different Soil Moisture Conditions—A Model Study under Simulated Soil Conditions. Plant Prod. Sci. 2015, 18, 118–127. [Google Scholar] [CrossRef][Green Version]
- Wasko, C.; Nathan, R. The local dependency of precipitation on historical changes in temperature. Clim. Change 2019, 156, 105–120. [Google Scholar] [CrossRef]
- Liu, X.; Wang, G.; Li, J.; Wang, Q. Nitrogen isotope composition characteristics of modern plants and their variations along an altitudinal gradient in Dongling Mountain in Beijing. Sci. China Ser. D Earth Sci. 2010, 53, 128–140. [Google Scholar] [CrossRef]
- Henriksson, N.; Lim, H.; Marshall, J.; Franklin, O.; McMurtrie, R.E.; Lutter, R.; Magh, R.; Lundmark, T.; Näsholm, T. Tree water uptake enhances nitrogen acquisition in a fertilized boreal forest—But not under nitrogen-poor conditions. New Phytol. 2021, 232, 113–122. [Google Scholar] [CrossRef]
- Song, H.; Yu, W.; Wang, L.; Jiao, W.; Dong, B. Influence of nitrogen input and sediment burial on biomass and nitrogen absorption characteristic of Suaeda salsa in the Yellow River estuary. Environ. Earth Sci. 2022, 81, 493. [Google Scholar] [CrossRef]
- Chen, C.; Li, J.; Wang, G.; Shi, M. Accounting for the effect of temperature in clarifying the response of foliar nitrogen isotope ratios to atmospheric nitrogen deposition. Sci. Total Environ. 2017, 609, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, L.; Li, Y. Ecological Stoichiometry within Plant Organs of Four Forest Trees in Sygera National Forest. Bioresources 2024, 19, 210–227. [Google Scholar] [CrossRef]
- Prieto, I.N.; Armas, C.; Pugnaire, F.I. Hydraulic lift promotes selective root foraging in nutrient-rich soil patches. Funct. Plant Biol. 2012, 39, 804–812. [Google Scholar] [CrossRef]
- Tang, B.; Yin, C.; Yang, H.; Sun, Y.; Liu, Q. The coupling effects of water deficit and nitrogen supply on photosynthesis, WUE, and stable isotope composition in Picea asperata. Acta Physiol. Plant. 2017, 39, 148. [Google Scholar] [CrossRef]
- Song, X.; Wan, F.; Chang, X.; Zhang, J.; Sun, M.; Liu, Y. Effects of Nutrient Deficiency on Root Morphology and Nutrient Allocation in Pistacia chinensis Bunge Seedlings. Forests 2019, 10, 1035. [Google Scholar] [CrossRef]
- Liu, J.; Gou, X.; Wang, F.; Zhang, F.; Zhang, J.; Xia, J.; Wang, Y. Nutrient allocation strategies of four conifers from semiarid to extremely arid environments. Plant Physiol. Biochem. 2022, 186, 257–265. [Google Scholar] [CrossRef]
- Kimmel, K.; Furey, G.N.; Hobbie, S.E.; Isbell, F.; Tilman, D.; Reich, P.B. Diversity-dependent soil acidification under nitrogen enrichment constrains biomass productivity. Glob. Change Biol. 2020, 26, 6594–6603. [Google Scholar] [CrossRef] [PubMed]
- Burnham, M.B.; Cumming, J.R.; Adams, M.B.; Peterjohn, W.T. Soluble soil aluminum alters the relative uptake of mineral nitrogen forms by six mature temperate broadleaf tree species: Possible implications for watershed nitrate retention. Oecologia 2017, 185, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Griffin-Nolan, R.J.; Bensaddek, L.; Decocq, G.; Hikosaka, K.; Kichey, T.; LeVonne, J.; Mishio, M.; Fridley, J. Away-range shifts in leaf function of a global invader: A case of resource reallocation? Biol. Invasions 2024, 26, 1489–1503. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, C.; Zheng, D.; Wang, E.; Liang, J.; Yin, C. Nitrogen uptake preference and allocation in Populus cathayana in response to drought stress. Environ. Exp. Bot. 2023, 213, 105415. [Google Scholar] [CrossRef]
- Li, X.; Li, M.; Xu, L.; Liu, C.; Zhao, W.; Cheng, C.; He, N. Allometry and Distribution of Nitrogen in Natural Plant Communities of the Tibetan Plateau. Front. Plant Sci. 2022, 13, 845813. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).